A 47-year-old man presented to a Michigan emergency department in January with a 1-day history of presyncope, swelling of the eyelids, and a diffuse pruritic rash. He also reported a 3-week history of postprandial watery bowel movements, without blood. Beginning 5 days before presentation, he had noted diffuse crampy abdominal pain and nausea with occasional nonbloody emesis.
The patient has progressive, multisystem symptoms. Presyncope may be caused by intravascular volume depletion, peripheral vasodilation, or both. The patient’s gastrointestinal losses may have caused volume depletion. Infectious causes of diarrhea such as giardia may be associated with urticaria. An autoimmune condition such as celiac disease may lead to abdominal symptoms and the diffuse pruritic rash of dermatitis herpetiformis. Swelling of the eyelids may be related to a protein-losing enteropathy, but there is no mention of peripheral edema. A potential unifying diagnosis would be mast-cell activation and histamine release. This condition may be caused by systemic or cutaneous mastocytosis, related to allergic disease or chronic inflammatory and neoplastic disorders, or it may be idiopathic.
The patient had a history of type 2 diabetes mellitus, nephrolithiasis, supraventricular tachycardia, hypertriglyceridemia, and attention deficit–hyperactivity disorder. His medications included metformin, saxagliptin, metoprolol, and atorvastatin, as well as amphetamine–dextroamphetamine on an as-needed basis. He had recently begun taking naproxen and calcium carbonate, both as needed for abdominal symptoms. He reported having an allergy to penicillin with unknown reaction. The patient had a 26-pack-year history of tobacco smoking but had quit 1 year earlier. He said that he did not use alcohol and illicit substances. He reported no recent travel outside Michigan, no new foods, and no sick contacts. There was no known family history of urticaria, allergies, or asthma.
On physical examination, the patient’s temperature was 36.8°C, the heart rate 134 beats per minute, the respiratory rate 16 breaths per minute, the blood pressure 95/52 mm Hg, and the oxygen saturation 99% while he was breathing ambient air. There was mild lip edema; the tongue appeared normal. Auscultation of the heart revealed regular tachycardia without extraneous sounds. Lung sounds were clear in both lungs with normal work of breathing. The abdomen was soft and diffusely tender to palpation; there was no rebound tenderness or guarding. There were no palpable lymph nodes and no hepatomegaly or splenomegaly. Skin examination revealed confluent urticaria on the scalp, neck, shoulders, abdomen, and inguinal regions. The remainder of the examination was normal.
The hypotension, tachycardia, confluent urticaria, and lip edema are consistent with anaphylaxis. Common causes of anaphylaxis include foods and food additives, medications, and insect bites or stings, often because of IgE-mediated reactions. Although food allergies are more common in children, they may also develop in adults, and a thorough review of dietary intake just before the patient became ill is crucial. He had recently started taking a nonsteroidal antiinflammatory drug (NSAID). Although this new medication can lead to an IgE-mediated process, the use of nonselective NSAIDs can also lead to a clinical syndrome similar to anaphylaxis that is not IgE-mediated. NSAIDs inhibit cyclooxygenase-1, and in some persons, this inhibition can increase proinflammatory mediators, thus triggering mast-cell activation and histamine release. The patient should be asked about recent insect bites or stings. Other considerations include bradykinin-mediated processes, such as hereditary angioedema, acquired C1-inhibitor deficiency, or drug-induced angioedema, but these would be unlikely to explain this patient’s urticaria and hypotension.
