Abstract
This review discusses the definition and major categories of cutaneous T-cell lymphoma, Sézary syndrome and mycosis fungoides, and the role of immunophenotyping in their diagnosis. The following key points are raised:
Sézary syndrome and mycosis fungoides cells most often have a characteristic CD3+ CD4+ CD7- and/or CD26- immunophenotype.
This immunophenotype is not specific, but can assist in the distinction from non-neoplastic T-cells and other subtypes of mature T-cell neoplasm.
However, small subsets of normal and reactive T-cells can have an overlapping immunophenotype, and can be distinguished by evaluating for additional changes in antigen expression.
Keywords: Sézary syndrome, mycosis fungoides, flow cytometry, T-cell, lymphocyte
Cutaneous T-cell lymphomas: Definition and major categories
Primary cutaneous lymphomas are a group of lymphomas which usually present in the skin with no evidence of extracutaneous involvement at the time of diagnosis (1,2). These lymphomas are a heterogeneous group of clinicopathologic entities characterized at a minimum by monoclonal T- or B-cell proliferations (1,3). The most up to date consensus classification system for these diseases is published as the 2018 WHO classification of Skin Tumours (4) which contemporizes the 2005 World Health Organization-European Organization for the Research and Treatment of Cancer (WHO-EORTC) consensus classification (1) and intervening Haematopoietic/Lymphoid (2017) publication from the WHO (5). Seventy-five to 80% of all primary cutaneous lymphomas are derived from mature T cells, while B-cell lymphomas account for the remaining 20–25% (1).
The term “cutaneous T-cell lymphoma” (CTCL) is commonly used to include any type of primary cutaneous lymphoma of T-cell origin. Historically, the term CTCL has been used when referring to mycosis fungoides (MF) which is the most common CTCL type, and its closely related leukemic counterpart Sézary syndrome (SS). MF and SS share a common staging system and often both entities are accepted in the same clinical trials (1,6). Distinctive clinicopathologic variants (subtypes) of MF that are recognized in the latest WHO classification of skin tumours (4) include folliculotropic MF, pagetoid reticulosis and granulomatous slack skin syndrome, although other less common clinical and pathologic variants are often described (7). Other CTCL types in the classification include the following entities: primary cutaneous CD30+ T-cell lymphoproliferative disorders (primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis), subcutaneous panniculitis-like T-cell lymphoma, and primary cutaneous peripheral T-cell lymphoma (rare subtypes of primary CTCL, including primary cutaneous gamma-delta T-cell lymphoma, primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma, primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder and primary cutaneous acral CD8+ T-cell lymphoma). Occasional cases do not fit into any of the above subtypes and are considered “primary cutaneous peripheral T-cell lymphoma NOS (not otherwise specified)” (4). There are other entities that often present in the skin and hence may be considered CTCL, but with rare exceptions should be considered systemic diseases. These entities include adult T-cell leukemia/lymphoma, cutaneous manifestations of chronic active EBV infection, and extranodal NK/T-cell lymphoma, nasal type (5).
MF is by far the most common type of primary cutaneous lymphoma, comprising approximately 50% of all cutaneous lymphomas. It is a CTCL which is postulated to derive from mature skin-resident effector memory (usually CD4+) T cells (8). Typically, MF has an indolent clinical course, with most patients (80%) presenting with early-stage disease, characterized by patches and plaques which may slowly progress to tumor lesions and systemic involvement. The initial patches and plaques are characterized by medium sized cerebriform lymphocytes mostly confined to the epidermis (epidermotropism) and papillary dermis. Disease progression tends to manifest with tumor lesions that show lesser epidermotropism and a tendency for large cell transformation (9). In addition, around one-third of patients present with advanced disease already at diagnosis (9,10) by virtue of either skin tumors and/or extracutaneous involvement presenting in the context of a vague history of a chronic dermatitis. Patients with MF can either present with, or develop, an erythrodermic appearance (TNMB stage T4), which may or may not be accompanied by peripheral blood (PB) involvement (stage ranges from absent PB involvement (B0/clinical stage IIIA) to low PB tumor burden (B1/IIIB), with or without T-cell clonality in the PB), but which does not meet hematologic criteria for Sézary syndrome (high PB tumor burden with >1,000/uL Sézary cells and T-cell clonality in the PB) (5,11,12). Importantly, the prognosis of patients with MF correlates with clinical stage, i.e. early-stage disease (stage IA-IIA) usually has a good long-term survival (5-year survival from 50 to 100%), whilst advanced-stage disease (stage IIB-IVB) is associated with a much worse prognosis and a 5-year survival lower than 40% (9).
