Abstract
Background
Five-fluorouracil, folinic acid, oxaliplatin and irinotecan (FOLFOXIRI) regimen is used as the first-line treatment for metastatic colorectal cancer (mCRC). The use of capecitabine, an oral fluoropyrimidine pro-drug, is feasible and safe; hence, it provides an interesting alternative to 5-fluorouracil in the abovementioned regimen. This study aimed to evaluate the efficacy and safety of capecitabine, oxaliplatin, and irinotecan (XELOXIRI) regimen use with or without targeted drugs in Chinese patients with mCRC.
Methods
We conducted a retrospective, longitudinal cohort study of patients with mCRC who received XELOXIRI regimen with or without targeted drugs (bevacizumab or cetuximab) every 2 weeks between January 2017 and November 2019 at the National Cancer Center/Cancer Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College. Treatment efficacy was assessed by investigators by evaluating the objective response rate (ORR) and disease control rate (DCR). Overall survival (OS) was assessed using Cox proportional hazards models. The adverse events were also analyzed.
Results
Sixty-one consecutive patients were examined and followed up for survival. As of November 8, 2021, the median follow-up time was 35.4 months. Disease progression and death occurred in 50 (82%) and 38 (62%) patients, respectively. The median treatment duration of XELOXIRI with or without bevacizumab or cetuximab was 10 cycles (range, 1–12 cycles). The median OS and PFS were 32.2 months (95%CI [24.8–39.6]) and 9.3 months (95% CI [8.1–10.5]), respectively. The ORR of 48 patients with measurable lesions was 70.8%, and the DCR was 89.6%. RAS/BRAF wild-type (HR 0.39; 95% CI [0.16–0.96], p = 0.04) and metastatic organs > 2 (HR 3.25; 95% CI [1.34–7.87], p = 0.009) were independent prognostic factors for OS. The incidence of any grade of adverse events (AEs) was 96.7% (59/61). Grade ≥ 3 AEs included neutropenia (19.7%), leukopenia (9.8%), diarrhea (3.3%), vomiting (3.3%), febrile neutropenia (1.6%), and thrombocytopenia (1.6%). No treatment-related death occurred.
Conclusion
The use of the XELOXIRI regimen with or without a targeted drug was effective, with a manageable toxicity profile in Chinese patients with mCRC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09889-3.
Keywords: Colorectal cancer, Triplet chemotherapy, Irinotecan, Oxaliplatin, Capecitabine
Background
Over the last decade, the triplet chemotherapy combining irinotecan, oxaliplatin, and 5-fluorouracil (FOLFOXIRI) with or without monoclonal antibodies (bevacizumab or cetuximab) as the first-line treatment of metastatic colorectal cancer (mCRC) has shown a significantly improved overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and R0 resection rate of liver metastases than standard doublet chemotherapy regimens [1–11]. Since 2015, FOLFOXIRI plus bevacizumab has been listed as a first-line treatment option for fit patients with mCRC in several clinical guidelines worldwide [12–15]. However, the use of this regimen is also characterized by high toxicity, even in well-designed clinical trials, most of which were conducted in European and American populations; additionally, there is no uniform dose level of the components of this regimen. Few studies in China and Japan have reported that Asian populations can tolerate lower doses of the component drugs in the FOLFOXIRI regimen, especially the dose of irinotecan (which is recommended to be 150 mg/m2). Moreover, indirect comparisons have shown a higher incidence of neutropenia in Asian patients than in Western patients [16, 17]. In a phase I dose-escalation study conducted in China, the maximum tolerated dose of irinotecan was only 150 mg/m2 in a single infusion on day 8 [17]. The dose-limited toxicities (DLTs) of irinotecan were diarrhea and febrile neutropenia. The FOLFOXIRI has a limited clinical application, as the original regimen is poorly tolerated, especially in Asian patients; hence, an expert consensus in China has recommended the use of modified FOLFOXIRI [18, 19]. The Chinese modified FOLFOXIRI (cmFOLFOXIRI) regimen consists of intravenous infusions of oxaliplatin 85 mg/m2 over 120 min, irinotecan 150–165 mg/m2 over 90 min, folinic acid 400 mg/m2 over 120 min, and 5-fluorouracil 2,400–2,800 mg/m2 over a 46–48 h continuous infusion every 2 weeks. Nevertheless, FOLFOXIRI or cmFOLFOXIRI use is inconvenient, as it necessitates a continuous infusion of 5-fluorouracil, which requires the placement of indwelling central venous catheters and portable infusion pumps.
Capecitabine, an oral fluoropyrimidine pro-drug that demonstrates a superior safety profile and convenience, can be used as an alternative to 5-fluorouracil [20–23]. The use of the triplet regimen (XELOXIRI, COI, CAPOXIRI, or XELIRINOX), with capecitabine replacing 5-fluorouracil as the fluoropyrimidine backbone with or without antibodies, has been investigated in several phases I and II clinical trials in the first line and in conversion setting with different dose schedules [24–29]. The DLTs were grade 4 neutropenia and grade 3 diarrhea. The incidence of grade 3/4 neutropenia (0–57%) with XELOXIRI use was as high as that with FOLFOXIRI use, while the incidence of grade 3/4 diarrhea (0–40%) seems higher than that of FOLFOXIRI use, especially in patients with mCRC in Western countries [24–29]; this demonstrates the ethnic differences in oral fluoropyrimidine metabolism and tolerability between Western and Eastern Asian populations.
This study aimed to retrospectively evaluate the efficacy and safety of XELOXIRI use with or without targeted drugs in Chinese patients with advanced colorectal cancer.
Methods
Study design and population
This was a retrospective, longitudinal cohort study conducted at the National Cancer Center/Cancer Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College.
From January 1, 2017, to November 30, 2019, we examined eligible patients who developed a histologically confirmed adenocarcinoma of the colon or rectum, with the first occurrence of metastatic disease deemed unresectable at the age of at least 18 years and the presence of measurable and/or assessable lesions according to RECIST1.1 criteria. Furthermore, we included patients who had initiated therapy with XELOXIRI alone or combined with bevacizumab or cetuximab every 2 weeks for mCRC and had at least one visit to the study center. Major exclusion criteria included patients with previous chemotherapy or targeted therapy history (excludes patients with recurrence and metastasis more than 6 months away from adjuvant or neoadjuvant chemotherapy) and other malignant tumours in the past 5 years (except for cervical carcinoma in situ, cutaneous squamous or basal cell carcinoma treated for radical purposes).
