Abstract
A hallmark of cancer is cell death evasion, underlying suboptimal responses to chemotherapy, targeted agents, and immunotherapies. The approval of the antiapoptotic BCL2 antagonist venetoclax has finally validated the potential of targeting apoptotic pathways in patients with cancer. Nevertheless, pharmacologic modulators of cell death have shown markedly varied responses in preclinical and clinical studies. Here, we review emerging concepts in the use of this class of therapies. Building on these observations, we propose that treatment-induced changes in apoptotic dependency, rather than pretreatment dependencies, will need to be recognized and targeted to realize the precise deployment of these new pharmacologic agents.
Significance:
Targeting antiapoptotic family members has proven efficacious and tolerable in some cancers, but responses are infrequent, particularly for patients with solid tumors. Biomarkers to aid patient selection have been lacking. Precision functional approaches that overcome adaptive resistance to these compounds could drive durable responses to chemotherapy, targeted therapy, and immunotherapies.
INTRODUCTION
The death of cancer cells is crucial for the durability of chemotherapy, targeted therapies, and immunotherapies (1). Adaptation to these therapies, despite dramatic initial responses, suggests that fully eradicating cancer cells could reduce the emergence of acquired resistance. Thus, understanding how malignant cells die, or more precisely, why they often fail to die in response to therapy, has the potential to improve responses to a wide variety of anticancer therapeutics.
Multiple types of cell death may be involved in cancer, including apoptosis, ferroptosis, necroptosis, pyroptosis, necrosis, and others (1–5). Although the relative frequency of these cell death pathways is still incompletely characterized, most cancers have dysregulated apoptosis. For example, gain-of-function mutations in RAS and RAF, t(14;18) translocations, or loss of common tumor suppressors such as RB or TP53 inhibit this type of programmed cell death to ensure survival (6–8).
Given its importance in cancer pathogenesis, significant efforts have been taken to understand how tumor cells protect themselves from apoptosis and develop therapies to reengage this process. The recent development of specific pharmacologic agents that directly target the apoptotic regulatory machinery has finally created an opportunity to target these pathways. Here, we summarize the preclinical and clinical data that illustrate the contexts in which these treatments will be applicable and propose strategies that overcome barriers to their successful clinical deployment.
BCL2 FAMILY PROTEINS REGULATE APOPTOTIC CELL DEATH IN CANCER CELLS
Apoptosis can be triggered by two different, well-characterized pathways: extrinsic and intrinsic. Extrinsic apoptosis is activated by the binding of death ligands such as Fas/APO-1, TNFα, TNF-related apoptosis-inducing ligand (TRAIL) to their respective death receptors (CD95/FasR, TNFR1, and DR4/DR5). The subsequent conformational changes lead to the formation of the dynamic multiprotein death-inducing signaling complex (DISC; refs. 9, 10). DISC enables dimerization of caspase-8, leading to its activation, which engages downstream executioner caspases and apoptosis. This process is complex and tightly regulated at several levels, as reviewed elsewhere (1).
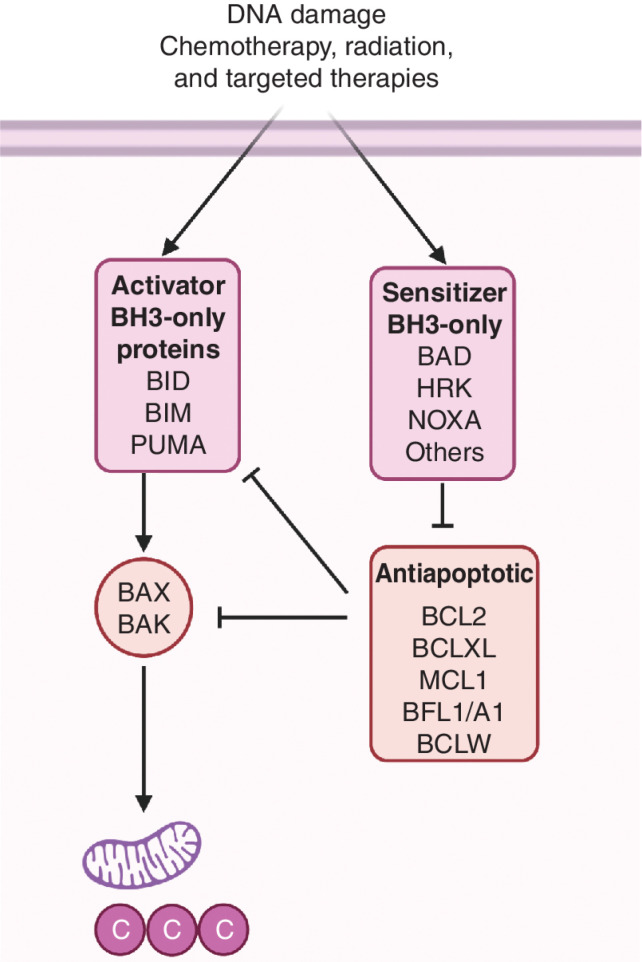
The intrinsic pathway, also known as the mitochondrial pathway, is regulated by the BCL2 family of proteins. This family has different members that can be classified based on structure, function, and BCL2 homology (BH) domains (11, 12): activator members, antiapoptotic members, sensitizers, and effectors (Fig. 1). Although the execution of this pathway requires the effector members BAX and BAK (13, 14), the three other groups determine the threshold required for their activation. First, the so-called BH3-only activator proteins (e.g., BIM, BID, and PUMA), each possessing a unique BH3 domain, directly bind to effector proteins promoting conformational changes that result in effector oligomerization and mitochondrial outer membrane permeabilization (MOMP; refs. 13, 15, 16). Second, antiapoptotic proteins such as BCL2, BCLXL, MCL1, BCLW, and BFL1/A1 can directly bind and sequester both activator and activated effector proteins, preventing apoptosis (17). In fact, antiapoptotic protein neutralization is sufficient to initiate this type of programmed cell death (18). There is a fourth group in the BCL2 family of proteins, the sensitizers, each of which also possesses a unique BH3 domain but cannot directly activate BAX and BAK. Sensitizers include BAD, HRK, BIK, NOXA, BMF, and BIK and exert a proapoptotic effect by competing for specific binding to antiapoptotic BCL2 family members and releasing the activators and effectors (19). For example, BAD has a high affinity for BCL2, BCLXL, and BCLW, but not for MCL1 or BFL1/A1. In contrast, HRK selectively binds to BCLXL, and NOXA specifically binds to MCL1 and BFL1/A1 (20). When activators outnumber and overcome inhibition by antiapoptotic proteins, they induce BAX and BAK oligomerization resulting in MOMP (21), and the release of cytochrome c, SMAC/DIABLO, and other proteins from the mitochondrial intermembrane space into the cytosol. Cytochrome c then binds to APAF1 and caspase-9 in the presence of dATP to trigger the apoptosome formation and activation of effector caspases, finally leading to apoptosis (13, 14). Inhibitor of apoptosis proteins (IAP) suppress this process by inhibiting caspases. SMAC/DIABLO in turn can block IAPs binding of caspases, allowing apoptosis to proceed (22, 23). The BCL2 family of proteins collectively represents an intricate interactome controlled at several levels: dynamic binding of its members, altered transcription and translation, and posttranslational modifications (12).
Figure 1.
The BCL2 interactome. The BCL2 family of proteins is comprised of four distinct subgroups: effectors, activators, antiapoptotics, and sensitizers. Once activated, effectors BAX and BAK induce mitochondrial outer membrane permeabilization (MOMP), leading to apoptosis. In response to therapy or oncogene activation, BH3-only activators (BID, BIM, or PUMA) engage effectors, promoting cell death. Antiapoptotic proteins (BCL2, BCLXL, MCL1, BFL1/A1, and BCLW) sequester activators or effector proteins to prevent apoptosis. BH3-only sensitizers act as selective antagonists of antiapoptotic proteins. For example, BAD has high affinity for BCL2, BCLXL, and BCLW, but not for MCL1 or BFL1. In contrast, HRK selectively binds to BCLXL, and NOXA specifically binds to MCL1. When proapoptotic members outnumber antiapoptotic, the mitochondria are permeabilized by BAX/BAK releasing cytochrome c and SMAC/DIABLO to the cytosol and engaging apoptosis.
Most cancer cells have dysregulated apoptotic signaling (11, 24). Dysregulation of antiapoptotic proteins in cancer cells was initially recognized following the discovery of BCL2 as an oncogene product of the t(14:18) chromosomal translocation found in malignant lymphomas (25–28). Based on their homology to the sequence of BCL2, other proteins were later identified (29). Among them, other antiapoptotic proteins were recognized as survival factors in cancer, notably BCLXL and MCL1 (30, 31). These proteins are upregulated in different types of cancers (32–34). In addition to their higher expression of antiapoptotic BCL2 family members, most cancer cells also have higher levels of proapoptotic proteins (35). The increased binding of antiapoptotic proteins to proapoptotic BCL2 members not only prevents cell death, but also makes cancer cells more vulnerable to apoptosis compared with normal cells. Accordingly, most tumors exhibit a higher state of readiness to die, or “priming,” compared to normal cells (36, 37). Cells that are more “primed” are closer to the apoptosis threshold and more sensitive to anticancer agents. Heightened apoptotic priming of cancer cells underlies the selective elimination by anticancer therapy compared with effects on most adult healthy tissues (with the notable exception of hematopoietic cells), which present low expression of apoptotic proteins and are refractory to apoptosis (38, 39).
Different anticancer therapies induce cell death through diverse effects on apoptotic signaling, including accumulation of proapoptotic BH3-only proteins, mostly activators (16, 36, 40, 41) but also sensitizers (42); decreases in antiapoptotic BCL2 family proteins (33, 43, 44); or both (45). Therefore, many anticancer agents prime cancer cells toward a proapoptotic phenotype through different mechanisms.
