This study found that weight loss improves response rates in women with obesity and atypical hyperplasia or low-risk endometrial cancer undergoing conservative management with intrauterine progestin. Given the additional benefits of weight loss for fertility, cardiovascular health andquality of life, future research should focus on how best to accomplish it.
Abstract
Intrauterine progestin is a treatment option for women with atypical hyperplasia or low-risk endometrial cancer who wish to preserve their fertility, or whose poor surgical fitness precludes safe hysterectomy. We hypothesized that in such women with obesity, weight loss during progestin treatment may improve oncological outcomes. We conducted a prospective nonrandomized study of women with obesity and atypical hyperplasia or low-grade stage 1a endometrial cancer undergoing progestin treatment. Women with a body mass index (BMI) ≥ 35 kg/m2 were offered bariatric surgery; those who declined and those with a BMI of 30 to 34.9 kg/m2 were encouraged to lose weight by low-calorie diet. We assessed uptake of bariatric surgery; weight lost during progestin treatment; and the impact of more than 10% total body weight loss on progestin treatment response at 12 months. 71 women [median age 58 years (interquartile range; IQR 35–65); mean BMI 48 kg/m2 (SD 9.3)] completed the study. Twenty-three women (32%) had bariatric surgery, on average 5 months (IQR 3–8) after progestin treatment commenced. Weight change during progestin treatment was −33.4 kg [95% confidence interval (CI) −42.1, −24.7] and −4.6 kg (95% CI −7.8, −1.4) in women receiving bariatric surgery and low-calorie diet, respectively (P < 0.001). Forty-three women (61%) responded to progestin, while 23 (32%) showed stabilized and 5 (7%) progressive disease. Response at 12 months was not predicted by age or baseline BMI, but women who lost more than 10% of their total body weight were more likely to respond to progestin than those who did not (adjusted odds ratio 3.95; 95% CI 1.3, 12.5; P = 0.02). Thus weight loss may improve oncological outcomes in women with obesity-associated endometrial neoplastic abnormalities treated with progestin.
Prevention Relevance:
This study found that weight loss improves response rates in women with obesity and atypical hyperplasia or low-risk endometrial cancer undergoing conservative management with intrauterine progestin. Given the additional benefits of weight loss for fertility, cardiovascular health and quality of life, future research should focus on how best to accomplish it.
Introduction
Obesity is the major risk factor for type I endometrial cancer and its precursor lesion, atypical hyperplasia (1). It has been estimated that every 5 kg/m2 increase in body mass index (BMI) confers a 60% higher lifetime risk of endometrial cancer (2), such that women with a BMI > 40 kg/m2 are seven times more likely to develop endometrial cancer than normal-weight women (3). With escalating obesity rates driving an epidemic of endometrial cancer in high-income countries (4), effective nonsurgical treatments are urgently needed for women who wish to preserve their fertility as well as those whose obesity and associated medical comorbidities make hysterectomy a hazardous procedure (5). The mechanisms underpinning obesity-driven endometrial carcinogenesis are incompletely understood, but include alterations to the hormonal, metabolic, and immunologic microenvironment of the endometrium that predispose it to inappropriate growth and persistence (6). High-dose oral or intrauterine progestin is the recommended treatment for women with endometrial neoplastic abnormalities wishing to avoid hysterectomy (7). It halts growth, reverses neoplastic change, and restores endometrial health in some women with low-grade early-stage endometrial cancer and atypical hyperplasia (8); however treatment is slow, response is unpredictable, and recurrence is common (9).
Bariatric surgery–induced weight loss is known to offer sustained risk reduction from obesity-driven endometrial cancer from both retrospective and prospective studies (10, 11). At the tissue level, bariatric surgery is rapidly followed by downregulation of proproliferative signaling pathways, reduced endometrial growth, and spontaneous clearance of both latent and precursor endometrial neoplastic lesions (12). Taken together, these findings provide a strong biological rationale for bariatric surgery–induced weight loss as a means of improving response rates in women with endometrial neoplastic abnormalities undergoing progestin treatment, however this strategy remains untested. There may be additional benefits to weight loss in this setting, including improved natural fecundity and successful assisted reproduction should progestin therapy be effective; reduced surgical morbidity and lower rates of conversion to open hysterectomy if not; reduced risk of recurrent disease following cessation of treatment; as well as established benefits for metabolic and cardiovascular health in women known to be at significant risk of cardiovascular events (13).
The aim of this study was to determine the effectiveness of weight-loss interventions offered to women undergoing progestin treatment for obesity-associated atypical hyperplasia and low-grade early-stage endometrial cancer. We hypothesized that bariatric surgery would be an acceptable, feasible, and effective weight-loss intervention when offered during the progestin treatment window. We further hypothesized that women who lost more than 10% of their total body weight by bariatric surgery or low-calorie diet would be more likely to respond to progestin than those who did not.
Materials and Methods
Study approvals
The MIrena for the Reduction of Endometrial Neoplastic Abnormalities (MIRENA) study is a prospective observational study conducted at St Mary's Hospital, Manchester University NHS Foundation Trust (MFT), a large gynecologic cancer center in the North-West of England, with the aim of identifying predictive biomarkers of progestin treatment response in women with atypical hyperplasia and low-grade early-stage endometrial cancer. All women gave written, informed consent to participate in the study. It was sponsored by MFT and approved by the North West Research Ethics Committee (14/NW/0056). The study was prospectively registered on the UK (ISRCTN 31662931) clinical trial database and is conducted in accordance with Good Clinical Practice guidelines.
