Abstract
PURPOSE:
To evaluate uptake and follow-up utilizing internet-assisted population genetic testing (GT) for BRCA1/2 Ashkenazi Jewish (AJ) founder mutations (Ashkenazi Jewish Pathogenic Variants: AJPVs).
METHODS:
Across four cities in the U.S. from December 2017-March 2020, individuals aged ≥25 with ≥1 AJ grandparent were offered enrollment. Participants consented and enrolled online with chatbot and video education, had BRCA1/2 AJPV GT, and chose to receive results from their primary care provider (PCP) or study staff. Surveys were at baseline, 12-weeks, and annually for 5 years.
RESULTS:
5,193 participants enrolled, and 4,109 (79.1%) were tested (median age=54, female=77.1%). Upon enrollment, 35.1% of participants selected a PCP to disclose results and 40.5% of PCPs agreed. Of those tested, 138 (3.4%) were AJPV heterozygotes, of whom 21 (15.2%) had no significant cancer family history (FH) while 86 (62.3%) had a known familial PV. At 12-weeks, 85.5% with AJPVs planned increased cancer screening; only 3.7% with negative results and a significant FH reported further testing.
CONCLUSIONS:
While continued follow-up is needed, internet-enabled outreach can expand access to targeted GT using a medical model. Observed challenges for population genetic screening efforts include recruitment barriers, improving PCP engagement, and increasing uptake of additional testing where indicated.
INTRODUCTION
Pathogenic variants (PVs) in the BRCA1 and BRCA2 (BRCA) genes are associated with elevated risks of breast, ovarian, and other cancers. Interventions including bilateral salpingo-oophorectomy (BSO) and/or mastectomy as well as enhanced screening can reduce cancer incidence and mortality among those with PVs.1,2 Despite the benefits of identifying BRCA PVs, testing rates are relatively low in the U.S. with <10% of individuals predicted to have a BRCA PV identified to date.3 Thus, there is a need to facilitate broader BRCA testing uptake in at-risk populations, including incorporating hereditary cancer risk assessment into the routine practice of healthcare providers.4
Traditional medical models of identifying individuals eligible for testing are time-consuming and limited by availability of trained providers.5,6 Many experts have called for other strategies including testing of all cancer patients and their relatives,7,8 or population-based genetic testing (GT) for common variants.9 Screening individuals of Ashkenazi Jewish (AJ) descent for the founder mutations (Ashkenazi Jewish Pathogenic Variants: AJPVs) accounting for >90% of BRCA PVs in that population represents one such strategy.10–12 This approach has been studied in Israel, Canada, England, Australia, and with a U.S. pilot study.13–18 The absence of universal healthcare in the U.S. presents unique barriers to both testing and monitoring of such programs, despite evidence of their cost-effectiveness.19 Both the National Comprehensive Cancer Network (NCCN) and the U.S. Preventive Services Task Force support GT for all individuals with AJ ancestry,20,21 recognizing that population screening faces barriers of access, cost, appropriate counseling and medical follow-up.22 Such testing has been included by some direct-to-consumer (DTC) for-profit laboratories. However, most DTC approaches raise concerns due to the lack of pre- and post-test counseling,23 occurrence of false-positive24 and false-negative25 results, and uncertain follow-up.26
The BRCA Founder OutReach (BFOR) study piloted a novel GT service delivery model that sought to combine the patient-centeredness and convenience of DTC testing through use of a digital portal with risk-adapted medical follow-up and engagement of primary care providers (PCPs) in results sharing and management. We report the initial experience of a demonstration study directed to individuals of AJ ancestry, and describe key outcomes related to uptake and early participant medical follow-up. We also define the challenges that emerged that will need to be addressed to ensure the safe, effective deployment of population-based GT.
MATERIALS AND METHODS
This observational cohort study was statistically powered to assess and measure completion of informed consent and GT, choice of receipt of GT results through PCPs or study-provided expert staff, and medical and psychological outcomes following testing. A separate aim, reported elsewhere,27 examined facilitators and barriers to engaging PCPs in the BRCA results disclosure process.28 The current study involved four geographic areas: New York City (Memorial Sloan Kettering Cancer Center), Boston (Beth Israel Deaconess Medical Center and Dana Farber Cancer Institute), Philadelphia (University of Pennsylvania), and Los Angeles (Cedars-Sinai Medical Center, transitioned to David Geffen School of Medicine at the University of California Los Angeles during study). Target accrual was 4,000 participants (approximately 1,000 per site). The study was approved by a central IRB, Advarra, and acknowledged by the local IRBs under reliance agreements.
Study Recruitment and Enrollment
Participants enrolled from December 2017-March 2020 (Figure 1). Recruitment primarily involved community outreach which included engagement of community leaders, participation in local events, educational talks by BFOR investigators, social media marketing, email blasts, and print media (Supplementary Methods 1a). Participant eligibility criteria included: age ≥25, ≥1 grandparent of AJ ancestry, no previous medical BRCA testing, healthcare insurance, residence within an eligible zip code, and English literacy. All prospective participants meeting these criteria were invited to enroll.