Sézary syndrome (SS) is a more aggressive type of CTCL, accounting for 3–5% of these disorders, and is characterized by the triad of: (i) erythroderma, usually with severely disabling pruritus, in conjunction with (ii) generalized lymphadenopathy and (iii) the presence of clonally related neoplastic cells (Sézary cells) in the skin, lymph nodes and PB. In addition, according to WHO 2017 classification of lymphoid malignancies (5), one or more of the following are required: an absolute Sézary cell count of ≥1,000/uL of PB; an expanded CD4+ T-cell population resulting in a CD4:CD8 ratio ≥10; or the presence of an aberrant phenotype (i.e. by flow cytometry), consisting of loss of one or more T-cell antigens (5). Clinically, SS is characterized by an aggressive disease course: 5-year overall survival ranges between 10% to 30% (depending on ISCL and EORTC clinical stage) and median survival is approximately 30 months (5,6,11). In contrast to MF cells, which have an immunophenotype characteristics of skin-resident effector memory T cells (i.e. , CD27-, CD62L-, CD45RA-, CD45RO+ and CD197-), Sézary cells show the phenotype of circulating central memory CD4+ T cells, characterized by expression of the nodal-homing chemokine CCR7 (CD197), together with a CD27+, CD62L+/−, CD45RA- and CD45RO+ phenotypic profile (8), expressing skin-homing molecules/receptors, such as CLA (cutaneous lymphocyte antigen) and the CCR4 (CD194) and CCR10 chemokine receptors (13).
The fact that cells from MF and SS show slight different phenotypic profiles for some maturational markers led some authors to postulate a different cell of origin for the two diseases (8). However, recent studies have found that circulating MF and SS cells are frequently heterogeneous in their maturation profiles (14), and SS cells show diversity in naïve/memory maturation phenotype and molecular signature, as well as cytokine/chemokine receptor expression (15).
Diagnosis of mycosis fungoides and Sézary syndrome: Role of immunophenotyping
The diagnosis of MF and SS is often challenging, particularly in their early stages, since they can masquerade clinically as inflammatory conditions, such as chronic eczematous dermatitis, psoriasis, pityriasis rubra pilaris, chronic actinic dermatitis, drug reactions, idiopathic erythroderma or fungal infections, among others (13,16). Particularly, the differential diagnosis between SS (and other erythrodermic CTCL) and erythrodermic inflammatory dermatoses is difficult, because: (i) T-cell clonality can be occasionally observed in inflammatory conditions, as well as in older subjects (progressive increase during aging); (ii) partial loss of T-cell markers is often noted in non-CTCL individuals, which may represent normal memory T-cell downregulation of signaling molecules, such as CD3, CD4 or CD7 (compared to naïve T cells); and (iii) not all SS patients have a CD4:CD8 ratio ≥10 at presentation (16,17). In such circumstances, the unequivocal identification of Sézary cells, either in blood or skin, becomes a crucial issue. Currently, flow cytometry (FC) is considered the ideal method for detecting Sézary cells in PB (and other tissues); morphology has largely been replaced by FC for this purpose, because of a high inter-observer variability in cell counts, and the fact that atypical lymphocytes with cerebriform nuclei can be found in PB from healthy subjects and patients with benign inflammatory skin diseases (13,17).
Immunophenotyping of MF cells in skin.