The white-cell count was 26,500 per cubic millimeter, with 91.0% neutrophils, 5.5% lymphocytes, 3.0% monocytes, 0.2% eosinophils, and 0.3% basophils. The hematocrit was 44.5%, and the platelet count was 541,000 per cubic millimeter. The serum sodium level was 130 mmol per liter, the chloride level 90 mmol per liter, the carbon dioxide level 25 mmol per liter, the blood urea nitrogen level 23 mg per deciliter (8.2 mmol per liter), the creatinine level 1.8 mg per deciliter (160 μmol per liter; baseline value [3 months before presentation], 0.7 mg per deciliter [62 μmol per liter]), the glucose level 264 mg per deciliter (14.7 mmol per liter), and the total bilirubin level 1.8 mg per deciliter (31 μmol per liter). The international normalized ratio was 1.4, and the partial-thromboplastin time was 27.0 seconds. The lactic acid level was 3.3 mmol per liter (normal range, 0.5 to 1.6). Urinalysis revealed 1+ glucose and 2+ blood; microscopy revealed 11 to 20 hyaline casts per low-power field and occasional calcium oxalate crystals. An electrocardiogram showed sinus tachycardia with inverted T waves in leads III and aVF. A radiograph of the chest was unremarkable.
The patient’s blood pressure rapidly decreased to 75/40 mm Hg. Four liters of intravenous crystalloid fluids were administered, which led to an increase in the blood pressure to 114/65 mm Hg, but there was persistent tachycardia with a heart rate of 100 to 110 beats per minute.
Signs of shock, including worsening hypotension, end-organ dysfunction, and an elevated lactate level, are developing in this patient. His constellation of symptoms could be explained by histamine release, either from an IgE-mediated or non–IgE-mediated process. Treatment with intramuscular epinephrine should be administered for possible anaphylaxis. Sepsis should also be considered, although it is unlikely to explain the entire clinical presentation.
Blood and urine cultures were obtained. The serum troponin I level was 0.17 ng per milliliter (normal range, 0 to 0.04), and the procalcitonin level was 0.16 ng per milliliter (normal range, 0.02 to 0.07). Computed tomography of the abdomen and pelvis with the use of oral contrast material revealed diverticulosis and a 0.2-cm nonobstructing calculus in the left kidney.
The patient’s heart rate again increased to 130 beats per minute with a blood pressure of 95/52 mm Hg. He received an additional 2 liters of intravenous fluids, oral vancomycin, and intravenous cefepime, metronidazole, famotidine, and methylprednisolone. His heart rate decreased to approximately 110 beats per minute, and his blood pressure increased to approximately 120/80 mm Hg.
Treatments for septic shock and anaphylaxis have been initiated. Epinephrine is the first-line therapy for anaphylaxis and should be administered as soon as possible. Epinephrine stimulates beta-adrenergic receptors, leading to increased levels of intracellular cyclic adenosine monophosphate and decreased release of histamine and other mediators. Its adrenergic activity also causes vasoconstriction and increased cardiac output, thereby mitigating the cardiovascular effects of anaphylaxis. Methylprednisolone and histamine H2-receptor blockers are considered to be second-line treatments for anaphylaxis, because they have a slower onset of action and are unable to rapidly stabilize further degranulation of mast cells.
Serum tryptase and histamine levels may help in diagnosis. Both should be obtained quickly to maximize their usefulness, given the short half-lives of serum tryptase and histamine. An acutely elevated tryptase level that returns to normal when reassessed 24 hours after the resolution of symptoms would be consistent with anaphylaxis. A persistently elevated tryptase level should prompt evaluation, including bone marrow biopsy, for mastocytosis. An elevated histamine level and normal tryptase level should prompt consideration of a histamine-producing source.
The serum C4 level was 70 mg per deciliter (normal range, 15 to 56). The serum IgA level was 140 mg per deciliter (normal range, 77 to 442), the IgG level 440 mg per deciliter (normal range, 703 to 1666), and the IgM level less than 25 mg per deciliter (normal range, 38 to 249); the IgE level was pending. A serum tryptase level was obtained. An additional 10 liters of intravenous fluids were infused, which resulted in improved blood pressure, kidney function, and lactate level. One dose of intramuscular epinephrine was administered. The patient was admitted to the intensive care unit.
Postprandial diarrhea progressing to abdominal pain and nausea suggests that the initial stages of illness were localized to mast cells within the gastrointestinal tract. The elevated C4 level rules out an acute complement-consumptive process and suggests that the abdominal pain is not due to hereditary angioedema. The progression and increasing severity of symptoms before admission may be due to allergic sensitization with repeated antigen exposures and an IgE-mediated response.