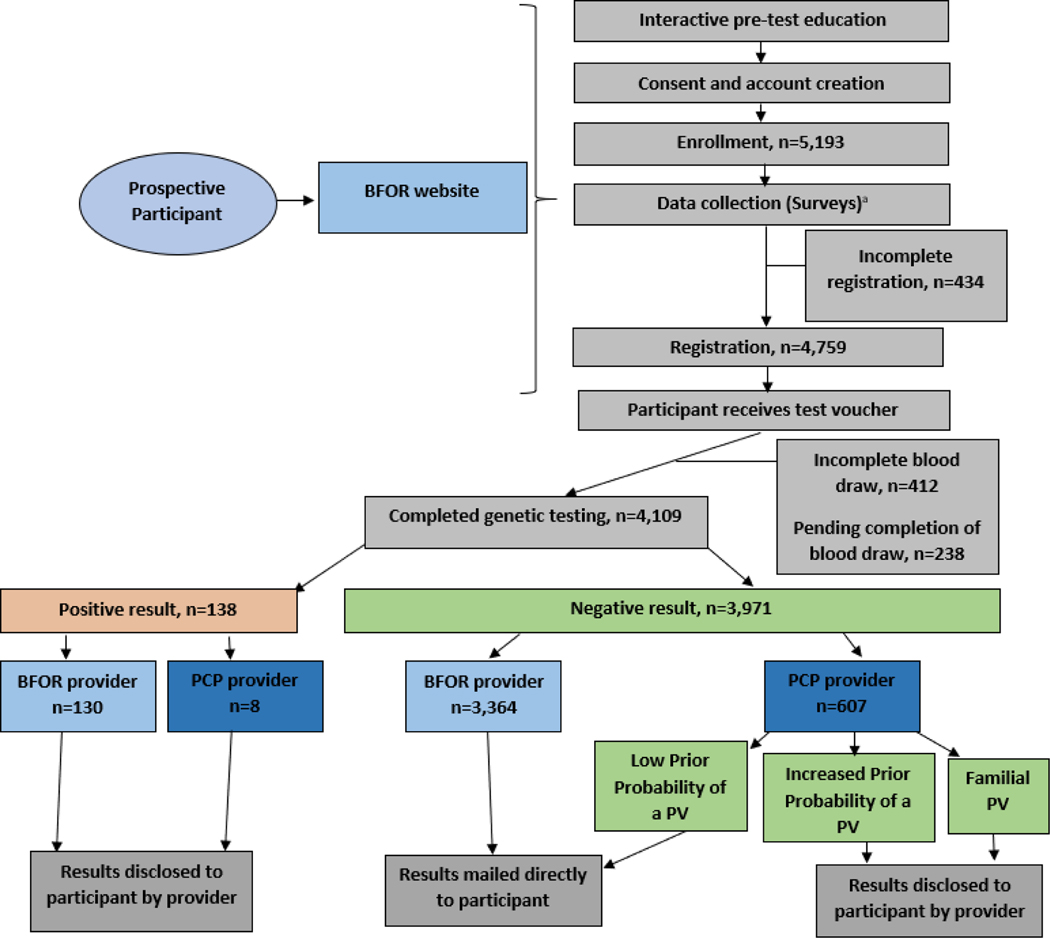
Figure 1.
Overall participant flow. PCP = Primary Care Provider; PV = Pathogenic Variant.
a. As a part of baseline data collection n=4,821 for participant responses to items assessing how they heard about the BFOR study and n=4,848 responded to items assessing from which provider they preferred to receive their results.
Participants completed pre-test and study education, provided consent, and answered surveys online. Following registration participants proceeded with genetic testing at Quest Diagnostics. Results disclosure method was stratified by results type, BRCA prior probability, and disclosing provider. Post-results survey data collection timepoints are not shown.
The study employed a chatbot-based digital health tool (LifeLink) accessed via www.bforstudy.com (Supplementary Figure 1), accompanied by a support hotline for participants and PCPs. Participants were enrolled upon eligibility confirmation, completion of interactive pre-test and study education, and informed e-consent, which approximated elements of pre-test counseling.29 Following e-consent, an online study account was created. To complete registration, enrollees answered personal and family history baseline questions. Following registration, participants were electronically provided with a requisition for BRCA AJPV GT to be performed at no-charge at Quest Diagnostics. Participants received up to three email reminders to complete registration and to have their blood drawn, and were considered to have not completed study GT if they did not complete registration within 90 days from enrollment or have their blood drawn within 180 days from registration.
Return of Results
During registration, participants elected to receive results from the BFOR team or their PCP. Nominated PCPs opted-in/out of disclosing results. Results and medical management recommendations were sent to disclosing PCPs to facilitate results transmission. For participants whose PCPs opted out or did not respond, BFOR disclosed results. Given the aim to assess PCP uptake at baseline, there was no systematic attempt to recruit PCPs to refer patients to the study.
Participants were categorized into BRCA prior probability categories based on an algorithm derived from NCCN BRCA guidelines at the time of study inception30 (Supplementary Methods 2). Enrollees were stratified into three categories based on their personal/family history of breast, ovarian, prostate, and pancreatic cancers and their family history of BRCA GT: 1) Low Prior Probability of a PV: individuals who did not meet NCCN guidelines without taking their AJ ancestry into account were deemed to be at low risk of having a BRCA PV; 2) Increased Prior Probability of a PV: individuals who met NCCN guidelines without taking their AJ ancestry into account were deemed to be at an increased risk of having a BRCA PV; and 3) Familial PV: individuals at risk for a reported familial BRCA PV. Method of results disclosure and recommended follow-up are detailed in Supplementary Methods 3. All participants who tested “positive” for a BRCA AJPV received post-test counseling from the BFOR team or their PCP supplemented with study provided post-test education; these individuals were also advised to complete no-cost confirmation testing (repeat testing of the AJ panel) through Quest Diagnostics. Individuals with an Increased Prior Probability of a PV who tested “negative” for a BRCA AJPV and who fulfilled general population guidelines for complete gene BRCA testing were recommended to undergo additional genetic testing outside of the study. In late 2019, a confirmation testing result discrepancy was identified. Following participant notification and remediation, all study participants were notified of these events and offered no-cost saliva-based confirmation testing.
Data Collection
Participants were surveyed upon enrollment (baseline), at 12-weeks post-disclosure, and will be surveyed annually for five years. Assessed variables included: 1) baseline: demographics, personal and family medical history, chatbot satisfaction, perceived risk; 2) baseline and follow-up: anxiety, cancer-specific distress, knowledge; and 3) follow-up: cancer risk management, completion of additional GT, perceived risk, GT decision satisfaction, GT concerns, family communication, self-concept (see details in Supplementary Table 1). Baseline and 12-week medical data are reported here. As described elsewhere,31 a subset of PCPs were surveyed at baseline, surveyed post-disclosure, and invited to participate in qualitative interviews to assess their BRCA GT knowledge and perspectives on and experiences with disclosing GT results.
Analyses
Descriptive statistics were computed for participant demographics, outcomes of recruitment efforts, participant preferences for and level of PCP involvement, and test results. To analyze impact of demographics on study endpoints, a multidimensional sociodemographic index reflecting income, education, employment, and housing quality was derived based on participant mailing address (Area Deprivation Index [ADI]).32,33 For analyses, we divided participants with complete ADI scores into quartiles based on the sample distribution from lowest to highest (1–100; higher scores indicating greater socioeconomic disadvantage). To evaluate participant characteristics associated with not completing study GT, receiving positive GT results, and selecting a PCP to disclose results, we conducted bivariate analyses followed by multivariable logistic regression models with forward stepwise selection (with models including variables significantly (p<0.05) associated in bivariate analyses with the outcome of interest). Multivariable models were confirmed to be a good fit to the data with no multicollinearity observed among model predictors (IBM SPSS Statistics V.26.0).
