Abstract
Introduction:
Obesity is an established risk factor for recurrent atrial fibrillation (AF) after ablation. The impact of pre-procedure weight changes on freedom from AF (FFAF) after ablation in obese and nonobese patients is unknown.
Methods:
A single-center retrospective cohort study of patients undergoing pulmonary vein isolation was performed. Before ablation, all candidates were encouraged to adopt healthy lifestyle habits according to American Heart Association guidelines, including weight loss, by their physician. The primary endpoint was FFAF through 1-year after completion of the 3-month blanking period.
Results:
Of the 601 patients (68% male; average age 62.1 ± 10.3 years) included in analysis, 234 patients (38.9%) were obese (body mass index ≥ 30) and 315 (52.4%) had paroxysmal AF. FFAF was observed in 420 patients (69.9%) at 15 months. Percent change in weight that occurred during the year before ablation independently predicted FFAF through 15-months in all patients (adjusted odds ratio = 1.17, 95% confidence interval: 1.11–1.23). Subgroup analyses based on paroxysmal vs persistent AF, presence of obesity, and history of prior ablation were performed. Percent change in weight over the year before ablation was independently associated with FFAF in all subgroups except nonobese patients with persistent AF.
Conclusion:
Pre-ablation weight loss was associated with FFAF in both obese and nonobese patients. Further studies are needed to define the optimal approach to weight loss before AF ablation.
Keywords: atrial fibrillation, catheter ablation, cryoballoon, pulmonary vein isolation, weight loss
1 |. INTRODUCTION
Catheter ablation of atrial fibrillation (AF) is supported by a class I indication for treatment of symptomatic paroxysmal AF in patients who are refractory or intolerant to antiarrhythmic medications, and a class IIa indication in symptomatic persistent AF or before initiation of antiarrhythmic medication.1,2 Despite advances in treatment strategies, rates of freedom from AF (FFAF) at 1 year following ablation have demonstrated a plateau in the range of 60%–80%.3,4 Obese patients have particularly low rates of FFAF 1 year after ablation with published rates ranging from 40% to 50%.5–7
In obese patients, structural and electrical remodeling of the atria may lead to decreased rates of FFAF after ablation.8–10 As the rate of obesity rises to nearly 50% in the United States, the impact of obesity on outcomes of catheter ablation must be factored into treatment considerations.11 Current guidelines recommend that all obese patients with AF pursue weight loss. However, the optimal magnitude and strategy to achieve weight loss are not defined, particularly in relation to ablation.2,12 There are currently few data on the impact of patient-directed pre-procedure weight loss on FFAF following ablation in both obese and nonobese patients.
The goal of the present study is to determine if patient-directed weight loss over the year before AF ablation is associated with increased rates of FFAF in both obese and nonobese patients.
2 |. METHODS
2.1 |. Patients
A retrospective analysis of a prospectively maintained, single center database of all AF ablation cases performed at Northwestern Memorial Hospital between 2012 and 2017 was conducted. Inclusion criteria included PVI using cryoballoon ablation (CBA), presence of a recorded weight in the electronic medical record 12 ± 6 months before ablation, and postablation follow up between 3 and 15 months. Before their ablation, and as part of comprehensive AF care, all patients were encouraged to adopt healthy lifestyle habits including weight loss, alcohol reduction, assessment for sleep apnea, and 150 min of moderate intensity exercise weekly, according to American Heart Association guidelines.13 Patients were encouraged to independently engage in these healthy lifestyle activities as part of routine counseling, and no patients were referred to a formal lifestyle modification program. The primary endpoint was FFAF, defined as freedom from documented AF/atrial tachycardia/atrial flutter greater than 30 s, from the end of a 90-day blanking period through 15 months postprocedure (12 months post-blanking period).1,14 This study was approved by the Institutional Review Board (IRB) at Northwestern University and adhered to guidelines set forth in the Helsinki Declaration.
2.2 |. Procedural details
The CBA procedural protocol used in this study has been published previously.5,15 All procedures were performed by board certified electrophysiologists with extensive ablation experience. Briefly, after transeptal catheterization, an Arctic Front Advance (Medtronic Inc.) cryoballoon catheter and Achieve catheter (Medtronic Inc.) were introduced into the left atrium (LA) using the FlexCath Advance Steerable Sheath (Medtronic Inc.). Electroanatomic mapping was performed at the discretion of the operator with CARTO3, (Biosense Webster) or EnSite (Abbott Laboratories). CBA was performed at the ostium of each pulmonary vein (PV), with a pulmonary venogram obtained before each lesion to confirm appropriate location and balloon occlusion. Lesion duration evolved over time from a minimum of two 4-min freezes per vein to one 3-min freeze per vein, based on time to PV isolation. A second generation cryoballoon was used in all cases. Cryoballoon size was selected based on PV anatomy on preoperative MRI. Target temperatures were −35 to −55°C for all patients and esophageal temperature monitoring was used for those patients receiving general anesthesia. Entrance block was confirmed after PVI. During isolation of the right-sided PVs, a catheter was positioned in the superior vena cava to perform high-output pacing to monitor for phrenic nerve injury. No provocative maneuvers were routinely performed after CBA to induce atrial arrhythmias. Cardioversion to sinus rhythm was performed if a patient remained in AF after CBA. If a patient developed early return of AF (ERAF) < 90 days post-CBA, the decision to perform a cardioversion or initiate a new antiarrhythmic drug was made by the treating physician.