A triggering event leading to new-onset allergy must be considered. Approximately 10% of patients have no specific allergic cause identified. Any food can cause IgE-mediated allergy, but fish (and other seafood) and peanuts (as well as tree nuts) are two groups that cause the majority of food allergies in adults and must be carefully explored. Systemic mastocytosis is a rare condition that may present as new-onset allergy and may be triggered by medications, stress, exercise, or emotional events. This condition is less likely because of the patient’s near-daily symptoms (mastocytosis causes intermittent systemic symptoms) and the absence of classic urticaria pigmentosa, hepatosplenomegaly, anemia, and lymphadenopathy related to mast-cell infiltration. The lone star tick, which is endemic in the East (especially the Southeast) United States, with expansion into the upper midwestern and northeastern United States and eastern Canada, has been associated with the development of allergy to “alpha-gal” (galactose-alpha-1,3-galactose), a carbohydrate moiety that is present in all nonprimate mammals. After a bite from this tick, humans may have new allergic responses to red meat.
On hospital day 2, the patient’s abdominal pain and rash abated considerably. His white-cell count was 14,400 per cubic millimeter. Blood and urine cultures were negative. Antimicrobial agents were discontinued.
The patient had no further symptoms until hospital day 4, at which time he reported chest tightness, dyspnea, and worsening pruritic rash. Examination revealed tachycardia, hypotension, hypoxemia, wheezing, and worsened urticaria. Another dose of epinephrine was administered, which resulted in the almost-immediate abatement of symptoms. Laboratory studies that had been obtained on admission showed a serum IgE level of 299 IU per milliliter (718 μg per liter; normal value, <214 IU per milliliter [514 μg per liter]) and a tryptase level of 33.5 μg per liter (normal value, <10.9). An allergy consultation was requested.
The elevated tryptase level suggests mast-cell involvement and, in the context of acute symptoms, supports the hypothesis of an IgE-mediated trigger. Recurrent symptoms probably represent repeated antigen exposure. A thorough history of food intake, medication intake, activities, and exposures before this event and leading up to admission is critical. Symptoms and signs develop rapidly with anaphylaxis, typically within 5 to 30 minutes after exposure, whereas there is often a delay of several hours in patients with alpha-gal allergy.
The patient was given medical therapy for anaphylaxis, with resolution of symptoms and signs. On further questioning, he reported having consumed beef spare ribs approximately 4 hours before his worsening symptoms began on hospital day 4. He noted that he had been hunting deer in rural southeast Michigan and had ingested venison 2 days before presentation. He had consumed venison in the past without difficulty, and his typical diet included red meat. He did not recall a history of tick bites.
The occurrence of allergic reactions after the ingestion of venison and beef, with symptoms during the most recent episode having begun 4 hours after ingestion, is highly suggestive of alpha-gal allergy. The patient is a deer hunter, and white-tailed deer serve as hosts for the lone star tick. Tick saliva may contain alpha-gal from other mammals on which the tick has fed, and this exposure appears to trigger the development of alpha-gal–directed IgE antibodies in humans. The time line of symptom onset suggests that the patient had received a tick bite that predated his most recent hunting endeavor.
The patient received a diagnosis of alpha-gal syndrome and was discharged with a tapering dose of glucocorticoid as well as an intramuscular epinephrine autoinjector and diphenhydramine to be used as needed, instructions to avoid eating red meat, and a follow-up appointment for formal testing in the allergy clinic. After being lost to follow-up for 15 months, he presented to the allergy clinic, at which point laboratory testing revealed a serum tryptase level within the normal range and positivity for alpha-gal IgE. He had avoided eating red meat and had had no further episodes of anaphylaxis.