RESULTS
Participant Recruitment, Enrollment, and Demographics
Throughout the study there were over 200 discrete outreach efforts (Supplementary Methods 1b). We observed a temporal relationship between enrollment and selected outreach efforts (Supplementary Figure 2) with the greatest enrollment seen following public endorsement by community leaders through email blasts or media. Not all community or religious leaders lent active support to study participation, with a small number citing concerns including potential for stigma and perceived lack of medical actionability.34 Outreach through three targeted email blasts to 307,264 individuals of probable AJ ancestry yielded 6,691 (0.7%) study website visits and no discernible increase in enrollment. Of the 4,864 participant who reported their mode of knowledge regarding the study, 1,353 (27.8%) indicated they were influenced by a friend, 962 (19.8%) by a family member, 641 (13.2%) by social/regular media ads, 610 (12.5%) by a member of their community, 316 (6.5%) by a PCP, and 1,158 (23.8%) by other contacts or organizations.
From December 2017-March 2020, there were a total of 61,605 unique visits to the BFOR study website, 8.4% of which resulted in study enrollment (n=5,193 enrolled participants). Of the 5,193 participants enrolled in the BFOR study, 846 (16.3%) did not complete study GT (of whom 8.4% did not complete registration; 7.9% registered but did not proceed with a blood draw), , 238 (4.6%) were active in the study but had not yet completed GT at the time of analysis, and 4,109 (79.1%) completed study GT. Although enrolled participants’ ADI scores ranged from 1–97, participants were generally of high socioeconomic status (median ADI=5), with 3,286 (70.5% of those with an ADI) having a score ≤10 reflecting the least disadvantaged decile of national scores. Among participants who completed GT, a minority, 511 (12.4%), reported a personal cancer history, and 1,532 (37.3%) had never previously considered any GT (Table 1). Further, among these participants the median age was 54 and 3,169 (77.1%) were female; 2,304 (56.1%) had a low prior probability of a PV, 1,490 (36.3%) had an increased prior probability of a PV, and 315 (7.7%) had a known familial PV. Following pre-test education, genetic knowledge was high among participants with an average of 90% of questions answered correctly. When asked about interest in using a chatbot again for a research study or healthcare experience, interest was moderate (rating= 7.2/10).
Table 1.
Characteristics of the Cohort
| All Enrolled Participants | 5,193 | |
| Region | Philadelphia | 1,037 (20.0%) |
| New York | 1,670 (32.2%) | |
| Boston | 1,207 (23.2%) | |
| Los Angeles | 1,271 (24.5%) | |
| No Region | 8 (0.1%) | |
| Median ADI, Range | 5, 1–97 | |
| ADI Quartile 1 (AD1=1–2) |
1,229 (23.7%) |
|
| ADI Quartile 2 (ADI=3–5) |
1,101 (21.2%) |
|
| ADI Quartile 3 (ADI=6–12) |
1,233 (23.7%) |
|
| ADI Quartile 4 (ADI=13–100) |
1,095 (21.1%) |
|
| Missing ADI |
535 (10.3%) |
|
| Incomplete registration | 434 (8.4%) | |
| Incomplete blood draw | 412 (7.9%) | |
| Active in study but pending GT | 238 (4.6%) | |
| Completed study GT | 4,109 (79.1%) | |
| Male/Female | 940 (22.9%) / 3,169 (77.1%) | |
| Median Age, Range | 54, 25–93 25–44: 33.4% 45–64: 40.2% 65–84: 25.8% Over 85: 0.5% |
|
| Personal history of cancer | 511 (12.4%) | |
| Low Prior Probability of a PV | 2,304 (56.1%) | 490 (21.3%) Male |
| 1,814 (78.7%) Female | ||
| Increased Prior Probability of a PV | 1,490 (36.3%) | 301 (20.2%) Male |
| 1,189 (79.8%) Female | ||
| Familial PV | 315 (7.7%) | 149 (47.3%) Male |
| 166 (52.7%) Female | ||
ADI = Area Deprivation Index; GT = Genetic Testing, PV = Pathogenic Variant. Characteristics of the study cohort are represented above. Region is shown for all participants who provided consent for the study (n=5,193). In March 2020, all participants pending phlebotomy were deferred due to the COVID-19 pandemic and deemed ‘pending’ at time of analysis. The remaining demographics are reported for all participants who completed GT (n=4,109). Missing ADI scores are due to either incomplete participant street addresses or unavailability of corresponding ADI scores due to neighborhood characteristics.32,33
To explore the particular applicability of this model for facilitating cascade testing, baseline characteristics of those with a known familial PV were compared to those without a known familial PV (i.e., those with a low or increased prior probability of a PV). Those with known familial PV were significantly younger (mean age: 50.5 vs. 53.2, p=0.005), more likely to be male (47.3% vs. 20.8%, p<0.001), more likely to have had a provider recommend GT and had planned to do it (11.4% vs. 6.1%) or to have thought about GT but not done it yet (40.6% vs. 30.0%; p<0.001), less likely to have a PCP (86.0% vs. 91.8%, p<0.001), and more likely to have elevated baseline levels of cancer-specific distress (48.6% vs. 41.2%, p=0.015).
Characteristics Associated with Not Completing Study GT
We examined which participant characteristics were associated with not completing study GT due to incomplete registration or blood draw. In the multivariable analysis, ADI score, age, and BRCA prior probability were significantly associated with completion of study GT (Table 2). Specifically, those with greater social disadvantage (i.e., ADI scores in quartile 2, 3, and 4 of the sample) had a higher likelihood of not completing study GT than those with the least social disadvantage (i.e., ADI scores in quartile 1). Older age was associated with a lower likelihood of not completing study GT. Compared to participants with a low prior probability of a PV, participants with a familial PV had a lower likelihood of not completing study GT.
Table 2.
