Abstract
Although STAT1 tyrosine-701 phosphorylation (designated STAT1-pY701) is indispensable for STAT1 function, the requirement for STAT1 serine-727 phosphorylation (designated STAT1-pS727) during systemic autoimmune and anti-pathogen responses remains unclear. Using autoimmune-prone B6.Sle1b mice expressing a STAT1-S727A mutant in which serine is replaced by alanine, we report here that STAT1-pS727 promotes autoimmune antibody-forming cell (autoimmune AFC) and germinal center (GC) responses, driving autoantibody production and systemic lupus erythematosus (SLE) development. In contrast, STAT1-pS727 is not required for GC, follicular helper T (Tfh) cell and antibody responses to various foreign-antigens including pathogens. STAT1-pS727 is also not required for gut microbiota and dietary antigen-driven GC and Tfh responses in B6.Sle1b mice. By generating B cell-specific bone marrow (BM) chimeras, we demonstrate that STAT1-pS727 plays an important B cell-intrinsic role in promoting autoimmune AFC, GC and Tfh responses, leading to SLE-associated autoantibody production. Our analysis of TLR7-accelerated B6.Sle1b.Yaa SLE disease model expressing a STAT1-S727A mutant reveals STAT1-pS727-mediated regulation of autoimmune AFC and GC responses, and lupus nephritis (LN) development. Together, we identify previously unrecognized differential regulation of systemic autoimmune and anti-pathogen responses by STAT1-pS727. Our data implicate STAT1-pS727 as a therapeutic target for SLE without overtly affecting STAT1-mediated protection against pathogenic infections.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a debilitating autoimmune disease characterized by the production of high-affinity antinuclear antibodies (ANA). SLE is associated with the loss of peripheral B cell tolerance in the extra-follicular antibody-forming cell (AFC) and follicular germinal center (GC) pathways (1-3). However, mechanisms by which altered AFC and GC responses lead to the development of autoreactive B cells, ANAs and SLE are incompletely understood. Identifying the specific signals that are critical for SLE-associated AFC and GC responses may inform the development of targeted therapies for SLE.
In addition to the cognate interactions between B cells and T helper cells, cytokines control the development of autoantibody (autoAb)-producing B cells. Previous studies have demonstrated the requirement of B cell-intrinsic type I (T1IFN) and II (IFNγ) interferon (IFN) signaling in SLE-associated AFC and GC responses (4-6). Both T1IFN and IFNγ that contribute to SLE development signal through the transcription factor signal transducer and activator of transcription 1 (STAT1). STAT1 was previously shown to play a critical role in the development of spontaneous GCs (Spt-GCs), T follicular helper cells (Tfh) and IgG autoAbs (5, 7). Thus, targeting STAT1 downstream of IFN signaling could be an attractive treatment strategy for SLE. However, deficiency of STAT1 in humans and mice results in impaired immune responses and an increased susceptibility to infections (8, 9). Therefore, it is crucial to develop highly specific therapeutics for STAT1 that would eliminate autoreactive B cells without overtly affecting STAT1-mediated anti-pathogen immunity.
Activation of interferon receptors triggers STAT1 phosphorylation at tyrosine 701 (STAT1-pY701) by receptor-associated JAK tyrosine kinases, causing STAT1 dimerization and nuclear translocation. However, for optimal transcriptional activity, STAT1 also needs to be phosphorylated at serine 727 (STAT1-pS727) in its C-terminal transactivation domain (TAD) (10-12). Serine phosphorylation of the STAT1 TAD, either induced by cytoplasmic or nuclear kinases (13, 14), regulates STAT1 function by allowing the recruitment of additional transcriptional coactivators to the promoters of STAT1 target genes (10-12). By expressing a STAT1-S727A mutant, in which serine is replaced by alanine, previous in vitro studies in macrophages have shown that serine phosphorylation of the STAT1 TAD (STAT1-pS727) is important for RNA polymerase II (RNAP II or Pol II) recruitment to the promoters of STAT1 target genes and the consequent regulation of 40-50% IFNγ-induced genes in response to IFNγ stimulation (14). The role of STAT1-pS727 in eliciting innate immune responses has also been described previously (10). However, the requirement of STAT1-pS727 in anti-pathogen AFC, GC and Ab responses is not clear. Moreover, the role of STAT1-pS727 in autoimmune AFC and GC responses, autoAb production and SLE pathogenesis is not known.
By crossing B6.STAT1-S727A (STAT1-SA) mutant mice (10) to the autoimmune-prone B6.Sle1b mouse model that develops a moderate level of autoimmunity without significant disease manifestations, we demonstrate a crucial role of STAT1-pS727 in autoimmune AFC and GC responses, and autoAb production (15, 16). We also observe an important B cell-specific function of STAT1-pS727 in promoting these autoimmune processes. These reduced autoimmune responses in SLE-prone B6.Sle1b mice in the absence of STAT1-S727 phosphorylation are not due to a defect in primary bone marrow and splenic B cell development in STAT1-S727A mutant mice. By analyzing the Toll like Receptor 7 (TLR7)-promoted SLE disease model expressing a STAT1-S727A mutant, we observe significantly reduced SLE-associated AFC, GC and autoAb responses, and ameliorated kidney pathology. STAT1-pS727, however, is not necessary for GC and Tfh responses to foreign-antigens, including NP-KLH, virus like particles (VLPs) and mouse polyomavirus infection. Interestingly, STAT1-pS727 is also not required for GC and Tfh responses to gut microbiota and dietary antigens. These data suggest a differential regulation of autoimmune and anti-pathogen responses by STAT1-pS727, and identify STAT1-pS727 as a potential therapeutic target for SLE that does not overtly compromise the protective immunity to pathogens in SLE patients.
MATERIALS AND METHODS
Mice
C57BL/6J (B6), B6.SB-Yaa/J (B6.yaa) and B6.129S2-Ighmtm1Cgn/J (μMT) mice were originally purchased from the Jackson Laboratory and bred in house. The B6.Sle1b mice (congenic for the Sle1b sublocus) were described previously (15). B6.Sle1b.yaa (designated Sle1byaa) mice were generated by crossing B6.Yaa mice to B6.Sle1b mice. Previously described B6.STAT1-S727A (B6.129P2-Stat1tm1Tdec) mice (10) were crossed to B6.Sle1b and B6.Sle1b.yaa background to generate B6.Sle1b.STAT1-S727A (Sle1b.STAT1-SA) and B6.Sle1byaa.STAT1-S727A (Sle1byaa.STAT1-SA) mice. All animal studies were conducted at Pennsylvania State University Hershey Medical Center in accordance with the guidelines approved by our Institutional Animal Care and Use Committee. Animals were housed in a specific pathogen free barrier facility.