We collected data on the following demographic and clinical characteristics at baseline: primary cancer site, sites of metastases, genetic mutation status, treatment type, dosing and drug dose modifications, tumour response, treatment after XELOXIRI use, survival, and patient conditions while on treatment, as documented in our medical record system. PFS was defined as the time period from the date of chemotherapy initiation to the date of imaging-confirmed disease progression or the death from any cause before disease progression. Disease-free survival (DFS) was defined as the time period from the day of R0 resection to imaging-confirmed disease progression or death due to any cause before cancer recurrence or the appearance of a second primary cancer. OS is defined as the time period from the date of chemotherapy initiation to death from any cause. ORR, DCR, PFS and DFS were evaluated by the investigator retrospectively. This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College.
Statistical analysis
Descriptive statistics were used to analyze demographic as well as clinical efficacy and safety data at baseline and during follow-up. The measurement data were analyzed using the t-test and count data using the χ2 test. The Wilcoxon rank-sum test was used for the comparison of grade data. The ORR was defined as the proportion of patients with the best response of complete response (CR) or partial response (PR), and disease control rate (DCR) was defined as the proportion of patients with the best response of CR, PR, or stable disease (SD) according to RECIST1.1 criteria. Survival analysis was performed using the Kaplan–Meier method to estimate the median and 95% confidence interval (CI) of the incidence of events. The log-rank test was used for subgroup analysis. Cox regression analysis was used to evaluate the impact of research factors on survival or risk rate. All statistical tests were two-sided, and p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, New York, USA).
Results
Patient characteristics
Patient clinical and pathological features are shown in Table 1. This study included 61 patients, 38 (62.3%) of whom were men. The median age was 50 years (range, 26–70 years). In 19(31.1%) patients, the primary tumour was in the right colon (from the cecum to the transverse colon). Forty-two (68.9%) patients had left-sided tumours, including 17 (27.9%) and 25 (41.0%) patients whose primary tumours were in the colon (from the splenic flexure to the sigmoid colon) and rectum, respectively. Moreover, 56 patients (91.8%) had synchronous distant metastases at diagnosis, 14 (23%) and 11 (18%) of whom had peritoneal metastases and more than two metastatic sites, respectively. Genetic mutation status was available in 54 patients. We found that 23 (37.7%) patients had RAS mutation, 12 (19.7%) patients had BRAFV600E mutation, and 19 (31.1%) patients had RAS/BRAFV600E wild-type (WT). No known high microsatellite instability (MSI-H) was present.
Table 1.
Patient characteristics
| N (%) | All | XELOXIRI | XELOXIRI + BEV | XELOXIRI + CET |
|---|---|---|---|---|
| 61 (100) | 39 (63.9) | 18a (29.5) | 4 (6.6) | |
| Sex | ||||
| Male | 38 (62.3) | 27 (44.3) | 9 (14.8) | 2 (3.3) |
| Female | 23 (37.7) | 12 (19.7) | 9 (14.8) | 2 (3.3) |
| Age | ||||
| Median (range) | 50 (26–70) | 60 (27–70) | 38 (36–66) | 29.5 (26–46) |
| ≤ 65 years | 52 (85.2) | 31 (50.8) | 17 (27.9) | 4 (6.6) |
| > 65 years | 9 (14.8) | 8 (13.1) | 1 (1.6) | 0 |
| ECOG | ||||
| 0 | 20 (32.8) | 15 (24.6) | 4 (6.6) | 1 (1.6) |
| 1 | 41 (68.3) | 24 (39.3) | 14 (23.0) | 3 (4.9) |
| Primary location | ||||
| Right colon | 19 (31.1) | 13 (21.3) | 6 (9.8) | 0 |
| Left colon | 42 (68.9) | 26 (42.6) | 12 (19.7) | 4 (6.6) |
| Colon | 17 (27.9) | 6 (9.8) | 9 (14.8) | 2 (3.3) |
| Rectum | 25 (41.0) | 20 (32.8) | 3 (4.9) | 2 (3.3) |
| Surgery on primary tumour | ||||
| Yes | 7 (11.5) | 4 (6.6) | 3 (4.9) | 0 |
| No | 54 (88.5) | 35 (57.4) | 15 (24.6) | 4 (6.6) |
| (Neo)adjuvant chemotherapy | ||||
| Yes | 6 (9.8) | 3 (4.9) | 3 (4.9) | 0 |
| No | 55 (90.2) | 36 (59.0) | 15 (24.6) | 4 (6.6) |
| (Neo)adjuvant radiotherapy | ||||
| Yes | 1 (1.6) | 1 (1.6) | 0 | 0 |
| No | 60 (98.4) | 38 (62.3) | 18 (29.5) | 4 (6.6) |
| Time to metastasis | ||||
| synchronous | 56 (91.8) | 35 (57.4) | 17 (27.9) | 4 (6.6) |
| Metachronous | 5 (8.2) | 4 (6.6) | 1 (1.6) | 0 |
| Metastasis sites | ||||
| Liver | 41 (67.2) | 27 (44.3) | 11 (18.0) | 3 (4.9) |
| Liver-only | 15 (24.6) | 9 (14.8) | 5 (8.2) | 1 (1.6) |
| Lung | 18 (29.5) | 12 | 6 (9.8) | 0 |
| Lung-only | 5 (8.2) | 4 (6.6) | 1 (1.6) | 0 |
| Peritoneum | 14 (23.0) | 8 (13.1) | 5 (8.2) | 1 (1.6) |
| Peritoneum-only | 7 | 3(4.9) | 3(4.9) | 1(1.6) |
| Numbers of metastatic organs | ||||
| 1 | 32 (52.5) | 20 (32.8) | 10 (16.4) | 2 (3.3) |
| 2 | 18 (29.5) | 13 (21.3) | 4 (6.6) | 1 (1.6) |
| > 2 | 11 (18.0) | 6 (9.8) | 4 (6.6) | 1 (1.6) |
| RAS and BRAF status | ||||
| RAS mutant | 23 (37.7) | 17 (27.9) | 6 (9.8) | 0 |
| BRAF mutant | 12 (19.7) | 4 (6.6) | 8 (13.1) | 0 |
| RAS/BRAF wild-type | 19 (31.1) | 13 (21.3) | 2a (3.3) | 4 (6.6) |
| Missing data | 7 (11.5) | 5 (8.2) | 2 (3.3) | 0 |
| Primary tumour site and RAS/BRAF status | ||||
| Right and RAS/BRAF wild-type | 2 (3.3) | 1 (1.6) | 1 (1.6) | 0 |
| Right and RAS mutant | 11 (18.0) | 8 (13.1) | 3 (4.9) | 0 |
| Right and BRAF mutant | 2 (3.3) | 2 (3.3) | 0 | 0 |
| Left and RAS/BRAF wild-type | 17 (27.9) | 12 (19.7) | 1 (1.6) | 4 (6.6) |
| Left and RAS mutant | 12 (19.7) | 9 (14.8) | 3 (4.9) | 0 |
| Left and BRAF mutant | 10 (16.4) | 2 (3.3) | 8 (13.1) | 0 |
| Missing data | 7 (11.5) | 5 (8.2) | 2 (3.3) | 0 |
BEV Bevacizumab, CET Cetuximab, ECOG Eastern cooperative oncology group, NA Not available, XELOXIRI Capecitabine, oxaliplatin and irinotecan
a One patient received XELOXIRI + CET therapy for three cycles after nine cycles of XELOXIRI + BEV
Treatment
We found that 39, 4, and 17 patients received XELOXIRI alone, XELOXIRI plus cetuximab, and XELOXIRI plus bevacizumab, respectively; moreover, one patient initially received a XELOXIRI-bevacizumab combination, which was later switched to a XELOXIRI-cetuximab combination (Table 1). The dose schedule of XELOXIRI was as follows: irinotecan infusion for 1 h (at a dose of 120–170 mg/m2), then oxaliplatin infusion for 2 h (at a dose of 70–100 mg/m2), and oral capecitabine (at a dose of 600–1000 mg/m2 twice daily) for 1–7 days every 2 weeks. For patients treated with targeted drugs, bevacizumab (5 mg/kg) or cetuximab (500 mg/m2) was administered on the same day ahead of XELOXIRI treatment, every 2 weeks.