Characteristics Associated with Not Completing Study Genetic Testing (GT), Provider Choice, and GT Results
| Baseline Characteristic | N (%) Incomplete study GT | BV p value | Multivariable (adjusted) Odds Ratio (95% CI) | N (%) Selected PCP | BV p value | Multivariable (adjusted) Odds Ratio (95% CI) | N (%) Positive GT result | BV p value | Multivariable (adjusted) Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Agea: <54 years | 362 (14.3) | <.001 | .97 (.96-.98) | 765 (30.4) | <.001 | 1.01 (1.01–1.02) | 82 (4.1) | .015 | .98 (.97–1.00) |
| ≥54 years | 171 (7.3) | 938 (40.3) | 56 (2.7) | ||||||
| Sex: Female | 361 (9.7) | .15 | N/A | 1319 (35.4) | .34 | N/A | 62 (2.0) | <.001 | 1.00 (reference) |
| Male | 87 (8.2) | 358 (33.8) | 76 (8.1) | 3.47 (2.26–5.34) | |||||
| ADI: Quartile 1 | 168 (12.7) | <.001 | 1.00 (reference) | 419 (33.4) | .052 | N/A | 30 (2.8) | .08 | N/A |
| Quartile 2 | 188 (15.4) | 1.38 (1.01–1.88) | 394 (34.4) | 30 (3.1) | |||||
| Quartile 3 | 224 (16.3) | 1.44 (1.06–1.95) | 464 (36.0) | 36 (3.3) | |||||
| Quartile 4 | 248 (19.8) | 1.74 (1.29–2.35) | 422 (36.8) | 42 (4.4) | |||||
| BRCA Prior Probability: Low | 268 (9.9) | .014 | 1.00 (reference) | 950 (35.1) | .003 | 1.00 (reference) | 21 (0.9) | <.001 | 1.00 (reference) |
| Familial PV | 18 (5.2) | .47 (.28-.78) | 93 (27.0) | .72 (.56-.94) | 86 (27.3) | 28.07 (16.19–48.67) | |||
| Increased | 152 (8.8) | .89 (.72–1.11) | 631 (36.6) | 1.02 (.89–1.16) | 31 (2.1) | 2.35 (1.29–4.29) | |||
| Cancer-specific distressa: ≤ 6.0 | 213 (9.0) | .43 | N/A | 834 (35.3) | .86 | N/A | 56 (2.8) | .005 | 1.02 (1.01–1.04) |
| > 6.0 | 179 (8.7) | 735 (35.9) | 70 (3.9) | ||||||
| Past GT experience: NC | 167 (9.4) | .96 | N/A | 655 (36.8) | <.001 | 1.00 (reference) | 35 (2.3) | <.001 | N/A |
| PH | 95 (9.8) | 292 (30.3) | .83 (.70-.99) | 17 (2.1) | |||||
| PR&P | 28 (9.0) | 141 (45.5) | 1.45 (1.13–1.87) | 15 (5.6) | |||||
| PR&DA | 1 (4.5) | 11 (50.0) | 1.40 (.60–3.27) | 0 (0) | |||||
| TABND | 134 (9.2) | 492 (33.6) | .93 (.80–1.08) | 61 (4.8) | |||||
| TABI | 23 (9.4) | 86 (35.2) | .97 (.73–1.30) | 10 (4.7) | |||||
| Has PCP: Yes | 465 (10.5) | .007 | N/A | 1682 (38.3) | <.001 | 10.98 (7.04–17.13) | 116 (3.1) | .002 | N/A |
| No | 67 (14.7) | 21 (4.6) | 1.00 (reference) | 22 (6.2) | |||||
BV=bivariate (unadjusted) analysis; PCP=Primary Care Provider; ADI=Area Deprivation Index; Low=Low prior probability of a pathogenic variant (PV); Increased=Increased prior probability of a PV; Past GT experience=defined as any GT other than medical BRCA testing; NC=never considered; PH= previously had; PR&P=provider recommended and planned to do it; PR&DA= provider recommended and decided against it; TABND=thought about it but did not do it; TABI=thought about it but insurance won’t cover it.
N (%) shown for the variable dichotomized at the median; however, variable was treated as continuous for BV and MV analyses. Characteristics associated with not completing study GT, selecting a PCP to disclose results, and positive GT results are shown above, based on BV analysis followed by MV logistic regression models with forward stepwise selection (with models including variables significantly (p<0.05) associated in BV analyses with the outcome of interest). Significant MV associations are shown in bolded text. N/A indicates variable was nonsignificant in BV and/or MV model. Analyses also evaluated associations between personal cancer history, chosen provider for disclosure, parental status, perceived cancer risk, and anxiety with the outcomes of interest (results not shown due to nonsignificance).