USING BH3 MIMETICS TO ENHANCE APOPTOTIC RESPONSES
Given the frequent dysregulation of the antiapoptotic BCL2 family members in cancer, several pharmaceutical companies have developed compounds that imitate BH3-only proteins—the so-called BH3 mimetics. These small molecules bind with high affinity and specificity to the hydrophobic groove of the antiapoptotic proteins, inhibiting them and displacing bound proapoptotic proteins by imitating the action of sensitizer BCL2 proteins (Fig. 2A). The first molecule described to target BCL2 was HA14-1, which showed in vitro and in vivo activity alone or in combination with cytotoxic therapy (46). Obatoclax, which originated from a development program to therapeutically modulate BCL2 family members, followed (47) but had low affinity, had limited clinical activity, and was found to act through a BAX/BAK-independent mechanism (48). Other early attempts to target BCL2 included gossypol and oblimersen sodium (Genasense; Genta, an antisense oligonucleotide), but both were also unsuccessful, in part due to their low specificity or potency (49, 50). Abbott Laboratories (now AbbVie) developed ABT-737, the first on-target specific BH3 mimetic inhibiting BCL2, BCLXL, and BCLW (51, 52). This compound was further refined to improve its oral bioavailability, leading to the analogue ABT-263 (navitoclax; ref. 53). Navitoclax showed promising results in multiple blood cancers, particularly chronic lymphocytic leukemia (CLL), which express high levels of BCL2 (54, 55). However, this agent caused thrombocytopenia due to platelets’ singular dependence on BCLXL (56). Navitoclax continues to be evaluated in multiple clinical trials but has yet to be approved by the FDA. To reduce the risk of thrombocytopenia, AbbVie developed a selective BCL2 inhibitor called ABT-199 (venetoclax; ref. 57). The clinical response of patients with CLL to venetoclax was evidence of impressive anticancer activity, leading to rapid debulking and even instances of tumor lysis syndrome (58).
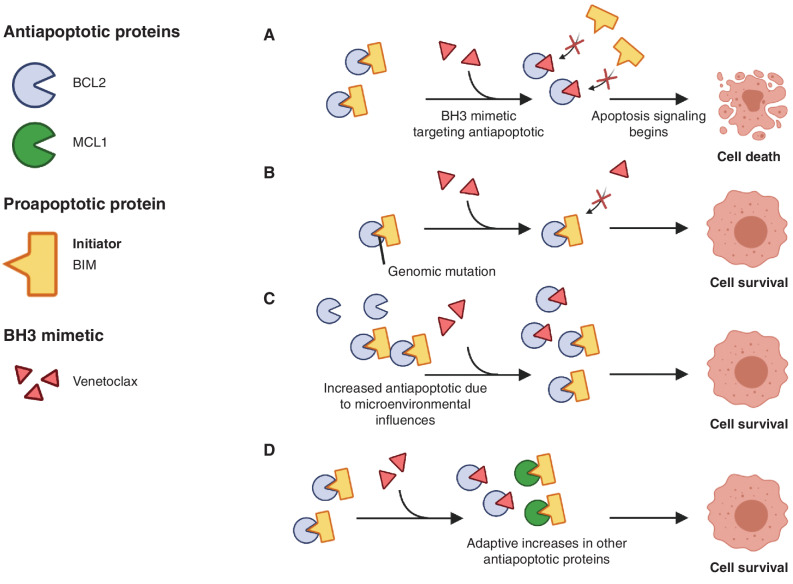
Figure 2.
Mechanisms of resistance to BH3 mimetics. For illustrative purposes, we present BCL2 as a prototypical antiapoptotic family member, BIM as a BH3-only protein, and venetoclax as the BH3 mimetic. A, The BH3 mimetic can disrupt binding of BCL2 to BIM, thereby enhancing BIM-dependent apoptosis. B, Genomic mutation that disrupts binding of the BH3 mimetic to the antiapoptotic family member. C, Microenvironmental influences such as IL10 or CD40 lead to enhanced expression of BCL2, limiting the effects of the BH3 mimetic. D, Cells adapt to conditions by upregulating alternative antiapoptotic family members, thereby reducing dependence on BCL2.
Encouraged by its clinical efficacy, several pharmaceutical companies are currently developing other BH3 mimetics (Table 1). Even though inhibition of BCLXL may lead to the reported on-target thrombocytopenia (56), selective BCLXL inhibitors have also been developed for their potential anticancer activity. One of the first compounds shown to be highly selective was WEHI-539 (59), which was also effective against solid tumors. More recent derivatives of this first BCLXL inhibitor, such as A-1155463 or A-1331852 (60), have also been studied, with promising preclinical results. In particular, the latter holds great promise due to its oral bioavailability (61, 62), yet none of these inhibitors have been assessed in clinical trials. In contrast, a new strategy targeting BCLXL degradation using a proteolysis-targeting chimera (PROTAC; DT2216), which has unique selectivity to target this antiapoptotic protein in tumor cells but not in platelets, is now under evaluation, with clinical potential (63). This strategy, if successful, could overcome one of the major clinical limitations of navitoclax in the treatment of patients with solid tumors.
Table 1.
BCL2 family member antagonists in clinical use or clinical trial development
| Drug | Developer | Type | Target(s) | Development status |
|---|---|---|---|---|
| ABT-199 (venetoclax) | AbbVie | Small molecule | BCL2 | Approved for CLL |
| ABT-263 (navitoclax) | AbbVie | Small molecule | BCL2, BCLXL, BCLW | Phase I/II |
| AMG-176 | Amgen | Small molecule | MCL1 | Phase I |
| APG-1252 | Ascentage Pharma | Small molecule | BCL2, BCLXL | Phase I, II |
| APG-2575 | Ascentage Pharma | Small molecule | BCL2 | Phase I, II |
| AT-101 | Ascenta Therapeutics | Small molecule | Pan-BCL2 | Phase II; terminated |
| AZD-5991 | AstraZeneca | Small molecule | MCL1 | Phase I, II; suspended |
| AZD0466 | AstraZeneca | Small molecule | BCL2, BCLXL | Phase I, II |
| BGB-11417 | BeiGene | Small molecule | BCL2 | Phase I/II |
| NU-0129 | Northwestern University | siRNA | BCL2L12 | Phase I; completed |
| Obatoclax | Teva | Small molecule | Pan-BCL2 | Phase III; terminated |
| Oblimersen | Genta | Antisense oligonucleotide | BCL2 | Phase III; halted |
| PRT1419 | Prelude Therapeutics | Small molecule | MCL1 | Phase I |
| S-055746 | Servier; Vernalis; Novartis | Small molecule | BCL2, MCL1 | Phase I; completed |
| S55746/BCI-201 | Servier; Novartis | Small molecule | BCL2 | Phase I; completed |
| S64315/MIK665 | Novartis | Small molecule | MCL1 | Phase I, II |
| SPC2996 | Santaris Pharma | Locked nucleic acid antisense | BCL2 | Phase I; completed |
NOTE: Data based on ClinicalTrials.gov as of December 2021.
The observation that the MCL1 antiapoptotic protein is commonly used by cancer cells to evade apoptosis (64–69) has also stimulated the development of novel targeted therapies. One of the first selective inhibitors was A-1210477, presenting excellent in vitro results in hematologic malignancies and solid tumors, such as breast and lung cancer cell lines (70), particularly in combination with navitoclax. Encouraged by the anticancer action of these molecules, a new generation of promising small-molecule MCL1 antagonists is now in clinical development. These include S64315/MIK665 (71), AZD-5991 (72), PRT1419 (73), and AMG-176 (74), among others, which are currently being explored in clinical trials (NCT02992483, NCT04629443, NCT03013998, NCT02675452, NCT04178902, NCT04543305, and others), mostly in hematologic malignancies. Other compounds that inhibit or degrade MCL1 have also demonstrated activity in preclinical models (75–78). Given that MCL1 is a short-lived protein (79), indirect targeting by CDK9 inhibitors, such as alvocidib, AZD4573 (NCT03263637), or voruciclib (NCT03547115), or protein selective degradation approaches (75) have emerged as alternative strategies (80). BFL1/A1 can also be similarly targeted using CDK9 inhibitors (80) or dual inhibitors of MCL1 and BFL1 (78).
The potential for toxicities associated with MCL1 inhibition has been raised (81, 82). Toxicities associated with MCL1 inhibition by S63845 could not be fully appreciated in initial studies due to the lower affinity of this drug for murine compared with human MCL1 (81). However, MCL1 inhibition led to only modest toxicity in a humanized murine model (83). Similarly, AMG-176 was relatively well tolerated alone or when combined with venetoclax in a humanized MCL1 knock-in model (74). In some early clinical trials of MCL1 inhibitors, drugs were generally tolerated, with mostly hematologic and gastrointestinal side effects. However, a trial of AMG-397 was suspended because of cardiac toxicity. These toxicities may be related to on-target effects on cardiac myocytes, potentially limiting the drug's therapeutic window.
Other alternative approaches to restoring apoptosis are being explored. Although antiapoptotic BCL2 inhibitors lead to indirect activation of BAX and BAK, direct pharmacologic activation of BAX has recently been described in preclinical models (84–86). This approach can overcome resistance to BH3 mimetics in some cancer cells that exhibit decreased levels of BH3-only proteins. BAX is present in most cancer cells (albeit in an inactive or inhibited state) and is infrequently inactivated in cancers. A pharmacologic activator of BAX exhibited cytotoxicity in AML cells while sparing healthy cells (84). BAX activators synergize with BH3 mimetics in tumors that have decreased levels of BH3-only proteins (87).
BH3 MIMETICS HAVE LIMITED CLINICAL ACTIVITY IN MOST CANCER TYPES
BH3 mimetics have been evaluated in hundreds of clinical trials. Though a description of all these studies is beyond the scope of this review, here we summarize key observations about their use as single agents in the treatment of both hematologic and solid tumors. Except in a few specific disease types, such as hematologic malignancies like CLL or blastic plasmacytoid dendritic cell neoplasm (BPDCN; refs. 58, 88), BH3 mimetics as monotherapy have not produced high response rates.
Navitoclax was the first BH3 mimetic tested in patients with CLL, given the high expression of the BCL2 protein in CLL. Although there was clear activity, the dose-limiting thrombocytopenia associated with BCLXL inhibition led AbbVie to later investigate the BCL2-specific venetoclax in patients with CLL. Venetoclax had impressive single-agent activity, causing tumor lysis syndrome and demanding a dose-escalation strategy and clinical surveillance. It showed outstanding activity even in relapsed patients with CLL, achieving an 80% response rate with tolerable secondary effects (58). It was later approved for the treatment of patients with CLL with 17p chromosomal deletion, becoming the first BH3 mimetic permitted in the clinic for cancer treatment. Currently, venetoclax has been approved for different clinical indications in CLL, including its combination with rituximab for relapsed/refractory (R/R) patients and with obinutuzumab for first-line treatment, and further evaluated in dozens of clinical trials (89). In acute myelogenous leukemia (AML), venetoclax has been approved in combination with other chemotherapeutic agents (azacitidine, decitabine, or low-dose cytarabine), but as monotherapy the overall response rate in R/R patients was only 19% (90, 91). The European Medicines Agency (EMA) recently approved the use of venetoclax in combination treatment with a hypomethylating agent in adult patients with newly diagnosed AML who are ineligible for intensive chemotherapy.
