Abstract
Background:
Human Endogenous Retroviruses (HERVs) are genetic elements in the human genome which resulted from ancient retroviral germline infections. HERVs have strong transcriptional promoters and enhancers that affect a cell’s transcriptome. They also encode proteins that can exert effects in human cells. This review examines how our increased understanding of HERVs have led to their potential use as biomarkers and immunologic targets.
Material and Methods:
PubMed/Medline, Embase, Web of Science and Cochrane databases were used in a systematic search to identify all articles studying the potential impact of HERVs on surgical diseases. The search included studies that involved clinical patient samples in diseases including cancer, inflammatory conditions and autoimmune disease. Articles focused on conditions not routinely managed by surgeons were excluded.
Results:
86 articles met inclusion and quality criteria for this review and were included. Breast cancer and melanoma have robust evidence regarding the use of HERVs as potential tumor markers and immunologic targets. Reported evidence of the activity of HERVs in colorectal cancer, pancreatic cancer, hepatocellular cancer, prostate and ovarian cancer, germ cell tumors as well as idiopathic pulmonary hypertension, and the inflammatory responds in burns was also reviewed.
Conclusions:
Increasingly convincing evidence indicates that HERVs may play a role in solid organ malignancy and present important biomarkers or immunologic targets in multiple cancers. Innovative investigation of HERVs is a valuable focus of translational research, and can deepen our understanding of cellular physiology and the effects of endogenous retroviruses on human biology. As strategies for treatment continue to focus on genome-based interventions, understanding the impact of endogenous retroviruses on human disease will be critical.
Keywords: Human Endogenous Retrovirus, Retrovirology, Cancer, Surgical Disease
Introduction
Human endogenous retroviruses (HERVs) represent a class of retrotransposable elements in the human genome derived from insertion of retroviral DNA in the human germline. They comprise 8% of the human genome and have broad effects on human biology [1]. An example of the biologic function of these viruses is the Syncitin protein, which is exapted from a HERV envelope gene that drives cell-cell fusion and the formation of syncitiotrophoblasts in the mammalian placenta [2]. Another biological function is a HERV promoter that activates the amylase gene in the human salivary gland which contributed to the expansion of the human diet to include starches [3].
Retroviruses are RNA viruses that replicate via a DNA intermediate and integrate permanently into a host’s genome. The retrovirus then utilizes the host transcriptional machinery to generate progeny virions. Though retroviral infection usually occurs in somatic cells, over evolutionary time, different retroviruses have infected and integrated into the genome of human germ cells. When the provirus becomes permanently integrated into the human germline, it is passed onto progeny in Mendelian fashion. After initial integration, the provirus is also capable of retrotransposition, which has the effect of amplifying the number of copies (100–1,000) of the provirus in the host genome. The viruses are eventually rendered replication-incompetent through accumulated deletions and nonsense mutations within the viral genome. In this way, the virus becomes fixed in the human genome. At this point, these retroviruses are “endogenized,” as they are no longer capable of horizontal infection of another host and are re-classified as HERVs. In addition to acquiring large deletions and nonsense mutations within the proviral genome, HERVs experience host transcriptional silencing through DNA methylation and chromatin remodeling [4, 5].
There have been approximately 40 independent, phylogenetically distinct HERV groups categorized in the human genome [6]. This has led to a classification system based on the tRNA primer binding site of the HERV reverse transcriptase [7]. For example, the most recently integrated HERV, which was acquired in the human genome after the divergence of chimpanzees and humans [8], has a lysine (amino-acid code K) tRNA binding site and is so classified as HERV-K. The HERV-K members are the most biologically active of the HERVs, as they were most recently acquired and have therefore undergone the least amount of genetic silencing.
Within every retroviral genome, including HERVs, two flanking long-terminal repeats (LTRs) contain promoters that drive transcription of viral sequences, which include at least 3 genetic regions common to all retroviruses: Gag, Pol, and Env (Figure 1). The Gag region encodes structural proteins for assembly and encapsulation, the Pol genes encode enzymes for replicating the genomic RNA, including retroviral enzymes protease, reverse transcriptase and integrase, and the Env region encodes envelope proteins that mediate viral fusion and infection.
Figure 1.
Schematic representation of a retrovirus genomic structure. This figure depicts the genes common to all retroviruses. In complex retroviruses, such as HIV and HERV-K, additional accessory genes are present.
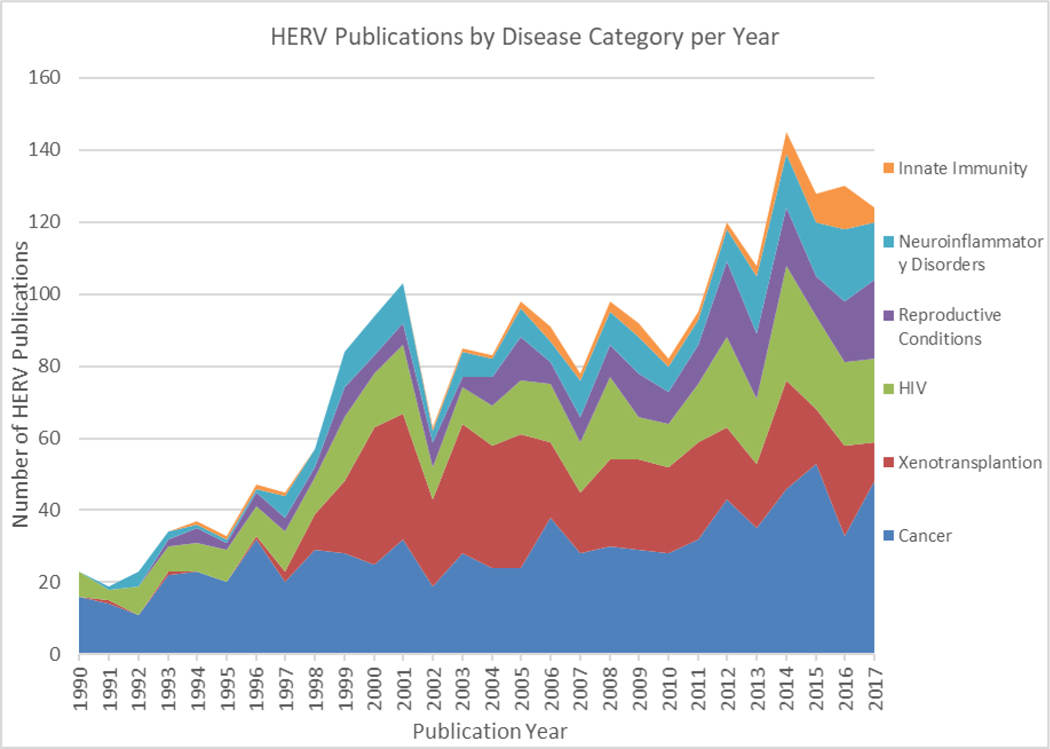
Investigators have been attempting to elucidate the functions, either biologic or pathophysiologic, for these endogenous sequences since their discovery [9]. As techniques for detecting HERVs have improved, so has their detection in human biology. They have correspondingly been increasingly studied. This is evidenced by the progressive expansion of HERV-related scientific publications since the early 1990’s (Figure 2), covering a diverse range of inquiry including cancer [10], neuroinflammatory disease [11], innate immunity [12], autoimmune disease [13], zoonosis in xenotransplantation [14] and activation by other viruses [15, 16]. For some disease processes like multiple sclerosis, robust evidence has demonstrated the Env protein from a HERV group is highly expressed in neural plaques and enacts a pro-inflammatory microenvironment in neurons [17, 18]. This has led to the development of a humanized monoclonal antibody to the Env protein and a current clinical Phase-2 randomized trial evaluating the monoclonal antibody efficacy in patients diagnosed with multiple sclerosis [19].