Characteristics Associated with Provider Involvement
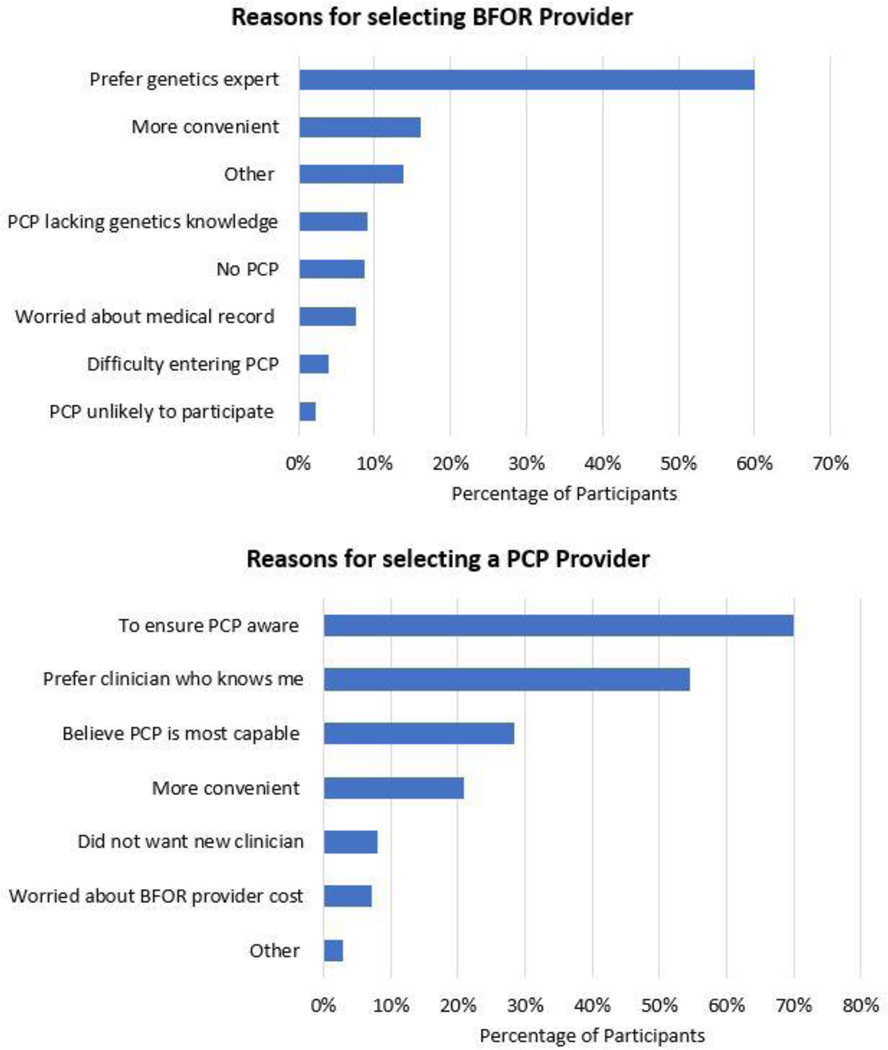
During registration, of the 4,848 participants who selected a provider to disclose results, 3,145 (64.9%) selected a BFOR provider and 1,703 (35.1%) nominated their PCP. Among the 2,336 participants who provided at least one reason for selecting a BFOR provider, the majority (60.1%) preferred to get results from a cancer genetics expert. Among the 1,387 participants who provided at least one reason for nominating their PCP, the majority (70.1%) wanted to ensure that their PCP was aware of their testing and results (Figure 2). In multivariable analysis, age, having an established PCP, BRCA prior probability, and past experience with GT (i.e., any GT other than medical BRCA testing) were significantly associated with the likelihood of choosing a PCP to return results (Table 2). Older age and having an established PCP were associated with a higher likelihood of choosing a PCP. Compared to participants with a low prior probability of a PV, participants with a familial PV had a lower likelihood of choosing a PCP. Compared to participants who had not considered any GT, those with any prior GT had a lower likelihood of choosing a PCP, while those whose provider had recommended testing and who were planning to have GT had a greater likelihood of choosing a PCP. Upon nomination, 690 (40.5%) PCP invitations to disclose results were accepted; for the remainder, results were disclosed by the BFOR team.
Figure 2.
Participant reported reason for provider choice. Key to full reasons below:
BFOR Choice: (n=2,336)
• Prefer genetics expert = I preferred to get my results from a clinician with expertise in cancer genetics
• More convenient = I thought it would be more convenient to see a BFOR-affiliated provider (for example, because of distance or scheduling)
• Other
• PCP lacking genetics knowledge = I did not think my primary care provider knows very much about BRCA or cancer genetics
• No PCP = I did not have a primary care provider
• Worried about medical record = I was worried about my BRCA results being in my medical record
• Difficulty entering PCP = I had difficulty entering my primary care provider’s information
• PCP unlikely to participate = I did not think my primary care provider would be willing to participate
PCP Choice: (n=1,387)
• To ensure PCP aware= I wanted to be sure my primary care provider is aware of the testing and results
• Prefer clinician who knows me= I preferred to get my results from a clinician who knows me
• Believe PCP is most capable= I believed my primary care provider is most capable of managing my healthcare
• More convenient= I thought it would be more convenient to see my primary care provider (for example, because of distance or scheduling)
• Did no want new clinician= I did not want to involve a new clinician in my healthcare
• Worried about BFOR provider cost= I was concerned about the potential costs (for example, insurance coverage for a BFOR-affiliated provider)
• Other
Characteristics Associated with GT Results
Of participants who completed study GT (n=4,109), 138 were found to have an AJPV (3.4%); of these individuals with a positive result, 21 (15.2%) had a low prior probability of a PV, 31 (22.5%) had an increased prior probability of a PV, and 86 (62.3%) had a familial PV (Table 3). Participants who had a low prior probability of a PV had a 0.9% chance of having an AJPV, compared to 2.1% of those who had an increased prior probability of a PV, and 27.3% of those who had a familial PV (Supplementary Table 2). Excluding those with a familial PV, 1.4% (52/3,794) of participants with either a low or increased prior probability of a PV had an AJPV. In total, 3,971 participants tested negative. In multivariable analysis, baseline cancer-specific distress, age, sex, and BRCA prior probability were significantly associated with result type (Table 2). Greater cancer-specific distress at baseline was associated with a greater likelihood of receiving a positive result. Older age was associated with a lower likelihood of receiving a positive result whereas male sex was associated with a higher likelihood. Compared to participants with a low prior probability of a PV, those with a familial PV or an increased prior probability of a PV had a greater likelihood of receiving a positive result.
Table 3.
BRCA Prior Probability, Sex, and Early Participant Outcomes according to Test Result
| Result | BRCA Prior Probability | Sex | Early Medical Follow-up and Intentions |
|---|---|---|---|
| 138 (3.4%) Positive GT | 21 (15.2%) Low Prior Probability of a PV | 8 (38.1%) Male | 59/69a (85.5%) of participants who tested positive reported planning to increase the frequency of their cancer screening based on their test results. |
| 13 (61.9%) Female | |||
| 31 (22.5%) Increased Prior Probability of a PV | 11 (35.5%) Male | ||
| 20 (64.5%) Female | |||
| 86 (62.3%) Familial PV | 57 (66.3%) Male | ||
| 29 (33.7%) Female | |||
| 3,971 (96.6%) Negative GT | 2,283 (57.5%) Low Prior Probability of a PV | 482 (21.1%) Male | |
| 1,801 (78.9%) Female | |||
| 1,459 (36.7%) Increased Prior Probability of a PV | 290 (19.9%) Male | 23/630a (3.7%) of participants with an increased prior probability of a PV who tested negative completed further recommended GT. | |
| 1,169 (80.1%) Female | |||
| 229 (5.8%) Familial PV | 92 (40.2%) Male | ||
| 137 (59.8%) Female |
GT= Genetic Testing, PV = Pathogenic Variant.