Efficacy
As of November 8, 2021, all patients were followed up for survival; the median duration of follow-up was 35.4 months (range, 3.9–52.9 months). Disease progression and death occurred in 50 (82.0%) and 38 (62.3%) patients, respectively. The median OS was 32.2 months (95% CI [24.8–39.6]), and the 1-, 2-, and 3-year OS rates were 84.6%, 55.1%, and 38.6%, respectively. The median PFS was 9.3 months (95% CI [8.1–10.5]), and the 1-year PFS rate was 35.0%. Regarding tumour response, assessed by investigators, none of the 48 patients with measurable lesions achieved CR, whereas 34 achieved PR, resulting in an objective response rate of 70.8% (95% CI [64.1%-77.5%]). The DCR was 89.6% (95% CI [82.9%-96.3%]) (Table 2). Of 15 patients with only liver metastases, 13 achieved PR and 2 had SD. The ORR and DCR were 86.7% and 100%, respectively. Twenty-four patients (39.3%) underwent surgery, including 16 (26.2%) patients who underwent combined resection of primary tumour and metastases and achieved no evidence of disease (NED). Five patients with synchronous unresectable distant metastases had only the primary tumour resected. Another three patients had the liver metastases resected but with residual. As of November 8, 2021, 13 out of 16 patients with NED had disease progression; 4 patients died, 11 survived, and 1 was lost to follow-up. The median PFS was 10.2 months (95% CI [6.6–13.7]), the median DFS was 5.7 months (95% CI [1.8–9.7]), 1-year DFS rate was 31.3%, and the median OS was not reached.
Table 2.
Response of patients with measurable disease
| Response | All | RAS mutant | RAS WT | BRAFV600E mutant | RAS/BRAFV600E WT |
|---|---|---|---|---|---|
| n = 48 | n = 18 | n = 24 | n = 8 | n = 16 | |
| PR | 34(70.8%) | 13 (72.2%) | 19 (79.2%) | 4 (50.0%) | 15 (93.8%) |
| SD | 9 (18.8%) | 4 (22.2%) | 3 (12.5%) | 2 (25.0%) | 1(6.3%) |
| PD | 4(8.3%) | 1 (5.6%) | 2 (8.3%) | 2 (25.0%) | 0 |
| NE | 1 (2.1%) | 0 | 0 | 0 | 0 |
| NED | 1(2.1%) | 0 | 0 | 0 | 0 |
| ORR | 70.8% | 72.2% | 79.2% | 50.0% | 93.8% |
| DCR | 89.6% | 94.4% | 91.7% | 75.0% | 100% |
Genotype and response rate, Fisher exact probability test (two-sided), BRAF V600E mutant vs. RAS/BRAFV600E WT, p = 0.028
DCR Disease control rate, NED No evidence of disease, ORR Objective response rate, PR Partial response, SD Stable disease, WT wild-type
Regarding genetic mutation status, there were significant differences in OS between patients with the RAS and BRAF, both WT and those with a RAS or BRAFV600E mutant (not reached vs. 24.0 months; hazard ratio [HR], 0.37; 95% CI [0.15–0.91]; p = 0.024) (Fig. 1). Furthermore, the median OS was 18.9 and 28 months for patients with RAS and BRAFV600E mutants, respectively (Fig. 2). The OS was significantly shorter in patients with more than two metastatic organs than in those with one or two metastatic organs (13.5 vs. 34.7 months; HR 3.46; 95% CI [1.02–11.8]; p = 0.001) (Fig. 3). A significant improvement in OS was also observed between patients who achieved NED and those who did not (not reached vs. 28.0 months; HR 0.35; 95% CI [0.16–0.77]; p = 0.04). Baseline neutrophil/lymphocyte ratio (NLR) values were available in 52 patients. The median NLR was 2.98 (0.87–31.81); moreover, no significant differences in PFS (HR 1.22; 95% CI [0.67–2.22]; p = 0.51) and OS (HR 1.21; 95% CI [0.58–2.50]; p = 0.62) were found between patients with NLR ≥ 3 and NLR < 3.
Fig. 1.