Evidence of a role for BCL2 family dependence in solid tumors from preclinical data has similarly failed to translate into significant responses in the clinic thus far. Among the first approaches to target BCL2 family members in solid tumors was an antisense oligonucleotide specific for BCL2 mRNA (oblimersen; ref. 92). Despite encouraging initial results in human tumors, a phase III trial found that the addition of oblimersen did not improve the overall survival of patients with melanoma treated with chemotherapy. A modest increase in progression-free survival and response rate was observed (93), but the toxicity and lack of survival benefit data led the FDA to reject its application. In lung cancer, venetoclax and navitoclax had cytotoxicity in small-cell lung cancers (SCLC; refs. 94, 95). Venetoclax is now being evaluated in pediatric and young adult patients with relapsed or refractory malignancies, which includes MYCN-amplified neuroblastoma (NCT03236857). Nevertheless, the activity of BH3 mimetics in solid tumors has been inadequately evaluated in the clinic. BCLXL inhibition is therapeutically challenging in patients with solid tumors because of on-target thrombocytopenia. Approaches such as PROTACs and antibody–drug conjugates such as ABBV-155 could circumvent these issues, as discussed above. Data from MCL1 inhibitor trials in solid patients are also eagerly awaited (NCT04837677).
These data collectively suggest that biomarkers to enrich populations more likely to respond to treatment or approaches that combine multiple drugs may be required for maximum efficacy (91, 96).
RESISTANCE MECHANISMS TO BH3 MIMETICS
Features of the tumor cell, such as genomic mutations, or microenvironmental influences have been investigated to determine their contribution to BH3 monotherapy resistance.
Genomic Mechanisms of Resistance to BCL2 Family Inhibitors
Both preclinical and clinical data indicate that somatic mutations in BCL2 family members may disrupt binding to BH3 mimetics, leading to therapeutic resistance (Fig. 2B). In vitro selection with continuous exposure to ABT-199 yielded two missense mutations in the BCL2 BH3 domain (F101C and F101L) that blocked drug binding. A missense mutation in the transmembrane domain of proapoptotic BAX was also observed (97). In an analysis of 15 patients with CLL treated with venetoclax, seven patients were found to have a G101V mutation in BCL2, which increased with the duration of treatment (98, 99). This mutation reduces the binding of venetoclax to BCL2 180-fold (99, 100). Other mutations in BCL2 in venetoclax-resistant patients have also been described, putatively affecting drug affinity (101, 102). In subsequent studies, patients resistant to venetoclax were found that have distinct mutations in different CLL cells, all of which were not observed before treatment (103), suggesting subclonal mechanisms of genomic resistance.
Besides mutations of BCL2 family members themselves, resistance mutations in other pathways have also been described in patients. In a study of six patients with CLL treated with venetoclax, resistance was associated with multiple genomic changes, including SF3B1 and TP53 mutations (104, 105). The deletion of CDKN2A/B has been observed in two studies of venetoclax resistance (104, 106). CRISPR-based screens have also nominated other pathways that contribute to resistance, some of which are dysregulated in cancer (104). For example, somatic mutation or the genomic loss of the SWI–SNF chromatin remodeling complex factors was found to facilitate the upregulation of BCLXL in patients, leading to resistance to ibrutinib/venetoclax combination (107). Clinical experience with other BH3 mimetics is more limited. However, preclinical data also suggest that somatic mutations may contribute to resistance to other BH3 mimetic inhibitors (107).
Translational studies have observed that FLT3 internal tandem duplication mutations as well as PTPN11 mutations are associated with resistance to venetoclax therapy (108), likely through their effects on MCL1 and/or BCLXL (108–111). Mutations in KRAS also conferred resistance to venetoclax alone or in combination, likely by downregulating BCL2 and BAX and upregulating MCL1 and BCL2A1/BFL1. SF3B1 as well as upregulation of BCL2A1 (BFL1) may also be potential mechanisms of resistance to venetoclax monotherapy or combinations.
Resistance Due to Alterations in the Tumor Microenvironment
The tumor microenvironment has been proposed to regulate the sensitivity of CLL cells to BH3 mimetics (Fig. 2C). One of the first pieces of evidence was described by Vogler and colleagues, who found that after ABT-737 treatment, resistant CLL cells developed in lymph nodes (112). Using in vitro assays, Thijssen and colleagues demonstrated that CD40 stimulation led to resistance to ABT-199 (113). Similarly, analysis of patients with MCL and CLL treated with ibrutinib and venetoclax demonstrated heterogeneous intrinsic resistance, which was associated with microenvironmental factors such as IL10 or the CD40 ligand (114). These agonists led to enhanced expression of MCL1 and BCLXL through NF-κB signaling.
The growing appreciation of the tumor microenvironment in modulating responses to BH3 mimetics has suggested that ex vivo culture systems may be helpful to understand the stromal components involved in resistance (115). One approach to target these stromal components was suggested by Davids and colleagues. The authors found that CLL cells from the peripheral blood are highly primed to undergo apoptosis (116), whereas stroma sharply reduced priming. Inhibition of stromal interactions using a PI3K δ-isoform or a BTK inhibitor displaced CLL cells into the blood and increased their priming (116, 117). Similar results were observed in follicular lymphoma (118).
ADAPTIVE RESISTANCE IS COMMONLY ASSOCIATED WITH BH3 MIMETICS
Despite the aforementioned genomic and microenvironmental influences on the response of tumor cells to BH3 mimetics, these mechanisms cannot explain widespread resistance in many cancer types, including most solid tumors. In contrast, adaptive changes to individual BH3 mimetics are commonly observed, contributing to resistance in many contexts (Fig. 2D). In such instances, inhibition of a specific antiapoptotic protein leads to rapid tumor-intrinsic nongenomic adaptation through a second antiapoptotic protein. Multiple studies describe compensatory mechanisms between antiapoptotic proteins as a cross-talk to ensure the tumor's survival. The potential of BH3 mimetics simultaneously targeting several antiapoptotic proteins or in combination with other anticancer agents is a burgeoning field of investigation that will affect the clinic in the coming years.
One of the first examples of this cross-compensating mechanism was described by Yecies and colleagues when exposing lymphoma cell lines to ABT-737 (119). They observed that BIM was displaced from BCL2 to MCL1 and BFL1/A1, resulting in therapy resistance (119). In patients with CLL, Haselager and colleagues have shown that proapoptotic BIM sequentially interacts with antiapoptotic BCL2 family members, and that BCLXL is more relevant for venetoclax resistance than MCL1 (120). In B-cell lymphoma models, transcriptional remodeling has been observed following ABT-199 treatment (121). These adaptive mechanisms are not restricted to hematologic malignancies. The combination of BH3 mimetics for solid tumors is a more recent field of study, but several reports demonstrated compensation between MCL1 and BCLXL. These codependencies have been described in multiple pediatric solid tumors (122), breast cancer (123, 124), melanoma (125), non–small cell lung cancer (NSCLC; ref. 66), cervical cancer (126), and many others.
Additionally, therapy-induced senescence is becoming an expanding field of study because it may promote resistance and ultimately tumor progression, as reviewed elsewhere (127). Paradoxically, these two distinct biological processes, senescence and oncogenesis, share a common vulnerability—antiapoptotic dependence—and BH3 mimetics such as navitoclax and others are extensively explored as senolytics (128, 129). Regardless of the exact mechanism underpinning persister cancer cells’ resistance to treatment, they rely on BCL2 antiapoptotic proteins for survival, making BH3 mimetics excellent candidates to enhance therapy effectiveness in combination.
Except in rare cases where the inhibition of a cell's basal apoptotic dependency is sufficient to achieve complete responses (Fig. 3A), resistance to BH3 mimetics will emerge through genetic or epigenetic changes (Fig. 3B). Recognizing the widespread adaptive changes in response to BH3 mimetics in many cancer types suggests that approaches that target adaptive changes could improve their efficacy (Fig. 3C). Here, we outline some key principles of these strategies.
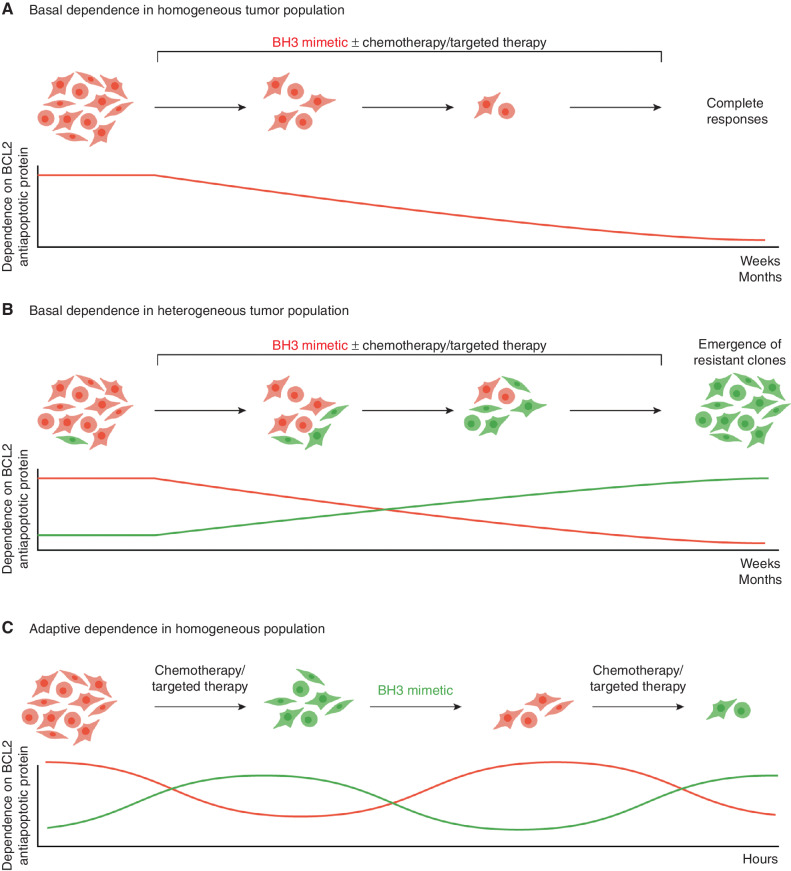
Figure 3.