Figure 2.
HERV-related Publications, 1990–2017. This figure depicts the numbers of publications per year related to HERVs since 1990, categorized into the different human diseases studied.
A major area of investigation has been in oncogenesis, where there is a well-established history of retroviruses associated with malignancy. [20]. Controversy remains whether the presence of HERV elements have a role in cancer initiation or progression, or if they are a bystander effect of epigenetic changes in the cancer cell [21]. Generally, HERV differential regulation in cancer cells is likely a result of the global genomic instability of tumors leading to hypomethylation of DNA and chromatin remodeling. These epigenetic changes can expose previously transcriptionally silent HERV LTRs which results in increased transcription and translation of HERV elements [21]. Though HERVs are no longer replication competent, they can have significant effects on transcription levels of human genes and oncogenic pathways through the insertional position of the LTRs near, for example, proto-oncogenes [22]. HERVs can also act as alternative promoters and polyadenylation signals resulting in alternative splicing of human genes, especially when they are inserted within intronic regions [23, 24].
HERV proteins have been shown to interact with other cytosolic proteins including zinc finger motifs on tumor suppressor genes [25], and are involved in tumor immune-evasion [26] as well as metastatic transformation through cell fusion [27]. HERV proteins are also immunogenic; a strong body of evidence demonstrates upregulation of an adaptive immune responses to HERVs in malignant tissue and serum. Such a response is absent in unaffected organs or serum from disease-free individuals. Consequently, HERVs are viewed as intriguing targets for immune therapy and as biomarkers for diagnosis and disease progression. It is important to emphasize that HERVs roles in cancer progression (or any disease) is not a result of an “infection” as HERV elements were permanently integrated into the human genome millions of years ago. Rather, these genomic elements are regulated by epigenetic phenomena and when expressed, seem to be important components in several disease pathways.
The purpose of this systematic review is to summarize the highest-quality data regarding the effects of HERVs on conditions commonly encountered by surgeons. Surgeons are distinctively situated to construct tissue data banks and follow patients longitudinally [28, 29]. They often meet patients early in their disease course and may acquire tissue on multiple occasions, thereby shedding light on how the host genome activity changes as a disease progress. Furthermore, understanding how HERVs behave in the genome will extend our understanding of the behavior of other highly repeated genomic elements and yield important new concepts in epigenetics, bioinformatics, and genome-wide studies. Though the scope of this report is not exclusive to a specific intervention, a systematic PRISMA approach was adopted to capture the full range of existing evidence, and provide objectivity to evaluation of studies [30].
Methods
This systematic review was registered on PROSPERO (Registration #CRD42015016330, http://www.crd.york.ac.uk/PROSPERO/) and conducted using PRISMA guidelines for systematic reviews [30]. We searched PubMed/Medline, Embase, Web of Science and the Cochrane Database on February 1, 2018 using the key terms Human Endogenous Retrovirus, HERV, Cancer, Autoimmune Disease, Inflammation, and Transplantation (search strategies for each database available in Supplementary Document). In addition, the reference sections of recent articles were individually reviewed to assure inclusion of the most up-to-date and clinically important articles. We included original articles that addressed HERV expression in surgical disease. We concentrated on solid organ malignancies as the majority of HERV research has been conducted in this field. In addition, surgeons are uniquely positioned to both acquire tumor samples and investigate the biologic role of HERVs in cancer.
We further concentrated on articles that directly implicated HERV pathology and/or indicated clinical relevance of HERVs in diagnosis, disease stratification, response to therapy, or as a target for immune therapy. We excluded all review articles, abstracts, papers published in a non-English language, papers published exclusively using animal tissue or cell lines, and papers addressing pathologies that are unlikely to require surgical care.
Titles were screened by DFG to eliminate articles concentrating on neurodegenerative diseases and porcine endogenous retrovirus involved in xenotransplantation. Abstracts and full articles were independently screened by the same authors (DFG and SKR). Conflicts regarding eligibility were resolved through discussion between authors. As the majority of the included studies are human tissue analyses, standard ratings of quality including the Oxford Centre for Evidence-base medicine rating were not applicable. We thus gauged and independently assigned a quality score (low, intermediate, high) of clinical applicability to each study based on number of patients included, experimental rigor and evidence of pathophysiologic role of HERVs. Articles with low quality scores were removed.
Results
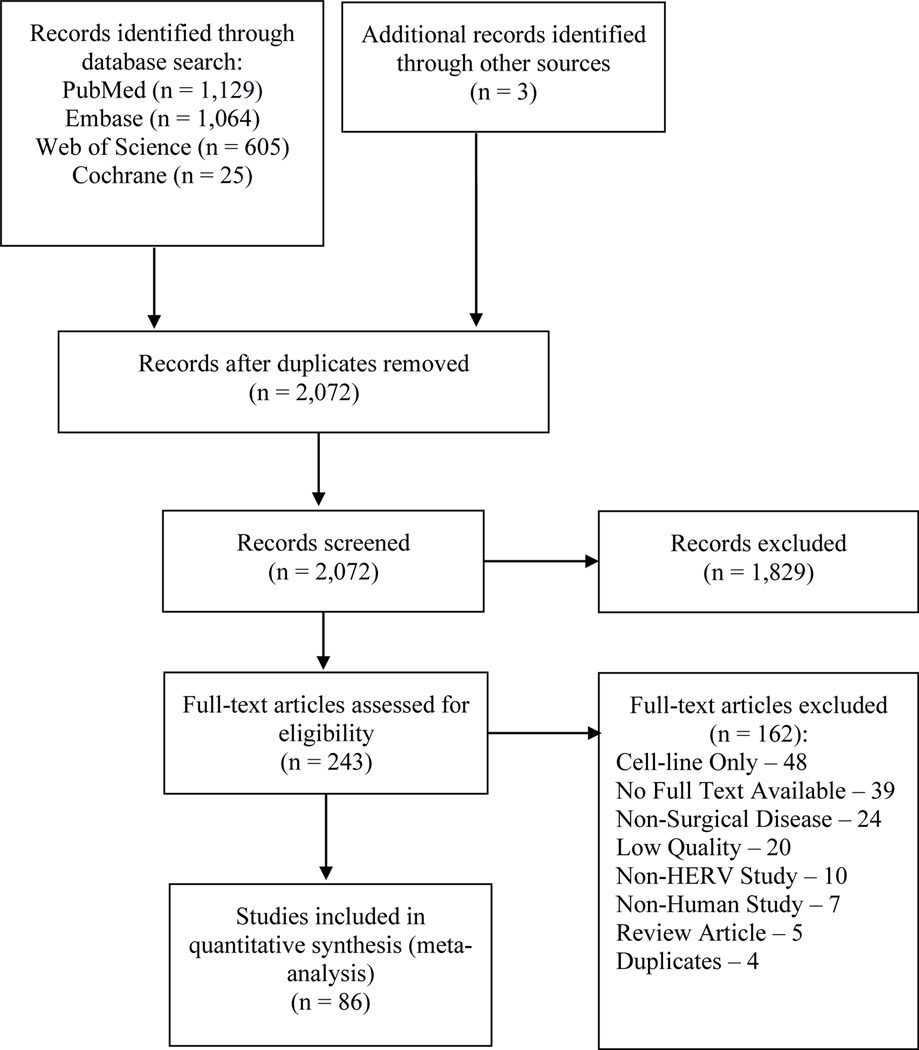
The initial literature search identified 2,072 studies of which 1,829 were eliminated by title and abstract screening. The majority of these articles were eliminated as they focused on neurodegenerative diseases, porcine endogenous retrovirus involved in xenotransplantation and activation of HERVs by other viruses. 243 full articles were reviewed and 86 met both inclusion and quality criteria for this review. The modified PRISMA flowchart showing study selection is presented in Figure 3. The majority of HERV-related literature for surgical disease has concentrated on cancer with a small subset on autoimmune and inflammatory conditions. The following sections summarize the included body of literature, grouped by disease. Also included is an abridged table (Table 1) of select references that highlight the clinical impact of HERVs on surgical disease (a summary table of all references is provided in the Supplementary Document).