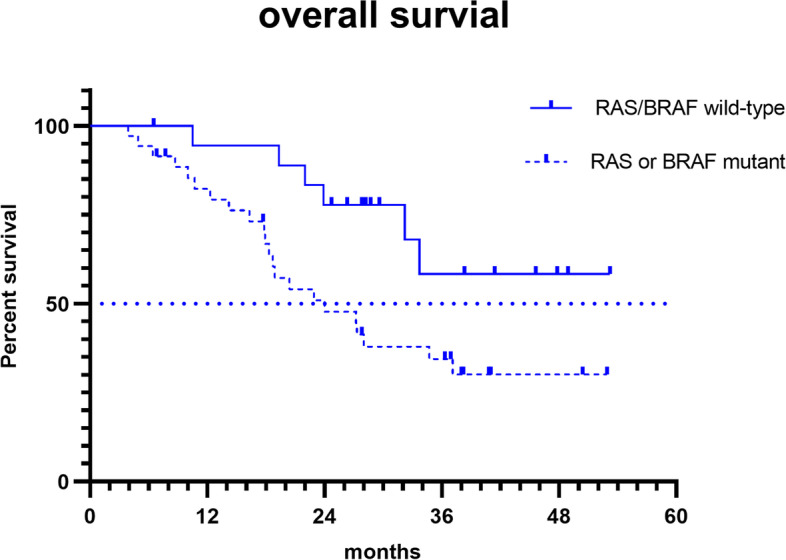
Kaplan Meier survival curves of overall survival (RAS/BRAFV600E wild-type; RAS or BRAF.V600E mutant)
Fig. 2.
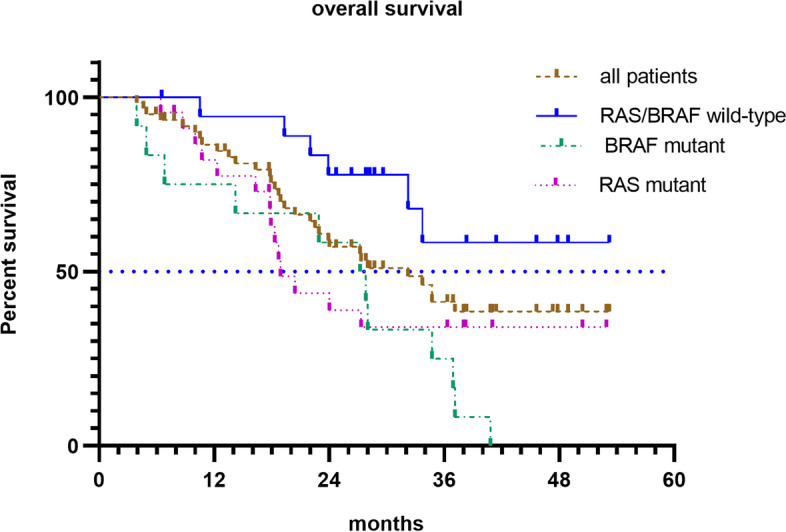
Kaplan Meier survival curves of overall survival (all patients; RAS/BRAF wild-type; BRAF mutant; RAS mutant)
Fig. 3.
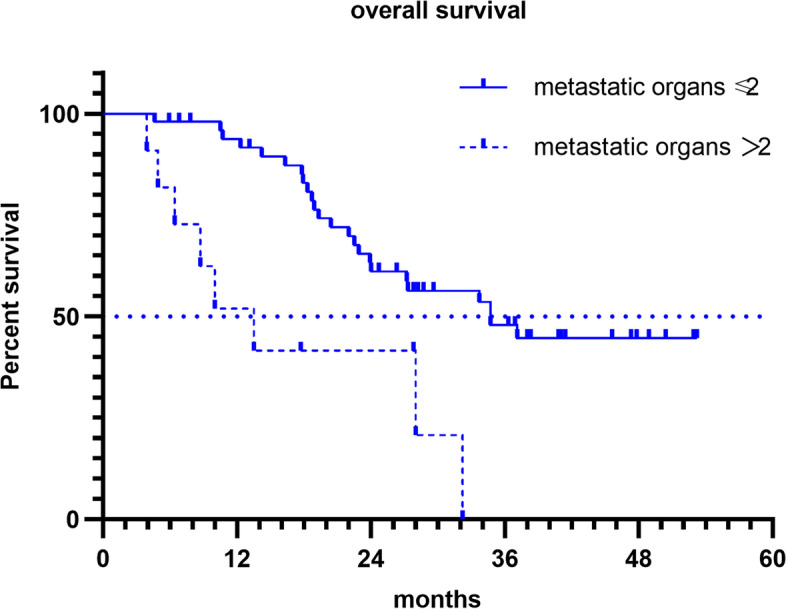
Kaplan Meier survival curves of overall survival (metastatic organs ≥ 2; metastatic organs < 2)
In the multivariable model, RAS/BRAF WT (HR 0.39; 95% CI [0.16–0.96], p = 0.04) and more than two metastatic organs (HR 3.25; 95% CI [1.34–7.87], p = 0.009) were independent prognostic factors for OS (Supplementary Fig. 1).
Tolerance and safety
The median number of treatment cycles of XELOXIRI with or without bevacizumab or cetuximab was 10 (range, 1–12). The main reasons for discontinuing XELOXIRI therapy were surgery (42.6%), switching to maintenance therapy (capecitabine with or without bevacizumab) (14.8%), drug toxicity (6.6%), disease progression (19.7%), loss to follow-up (8.2%), receiving other location therapies (ablation [1.64%] and radiotherapy [4.92%]).
During treatment, 19 patients (31.2%) underwent drug discontinuation, of whom 10 (16.4%), 5 (8.2%), 1 (1.6%), 2 (3.3%), and 1 (1.6%) patients discontinued irinotecan, oxaliplatin, capecitabine, both oxaliplatin and irinotecan, and both irinotecan and capecitabine, respectively. The mean and median dose intensities of oxaliplatin in the combined treatment regimen were 36.95 mg/m2/week and 37.31 mg/m2/week, respectively. Both the mean and median dose intensities of irinotecan were 71.41 mg/m2/week. The mean and median dose intensities of capecitabine were 5599.75 mg/m2/week and 5599.75 mg/m2/week, respectively (Table 3).
Table 3.
Treatment exposure
| Reasons for discontinuing XELOXIRI | n = 61 (%) |
|---|---|
| Surgery | 26 (42.62%) |
| Maintenance treatment | 9 (14.75%) |
| AEs | 4 (6.56%) |
| Disease progression | 12 (19.67%) |
| Loss to follow-up | 5 (8.20%) |
| Radiotherapy | 3 (4.92%) |
| Ablation | 1 (1.64%) |
| Drug discontinuation | n (%) |
| Any drug discontinuation | 19 (31.15%) |
| Oxaliplatin | 5 (8.20%) |
| Irinotecan | 10 (16.39%) |
| Capecitabin | 1 (1.64%) |
| Oxaliplatin and irinotecan | 2 (3.28%) |
| Irinotecan and capecitabin | 1 (1.64%) |
| Dose intensity | mg/m2/week |
| Oxaliplatin | |
| mean dose intensity | 36.95 |
| median dose intensity | 37.31 |
| Irinotecan | |
| mean dose intensity | 71.41 |
| median dose intensity | 71.41 |
| Capecitabin | |
| mean dose intensity | 5599.75 |
| median dose intensity | 5599.75 |
AE Adverse event, XELOXIRI Capecitabine, oxaliplatin and irinotecan
Table 4 shows the incidences of adverse events (AEs) related to the treatment grade. The incidence of all grades of AEs was 96.7%, and that of grade 3/4 AEs was 32.8%. The most common grade 3/4 AEs included neutropenia (12 [19.7%]), febrile neutropenia (2 [3.3%]), leukopenia (6 [9.8%]), thrombocytopenia (1 [1.6%]), diarrhea (2 [3.3%]), and vomiting (2 [3.3%]). In 8 patients, UGT1A1*6 and UGT1A1*28 genotypes were tested, and 4 had double WT genotypes. Only 1 patient with a heterozygous UGT1A1*28 genotype developed grade 3 neutropenia. No treatment-related death occurred.