In general, MF in the skin compartment is a CD4+ memory T-helper cell process in which pan-T-cell antigens CD2, CD3 and CD5 are retained in early lesions and occasionally lost in more advanced disease; CD7 tends to be lost early in the disease, by both routine immunohistochemistry and FC (5,13). (Figure 1) However, loss of CD7 is not specific to MF, and may occur in inflammatory dermatoses. Thus, to achieve a diagnostic specificity of 93% using CD7 in patch stage MF, at least 90% of T cells should be negative for CD7 by immunohistochemistry (18). Loss of at least 70% CD5 may also help confirm MF in these challenging cases. CD26 is usually negative in MF, as in Sézary cells, though cases with cutaneous CD26+ CD4+ tumor T cells have been described in the skin (19), and blood (20). T-cell receptor (TCR)-αβ is typically expressed on the tumor population, reflecting a monoclonal rearrangement of the TCR alpha and beta chains of tumor CD4+ T cells and highlighted immunohistochemically by antibodies, such as betaF1- to a common framework determinant on the beta-subunit of the T-cell receptor (21). Rare variants of MF cells showing CD8+ or double CD4-CD8- negative TCRαβ phenotypes have been described (5,7). Also, TCRγδ expressing tumor cells have been described as uncommon variants of MF (22,23), which may be identified using antibodies to either TCRδ or TCRγ chains of the T-cell receptor. Ki-67 proliferation indices increase as the disease progresses, with tumor stage evolution or transformation to larger cells and may correlate with prognosis (24,25). CD25 can also be identified in later stage MF, and shows a dim cytoplasmic staining pattern, usually in only a subset of the atypical cells. MF is typically CD30 negative by immunohistochemistry and FC, although patch, plaque, tumor, erythrodermic, nontransformed and transformed MF can each show CD30 positivity to varying degrees and in different anatomic compartments and cell subsets (5). Programmed death receptor 1 (PD-1, CD279) may be also (dimly) expressed in MF, but less often than in SS, as described below. Other T-cell follicle helper (TFH) markers, including ICOS, CXCL-13, Bcl-6 and CD10 are expressed at some level in many cases of MF, but these molecules are positive in less than 50% of neoplastic cells (26). TFH phenotype in MF or SS, has been shown to remain concordant among MF biopsies from the same patient over time, and it is not dependent on disease stage, with a cut-off of at least 10% of neoplastic cells expressing at least 3 antigens (26). FC analysis of lymphocytes from mechanically extracted and digested skin biopsy specimens has shown diagnostic T-cell aberrancies in patients with clinical and histologic findings consistent with MF, and not with indeterminate or negative findings (27,28). While not routinely used at this time, FC of skin specimens may be able to distinguish between tumor cell and tumor infiltrating lymphocyte “immune escape” phenotypes using clonotypic TCR-Vβ staining and activation/exhaustion markers such as PD-1 and programmed death receptor ligand-1 (PD-L1), with the potential for eventual therapeutic stratification (29). FC has identified both small and large cell shared monoclonal populations in MF not seen by immunohistochemistry, with CD3+/CD26- T cells accounting for 70% of MF cases, and absent CD7 only in 57% of cases. However, in some cases of erythrodermic MF, FC analysis of PB may be the most concretely diagnostic method of confirming lymphoma.
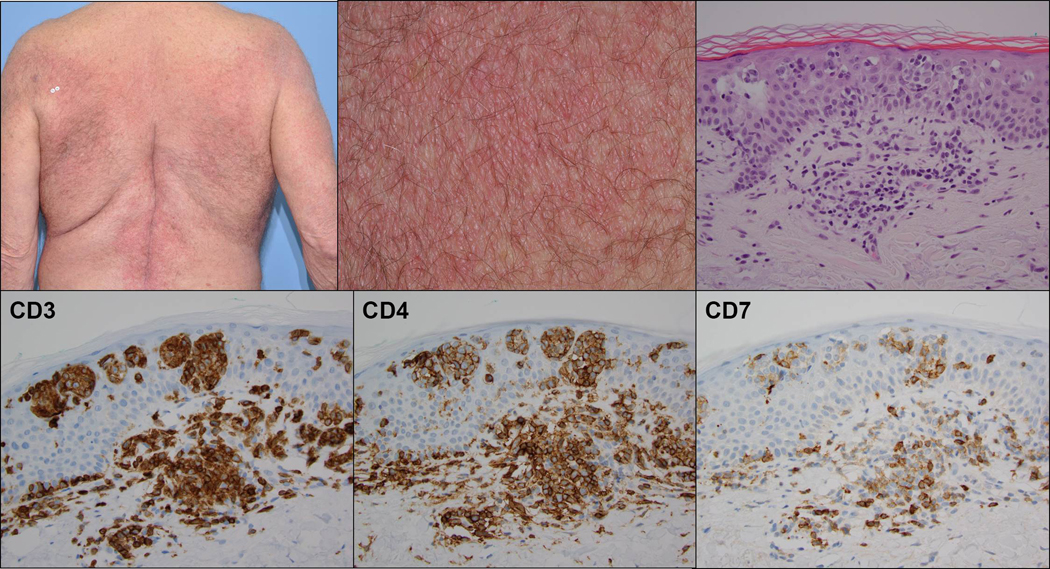
Figure 1:
Clinical photographs (low and high power) demonstrating broad symmetrical erythematous plaques with fine scale in a “bathing trunk” distribution (upper row left and middle). H&E stained histologic section demonstrates aggregates of atypical epidermotropic lymphocytes with superficial dermal perivascular involvement (upper row right). Immunohistochemical stains demonstrating staining of the infiltrate for CD3 and CD4, including many epidermotropic cells, and loss of staining for CD7 (lower row).
Immunophenotype of SS/MF cells in peripheral blood.