Imiquimod treatment, viral infection and immunization
For epicutaneous imiquimod treatment, 5% imiquimod cream (Glenmark Pharmaceuticals) was applied on the ears of mice, 3 times weekly for 4-12 wks based on the experimental design as previously described (17-19). To study the systemic autoimmune responses, such as AFC, GC, Tfh and antibody responses, mice were treated for 8 wks. For viral infection, 10-12 wk old mice were inoculated intracerebrally (i.c.) with 3x105 pfu of mouse polyomavirus (MuPyV) strain and analyzed 12d post-infection. For immunization studies, 10-12 wk old mice were immunized with 200μg/mouse of NP-KLH (Biosearch Technologies) intraperitoneally (i.p) in Complete Freund’s Adjuvant (Sigma Aldrich) followed by immunization with 100μg of NP-KLH in incomplete Freund’s adjuvant on day 7. Spleen cells were prepared from these mice and analyzed on 14d post primary immunization. 10-12 wk old mice were immunized (i.p) with 25 μg of purified Qβ-VLPs in 250 μl of PBS as previously described (20) and spleen cells were analyzed 10d post-immunization.
Flow Cytometry
Flow cytometric analysis of total mouse splenocytes or bone marrow cells was performed using the following antibodies: B220-BV605 (RA3-6B2), CD4-AF700 (RMP4-5), CD44-APC (IM7), CD62L-PECy7 (MEK-14), PD1-PE (29F.1A12), IgM-BV605 (RMM-1), IgD-BV711 (11-26c2a), CD93-PE (AA4.1), Streptavidin-PECy5, MHCII-PECy7 (M5/114.15.2), Ly51-biotin (6C3), CD24-APC (M1/69), CD23-biotin (B3H4). GL7-FITC (GL-7), CD95- PeCy7, CXCR5-biotin (2G8), CD43-FITC (S7), CD19-biotin (1D3), CD90.2-biotin (53-2.1), CD11b-AF700 (M1/70), CD11c-FITC (HL3) (BD Biosciences). CD8α (clone 53–6.7) (eBioscience). All cells were stained with fixable viability dye-eFluor780 (Invitrogen) prior to surface staining. Stained cells were analyzed using the BD LSR II flow cytometer (BD Biosciences). Data were acquired using FACSDiva software (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Immunofluorescence and ANA staining
Mouse spleens or kidneys were embedded in OCT compound and snap frozen over liquid nitrogen. 5μm thin sections were cut on a cryostat, mounted on ColorFrost plus microscope slides (Fisher Scientific, PA) and fixed in cold acetone for 20 min. The following antibodies and reagents were utilized for immunofluorescence staining of mouse spleen sections for germinal centers: PE-anti-CD4 (GK1.5, Biolegend); FITC-GL7 (RA3-6B2, BD Biosciences); APC-anti-IgD (11-26c2a, BD Biosciences). Kidney sections were stained for C3 using FITC-anti-C3 (ICL Laboratories) or Biotin-anti-IgG (Jackson Immunoresearch) followed by SA-PE. Anti-nuclear Ab (ANA) reactivity was detected by indirect immunofluorescence staining of HEp-2 cell slides using sera from indicated mice at a 1:50 dilution, and probed with FITC-rat anti-mouse Kappa (H139-52.1). The images of stained spleen and kidney sections were captured using the Leica DM4000 fluorescence microscope and analyzed using a Leica application suite-advanced fluorescence software (LAS-AF, Leica Microsystems). For the measurement of the GC area randomly selected germinal centers (GL-7+) were measured for total area (μm2) using the LAS-AF quantitation tool.
Kidney histopathology
Kidneys from 6 mo old mice were fixed in 10% neutral buffered formalin and embedded in paraffin. Kidney sections were cut at 3 μm and 6 μm thickness for Periodic Acid-Schiff (PAS) and H&E staining, respectively. All images were obtained with an Olympus BX51 microscope and DP71 digital camera using cellSens Standard 1.12 imaging software (Olympus America, Center Valley, PA). Two pathologists blinded to the genotype of mice evaluated the kidney sections. One kidney section per mouse was evaluated: each glomerulus was examined at 400x magnification and scored from 0 (normal) to 4 (severe) based on glomerular size and lobulation, presence of karyorrhectic nuclear debris, capillary basement membrane thickening, and the degree of mesangial matrix expansion and mesangial cell proliferation (21). H&E sections were scored for overall glomerular damage and interstitial inflammation as described (22).
ELISpot assay
ELISpot assays were performed as previously described (5, 6). Briefly, splenocytes in RPMI containing 10% fetal bovine serum were plated at a concentration of 1 x 106 cells/well onto salmon sperm dsDNA- (Invitrogen), nucleosome- (histone from Sigma Aldrich plated on a layer of dsDNA coating)- or SmRNP- (Arotec Diagnostics) coated multiscreen 96-well filtration plates (Millipore, Bedford, MA). Serially diluted (1:2) cells were incubated for 12 h at 37°C. dsDNA-, nucleosome-, and smRNP-specific AFCs were detected by biotinylated anti-kappa Ab (Invitrogen) and streptavidin (SA)-alkaline phosphatase (Vector Laboratories) or alkaline phosphatase-conjugated anti-mouse IgG (Molecular Probes). Plates were developed using the Vector Blue Alkaline phosphatase Substrate Kit III (Vector Laboratories). ELISpots were enumerated and analyzed using a computerized ELISpot plate imaging/ analysis system (Cellular Technology).
ELISA
Serum autoAbs were measured using standard ELISA protocols as described (16). Briefly, total IgG autoAb titers were measured in ELISA plates coated with salmon sperm dsDNA, nucleosome or smRNP and detected with biotinylated secondary Ab followed by streptavidin (SA)-alkaline phosphatase (Vector Laboratories). Plates were developed using PNPP (p-Nitrophenyl Phosphate, Disodium Salt) (Thermo Fisher Scientific) substrates for alkaline phosphatase and read at λ405 nm on Synergy H1 (BioTek Instruments).
Generation of mixed bone marrow (BM) chimeric mice
12 wk old female B6.μMT mice were lethally irradiated with two doses of 450 rads of x-rays (X-RAD 320iX Research Irradiator; Precision X-Ray) within a 4h interval. Within few hours of the second irradiation, each B6.μMT recipient mice received intravenously (tail vein) 10x106 T cell-depleted BM cells isolated from 10 wk old female donor mice with 80% of cells from B6.μMT mice and 20% from B6.Sle1b or Sle1b.STAT1-SA mice. Recipients were analyzed for spontaneous GC B cell and Tfh cell development, ANA-specific AFC and autoAb responses 11 wks after BM cell transfer.
Statistical analysis
P values were calculated using unpaired, nonparametric, Mann–Whitney, Student’s t-test (for comparison of two groups) or one-way or two-way ANOVA, with a follow-up Tukey multiple-comparison test (for comparison of more than two groups) or two-way ANOVA, with a follow-up Sidak multiple-comparison test (for comparison of two groups). Ns = non-significant, * P < 0.05, ** P <0.01, *** P <0.001 and **** P <0.0001. GraphPad Prism 6 software was used.