Adaptive resistance to BH3 mimetics. A, In the traditional model, the dependence of each cell to each antiapoptotic BCL2 member is established, thereby predicting response to a BH3 mimetic targeting this dependency. B, However, in a heterogeneous group of cells with different BCL2 dependencies (depicted as red or green), continuous treatment with a BH3 mimetic targeting a specific dependency (with or without targeted therapy) leads to cytotoxic responses of a subset of cells. Resistant clones with a different dependency emerge in weeks to months due to impaired binding of the drug to its target. Profiling of BCL2 dependence at the time of resistance would enable rational selection of alternative therapeutic approaches. C, In an adaptive model of resistance, apoptotic BCL2 member dependence is plastic. Chemotherapy or targeted therapy induces a change in the dependency of the cell to specific BH3 mimetics within hours. Profiling of the tumor will identify the specific dependency, which can then be targeted using a specific BH3 mimetic. In this model, dependence on specific BCL2 members evolves without genomic changes. Approaches that recognize and target these rapid adaptive changes may overcome resistance. In practice, both clonal evolution and adaptive resistance contribute to resistance to BH3 mimetics, although we posit that adaptive changes are more ubiquitous.
Targeting Multiple Antiapoptotic BCL2 Family Members
Combining several BH3 mimetics, such as MCL1 and BCL2/BCLXL inhibitors, has had significant efficacy in preclinical in vitro and in vivo models (70, 130), leading to their current evaluation in clinical trials (e.g., NCT0321683 and NCT03672695). Even pan-BCL2 inhibition combining BH3 mimetics is now being explored for different types of cancers (70, 130, 131). Whether targeting multiple BCL2 inhibitors can safely be administered concomitantly remains to be determined. Pan-BCL2 inhibition may be tolerable because normal adult tissues are relatively refractory to apoptosis with the exception of the hematopoietic system (38, 39). However, patients will likely need to be closely monitored to control myelosuppression, cardiotoxicity, and tumor lysis syndrome in hematologic lineages (56, 57).
Combining Anticancer Therapies with BH3 Mimetics
Undoubtfully, the most studied strategy regarding BH3 mimetics is their combination with current therapies. The fundamental idea behind this approach is that tumors rapidly adapt to current treatments, both conventional chemotherapy and targeted agents, and persister cancer cells survive leading to relapse (132). The primary mechanism by which chemotherapy may synergize with BH3 mimetics is by lowering the apoptotic threshold of cells (11). Preclinical data have shown synergy between cytotoxic agents such as cytarabine and venetoclax by enhancing BH3 activity and/or suppressing MCL1 to promote apoptosis. Consistent with these observations, venetoclax was associated with deeper remissions in patients with AML when combined with chemotherapy (133, 134), and MEK inhibition synergizes with ABT-737 (135) or venetoclax (136). A recent study by Flanagan and colleagues also demonstrated the potential of combining epigenetic modulators with venetoclax to treat multiple myeloma (137). Other combinations of these agents with BH3 mimetics have been described for multiple hematologic malignancies (138).
Apart from the previously cited examples of BH3 mimetic combinations in hematologic malignancies, multiple reports demonstrated that BCL2/BCLXL inhibition combined with conventional chemotherapy was effective against many solid tumors, including breast cancer, ovarian cancer, neuroblastoma, NSCLC, and many others (32, 44, 130, 139–144). Also, as new compounds emerge, MCL1 inhibition has been successfully used in preclinical studies to boost standard-of-care treatments in hepatocellular carcinoma (68), breast cancer (145), ovarian cancer (130), and rhabdomyosarcoma (146), among others.
Many oncogenic kinases lead to the phosphorylation-dependent ubiquitination of BIM (147–150), suggesting that their upregulation by kinase inhibitors could enhance its levels. The stabilization of BIM through oncogenic kinase inhibitors could prime the cells toward apoptosis. This approach has the advantage of targeting cancer cells with dysregulated oncogenic signaling, potentially improving the therapeutic index over nonmalignant tissues. Many reports demonstrate that therapies that target kinase vulnerabilities in tumor cells may paradoxically induce prosurvival adaptations by antiapoptotic BCL2 family proteins (151, 152). In consequence, rational combinations with BH3 mimetics often enhance the cytotoxic effect of these targeted drugs. For instance, PIK3CA-mutant breast cancer is sensitive to mTOR and BCLXL inhibition (153), estrogen receptor–positive (ER+) breast cancer to tamoxifen plus venetoclax (143), and HER2-amplified breast cancer to lapatinib with the MCL1 inhibitor S63845 (145). For NSCLC, several combinations have been reported: gefitinib plus ABT-737 in EGFR-mutant tumors (36, 40), EGFR and MCL1 dual inhibition in drug-tolerant cells (154), third-generation EGFR inhibitors that target EGFR T790M with navitoclax (142), and dual MEK and MCL1 inhibition for KRAS-mutant tumors (155). Other examples include the aurora kinase A inhibitor MLN8237 combined with venetoclax for MYCN-amplified neuroblastoma (156), TORC1/2 inhibitors with navitoclax for KRAS- and BRAF-mutant colorectal cancer (43), PI3K inhibitors with BH3 mimetics against SCLC (157) and ER+ breast cancer (158), sorafenib/regorafenib plus navitoclax for hepatocellular carcinoma (159), dabrafenib/trametinib with MCL1 inhibitors for melanoma (151), and many others. Thus, the comprehensive identification of these prosurvival adaptations could enable new therapeutic strategies combining anticancer agents with BH3 mimetics to improve cancer elimination (see Fig. 3C). Although this approach seems especially promising to treat multiple solid tumors, their molecular plasticity complicates a correct prediction for treatment success (160, 161).
Targeting Mitochondrial Adaptation
The observation that mitochondrial metabolism is associated with changes in sensitivity to venetoclax/cytarabine therapy provides a rationale for using electron transport chain complex inhibitors (162, 163) and suggests that these inhibitors could overcome adaptation. Alternatively, switching combination therapies to circumvent these changes could be implemented using currently approved therapies (162).
Despite the improved understanding of the mechanisms underlying the sensitivity of some cancers to these agents, major questions remain. For example, it will be essential to evaluate if BH3 mimetics could adversely affect antitumor immunity due to some immune cells’ dependency on BCL2 antiapoptotic family members. BH3 mimetics were observed to reduce selected CD4+ and CD8+ T cells, B cells, and some dendritic cells (164, 165). Nevertheless, the treatment of immunocompetent mice with MYC dysregulation with navitoclax and metformin increased the levels of tumor-infiltrating T cells and improved treatment outcomes in immunocompetent murine models (166). A recent study also demonstrated that venetoclax increases intratumoral effector T cells and cancer elimination when combined with immune-checkpoint inhibitors (167). Sharma and Allison have suggested that the killing of tumor cells could release tumor antigens, enhancing the efficacy of immunologic agents (168). Thus, there may also be a rationale for combining BCL2 inhibitors with chimeric antigen receptor (CAR) T-cell therapy, as this immune-based therapy kills tumor cells via apoptosis induction (169). Undoubtedly, the effects of BH3 mimetics on tumor immunity will add to the enormous complexity of apoptotic regulation. Clarification of the impact of apoptotic priming on tumor immunity could define specific approaches to enhance antitumor immune responses.
Overall, the clinical implementation of BH3 mimetics, as single agents but especially in combination, faces a clear problem: how to guide their use. In the next section, we cover some of the most recent approaches to answer this clear unmet need.
A NEW TYPE OF BIOMARKERS IS NEEDED FOR THE SELECTION OF BH3 MIMETICS
As described above, genomic biomarkers have not been strongly associated with clinical responses to BH3 mimetics. For example, CLL cells do not exhibit genetic alterations in BCL2, despite being strongly sensitive to venetoclax. Conversely, follicular lymphomas, which show dysregulated BCL2 expression due to the t(14;18) translocation driving the BCL2 gene from the immunoglobulin promoter, are infrequently responsive to venetoclax as monotherapy (170). Transcripts of BCL2 family members may, to a limited extent, predict response to BH3 mimetics: navitoclax or venetoclax cytotoxicity is partially correlated with BCL2 mRNA expression (171) but anticorrelated with MCL1 expression (52, 172, 173). The expression of the BCL2 family of sensitizers also contributes to sensitivity. For instance, high expression of the MCL1 antagonist NOXA (gene name, PMAIP1) is associated with resistance to BCL2L1 knockout (174). Yet these correlations are far from perfect, likely due to the multiple complex interactions of BCL2 family members as well as their numerous forms of regulation, including posttranslational modification. Measurements of these parameters, either alone or in combination, are not sufficient to predict the apoptotic response of cancer cells or the dependence on specific family BCL2 family members (175, 176). Given the relatively weak predictive capacity of individual features of the BCL2 family members, we posit that a new generation of functional biomarkers will be needed to predict anticancer therapy induction of apoptosis.
Directly exposing patient-isolated living cancer cells to therapeutic agents ex vivo to determine chemosensitivity has historically been explored, but the development of novel technologies has fostered functional precision medicine. Some exciting new approaches include the direct evaluation in blood cancers (177, 178), organoids (179), pharmacoscopy (180), and cancer cell mass measurements (181). Functional assays may be particularly crucial for selecting individualized BH3 mimetics in solid tumors compared with hematologic malignancies because fewer respond to single-agent BH3 mimetics. However, a limiting factor is consistent ex vivo culture assays because compromised cell viability could lead to undesired phenotypic changes (182).