Commentary
Our patient presented with several weeks of nonspecific gastrointestinal symptoms punctuated by acute cardiovascular, mucosal, and dermatologic findings, which were consistent with anaphylaxis. Worsening symptoms after the ingestion of red meat, a personal history of hunting and of consuming venison before symptoms, and delayed onset of anaphylaxis after meat exposure all supported the diagnosis of alpha-gal syndrome.
Alpha-gal syndrome first came to broad attention in the mid 2000s1 when a number of patients who had been enrolled in clinical trials of cetuximab (an agent now approved for use in patients with metastatic colorectal cancer or squamous-cell carcinoma of the head and neck) had anaphylaxis or urticaria during the first infusion.2,3 Although severe hypersensitivity reactions have since been noted to occur in 3% of patients receiving cetuximab overall, 22% of the patients who received it in late-phase trials in Tennessee and North Carolina had severe hypersensitivity reactions.3 The regional predominance of the reactions prompted the consideration of an environmental factor. Cetuximab includes at least seven distinct alpha-gal epitopes.4 IgE antibodies against alpha-gal were found to be common in the southeastern United States, where the tickborne disease Rocky Mountain spotted fever is well recognized; reports of allergies to red meat were also increasingly reported in that area.1,6 Alpha-gal is found in the tissues of all mammals except primates. This led to the hypothesis that bites from the Amblyomma americanum (lone star) species of tick deposited stomach contents (from previous mammalian meals) into human skin, leading to the development of IgE against alpha-gal.5 Once sensitization has occurred, ingestion of red meat triggers hypersensitivity reactions (Fig. 1).
Figure 1. Sensitization and Mechanism Leading to Clinical Appearance of Alpha-gal Syndrome.
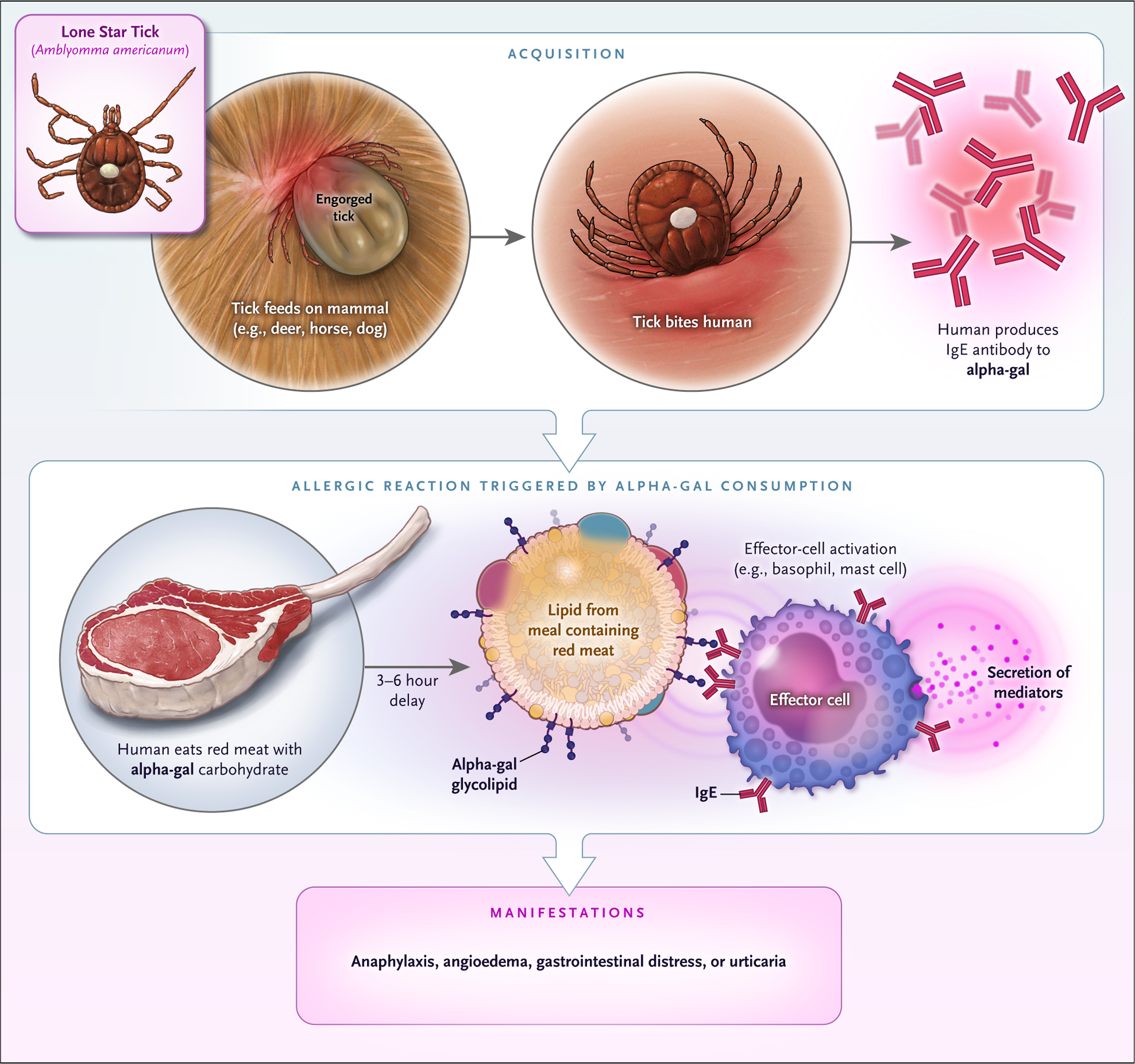
The current hypothesis is that a person is bitten by a lone star tick that has had a previous blood meal containing galactose-alpha-1,3-galactose (alpha-gal). Alternatively, some evidence suggests that alpha-gal can be passed through tick life stages or may be present endogenously in tick saliva. After a period of time, IgE to alpha-gal develops. Once IgE to alpha-gal populates effector cells, the ingestion of red meat can trigger reactions. Alpha-gal that is contained in glycolipids is suspected to be the triggering antigen, and the slow gut absorption of lipid accounts for the delay in reaction. Some patients have only one symptom such as hives or gastrointestinal distress, but most report multiple symptoms.