Figure 3.
Prisma Diagram of Search Methodology. This figure depicts the PRISMA pathway applied to the literature search and the criteria used for article inclusion and exclusion.
Table 1:
Summary of Key HERV Investigations in Surgical Disease by Disease Type
| Disease | Review Number | Year | First Author | N | Tissues examined | Role for HERV | Application |
|---|---|---|---|---|---|---|---|
| Breast cancer | [38] | 2015 | Zhou | 21 | Tumor tissue | Yes | Immunologic therapy |
| Breast cancer | [39] | 2012 | Wang-Johanning | 223 | Tumor tissue | Yes | Immunologic target |
| Breast cancer | [44] | 2014 | Wang-Johanning | 155 | Tumor tissue, serum | N/A | Screening Biomarker |
| Melanoma | [53] | 2008 | Hahn | 312 | Serum | N/A | Biomarker |
| Melanoma | [56] | 2015 | Krishnamurthy | 359 | Tumor Tissue | N/A | Immunologic Target |
| Colorectal cancer | [63] | 2015 | Perot | 139 | Tumor tissue | Yes | Biomarker |
| Pancreatic cancer | [66] | 2017 | Li | 30 | Tumor tissue and Serum | Yes | Immunologic Target |
| Hepatocellular Carcinoma | [68] | 2016 | Ma | 84 | Tumor tissue | No | Biomarker for disease progression |
| Renal cell carcinoma | [82] | 2011 | Cherkasova | 67 | Tumor tissue | Yes | Immunologic Target |
| Prostate cancer | [91] | 2013 | Reis | 370 | Serum, tissue | Yes | Biomarker |
| Pulmonary Arterial Hypertension | [107] | 2017 | Saito | 18 | Disease tissue | Yes | Therapeutic target |
| Burns | [109] | 2014 | Lee | 11 | Serum | Yes | Biomarker |
Breast Cancer
HERV impact on the pathogenesis of breast cancer has been extensively studied. The course of endogenous retrovirus research and its effects on breast cancer mirrors the activity of the entire field with early studies focused on HERV identification and characterization in breast cancer tumors samples [31] [32, 33], and later studies focused on biologic roles. As techniques for studying HERV elements became more developed, investigations transitioned to identifying an oncologic mechanism for HERVs in breast cancer [34] and later its clinical utility as a tumor marker [35–37] and therapeutic target [38, 39].
A study by Golan and colleagues measured HERV Reverse Transcriptase (RT) protein in over 100 patients with 5–10-year follow-up and found a negative correlation between HERV transcription and disease-free survival (p< 0.004) as well as overall survival (p <0.0034). Additionally, the RT over-expression was associated with distant metastases indicating a possible prognostic role [40]. Another study by Zhao et. al. found that HERV envelope mRNA and protein expression were increased in breast cancer compared to normal adjacent tissue and was undetectable in healthy controls [41]. In this study, 70% of tumors had measurable HERV mRNA, and 86% had positive immunohistochemical staining for viral envelope protein. Moreover, the authors demonstrated a significant correlation between increased HERV expression and tumor size, lymph node metastases, and in turn overall survival. Interestingly, two studies detected no statistical correlation between the presence of genomic insertional polymorphic HERV-Ks and breast cancer risk [42, 43]. These studies, however, did not examine the transcriptional activity of the proviruses or test for differential gene expression between normal tissue and cancerous breast tissue.
In a separate study, HERV activity was examined as a possible prognostic biomarker for early stage breast cancer, somewhat akin to the use of measuring Human Papilloma Virus (HPV) in early detection of cervical cancer. This study utilized real-time reverse-transcriptase PCR to quantitate expression of HERV RNA in patients with breast cancer and examined if HERV mRNA levels in tumors correlated with eventual development of distant metastasis. Additionally, the authors of this study measured antibody responses in the sera of breast cancer patients and patients with ductal carcinoma in situ. The diagnostic performance of antibody titer was favorable to mammograms. Furthermore, HERV mRNA and antibody response did serve as a sensitive and specific screening test for early stage and preneoplastic breast cancer markers. Lastly, it was proposed that HERV proteins may make attractive vaccines against breast cancer [44]. Interestingly, Johanning and colleagues looked at HERV-K expression in different intraductal carcinoma subtypes and found significant overexpression of HERV-K in basal cell subtype [45], the subtype with the poorest prognosis.
Breast cancer patients have also been shown to possess HERV-specific antibody in serum and cytotoxic T-cell responses, as well as HERV-induced cytokine up-regulation [46]. This has led to recent studies evaluating HERV-K as a potential target for immunotherapy. Wang-Johanning et al. developed an anti-HERV-K Env antibody and demonstrated significant reduction in growth of xenograft tumors in an in-vivo mouse experiment [39] confirming the role of HERV-K Env as a tumor-associated antigen. This group then developed a chimeric antigen receptor (CAR) T cell specific for the HERV-K Env protein. Administration of the HERV-K CAR T-cells exhibited significant, tumor-specific cytotoxicity toward breast cancer cells in a mouse xenograft-model. It also prevented tumor metastasis [38]. Continued analyses from this investigation indicate HERV-K Env may act as a oncoprotein with effects on both the p53 and RAS signaling pathways [47].
Melanoma
An early study by Muster and colleagues assessed 50 clinical samples of melanoma, metastatic lymph nodes and corresponding normal tissue. They found highly specific expression of HERV sequences and viral proteins in all tumor samples and metastatic lymph nodes but only 1 of 29 normal tissues including non-metastatic lymph nodes [48]. In a study by Singh et al, patient samples from melanoma, benign nevi, and normal controls were studied [49]. Although all samples contained transcribed HERV mRNA, the Rec gene was only transcribed in melanoma, and its expression was associated with thicker lesions. HERV Env protein has also been observed in melanomas and atypical nevi, and in vitro experiments have suggested that a functional Env protein contributes to fusogenic activity in melanoma cells and may directly contribute to melanoma metastasis [50].
Though early studies by Buscher found variable expression of HERV products and poor humoral response in melanoma samples and patients [51, 52]; more recent studies by Hahn and colleagues demonstrated a clear serologic response to HERV antigens and observed a correlation between increased HERV antibody response and a decrease in both disease-specific (stage I-IV, p < 0.001) and overall survival (stage I-III, p = 0.005) [53]. Additional tissue and serum-based studies also demonstrated an immune response to HERV-derived epitopes, including a HERV-K env splice variant name HERV-K-Mel, indicating that HERVs expressed in melanoma do function as immune targets and could potentially serve as targets for cancer vaccination or immunotherapy [54, 55]. Krishnamurthy et al. confirmed that HERV-K Env is a tumor associated antigen in 220 melanoma samples compared to 139 normal tissue controls. The group developed a Chimeric Antigen Receptor (CAR) T cell specific to the HERV-K Env protein. Administration of the HERV-K Env CAR T cell demonstrated significant anti-tumoral effect in a melanoma in-vivo mouse xenograft model, including a reduction in overall tumor burden (p < 0.0001) and number of liver metastatic colonies (p < 0.05) [56].