In this regard, the Letai laboratory developed the BH3 profiling method to rapidly identify antiapoptotic dependencies in cancer cells (176, 183–185). This functional assay uses synthetic ~20-mer BH3 peptides, mimicking BH3-only proteins, acting as a prodeath signal to induce MOMP. By using different peptides, BH3 profiling can interrogate the cancer cells and obtain precise information regarding the apoptotic status of the cell. For example, the BH3 peptides BIM and BID, which have the capacity to bind to all antiapoptotic proteins and also directly activate BAX and BAK, measure overall apoptotic priming (how close cancer cells are to the apoptotic threshold; ref. 16). Interestingly, overall pretreatment apoptotic priming by itself is a good indicator of clinical response to conventional chemotherapy (38, 39, 186). Furthermore, using peptides recapitulating the BH3 domain sequence of sensitizer proteins—such as BAD, HRK, or NOXA—BH3 profiling can identify specific antiapoptotic dependencies (21) and accurately identify BH3 mimetic cytotoxicity (187). For instance, a cell that is primed when exposed to the BAD BH3 peptide indicates BCL2/BCLXL dependence. Similarly, cytochrome c release following exposure to the HRK peptide points to BCLXL dependence. BH3 profiling analyses have evolved, and several technologies have been adapted to perform these measurements, such as fluorimetry (38, 183), flow cytometry (176, 185), microscopy (188), and even microfluidics (J. Montero; manuscript submitted). Numerous studies utilized BH3 profiling to determine the therapeutic use of BH3 mimetics for hematologic malignancies (44, 119, 184, 189–194). Therefore, the current deployment of BH3 profiling in clinical trials (NCT03593915, NCT01523977, NCT04898894, NCT03709758, and others) offers the possibility of patient stratification.
As mentioned earlier, cancer cells are overall more primed for apoptosis than normal adult tissues (38) and often rely on antiapoptotic proteins for their survival. Identifying vulnerabilities of individual tumors may be essential to guide implementation of BH3 mimetics. However, an alternative approach is to target them sequentially due to potential toxicities of simultaneous targeting of BCL2 family members. A key question with this approach is the timing and optimal sequencing of each agent and BH3 mimetic. The rationale development of such approaches will likely be aided by functionally monitoring these adaptations. Accordingly, the development of dynamic biomarkers for these combination therapies and their deployment in selected populations will likely be required to maximize the potential of these therapeutics for patients.
Dynamic Biomarkers for BH3 Mimetics
One approach to generating novel predictive biomarkers for therapy response is termed dynamic BH3 profiling, or DBP (ref. 195; Fig. 4). DBP measures how much a given treatment primes cancer cells for apoptosis (Δ% priming) by measuring early changes in the apoptotic signaling preceding frank cell death days/weeks in advance. DBP represents an enormous technical advantage because it avoids sample deterioration due to the short ex vivo culture and permits directly testing therapies on patient-isolated cancer cells in less than a day. This assay allows for rapid functional analysis of many samples and treatments and has been successfully tested in vitro, in murine models, and on patient samples (88, 185, 195–199). DBP is superior to prior attempts to guide cancer treatment, as it is performed within a day, can be high- throughput (188), has an excellent predictive capacity, and has been validated clinically (88, 195, 198).
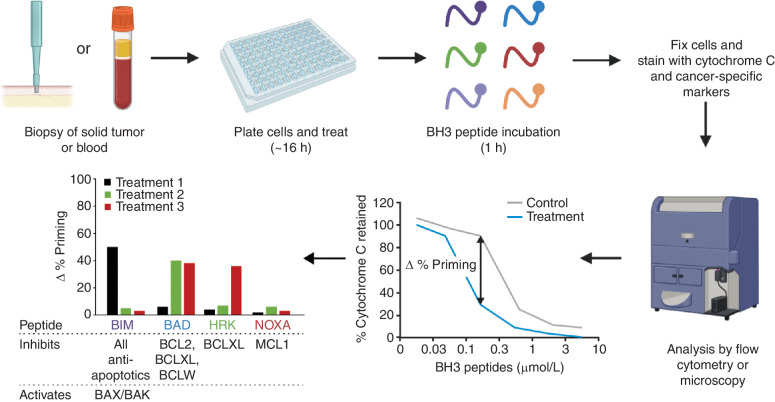
Figure 4.
Scheme for the dynamic BH3 profiling of primary clinical samples. Tumor cells are isolated from either a solid tumor biopsy or blood. Cells are treated with an anticancer agent such as chemotherapy, targeted therapy, or a BH3 mimetic, followed by the addition of BH3 peptides. Mitochondrial depolarization (cytochrome c release) on tumor cells is analyzed by flow cytometry or microscopy. The percentage of change in priming is determined by comparing treated versus control cells, and this parameter predicts response to the agent. By using different BH3 peptides, BH3 profiling can predict response to treatment (using the BIM peptide) or changes in antiapoptotic dependencies (using specific BH3 peptides such as BAD, HRK, or NOXA/MCL1), which indicate potential combination therapies with BH3 mimetics. The overall time from biopsy to results is approximately 24 hours. Adapted from ref. 11 and used under a CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/).
Furthermore, by using specific BH3 peptides mimicking sensitizer proteins (like BAD, HRK, NOXA, and others), DBP can also rapidly identify antiapoptotic adaptations upon treatment (Fig. 4). Several laboratories used this approach to determine different combinations with BH3 mimetics now explored in the clinic. For instance, DBP identified that CLL patient cells acquired resistance to BTK inhibitors, such as ibrutinib and acalabrutinib, by BCL2 adaptation, pointing to their combination with venetoclax (152). Recently, this same strategy was used to detect a rapid MCL1-mediated protection in melanoma when using MAPK inhibitors and the enhanced synergistic cytotoxicity when combined with BH3 mimetics such as S63845 or AZD-5991. Interestingly, this study showed that the observed acquired antiapoptotic dependence to the BRAF inhibitor dabrafenib was not mediated by an increase in MCL1 expression, but through a NOXA mRNA destabilization leading to protein decrease (151). Other novel therapeutic combinations with BH3 mimetics were recently identified using this approach in NSCLC (200), breast cancer (123, 158, 201, 202), esophageal cancer/mesothelioma (203), and pediatric cancers (146, 204), among others. Depicting the complexity of these prosurvival adaptations and antiapoptotic cross-talk is key to accurately identifying the right drug combination and the optimal sequential administration to maximize the cytotoxic effect in tumors. In this regard, DBP can be uniquely used as a predictive biomarker to track tumor adaptation to treatment and guide the combined metronomic use of anticancer agents and BH3 mimetics to increase effectiveness, avoid relapse, and reduce undesired secondary effects in patients.
Implementation of DBP into clinical practice to predict individualized responses to BH3 mimetics is feasible at present, particularly in patients with hematologic malignancies. Recently, Garcia and colleagues reported that DBP strongly predicted response of patients with AML to therapy (198). The application of similar approaches in patients with solid tumors is nonetheless more challenging because resection of metastasis with solid tumors is typically not performed prior to treatment. Adaptation of DBP for a small number of cells (e.g., from fine needle or core biopsies) will be required. Alternatively, it may be possible to propagate cells as organoids to improve the yield of tumor cells prior to profiling. Additional challenges for DBP and other functional predictive biomarkers for precision medicine are their clinical implementation that will undoubtfully demand regulatory approval, evaluation in clinical trials, and interdisciplinary work from research laboratories, industry, and agencies.
SUMMARY AND FUTURE DIRECTIONS
Overall, the relative safety of BH3 mimetics thus far has established that targeting apoptotic signaling may be a successful approach to enhance the treatment of patients with cancer. Their combination with current anticancer therapies, conventional chemotherapy or targeted therapies, and possibly with immunotherapies or CAR T cells, could significantly improve clinical outcomes. Moreover, these therapeutic strategies could also avoid relapse and diminish undesired secondary effects by sparing nontumoral tissues protected against apoptosis.
However, the key challenge of optimizing the opportunity provided by these apoptosis-inducing drugs is their individualized deployment. Precision medicine demands new therapeutic strategies and effective biomarkers to guide their use in the clinic; however, most efforts have been guided by analyses on DNA, RNA, and cancers’ molecular components in general combined with sophisticated bioinformatic evaluations. Undoubtfully, “omics” have changed how we stratify and treat patients in the clinic, but they still present some limitations. For instance, these measurements are static, as they isolate and analyze cancer cell constituents at a given time. Therefore, by definition, omics cannot evaluate dynamic cell changes in response to a perturbation.
Accordingly, deconvolution of BCL2 family member regulation and their variable dependencies across tumor types and cells emphasize the need for rational approaches to their targeting. Biomarker-driven approaches, especially functional assays, could enable efficient strategies to overcome resistance to current generation therapies. Targeting adaptation in BCL2 family members may help realize the unprecedented opportunity to target cell death in patients with cancer. By monitoring antiapoptotic adaptations to therapy with functional assays that track how living cancer cells respond to treatment, such as DBP, we could anticipate the appearance of persister cancer cells and overcome tumor cell death evasion. This approach could identify drugs that prime cancer cells to BH3 mimetics, mostly undetectable for other technologies, making possible personalized rational combinations with these agents. Utilizing functional assays such as BH3 profiling thus may be extremely valuable to guide BH3 mimetic clinical implementation. Precision medicine is entering a new era where clinicians can rationally design sequential combinations of drugs and BH3 mimetics to maximize cancer cell killing to benefit patients.
Acknowledgments
R. Haq acknowledges funding from the Melanoma Research Alliance and the O'Connor-Macgregor Fund for Melanoma Research. J. Montero acknowledges the Ramon y Cajal Programme, Ministerio de Economia y Competitividad (RYC-2015-18357), Ministerio de Ciencia, Innovación y Universidades (RTI2018-094533-A-I00), and the Cellex Foundation. The authors thank Prof. A. Letai for helpful discussions, comments, and suggestions that greatly helped improve this article. Some of the figures in this article were created with BioRender.
Authors’ Disclosures
J. Montero reports grants from Ramon y Cajal Programme, Ministerio de Economia y Competitividad (RYC-2015-18357), Ministerio de Ciencia, Innovación y Universidades (RTI2018-094533-A-I00), and the Cellex Foundation during the conduct of the study; personal fees from Oncoheroes Biosciences and Vivid Biosciences, and other support from AstraZeneca outside the submitted work; a patent for dynamic BH3 profiling (WO2014047342A1) owned by Dana-Farber Cancer Institute, licensed, and with royalties paid; and is an unpaid board member for The Society for Functional Precision Medicine. R. Haq reports personal fees from Tango Therapeutics, and grants from Novartis and Bristol-Myers-Squibb outside the submitted work.