Participant selection
Women who wished to undergo fertility-sparing treatment or who were considered at high surgical or anesthetic risk for standard surgical management of their endometrial neoplastic abnormality were invited to participate in the MIRENA study. Eligible women were aged 18 years or older with biopsy-proven atypical hyperplasia or grade 1 or 2 endometrioid adenocarcinoma with less than 50% myometrial invasion on MRI [Federation Internationale des Gynaecologistes et Obstetristes (FIGO) 2009 stage 1a endometrial cancer]. Women were excluded from the study if central pathology review showed high-grade or nonendometrioid histology; central radiology review showed ≥50% myometrial invasion, extra-uterine disease, or adnexal masses suspicious for malignancy; or if, after informed discussion with specialists in the cancer fertility clinic and/or high-risk anesthetic clinic, women chose to undergo standard surgical management or primary radiotherapy instead.
Study procedures
At baseline, women were screened for eligibility. Height was measured using a stadiometer; weight using electronic scales after removal of bulky clothing, and BMI determined (kg/m2). Baseline blood tests included fasting glucose, cholesterol, glycosylated hemoglobin A1c (HbA1c), and C-reactive protein (CRP). All analytes were measured using standard automated clinical service protocols in the MFT Clinical Biochemistry Laboratory. Clinically abnormal results were managed as per hospital policy.
Formalin fixed, paraffin embedded (FFPE) blocks of the index biopsy were obtained from the referring hospital for pathology review by at least two specialist gynecologic pathologists, according to the World Health Organization (WHO) classification system (14, 15). Baseline MRI scans were performed for all participants unless contraindicated, and the digitalized images were reviewed by specialist gynecologic radiologists at the Gynecological Oncology Multidisciplinary Team meeting. Where an MRI scan was contraindicated, a CT scan confirmed no extrauterine disease. All women requesting fertility-sparing management were seen in St Mary's specialist cancer fertility clinic to discuss pregnancy expectations, challenges, and opportunities. Those women who were deemed unsuitable for hysterectomy on account of their poor surgical fitness were reviewed in St Mary's high-risk anesthetic clinic for preoperative assessment, investigations, and optimization.
All women underwent baseline endometrial biopsy to confirm diagnosis prior to commencing progestin treatment. This reduced the potential for misclassification of disease status secondary to sampling issues and challenges of pathologic interpretation. Biopsies were reviewed by at least two specialist gynecologic pathologists (14, 15). Where possible, biopsies were taken in the outpatient department using a Pipelle© (Carefusion, UK) sampling device immediately prior to insertion of the levonorgestrel-releasing intrauterine system (LNG-IUS, 52 mg intrauterine progestin, Mirena). The Pipelle© is rotated through 360 degrees whilst sampling the full length of the endometrial cavity to obtain a representative sample. Where outpatient sampling was not possible or poorly tolerated, sampling was performed under general anesthetic. Women were advised to have their IUS threads checked at 1 month to ensure correct intrauterine placement. Women experiencing pelvic pain, heavy bleeding, or lost threads in the weeks immediately following LNG-IUS insertion were assessed by transvaginal ultrasound scan. A misplaced or lost IUS was replaced once. In the event of further IUS displacement, women were instead treated with 200-mg medroxyprogesterone acetate twice daily.
Women were seen in gynecology clinic at 3 months, 6 months, 9 months, and 12 months following placement of the intrauterine progestin device. At every visit, an endometrial sample was taken to exclude progressive disease. An MRI scan was performed at 3 and/or 6 months to check for interval change in women with endometrial cancer at baseline, and in all women at 12 months, to assess disease response, wherever possible. Women with progressive disease were withdrawn from the study at the earliest indication and underwent hysterectomy or radiotherapy. The remaining participants had their intrauterine progestin device removed at 12 months for a 6-week ‘wash out’ period. A further endometrial sample was taken 6 weeks later and the intrauterine progestin device replaced. This was done in the outpatient clinic for women with atypical hyperplasia at baseline and a reassuring 9- and 12-month biopsy. For all other women, hysteroscopy, dilatation and curettage, and replacement of the intrauterine progestin device was carried out under general or spinal anesthetic, where possible.
Progestin treatment response
Progestin treatment response was defined as complete if both end-of-treatment biopsies (at 12 months and following the 6-week ‘wash out’ period) showed no hyperplasia or neoplasia and the 12-month MRI scan ± hysteroscopic appearance of the uterine cavity was normal (no endometrial mass lesions, endometrium thin and regular, and no myometrial invasion). Partial response was defined as substantial improvement in endometrial morphology (e.g., posttreatment low-volume abnormality with minimal/mild cytological atypia) on posttreatment biopsies and a normal hysteroscopy/MRI scan. Stable disease was defined as posttreatment biopsies and scan showing similar appearances to those at baseline. Progressive disease was defined as worsening endometrial morphology (e.g., from atypical hyperplasia to definite grade 1 endometrioid adenocarcinoma, or from grade 1 to grade 2 disease) or scan appearances (e.g., larger endometrial lesion, new or deeper myometrial invasion). Responders showed complete or partial responses; nonresponders had stable or progressive disease.
Weight loss interventions
Women with a BMI ≥35 kg/m2 were offered referral to the Specialized and Complex Obesity Service at Salford Royal Foundation NHS Trust for consideration of expedited bariatric surgery (laparoscopic gastric bypass or sleeve gastrectomy). Those who declined, those in whom bariatric surgery was contraindicated, and those with a BMI of 30 to 34.9 kg/m2 were encouraged to lose weight through a low-calorie diet of their choosing (for example, by joining a local slimming club or community weight management program). Women were advised that losing weight not only has proven benefits for their long-term health, mobility, quality of life, and fertility, as appropriate, but may also improve their progestin treatment response. Women were encouraged to take control of their own weight by setting themselves ambitious but achievable weight-loss targets (initially 1 kg/week). The 12-month target was ≥10% total body weight lost and/or an end of treatment BMI of less than 30 kg/m2 (16–18). Women were weighed at every clinic visit, weight loss goals revisited, and new targets set. Women referred for bariatric surgery underwent laparoscopic gastric bypass or sleeve gastrectomy depending on clinical factors and personal preference. Bariatric surgery was expedited to enable rapid-onset weight loss during the intrauterine progestin window, where possible.