Colorectal Cancer
Recent attention has turned to the HERV-H provirus in both colorectal and gastrointestinal cancer and its candidacy for targeted immune therapy. Wentzensen and colleagues first noted that between 40–50% of all gastric and colorectal cancers expressed HERV-H transcription secondary to CpG hypomethylation of the provirus’ viral promoter [57]. This finding was corroborated by Liang et al. [58]. Mullins et al. later noted that HERV-H Gag protein was expressed in colorectal cancer [59]. Gibbs and colleagues noted that a long non-coding RNA produced from a HERV has been evolutionarily conserved and is upregulated in 63 colorectal carcinomas when compared against matched controls [60]. Alves et al. followed with an evaluation of colorectal cancer to determine candidate genes for immunologic therapy and determined that the HERV-H provirus was the most promising candidate of 2100 genes that were differentially expressed [61]. Mechanistic evaluations of HERV-H in colorectal cancer showed that its transcription was strongly associated with immune invasion [62], microsatellite instability and early lymph node invasion [63]. Lastly, Diaz-Carballo and colleagues noted that HERV expression was strongly associated with chemotherapy resistance. In a corresponding cell-line experiment, the addition of anti-viral therapy in combination with cytotoxic chemotherapy demonstrated a synergistic antiproliferative effect [64]. Additional investigations are needed to validate the clinical impact of HERV-H blockade on colorectal cancer as an intervention.
Hepatobiliary Cancer
Schmitz-Winnenthal and colleagues evaluated 130 pancreatic adenocarcinoma tumor samples compared to 23 normal tissue controls to screen for novel targets for immunotherapy. The investigators found that HERV-K-MEL was expressed in >20% of pancreatic tumors but not in normal pancreatic tissue and may present a novel target for immunotherapy [65]. An investigation of the role of HERV-K Env in pancreatic cancer by Li et al. noted that HERV-K env was expressed in 80% of pancreatic adenocarcinoma but not in normal tissue and that serum antibody levels were significantly higher in patients diagnosed with the disease [66]. Through an in-vivo mouse model, the authors demonstrated that shRNA knock-down of HERV-K env reduced tumor growth rates and lung metastases. An RNA-seq analysis determined that HERV-K-env knock-down decreased expression of the RAS-ERK-RSK pathway. HERV-K expression has also been demonstrated in normal liver tissue as well as hepatocellular carcinoma [67]. In an investigation of hepatocellular carcinoma and surrounding normal tissue, Ma et al. noted that increased transcription of HERV-K was an independent prognostic factor for lower overall survival rates in hepatocellular carcinoma [68].
Germ Cell Tumors
Germ cell tumors (GCT) represent one of the earliest tissues studied for the clinical impact of HERVs. Multiple investigations completed in the mid-1990s demonstrated the presence of HERV protein transcription and the development of highly specific antibodies to HERV-K Env protein in various GCTs [69–71]. However, HERV Gag protein expression has been found in normal testes as well disease tissue, potentially reducing its utility as a biomarker [72]. Conversely, during this same period, a separate group noted that serum titers for HERV-K proteins resolved with resection of the GCTs and proposed HERV-K antibodies as a tumor marker for disease response and surveillance [73]. Similarly, Kleiman and colleagues noted that a decrease in HERV-K Gag and Env antibody titers after the first round of chemotherapy in patients with GCTs directly correlated with disease free survival (p = 0.01) [74].
More recently, investigations of patients with seminomas have noted a strong adaptive immune response to HERV-K [75] and differential expression of syncytin-1, a HERV-W env protein [76]. The latter group noted that syncytin-1 may be a useful and specific biomarker for pure seminomas or GCT with a seminoma component. Though there is strong evidence that HERV proteins generate disease specific immune responses, no clinical evaluation of their utility against other GCT disease markers has been conducted to date, likely because of GCT’s relative rarity.
Renal Cell and Urothelial Carcinoma
HERV-E env is a potential therapeutic target for urothelial and clear cell renal cell carcinoma (ccRCC). Early investigations in RCC explore multiple different HERV families. Florl and colleagues demonstrated that HERV transcription was upregulated in urothelial carcinoma as a result of genomic instability and hypomethylation of retrotransposable elements [77]. Haupt et. al noted that overall HERV transcription was upregulated in RCC cancer cell lines but not RCC tissue as compared to normal controls [78]. Yu and colleagues noted that an increased expression of HERV-W env expression in urothelial carcinoma was a result of a mutation in the 3’LTR allowing increased binding of the C-Myb transcription factor [79].
A more recent investigation by Gosenca determined that a specific HERV-E provirus is located in an antisense orientation of an intron in the cytosolic phospholipase A2 gene (cPLA2), a common cancer pathway. The authors found that upregulation of the HERV-E provirus corresponded to down-regulation of the cPLA2, which the authors suggest may potentially contribute to tumorigenesis [80]. The finding of strong differential expression of HERV-E in renal cell carcinoma [81] led to the discovery by Cherkasova and colleagues that HERV-E env upregulation is a result of inactivation of the von Hippel Lindau tumor suppressor gene. This leads to over-expression of hypoxia-inducible transcription factors 1α and 2α and subsequent hypomethylation of the HERV-E viral promoter [82]. The same group later described the presence of HERV-E Env on the cell surface of ccRCC tumors. By creating stimulated CD8+ T cells that responded to the HERV-E env as an antigen, the group identified this as a potential target for T cell-based immunotherapy [83, 84].
Prostate Cancer
HERV activity within tumor tissue and serum from prostate cancer patients have been compared against that of healthy controls and patients with benign prostate conditions [85–90]. These studies demonstrate a correlation between expression and translation of HERV-K gag and antibody response, and propose the application of HERVs as novel biomarkers and/or immunotherapeutic targets in prostate cancer. Furthermore, Reis and colleagues noted that HERV-K- Gag antibody expression levels closely correlate with disease progression and mortality [91]. Wallace et al. corroborated these findings and noted that the specificity of HERV-K gag expression performed better as a disease biomarker in older men as opposed to younger men. This is in contrast to prostate-specific antigen (PSA) where sensitivity decreases with age [92].
Ovarian Cancer
A study by Rycaj et al, examined tumor tissue, benign tissue, ascites fluid, and serum from healthy patients, patients with ovarian cancer, and patients with benign ovarian disease [93]. They detected reverse transcriptase activity in tumors and in the serum of patients with ovarian cancer, as well as expression of envelope protein on the surface of tumor cells. This study also identified human antibody responses to envelope proteins from HERVs and proposed that these antigens could be potential targets for immunotherapy. Three additional papers examining HERV activity in ovarian cancer [5, 94, 95] showed that patients with ovarian cancer expressed higher levels of anti-HERV antibodies than healthy controls, and ovarian cancer samples stained positive for envelope protein. Hypomethylation of HERV-K transcripts was also shown to correlate with inferior response to platinum-based chemotherapy and decreased overall survival. However, it was unclear if HERVs appeared to be specifically targeted for hypomethylation in the genomes of these tumors, or whether there was global hypomethylation in tumors. Regardless, only HERV hypomethylation was correlative with clinical outcome.