Table 4.
Adverse events
| Adverse event | n (%) | ||||
|---|---|---|---|---|---|
| Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Any AEs | 59 (96.7) | 58 (95.1) | 29 (47.5) | 17 (27.9) | 3 (4.9) |
| Decreased appetite | 43 (70.5) | 43 (70.5) | 0 | 0 | 0 |
| Nausea | 46 (75.4) | 32 (52.5) | 14 (23.0) | 0 | 0 |
| Vomiting | 20 (32.8) | 7 (11.5) | 11 (18.0) | 2 (3.3) | 0 |
| Diarrhea | 13 (21.3) | 9 (14.8) | 2 (3.3) | 2 (3.3) | 0 |
| Stomach ache | 6 (9.8) | 6 (9.8) | 0 | 0 | 0 |
| Peripheral neurotoxicity | 19 (31.1) | 17 (27.9) | 2 (3.3) | 0 | 0 |
| Hand-foot skin reaction | 10 (16.4) | 10 (16.4) | 0 | 0 | 0 |
| Fatigue | 24 (39.3) | 23 (37.7) | 1 (1.6) | 0 | 0 |
| Rash | 4 (6.6) | 4 (6.6) | 0 | 0 | 0 |
| Leukopenia | 30 (49.2) | 12 (19.7) | 12 (19.7) | 6 (9.8) | 0 |
| Neutropenia | 32 (52.5) | 11 (18.0) | 9 (14.8) | 9 (14.8) | 3 (4.9) |
| Febrile neutropenia | 2 (3.3) | 0 | 0 | 1 (1.6) | 1 (1.6) |
| Anemia | 14 (23.0) | 12 (19.7) | 2 (3.3) | 0 | 0 |
| Thrombocytopenia | 6 (9.8) | 3 (4.9) | 2 (3.3) | 1 (1.6) | 0 |
| Elevated ALT | 4 (6.6) | 4 (6.6) | 0 | 0 | 0 |
| Elevated AST | 4 (6.6) | 4 (6.6) | 0 | 0 | 0 |
| Anaphylactic reaction | 1 (1.6) | 0 | 0 | 1 (1.6) | 0 |
AE Adverse event, ALT Alanine aminotransferase, AST Aspartate aminotransferase
Discussion
This retrospective study showed that the use of the triplet XELOXIRI combination with or without an antibody (bevacizumab or cetuximab) as first-line therapy in Chinese patients with mCRC produces a comparable safety and efficacy to that of the FOLFOXIRI regimen.
The median OS of our cohort was 32.2 months, which is higher than that reported in serial phase III trials conducted by the GONO group (22.6–29.8 months) [1–6]. Notably, the proportion of patients harboring the BRAFV600E mutant in our cohort was higher (19%) than that in the GONO trials (4.8%-10%)2, 3, 5–7. Moreover, 23% of patients in the present cohort had peritoneal metastases; mCRC was most difficult to treat in these patients.
A large amount of evidence indicates that the presence of BRAFV600E mutation is related to the poor prognosis of patients with colorectal cancer, which is non-responsive to anti-EGFR treatment [30–38]. The use of cetuximab or panitumumab plus chemotherapy cannot obviously improve the survival of patients with BRAFV600E mutation [39]. In the TRIBE trial, the use of bevacizumab plus FOLFOXIRI showed statistically significant advantages in ORR (56% vs. 42%; OR: 1.82; 95% CI [0.38–8.78]) and OS (19.0 vs. 10.7 months; HR 0.54; 95% CI [0.24–1.20]) compared with the use of bevacizumab plus FOLFIRI in patients with mCRC harboring BRAFV600E mutation, which motivated the recommendation of the FOLFOXIRI-bevacizumab combination use by international guidelines. However, in the post-hoc subgroup analyses of the TRIBE2 trial based on the BRAFV600Emutational status, no significant differences in efficacy were detected between the use of FOLFOXIRI plus bevacizumab and that of the two-drug chemotherapy plus bevacizumab [6]. In this study, 10 of the 12 patients with BRAFV600E mutation received XELOXIRI plus bevacizumab therapy; the ORR was 50%, and the mOS of 28 months seemed more promising than that reported in the previous trials3, 6 (Supplementary Table 1).
According to a previous study, patients with mCRC having left-sided primary tumours have a better prognosis than those with right-sided primary tumours [40]. Approximately 5% of patients with mCRC have BRAFV600E mutation, most of which occur in the right colon. Notably, the proportion of patients with BRAF mutations in this study is relatively high, and these patients are not known to have MSI-H; interestingly, most of them have left-sided colon tumours. The left-sided primary tumour may be an explanation for the promising survival of the patients with BRAFV600E mutation. However, this result needs to be interpreted carefully and verified further, given the small sample size of this study. Furthermore, it is not surprising that some patients with mCRC having BRAFV600E mutations show an indolent clinical course and a relatively favorable prognosis [41].Several studies have demonstrated that 10–20% of patients with BRAF mutations can survive for more than 2 years. Moreover, a high level of biological heterogeneity has been revealed in patients with mCRC with BRAF mutations (both V600E and beyond V600E), including clinical characteristics, pathological features, and molecular alterations [42–44]. At present, several methods have been established for further stratification of BRAF mutations, including subtypes based on signaling mechanisms (classes I, II, and III) [45], molecular consensus subtypes (CMS) (CMS1, CMS2, CMS3, and CMS4) [46], and the transcriptional subtypes of BRAFV600E (BRAFV600E mutant 1 and BRAFV600E mutant 2) [47]. Nevertheless, continuous exploration is needed to reveal the association between molecular profiles and clinical outcomes and to further guide precise treatments using these methods.