Note that 50% of participants who tested positive have completed the 12-week follow-up survey, and 43.2% of Increased Prior Probability of a PV participants who tested negative have completed the follow-up survey.
Participant Reported Early Medical Follow-up and Intentions
At time of analysis, the 12-week follow-up survey had been sent to and completed by 52.6% (2,069/3,932) participants, with completion differing by region (Philadelphia: 56.8%, Boston: 53.9%, New York City: 51.4%, Los Angeles: 50.0%, p=0.021), BRCA prior probability (low prior probability: 56.6%, familial PV: 49.2%, increased prior probability: 47.2%, p<0.001), and age (OR=1.01, 95% CI 1.00–1.01, p=0.03). At 12-weeks post-results disclosure, 44.6% (923/2,069) of participants reported referring at least one family member to participate in the BFOR study. Of participants with an increased prior probability of a PV who tested negative and completed the 12-week survey (n=630), 23 (3.7%) reported following the recommendation to complete further GT; 21 of these participants received results from a BFOR provider while two received results from their PCP (given limited sample size, no significant difference was observed, X2(1)=1.50, p=0.28). Among the 69 participants who tested positive and completed the 12-week survey, 59 (85.5%) reported planning to increase their cancer screening frequency based on their results (Table 3). One participant with a breast cancer diagnosis 25 years earlier tested positive for a BRCA2 AJPV and immediately underwent BSO with an early-stage invasive fallopian tube carcinoma identified; she received adjuvant chemotherapy and remains disease free. Her daughter then tested positive and underwent preventive surgeries. Another participant who tested positive for a BRCA1 AJPV underwent BSO in which a serous tubal intraepithelial carcinoma was identified.
Confirmation Testing and Expansion
During routine confirmation testing of participants with AJPVs a discordant result was detected, determined by the laboratory to be due to a processing issue affecting a single batch of samples with two total result discrepancies. Confirmation testing was then extended to all participants. With over 1,000 samples returned thus far, all are concordant with the original report.
DISCUSSION
Over a 27-month period the BFOR study achieved its target accrual of 4,000 participants, demonstrating the uptake of web-enabled population genetic screening. Among expected findings were the preponderance of female enrollees13,16,17 and the detection of an AJPV in 0.9% of those with a low prior probability of a PV.35 While the study identified AJPV heterozygotes who did not meet clinical family history criteria for GT in the general population (n=21, 15.2%), most participants with an AJPV met such criteria. Thus, 60% (31/52) of those without a known familial PV and 84.8% (117/138) overall had an AJPV detected in the presence of a family history, demonstrating the predictive importance of family history even in the AJ population. Rates of GT completion and participant knowledge following pre-test education were comparable to studies including traditional pre-test counseling,36 demonstrating effectiveness of this digital approach in a real-world setting. Over a quarter of enrollees were >65 years old, demonstrating that older age was not an inherent barrier to a web-based initiative. Although older participants were less likely to test positive, we observed a significant association between increasing age and GT completion. Older individuals may have been more inclined to have GT, perhaps due to lack of insurance coverage for BRCA testing, had more time to complete the study, or had fewer life insurance concerns. The finding that participants with a familial PV were more likely to complete GT may reflect a higher motivation for these participants based on their increased likelihood of testing positive.
This study also elucidated many challenges facing web-enabled community-based service delivery models. Only 8.4% of website visits resulted in study enrollment. Once enrolled, 16.3% of participants did not complete study GT, not completing either registration (8.4%) or blood draw (7.9%). Study enrollment and registration took approximately one hour, with participants reporting moderate interest in using a similar chatbot again. Blood draw was the primary mechanism of specimen collection, as saliva-based analysis was not available at study commencement and used only for confirmation testing, also perhaps limiting enrollment. Although the high number of website visits compared to study consents may be partially accounted for by individuals who were ineligible for the study as well as visitors returning on different devices, an incremental increase in the conversion of website visits to study enrollment would substantially increase uptake. Future approaches could utilize briefer pre-test education, less time-intensive research instruments, and expanded specimen collection options (e.g., at-home saliva collection).
Despite the large numbers of individuals of AJ ancestry in the recruitment areas, it took over two years to enroll the projected 4,000 participants. Even with substantial community outreach, multiple barriers impacted timely recruitment. Outreach through paid social media marketing was extremely low yield; referral from friends, family members, or other contacts or organizations was more effective and cited as primary motivators for participation. Endorsements from community leaders and religious organizations were generally but not universally favorable or occurred toward the end of the enrollment period. As this study did not systematically recruit PCPs to refer patients for testing, interventions that target PCPs could be explored as potential strategies to increase GT uptake. Although BFOR study testing was provided at no cost to participants, the study population was characterized as predominantly high socioeconomic status. However, not completing study GT was more common among participants with greater social disadvantage. Although the potential barrier of cost to testing uptake was likely ameliorated by the no-cost provision of testing in this study, these findings suggest that economic constraints remain a challenge for completion of and follow-up of population genetic screening.
The BFOR study demonstrated important challenges to scaling up a medical GT model that utilizes healthcare providers to share results and direct management. The finding that most participants (64.9%) chose to get their results from a BFOR provider, attributing this choice to their perceived expertise, underscores the continued need to expand the number of cancer genetics specialists and/or increase PCPs’ recognized roles in the field. Older participants were more likely to select PCPs to convey results, perhaps due to established relationships with their providers. In contrast, participants who had a personal experience with GT were less likely to select a PCP to disclose results, perhaps reflecting increased familiarity with the role of genetic counselors. Notably, PCP acceptance of the minority (40.5%) of invitations to disclose results demonstrates a critical need to develop strategies to better engage PCPs in genetic medicine; such strategies could involve professional and academic organizations of internists, generalists, and other health providers.37,38
Although this study demonstrates that access to genetic counseling and testing services could be improved with web-based tools, barriers to post-test preventive care remain, many of which have been identified in a prior study in Israel.13 In addition to analytic validity, accurate test interpretation critically depends on context. Early study assessments of patient management reveal low adherence to recommendations for additional GT in participants with an increased prior probability of a PV and negative AJPV testing. While the need for additional personal/family history-directed testing in the context of population screening has not been fully addressed in prior studies,14,17 this issue was addressed and highlighted in the study in Israel where among the 26% of participants who tested negative for an AJPV with a suggestive family history, 18% of those enrolled in the population screening arm did not comply with post-test recommendations.13 This issue of post-test compliance poses a significant challenge to avoid false reassurance from negative results.39,40 These observations also suggest that broader, panel-based initial testing, although more costly and likely to yield variants of uncertain significance, may be more efficient. However, there is no consensus about which genes such a panel should include, and broader panels would also make PCP engagement more challenging. Although individuals with familial PVs were not targeted for recruitment, it is notable that they represented 7.7% of all participants tested. The findings that study participants with a familial PV were more likely to be younger and male, and that the majority of AJPV heterozygotes, predominantly men, had a familial PV, supports the particular utility of this web-based model in facilitating cascade GT.8 These findings are relevant to future population screening models which will include individuals aware and unaware of a history of a familial PV. Confirmation testing, built into study procedures, detected two discordant results due to a processing issue affecting a single batch of samples, which underscores the importance of assuring the accuracy of GT administered on a population scale.