2.3 |. Clinical follow up
Antiarrhythmic drugs were routinely stopped after a 3-month postprocedure blanking period. At a minimum, guideline recommendations for postablation arrhythmia monitoring were followed.16 All patients had scheduled clinical follow up at the end of the blanking period and every 6 months thereafter for at least 2 years. Routine ECGs at the time of office visits, external monitoring for 7–21 days, downloads from implanted devices and readings from Kardia smartphone monitors (AliveCor) were used for rhythm assessment. Additional rhythm assessments were performed in response to symptoms suggestive of arrhythmia recurrence, or at the discretion of the treating physician.
2.4 |. Endpoints and data analysis
The primary endpoint for this study was FFAF from the end of a 3-month blanking period through 15 months postprocedure.17 Patient demographics, arrhythmia evaluation, weight values and procedural complications were abstracted from the electronic medical record, and data was entered into an IRB approved database. Two values for weight (kg) were used for each patient: one from the date of ablation and one from a time period 12 ± 6 months before ablation. If multiple weight measurements were present 12 ± 6 months before ablation, the value closest to 12 months before ablation was recorded for analysis. Numerical results are reported as mean ± standard deviation, median (interquartile range), or number (%). Univariate analyses were completed using SPSS Version 26 (IBM) to complete χ2 or Fisher exact tests for categorical variables, and Student’s t tests or Mann–Whitney U Tests for continuous variables as appropriate. Multivariate analyses were performed on variables found to have a p < .1 according to univariate analysis, and pre-determined variables of interest, to identify independent factors associated with FFAF within 15 months after CBA. Kaplan–Meier curves were created to compare FFAF rates over time among patients with various degrees of weight changes using Prism Software Version 8 (GraphPad). p values less than 0.05 were considered to be significant in this study.
3 |. RESULTS
3.1 |. Patient characteristics
Of 747 total CBA procedures performed during the study period, 601 patients (68% male; age 62.1 ± 10.3 years, 52.4% paroxysmal AF) met inclusion criteria (Table 1). Twenty-seven patients who did not have follow up data between 3 and 15 months postprocedure, and 119 patients who did not have a recorded weight 12 ± 6 months before their procedure were excluded from analysis (Figure 1). The median (IQR) amount of time between initial pre-procedure weight measurement and ablation was 363 (313–406) days. The median duration of time between diagnosis of AF and ablation was 34 (12–66) months, and 79 patients (13.1%) had a prior ablation for AF. Of the patients who had a prior ablation for AF, 60 (75.9%) had a prior radiofrequency (RF) ablation, 18 patients (22.8%) had a prior CBA, and 1 patient (1.3%) had a prior surgical MAZE procedure. The median (IQR) follow up time for patients included in this study was 15 (7.8–15) months.
TABLE 1.
Baseline demographics and freedom from atrial fibrillation through 15-month postablation
| Parameter | Total (n = 601) | No FFAF (n = 181) | FFAF (n = 420) | p |
|---|---|---|---|---|
| Male sex | 407 (67.7%) | 120 (66.3%) | 287 (68.3%) | 0.63 |