FC evaluation of PB is useful in the differential diagnoses of erythroderma, i.e. for the diagnosis of SS vs. reactive/inflammatory erythrodermic conditions (16,17), and for staging MF (12). Since the clinical presentation of erythroderma is generally not discriminative and skin biopsies of SS patients may show only reactive changes in up to one third of cases, the diagnosis greatly depends on the detection of Sézary cells in PB by FC. Several FC-based reports have shown that SS patients show higher percentages of circulating CD4+CD7- and CD4+CD26- T cells compared to patients with benign dermatoses, and a significantly higher CD4:CD8 ratio than patients with benign dermatoses or no lymphoproliferative disorders (12,13). (Figure 2) Accordingly, a recent multicenter study found that loss of CD26 in ≥80% CD4+ T cells and/or loss of CD7 in ≥40% CD4+ T cells could distinguish most SS patients (>80%) from patients with erythrodermic inflammatory dermatoses with 100% specificity (16) (Figure 2). Also, aberrant lower expression of CD2, CD3 or CD5 and less frequently CD4 is identified in around 66% of SS; therefore, in conjunction with other findings, decreased expression of these markers (i.e. losses of 25 or 50% or more in intensity of expression) would support the diagnosis of a T-cell lymphoproliferative disorder (5) (Figure 2).
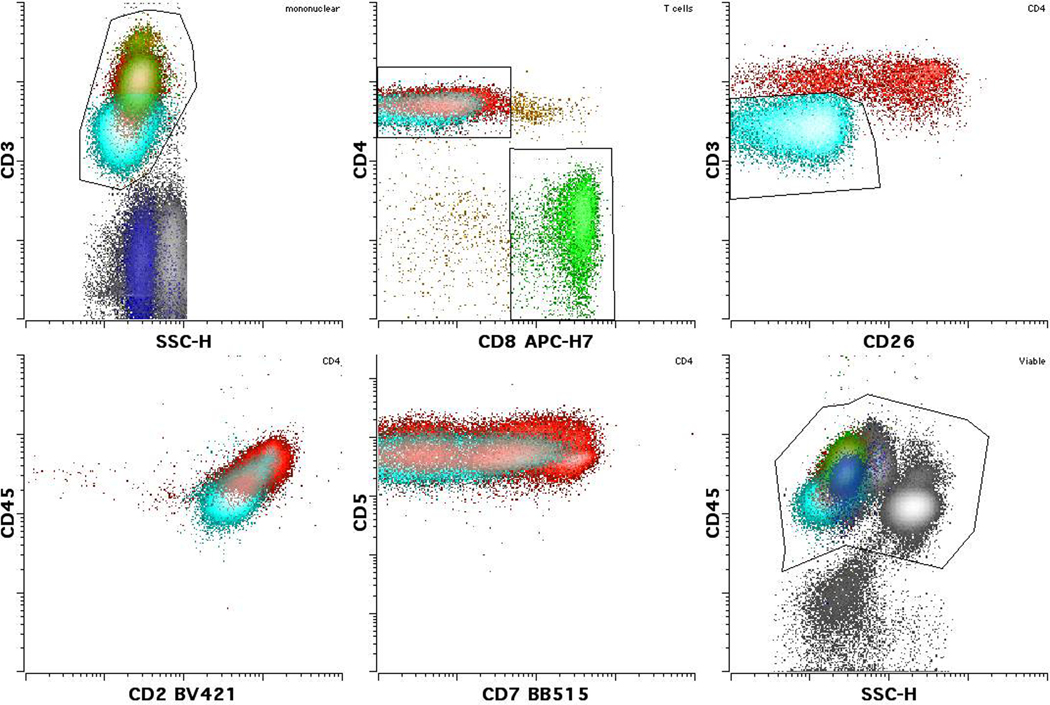
Figure 2:
Sezary/MF cells (light blue) often show a change in CD3 expression as seen in this case compared to either normal CD4 (red) or CD8 (green) positive T cells. Dimmer than normal CD3 expression is seen in this case, although (less frequently) brighter than normal expression may also be seen in other cases. Typically reduced or negative expression of CD26 and variably reduced CD7 are also present. Please note that normal T cells with reduced CD26 and CD7 may also be seen. Some cases also show reduced CD2 and altered CD45 (dim as in this case, or occasionally bright). CD5 is rarely altered. (Images courtesy Dr. M. Roshal, Memorial Sloan Kettering Cancer Center)
More recent studies described expression of killer-cell immunoglobulin-like receptors (CD158a, CD158b, and CD158k), the CD164 adhesion molecule or the actin-bundling protein T-plastin on Sézary cells (16,30,31,32), but results are discrepant and not conclusive, in the sense that the percentage of SS cases found to express these markers is variable among the different studies and usually not all SS cells in positive cases express them; additionally, the expression of CD158k and particularly CD164 has not been adequately analyzed in normal CD4+ T cells, to identify overlapping phenotypes. Accordingly, it should be taken into account that there is no specific marker for Sézary cells, as normal CD4+ T cells -both in basal and particularly in reactive conditions-, may show phenotypes that resemble that of Sézary cells, mainly when FC analysis is based on single or individual markers (17). Therefore, none of these abnormalities is specific or sensitive enough to be used as the sole diagnostic criterion to establish a differential diagnosis between SS and reactive erythroderma. As an example, CD7 expression also defines a subset of normal memory CD4+ T cells, which actually can be increased in patients with benign skin diseases; though these cells usually express high levels of CD2, a minor subset of (central) memory CD4+ T cells may also show lower levels of expression of CD2, together