Finally, continued monitoring of participants through annual surveys will be important. Longer-term follow-up will allow for evaluation of the appropriateness of medical interventions and participant outcomes as well as comparison of adherence to recommendations according to disclosing provider.
Thus, early results from the BFOR study demonstrate the potential impact of integrating a web-enabled digital tool into GT delivery. This approach effectively provided targeted GT to a population known to be at increased risk for inherited PVs and serves as an additional rationale for insurance providers to recognize AJ ancestry as a stand-alone criterion for AJPV testing regardless of family history. This preliminary report does not address clinical endpoints, psychological impact, or measures of the provider experience which will be addressed in subsequent papers. However, this study has revealed challenges relating to community uptake, engagement of PCPs in delivery of GT services, laboratory testing and logistics, and the need for continued outreach to participants who tested negative but may require further GT or enhanced screening. Based on these findings, certain modifications to subsequent population genetic screening efforts should be considered. Future study designs could include: recruitment of high-risk individuals based on criteria not limited to ancestry, enhanced community and PCP outreach to facilitate participation, facilitated cascade testing, use of at-home saliva testing and broader panel-based GT, and augmented educational and engagement efforts targeted to a broad spectrum of healthcare providers.
Supplementary Material
ACKNOWLEDGEMENTS:
This study was funded by the Sharon Levine Corzine Research Fund, Breast Cancer Research Foundation, The Basser Center for BRCA, The Nancy Ann Mellen Fund for Hereditary Cancer Research, Robin and Ken Isaacs, Brooke and Eric Meltzer, Jerold O. and Abbe Beth Young, Michael Stoler, Anonymous Donors, American Cancer Society Cancer Control Career Development Award for Primary Care Physicians (LEP, grant number 130741-CCCDA-17–072-01-CCCDA), American Cancer Society Mentored Research Scholar Grants in Applied and Clinical Research (JGH, grant number MRSG-16–020-01-CPPB), NCI P30 CA008748 (JGH, JDL, PRT, VM, TW, BD, MER, KMM, KO), NCI P30 CA016520 (SMD, KLN, HS, KS), NCI P30CA016042 (BYK, DK, JL, AB) We would like to acknowledge Oneinforty and its founder, the late Lauren M. Corduck (1971–2020), as well as all BFOR study participants. The BFOR study paid for services by vendor agreement with LifeLink Inc. and an agreement with Quest Diagnostics who provided testing at discounted costs to the BFOR study and no cost to participants.
ETHICS DECLARATION:
The BFOR study was approved by a central IRB (cIRB), Advarra (formerly Quorum) and acknowledged by the local IRBs (MSKCC, DFCI, BI, UCLA, UPENN) under reliance agreements. Informed consent was required and obtained from all participants. Identifying information was collected with access restricted to study staff, with de-identified data used for purposes of analysis.
Footnotes
AUTHOR INFORMATION:
Conceptualization: JGH, LEP, RCB, JNB, MER, KLN, NT, BYK, SD, JEG, KO; Data Curation: KMM, JDL, PRT; Formal Analysis: KMM, JGH, PRT; Funding Acquisition: JGH, LEP, NT, BYK, SD, JEG, KO; Investigation: JGH, LEP, KLN, NT, BYK, SD, JEG, KO; Methodology: JGH, LEP, KLN, NT; BYK, SD, JEG, KO; Resources: YF, KB, VH, JH; Project Administration: KMM, HS, DK, CJ, JL, KS, CG, JDL, AB, VM, TW, BD, RCB, JNB; Software: JH; Supervision: KLN, NT, BYK, SD, JEG, KO; Writing – original draft: KMM, JGH, KO; Writing – review & editing: HS, DK, CJ, JL, KS, LEP, CG, JDL, PRT, AB, VM, TW, BD, RCB, JNB, YF, KB, VH, JH, MER, KLN, NT, BYK, SD, JEG
Trial Registration: clinicaltrials.gov Identifier: NCT03351803
DATA AVAILABILITY:
Data supporting this manuscript as reported in Tables 1–3, Figure 2, Supplementary Table 2, and Supplementary Figure 2 are not publicly available in order to protect patient privacy. Deidentified aggregate data is available upon request. Data requests can be made via email to the corresponding author and will be pending data use agreements: Offitk@mskcc.org.