| Age (years) | 62.1 ± 10.3 | 63.0 ± 10.6 | 61.7 ± 10.1 | 0.14 |
| AF type | ||||
| Paroxysmal AF | 315 (52.4%) | 82 (45.3%) | 233 (55.5%) | 0.02* |
| Time since AF diagnosis (months) | 52.4 ± 65.9 | 59.3 ± 64.7 | 49.5 ± 66.3 | 0.10 |
| Left atrial diameter (mm) | 39.5 ± 6.9 | 40.2 ± 6.7 | 39.2 ± 7.0 | 0.09 |
| LVEF (%) | 56.4 ± 9.2 | 55.4 ± 10.5 | 56.8 ± 8.6 | 0.11 |
| Prior ablation for AF | 79 (13.1%) | 21 (11.6%) | 58 (13.8%) | 0.46 |
| Comorbid conditions | ||||
| Diabetes | 89 (14.8%) | 25 (13.8%) | 64 (15.2%) | 0.65 |
| Hypertension | 287 (47.8%) | 85 (47.0%) | 202 (48.1%) | 0.80 |
| Prior TIA/CVA | 47 (7.8%) | 14 (7.7%) | 33 (7.9%) | 0.96 |
| COPD | 8 (1.3%) | 4 (2.2%) | 4 (1.0%) | 0.22 |
| OSA | 101 (16.8%) | 31 (17.1%) | 70 (16.7%) | 0.89 |
| Current smoker | 43 (7.2%) | 12 (6.6%) | 31 (7.4%) | 0.74 |
| CAD | 99 (16.5%) | 29 (16.0%) | 70 (16.7%) | 0.85 |
| Structural heart disease | 82 (13.6%) | 34 (18.8%) | 48 (11.4%) | 0.02* |
| CHA2DS2-VASc | 2.0 ± 1.4 | 2.1 ± 1.4 | 2.0 ± 1.4 | 0.21 |
| Pre-procedure medications | ||||
| ACE-i/ARB | 209 (34.8%) | 67 (37.0%) | 142 (33.8%) | 0.45 |
| Statin | 271 (45.1%) | 72 (39.8%) | 199 (47.4%) | 0.09 |
| Calcium channel blocker | 113 (18.8%) | 38 (21.0%) | 75 (17.9%) | 0.37 |
| Beta blocker | 349 (58.1%) | 103 (56.9%) | 246 (58.6%) | 0.70 |
| Increase in number antihypertensives in year before ablation | 47 (7.8%) | 16 (8.8%) | 31 (7.4%) | 0.62 |
| CIED | 39 (6.5%) | 21 (11.6%) | 18 (4.3%) | 0.001* |
| RBBB pre-ablation | 35 (5.8%) | 8 (4.4%) | 27 (6.4%) | 0.34 |
| LBBB pre-ablation | 19 (3.2%) | 7 (3.9%) | 12 (2.9%) | 0.52 |
| Ablation characteristics | ||||
| Presenting rhythm AF | 176 (29.3%) | 64 (35.4%) | 112 (26.7%) | 0.04* |
| Ablation lines in addition to PVI | 81 (13.5%) | 24 (13.3%) | 57 (13.6%) | 1.00 |
| Total CBA freezes | 9.4 ± 2.8 | 9.8 ± 2.7 | 9.2 ± 2.8 | 0.03* |
| ERAF | 133 (22.1%) | 76 (42.0%) | 57 (13.6%) | <0.001* |
| Weight characteristics | ||||
| BMI 1-year before ablation (kg/m2) | 29.5 ± 6.3 | 29.2 ± 5.9 | 29.7 ± 6.5 | 0.45 |
| BMI at time of ablation (kg/m2) | 29.2 ± 6.2 | 29.5 ± 6.1 | 29.0 ± 6.2 | 0.38 |
| Lost weight pre-ablation | 349 (58.1%) | 61 (33.7%) | 288 (68.6%) | <0.001* |
| % Change weight over 1-year before ablation | −0.97 ± 5.2 | 1.1 ± 4.5 | −1.8 ± 5.2 | <0.001* |
Abbreviations: ACE-i, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; CBA, cryoballoon ablation; CIED, cardiovascular implantable electronic device; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ERAF, early return of AF < 90 days postablation; FFAF, freedom from atrial fibrillation; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; PVI, pulmonary vein isolation; RBBB, right bundle branch block; TIA, transient ischemic attack.
p < 0.05.
FIGURE 1.
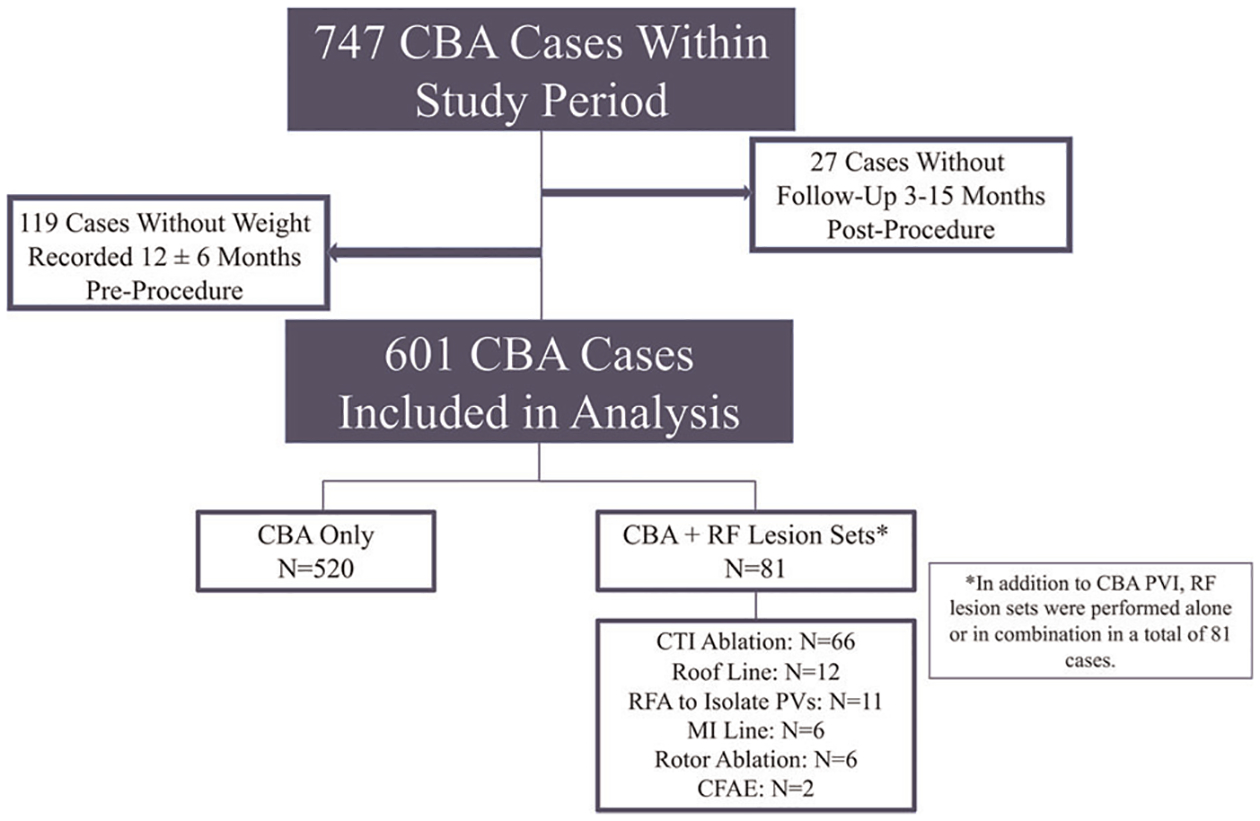
CONSORT diagram of exclusion criteria and ablation techniques. Among 747 cryoballoon cases completed during the study period, 601 were included in analysis. There were 520 (86.5%) cases exclusively consisting of cryoballoon pulmonary vein isolation. Additional radiofrequency lesions were delivered in 81 (13.5%) cases, of which 16 cases included ≥2 unique radiofrequency ablation targets. CBA, cryoballoon ablation; CFAE, complex fractionated atrial electrogram; CTI, cavotricuspid isthmus; LA, left atrium; MI line, mitral isthmus line; PVs, pulmonary veins; RF, radiofrequency; RFA, radiofrequency ablation