with negativity of CD26 (Figure 3) (Julia Almeida and EuroFlow Consortium, unpublished observations); along the same lines, other signaling molecules (such as CD3 and CD4) are downregulated after T-cell activation (i.e. in reactive conditions). Nevertheless, distinction between Sézary cells and normal/reactive CD4+ T cells significantly improves when a more extensive phenotypic profile (and not just individual parameters) is considered. Accordingly, CD26 together with CD7 can diagnose almost 90% of SS cases (16), and this reaches 93% with the addition of CD2 (16). Furthermore, it has been shown that a CD3/TCRαβlo, CD4lo, CD2-/lo, CD7-/lo and CD26-/lo profile clearly distinguish Sézary cells from normal/reactive CD4+ T cells (17), even within the memory CD4+ T-cell compartment. The former cells also systematically show dim but clear expression of PD-1 (CD279) (5,33), in the absence of cytotoxic markers (17). Therefore, the widely adopted criteria recommended by the EORTC Cutaneous Lymphoma Task Force, -based only on CD3+CD7- and/or CD3+CD26- CD4+ T cells (16)- would be not adequate for the specific identification (and hence, accurate enumeration) of SS/MF cells in PB, since small subsets of normal and particularly reactive T cells may have an overlapping immunophenotype. Then, a more appropriate strategy for the identification of SS/MF cells (i.e. to focus on the whole phenotype for all markers, and/or to include additional markers) would be followed. To further improve the specificity of FC in the diagnosis of SS (vs. reactive erythrodermic conditions), the TCR-Vβ repertoire of CD4+ T cells showing a suspect phenotype can be analyzed using monoclonal antibodies to 24 TCR-Vβ segments, which cover 70% of the known TCR-Vβ repertoire: in virtually all cases, this assay will show either restriction of one of these TCR-Vβ families or will be negative for all tested antibodies, in both cases confirming monoclonality (17,34). However, it should be taken into account that the large number of potential Vβ chains makes the TCR-Vβ repertoire analysis by flow cytometry challenging (and expensive) to test these markers in combination with other markers of MF/SS in routine clinical practice; interestingly, recent studies have preliminarily show the potential utility of one single antibody to confirm clonality by flow cytometry (an anti-TCR-Cβ1 fluorochrome-conjugated antibody) -in addition to typical panels for identifying MF/SS cells- for diagnosis and monitoring of SS (35,36), although the published data with this novel antibody is limited so far, and therefore more studies are needed specifically addressing its value for MF/SS diagnosis.
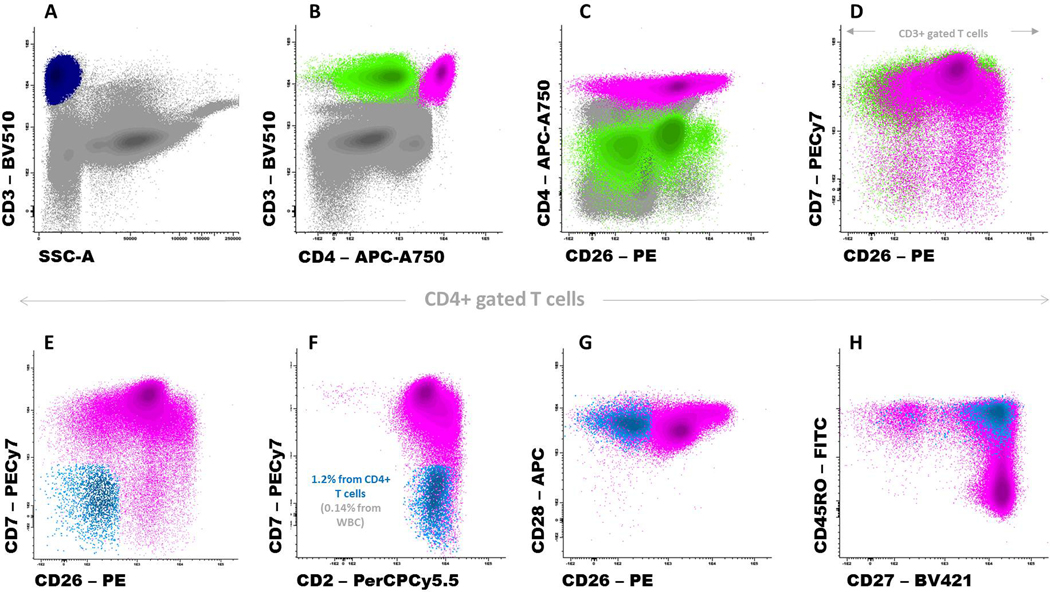
Figure 3:
A representative peripheral blood sample from an adult healthy donor stained with a combination of monoclonal antibodies designed for the identification of Sézary cells. Panel A shows total T cells (dark blue dots) while Panels B and C show the CD4+ T-cell (pink dots) and CD8+ T-cell subsets (green dots); non-T-cell events are displayed in gray. In Panel D, only T cells are displayed. Panels E-H show that a minor population of CD4+ T cells (displayed in light blue) has overlapping phenotypic features with Sézary cells: CD7-, CD26-, CD28+ and a mostly central memory (CD27+ and CD45RO+) phenotypic profile. Cells were stained using BulkLysis-based EuroFlow SOPs (www.euroflow.org); a total of 7×106 leukocyte events are shown in Panels A-C.