Other Cancers
HERV transcription has been reported in several other cancers. HERV-K polymorphisms at specific loci were found in female never-smokers over 60 who developed lung adenocarcinoma [96]. HERV-K-MEL transcription is expressed in 40% of tumor samples in head and neck squamous cell carcinoma but correlation with clinical-pathologic parameters have not been proven [97]. Buslei and colleagues reviewed Syncytin-1 expression in pituitary adenomas and noted that deregulation of Syncytin-1 leads to an increased expression in all subtypes of pituitary adenomas [98]. In a comparison between osteosarcomas and normal bone, HERVs were noted as being the most differentially expressed repetitive elements, suggesting a pathophysiologic role [99]. Strissel and colleagues examined the role of Syncytin-1 in 10 endometrial carcinomas with patient-matched controls and noted that hypomethylation of the HERV-W LTR opened a transcription factor binding site that leads to up-regulation of the transcript in disease tissue [100].
Several investigations have demonstrated increased expression of HERVs in combined leukemia and lymphoma experimental populations when compared to normal controls [101, 102]. However, recent investigations of HERV expression in leukemia and lymphoma have found mixed results. Bergallo et al. found that HERV-K is over expressed in Acute Lymphoblastic Leukemia (ALL) compared to unaffected patient controls, while Acute Myeloid Leukemia (AML) was not differentially expressed [103]. Conversely, in a separate investigation, authors noted that HERV-K env is over-expressed in AML but not in ALL [104]. Preliminary studies in Cutaneous T Cell Lymphoma have found that HERV-W and HERV-K is over expressed compared to normal controls [105] and investigators have demonstrated increased HERV-K expression in the serum of lymphoma patients [35]. However, to date, there have been few clinical investigations in hematologic malignancies that link HERV activity with malignant transformation.
HERV Activity in Other Surgical Disease
HERV activity has been investigated in both inflammatory and autoimmune disease. Low expression of HERVs in peripheral blood mononuclear cells (PBMCs) has been correlated with up-regulation of toll-like receptors in patients with idiopathic nephrotic syndrome [106]. HERV-K env is also upregulated in patients with idiopathic pulmonary hypertension and induces pro-inflammatory cytokines (interleukin 6, interleukin 1β, tumor necrosis factor α) [107]. One group has examined the transcriptional profile of HERVs in major burn patients [108, 109]. Their work indicated a correlation between burn severity, HERV transcription, and inflammatory mediator expression, suggesting a possible application of HERVs as inflammatory biomarkers. The effect HERV transcription plays on divergent injury response has been extensively studied in animals [110], but has yet to be fully understood in humans.
HERV activity and expression have been linked to multiple human reproductive pathologies encountered by obstetricians, gynecologist and urologists including pre-eclampsia [111], endometriosis [112], placentation [113], hydatidiform moles [114] and azoospermia [115]. Several studies have noted decreased expression of human syncitin-2 protein (encoded by HERV-W sequences) in patients with pre-eclampsia. Moreover, the severity of the condition appears to correlate with the magnitude of down-regulation [111, 116]. The decreased levels of syncitin expression are measurable in-patient serum, indicating its potential use as a biomarker in a disease that often has an insidious and silent presentation. Subsets of males in Japan with azoospermia and oligospermia were found to have HERV-mediated deletion of testis-specific transcripts of the Y-chromosome [115], leading the authors to suggest that HERV-mediated recommendation events could be a cause of idiopathic male infertility.
Discussion:
Evidence that HERV activity in the human genome has an impact on the pathogeneses of human diseases is growing rapidly. In breast cancer, melanoma, and colorectal cancer, studies are now concentrating on immunologic response and targeted therapy. Several other diseases regularly treated by surgeons including germ cell tumors, prostate cancer, ovarian cancer and hepatobiliary cancer have growing evidence that HERVs may serve as clinically important tumor markers or play a role in tumorigenesis.
One crucial factor that makes the impact of HERVs on human biology difficult to quantitate is their highly repetitive nature and polymorphism in the human population. Because of their genetic amplification and properties of transposability, they have been traditionally overlooked in genome-wide association studies. They also remain largely unannotated in the human genome. Because of this, many of the early publications reporting HERV detection in human disease were descriptive and qualitative in nature. As HERV detection has increased, so has the awareness that these elements have important influence on cell biology and our understanding of the basic mechanisms of these elements’ activity is lacking. With genomic and transcriptomic technology becoming less expensive and higher in quality, it is becoming easier to look at individual patients’ expression profiles. This growing biologic data set is allowing more sophisticated molecular subtyping of tumors, including HERVs, which will undoubtedly benefit immunotherapy and/or targeted therapy in the future. As such, we believe it is critical for surgeons to work closely with the biorepository and pathology department at their local institution to preserve fresh frozen tissue for exome and transcriptome analysis [117].
Nevertheless, it remains unproven whether changes in HERV expression and translation are truly causal and impactful in disease pathogenesis. While the body of evidence is significant, it is also heterogeneous, as different investigators have emphasized the study of different HERV groups and specific elements. Furthermore, most studies in this review do not include normal human tissue from patients without a history of the disease being studied, instead using normal tissue from the afflicted patient. While noting a difference in HERV activity between normal and diseased tissues within the same patient does lend support to the hypothesis that HERVs play a role in disease, tissue from unaffected patients remains an important negative control. This presents a potential challenge, as the collection of solid organ tissue samples from healthy individuals is fraught with ethical considerations. It is possible that HERV activity correlates with some host factor not yet identified that activates HERV transcription prior to malignant transformation or disease presentation. Moving forward, a more complete understanding of the basic mechanisms of these observations will be paramount in order for the clinical impact of HERV research to be fully realized. This includes a detailed understanding of the mechanisms and interactions of different HERV elements in both a tissue- and disease-specific manner.
Conclusion:
Human endogenous retroviruses comprise roughly 8% of the human genome and contain transcriptional promoters and open reading frames for viral proteins. The evidence that HERVs are differentially expressed in many human diseases is strong. Future studies of these elements’ effects on human disease should focus on broad populations of patients, given that individual HERV polymorphisms may have an epigenetic clinical impact. Tissue- and disease-specific databanks are ideal for studying these elements. Surgeons can play a vital role in the investigation of HERVs because of their unique access to tissue and their longitudinal patient interactions. As genomics becomes an increasingly important part of an individualized approach to treatment, elucidating the roles of these endogenous sequences in human disease may guide new therapies or provide new biomarkers for disease progression. Toward this overarching goal, collaboration among clinicians, surgeons, retrovirologists, geneticists and other basic scientists will be crucial.
Supplementary Material
Acknowledgements:
The authors wish to thank Drs. Marie-Louise Hammarskjold and David Rekosh in the Department of Microbiology, Immunology and Cancer Biology and the Thaler Center for HIV Research at the University of Virginia for their insight into HERV biology. The authors also wish to thank Mrs. Megan Nunemaker and Kelly Near of the University of Virginia Claude Moore Health Sciences Library for assistance building the search protocols.
Funding:
This work was supported by The National Cancer Institute of the National Institutes of Health (Grant number: T32 CA163177).