3.2 |. Procedural characteristics
At the time of ablation, AF was the presenting rhythm in 176 (29.3%) cases, and 396 patients (65.9%) presented in normal sinus rhythm. There were 21 patients (3.5%) who presented in atrial flutter, and 8 (1.3%) presented in an atrial paced rhythm. Patients required an average of 9.4 ± 2.8 CBA freezes per case. Additional RF ablation lesions were delivered in 81 (13.5%) cases, consisting of 11 cases (1.8%) that required additional RF lesions to achieve PV isolation, 66 (11.0%) cases of cavotricuspid (CTI) ablation, 12 cases (2.0%) with a LA roof line, 6 cases (1.0%) with a mitral isthmus line, 3 cases (0.5%) with rotor ablation, and 2 cases (0.3%) with ablation of complex fractionated atrial electrograms. Of the 81 cases with RF ablation lesions, 16 cases (2.7%) included at least two unique RF ablation targets (Figure 1).
3.3 |. Procedure results
Through 15 months of follow-up, and excluding the 3-month blanking period, FFAF was observed in 420/601 (69.9%) patients. Of the 181 patients who had recurrent AF, the methods for detecting AF were as follows: ECG (53.0%), ambulatory cardiac monitor (37.0%), dual chamber intracardiac device (4.4%), implantable cardiac monitor (Reveal LINQ; Medtronic Inc.) (4.4%), and Kardia Smartphone Monitor (1.1%). There was no difference in the rate of ambulatory rhythm monitoring between the patients with and without FFAF through 15 months (p = 0.28).
3.4 |. Weight characteristics
Patients had an average body mass index (BMI) of 29.5 ± 6.3 kg/m2 1-year before ablation, and 29.2 ± 6.2 kg/m2 on the day of ablation. There were 234 patients (38.9%) who were obese (BMI ≥ 30 kg/m2) and 367 patients (61.1%) who were not obese (BMI < 30 kg/m2) 1-year before ablation. The average BMI of obese patients was 35.8 ± 5.0 kg/m2 and the average BMI of nonobese patients was 25.5 ± 2.9 kg/m2 1-year before ablation. Patients with paroxysmal AF (pAF) had a lower BMI than patients with persistent AF (pAF: 28.5 ± 6.3 kg/m2; persistent AF: 30.6 ± 6.2 kg/m2; p < .001). Obese patients had higher rates of diabetes mellitus, hypertension, persistent AF, and obstructive sleep apnea (Table 2). In the year before ablation, 144 (61.5%) obese and 206 (56.1%) nonobese patients lost weight. Of those patients who lost weight during the year before ablation, obese and nonobese patients lost a median of 3.2% (1.8%–5.9%) and 2.7% (1.4%–4.3%) of their body weight, respectively.
TABLE 2.
Comorbidities in obese versus nonobese patients
| Comorbidity | Obese patients (n = 234) |
Nonobese patients (n = 367) | p |
|---|---|---|---|
| Diabetes | 57 (24.4%) | 32 (8.7%) | <0.001* |
| Hypertension | 138 (59.0%) | 149 (40.6%) | <0.001* |
| Active alcohol use | 114 (48.7%) | 193 (52.6%) | 0.36 |
| Prior TIA/CVA | 14 (6.0%) | 33 (9.0%) | 0.21 |
| Chronic obstructive pulmonary disease | 5 (2.1%) | 3 (0.8%) | 0.27 |
| Obstructive sleep apnea | 76 (32.5%) | 25 (6.8%) | <0.001* |
| Coronary artery disease | 43 (18.4%) | 56 (15.3%) | 0.31 |
| Persistent AF | 126 (53.8%) | 160 (43.6%) | 0.02* |
| Months of AF diagnosis | 49.7 ± 57.8 | 54.1 ± 70.6 | 0.78 |
Abbreviations: AF, atrial fibrillation; CVA, cerebrovascular accident; TIA, transient ischemic attack.