Associations among tick bites, IgE against alpha-gal, and hypersensitivity reactions to meat have been documented in Asia, Australia, Europe, and the United States.6,7 In these geographic regions, various ticks have been associated with alpha-gal syndrome; however, other species of ticks, including Ixodes scapularis, have not been implicated, which suggests that there may be specific tick factors that lead to sensitization. Our patient lived and hunted in southeastern Michigan. Previously rare in this region, the lone star tick is now the third most common tick in Michigan, and its numbers have been increasing.8 Greater populations of lone star ticks in new regions may be explained by changing patterns of land use, an abundance of mammalian hosts such as white-tailed deer, and climate change, which is causing conditions that are increasingly hospitable for lone star ticks and their hosts.9
Alpha-gal syndrome is an increasingly recognized public health issue. Serum testing of specific IgE against alpha-gal is the confirmatory test of choice, whereas intradermal testing is generally not recommended. Between 2010 and 2018, more than 34,000 persons tested positive for alpha-gal IgE in the United States.10 The most common foods precipitating allergy are mammalian meats, especially beef. Reactions to pork, lamb, rabbit, chicken, turkey, and kangaroo have also been described.6 Although most patients with alpha-gal syndrome can safely consume dairy products, some have reactions; the removal of cow’s milk from the diet is recommended on an individualized basis.11 Alpha-gal syndrome may result in myriad symptoms after the ingestion of meat. In a small study, the most common manifestations were pruritus or urticaria (in 70% of patients), gastrointestinal symptoms (in 40%), angioedema, contact urticaria of the oral mucosa, and anaphylaxis.12 However, symptoms and signs may be more insidious, and cases that involve predominantly gastrointestinal symptoms, presyncope, or syncope may be particularly challenging to recognize.11,13
An unusual feature of the IgE-mediated hypersensitivity reaction to alpha-gal is its timing. Symptoms are delayed in onset, often occurring 3 to 6 hours after ingestion.6 The delay, which can complicate diagnosis, may be related to the fact that the carbohydrate allergen is bound to lipid. Lipids are absorbed into the gut and circulation more slowly than protein allergens, which are associated with rapid-onset reactions.14 Many patients have awoken from sleep with allergic or anaphylactic symptoms after consuming red meat for dinner. In our patient, unequivocal manifestations of anaphylaxis recurred 4 hours after the ingestion of red meat, which supported the diagnosis of alpha-gal syndrome.