RESULTS
STAT1-pS727 promotes autoimmune AFC and GC responses
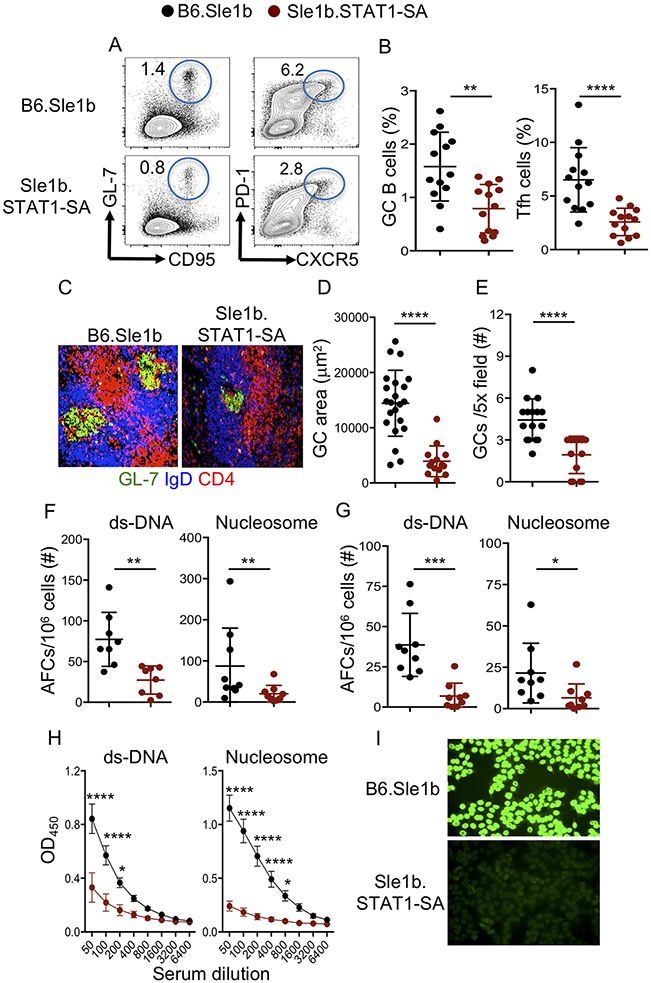
Although the role of STAT1 tyrosine 701 phosphorylation (designated STAT1-pY701) in nuclear translocation and transcriptional activity is well described, how STAT1 serine 727 phosphorylation (designated STAT1-pS727) may regulate STAT1 function in the in vivo systems has been underexplored. Especially, the involvement of STAT1-pS727 in the regulation of autoimmune AFC, GC, Tfh and autoantibody (autoAb) responses is not known. To determine the role of STAT1-pS727 in autoimmune AFC, GC and Tfh responses, we crossed SLE-prone B6.Sle1b mice to B6.STAT1-S727A mutant mice (10) to generate B6.Sle1b.STAT1-S727A mice (designated Sle1b.STAT1-SA) in which serine 727 in STAT1 is replaced with alanine. Spontaneous autoimmune responses assessed in 5-6 mo old Sle1b.STAT1-SA female mice showed a reduced frequency of GC B cells and Tfh cells (Figure 1A-B), and reduced size and number of splenic GCs (Figure 1C-E) compared to B6.Sle1b control mice. Sle1b.STAT1-SA mice also had a reduced number of ds-DNA and nucleosome-specific splenic (Figure 1F) and bone marrow (BM, Figure 1G) AFCs that strongly correlated with reduced serum autoAb titers and ANA-seropositivity (Figure 1H-I). Together, these data highlight the critical role of STAT1-pS727 in autoreactive B cell differentiation into AFCs and GC B cells and autoAb production.
Figure 1. STAT1-pS727 regulation of autoimmune GC, AFC and autoAb responses.
Representative flow cytometry plots (A) and the percentage (B) of splenic B220+GL7hiCD95hi GC B cells of total B220+ B cells and CD4+CD44hiPD-1hiCXCR5hi Tfh cells of total CD4+ T cells. Representative histological images show GCs (C), quantification of GC area (D) and GC frequency (E) in spleens. Quantification of splenic (F) and BM (G) dsDNA- and nucleosome-specific AFCs by ELIspot. (H) dsDNA- and nucleosome-specific IgG Ab titers were measured by ELISA. (I) Representative images of serum ANA reactivity measured by Hep-2 clinical assay. The data shown were the cumulative results of three independent experiments generated from 5-6 mo old B6.Sle1b and Sle1b.STAT1-SA female mice. 3-5 mice were analyzed in each experiment. Each symbol indicates an individual mouse (B, F-G) or GC (D, E). Statistical analysis was performed by two-way ANOVA, with a follow-up Sidak multiple-comparison test (H) or unpaired, nonparametric Mann-Whitney Student’s t-test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Reduced autoimmune responses in STAT1-S727A mutant mice are not due to a defect in primary B cell development
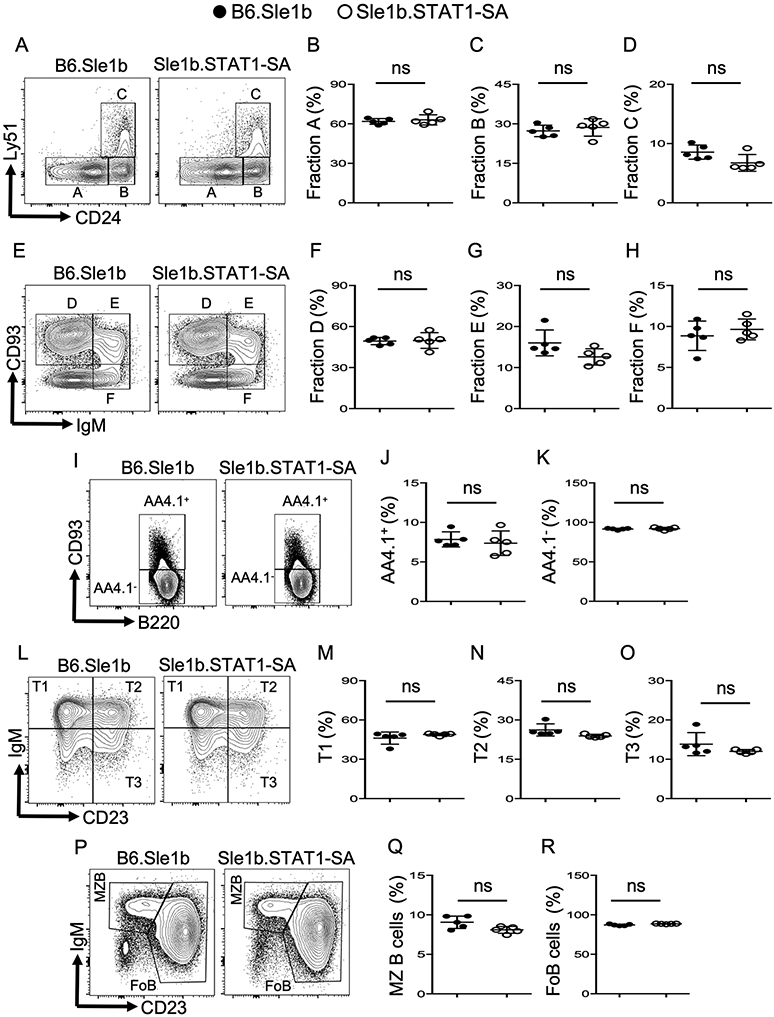
Next, we assessed whether the reduced autoimmune GC and AFC responses in Sle1b.STAT1-SA mice were due to defects in primary B cell development in the bone marrow (BM) and peripheral secondary lymphoid organs. We analyzed subpopulations of the early B cell progenitors such as B220+CD43+HSA−BP-1− fraction A, B220+CD43+HSA+BP-1− fraction B, B220+CD43+HSA+BP-1+ fraction C, B220+CD43−IgM−CD93+ fraction D, B220+CD43−IgM+CD93+ fraction E, and B220+CD43−IgM+CD93− fraction F in BM and found no significant differences in fractions A to F between Sle1b.STAT1-SA and B6.Sle1b control mice (Figure 2A-H). We also analyzed peripheral B cell developmental stages in spleens including B220+AA4.1+CD23−IgM+ transitional type 1- T1, B220+AA4.1+CD23+IgM+ T2, and B220+AA4.1+CD23+IgM− T3 cells, B220+AA4.1-CD93−CD23−IgM+ marginal zone (MZ) B cells and B220+AA4.1-CD93−CD23+IgM+ mature/follicular B cells (Figure 2I-R). We observed no significant differences in splenic B cell development between Sle1b.STAT1-SA and B6.Sle1b control mice (Figure 2I-R), indicating that STAT1-pS727 is not required for either BM or peripheral B cell development. These data suggest that reduced autoimmune responses in SLE-prone B6.Sle1b mice in the absence of STAT1-S727 phosphorylation are not due to a defect in primary B cell development in STAT1-S727A mutant mice.