REFERENCES:
- 1.Nelson HD, Fu R, Goddard K, et al. U.S. Preventive Services Task Force Evidence Synthesis, formerly Systematic Evidence Reviews. In: Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 2.Sigal BM, Munoz DF, Kurian AW, Plevritis SK. A simulation model to predict the impact of prophylactic surgery and screening on the life expectancy of BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drohan B, Roche CA, Cusack JC Jr., Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: Using technology to identify carriers. Ann Surg Oncol. 2012;19(6):1732–1737. [DOI] [PubMed] [Google Scholar]
- 4.PDQ® Cancer Genetics Editorial Board. PDQ Cancer Genetics Risk Assessment and Counseling. Bethesda, MD: National Cancer Institute. 2021. https://www.cancer.gov/about-cancer/causes-prevention/genetics/risk-assessment-pdq. Accessed 15 September 2020. [Google Scholar]
- 5.Cohen SA, Huziak RC, Gustafson S, Grubs RE. Analysis of advantages, limitations, and barriers of genetic counseling service delivery models. J Genet Couns. 2016;25(5):1010–1018. [DOI] [PubMed] [Google Scholar]
- 6.Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the supply and demand for certified genetic counselors: A workforce study. J Genet Couns. 2018;27(1):16–20. [DOI] [PubMed] [Google Scholar]
- 7.Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;318(9):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: An alternative to population-based screening. J Clin Oncol. 2020;38(13):1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy-Lahad E, Lahad A, King MC. Precision medicine meets public health: Population screening for BRCA1 and BRCA2. J Natl Cancer Inst. 2015;107(1):420. [DOI] [PubMed] [Google Scholar]
- 10.Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14(2):185–187. [DOI] [PubMed] [Google Scholar]
- 11.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408. [DOI] [PubMed] [Google Scholar]
- 12.Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. 2000;83(10):1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman S, Tomer A, Ben-Chetrit A, et al. Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: Proactive recruitment compared with self-referral. Genet Med. 2017;19(7):754–762. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe KA, Poll A, Royer R, et al. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28(3):387–391. [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe KA, Poll A, Royer R, et al. A comparison of the detection of BRCA mutation carriers through the provision of Jewish population-based genetic testing compared with clinic-based genetic testing. Br J Cancer. 2013;109(3):777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manchanda R, Loggenberg K, Sanderson S, et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: A randomized controlled trial. J Natl Cancer Inst. 2015;107(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesman C, Rose E, Grant A, Zimilover A, Klugman S, Schreiber-Agus N. Experiences from a pilot program bringing BRCA1/2 genetic screening to the US Ashkenazi Jewish population. Genet Med. 2017;19(5):529–536. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman S, Lahad A, Tomer A, Cohen C, Levy-Lahad E, Raz A. Population screening for BRCA1/BRCA2 mutations: Lessons from qualitative analysis of the screening experience. Genet Med. 2017;19(6):628–634. [DOI] [PubMed] [Google Scholar]
- 19.Manchanda R, Patel S, Antoniou AC, et al. Cost-effectiveness of population based BRCA testing with varying Ashkenazi Jewish ancestry. Am J Obstet Gynecol. 2017;217(5):578 e571–578 e512. [DOI] [PubMed] [Google Scholar]
- 20.Daly MB, Pilarski R, Yurgelun MB, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18(4):380–391. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopal PS, Nielsen S, Olopade OI. USPSTF Recommendations for BRCA1 and BRCA2 testing in the context of a transformative national cancer control plan. JAMA Netw Open. 2019;2(8):e1910142. [DOI] [PubMed] [Google Scholar]
- 22.Swink A, Nair A, Hoof P, et al. Barriers to the utilization of genetic testing and genetic counseling in patients with suspected hereditary breast and ovarian cancers. Proc (Bayl Univ Med Cent). 2019;32(3):340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borry P, Cornel MC, Howard HC. Where are you going, where have you been: A recent history of the direct-to-consumer genetic testing market. J Community Genet. 2010;1(3):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tandy-Connor S, Guiltinan J, Krempely K, et al. False-positive results released by direct-to-consumer genetic tests highlight the importance of clinical confirmation testing for appropriate patient care. Genet Med. 2018;20(12):1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton R, Crawford G, Freeman L, Fenwick A, Wright CF, Lucassen A. Direct-to-consumer genetic testing. BMJ. 2019;367:l5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Academies of Sciences, Enginnering and Medicine. Exploring the Current Landscape of Consumer Genomics: Proceedings of a Workshop. Washington, DC: The National Academies Press; 2020. [PubMed] [Google Scholar]
- 27.Pace L, Tung N, Hamilton JG, et al. Challenges and opportunities in engaging primary care providers in BRCA testing: Early results from the BFOR study. Poster Presented at the Society for General Internal Medicine Annual Meeting; May 8–11, 2019; Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pace LE, Tung N, Lee YS, et al. Challenges and opportunities in engaging primary care providers in BRCA testing: Results from the BFOR study. J Gen Intern Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeCompte LL, Young SJ. Revised common rule changes to the consent process and consent form. Ochsner J. 2020;20(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly MB, Pilarski R, Axilbund JE, et al. Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2015. J Natl Compr Canc Netw. 2016;14(2):153–162. [DOI] [PubMed] [Google Scholar]
- 31.Pace LE, Lee YS, Tung N, et al. Comparison of up-front cash cards and checks as incentives for participation in a clinician survey: A study within a trial. BMC Med Res Methodol. 2020;20(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.University of Wisconsin School of Medicine and Public Health. 2018. Area Deprivation Index V3.0. Downloaded from https://www.neighborhoodatlas.medicine.wisc.edu/ May 1, 2021.
- 34.Eckstein Y.Genetic Testing? Only When It Will Do No Harm. In. AMI 2016:10–11. [Google Scholar]
- 35.Lynce F, Isaacs C. Population-based BRCA1/2 testing in Ashkenazi Jews: Ready for prime time. J Natl Compr Canc Netw. 2016;14(6):809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manchanda R, Burnell M, Gaba F, et al. Attitude towards and factors affecting uptake of population-based BRCA testing in the Ashkenazi Jewish population: A cohort study. BJOG. 2019;126(6):784–794. [DOI] [PubMed] [Google Scholar]
- 37.Trepanier AM, Supplee L, Blakely L, McLosky J, Duquette D. Public health approaches and barriers to educating providers about hereditary breast and ovarian cancer syndrome. Healthcare (Basel). 2016;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weitzel JN, Blazer KR, MacDonald DJ, Culver JO, Offit K. Genetics, genomics, and cancer risk assessment: State of the art and future directions in the era of personalized medicine. CA Cancer J Clin. 2011;61(5):327–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath SP, Walton N, Williams MS, Kim KK, Bastola K. Are providers prepared for genomic medicine: Interpretation of direct-to-consumer genetic testing (DTC-GT) results and genetic self-efficacy by medical professionals. BMC Health Serv Res. 2019;19(1):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller CE, Krautscheid P, Baldwin EE, et al. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A. 2014;164A(5):1094–1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this manuscript as reported in Tables 1–3, Figure 2, Supplementary Table 2, and Supplementary Figure 2 are not publicly available in order to protect patient privacy. Deidentified aggregate data is available upon request. Data requests can be made via email to the corresponding author and will be pending data use agreements: Offitk@mskcc.org.