p < 0.05
3.5 |. Factors associated with freedom from AF:
Of the variables tested, univariate analysis revealed that pAF, absence of structural heart disease, absence of ERAF, absence of a cardiovascular implantable electronic device (CIED), and percent of weight change in the year before ablation were associated with FFAF 15-months after CBA (Table 1). Multivariate analysis of pre-selected variables and variables with p < .1 on univariate analysis demonstrated that percent change in weight during the year before ablation was independently associated with FFAF through 15 months post-CBA in all patients (adjusted odds ratio = 1.17, 95% confidence interval: 1.11–1.23). Multivariate analysis also demonstrated that shorter duration of AF diagnosis, absence of a CIED, and absence of ERAF were independently associated with FFAF. Kaplan–Meier estimates of FFAF by pre-ablation weight changes in obese and nonobese patients demonstrated that percent weight loss in the year before ablation was associated with FFAF through 15 months (Figures 2 and 3). In both groups, weight gain before ablation was associated with decreased rates of FFAF.
FIGURE 2.
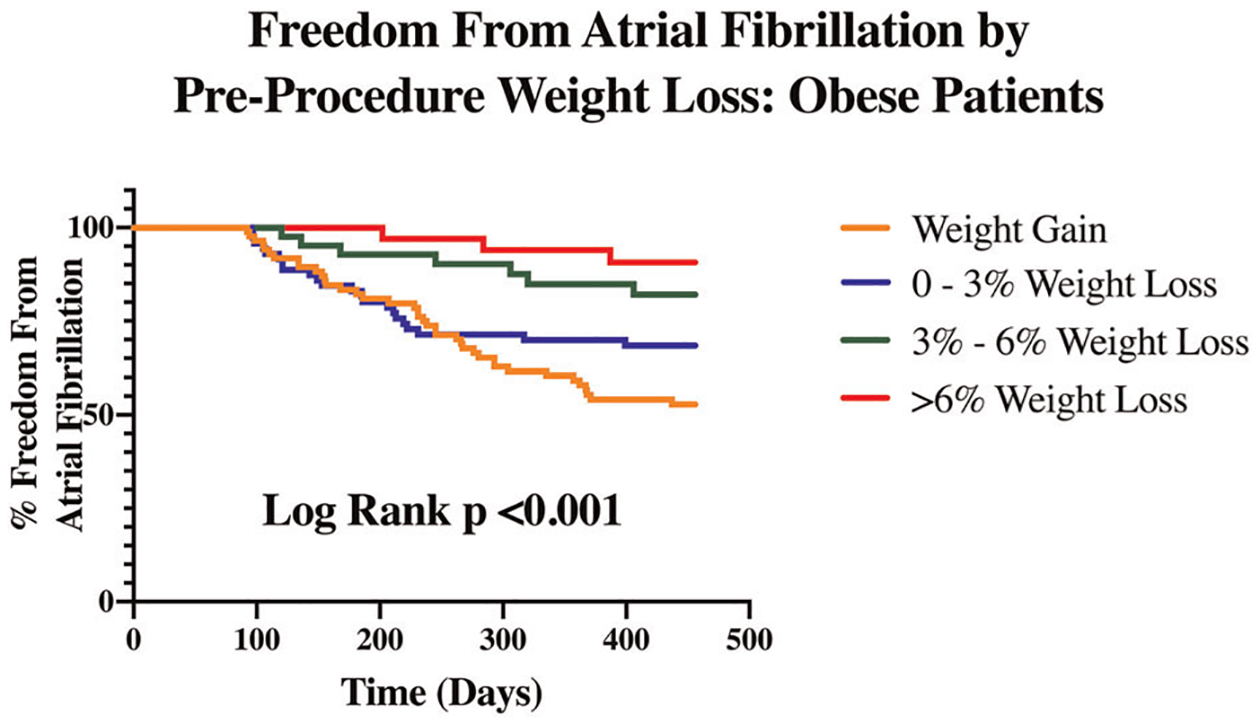
Freedom from atrial fibrillation by preprocedure weight loss: obese patients. Among obese patients who underwent cryoballoon ablation, survival analysis demonstrates that percent weight loss in the year before ablation is associated with freedom from atrial fibrillation through 15-months postablation
FIGURE 3.