Recent international guidelines have recommended the use of NED to evaluate therapeutic efficacy in patients with colorectal liver-limited metastases [8, 48–55]. The use of an effective systemic regimen is the cornerstone for conversions. In this study, the ORR reached 86.7% (13/15) among patients with only liver metastases. Due to the high rate of tumour shrinkage, 26.2% of patients underwent tumour resection and achieved NED and long-term survival. The ORR and R0 resection rates were numerically comparable to those in the FOCULM (95.5% and 55.2%, respectively) and VOLFI (87% and 33%, respectively) trials [8, 9].
NLR has been reported as a poor prognostic factor in several gastrointestinal tumours [56–59]. A retrospective analysis of the TRIBE study found that patients with mCRC with high NLR ≥ 3 had significantly decreased survival benefits (PFS, HR 1.27; OS HR 1.56) compared to patients with NLR < 3 [59]. Inconsistent with this, there was no significant association between NLR and survival in this study. The prognostic value of NLR in patients with mCRC needs to be further evaluated using studies with expanded samples.
So far, XELOXIRI administered every 2 weeks with or without bevacizumab has been investigated on patients with different dose schedules in three phase II and one phase I trials conducted in Western countries; the efficacy was found to be promising [27, 28, 60]; the most common AEs or/ and DLTs were neutropenia and diarrhea (Table 5).
Table 5.
Similar clinical studies on XELOXIRI
| Study and population | Schedule | Irinotecan (mg/m2) | Oxaliplatin (mg/m2) |
Capecitabine (mg/m2/d) |
Targeted drug | Grade 3–4 AEs | DLT | ORR (%) | PFS (months) |
OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
|
Italy-GONO (2009) Phase 2, N = 36 |
Q2w | 165 | 85 | 2000 | - | Neutropenia (30%), febrile neutropenia (11%), diarrhea (30%) | - | 67 | 10.1 | 17.9 |
|
Italy-ITMO (2007) phase 2, N = 38 |
Q2w | 180 | 85 | 2000 | - | Diarrhea (24%), nausea (16%) | Diarrhea | 63 | 8.5 | 23.5 |
|
Italy (2015) phase 2, N = 51 |
Q2w | 180 | 85 | 2000 | - | Neutropenia (6%), diarrhea (31%), mucositis (4%) | - | 62 | 10.3 | 22 |
|
Spain (2010) phase 1, N = 87 |
Q2w | 150 | 85 | 2000 | - | Neutropenia (27%), diarrhea (11%) and leukopenia (8%) | Neutropenia, diarrhea | 66.6 | 12 | 27 |
|
Canada (2018) phase 1, N = 39 |
Q3w | 160 | 100 | 1900 | - | Neutropenia (56%), diarrhea (15%) | Febrile neutropenia, diarrhea | 67 | 11 | 25 |
|
Japan QUATTRO-II (2021), phase 2, N = 9 (Safety lead-in results) |
Q3w | 200 | 130 | 1600 |
Bev 7.5 mg/kg |
Neutropenia (44%) | Febrile neutropenia | 89 | - | - |
|
Japan (2015) Phase 1, N = 12 |
Q3w | 150 | 100 | 1700 |
Bev 7.5 mg/kg |
Neutropenia (41%), febrile neutropenia (8%), diarrhea (8%) | Neutropenia | 83 | 15 | - |
|
Japan (2017) Phase 1, N = 12 |
Q3w | 150 | 100 | 1700 |
Cet 250 mg/m2 qw (Increase the first dose) |
Neutropenia (50%), febrile neutropenia (8%), diarrhea (17%) | Neutropenia | 83 | 14.5 | - |
|
Japan (2019) phase 1, N = 6 |
Q2W | 150 | 85 | 2000 | - | Febrile neutropenia (2/6) | Febrile neutropenia | - | - | - |
AE Adverse event, Bev Bevacizumab, Cet Cetuximab, DLT Dose-limited toxicity, ORR Objective response rate, OS Overall survival, PR Partial response, PFS Progression-free survival
Italian regimens composed of irinotecan (165 or 180 mg/m2), oxaliplatin (85 mg/m2), and 1–7 or 2–6 days of capecitabine (2000 mg/m2/day) every 2 weeks. The incidences of grade 3/4 neutropenia and diarrhea ranged from 6%-30% and 24%-31%, respectively [27, 28, 60]. Spain’s study showed that a lower dose of irinotecan (150 mg/m2) might result in a lower incidence of grade ≥ 3 diarrhea (11%) and a comparable incidence of grade ≥ 3 neutropenia [29] (Table 5). To date, studies concerning the use of triplet drug regimens in Asian populations are limited. Notably, the use of irinotecan-containing regimens seems to increase the incidence of neutropenia in Asian populations. For example, the incidence of grade 3/4 hematologic toxicities with FOLFOXIRI-bevacizumab combination use reached 72.5% in the phase II QUATTRO trial, which was conducted in Japan. However, the incidence of grade 3/4 neutropenia was decreased to less than 50% without compromised efficacy when the dose of irinotecan was modified [8, 61–63]. For XELOXIRI regimens with the 3 weeks schedule, three trials in Japan showed the incidences of grade 3/4 neutropenia ranged from 41%-50% [25, 26, 64] (Table 5). The only published clinical trial from an Asian population using the XELOXIRI regimen (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, and 1–7 days of capecitabine 2000 mg/m2/day) every 2 weeks was a phase I trial conducted on 6 Japanese patients in the neoadjuvant setting. Grade 3/4 neutropenia occurred in 2/6 (33.3%) patients [24] (Table 5). Similar to our study findings.
Our study had some limitations. This is a retrospective study and thus is inevitably affected by confounding factors. Specifically, the AEs were mainly obtained from patient medical records; hence, these AEs may be missed. Furthermore, due to the small sample size, some patients lacked information related to genetic testing. Moreover, the doses of drugs used in this study were not uniform. However, this study adds efficacy and safety data on the chemotherapy regimens that can be used in the Asian population. We showed that the use of the XELOXIRI regimen, which involves the replacement of 5-fluorouracil with capecitabine, is well tolerated and safe. A phase I/II trial is currently underway to evaluate the appropriate doses, efficacy, safety, and potential predictive or prognostic factors associated with the use of the XELOXIRI-bevacizumab combination regimen as first-line treatment for metastatic colorectal cancer (ChiCTR2000032590, NCT04380103).