References
- 1. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis Pet al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CEet al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima Net al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005;1:112–9. [DOI] [PubMed] [Google Scholar]
- 4. Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 2013;38:209–23. [DOI] [PubMed] [Google Scholar]
- 5. Izzo V, Bravo-San Pedro JM, Sica V, Kroemer G, Galluzzi L. Mitochondrial permeability transition: new findings and persisting uncertainties. Trends Cell Biol 2016;26:655–67. [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 7. Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26:1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature 2004;432:307–15. [DOI] [PubMed] [Google Scholar]
- 9. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998;281:1305–8. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012;119:651–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ 2018;25:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell 2010;37:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya Met al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 2000;14:2060–71. [PMC free article] [PubMed] [Google Scholar]
- 14. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJet al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001;292:727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SGet al. BAX activation is initiated at a novel interaction site. Nature 2008;455:1076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarosiek KA, Chi X, Bachman JA, Sims JJ, Montero J, Patel Let al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol Cell 2013;51:751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten Tet al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 2001;8:705–11. [DOI] [PubMed] [Google Scholar]
- 18. O'Neill KL, Huang K, Zhang J, Chen Y, Luo X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev 2016;30:973–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002;2:183–92. [DOI] [PubMed] [Google Scholar]
- 20. Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MGet al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005;17:393–403. [DOI] [PubMed] [Google Scholar]
- 21. Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SAet al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006;9:351–65. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GSet al. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 1998;273:7787–90. [DOI] [PubMed] [Google Scholar]
- 23. Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GEet al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000;102:43–53. [DOI] [PubMed] [Google Scholar]
- 24. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 2019;20:175–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988;335:440–2. [DOI] [PubMed] [Google Scholar]
- 26. McDonnell TJ, Nunez G, Platt FM, Hockenberry D, London L, McKearn JPet al. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol 1990;10:1901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 1990;348:331–3. [DOI] [PubMed] [Google Scholar]
- 28. Weiss LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med 1987;317:1185–9. [DOI] [PubMed] [Google Scholar]
- 29. Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends Biochem Sci 2014;39:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LAet al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993;74:597–608. [DOI] [PubMed] [Google Scholar]
- 31. Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A 1993;90:3516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJet al. Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol Cancer Ther 2012;11:1026–35. [DOI] [PubMed] [Google Scholar]
- 33. Heere-Ress E, Thallinger C, Lucas T, Schlagbauer-Wadl H, Wacheck V, Monia BPet al. Bcl-X(L) is a chemoresistance factor in human melanoma cells that can be inhibited by antisense therapy. Int J Cancer 2002;99:29–34. [DOI] [PubMed] [Google Scholar]
- 34. Haq R, Yokoyama S, Hawryluk EB, Jonsson GB, Frederick DT, McHenry Ket al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc Natl Acad Sci U S A 2013;110:4321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung Eet al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov 2011;1:352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GIet al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res 2007;67:11867–75. [DOI] [PubMed] [Google Scholar]
- 37. Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie Bet al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017;130:2401–9. [DOI] [PubMed] [Google Scholar]
- 38. Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel Get al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 2011;334:1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarosiek KA, Fraser C, Muthalagu N, Bhola PD, Chang W, McBrayer SKet al. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell 2017;31:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med 2007;4:1681–89; discussion 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 2008;118:3651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winter PS, Sarosiek KA, Lin KH, Meggendorfer M, Schnittger S, Letai Aet al. RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis. Sci Signal 2014;7:ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faber AC, Coffee EM, Costa C, Dastur A, Ebi H, Hata ANet al. mTOR inhibition specifically sensitizes colorectal cancers with KRAS or BRAF mutations to BCL-2/BCL-XL inhibition by suppressing MCL-1. Cancer Discov 2014;4:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bah N, Maillet L, Ryan J, Dubreil S, Gautier F, Letai Aet al. Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death Dis 2014;5:e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RTet al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A 2009;106:19503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SMet al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A 2000;97:7124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SRet al. Small molecule obatoclax (GX15–070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 2007;104:19512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Zet al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15–070 (obatoclax). Cancer Res 2008;68:3413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Brien SM, Cunningham CC, Golenkov AK, Turkina AG, Novick SC, Rai KR. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol 2005;23:7697–702. [DOI] [PubMed] [Google Scholar]
- 50. Schlagbauer-Wadl H, Klosner G, Heere-Ress E, Waltering S, Moll I, Wolff Ket al. Bcl-2 antisense oligonucleotides (G3139) inhibit Merkel cell carcinoma growth in SCID mice. J Invest Dermatol 2000;114:725–30. [DOI] [PubMed] [Google Scholar]
- 51. Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BAet al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005;435:677–81. [DOI] [PubMed] [Google Scholar]
- 52. van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PEet al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006;10:389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin Set al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008;68:3421–8. [DOI] [PubMed] [Google Scholar]
- 54. Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JPet al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 2010;11:1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SLet al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 2012;30:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJet al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011;118:1663–74. [DOI] [PubMed] [Google Scholar]
- 57. Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen Jet al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013;19:202–8. [DOI] [PubMed] [Google Scholar]
- 58. Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JFet al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JMet al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol 2013;9:390–7. [DOI] [PubMed] [Google Scholar]
- 60. Wang L, Doherty GA, Judd AS, Tao ZF, Hansen TM, Frey RRet al. Discovery of A-1331852, a first-in-class, potent, and orally-bioavailable BCL-XL inhibitor. ACS Med Chem Lett 2020;11:1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tao ZF, Hasvold L, Wang L, Wang X, Petros AM, Park CHet al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med Chem Lett 2014;5:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SKet al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 2015;7:279ra40. [DOI] [PubMed] [Google Scholar]
- 63. Zhang X, Thummuri D, He Y, Liu X, Zhang P, Zhou Det al. Utilizing PROTAC technology to address the on-target platelet toxicity associated with inhibition of BCL-XL. Chem Commun 2019;55:14765–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan Jet al. The landscape of somatic copy-number alteration across human cancers. Nature 2010;463:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Koss B, Morrison J, Perciavalle RM, Singh H, Rehg JE, Williams RTet al. Requirement for antiapoptotic MCL-1 in the survival of BCR-ABL B-lineage acute lymphoblastic leukemia. Blood 2013;122:1587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang H, Guttikonda S, Roberts L, Uziel T, Semizarov D, Elmore SWet al. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene 2011;30:1963–8. [DOI] [PubMed] [Google Scholar]
- 67. Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JLet al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood 2002;100:194–9. [DOI] [PubMed] [Google Scholar]
- 68. Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub Set al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol 2006;44:151–7. [DOI] [PubMed] [Google Scholar]
- 69. Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WDet al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev 2012;26:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue Jet al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis 2015;6:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat Get al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016;538:477–82. [DOI] [PubMed] [Google Scholar]
- 72. Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin Eet al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun 2018;9:5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bhagwat N, Grego A, Gowen-MacDonald W, Wang M, Cowart M, Wu Xet al. Abstract 983: preclinical characterization of PRT1419, a potent, selective and orally available inhibitor of MCL1. Exp Mol Therapeut 2021;81:983. [Google Scholar]
- 74. Caenepeel S, Brown SP, Belmontes B, Moody G, Keegan KS, Chui Det al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov 2018;8:1582–97. [DOI] [PubMed] [Google Scholar]
- 75. Wang Z, He N, Guo Z, Niu C, Song T, Guo Yet al. Proteolysis targeting chimeras for the selective degradation of Mcl-1/Bcl-2 derived from nonselective target binding ligands. J Med Chem 2019;62:8152–63. [DOI] [PubMed] [Google Scholar]
- 76. Lu X, Liu YC, Orvig C, Liang H, Chen ZF. Discovery of beta-carboline copper(II) complexes as Mcl-1 inhibitor and in vitro and in vivo activity in cancer models. Eur J Med Chem 2019;181:111567. [DOI] [PubMed] [Google Scholar]
- 77. Liu J, Tian Z, Zhou N, Liu X, Liao C, Lei Bet al. Targeting the apoptotic Mcl-1-PUMA interface with a dual-acting compound. Oncotarget 2017;8:54236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kump KJ, Miao L, Mady ASA, Ansari NH, Shrestha UK, Yang Yet al. Discovery and characterization of 2,5-substituted benzoic acid dual inhibitors of the anti-apoptotic Mcl-1 and Bfl-1 proteins. J Med Chem 2020;63:2489–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Senichkin VV, Streletskaia AY, Zhivotovsky B, Kopeina GS. Molecular comprehension of Mcl-1: from gene structure to cancer therapy. Trends Cell Biol 2019;29:549–62. [DOI] [PubMed] [Google Scholar]
- 80. Cidado J, Boiko S, Proia T, Ferguson D, Criscione SW, San Martin Met al. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin Cancer Res 2020;26:922–34. [DOI] [PubMed] [Google Scholar]