Footnotes
Disclosures: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Griffiths DJ, Endogenous retroviruses in the human genome sequence. Genome Biol, 2001. 2(6): p. Reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rote NS, Chakrabarti S, and Stetzer BP, The role of human endogenous retroviruses in trophoblast differentiation and placental development. Placenta, 2004. 25(8–9): p. 673–683. [DOI] [PubMed] [Google Scholar]
- 3.Samuelson LC, et al. , Retroviral and pseudogene insertion sites reveal the lineage of human salivary and pancreatic amylase genes from a single gene during primate evolution. Molecular and cellular biology, 1990. 10(6): p. 2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt K, et al. , Transcriptional profiling of human endogenous retrovirus group HERV-K(HML-2) loci in melanoma. Genome biology and evolution, 2013. 5(2): p. 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menendez L, Benigno BB, and McDonald JF, L1 and HERV-W retrotransposons are hypomethylated in human ovarian carcinomas. Molecular cancer, 2004. 3: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer J, Blomberg J, and Seal RL, A revised nomenclature for transcribed human endogenous retroviral loci. Mob DNA, 2011. 2(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomberg J, et al. , Classification and nomenclature of endogenous retroviral sequences (ERVs): problems and recommendations. Gene, 2009. 448(2): p. 115–23. [DOI] [PubMed] [Google Scholar]
- 8.Sverdlov ED, Retroviruses and primate evolution. Bioessays, 2000. 22(2): p. 161–71. [DOI] [PubMed] [Google Scholar]
- 9.Voisset C, Weiss RA, and Griffiths DJ, Human RNA “rumor” viruses: the search for novel human retroviruses in chronic disease. Microbiology and molecular biology reviews : MMBR, 2008. 72(1): p. 157–96, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downey RF, et al. , Human endogenous retrovirus K and cancer: Innocent bystander or tumorigenic accomplice? International journal of cancer.Journal international du cancer, 2014. [DOI] [PMC free article] [PubMed]
- 11.Kury P, et al. , Human Endogenous Retroviruses in Neurological Diseases. Trends Mol Med, 2018. 24(4): p. 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst T and Magiorkinis G, Activation of the innate immune response by endogenous retroviruses. The Journal of general virology, 2014. [DOI] [PubMed]
- 13.Nakagawa K and Harrison LC, The potential roles of endogenous retroviruses in autoimmunity. Immunological reviews, 1996. 152: p. 193–236. [DOI] [PubMed] [Google Scholar]
- 14.Brown J, et al. , Xenotransplantation and the risk of retroviral zoonosis. Trends in microbiology, 1998. 6(10): p. 411–415. [DOI] [PubMed] [Google Scholar]
- 15.Bergallo M, et al. , CMV induces HERV-K and HERV-W expression in kidney transplant recipients. Journal of Clinical Virology, 2015. 68((Mareschi K, katia.mereschi@unito.it; Fagioli F, franca.fagioli@unito.it) Pediatric Onco-Hematology, Stem Cell Transplantation and Cellular Therapy Division, City of Science and Health of Turin, Regina Margherita Children’s Hospital, Turin, Italy: ): p. 28–31. [DOI] [PubMed] [Google Scholar]
- 16.van der Kuyl AC, HIV infection and HERV expression: a review. Retrovirology, 2012. 9: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perron H, et al. , Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler, 2012. 18(12): p. 1721–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolland A, et al. , The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol, 2006. 176(12): p. 7636–44. [DOI] [PubMed] [Google Scholar]
- 19.Curtin F, et al. , A placebo randomized controlled study to test the efficacy and safety of GNbAC1, a monoclonal antibody for the treatment of multiple sclerosis - Rationale and design. Mult Scler Relat Disord, 2016. 9: p. 95–100. [DOI] [PubMed] [Google Scholar]
- 20.Rous P, A SARCOMA OF THE FOWL TRANSMISSIBLE BY AN AGENT SEPARABLE FROM THE TUMOR CELLS. J Exp Med, 1911. 13(4): p. 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassiotis G and Stoye JP, Making a virtue of necessity: the pleiotropic role of human endogenous retroviruses in cancer. Philos Trans R Soc Lond B Biol Sci, 2017. 372(1732). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebollo R, Romanish MT, and Mager DL, Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu Rev Genet, 2012. 46: p. 21–42. [DOI] [PubMed] [Google Scholar]
- 23.Schulte AM, et al. , Human trophoblast and choriocarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. Proceedings of the National Academy of Sciences of the United States of America, 1996. 93(25): p. 14759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen CJ, Lock WM, and Mager DL, Endogenous retroviral LTRs as promoters for human genes: a critical assessment. Gene, 2009. 448(2): p. 105–14. [DOI] [PubMed] [Google Scholar]
- 25.Boese A, et al. , Human endogenous retrovirus protein cORF supports cell transformation and associates with the promyelocytic leukemia zinc finger protein. Oncogene, 2000. 19(38): p. 4328–36. [DOI] [PubMed] [Google Scholar]
- 26.Kudo-Saito C, et al. , Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Res, 2014. 74(5): p. 1361–70. [DOI] [PubMed] [Google Scholar]
- 27.Bjerregaard B, et al. , Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci, 2006. 63(16): p. 1906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis ST, et al. , Tumor banks: the cornerstone of basic research in urology. International braz j urol : official journal of the Brazilian Society of Urology, 2010. 36(3): p. 348–354. [DOI] [PubMed] [Google Scholar]
- 29.Tsikitis VL, et al. , Surgeon leadership enables development of a colorectal cancer biorepository. American Journal of Surgery, 2013. 205(5): p. 563–5; discussion 565. [DOI] [PubMed] [Google Scholar]
- 30.Shamseer L, et al. , Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed.), 2015. 349: p. g7647. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, et al. , Detection of mammary tumor virus env gene-like sequences in human breast cancer. Cancer research, 1995. 55(22): p. 5173–5179. [PubMed] [Google Scholar]
- 32.Wang-Johanning F, et al. , Quantitation of HERV-K env gene expression and splicing in human breast cancer. Oncogene, 2003. 22(10): p. 1528–1535. [DOI] [PubMed] [Google Scholar]
- 33.Wang-Johanning F, et al. , Expression of human endogenous retrovirus k envelope transcripts in human breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 2001. 7(6): p. 1553–1560. [PubMed] [Google Scholar]
- 34.Frank O, et al. , Variable transcriptional activity of endogenous retroviruses in human breast cancer. Journal of virology, 2008. 82(4): p. 1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contreras-Galindo R, Human endogenous retrovirus-K (HML-2) elements in the plasma of people with lymphoma and breast cancer. Journal of Acquired Immune Deficiency Syndromes, 2009. 51((Contreras-Galindo R) University of Michigan, Department of Internal Medicine, Division of Infectious Diseases, Ann Arbor, United States: ): p. 52. [Google Scholar]
- 36.Armbruester V, et al. , A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clinical cancer research : an official journal of the American Association for Cancer Research, 2002. 8(6): p. 1800–1807. [PubMed] [Google Scholar]
- 37.Rhyu DW, et al. , Expression of human endogenous retrovirus env genes in the blood of breast cancer patients. International journal of molecular sciences, 2014. 15(6): p. 9173–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, et al. , Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of Ras. Oncoimmunology, 2015. 4(11): p. e1047582-e1047582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang-Johanning F, et al. , Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. Journal of the National Cancer Institute, 2012. 104(3): p. 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golan M, et al. , Human endogenous retrovirus (HERV-K) reverse transcriptase as a breast cancer prognostic marker. Neoplasia (New York, N.Y.), 2008. 10(6): p. 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J, et al. , Expression of Human Endogenous Retrovirus Type K Envelope Protein is a Novel Candidate Prognostic Marker for Human Breast Cancer. Genes & cancer, 2011. 2(9): p. 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burmeister T, et al. , Insertional polymorphisms of endogenous HERV-K113 and HERV-K115 retroviruses in breast cancer patients and age-matched controls. AIDS Research and Human Retroviruses, 2004. 20(11): p. 1223–1229. [DOI] [PubMed] [Google Scholar]