The above referred phenotypic profile (CD3/TCRαβlo, CD4lo, CD2-/lo, CD7-/lo and CD26-/lo together with CD45ROhi) is the most common for tumor cells from both MF and SS cases. No major evident phenotypical differences (both in skin and blood) have been described in pathological cells between MF and SS that can be applied for differential diagnosis in routine settings (i.e. in both diseases, cells show an overlapping phenotype). Despite this, some (minor) phenotypic differences of cells for both diseases have been documented; accordingly, it is interesting to note that in MF it is more frequent to find cells negative for CD27, CD62L and CD197 than in SS (which would reflect differences in their maturation stages, as mentioned above) (8), together with a higher percentage of MF cases showing a CD26 positive phenotype -particularly in early stages- as well as a CD279- profile, vs. SS, in addition to the fact that certain phenotypic variants of MF, particularly “non-CD4+” (i.e. CD4−CD8−, and mainly gamma-delta and CD8+ cells) are rare in SS (5,19,20,22,23). Also, it is important to note that immunophenotypic shifts in individuals’ disease have been shown to occur, at different skin sites, over time, and after the administration of various therapeutic agents (17). On the other hand, it should be taken into account that certain phenotypic features of Sézary cells can be also observed in T-cell chronic lymphoproliferative disorders other than SS/MF. In turn, tumor cells from erythrodermic adult T-cell leukemia/lymphoma, and even from T-cell prolymphocytic leukemia (that can also rarely present with erythroderma and in some cases with the cells showing dim expression of CD3 and absent CD7) may have a similar phenotype as Sézary cells, as well as many peripheral T-cell lymphomas, not otherwise specified. Ultimately, the general disease categorization could be established by FC, but the final diagnosis requires clinical correlation, in accordance with the WHO criteria (5), so that the integrated data may help clarify the diagnosis.
REFERENCES
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005. May 15;105(10):3768–3785. [DOI] [PubMed] [Google Scholar]
- 2.Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, Jaffe ES. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. lymphomas. Blood. 2019. Apr 18;133(16):1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burg G, Kempf W, Cozzio A, Feit J, Willemze R, S Jaffe E, Dummer R, Berti E, Cerroni L, Chimenti S, Diaz-Perez JL, Grange F, Harris NL, Kazakov DV, Kerl H, Kurrer M, Knobler R, Meijer CJ, Pimpinelli N, Ralfkiaer E, Russell-Jones R, Sander C, Santucci M, Sterry W, Swerdlow SH, Vermeer MH, Wechsler J, Whittaker S. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J. Cutan. Pathol. 2005. Nov;32(10):647–674. [DOI] [PubMed] [Google Scholar]
- 4.Elder DE, Massi D, Scolyer R, Willemze R (Eds). WHO Classification of Skin Tumours (4th edition). Lyon: 2018. (ISBN-13: 978–92-832–2440-2). [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pireli SA, Stein H, Thiele J (Eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). IARC; Lyon; 2017. (ISBN 13: 978–92-832–4494-3). [Google Scholar]
- 6.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S, Wood G, Dummer R, Ranki A, Burg G, Heald P, Pittelkow M, Bernengo MG, Sterry W, Laroche L, Trautinger F, Whittaker S; ISCL/EORTC. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007. Sep 15;110(6):1713–1722. [DOI] [PubMed] [Google Scholar]
- 7.Virmani P, Myskowski P, Pulitzer M. Unusual Variants of mycosis fungoides. Diagnostic Histopathology; 2016. April 22(4); 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sézary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010. Aug 5;116(5):767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarisbrick JJ, Kim YH, Whittaker SJ, Wood GS, Vermeer MH, Prince HM, Quaglino P. Prognostic factors, prognostic indices and staging in mycosis fungoides and Sézary syndrome: where are we now?. Br J Dermatol. 2014. Jun;170(6):1226–1236. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–866. [DOI] [PubMed] [Google Scholar]