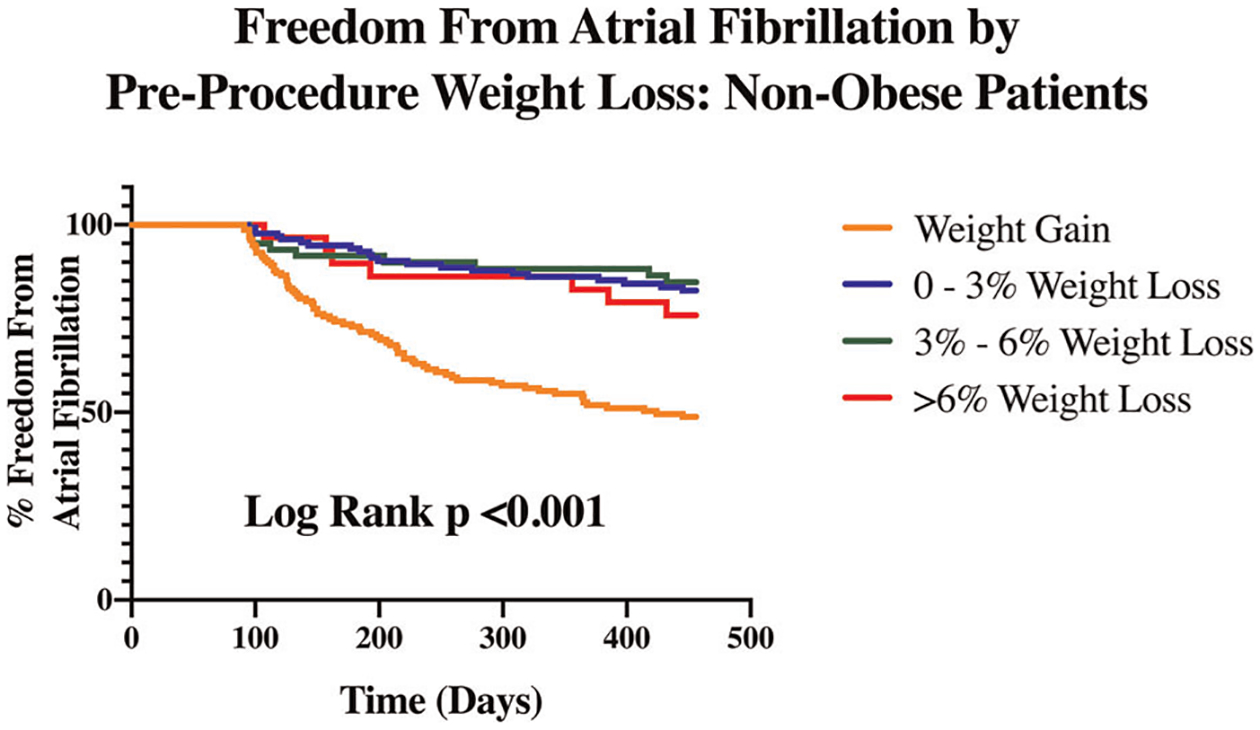
Freedom from atrial fibrillation by preprocedure weight loss: nonobese patients. Among nonobese patients who underwent cryoballoon ablation, survival analysis demonstrates that percent weight loss in the year before ablation is associated with freedom from atrial fibrillation through 15-months postablation
Subgroup analyses based on paroxysmal versus persistent AF, presence of obesity pre-ablation, and history of prior ablation were also performed. Percent change in weight over the year before ablation was independently associated with FFAF at 15 months in all subgroups except nonobese patients with persistent AF (Figure 4).
FIGURE 4.
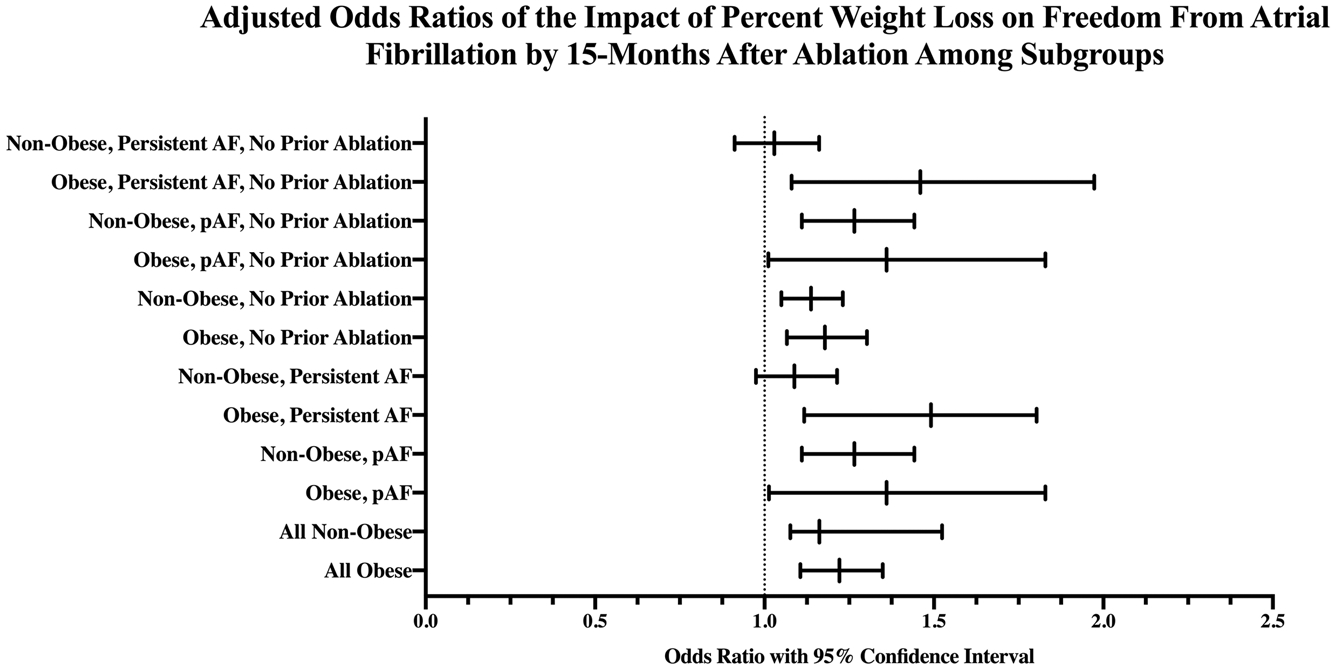
Adjusted odds ratios of the impact of percent weight loss on freedom from atrial fibrillation through 15-month after ablation among subgroups. Adjusted odds ratios demonstrate that percentage of weight loss during the year before ablation for atrial fibrillation independently predicts freedom from atrial fibrillation through 15-month postablation in all subgroups analyzed with the exception of nonobese patients with persistent AF. AF, atrial fibrillation; pAF, paroxysmal atrial fibrillation
4 |. DISCUSSION
Obesity is an established risk factor for development of AF, and overweight patients who undergo catheter ablation have lower success rates than non-overweight patients.6,18 Current guidelines recommend risk factor modification, including weight loss, for overweight and obese patients with AF.2,12 However, the extent and method of weight loss for patients to target before AF ablation has not been specified. In the present study, patient-directed modest weight loss in the year before ablation was associated with FFAF through 15 months post-CBA in both obese and nonobese patients undergoing ablation for AF. With the exception of nonobese patients with persistent AF, all subgroups analyzed showed benefit of weight loss over the year before ablation on FFAF.