- 43.Wildschutte JH, et al. , The distribution of insertionally polymorphic endogenous retroviruses in breast cancer patients and cancer-free controls. Retrovirology, 2014. 11: p. 62–1720768941312026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang-Johanning F, et al. , Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. International journal of cancer.Journal international du cancer, 2014. 134(3): p. 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johanning GL, et al. , Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Scientific Reports, 2017. 7(Journal Article): p. 41960–41960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang-Johanning F, et al. , Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer research, 2008. 68(14): p. 5869–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou F, et al. , Activation of HERV-K Env protein is essential for tumorigenesis and metastasis of breast cancer cells. Oncotarget, 2016. 7(51): p. 84093–84117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muster T, et al. , An endogenous retrovirus derived from human melanoma cells. Cancer research, 2003. 63(24): p. 8735–8741. [PubMed] [Google Scholar]
- 49.Singh S, et al. , Human endogenous retrovirus K (HERV-K) rec mRNA is expressed in primary melanoma but not in benign naevi or normal skin. Pigment cell & melanoma research, 2013. 26(3): p. 426–428. [DOI] [PubMed] [Google Scholar]
- 50.Huang G, et al. , Human endogenous retroviral K element encodes fusogenic activity in melanoma cells. Journal of carcinogenesis, 2013. 12: p. 5–3163.109032. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buscher K, et al. , Expression of human endogenous retrovirus K in melanomas and melanoma cell lines. Cancer research, 2005. 65(10): p. 4172–4180. [DOI] [PubMed] [Google Scholar]
- 52.Büscher K, et al. , Expression of the human endogenous retrovirus-K transmembrane envelope, Rec and Np9 proteins in melanomas and melanoma cell lines. Melanoma research, 2006. 16(3): p. 223–34. [DOI] [PubMed] [Google Scholar]
- 53.Hahn S, et al. , Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Research and Human Retroviruses, 2008. 24(5): p. 717–723. [DOI] [PubMed] [Google Scholar]
- 54.Humer J, et al. , Identification of a melanoma marker derived from melanoma-associated endogenous retroviruses. Cancer research, 2006. 66(3): p. 1658–1663. [DOI] [PubMed] [Google Scholar]
- 55.Schiavetti F, et al. , A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer research, 2002. 62(19): p. 5510–6. [PubMed] [Google Scholar]
- 56.Krishnamurthy J, et al. , Genetic engineering of T cells to target HERV-K, an ancient retrovirus on melanoma. Clinical Cancer Research, 2015. 21(14): p. 3241–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wentzensen N, et al. , Expression of an endogenous retroviral sequence from the HERV-H group in gastrointestinal cancers. International journal of cancer.Journal international du cancer, 2007. 121(7): p. 1417–1423. [DOI] [PubMed] [Google Scholar]
- 58.Liang Q, et al. , Expression patterns of non-coding spliced transcripts from human endogenous retrovirus HERV-H elements in colon cancer. PLoS ONE, 2012. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mullins CS, et al. , Generation, Characterization and Application of Antibodies Directed against HERV-H Gag Protein in Colorectal Samples. PLoS One, 2016. 11(4): p. e0153349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibb EA, et al. , Activation of an endogenous retrovirus-associated long non-coding RNA in human adenocarcinoma. Genome Medicine, 2015. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alves PM, et al. , Identification of tumor-associated antigens by large-scale analysis of genes expressed in human colorectal cancer. Cancer immunity, 2008. 8: p. 11. [PMC free article] [PubMed] [Google Scholar]
- 62.Kudo-Saito C, et al. , Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Research, 2014. 74(5): p. 1361–1370. [DOI] [PubMed] [Google Scholar]
- 63.Pérot P, et al. , Expression of young HERV-H loci in the course of colorectal carcinoma and correlation with molecular subtypes. Oncotarget, 2015. 6(37): p. 40095–40111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Díaz-Carballo D, et al. , Therapeutic potential of antiviral drugs targeting chemorefractory colorectal adenocarcinoma cells overexpressing endogenous retroviral elements. Journal of experimental & clinical cancer research : CR, 2015. 34: p. 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmitz-Winnenthal FH, et al. , Potential target antigens for immunotherapy in human pancreatic cancer. Cancer letters, 2007. 252(2): p. 290–8. [DOI] [PubMed] [Google Scholar]
- 66.Li M, et al. , Downregulation of human endogenous retrovirus type K (HERV-K) viral env RNA in pancreatic cancer cells decreases cell proliferation and tumor growth. Clinical Cancer Research, 2017. 23(19): p. 5892–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cha H, et al. , Expression profiles of HERV-R and HERV-K Env proteins in various cancerous tissues. FEBS Journal, 2017. 284((Kim H) Department of Biological Science, Pusan National University, Busan, South Korea: ): p. 264. [Google Scholar]
- 68.Ma W, et al. , Human Endogenous retroviruses-k (HML-2) expression is correlated with prognosis and progress of hepatocellular carcinoma. BioMed Research International, 2016. 2016((Ding L; Zhou F) Department of Clinical Hematology, Zhongnan Hospital, Wuhan University, Wuhan, China: ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sauter M, et al. , Human endogenous retrovirus K10: expression of Gag protein and detection of antibodies in patients with seminomas. Journal of virology, 1995. 69(1): p. 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sauter M, et al. , Specificity of antibodies directed against Env protein of human endogenous retroviruses in patients with germ cell tumors. Cancer research, 1996. 56(19): p. 4362–4365. [PubMed] [Google Scholar]
- 71.Herbst H, Sauter M, and Mueller-Lantzsch N, Expression of human endogenous retrovirus K elements in germ cell and trophoblastic tumors. The American journal of pathology, 1996. 149(5): p. 1727–35. [PMC free article] [PubMed] [Google Scholar]
- 72.Roelofs H, et al. , Detection of human endogenous retrovirus type K-specific transcripts in testicular parenchyma and testicular germ cell tumors of adolescents and adults: clinical and biological implications. The American journal of pathology, 1998. 153(4): p. 1277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boller K, et al. , Characterization of the antibody response specific for the human endogenous retrovirus HTDV/HERV-K. Journal of virology, 1997. 71(6): p. 4581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleiman A, et al. , HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in patients with germ cell tumors. International journal of cancer.Journal international du cancer, 2004. 110(3): p. 459–461. [DOI] [PubMed] [Google Scholar]
- 75.Rakoff-Nahoum S, et al. , Detection of T lymphocytes specific for human endogenous retrovirus K (HERV-K) in patients with seminoma. AIDS Research and Human Retroviruses, 2006. 22(1): p. 52–56. [DOI] [PubMed] [Google Scholar]
- 76.Benešová M, et al. , DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas. Retrovirology, 2017. 14(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Florl AR, et al. , DNA methylation and expression of LINE-1 and HERV-K provirus sequences in urothelial and renal cell carcinomas. British journal of cancer, 1999. 80(9): p. 1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haupt S, et al. , Human endogenous retrovirus transcription profiles of the kidney and kidney-derived cell lines. The Journal of general virology, 2011. 92(Pt 10): p. 2356–2366. [DOI] [PubMed] [Google Scholar]