Figure 2. Mice expressing STAT1-SA mutant have normal B cell development.
Flow cytometry analysis showing gating strategies (A, E, I, L, P) and percentages of B cell developmental fraction A (B220+CD43+HSA−BP-1−) (A, B), fraction B (B220+CD43+HSA+BP-1−) (A, C), fraction C (B220+CD43+HSA+BP-1+) (A, D), fraction D (B220+CD43−IgM−CD93+) (E, F), fraction E (B220+CD43−IgM+CD93+) (E, G), and fraction F (B220+CD43−IgM+CD93−) (E, H) of live cell gated BM cells from B6.Sle1b and Sle1b.STAT1-SA mice. Splenocytes from the same mice were characterized for peripheral B cell developmental stages in spleens including AA4.1+ (I, J), AA4.1- (I, K), transitional type 1- T1 (B220+AA4.1+CD23−IgM+) (L, M), T2 (B220+AA4.1+CD23+IgM+) (L, N), and T3 (B220+AA4.1+CD23+IgM−) (L, O), marginal zone (MZ) B cells (B220+CD93−CD23−IgM+) (P, Q) and mature/follicular B cells (FoB) (B220+CD93−CD23+IgM+) (P, R) of total B220+ B cells. These data are representative of two independent experiments (4-5 mice in each experiment). Each symbol in each panel represents a mouse. Statistical analysis was performed by unpaired, nonparametric Mann-Whitney Student’s t-test. ns, non-significant.
STAT1-pS727 is dispensable for foreign antigen, gut microbiota or dietary antigen-driven GC, Tfh and Ab responses
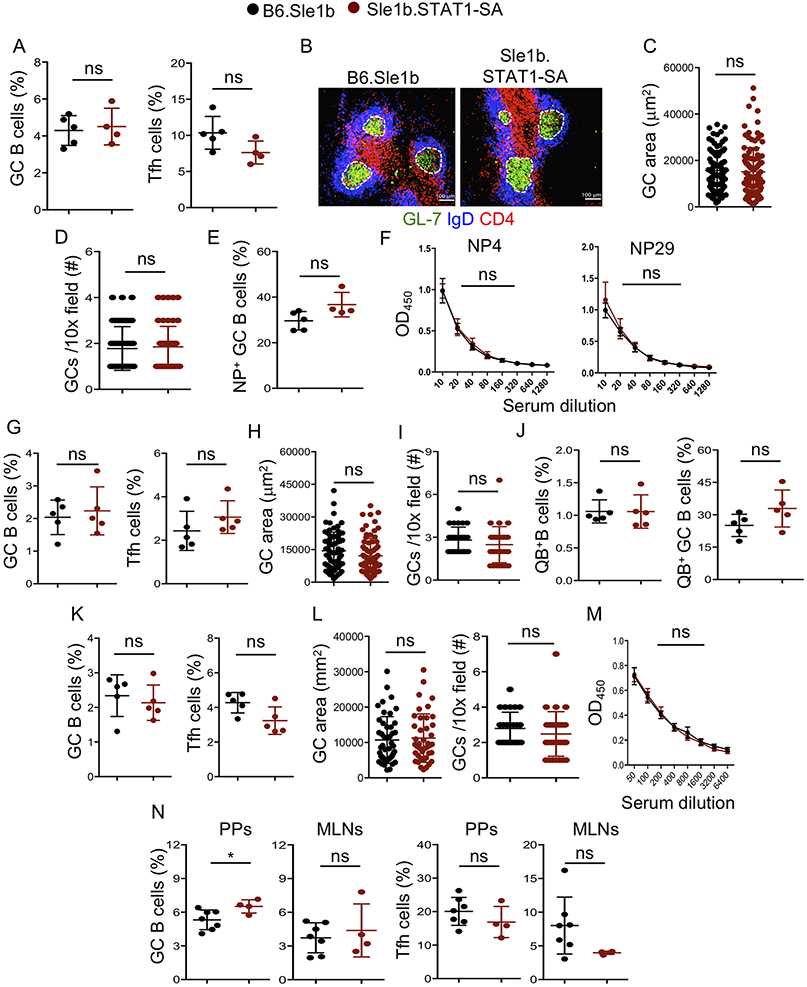
Given the impact of STAT-pS727 on the regulation of autoimmune responses, we investigated the requirement of STAT-pS727 in immune responses to foreign antigens including viral infection. We first immunized mice with the T cell-dependent antigen, NP-KLH. Surprisingly, GC and Tfh responses were similar between Sle1b.STAT1-SA and B6.Sle1b control mice 14d post-immunization (Figure 3A). The GC size, GC number and NP-specific GC B cell frequency were also comparable between the strains (Figure 3B-E). Likewise, we observed comparable high (NP4) and low (NP29) affinity NP-specific Ab responses between Sle1b.STAT1-SA and control mice (Figure 3F). B cells have recently been shown to be the dominant antigen presenting cells (APCs) involved in the induction of Tfh differentiation in virus derived nanoparticle immunization (23). Thus, next we analyzed B cell responses to virus like particles (VLP). Upon VLP immunization, Sle1b.STAT1-SA mice showed identical GC and Tfh frequencies compared to control mice (Figure 3G-l). The VLP specific (QB+) total and GC B cells were also similar between the groups (Figure 3J). To further explore B cell responses to a pathogen, we utilized an infection model, where we infected mice intracerebrally with mouse polyoma virus (muPyV). muPyV also induced comparable GC and Tfh responses in both the groups (Figure 3K-L). Virus specific Ab titers (Figure 3M), cytokine producing and virus specific effector CD8+ T cells, and viral titers in spleen and brain were similar between the groups (not shown). Additionally, gut microbiota and dietary antigen-driven GC and Tfh responses in Peyer’s patches (PPs) and mesenteric LNs (MLNs) in Sle1b.STAT1-SA mice were comparable to B6.Sle1b control groups (Figure 3N). Together, these data utilizing multiple models of foreign antigenic challenge or gut microbiota highlight no significant role for STAT-pS727 in foreign antigen-, gut microbiota- or dietary antigen-driven GC B cell, Tfh and Ab responses.
Figure 3. STAT1-pS727 deficiency does not alter immune response to T dependent antigens, VLPs and viral infection.