End-of-study management and follow-up procedures
Women with histologic and/or radiological evidence of progressive disease were offered total laparoscopic hysterectomy ± bilateral salpingo-oophorectomy, depending on their age and diagnosis. Women with stable disease were offered hysterectomy wherever possible. Young women who had shown complete responses to intrauterine progestin were offered assisted reproduction if there was a history of primary infertility, or encouraged to keep their intrauterine progestin device in situ until they were ready to conceive. All other women were offered hysterectomy at the end of the study, if feasible. Women who did not undergo hysterectomy were encouraged to keep their intrauterine progestin device in situ indefinitely with 3 to 6–monthly then annual symptom appraisal, biopsies, and/or transvaginal ultrasound, to 5 years.
Statistical analyses
This is an interim, posthoc analysis of women who were recruited to the MIRENA study between June 2014 and July 2019. The main outcomes were (i) uptake of bariatric surgery; (ii) weight lost during progestin treatment; and (iii) the impact of more than 10% total body weight loss on progestin treatment response at 12 months. All participants were included in the primary analysis of progestin response and uptake of bariatric surgery. Weight loss at 12 months and the impact of weight loss on progestin treatment response excluded those who progressed. Continuous data were normally distributed according to the Shapiro–Wilk test, with P > 0.05 indicating normality. Descriptive statistics are reported as means with ranges for parametric data, medians with interquartile ranges (IQR) for nonparametric data, and counts alongside percentages for categorical data. Differences between two continuous groups were tested using independent samples t test, paired t test, or Mann–Whitney U test as appropriate. Differences between 2 categorical groups were assessed using χ2 test. To test the strength of association between variables and progestin treatment response at 12 months, univariable and multivariable logistic regression analyses were performed. Univariable and multivariable analyses are reported as OR with 95% confidence intervals (95% CI). In the multivariable analysis we adjusted for factors that were significantly associated with progestin treatment response (histology and % change in total body weight) in the univariable analysis, as well as clinical factors known to be associated with clinical outcomes in endometrial cancer (age and type 2 diabetes mellitus status). A p value of ≤0.05 was considered as statistically significant. All data analyses were performed using STATA (StataCorp. 2015. Stata Statistical Software: Release 14, StataCorp LLC).
Results
In total, 119 women were screened for the MIRENA study (Fig. 1). Of these, 48 (40%) women were withdrawn because they had a BMI <30 kg/m2 (14), were upstaged (12), down-staged (3), declined intrauterine progestin (4), could not comply with study procedures (1), did not complete treatment (9), or had not completed 12-month follow up at study censor (5), leaving 71 women eligible for analysis. Their median age, mean weight, and mean BMI was 58 years (IQR 35–65), 126 kg (SD 23.8), and 48 kg/m2 (SD 9.3), respectively (Table 1). All women received a LNG-IUS at baseline, 65 (92%) of these were successfully inserted in the outpatient clinic. One woman was treated with oral medroxyprogesterone acetate after her LNG-IUS fell out twice. Bleeding, pain, and LNG-IUS expulsion affected 25 (35%), 5 (7%), and 1 (1%) women respectively; most bleeding settled by 3 (11; 44%) or 6 months (21; 84%) postinsertion, consistent with previous studies (19).
Figure 1.
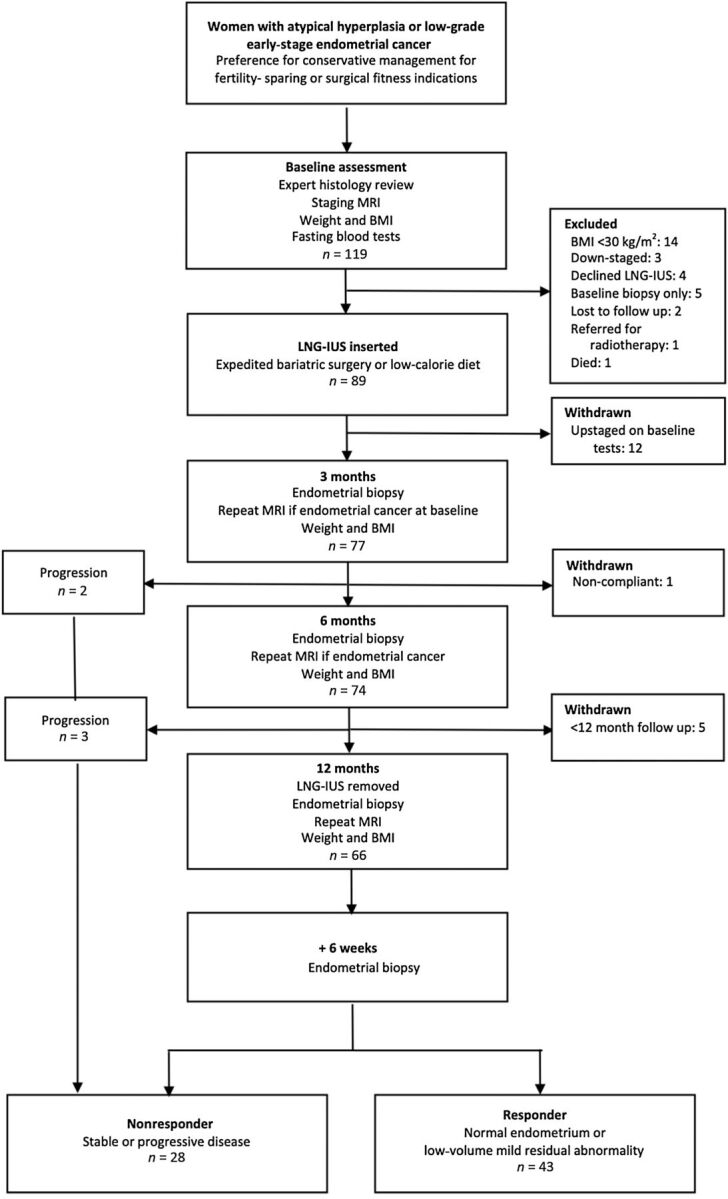
Study flow chart.