- 81. Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase Set al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev 2013;27:1351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina Met al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol 2012;14:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brennan MS, Chang C, Tai L, Lessene G, Strasser A, Dewson Get al. Humanized Mcl-1 mice enable accurate preclinical evaluation of MCL-1 inhibitors destined for clinical use. Blood 2018;132:1573–83. [DOI] [PubMed] [Google Scholar]
- 84. Reyna DE, Garner TP, Lopez A, Kopp F, Choudhary GS, Sridharan Aet al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 2017;32:490–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brahmbhatt H, Uehling D, Al-Awar R, Leber B, Andrews D. Small molecules reveal an alternative mechanism of Bax activation. Biochem J 2016;473:1073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pritz JR, Wachter F, Lee S, Luccarelli J, Wales TE, Cohen DTet al. Allosteric sensitization of proapoptotic BAX. Nat Chem Biol 2017;13:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li R, Ding C, Zhang J, Xie M, Park D, Ding Yet al. Modulation of bax and mTOR for cancer therapeutics. Cancer Res 2017;77:3001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Montero J, Stephansky J, Cai T, Griffin GK, Cabal-Hierro L, Togami Ket al. Blastic plasmacytoid dendritic cell neoplasm is dependent on BCL2 and sensitive to venetoclax. Cancer Discov 2017;7:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ryan CE, Davids MS. BCL-2 inhibitors, present and future. Cancer J 2019;25:401–9. [DOI] [PubMed] [Google Scholar]
- 90. Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman Tet al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 2016;6:1106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PSet al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Banerjee D. Genasense (Genta Inc). Curr Opin Investig Drugs 2001;2:574–80. [PubMed] [Google Scholar]
- 93. Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer Uet al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol 2006;24:4738–45. [DOI] [PubMed] [Google Scholar]
- 94. Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJet al. Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin Cancer Res 2018;24:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira Det al. Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 2011;29:909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Konopleva M, Letai A. BCL-2 inhibition in AML: an unexpected bonus? Blood 2018;132:1007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fresquet V, Rieger M, Carolis C, Garcia-Barchino MJ, Martinez-Climent JA. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood 2014;123:4111–9. [DOI] [PubMed] [Google Scholar]
- 98. Thangavadivel S, Byrd JC. Gly101Val BCL2 mutation: one step closer to understanding venetoclax resistance in CLL. Cancer Discov 2019;9:320–2. [DOI] [PubMed] [Google Scholar]
- 99. Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ERet al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov 2019;9:342–53. [DOI] [PubMed] [Google Scholar]
- 100. Birkinshaw RW, Gong JN, Luo CS, Lio D, White CA, Anderson MAet al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat Commun 2019;10:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tausch E, Close W, Dolnik A, Bloehdorn J, Chyla B, Bullinger Let al. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019;104:e434–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Blombery P, Birkinshaw RW, Nguyen T, Gong JN, Thompson ER, Xu Zet al. Characterization of a novel venetoclax resistance mutation (BCL2 Phe104Ile) observed in follicular lymphoma. Br J Haematol 2019;186:e188–e91. [DOI] [PubMed] [Google Scholar]
- 103. Blombery P, Thompson ER, Nguyen T, Birkinshaw RW, Gong JN, Chen Xet al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood 2020;135:773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guieze R, Liu VM, Rosebrock D, Jourdain AA, Hernandez-Sanchez M, Martinez Zurita Aet al. Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell 2019;36:369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nechiporuk T, Kurtz SE, Nikolova O, Liu T, Jones CL, D'Alessandro Aet al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov 2019;9:910–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Herling CD, Abedpour N, Weiss J, Schmitt A, Jachimowicz RD, Merkel Oet al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat Commun 2018;9:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Agarwal R, Chan YC, Tam CS, Hunter T, Vassiliadis D, Teh CEet al. Dynamic molecular monitoring reveals that SWI-SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat Med 2019;25:119–29. [DOI] [PubMed] [Google Scholar]
- 108. Zhang H, Nakauchi Y, Kohnke T, Stafford M, Bottomly D, Thomas Ret al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat Cancer 2020;1:826–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen L, Chen W, Mysliwski M, Serio J, Ropa J, Abulwerdi FAet al. Mutated Ptpn11 alters leukemic stem cell frequency and reduces the sensitivity of acute myeloid leukemia cells to Mcl1 inhibition. Leukemia 2015;29:1290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zaanan A, Okamoto K, Kawakami H, Khazaie K, Huang S, Sinicrope FA. The mutant KRAS gene up-regulates BCL-XL protein via STAT3 to confer apoptosis resistance that is reversed by BIM protein induction and BCL-XL antagonism. J Biol Chem 2015;290:23838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EMet al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 2013;23:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJet al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood 2009;113:4403–13. [DOI] [PubMed] [Google Scholar]
- 113. Thijssen R, Slinger E, Weller K, Geest CR, Beaumont T, van Oers MHet al. Resistance to ABT-199 induced by microenvironmental signals in chronic lymphocytic leukemia can be counteracted by CD20 antibodies or kinase inhibitors. Haematologica 2015;100:e302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jayappa KD, Portell CA, Gordon VL, Capaldo BJ, Bekiranov S, Axelrod MJet al. Microenvironmental agonists generate de novo phenotypic resistance to combined ibrutinib plus venetoclax in CLL and MCL. Blood Adv 2017;1:933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chiron D, Bellanger C, Papin A, Tessoulin B, Dousset C, Maiga Set al. Rational targeted therapies to overcome microenvironment-dependent expansion of mantle cell lymphoma. Blood 2016;128:2808–18. [DOI] [PubMed] [Google Scholar]
- 116. Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJet al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood 2012;120:3501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pavlasova G, Borsky M, Seda V, Cerna K, Osickova J, Doubek Met al. Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood 2016;128:1609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Serrat N, Guerrero-Hernandez M, Matas-Cespedes A, Yahiaoui A, Valero JG, Nadeu Fet al. PI3Kdelta inhibition reshapes follicular lymphoma-immune microenvironment cross talk and unleashes the activity of venetoclax. Blood Adv 2020;4:4217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 2010;115:3304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Haselager MV, Kielbassa K, Ter Burg J, Bax DJC, Fernandes SM, Borst Jet al. Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood 2020;136:2918–26. [DOI] [PubMed] [Google Scholar]
- 121. Zhao X, Ren Y, Lawlor M, Shah BD, Park PMC, Lwin Tet al. BCL2 amplicon loss and transcriptional remodeling drives ABT-199 resistance in B cell lymphoma models. Cancer Cell 2019;35:752–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kehr S, Haydn T, Bierbrauer A, Irmer B, Vogler M, Fulda S. Targeting BCL-2 proteins in pediatric cancer: dual inhibition of BCL-XL and MCL-1 leads to rapid induction of intrinsic apoptosis. Cancer Lett 2020;482:19–32. [DOI] [PubMed] [Google Scholar]
- 123. Alcon C, Gomez Tejeda Zanudo J, Albert R, Wagle N, Scaltriti M, Letai Aet al. ER+ breast cancer strongly depends on MCL-1 and BCL-xL anti-apoptotic proteins. Cells 2021;10:1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Goodwin CM, Rossanese OW, Olejniczak ET, Fesik SW. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ 2015;22:2098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lee EF, Harris TJ, Tran S, Evangelista M, Arulananda S, John Tet al. BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis 2019;10:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Abdul Rahman SF, Muniandy K, Soo YK, Tiew EYH, Tan KX, Bates TEet al. Co-inhibition of BCL-XL and MCL-1 with selective BCL-2 family inhibitors enhances cytotoxicity of cervical cancer cell lines. Biochem Biophys Rep 2020;22:100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst 2010;102:1536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi Jet al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016;22:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Prasanna PG, Citrin DE, Hildesheim J, Ahmed MM, Venkatachalam S, Riscuta Get al. Therapy-induced senescence: opportunities to improve anticancer therapy. J Natl Cancer Inst 2021;113:1285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Stover EH, Baco MB, Cohen O, Li YY, Christie EL, Bagul Met al. Pooled genomic screens identify anti-apoptotic genes as targetable mediators of chemotherapy resistance in ovarian cancer. Mol Cancer Res 2019;17:2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Prukova D, Andera L, Nahacka Z, Karolova J, Svaton M, Klanova Met al. Cotargeting of BCL2 with venetoclax and MCL1 with S63845 is synthetically lethal in vivo in relapsed mantle cell lymphoma. Clin Cancer Res 2019;25:4455–65. [DOI] [PubMed] [Google Scholar]
- 132. Shen S, Vagner S, Robert C. Persistent cancer cells: the deadly survivors. Cell 2020;183:860–74. [DOI] [PubMed] [Google Scholar]
- 133. DiNardo CD, Lachowiez CA, Takahashi K, Loghavi S, Xiao L, Kadia Tet al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol 2021;39:2768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chua CC, Roberts AW, Reynolds J, Fong CY, Ting SB, Salmon JMet al. Chemotherapy and venetoclax in elderly acute myeloid leukemia trial (CAVEAT): a phase Ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J Clin Oncol 2020;38:3506–17. [DOI] [PubMed] [Google Scholar]
- 135. Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin Bet al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia 2012;26:778–87. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136. Han L, Zhang Q, Dail M, Shi C, Cavazos A, Ruvolo VRet al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica 2020;105:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Flanagan L, O'Dwyer ME, Murphy P, Quinn J, Glavey S, Ni Chonghaile T. Venetoclax and epigenetic modifiers: promising novel combinations for the treatment of multiple myeloma. Blood 2021;138:4703. [Google Scholar]
- 138. Prado G, Kaestner CL, Licht JD, Bennett RL. Targeting epigenetic mechanisms to overcome venetoclax resistance. Biochim Biophys Acta Mol Cell Res 2021;1868:119047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cleary JM, Lima CM, Hurwitz HI, Montero AJ, Franklin C, Yang Jet al. A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Invest New Drugs 2014;32:937–45. [DOI] [PubMed] [Google Scholar]
- 140. Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa Fet al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A 2012;109:2766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kim EY, Jung JY, Kim A, Chang YS, Kim SK. ABT-737 synergizes with cisplatin bypassing aberration of apoptotic pathway in non-small cell lung cancer. Neoplasia 2017;19:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HEet al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 2016;22:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie MEet al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 2013;24:120–9. [DOI] [PubMed] [Google Scholar]