(A-F) These data were generated from spleens of NP-KLH immunized B6.Sle1b and Sle1b.STAT1-SA female mice. (A) Quantification of B220+GL7hiCD95hi GC B cells and CD4+CD44hiPD-1hiCXCR5hi Tfh cells that were gated on total B220+ B and CD4+ T cells, respectively. Representative histological images show GCs (B), GC area (C) and GC frequency (D) in spleen sections. (E) Percentage of NP18-specific B220+GL7hiCD95hi GC B cells that were gated on total B220+ B cells. (F) NP4- and NP29-specific IgG titers were measured by ELISA. (G-J) These data were derived from VLP immunized B6.Sle1b and Sle1b.STAT1-SA female mice. (G) Flow cytometry data depicts frequency of splenic B220+GL7hiCD95hi GC B cells and CD4+CD44hiPD-1hiCXCR5hi Tfh cells that were gated on total B220+ B and CD4+ T cells, respectively. Quantification of GC area (H) and GC frequency (I) in spleen sections. (J) Percentage of splenic VLP-specific Qβ+ B and GC B cells. (K-M) These data were derived from mouse polyoma virus (mPyV) infected B6.Sle1b and Sle1b.STAT1-SA female mice. (K) Frequency of splenic B220+GL7hiCD95hi GC B cells and CD4+CD44hiPD-1hiCXCR5hi Tfh cells. (L) Quantification of GC area and GC frequency in spleen sections. (M) VP1 specific IgG titers measured by ELISA. (N) Quantitation of GC B cells and Tfh cells from total B220+ B and CD4+ T cells, respectively, in Peyer’s patches (PPs) and mesenteric lymph nodes (MLNs) in unimmunized mice. These data represent 2-3 experiments and each symbol indicates an individual mouse or GC. 3-5 mice were analyzed in each experiment. Statistical analysis was performed by two-way ANOVA, with a follow-up Sidak multiple-comparison test (F, M) or unpaired, nonparametric Mann-Whitney Student’s t-test. ns, non-significant; *, P < 0.05.
B cell-intrinsic STAT1-pS727 regulates autoimmune AFC, GC and Tfh responses
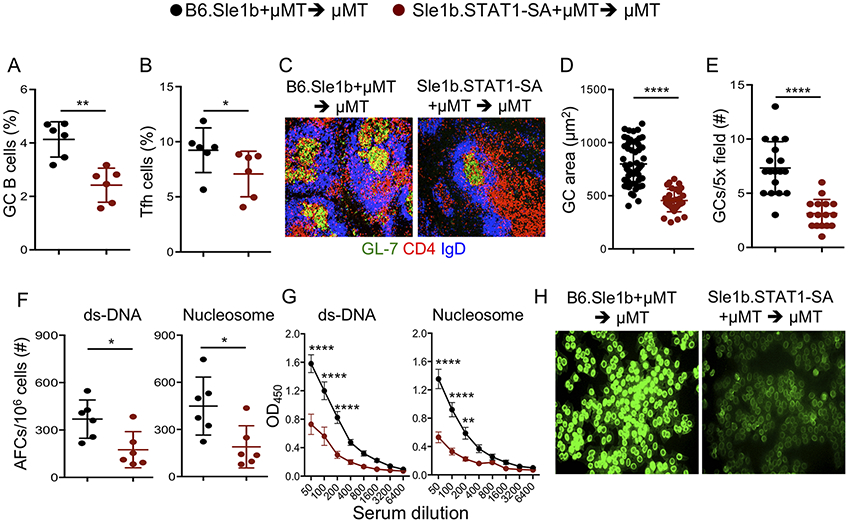
Next, to define the B cell-intrinsic role of STAT1-pS727 in regulating autoimmune AFC, GC and Tfh responses, we generated mixed bone marrow (BM) chimeras by reconstituting lethally irradiated B6.μMT mice, which lack mature B cells, with a mixture of BM cells from B6.μMT mice and B6.Sle1b or Sle1b.STAT1-SA mice as described (4, 5). Analysis of chimeras post-BM transfer revealed a significantly lower percentage of GC B cells and Tfh in Sle1b.STAT1-SA +μMT > μMT mice than B6.Sle1b +μMT > μMT control mice (Figure 4A, B). Sle1b.STAT1-SA +μMT > μMT mice had a lower frequency of and smaller GCs than those in B6.Sle1b +μMT > μMT control mice (Figure 4C-E). Sle1b.STAT1-SA +μMT > μMT mice had significantly decreased autoAb-producing AFCs (Figure 4F), serum autoAb titers (Figure 4G) and ANA seropositivity (Figure 4H) than B6.Sle1b +μMT > μMT control chimeras. These data demonstrate an important B cell-intrinsic role for STAT1-pS727 in the regulation of autoimmune AFC, GC and Tfh responses.
Figure 4. B cell-intrinsic role of STAT1-pS727 in autoimmune AFC, GC and Tfh responses.
Quantitation of B220+GL7hiCD95hi GC B cells gated on total B220+ B cells (A) and CD4+CD44hiPD-1hiCXCR5hi Tfh cells gated on total CD4+ T cells (B) in splenocytes isolated from BM chimeric B6.Sle1b + μMT > μMT and Sle1b.STAT1-SA + μMT > μMT female mice 10 wk post BM cell transfer. Representative histological images of splenic GCs (C) and GC area (D) and frequency (E) were quantified. dsDNA- and nucleosome-specific splenic AFCs (F), serum dsDNA- and nucleosome-specific Abs (G), and serum ANA reactivity (H) are shown. Each symbol represents a mouse (A, B, F) or a GC (D, E). These data represent one experiment with 6 recipient mice per group. Statistical analysis was performed by two-way ANOVA, with a follow-up Sidak multiple-comparison test (G) or unpaired, nonparametric Mann-Whitney Student’s t-test. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
STAT1-pS727 regulates TLR7-accelerated autoimmune AFC, GC and autoantibody responses
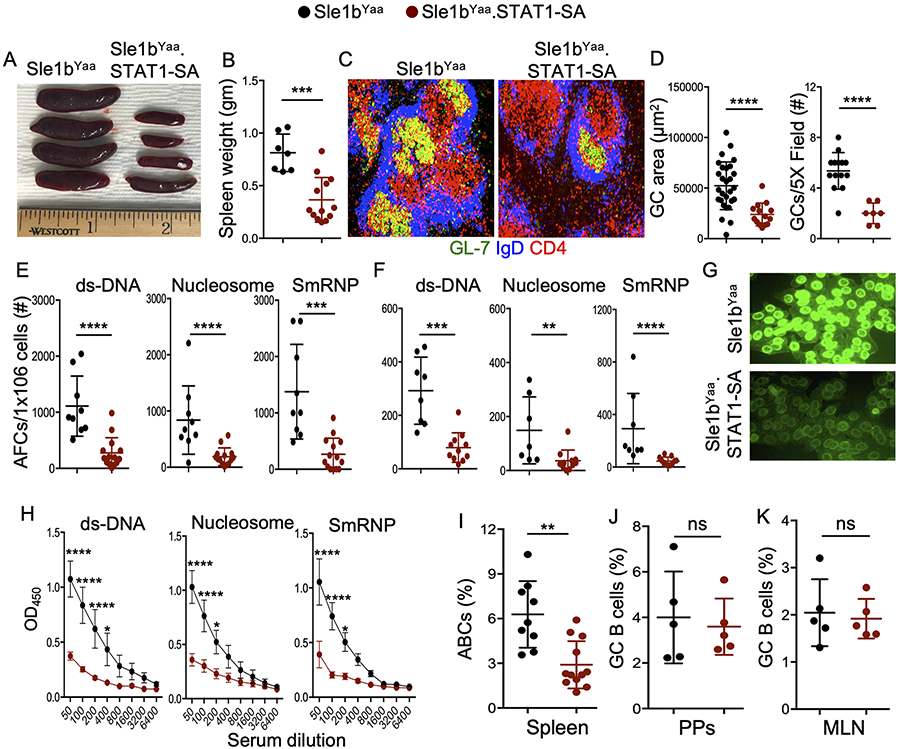
Having demonstrated the requirement of STAT1-pS727 in regulating moderate levels of autoimmune responses in B6.Sle1b mice which do not develop SLE disease, we investigated the role of STAT1-pS727 in TLR7-accelerated autoimmune AFC and GC responses in B6.Sle1b.Yaa SLE disease model. We crossed Sle1b.STAT1-SA mice to the B6.Sle1b.Yaa (Sle1bYaa) SLE disease model in which male mice overexpress TLR7 due to a translocation of a section of the X chromosome containing the Tlr7 gene (Sle1bYaa.STAT1-SA) (24-26). Male Sle1bYaa.STAT1-SA mice showed reduced splenomegaly compared to Sle1bYaa control mice (Figure 5A, B). The reduction in splenomegaly was accompanied by a lower frequency of and reduced size of GCs (Figure 5C, D) in Sle1bYaa.STAT1-SA mice compared to Sle1bYaa control mice. The number of autoAb-producing splenic (Figure 5E) and BM (Figure 5F) AFCs, serum ANA reactivity (Figure 5G), and autoAb titers (Figure 5H) in Sle1bYaa.STAT1-SA mice were also much lower than Sle1bYaa control mice.