Conclusions
The use of the XELOXIRI regimen with or without a targeted drug was effective, with a manageable toxicity profile in Chinese patients with mCRC.
Supplementary Information
Additional file 1: Supplementary Table 1. Patient survival. Supplementary Fig. 1. Cox metanalysis of the impact of research factors on survival or risk rate.
Acknowledgements
We are grateful to all study patients and their families who gave their consent for the use of their data. Moreover, we sincerely thank all the team members who provided help in the course of this study. We also would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- AEs
Adverse events
- CR
Complete response
- FOLFOXIRI
Irinotecan, oxaliplatin, and 5-fluorouracil
- cmFOLFOXIRI
Chinese modified FOLFOXIRI
- DCR
Disease control rate
- DLTs
Dose-limited toxicities
- PR
Partial response
- CI
Confidence interval
- mCRC
Metastatic colorectal cancer
- NED
No evidence of disease
- NLR
Neutrophil/lymphocyte ratio
- ORR
Objective response rate
- OS
Overall survival
- PFS
Progression-free survival
- SD
Stable disease
- WT
Wild-type
- XELOXIRI
Capecitabine, oxaliplatin, and irinotecan
Authors’ contributions
X.L. designed the study and completed the manuscript; K.O. and XT.M. reviewed and edited the manuscript; LZ.G., Q.W., and HZ.Z. made substantial contributions to the acquisition of data; L.Y. agreed to take responsibility for all aspects of the work. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
All data used during this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments. This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, the Chinese Academy of Medical Sciences and Peking Union Medical College (approval number: 2022011210085902). As this study is retrospective, the requirement for informed consent was waived by the Ethics Committees of the National Cancer Center/Cancer Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/jco.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 2.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 3.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/s1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberger T, Bridgewater J, Chau I, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702–708. doi: 10.1093/annonc/mdu580. [DOI] [PubMed] [Google Scholar]
- 5.Cremolini C, Marmorino F, Loupakis F, et al. TRIBE-2: a phase III, randomized, open-label, strategy trial in unresectable metastatic colorectal cancer patients by the GONO group. BMC Cancer. 2017;17:408. doi: 10.1186/s12885-017-3360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507. doi: 10.1016/s1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz HI, Tan BR, Reeves JA, et al. Phase II randomized trial of sequential or concurrent FOLFOXIRI-Bevacizumab versus FOLFOX-Bevacizumab for metastatic colorectal cancer (STEAM) Oncologist. 2019;24:921–932. doi: 10.1634/theoncologist.2018-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H, Wang K, Huang M, et al. Modified FOLFOXIRI with or without Cetuximab as conversion therapy in patients with RAS/BRAF wild-type unresectable liver metastases colorectal cancer: the FOCULM multicenter phase ii trial. Oncologist. 2021;26:e90–e98. doi: 10.1634/theoncologist.2020-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modest DP, Martens UM, Riera-Knorrenschild J, et al. FOLFOXIRI Plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109) J Clin Oncol. 2019;37:3401–3411. doi: 10.1200/jco.19.01340. [DOI] [PubMed] [Google Scholar]
- 10.Aranda E, Viéitez JM, Gómez-España A, et al. FOLFOXIRI plus bevacizumab versus FOLFOX plus bevacizumab for patients with metastatic colorectal cancer and ≥3 circulating tumour cells: the randomised phase III VISNÚ-1 trial. ESMO Open. 2020;5:e000944. doi: 10.1136/esmoopen-2020-000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folprecht G, Mende M, Liersch T, et al. Cetuximab/ irinotecan/ 5-FU +/- oxaliplatin or FOLFOXIRI +/- bevacizumab in patients with colorectal cancer and nonresectable liver metastases (AIO CELIM2-study) 2020: 4024; NCT01802645.
- 12.Benson AB 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, Engstrom PF, Enzinger PC, Fenton MJ, Fuchs CS, Grem JL, Hunt S, Kamel A, Leong LA, Lin E, Messersmith W, Mulcahy MF, Murphy JD, Nurkin S, Rohren E. National Comprehensive Cancer Network. Colon cancer, version 3.2014. J Natl Compr Cancer Netw. 2014;12(7):1028–59. 10.6004/jnccn.2014.0099. [DOI] [PubMed]
- 13.Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii1–9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnosis and Treatment Guidelines For Colorectal Cancer Working Group C Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version) Chin J Cancer Res. 2019;31:117–134. doi: 10.21147/j.issn.1000-9604.2019.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunakawa Y, Fujita K, Ichikawa W, et al. A phase I study of infusional 5-fluorouracil, leucovorin, oxaliplatin and irinotecan in Japanese patients with advanced colorectal cancer who harbor UGT1A1*1/*1,*1/*6 or *1/*28. Oncology. 2012;82:242–248. doi: 10.1159/000337225. [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Li WW, Huang J. Safety and efficacy of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) in Chinese patients with advanced colorectal cancer. Zhonghua Zhong Liu Za Zhi. 2017;39:380–383. doi: 10.3760/cma.j.issn.0253-3766.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Committee of Colorectal Cancer, Chinese Southern Oncology Group. Zhonghua wei chang wai ke za zhi = Chinese J Gastrointest Surg. 2021;24(6):473–9. 10.3760/cma.j.cn.441530-20210209-00060. [DOI] [PubMed]
- 19.Y Deng. The chinese expert consensus on the clinical application of the chinese modified triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil, and leucovorin for colorectal cancer. Gastroenterol Rep (Oxf) 2021;9:279–289. doi: 10.1093/gastro/goab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–1281. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 21.Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–2292. doi: 10.1200/jco.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy J, Twelves C, Van Cutsem E, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–575. doi: 10.1093/annonc/mdf089. [DOI] [PubMed] [Google Scholar]
- 23.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 24.Kudo T, Takemasa I, Hata T, et al. A phase i study of Neoadjuvant Capecitabine, Oxaliplatin, and Irinotecan (XELOXIRI) in patients with locally advanced rectal cancer. Oncology. 2019;97:211–216. doi: 10.1159/000500677. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Ohnuma H, Hirakawa M, et al. A dose-escalation study of oxaliplatin/capecitabine/irinotecan (XELOXIRI) and bevacizumab as a first-line therapy for patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2015;75:587–594. doi: 10.1007/s00280-014-2672-9. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Hirakawa M, Ohnuma H, et al. A triplet combination with capecitabine/oxaliplatin/irinotecan (XELOXIRI) plus cetuximab as first-line therapy for patients with metastatic colorectal cancer: a dose escalation study. Cancer Chemother Pharmacol. 2017;80:1133–1139. doi: 10.1007/s00280-017-3458-7. [DOI] [PubMed] [Google Scholar]