- 144. Tanos R, Karmali D, Nalluri S, Goldsmith KC. Select Bcl-2 antagonism restores chemotherapy sensitivity in high-risk neuroblastoma. BMC Cancer 2016;16:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Merino D, Whittle JR, Vaillant F, Serrano A, Gong JN, Giner Get al. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med 2017;9:eaam7049. [DOI] [PubMed] [Google Scholar]
- 146. Alcon C, Manzano-Munoz A, Prada E, Mora J, Soriano A, Guillen Get al. Sequential combinations of chemotherapeutic agents with BH3 mimetics to treat rhabdomyosarcoma and avoid resistance. Cell Death Dis 2020;11:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem 2003;278:18811–6. [DOI] [PubMed] [Google Scholar]
- 148. Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A 2004;101:15313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJet al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res 2008;21:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sheridan C, Brumatti G, Martin SJ. Oncogenic B-RafV600E inhibits apoptosis and promotes ERK-dependent inactivation of Bad and Bim. J Biol Chem 2008;283:22128–35. [DOI] [PubMed] [Google Scholar]
- 151. Montero J, Gstalder C, Kim DJ, Sadowicz D, Miles W, Manos Met al. Destabilization of NOXA mRNA as a common resistance mechanism to targeted therapies. Nat Commun 2019;10:5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 2017;31:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Anderson GR, Wardell SE, Cakir M, Crawford L, Leeds JC, Nussbaum DPet al. PIK3CA mutations enable targeting of a breast tumor dependency through mTOR-mediated MCL-1 translation. Sci Transl Med 2016;8:369ra175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Song KA, Hosono Y, Turner C, Jacob S, Lochmann TL, Murakami Yet al. Increased synthesis of MCL-1 protein underlies initial survival of EGFR-mutant lung cancer to EGFR inhibitors and provides a novel drug target. Clin Cancer Res 2018;24:5658–72. [DOI] [PubMed] [Google Scholar]
- 155. Nangia V, Siddiqui FM, Caenepeel S, Timonina D, Bilton SJ, Phan Net al. Exploiting MCL1 dependency with combination MEK + MCL1 inhibitors leads to induction of apoptosis and tumor regression in KRAS-mutant non-small cell lung cancer. Cancer Discov 2018;8:1598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ham J, Costa C, Sano R, Lochmann TL, Sennott EM, Patel NUet al. Exploitation of the apoptosis-primed state of MYCN-amplified neuroblastoma to develop a potent and specific targeted therapy combination. Cancer Cell 2016;29:159–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Potter DS, Galvin M, Brown S, Lallo A, Hodgkinson CL, Blackhall Fet al. Inhibition of PI3K/BMX cell survival pathway sensitizes to BH3 mimetics in SCLC. Mol Cancer Ther 2016;15:1248–60. [DOI] [PubMed] [Google Scholar]
- 158. Gomez Tejeda Zanudo J, Mao P, Alcon C, Kowalski K, Johnson GN, Xu Get al. Cell line-specific network models of ER(+) breast cancer identify potential PI3Kalpha inhibitor resistance mechanisms and drug combinations. Cancer Res 2021;81:4603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tutusaus A, Stefanovic M, Boix L, Cucarull B, Zamora A, Blasco Let al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget 2018;9:16701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Garraway LA, Janne PA. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov 2012;2:214–26. [DOI] [PubMed] [Google Scholar]
- 161. Letai A. Functional precision cancer medicine-moving beyond pure genomics. Nat Med 2017;23:1028–35. [DOI] [PubMed] [Google Scholar]
- 162. Bosc C, Saland E, Bousard A, Gadaud N, Sabatier M, Cognet Get al. Mitochondrial inhibitors circumvent adaptive resistance to venetoclax and cytarabine combination therapy in acute myeloid leukemia. Nature Cancer 2021;2:1204–23. [DOI] [PubMed] [Google Scholar]
- 163. Garciaz S, Guirguis AA, Muller S, Brown FC, Chan YC, Motazedian Aet al. Pharmacologic reduction of mitochondrial iron triggers a noncanonical BAX/BAK-dependent cell death. Cancer Discov 2022;12:774–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Carrington EM, Tarlinton DM, Gray DH, Huntington ND, Zhan Y, Lew AM. The life and death of immune cell types: the role of BCL-2 anti-apoptotic molecules. Immunol Cell Biol 2017;95:870–7. [DOI] [PubMed] [Google Scholar]
- 165. Carrington EM, Zhan Y, Brady JL, Zhang JG, Sutherland RM, Anstee NSet al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ 2017;24:878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Haikala HM, Anttila JM, Marques E, Raatikainen T, Ilander M, Hakanen Het al. Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti–PD-1 immunotherapy. Nat Commun 2019;10:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kohlhapp FJ, Haribhai D, Mathew R, Duggan R, Ellis PA, Wang Ret al. Venetoclax increases intratumoral effector T cells and antitumor efficacy in combination with immune checkpoint blockade. Cancer Discov 2021;11:68–79. [DOI] [PubMed] [Google Scholar]
- 168. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Karlsson H. Approaches to augment CAR T-cell therapy by targeting the apoptotic machinery. Biochem Soc Trans 2016;44:371–6. [DOI] [PubMed] [Google Scholar]
- 170. Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WGet al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 2017;35:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJet al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia 2014;28:210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Williams MM, Lee L, Hicks DJ, Joly MM, Elion D, Rahman Bet al. Key survival factor, Mcl-1, correlates with sensitivity to combined Bcl-2/Bcl-xL blockade. Mol Cancer Res 2017;15:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada Set al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006;10:375–88. [DOI] [PubMed] [Google Scholar]
- 174. DeWeirdt PC, Sangree AK, Hanna RE, Sanson KR, Hegde M, Strand Cet al. Genetic screens in isogenic mammalian cell lines without single cell cloning. Nat Commun 2020;11:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lucantoni F, Lindner AU, O'Donovan N, Dussmann H, Prehn JHM. Systems modeling accurately predicts responses to genotoxic agents and their synergism with BCL-2 inhibitors in triple negative breast cancer cells. Cell Death Dis 2018;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ryan J, Letai A. BH3 profiling in whole cells by fluorimeter or FACS. Methods 2013;61:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dietrich S, Oles M, Lu J, Sellner L, Anders S, Velten Bet al. Drug-perturbation-based stratification of blood cancer. J Clin Invest 2018;128:427–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Pemovska T, Kontro M, Yadav B, Edgren H, Eldfors S, Szwajda Aet al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov 2013;3:1416–29. [DOI] [PubMed] [Google Scholar]
- 179. Shi R, Radulovich N, Ng C, Liu N, Notsuda H, Cabanero Met al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res 2020;26:1162–74. [DOI] [PubMed] [Google Scholar]
- 180. Snijder B, Vladimer GI, Krall N, Miura K, Schmolke AS, Kornauth Cet al. Image-based ex-vivo drug screening for patients with aggressive haematological malignancies: interim results from a single-arm, open-label, pilot study. Lancet Haematol 2017;4:e595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Stevens MM, Maire CL, Chou N, Murakami MA, Knoff DS, Kikuchi Yet al. Drug sensitivity of single cancer cells is predicted by changes in mass accumulation rate. Nat Biotechnol 2016;34:1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Burstein HJ, Mangu PB, Somerfield MR, Schrag D, Samson D, Holt Let al. American Society of Clinical Oncology clinical practice guideline update on the use of chemotherapy sensitivity and resistance assays. J Clin Oncol 2011;29:3328–30. [DOI] [PubMed] [Google Scholar]
- 183. Ryan JA, Brunelle JK, Letai A. Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc Natl Acad Sci U S A 2010;107:12895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 2007;12:171–85. [DOI] [PubMed] [Google Scholar]
- 185. Ryan J, Montero J, Rocco J, Letai A. iBH3: simple, fixable BH3 profiling to determine apoptotic priming in primary tissue by flow cytometry. Biol Chem 2016;397:671–8. [DOI] [PubMed] [Google Scholar]
- 186. Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RMet al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 2012;151:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Villalobos-Ortiz M, Ryan J, Mashaka TN, Opferman JT, Letai A. BH3 profiling discriminates on-target small molecule BH3 mimetics from putative mimetics. Cell Death Differ 2020;27:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bhola PD, Ahmed E, Guerriero JL, Sicinska E, Su E, Lavrova Eet al. High-throughput dynamic BH3 profiling may quickly and accurately predict effective therapies in solid tumors. Sci Signal 2020;13:eaay1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Murase K, Kim HT, Bascug OR, Kawano Y, Ryan J, Matsuoka Ket al. Increased mitochondrial apoptotic priming of human regulatory T cells after allogeneic hematopoietic stem cell transplantation. Haematologica 2014;99:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur Get al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov 2014;4:362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood 2008;111:2300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LBet al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov 2014;4:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Touzeau C, Ryan J, Guerriero J, Moreau P, Chonghaile TN, Le Gouill Set al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 2016;30:761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Seyfried F, Demir S, Horl RL, Stirnweiss FU, Ryan J, Scheffold Aet al. Prediction of venetoclax activity in precursor B-ALL by functional assessment of apoptosis signaling. Cell Death Dis 2019;10:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan Det al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 2015;160:977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Townsend EC, Murakami MA, Christodoulou A, Christie AL, Koster J, DeSouza TAet al. The public repository of xenografts enables discovery and randomized phase II-like trials in mice. Cancer Cell 2016;30:183. [DOI] [PubMed] [Google Scholar]
- 197. Wu SC, Li LS, Kopp N, Montero J, Chapuy B, Yoda Aet al. Activity of the type II JAK2 inhibitor CHZ868 in B cell acute lymphoblastic leukemia. Cancer Cell 2015;28:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Garcia JS, Bhatt S, Fell G, Sperling AS, Burgess M, Keshishian Het al. Increased mitochondrial apoptotic priming with targeted therapy predicts clinical response to re-induction chemotherapy. Am J Hematol 2020;95:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Etchin J, Montero J, Berezovskaya A, Le BT, Kentsis A, Christie ALet al. Activity of a selective inhibitor of nuclear export, selinexor (KPT-330), against AML-initiating cells engrafted into immunosuppressed NSG mice. Leukemia 2016;30:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Potter DS, Du R, Bhola P, Bueno R, Letai A. Dynamic BH3 profiling identifies active BH3 mimetic combinations in non-small cell lung cancer. Cell Death Dis 2021;12:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Daniels VW, Zoeller JJ, van Gastel N, McQueeney KE, Parvin S, Potter DSet al. Metabolic perturbations sensitize triple-negative breast cancers to apoptosis induced by BH3 mimetics. Sci Signal 2021;14:eabc7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Watt AC, Cejas P, DeCristo MJ, Metzger-Filho O, Lam EYN, Qiu Xet al. CDK4/6 inhibition reprograms the breast cancer enhancer landscape by stimulating AP-1 transcriptional activity. Nat Cancer 2021;2:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Surman DR, Xu Y, Lee MJ, Trepel J, Brown K, Ramineni Met al. Therapeutic synergy in esophageal cancer and mesothelioma is predicted by dynamic BH3 profiling. Mol Cancer Ther 2021;20:1469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Manzano-Munoz A, Alcon C, Menendez P, Ramirez M, Seyfried F, Debatin KMet al. MCL-1 inhibition overcomes anti-apoptotic adaptation to targeted therapies in B-cell precursor acute lymphoblastic leukemia. Front Cell Dev Biol 2021;9:695225. [DOI] [PMC free article] [PubMed] [Google Scholar]