Figure 5. STAT1-pS727 promotes TLR7-accelerated systemic autoimmune responses and lupus nephritis.
Representative spleen size (A) and weight of spleens (B) from 6 mo old Sle1bYaa and Sle1bYaa.STAT1-SA male mice. Representative histological images of splenic GCs (C), and quantified GC area and their frequency (D) are shown. Numbers of dsDNA-, nucleosome- and SmRNP-specific splenic (E) and BM (F) AFCs; ANA reactivity (G); serum Abs against ds-DNA, nucleosome and SmRNP (H) were measured by ELISA. The percentages of splenic B220+CD11b+CD11c+ ABCs (I); GC B cells in Peyer’s patches (J) and mesenteric lymph nodes (K) that were pre-gated on total B220+ B cells from 6 mo old mice were measured by flow cytometry analysis. These data represent 2-4 experiments and each symbol indicates an individual mouse (B, E-N) or a GC (D). 4-5 mice were analyzed in each experiment. Statistical analysis was performed by two-way ANOVA, with a follow-up Sidak multiple-comparison test (h) or unpaired, nonparametric Mann-Whitney Student’s t-test. Ns, non-significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
TLR7-driven accumulation of age associated B cells (ABCs) has recently been shown to be important for the development of SLE-like autoimmunity (27). Interestingly, we observed a reduced percentage of ABCs in Sle1bYaa.STAT1-SA mice compared to control mice (Figure 5I). However, the gut microbiota and dietary antigen-driven GC (Figure 5J, K) and Tfh (not shown) responses in Peyer’s patches and mesenteric lymph nodes of Sle1bYaa.STAT1-SA mice were similar to Sle1bYaa control mice. To further validate our findings, we utilized the TLR7 ligand imiquimod (IMQ) treatment model, in which female mice were treated epicutaneously to accelerate autoimmune responses in the B6.Sle1b model following a protocol previously described (17-19). We found reduced splenomegaly (Figure S1A, B), and significantly lower number of dsDNA- and nucleosome-specific splenic (Figure S1C) and BM (Figure S1D) AFCs in Sle1b.STAT1-SA mice than IMQ-treated B6.Sle1b control mice. IMQ-treated Sle1b.STAT1-SA mice also had reduced serum autoAb titers (Figure S1E) than treated B6.Sle1b control mice. These findings together demonstrate that STAT1-pS727 regulates TLR7-accelerated autoimmune AFC and GC responses, and autoantibody production.
STAT1-pS727 promotes TLR7-accelerated SLE pathogenesis
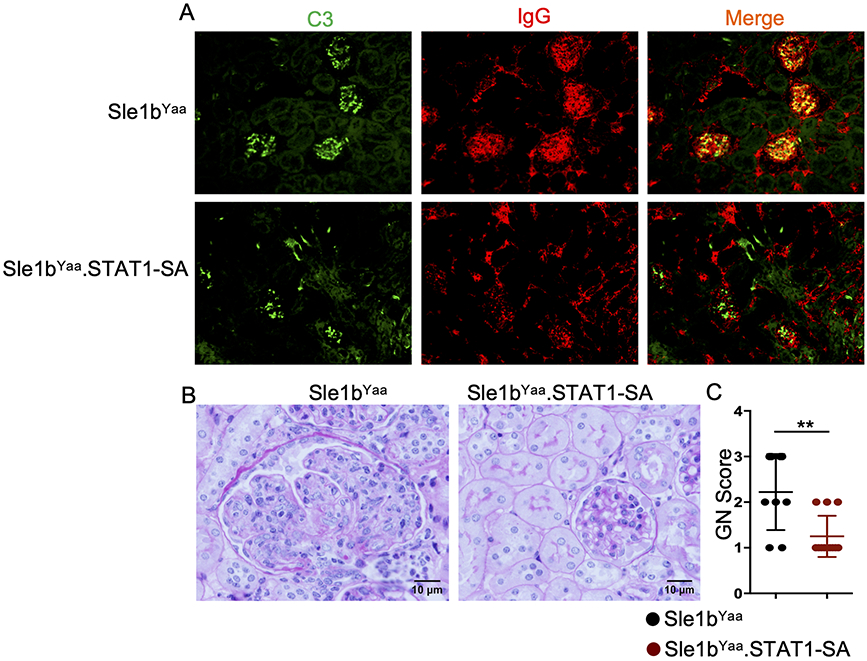
Having demonstrated the role of STAT1-pS727 in autoimmune GC and AFC responses and autoAb production, we next investigated the importance of STAT1-pS727 in SLE pathogenesis, focusing on kidney pathology using TLR7-accelerated Sle1bYaa SLE disease model. We found that reduced autoimmune GC, AFC, ABC and autoAb responses in Sle1bYaa.STAT1-SA mice strongly correlated with reduced immune complex (IC) deposition in the kidney glomeruli of Sle1bYaa.STAT1-SA mice compared to Sle1bYaa control mice as evaluated by immunofluorescent staining of kidney sections for anti-C3 and anti-IgG (Figure 6A). Consistent with reduced IC deposition, we observed significantly reduced glomerulonephritis (GN) in Sle1bYaa.STAT1-SA mice compared to Sle1bYaa control mice (Figure 6B, C). These findings demonstrate that STAT1-pS727 regulates TLR7-accelerated development of SLE.