Obese patients have structural and electrical remodeling of the LA which may contribute to the substrate for AF.9 Proliferation of atrial adipocytes and increased accumulation of epicardial and pericardial fat surrounding the LA leads to an inflammatory response, fibrosis, paracrine effects, and oxidative stress.10,19,20 The combination of these factors may mediate atrial remodeling, predispose to AF, and confer a resistance to treatment.19–21
Significant weight loss and risk factor modification are beneficial in patients with AF who do not undergo ablation.12,22–24 The LEGACY trial demonstrated that overweight patients who lost greater than 10% of their body weight after undergoing frequent counseling and diet modifications had significantly lower AF burden and symptom scores than patients who lost less than 9% of their body weight.23 Weight loss may be associated with improvement in AF via a reduction in LA area, deactivation of atrial adipocytes, and decrease in atrial inflammation and fibrosis.10,24 These changes associated with weight loss can decrease the arrhythmogenic potential of atrial tissue.
The ARREST AF Cohort study recently assessed the impact of physician-directed cardiometabolic risk factor modification on AF frequency, duration, and symptoms after radiofrequency catheter ablation.7 Among 149 patients with a BMI > 27 kg/m2, 61 self-selected patients participated in comprehensive physician-directed risk factor modification including weight loss, lipid management, glycemic control, smoking cessation, and sleep disordered breathing management. Patients in the risk factor modification cohort lost approximately 13% of their body weight, and were found to have greater FFAF after radiofrequency ablation compared to a control cohort.7
The present study assesses the impact of modest, patient-directed, weight loss on outcomes after CBA for AF. While prior work has demonstrated that significant physician-directed weight loss increases FFAF after ablation, frequent physician visits, weight loss greater than 10% and dietary changes may not be feasible for most patients. Patients in the present study were encouraged to lose weight by counselling from treating physicians during regularly scheduled appointments, rather than through inclusion in an integrated weight loss program. In addition, the current study is the first to show the impact of weight loss on FFAF after CBA. It has been suggested that the use of CBA may be favorable in an obese population due to the larger area of circumferential ablation.25 Finally, the present study demonstrates that any weight loss increases FFAF after ablation in nonobese patients, as well. Prior work has not assessed the impact of weight loss on patients with BMI < 27 kg/m2.
Regardless of baseline BMI, type of AF or absence of a prior ablation, all subgroups analyzed demonstrated benefit of weight loss on FFAF through 15 months with the exception of nonobese patients with persistent AF. This finding may be expected since the more advanced substrate in persistent atrial fibrillation would not necessarily reverse with weight loss in nonobese patients. Additionally, the number of patients in this subgroup may be underpowered to detect a statistically significant difference in outcomes.
There are several limitations of the current study including its retrospective nature and focus on CBA. Due to the intermittent nature of arrhythmia monitoring postablation, it is possible that asymptomatic AF episodes were missed. However, at a minimum, postablation rhythm monitoring was performed equally in obese and nonobese patients according to Heart Rhythm Society recommendations.16 While there was no difference in the rates of ambulatory rhythm monitoring between study groups, absence of a CIED was associated with FFAF in the present study. This finding may be expected as recent work by members of our group has demonstrated that a single 7-day ambulatory rhythm monitor has less than 50% sensitivity in detecting recurrent AF after ablation.26 Future studies may assess the impact of pre-ablation weight changes on postablation AF burden using continuous long-term monitoring for a more precise estimation of arrhythmia recurrence. Furthermore, while we routinely screened for sleep disordered breathing, we do not universally test for obstructive sleep apnea before ablation. This may have led to underestimation and undertreatment of obstructive sleep apnea in our sample. Due to the retrospective nature of this study, it was not possible to account for fluctuations in weight during the year before ablation.23 Finally, the favorable impact of weight loss on FFAF after ablation may have been affected by improvement in blood pressure, glycemic control, sleep disordered breathing, alcohol consumption, or cardiometabolic fitness. While the number of antihypertensive medications prescribed to each patient was collected, specific data on average blood pressure, blood glucose, sleep disordered breathing and overall fitness were not routinely recorded 1 year before ablation. Accordingly, they could not be evaluated in the current study but may serve as additional mechanisms by which weight loss improves ablation outcomes. Future studies should evaluate changes in these cardiometabolic factors, along with weight, before ablation in a prospective manner.