- 27.Vasile E, Masi G, Fornaro L, et al. A multicenter phase II study of the combination of oxaliplatin, irinotecan and capecitabine in the first-line treatment of metastatic colorectal cancer. Br J Cancer. 2009;100:1720–1724. doi: 10.1038/sj.bjc.6605075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajetta E, Celio L, Ferrario E, et al. Capecitabine plus oxaliplatin and irinotecan regimen every other week: a phase I/II study in first-line treatment of metastatic colorectal cancer. Ann Oncol. 2007;18:1810–1816. doi: 10.1093/annonc/mdm347. [DOI] [PubMed] [Google Scholar]
- 29.Zarate R, Rodríguez J, Bandres E, et al. Oxaliplatin, irinotecan and capecitabine as first-line therapy in metastatic colorectal cancer (mCRC): a dose-finding study and pharmacogenomic analysis. Br J Cancer. 2010;102:987–994. doi: 10.1038/sj.bjc.6605595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonsalves WI, Mahoney MR, Sargent DJ, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106(7):106–dju106. doi: 10.1093/jnci/dju106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modest DP, Ricard I, Heinemann V, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27:1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–5937. doi: 10.1200/jco.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 34.Taieb J, Le Malicot K, Shi Q, et al. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. J Natl Cancer Inst. 2017;109(5):djw272. doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taieb J, Jung A, Sartore-Bianchi A, et al. The evolving biomarker landscape for treatment selection in metastatic colorectal cancer. Drugs. 2019;79:1375–1394. doi: 10.1007/s40265-019-01165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JC, Renfro LA, Al-Shamsi HO, et al. (Non-V600) BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J Clin Oncol. 2017;35:2624–2630. doi: 10.1200/jco.2016.71.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu JE, Johnson B, Kugathasan L, et al. Population-based screening for BRAF (V600E) in metastatic colorectal cancer reveals increased prevalence and poor prognosis. Clin Cancer Res. 2020;26:4599–4605. doi: 10.1158/1078-0432.Ccr-20-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karapetis CS, Jonker D, Daneshmand M, et al. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer–results from NCIC CTG/AGITG CO.17. Clin Cancer Res. 2014;20:744–753. doi: 10.1158/1078-0432.Ccr-13-0606. [DOI] [PubMed] [Google Scholar]
- 39.Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 40.Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. doi: 10.1016/j.ejca.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Loupakis F, Intini R, Cremolini C, et al. A validated prognostic classifier for (V600E)BRAF-mutated metastatic colorectal cancer: the 'BRAF BeCool' study. Eur J Cancer. 2019;118:121–130. doi: 10.1016/j.ejca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Rodriquenz MG, Ciardiello D, Latiano TP, et al. Exploring biological heterogeneity and implications on novel treatment paradigm in BRAF-mutant metastatic colorectal cancer. Crit Rev Oncol Hematol. 2022;173:103657. doi: 10.1016/j.critrevonc.2022.103657. [DOI] [PubMed] [Google Scholar]
- 43.Angerilli V, Sabella G, Centonze G, et al. BRAF-mutated colorectal adenocarcinomas: Pathological heterogeneity and clinical implications. Crit Rev Oncol Hematol. 2022;172:103647. doi: 10.1016/j.critrevonc.2022.103647. [DOI] [PubMed] [Google Scholar]
- 44.Fanelli GN, Dal Pozzo CA, Depetris I, et al. The heterogeneous clinical and pathological landscapes of metastatic Braf-mutated colorectal cancer. Cancer Cell Int. 2020;20:30. doi: 10.1186/s12935-020-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Z, Torres NM, Tao A, et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell. 2015;28:370–383. doi: 10.1016/j.ccell.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barras D, Missiaglia E, Wirapati P, et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res. 2017;23:104–115. doi: 10.1158/1078-0432.Ccr-16-0140. [DOI] [PubMed] [Google Scholar]
- 48.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 51.Symonds LK, Cohen SA. Use of perioperative chemotherapy in colorectal cancer metastatic to the liver. Gastroenterol Rep (Oxf) 2019;7:301–311. doi: 10.1093/gastro/goz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruers T, Van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst. 2017;109(9):015–djx015. doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/jco.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 54.Adam R, Haller DG, Poston G, et al. Toward optimized front-line therapeutic strategies in patients with metastatic colorectal cancer–an expert review from the International Congress on Anti-Cancer Treatment (ICACT) 2009. Ann Oncol. 2010;21:1579–1584. doi: 10.1093/annonc/mdq043. [DOI] [PubMed] [Google Scholar]
- 55.Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS. SSO and TOS. Ann Oncol. 2018;29:44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 56.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Wei Q, Fan J, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta. 2018;479:181–189. doi: 10.1016/j.cca.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Xiao WK, Chen D, Li SQ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dell'Aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol. 2018;29:924–930. doi: 10.1093/annonc/mdy004. [DOI] [PubMed] [Google Scholar]
- 60.Di Bartolomeo M, Ciarlo A, Bertolini A, et al. Capecitabine, oxaliplatin and irinotecan in combination, with bevacizumab (COI-B regimen) as first-line treatment of patients with advanced colorectal cancer. An Italian Trials of Medical Oncology phase II study. Eur J Cancer. 2015;51:473–481. doi: 10.1016/j.ejca.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Xu RH, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018;19:660–671. doi: 10.1016/s1470-2045(18)30140-2. [DOI] [PubMed] [Google Scholar]
- 62.Oki E, Kato T, Bando H, et al. A multicenter clinical phase ii study of FOLFOXIRI Plus Bevacizumab as first-line therapy in patients with metastatic colorectal cancer: QUATTRO study. Clin Colorectal Cancer. 2018;17:147–155. doi: 10.1016/j.clcc.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Zhou H, Song Y, Jiang J, et al. A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res. 2016;28:598–605. doi: 10.21147/j.issn.1000-9604.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotani D, Yoshino T, Kotaka M, et al. Combination therapy of capecitabine, irinotecan, oxaliplatin, and bevacizumab as a first-line treatment for metastatic colorectal cancer: Safety lead-in results from the QUATTRO-II study. Invest New Drugs. 2021;39:1649–1655. doi: 10.1007/s10637-021-01125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Patient survival. Supplementary Fig. 1. Cox metanalysis of the impact of research factors on survival or risk rate.
Data Availability Statement
All data used during this study are available from the corresponding author upon reasonable request.