Table 1.
Baseline characteristics of study participants.
| Fertility-sparing (n = 23) | Poor surgical fitness (n = 48) | All participants (n = 71) | |
|---|---|---|---|
| Demographics | |||
| Median age (IQR), years | 31 (28–35) | 62 (58–68) | 58 (35–65) |
| White British, n (%) | 18 (78) | 47 (98) | 65 (92) |
| Asian, n (%) | 5 (22) | 0 (0) | 5 (7) |
| Black, n (%) | 0 (0) | 1 (2) | 1 (1) |
| Mean weight (SD), kg | 119 (24.11) | 129 (23.13) | 126 (23.81) |
| Mean BMI (SD), kg/m2 | 43 (7.30) | 50 (9.29) | 48 (9.34) |
| Menopausal status, n (%) | |||
| Premenopausal | 23 (100) | 4 (8) | 27 (38) |
| Postmenopausal | 0 (0) | 44 (92) | 44 (62) |
| Median parity (IQR) | 0 (0–0) | 2 (1–3) | 1 (0–2) |
| Medical status | |||
| PCOS, n (%) | 16 (70) | 0 (0) | 16 (23) |
| T2DM, n (%) | 3 (13) | 19 (40) | 22 (31) |
| Mean HbA1C (range), mmol/mol | 38 (25–61) | 45 (29–102) | 43 (25–102) |
| Mean Fasting glucose (range), mmol/L | 5.8 (4.0–9.9) | 6.6 (4.3–15.5) | 6.3 (4.0–15.5) |
| Hypertension, n (%) | 1 (4) | 39 (81) | 40 (56) |
| Hypercholesterolemia, n (%) | 0 (0) | 20 (42) | 20 (28) |
| Smoker, n (%) | 3 (13) | 5 (10) | 8 (11) |
| Cardiovascular disease, n (%) | 0 (0) | 12 (25) | 12 (17) |
| Mean CRP (range), mg/L | 8.7 (1–20) | 12.0 (1–52) | 10.9 (1–52) |
| Pathologic findings | |||
| Atypical hyperplasia, n (%) | 8 (35) | 23 (48) | 31 (44) |
| Endometrioid adenocarcinoma, n (%) | 15 (65) | 25 (52) | 40 (56) |
| Grade 1, n (%) | 15 (100) | 24 (96) | 39 (98) |
| Grade 2, n (%) | 0 (0) | 1 (4) | 1 (2) |
| Radiologic findings | |||
| Median endometrial thickness (IQR), mm | 12 (9–15) | 8 (5–12) | 10 (6–14) |
| Endometrial mass lesion, n (%) | 3 (13) | 5 (10) | 8 (11) |
| Myometrial invasion, n (%) | 1 (4) | 5 (10) | 6 (8) |
| Benign adnexal mass, n (%) | 3 (13) | 4 (8) | 7 (10) |
Abbreviations: PCOS, Polycystic ovary syndrome; T2DM, Type 2 diabetes mellitus.
In total, 64 of 71 women had a BMI > 35 kg/m2 and were offered bariatric surgery, of whom 23 (36%) underwent gastric bypass (12/23; 52%) or sleeve gastrectomy (11/23; 48%) on average 5 months (IQR 3–8) after progestin treatment commenced. One woman was referred but was declined bariatric surgery due to poor anesthetic fitness. One woman had an intraoperative cancellation due to suspicious liver lesions and was rescheduled when her biopsy identified sarcoidosis, not metastatic cancer. There were no intraoperative complications, conversion to open surgery, or returns to theatre. Five women received hysterectomy or primary radiotherapy prior to 12 months due to progression of their endometrial abnormality. For the remaining 66 women, weight change during the 12-month progestin treatment window was −33.4 kg (95% CI −42.1, −24.7) and −4.6 kg (95% CI −7.8, −1.4) following bariatric surgery and low-calorie diet, respectively (P < 0.001). Nineteen of 22 (86%) women who received bariatric surgery lost more than 10% of their total body weight by 12 months, while this was true for only 10 of 44 (23%) women who tried to lose weight by low-calorie diet (P < 0.001; Table 2). The 10 women who lost more than 10% of their total body weight by low-calorie diet lost an average 18.8 kg (95% CI −23.9, −13.7 kg weight change) whilst the remaining 34 lost an average 0.4 kg (95% CI −2.9, 2.0 kg weight change) during the 12-month progestin treatment window. Women with a baseline BMI of 30 to 35 kg/m2 lost on average 1.9 kg (95% CI −5.7, 1.9 kg weight change) by 12 months. No particular nonsurgical weight-loss intervention was more effective than another at achieving weight loss, however, initial weight loss (within the first 3 months) predicted long-term success (at 12 months). Women who lost more than 10% of their total body weight by low-calorie diet at 12 months regained some weight by 24 months (n = 8, mean +3.9 kg, 95% CI 9.8, −2.1), while those who received bariatric surgery showed ongoing weight loss at 24 months (n = 8, mean −7.6 kg, 95% CI −13.3, −2.0).