Anaphylaxis is a multisystem syndrome arising from the sudden release of mast-cell mediators into the systemic circulation.15 It usually results from IgE-mediated reactions to foods, medications, or insect bites or stings. Anaphylaxis is diagnosed clinically,15 although rapid assays of serum tryptase or histamine levels may assist in ruling out other disorders. Management includes intramuscular epinephrine, fluid resuscitation, supplemental oxygen and bronchodilator therapy, avoidance of the offending allergen, and development of a home emergency action plan.
This patient’s initial nonspecific gastrointestinal symptoms progressed to systemic features of anaphylaxis. The abrupt worsening of symptoms after presumed resolution prompted the consideration of allergen reexposure, and it became clear that he had had a delayed hypersensitivity reaction to red meat. The patient did not recall having received any tick bites, and the medical team did not find evidence of bites or attached ticks on examination. Yet, he had two episodes of anaphylaxis in our health care system, both after he had ingested red meat, and he did not have any additional anaphylaxis episodes after eliminating meat from his diet. He had probably received unrecognized tick bites, perhaps from small larval (“seed”) ticks, in the weeks before the symptoms of alpha-gal syndrome manifested, since he had previously been able to eat venison and other meats without reaction.
A careful review of exposures including foods and drugs as well as timing of symptom onset are key pieces of information to gather in patients with anaphylaxis of unclear cause. Such history is the key weapon when hunting for a diagnosis.
Footnotes
Conflict
Dr. Commins reports receiving fees for serving on a speakers’ bureau from Genentech; and Dr. Saint, receiving honoraria from ISMIE Mutual Insurance Company and advisory board fees from Doximity and Jvion. No other potential conflict of interest relevant to this article was reported.
Financial disclosure
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Steinke JW, Platts-Mills TAE, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol 2015;135:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008;358:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 2007;25:3644–8. [DOI] [PubMed] [Google Scholar]
- 4.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem 2007;364:8–18. [DOI] [PubMed] [Google Scholar]
- 5.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α−1,3-galactose. J Allergy Clin Immunol 2011;127:1286–93.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2009;123:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Nunen SA. Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Med J Aust 2018;208:316–21. [DOI] [PubMed] [Google Scholar]
- 8.Ticks and your health: preventing tick-borne illness in Michigan. Michigan Department of Health and Human Services, Michigan Department of Natural Resources, Michigan State University; (https://www.michigan.gov/documents/emergingdiseases/resize_307382_7.pdf). [Google Scholar]
- 9.Molaei G, Little EAH, Williams SC, Stafford KC. Bracing for the worst — range expansion of the lone star tick in the northeastern United States. N Engl J Med 2019;381:2189–92. [DOI] [PubMed] [Google Scholar]
- 10.Binder AM, Commins S, Altrich ML, et al. Diagnostic testing for galactose-alpha-1,3-galactose (alpha-gal), United States, 2010–2018. Ann Allergy Asthma Immunol 2021. January 07 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol 2020;16:667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi G-S, Kim J-H, Shin Y-S, Nahm D-H, Park H-S. Food allergy to meat and milk in adults. Allergy 2010;65:1065–7. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JM, Schuyler AJ, Workman L, et al. Investigation into the α-gal syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract 2019;7(7):2348–2358.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Román-Carrasco P, Lieder B, Somoza V, et al. Only α-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy 2019;74:1956–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report — Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117:391–7. [DOI] [PubMed] [Google Scholar]