Figure 6. STAT1-pS727 promotes TLR7-accelerated lupus nephritis.
(A) Kidney sections from 6-8 mo old Sle1bYaa and Sle1bYaa.STAT1-SA male mice were stained with anti-C3 (green) and anti-IgG (red). Representative images (B) of PAS stained kidney sections and the glomerulonephritis (GN) score (C) are shown from these mice. These data represent 7-8 mice from each group that was collected from two independent experiments. Statistical analysis was performed by two-way ANOVA, with a follow-up Sidak multiple-comparison test (h) or unpaired, nonparametric Mann-Whitney Student’s t-test. Ns, non-significant; **P < 0.01.
DISCUSSION
Our findings here provide novel insights into the role of STAT1-pS727 in promoting systemic autoimmunity and SLE disease development by regulating AFC, GC, Tfh and autoAb responses. Through the generation of B cell-specific BM chimeras we demonstrated the B cell-intrinsic role of STAT1-pS727 in autoimmune AFC and GC responses, promoting autoAb production. In a marked contrast, we found no contribution of STAT1-pS727 to foreign-antigen- or pathogen-driven GC, Tfh and Ab responses. STAT1-pS727 also did not play a significant role in GC and Tfh responses in gut associated lymphoid tissue (GALT) in B6.Sle1b mice, which were previously shown to be mediated by gut microbiota and dietary antigens (28-30). Together, these data collectively highlight the importance of STAT1-pS727 in promoting SLE-associated AFC, GC and SLE development, and the dispensability of this mechanism for GC B cell, Tfh and Ab responses to foreign antigens, including pathogenic infection, or gut microbiota and dietary antigens. Importantly, our findings indicate a differential requirement for STAT1-pS727 between autoimmune and pathogen driven responses, which maybe the ideal scenario for the implementation of targeted SLE therapeutics that preserve anti-microbial immunity.
While STAT1 downstream of type I and II interferon (IFN) signaling promotes systemic autoimmunity and SLE disease, it also plays an important role in anti-pathogen responses (31). A number of patients with STAT1 deficiencies are highly susceptible to viral infections (8). Mice deficient in STAT1 are also highly sensitive to microbial and viral infections (32). STAT1 is expressed as α and β isoforms. Unlike STAT1α, STAT1β lacks the C-terminal transactivation domain (TAD) including serine-727 and shows attenuated function compared to STAT1α. Attenuated function of the β isoform is likely due to the ability of the TAD in the α isoform to interact with other transcriptional co-regulators and promote maximal transcriptional activity of STAT1 (10, 11, 33). STAT1 deficiency in the TAD showed a significant attenuation of its interactions with other proteins (34, 35). Therefore, targeting entire STAT1 or the STAT1 TAD for treating SLE may predispose patients to lethal infections and death. We found significantly reduced autoimmune responses and ameliorated SLE pathogenesis, but intact immune responses to foreign antigens including mouse polyoma virus infection in S727A mutant mice. These data suggest that the suboptimal activity of STAT1 in the absence of STAT1-pS727 is sufficient for mounting anti-pathogen, but not autoimmune responses. Although inhibition of STAT1-pS727 by flavopiridol or by S727A mutation in a previous in vitro study did not change the amount of promoter bound STAT1, it affected the expression of 40-50% IFNγ-induced genes (14). Previously published and our current data together suggest that STAT1-pS727 is required to regulate the expression of genes that are important for autoimmune responses, but dispensable for foreign antigen driven responses.
In addition to IFN signaling other factors were also previously shown to induce and regulate STAT1-pS727 function. One of the critical regulatory mechanisms that inhibits B cell responses is the inhibitory Fc receptor, FcγRIIb (36). Concurrent engagement of FcγRIIb and BCR with antigen and antibody complexes recruits the FcγRIIb into BCR signaling complex to negatively regulate BCR signaling (37). Polymorphisms in the Fcgr2b gene or the absence of FcγRIIb signaling contributes to SLE development (38). Interestingly, BCR stimulation induces sustained STAT1-pS727 in B cells whereas FcγRIIb inhibits BCR-induced STAT1-pS727 (39). Previous studies also highlighted the role of TLRs in inducing STAT1-pS727 in myeloid cells independent of IFN signaling (40), although TLR-mediated induction of STAT1-pS727 in B cells was previously not well-explored. Using an in vitro stimulation system, a previous study demonstrated the role of TLR7 in the induction of phosphorylation of STAT1 at both Y701 and S727 (41). It was suggested that STAT1-pY701, but not STAT1-pS727, downstream of TLR7 and Bank1 in type I IFN response in B cells, contributed to SLE development in the TLR7-accelerated B6.Sle1b.yaa model (41). Consistent with this previous report, we have identified a role for TLR7 stimulation in the induction of STAT1-pS727 in B cells independent of IFN (both type I and II IFN) and IL21 receptor signaling (S.C. et al., unpublished). However, as opposed to the previous report (41), our findings demonstrate a role for STAT1-pS727 in TLR7-promoted SLE autoimmunity and disease development. These data indicate that STAT1-pS727 is at the intersection of several critical signaling pathways involved in immune cell function and signaling, and BCR, TLR and IFN signaling in part promote autoimmunity and SLE disease development through regulation of STAT1-pS727. Furthermore, the kinase(s) responsible for STAT1-pS727 following activation of these pathways and the subcellular location of this phosphorylation event are unclear at this time (12, 14). Our future studies will be focused on pursuing a deeper mechanistic understanding of differential regulation of anti-pathogen and autoimmune responses by STAT1 serine 727 phosphorylation.
In conclusion, our data highlight the importance of STAT1-pS727 downstream of several signaling pathways such as BCR, TLR and IFN signaling in autoimmune AFC, GC and Tfh responses, leading to autoantibody production and development of SLE pathogenesis. Our data further indicate that STAT1-pS727 is not required for foreign-antigen driven responses including pathogens. Future efforts should focus on the identification of kinase(s) involved in STAT1 serine phosphorylation and development of therapeutics to block STAT1-pS727 as a treatment for SLE that can maintain protective immunity to pathogens in SLE patients.
Supplementary Material
Key points.
STAT1-pS727 is required for SLE-associated AFC, GC and autoantibody responses
STAT1-pS727 in B cells promotes autoimmune AFC, GC and autoantibody responses
STAT1-pS727 is not required for foreign-antigen- or gut microbiota-driven responses
ACKNOWLEDGMENTS
We thank the PSUHMC flow cytometry core facility for their assistance. We thank the PSUMHC Department of Comparative Medicine for animal housing and care.