Table 2.
Weight change during 12-month progestin treatment window.
| Bariatric surgery | Low-calorie diet | All participants | ||
|---|---|---|---|---|
| (n = 22) | (n = 44) | (n = 66)a | ||
| Mean weight (SD), kg | Baseline | 137.7 (19.1) | 118.8 (23.4) | 125.1 (23.7) |
| 12 months | 104.3 (17.1) | 114.2 (21.9) | 110.9 (20.8) | |
| Mean difference (95% CI), 12 months vs. baseline | −33.4 (−42.1 to −24.7) | −4.6 (−7.8 to −1.4) | −14.2 (−19.0 to −9.4) | |
| P < 0.001 | P = 0.005 | P < 0.001 | ||
| Mean BMI (SD), kg/m2 | Baseline | 51.9 (7.5) | 45.1 (9.0) | 47.3 (9.0) |
| 12 months | 39.1 (5.5) | 43.4 (8.5) | 41.9 (7.8) | |
| Mean difference (95% CI), 12 months vs. baseline | −12.8 (−16.2 to −9.5) | −1.7 (−2.9 to −0.5) | −5.4 (−8.3 to −2.5) | |
| P < 0.001 | P = 0.006 | P < 0.001 | ||
| Mean change in total body weight (SD), % 12 months vs. baseline | −23.6 (13.0) | −3.4 (8.0) | −10.2 (13.7) | |
| Mean change in total body weight at 12 months, bariatric surgery vs. low-calorie diet | P < 0.001 | |||
| Mean change in BMI (SD), kg/m2 12 months vs. baseline | −12.8 (7.5) | −1.7 (4.0) | −5.4 (7.5) | |
| Mean change in BMI at 12 months, bariatric surgery vs. low-calorie diet | P < 0.001 | |||
| Weight loss >10% total body weight, n (%) | 19 (86) | 10 (23) | 29 (44) | |
| Proportion of women losing >10% total body weight at 12 months, bariatric surgery vs. low-calorie diet | P < 0.001 | |||
a12-month assessments are not included for the n = 5 women who progressed during progestin treatment, whose mean weight loss at progression was −6.4 kg (95% CI −18.2, −1.0); 1 had lost >10% of their total body weight.
Of the 71 participants, 43 (61%) showed progestin treatment responses, 23 (32%) had stable disease, and 5 (7%) progressed during progestin treatment. Progression was at 3 (n = 2) or 6 months (n = 3) and was treated by hysterectomy (n = 4) or primary radiotherapy (n = 1). Progestin treatment response was not predicted by age or baseline BMI, but women with hyperplasia were more likely to respond than those with cancer (adjusted OR 3.52; 95% CI 1.1, 11.0; P = 0.03). After excluding the 5 women who progressed, women who lost more than 10% of their total body weight were more likely to respond to progestin than those who did not (adjusted OR 3.95; 95% CI 1.3, 12.5; P = 0.02; Tables 3 and 4).
Table 3.
Clinical correlates of progestin treatment response.
| Clinical feature | Responder (n = 43) | Nonresponder (n = 28) | Responder vs. nonresponder (P) |
|---|---|---|---|
| Demographic | |||
| Median age (IQR), years | 56 (32–64) | 59 (45–68) | 0.12 |
| Premenopausal, n (%) | 19 (44) | 8 (29) | 0.19 |
| Mean baseline weight (SD), kg | 127.8 (21.3) | 122.9 (27.4) | 0.40 |
| Mean baseline BMI (SD), kg/m2 | 48 (8) | 48 (11) | 0.98 |
| Medical status | |||
| PCOS, n (%) | 11 (26) | 5 (18) | 0.45 |
| T2DM, n (%) | 12 (28) | 10 (36) | 0.49 |
| Endometrial abnormality | |||
| Endometrial cancer, n (%) | 20 (47) | 20 (71)c | 0.04 b |
| Myometrial invasion, n (%) | 3 (7) | 3 (11) | 0.75 |
| Benign adnexal mass, n (%) | 4 (9) | 3 (11) | 0.95 |
| Weight change at 12 months | |||
| Bariatric surgery, n (%) | 16 (37) | 7 (25) | 0.28 |
| Mean weight change (95% CI), kga | −17.0 (−22.7 to −11.3) | −9.0 (−18.1 to 0.1) | 0.11 |
| Mean BMI change (95% CI), kg/m2a | −6.4 (−8.6 to −4.3) | −3.5 (−7.0 to 0.5) | 0.13 |
| Mean change total body weight (SD), %a | −12.3 (12.9) | −6.2 (14.7) | 0.09 |
| Weight loss >10% total body weight, n (%)a | 23 (53) | 6 (26) | 0.03 b |
aExcludes the n = 5 women who progressed during progestin treatment.
bSignificant at P < 0.05.
cIncludes the n = 1 participant who was treated with medroxyprogesterone acetate instead of the LNG-IUS.
Table 4.