- 11.Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, Wood GS, Willemze R, Demierre MF, Pimpinelli N, Bernengo MG, Ortiz-Romero PL, Bagot M, Estrach T, Guitart J, Knobler R, Sanches JA, Iwatsuki K, Sugaya M, Dummer R, Pittelkow M, Hoppe R, Parker S, Geskin L, Pinter-Brown L, Girardi M, Burg G, Ranki A, Vermeer M, Horwitz S, Heald P, Rosen S, Cerroni L, Dreno B, Vonderheid EC; International Society for Cutaneous Lymphomas; United States Cutaneous Lymphoma Consortium; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011. Jun 20;29(18):2598–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarisbrick JJ, Hodak E, Bagot M, Stranzenbach R, Stadler R, Ortiz-Romero PL, Papadavid E, Evison F, Knobler R, Quaglino P, Vermeer MH. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: recommendations from the EORTC cutaneous lymphoma task force. Eur J Cancer. 2018. Apr;93:47–56. [DOI] [PubMed] [Google Scholar]
- 13.Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T- cell lymphoma (mycosis fungoides and Sézary syndrome). Part I: Diagnosis: clinical and histopathologic features and new molecular biologic markers. J Am Acad Dermatol. 2014. Feb;70(2):205.e1–16; quiz 221–222. [DOI] [PubMed] [Google Scholar]
- 14.Horna P, Moscinski LC, Sokol L, Shao H. Naïve/Memory T-Cell Phenotypes in Leukemic Cutaneous T-Cell Lymphoma: Putative Cell of Origin Overlaps Disease Classification. Cytometry B Clin Cytom. 2019. May;96(3):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roelens M, Delord M, Ram-Wolff C, Marie-Cardine A, Alberdi A, Maki G, Homyrda L, Bensussan A, Bagot M, Toubert A, Moins-Teisserenc H. Circulating and skin-derived Sézary cells: clonal but with phenotypic plasticity. Blood. 2017. Sep 21;130(12):1468–1471. [DOI] [PubMed] [Google Scholar]
- 16.Boonk SE, Zoutman WH, Marie-Cardine A, van der Fits L, Out-Luiting JJ, Mitchell TJ, Tosi I, Morris SL, Moriarty B, Booken N, Felcht M, Quaglino P, Ponti R, Barberio E, Ram-Wolff C, Jäntti K, Ranki A, Bernengo MG, Klemke CD, Bensussan A, Michel L, Whittaker S, Bagot M, Tensen CP, Willemze R, Vermeer MH.: A Multicenter Study of 59 Patients. J Invest Dermatol. 2016. Jul;136(7):1364–1372. [DOI] [PubMed] [Google Scholar]
- 17.Lima M, Almeida J, dos Anjos Teixeira M, Queiros ML, Santos AH, Fonseca S, Balanzategui A, Justica B, Orfao A. Utility of flow cytometry immunophenotyping and DNA ploidy studies for diagnosis and characterization of blood. Evaluation of Immunophenotypic and Molecular Biomarkers for Sézary Syndrome using Standard Operating Procedures involvement in CD4+ Sézary’s syndrome. Haematologica. 2003. Aug;88(8):874–887. [PubMed] [Google Scholar]
- 18.Murphy M, Fullen D, Carlson JA. Low CD7 expression in benign and malignant cutaneous lymphocytic infiltrates: experience with an antibody reactive with paraffin-embedded tissue. Am J Dermatopathol. 2002. Feb 24(1):6–16. [DOI] [PubMed] [Google Scholar]
- 19.Horna P, Kurant D, Sokol L, Sotomayor EM, Moscinski L, Glass LF. Flow cytometric identification of immunophenotypically aberrant T-cell clusters on skin shave biopsy specimens from patients with mycosis fungoides. Am J Clin Pathol. 2015. Jun;143(6):785–796. [DOI] [PubMed] [Google Scholar]
- 20.Bernengo MG, Novelli M, Quaglino P, Lisa F, De Matteis A, Savoia P, Cappello N, Fierro MT. The relevance of the CD4+ CD26- subset in the identification of circulating Sézary cells. Br J Dermatol. 2001. Jan;144(1):125–135. [DOI] [PubMed] [Google Scholar]
- 21.Ng C, Chan J, Hui P, Chan W, Lo s. Application of a T cell receptor antibody beta F1 for immunophenotypic analysis of malignant lymphomas. Am J Pathol. 1988. Aug: 132(2): 365–371. [PMC free article] [PubMed] [Google Scholar]
- 22.Hodak E, David M, Maron L, Aviram A, Kaganovsky E, Feinmesser M. CD4/CD8 double-negative epidermotropic cutaneous T-cell lymphoma: an immunohistochemical variant of mycosis fungoides. J Am Acad Dermatol. 2006. Aug;55(2):276–284. [DOI] [PubMed] [Google Scholar]