This work was supported by the National Institutes of Health RO1AI091670 to Z.S.M.R; LRA 548931 grant to Z.S.M.R and the Finkelstein Memorial award to S.C.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Cappione A, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, and Sanz I. 2005. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest 115: 3205–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinuesa CG, Sanz I, and Cook MC. 2009. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 9: 845–857. [DOI] [PubMed] [Google Scholar]
- 3.William J, Euler C, Christensen S, and Shlomchik MJ. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 297: 2066–2070. [DOI] [PubMed] [Google Scholar]
- 4.Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, Hou B, Buckner JH, and Rawlings DJ. 2016. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med 213: 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domeier PP, Chodisetti SB, Soni C, Schell SL, Elias MJ, Wong EB, Cooper TK, Kitamura D, and Rahman ZS. 2016. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med 213: 715–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domeier PP, Chodisetti SB, Schell SL, Kawasawa YI, Fasnacht MJ, Soni C, and Rahman ZSM. 2018. B-Cell-Intrinsic Type 1 Interferon Signaling Is Crucial for Loss of Tolerance and the Development of Autoreactive B Cells. Cell Rep 24: 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thibault DL, Chu AD, Graham KL, Balboni I, Lee LY, Kohlmoos C, Landrigan A, Higgins JP, Tibshirani R, and Utz PJ. 2008. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest 118: 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, and Casanova JL. 2012. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol 24: 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy DE, and Darnell JE. 2002. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3: 651–662. [DOI] [PubMed] [Google Scholar]
- 10.Varinou L, Ramsauer K, Karaghiosoff M, Kolbe T, Pfeffer K, Müller M, and Decker T. 2003. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 19: 793–802. [DOI] [PubMed] [Google Scholar]
- 11.Wen Z, Zhong Z, and Darnell JE. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82: 241–250. [DOI] [PubMed] [Google Scholar]
- 12.Decker T, and Kovarik P. 2000. Serine phosphorylation of STATs. Oncogene 19: 2628–2637. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Wen Z, Xu LZ, and Darnell JE. 1997. Stat1 serine phosphorylation occurs independently of tyrosine phosphorylation and requires an activated Jak2 kinase. Mol Cell Biol 17: 6618–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancerek J, Poss ZC, Steinparzer I, Sedlyarov V, Pfaffenwimmer T, Mikulic I, Dölken L, Strobl B, Müller M, Taatjes DJ, and Kovarik P. 2013. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 38: 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, and Wakeland EK. 2000. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A 97: 6670–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong EB, Khan TN, Mohan C, and Rahman ZS. 2012. The lupus-prone NZM2410/NZW strain-derived Sle1b sublocus alters the germinal center checkpoint in female mice in a B cell-intrinsic manner. J Immunol 189: 5667–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokogawa M, Takaishi M, Nakajima K, Kamijima R, Fujimoto C, Kataoka S, Terada Y, and Sano S. 2014. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: a new model of systemic Lupus erythematosus. Arthritis Rheumatol 66: 694–706. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Seto NL, Carmona-Rivera C, and Kaplan MJ. 2018. Accelerated model of lupus autoimmunity and vasculopathy driven by toll-like receptor 7/9 imbalance. Lupus Sci Med 5: e000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chodisetti SB, Fike AJ, Domeier PP, Singh H, Choi NM, Corradetti C, Kawasawa YI, Cooper TK, Caricchio R, and Rahman ZSM. 2020. Type II but Not Type I IFN Signaling Is Indispensable for TLR7-Promoted Development of Autoreactive B Cells and Systemic Autoimmunity. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao W, Hua Z, Liu C, Lin L, Chen R, and Hou B. 2017. Characterization of T-Dependent and T-Independent B Cell Responses to a Virus-like Particle. J Immunol 198: 3846–3856. [DOI] [PubMed] [Google Scholar]
- 21.Soni C, Domeier PP, Wong EB, Shwetank, Khan TN, Elias MJ, Schell SL, Lukacher AE, Cooper TK, and Rahman ZS. 2015. Distinct and synergistic roles of FcγRIIB deficiency and 129 strain-derived SLAM family proteins in the development of spontaneous germinal centers and autoimmunity. J Autoimmun 63: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corradetti C, Jog NR, Cesaroni M, Madaio M, and Caricchio R. 2018. Estrogen Receptor α Signaling Exacerbates Immune-Mediated Nephropathies through Alteration of Metabolic Activity. J Immunol 200: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong S, Zhang Z, Liu H, Tian M, Zhu X, Wang W, Zhou X, Zhang F, Ge Q, Zhu B, Tang H, Hua Z, and Hou B. 2018. B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4. Immunity 49: 695–708.e694. [DOI] [PubMed] [Google Scholar]
- 24.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, Casco J, Li QZ, Connolly JE, and Wakeland EK. 2008. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol 38: 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, and Bolland S. 2007. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, and Wakeland EK. 2006. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A 103: 9970–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, and Marrack P. 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood 118: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cebra JJ, Schrader CE, Shroff KE, and Weinstein PD. 1991. Are Peyer's patch germinal centre reactions different from those occurring in other lymphoid tissues? Res Immunol 142: 222–226. [DOI] [PubMed] [Google Scholar]
- 29.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, and Rajewsky K. 2004. B cell receptor signal strength determines B cell fate. Nat Immunol 5: 317–327. [DOI] [PubMed] [Google Scholar]
- 30.Hara S, Sasaki T, Satoh-Takayama N, Kanaya T, Kato T, Takikawa Y, Takahashi M, Tachibana N, Kim KS, Surh CD, and Ohno H. 2019. Dietary Antigens Induce Germinal Center Responses in Peyer's Patches and Antigen-Specific IgA Production. Front Immunol 10: 2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee AJ, and Ashkar AA. 2018. The Dual Nature of Type I and Type II Interferons. Front Immunol 9: 2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, and Schreiber RD. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84: 431–442. [DOI] [PubMed] [Google Scholar]
- 33.Pilz A, Ramsauer K, Heidari H, Leitges M, Kovarik P, and Decker T. 2003. Phosphorylation of the Stat1 transactivating domain is required for the response to type I interferons. EMBO Rep 4: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, and Lee MS. 2007. STAT1 as a key modulator of cell death. Cell Signal 19: 454–465. [DOI] [PubMed] [Google Scholar]
- 35.Kovarik P, Mangold M, Ramsauer K, Heidari H, Steinborn R, Zotter A, Levy DE, Müller M, and Decker T. 2001. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. EMBO J 20: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coggeshall KM 1998. Inhibitory signaling by B cell Fc gamma RIIb. Curr Opin Immunol 10: 306–312. [DOI] [PubMed] [Google Scholar]
- 37.Ravetch JV 1997. Fc receptors. Curr Opin Immunol 9: 121–125. [DOI] [PubMed] [Google Scholar]
- 38.Espéli M, Smith KG, and Clatworthy MR. 2016. FcγRIIB and autoimmunity. Immunol Rev 269: 194–211. [DOI] [PubMed] [Google Scholar]
- 39.Lafont V, Decker T, and Cantrell D. 2000. Antigen receptor signal transduction: activating and inhibitory antigen receptors regulate STAT1 serine phosphorylation. Eur J Immunol 30: 1851–1860. [DOI] [PubMed] [Google Scholar]
- 40.Luu K, Greenhill CJ, Majoros A, Decker T, Jenkins BJ, and Mansell A. 2014. STAT1 plays a role in TLR signal transduction and inflammatory responses. Immunol Cell Biol 92: 761–769. [DOI] [PubMed] [Google Scholar]
- 41.Wu YY, Kumar R, Iida R, Bagavant H, and Alarcón-Riquelme ME. 2016. BANK1 Regulates IgG Production in a Lupus Model by Controlling TLR7-Dependent STAT1 Activation. PLoS One 11: e0156302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.