Clinical predictors of progestin treatment response in total cohort (n = 71).
| OR (95% CI) | P | |
|---|---|---|
| Univariable analysis | ||
| Age (years) | 0.98 (0.9–1.0) | 0.11 |
| Baseline BMI (kg/m2) | 1.00 (0.9–1.1) | 0.98 |
| Postmenopausal | ||
| No | 1 | |
| Yes | 0.51 (0.2–1.4) | 0.19 |
| PCOS | ||
| No | 1 | |
| Yes | 1.58 (0.5–5.2) | 0.45 |
| T2DM | ||
| No | 1 | |
| Yes | 0.70 (0.3–2.0) | 0.49 |
| Histology | ||
| Endometrial cancer | 1 | |
| Atypical hyperplasia | 2.88 (1.0–8.0) | 0.04 b |
| Endometrial mass lesion | ||
| No | 1 | |
| Yes | 0.68 (0.2–3.1) | 0.61 |
| Myometrial invasion | ||
| No | 1 | |
| Yes | 0.76 (1.4–4.2) | 0.75 |
| Bariatric surgery | ||
| No | 1 | |
| Yes | 1.78 (0.6–5.2) | 0.29 |
| % change in total body weighta | ||
| <10% | 1 | |
| ≥10% | 3.26 (1.1–9.9) | 0.04 b |
| Multivariable analysis adjusted for age, T2DM, histology, % change in total body weight | ||
| Histology | ||
| Endometrial cancer | 1 | |
| Atypical hyperplasia | 3.52 (1.1–11.0) | 0.03 b |
| % change in total body weighta | ||
| <10% | 1 | |
| >10% | 3.95 (1.3–12.5) | 0.02 b |
aExcludes the n = 5 women who progressed during progestin treatment.
bSignificant at P < 0.05.
The median follow up was 40 months (IQR 24, 57), during which time 22 (31%) had a hysterectomy either for reassurance (5, 23%), stable/progressive disease (16, 73%), or recurrence (1, 4%). A further 4 (6%) recurred after initially responding to progestin but did not have a hysterectomy; all had a BMI > 30 kg/m2 and atypical hyperplasia at recurrence and received intrauterine progestin treatment. Six of 23 (26%) women attempted pregnancy, 2 by assisted reproduction, with 3 pregnancies in 2 women and 1 live birth (1/6, 17%). Six women (8%) died during follow up; one was an endometrial cancer–specific death (at 50 months), the other 5 deaths (at 12, 29, 31, 52, and 54 months) were unrelated to endometrial cancer or its treatment; none had received bariatric surgery.
Discussion
In this pragmatic, nonrandomized study, we assessed the potential for weight loss to improve progestin treatment response in women undergoing conservative management of obesity-associated atypical hyperplasia and low-grade early-stage endometrial cancer. Bariatric surgery was acceptable to a third of eligible women, received on average 5 months after progestin treatment commenced and induced an average total body weight loss of 23.6% during progestin treatment. The remaining two thirds of women were encouraged to lose weight by low-calorie diet; one quarter lost an average 13.7% of their total body weight by 12 months, while the other three quarters remained roughly weight stable. There were responses to intrauterine progestin in 61% women. Neither age nor baseline BMI predicted response, but women who lost more than 10% of their total body weight were nearly 4 times more likely to respond to intrauterine progestin than those who did not (adjusted OR 3.95; 95% CI 1.3, 12.5; P = 0.02).
There is a growing need for effective nonsurgical treatments for atypical hyperplasia and endometrial cancer given its rising incidence, particularly amongst younger women. Previous studies report high success rates from oral or intrauterine progestin (20–22), however most are retrospective case series and therefore prone to selection and reporting bias. Most experience is with oral progestin but its systemic side effects, including thromboembolic disease and weight gain (23, 24), compromise its suitability for an obesity-driven cancer. The LNG-IUS delivers progestin directly to the endometrium at a steep concentration gradient and constant rate that keeps serum levels low and avoids the peaks and troughs of oral administration (25), with high success rates (24, 26–28). A prospective study of intrauterine progestin reported treatment responses in 91% of 37 patients with atypical hyperplasia and 67% of 20 patients with endometrial cancer at 12 months (8). The higher proportion of cancers in our study, inclusion of stage 1a disease with myometrial invasion, and stringent criteria for treatment response may explain our lower response rates.
Identifying subgroups of women who might respond to intrauterine progestin is important to guide the safe management of atypical hyperplasia and low-grade early-stage endometrial cancer (9). It is not known whether clinical factors or tumor phenotype predominate as predictors of progestin response. Baseline BMI has not shown a consistent relationship with outcomes (20, 23, 29, 30) but posttreatment, obesity appears to increase the risk of recurrence (20, 23). We hypothesized that successful weight loss, defined by WHO as more than 10% total body weight (16), would improve oncological (31, 32), fertility (29), and general health outcomes in this population (17, 18, 33). Such ambitious weight-loss targets are achievable through gastric bypass or sleeve gastrectomy (34), however 68% of our participants declined or were unsuitable for bariatric surgery. We therefore took the pragmatic approach that a low-calorie diet of their own choosing was a suitable alternative, albeit expecting low success rates amongst women who were unable to engage with the lifestyle, dietary, and behavioral changes required. We were able to motivate a quarter of those who declined bariatric surgery to lose more than 10% of their total body weight simply by stating the need, setting targets, and monitoring progress. This so called ‘teachable moment’ (35) may be particularly powerful amongst those facing sterilizing hysterectomy and childlessness, and offers an opportunity to explore potential causes of obesity and prospects for its reversal.
To our knowledge, only one previous study has assessed the uptake, feasibility, and success of weight-loss interventions offered to women during the progestin treatment window (36). The feMMe trial reported an overall progestin response rate of 61%, and successful weight loss (defined as >7% total body weight at 6 months) in 25% of women randomized to the low-calorie diet arm, but the study was underpowered to assess the oncological benefit of weight loss for this indication.