- 23.Pulitzer M, Geller S, Kumar E, Frosina D, Moskowitz A, Horwitz S, Myskowski P, Kheterpal M, Chan A, Dogan A, Jungbluth A. T-cell receptor-δ expression and γδ+ T-cell infiltrates in primary cutaneous γδ T-cell lymphoma and other cutaneous T-cell lymphoproliferative disorders. Histopathology. 2018. Oct; 73(4):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edinger JT, Clark BZ, Pucevich BE, Geskin LJ, Swerdlow SH. CD30 Expression and proliferative fraction in nontransformed mycosis fungoides. Am J. Surg Pathol 2009. Dec; 33(12):1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghavan SS, Hong EK, Kim YH, Kim J. Utility of CD30, Ki-67, and p53 in assisting with the diagnosis of mycosis fungoides with large cell transformation. Cutan Pathol. 2019;46(1):33–43. [DOI] [PubMed] [Google Scholar]
- 26.Bosisio FM, Cerroni L. Expression of T-follicular helper markers in sequential biopsies of progressive mycosis fungoides and other primary cutaneous T-cell lymphomas. Am J Dermatopathol 2015. Feb; 37 (2):115–121. [DOI] [PubMed] [Google Scholar]
- 27.Oshtory S, Apisarnthanarax N, Gilliam A, Cooper K, Meyerson H. Usefulness of flow cytometry in the diagnosis of mycosis fungoides. JAAD. 2007. September; 57(3): 454–462. [DOI] [PubMed] [Google Scholar]
- 28.Jokinen C, Fromm J, Argenyi Z, Olerud J, Wood B, Greisman H. Flow cytometric evaluation of skin biopsies for mycosis fungoides. Am J Dermatopathol. 2011. July 33(5):483–491. [DOI] [PubMed] [Google Scholar]
- 29.Murray D, McMurray J, Eldershaw S, Pearce H, Scarisbrick J, Moss P. Progression of mycosis fungoides through divergence of tumor immunophenotype by differential expression of HLA-DR. Blood Advances. 2019. February 3(4):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benoit BM, Jariwala N, O’Connor G, Oetjen LK, Whelan TM, Werth A, Troxel AB, Sicard H, Zhu L, Miller C, Takeshita J, McVicar DW, Kim BS, Rook AH, Wysocka M. CD164 identifies CD4+ T cells highly expressing genes associated with malignancy in Sézary syndrome: the Sézary signature genes, FCRL3, Tox, and miR-214. Arch Dermatol Res. 2017. Jan;309(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones CL, Ferreira S, McKenzie RC, Tosi I, Caesar JA, Bagot M, Whittaker SJ, Mitchell TJ. Regulation of T-plastin expression by promoter hypomethylation in primary cutaneous T-cell lymphoma. J Invest Dermatol. 2012. Aug;132(8):2042–2049. [DOI] [PubMed] [Google Scholar]
- 32.Hurabielle C, Thonnart N, Ram-Wolff C, Sicard H, Bensussan A, Bagot M, Marie-Cardine A. Usefulness of KIR3DL2 to Diagnose, Follow-Up, and Manage the Treatment of Patients with Sézary Syndrome. Clin Cancer Res. 2017. Jul 15;23(14):3619–3627. [DOI] [PubMed] [Google Scholar]
- 33.Samimi S, Benoit B, Evans K, Wherry EJ, Showe L, Wysocka M, Rook AH. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression. Arch Dermatol. 2010. Dec;146(12):1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson JF, Huang J, Liu KJ, Carlson KR, Foss F, Choi J, Edelson R, Hussong JW, Mohl R, Hill S, Girardi M. Cutaneous T-cell lymphoma (CTCL): Current practices in blood assessment and the utility of T-cell receptor (TCR)-Vβ chain restriction. J Am Acad Dermatol. 2016;74(5):870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novikov ND, Griffin GK, Dudley G, Drew M, Rojas-Rudilla V, Lindeman NI, Dorfman DM. Utility of a Simple and Robust Flow Cytometry Assay for Rapid Clonality Testing in Mature Peripheral T-Cell Lymphomas. Am J Clin Pathol. 2019. Apr 2;151(5):494–503. [DOI] [PubMed] [Google Scholar]
- 36.Shi M, Jevremovic D, Otteson GE, Timm MM, Olteanu H, Horna P. Single Antibody Detection of T-Cell Receptor αβ Clonality by Flow Cytometry Rapidly Identifies Mature T-Cell Neoplasms and Monotypic Small CD8-Positive Subsets of Uncertain Significance. Cytometry B Clin Cytom. 2020. Jan;98(1):99–107. [DOI] [PubMed] [Google Scholar]