5 |. CONCLUSION
Regardless of baseline BMI, type of AF or history of prior ablation, percent weight loss in the year before ablation significantly predicted FFAF through 15 months in all patients undergoing ablation for AF except nonobese patients with persistent AF. Conversely, weight gain in the year before AF ablation was associated with lower rates of FFAF. Patient directed weight loss should be encouraged before AF ablation.
DISCLOSURE
Nishant Verma receives honoraria for speaking from Medtronic, Inc. Bradley P. Knight receives honoraria for consulting and speaking for Medtronic Inc. Rod S. Passman receives research support, consulting fees and speaker fees from Medtronic, research support from Abbott, and royalties from UpToDate; Northwestern University receives fellowship support from Medtronic, Inc.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. [DOI] [PubMed] [Google Scholar]
- 3.Knight BP, Novak PG, Sangrigoli R, et al. Long-Term Outcomes After Ablation for Paroxysmal Atrial Fibrillation Using the Second-Generation Cryoballoon: Final Results From STOP AF Post-Approval Study. JACC Clin Electrophysiol. 2019;5(3):306–314. [DOI] [PubMed] [Google Scholar]
- 4.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peigh G, Kaplan RM, Bavishi A, et al. A novel risk model for very late return of atrial fibrillation beyond 1 year after cryoballoon ablation: the SCALE-CryoAF score. J Interv Card Electrophysiol. 2020;58(2): 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sivasambu B, Balouch MA, Zghaib T, et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol. 2018;29(2): 239–245. [DOI] [PubMed] [Google Scholar]
- 7.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64(21):2222–2231. [DOI] [PubMed] [Google Scholar]
- 8.Pathak RK, Mahajan R, Lau DH, Sanders P. The implications of obesity for cardiac arrhythmia mechanisms and management. Can J Cardiol. 2015;31(2):203–210. [DOI] [PubMed] [Google Scholar]
- 9.Munger TM, Dong YX, Masaki M, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol. 2012;60(9):851–860. [DOI] [PubMed] [Google Scholar]
- 10.Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2016;37(20):1565–1572. [DOI] [PubMed] [Google Scholar]
- 11.Hales CMCM, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. Vol 360 Hyattsville, MD: National Center for Health Statistics; 2020. [Google Scholar]
- 12.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association. Circulation. 2020; 141(16):e750–e772. [DOI] [PubMed] [Google Scholar]
- 13.Azar AMOR, Piercy KL, Troiano RP, et al. Physical Activity Guidelines for Americans. Services UDoHaH. ed. 2 ed. Washington DC, 2018. [Google Scholar]
- 14.Mansour M, Calkins H, Osorio J, et al. Persistent Atrial Fibrillation Ablation With Contact Force-Sensing Catheter: The Prospective Multicenter PRECEPT Trial. JACC Clin Electrophysiol. 2020;6(8): 958–969. [DOI] [PubMed] [Google Scholar]
- 15.Peigh G, Wasserlauf J, Kaplan RM, et al. Repeat pulmonary vein isolation with or without FIRM-guided ablation for recurrent atrial fibrillation with pulmonary vein reconnection. J Cardiovasc Electrophysiol. 2020;31(5):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J Arrhythm. 2017;33(5):369–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-76–e1-776. [DOI] [PubMed] [Google Scholar]
- 18.Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10(1):90–100. [DOI] [PubMed] [Google Scholar]
- 19.Goudis CA, Vasileiadis IE, Liu T. Epicardial adipose tissue and atrial fibrillation: pathophysiological mechanisms, clinical implications, and potential therapies. Curr Med Res Opin. 2018;34(11):1933–1943. [DOI] [PubMed] [Google Scholar]
- 20.Ariyaratnam JPMM, Thomas G, Noubiap JJ, Lau D, Sanders P. Risk Factor Management Before and After Atrial Fibrillation Ablation. Cardiac Electrophysiology Clinics. 2020;12(2):141–154. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan R, Lau DH, Brooks AG, et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J Am Coll Cardiol. 2015;66(1):1–11. [DOI] [PubMed] [Google Scholar]
- 22.Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20(12): 1929–1935. [DOI] [PubMed] [Google Scholar]
- 23.Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015; 65(20):2159–2169. [DOI] [PubMed] [Google Scholar]
- 24.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–2060. [DOI] [PubMed] [Google Scholar]
- 25.Providência R, Adragão P, de Asmundis C, et al. Impact of Body Mass Index on the Outcomes of Catheter Ablation of Atrial Fibrillation: A European Observational Multicenter Study. J Am Heart Assoc. 2019;8(20):e012253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohrmann G, Kaplan R, Ziegler PD, Monteiro J, Passman R. Atrial fibrillation ablation success defined by duration of recurrence on cardiac implantable electronic devices. J Cardiovasc Electrophysiol. 2020;31(12):3124–3131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