We have previously shown that weight loss is associated with spontaneous clearance of latent precursors and occult atypical hyperplasia in women undergoing bariatric surgery (12). Weight loss reduces systemic hormonal, metabolic, and inflammatory stimulators of endometrial proliferation and may act synergistically with intrauterine progestin to induce neoplastic cell death (13). The natural history of obesity-driven endometrial cancer is incompletely understood but its generally indolent behavior provides the opportunity for curative treatment (37). Indeed, progression of atypical hyperplasia to cancer is neither inevitable nor rapid (38). We and others have shown that most low-grade, early-stage cancers can be conservatively managed with progestin but intensive monitoring is crucial to identify the subset of women with biologically aggressive disease who need radical treatment. Temporary remission may be sufficient to enable pregnancy, however the avoidance of hysterectomy requires long-term control. In this study, 5 women (7%) progressed during progestin treatment; 4 underwent laparoscopic hysterectomy or primary radiotherapy and remain alive and well. One woman progressed at 6 months and underwent a hysterectomy and vaginal brachytherapy for Stage 1b grade 2 endometrial cancer, developed widespread recurrence at 43 months, and died at 50 months. A further 5 women died during follow up from their medical comorbidities, reinforcing the need for evidence-based nonsurgical treatment options for women with endometrial cancer and obesity (39).
Strengths of our work include its prospective design, standardized treatment protocol, and long duration of follow up. We report outcomes on a relatively large number of participants given the single-center nature of our study. Our heterogeneous cohort of fertility-sparing and surgically unfit participants reflects the complexity and diversity of the real world experience of managing this condition. Limitations include the small study size, absence of data relating to the molecular subgroup of included endometrial tumors, and overall poor compliance with low-calorie diet interventions. The lack of racial and ethnic diversity in our study population precludes any insight into the feasibility and effectiveness of weight loss during progestin treatment in non-White British women. Our participants had very strong views about bariatric surgery, making a randomized trial neither feasible nor practicable. Nevertheless, we have shown that successful weight loss can be achieved within the confines of the progestin treatment window, which may have multiple benefits for future health and well-being (40, 41). Given the considerable advantages of weight loss in this context, future research should focus on how best to accomplish it.
Authors’ Disclosures
No disclosures were reported.
Role of Funder/Sponsor
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We would like to thank the women who participated in this study. We would also like to thank Joe Woods, Leo Sutcliffe, Tina Pritchard, Linsey Nelson, and Suzanne Carter for their help with patient recruitment, data acquisition, data management, and obtaining regulatory approvals. Special thanks to Arya Pontula and Zoe Maskell, who helped with database entry. We are grateful for the invaluable assistance of Gill Hesketh who sadly passed away before this study was completed. C.E. Barr, A.E. Derbyshire, and M.L. MacKintosh were supported by Manchester University NHS Foundation Trust Clinical Research Fellowships. N.A.J. Ryan was supported by a Medical Research Council Doctoral Training Fellowship (MR/M018431/1). E.J. Crosbie was supported through a National Institute for Health Research (NIHR) Clinician Scientist (NIHR-CS-012-009) and this article presents independent research funded by the NIHR, supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007) and facilitated by the Greater Manchester Local Clinical Research Network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Authors' Contributions
C.E. Barr: Data curation, formal analysis, writing–review and editing. N.A.J. Ryan: Data curation, writing–review and editing. A.E. Derbyshire: Data curation, writing–review and editing. Y.L. Wan: Data curation, writing–review and editing. M.L. MacKintosh: Data curation, writing–review and editing. R.J. McVey: Data curation, formal analysis, writing–review and editing. J. Bolton: Data curation, formal analysis, writing–review and editing. C. Fitzgerald: Data curation, writing–review and editing. D. Awad: Data curation, writing–review and editing. R.J. Slade: Data curation, writing–review and editing. A.A. Syed: Data curation, writing–review and editing. B.J. Ammori: Data curation, writing–review and editing. E.J. Crosbie: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet 2016;387:1094–108. [DOI] [PubMed] [Google Scholar]
- 2. Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body Mass Index, hormone replacement therapy and endometrial cancer risk: a meta analysis. Cancer Epidemiol Biomark Prev 2010;19:3119–30. [DOI] [PubMed] [Google Scholar]
- 3. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31:2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crosbie E, Morrison J. The emerging epidemic of endometrial cancer: Time to take action. Cochrane Database Syst Rev 2014;(12):ED000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obermair A, Baxter E, Brennan DJ, McAlpine JN, Muellerer JJ, Amant F, et al. Fertility-sparing treatment in early endometrial cancer: current state and future strategies. Obstet Gynecol Sci 2020;63:417–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2014;4:579–91. [DOI] [PubMed] [Google Scholar]
- 7. Sundar S, Balega J, Crosbie E, Drake A, Edmondson R, Fotopoulou C, et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur J Obstet Gynecol Reprod Biol 2017;213:71–97. [DOI] [PubMed] [Google Scholar]
- 8. Westin SN, Fellman B, Sun CC, Broaddus RR, Woodall ML, Pal N, et al. Prospective phase II trial of levonorgestrel intrauterine device: nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am J Obstet Gynecol 2021;224:191.e1–191.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derbyshire AE, Ryan N, Crosbie EJ. Biomarkers needed to predict progestin response in endometrial cancer. BJOG 2017;124:1584. [DOI] [PubMed] [Google Scholar]