- 79.Yu H, et al. , Mutations in 3’-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene, 2014. 33(30): p. 3947–3958. [DOI] [PubMed] [Google Scholar]
- 80.Gosenca D, et al. , HERV-E-mediated modulation of PLA2G4A transcription in urothelial carcinoma. PloS one, 2012. 7(11): p. e49341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi Y, et al. , Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. The Journal of clinical investigation, 2008. 118(3): p. 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cherkasova E, et al. , Inactivation of the von Hippel-Lindau tumor suppressor leads to selective expression of a human endogenous retrovirus in kidney cancer. Oncogene, 2011. 30(47): p. 4697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cherkasova E, et al. , Identification of a human endogenous retrovirus type-e (HERV-E) envelope with selective expression in clear cell kidney cancer that is immunogenic in vitro. Blood, 2012. 120(21). [Google Scholar]
- 84.Cherkasova E, et al. , Detection of an immunogenic HERV-E envelope with selective expression in clear cell kidney cancer. Cancer Research, 2016. 76(8): p. 2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang-Johanning F, et al. , Detecting the expression of human endogenous retrovirus E envelope transcripts in human prostate adenocarcinoma. Cancer, 2003. 98(1): p. 187–197. [DOI] [PubMed] [Google Scholar]
- 86.Perot P, et al. , Microarray-based identification of individual HERV loci expression: application to biomarker discovery in prostate cancer. Journal of visualized experiments : JoVE, 2013. (81):e50713. doi (81): p. e50713. [DOI] [PMC free article] [PubMed]
- 87.Rastogi A, et al. , Immunobiomarkers: Novel autoantibody panel comprising oncogenic ERG, CMYC, AMACR and HERV-K Gag for the detection of prostate cancer. Cancer Research, 2016. 76(14). [Google Scholar]
- 88.Goering W, Ribarska T, and Schulz WA, Selective changes of retroelement expression in human prostate cancer. Carcinogenesis, 2011. 32(10): p. 1484–92. [DOI] [PubMed] [Google Scholar]
- 89.Goering W, et al. , Human endogenous retrovirus HERV-K(HML-2) activity in prostate cancer is dominated by a few loci. Prostate, 2015. 75(16): p. 1958–1971. [DOI] [PubMed] [Google Scholar]
- 90.Ishida T, et al. , Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer immunity, 2008. 8: p. 15. [PMC free article] [PubMed] [Google Scholar]
- 91.Reis BS, et al. , Prostate cancer progression correlates with increased humoral immune response to a human endogenous retrovirus GAG protein. Clinical Cancer Research, 2013. 19(22): p. 6112–6125. [DOI] [PubMed] [Google Scholar]
- 92.Wallace TA, et al. , Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis, 2014. 35(9): p. 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rycaj K, et al. , Cytotoxicity of Human Endogenous Retrovirus K-Specific T Cells toward Autologous Ovarian Cancer Cells. Clinical cancer research : an official journal of the American Association for Cancer Research, 2015. 21(2): p. 471–483. [DOI] [PubMed] [Google Scholar]
- 94.Iramaneerat K, et al. , HERV-K hypomethylation in ovarian clear cell carcinoma is associated with a poor prognosis and platinum resistance. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society, 2011. 21(1): p. 51–57. [DOI] [PubMed] [Google Scholar]
- 95.Wang-Johanning F, et al. , Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. International journal of cancer.Journal international du cancer, 2007. 120(1): p. 81–90. [DOI] [PubMed] [Google Scholar]
- 96.Kahyo T, et al. , Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis, 2013. 34(11): p. 2531–2538. [DOI] [PubMed] [Google Scholar]
- 97.Cuffel C, et al. , Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. International journal of cancer, 2011. 128(11): p. 2625–34. [DOI] [PubMed] [Google Scholar]
- 98.Buslei R, et al. , Activation and regulation of endogenous retroviral genes in the human pituitary gland and related endocrine tumours. Neuropathology and applied neurobiology, 2015. 41(2): p. 180–200. [DOI] [PubMed] [Google Scholar]
- 99.Ho XD, et al. , Analysis of the Expression of Repetitive DNA Elements in Osteosarcoma. Frontiers in Genetics, 2017. 8(Journal Article): p. 193–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strissel PL, et al. , Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: Emergence of new molecular targets. Oncotarget, 2012. 3(10): p. 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Depil S, et al. , Expression of a human endogenous retrovirus, HERV-K, in the blood cells of leukemia patients. Leukemia, 2002. 16(2): p. 254–9. [DOI] [PubMed] [Google Scholar]
- 102.Sun Y, et al. , Expression of syncytin in leukemia and lymphoma cells. Leuk Res, 2010. 34(9): p. 1195–202. [DOI] [PubMed] [Google Scholar]
- 103.Bergallo M, et al. , Expression of the pol gene of human endogenous retroviruses HERV-K and -W in leukemia patients. Arch Virol, 2017. 162(12): p. 3639–3644. [DOI] [PubMed] [Google Scholar]
- 104.Januszkiewicz-Lewandowska D, et al. , Env gene expression of human endogenous retrovirus-k and human endogenous retrovirus-w in childhood acute leukemia cells. Acta Haematol, 2013. 129(4): p. 232–7. [DOI] [PubMed] [Google Scholar]
- 105.Fava P, et al. , Human Endogenous Retrovirus Expression in Primary Cutaneous T-Cell Lymphomas. Dermatology, 2016. 232(1): p. 38–43. [DOI] [PubMed] [Google Scholar]
- 106.Bergallo M, et al. , Toll-like receptors are essential for the control of endogenous retrovirus expression in Idiopathic Nephrotic Syndrome. Minerva urologica e nefrologica = The Italian journal of urology and nephrology, 2017. 69(2): p. 201–208. [DOI] [PubMed] [Google Scholar]
- 107.Saito T, et al. , Upregulation of Human Endogenous Retrovirus-K Is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension. Circulation, 2017. 136(20): p. 1920–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee YJ, et al. , The prevalence of human endogenous retroviruses in the plasma of major burn patients. Burns : journal of the International Society for Burn Injuries, 2013. 39(6): p. 1200–1205. [DOI] [PubMed] [Google Scholar]
- 109.Lee KH, et al. , Divergent and dynamic activity of endogenous retroviruses in burn patients and their inflammatory potential. Experimental and molecular pathology, 2014. 96(2): p. 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho K, Lee YK, and Greenhalgh DG, Endogenous retroviruses in systemic response to stress signals. Shock (Augusta, Ga.), 2008. 30(2): p. 105–116. [DOI] [PubMed] [Google Scholar]
- 111.Vargas A, et al. , Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reproductive sciences (Thousand Oaks, Calif.), 2011. 18(11): p. 1085–1091. [DOI] [PubMed] [Google Scholar]
- 112.Oppelt P, et al. , Expression of the human endogenous retroviruse-W envelope gene syncytin in endometriosis lesions. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 2009. 25(11): p. 741–747. [DOI] [PubMed] [Google Scholar]
- 113.Ruebner M, et al. , Regulation of the human endogenous retroviral Syncytin-1 and cell-cell fusion by the nuclear hormone receptors PPARgamma/RXRalpha in placentogenesis. Journal of cellular biochemistry, 2012. 113(7): p. 2383–2396. [DOI] [PubMed] [Google Scholar]
- 114.Bolze PA, et al. , Expression patterns of ERVWE1/Syncytin-1 and other placentally expressed human endogenous retroviruses along the malignant transformation process of hydatidiform moles. Placenta, 2016. 39((Mallet F, francois.mallet@biomerieux.com) EA Pathophysiology of Injury-induced Immunosuppression, University of Lyon1-Hospices Civils de Lyon-bioMérieux, Hôpital Edouard Herriot, Lyon Cedex 3, France: ): p. 116–124. [DOI] [PubMed] [Google Scholar]
- 115.Sin HS, et al. , A novel Y chromosome microdeletion with the loss of an endogenous retrovirus related, testis specific transcript in AZFb region. The Journal of urology, 2011. 186(4): p. 1545–1552. [DOI] [PubMed] [Google Scholar]
- 116.Vargas A, et al. , Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 2014. 28(8): p. 3703–3719. [DOI] [PubMed] [Google Scholar]
- 117.Lewis C, et al. , Building a ‘Repository of Science’: The importance of integrating biobanks within molecular pathology programmes. Eur J Cancer, 2016. 67: p. 191–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.