- 10. Ward KK, Roncancio AM, Shah NR, Davis M-A, Saenz CC, McHale MT, et al. Bariatric surgery decreases the risk of uterine malignancy. Gynecol Oncol 2014;133:63–6. [DOI] [PubMed] [Google Scholar]
- 11. Anveden Å, Taube M, Peltonen M, Jacobson P, Andersson-Assarsson JC, Sjöholm K, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol 2017;145:224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacKintosh ML, Derbyshire AE, McVey RJ, Bolton J, Nickkho-Amiry M, Higgins CL, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer 2019;144:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackintosh ML, Crosbie EJ. Obesity-driven endometrial cancer: is weight loss the answer? BJOG 2013;120:791–4. [DOI] [PubMed] [Google Scholar]
- 14. Emons G, Beckmann MW, Schmidt D, Mallmann P. Uterus commission of the gynecological oncology working group (AGO). New WHO classification of endometrial hyperplasias. Geburtshilfe Frauenheilkd 2015;75:135–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurman RJ, Carcangiu M, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: IARC; 2014. [Google Scholar]
- 16. World Health Organization (WHO). Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Geneva (Switzerland): World Health Organization; 2000. Available from https://apps.who.int/iris/handle/10665/63854. [PubMed] [Google Scholar]
- 17. Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: Differences at 5%, 10%, 15%, and over. Curr Obes Rep 2017;6:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive clinical practice guidelines for medical care of patients with obesity—executive summary. Endocr Pract 2016;22:842–84. Available from: https://pro.aace.com/publications/guidelines. [DOI] [PubMed] [Google Scholar]
- 19. Bofill Rodriguez M, Lethaby A, Jordan V. Progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev 2020;6:CD002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li M, Guo T, Cui R, Feng Y, Bai H, Zhang Z. Weight control is vital for patients with early-stage endometrial cancer or complex atypical hyperplasia who have received progestin therapy to spare fertility: a systematic review and meta-analysis. Cancer Manag Res 2019;11:4005–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012;125:477–82. [DOI] [PubMed] [Google Scholar]
- 22. Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol 2004;95:133–8. [DOI] [PubMed] [Google Scholar]
- 23. Park JY, Seong SJ, Kim TJ, Kim JW, Bae DS, Nam JH. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol Oncol 2017;146:39–43. [DOI] [PubMed] [Google Scholar]
- 24. Cholakian D, Hacker K, Fader AN, Gehrig PA, Tanner EJ 3rd. Effect of oral versus intrauterine progestins on weight in women undergoing fertility preserving therapy for complex atypical hyperplasia or endometrial cancer. Gynecol Oncol 2016;140:234–8. [DOI] [PubMed] [Google Scholar]
- 25. Edelman AB, Cherala G, Munar MY, Dubois B, McInnis M, Stanczyk FZ, et al. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an altered pharmacokinetic profile in obese oral contraceptives users. Contraception 2013;87:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim MK, Seong SJ, Kim J-W, Jeon S, Choi HS, Lee I-H, et al. Management of endometrial hyperplasia with a levonorgestrel-releasing intrauterine system: a korean gynecologic-oncology group study. Int J Gynecol Cancer 2016;26:711. [DOI] [PubMed] [Google Scholar]
- 27. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol 2015;213:469–78. [DOI] [PubMed] [Google Scholar]
- 28. Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev 2020;9:Cd012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonthier C, Walker F, Luton D, Yazbeck C, Madelenat P, Koskas M. Impact of obesity on the results of fertility-sparing management for atypical hyperplasia and grade 1 endometrial cancer. Gynecol Oncol 2014;133:33–7. [DOI] [PubMed] [Google Scholar]
- 30. Ciccone MA, Whitman SA, Conturie CL, Brown N, Dancz CE, Özel B, et al. Effectiveness of progestin-based therapy for morbidly obese women with complex atypical hyperplasia. Arch Gynecol Obstet 2019;299:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barr CE, Crosbie EJ. The Mirena coil is a suitable treatment of early-stage endometrial cancer in obese women: FOR: Careful selection and monitoring is key. BJOG 2020;127:1001. [DOI] [PubMed] [Google Scholar]
- 32. Barr CE, Crosbie EJ. Authors' reply re: Mirena Coil is a suitable treatment of early-stage endometrial cancer in obese women: FOR or AGAINST?: Treat the patient, not the disease. BJOG 2020;127:1300–1. [DOI] [PubMed] [Google Scholar]
- 33. Kitson S, Crosbie EJ. Endometrial cancer and obesity. The Obstetrician and Gynaecologist 2019;21:237–45. [Google Scholar]
- 34. Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, YWS Jr, Weidenbacher HJ, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg 2016;151:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark LH, Ko EM, Kernodle A, Harris A, Moore DT, Gehrig PA, et al. Endometrial cancer survivors' perceptions of provider obesity counseling and attempted behavior change: are we seizing the moment? Int J Gynecol Cancer 2016;26:318–24. [DOI] [PubMed] [Google Scholar]
- 36. Janda M, Robledo KP, Gebski V, Armes JE, Alizart M, Cummings M, et al. Complete pathological response following levonorgestrel intrauterine device in clinically stage 1 endometrial adenocarcinoma: Results of a randomized clinical trial. Gynecol Oncol 2021;161:143–51. [DOI] [PubMed] [Google Scholar]
- 37. Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med 2020;383:2053–64. [DOI] [PubMed] [Google Scholar]
- 38. Lacey JV Jr, Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J Clin Oncol 2010;28:788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wan YL, Beverley-Stevenson R, Carlisle D, Clarke S, Edmondson RJ, Glover S, et al. Working together to shape the endometrial cancer research agenda: The top ten unanswered research questions. Gynecol Oncol 2016;143:287–93. [DOI] [PubMed] [Google Scholar]
- 40. Kitson SJ, Lindsay J, Sivalingam VN, Lunt M, Ryan NAJ, Edmondson RJ, et al. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: A prospective case control study. Gynecol Oncol 2018;148:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kitson S, Ryan N, MacKintosh ML, Edmondson R, Duffy JM, Crosbie EJ. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database Syst Rev 2018;2:CD012513. [DOI] [PMC free article] [PubMed] [Google Scholar]


