Abstract
Background:
Clindamycin serves as an alternative surgical prophylactic antibiotic in patients with penicillin (PCN) or cephalosporin allergy labels. In the previous reports, the use of clindamycin was associated with higher incidences of surgical site infections (SSIs). We aimed to determine the characteristics of PCN or cephalosporin allergic reactions to stratify patient’s risk and indicate subsequent management; leading to de-labeling of PCN or cephalosporin allergy.
Methods:
We conducted a prospective cohort study of patients receiving clindamycin as surgical antibiotic prophylaxis from September 2021 to March 2022. Data were collected from electronic medical records; included demographic data, antibiotic allergy labels, allergic reaction, and allergy testing.
Results:
Clindamycin was administered in 445 patients who underwent 451 operations. Among these patients, 53.0% (n = 236) were female with a median age of 15 years (range; 0.5–57.0 years). PCN and cephalosporin allergies were labelled in 83.8% (n = 373) and 25.6% (n = 114) patients, respectively; 11.4% (n = 51) of patients were allergic to both classes of the antibiotics. There were 191 (51.2%) and 73 (64.0%) possible hypersensitivity reactions (HSRs) in PCN and cephalosporin groups, respectively. The most common reactions were rash (PCN: n = 99, 26.5%; cephalosporin: n = 35, 30.7%), and hives (PCN: n = 71, 19.0%; cephalosporin: n = 24, 21.1%). Severe reactions included angioedema (PCN: n = 7, 1.9%; cephalosporin: n = 5, 4.4%), anaphylaxis (PCN: n = 8, 2.1%; cephalosporin: n = 7, 6.1%), bronchospasm (cephalosporin: n = 1, 0.9%), airway involvement (PCN: n = 1, 0.3%; cephalosporin: n = 1, 0.9%), serum sickness (PCN: n = 1, 0.3%), blisters (PCN: n = 1, 0.3%), and drug reaction with eosinophilia and systemic symptoms (DRESS) (PCN: n = 1, 0.3%). Low-risk history of allergy included gastrointestinal side effects (PCN: n = 9, 2.4%; cephalosporin: n = 3, 2.7%), positive family history (PCN: n = 7, 1.9%; cephalosporin: n = 1, 0.9%), and remote history of allergy (PCN: n = 2, 0.5%). There were 201 (53.9%) and 53 (46.5%) unknown reactions in PCN and cephalosporin groups, respectively. In the overall cohort, 3 patients (0.7%) were skin tested for drug allergy (PCN: n = 2, 0.5%; cephalosporin: n = 2, 1.8%).
Conclusion:
Clindamycin was largely administered in patients with non-severe HSRs, low-risk history or unknown reactions to PCN or cephalosporin, whom cefazolin could have been administered safely. Obtaining a detailed history of antibiotic allergy, allergy testing and/or direct oral challenge can de-label unsubstantiated PCN or cephalosporin allergy and ultimately reduce the incidence of SSIs by optimizing the rate of more effective antibiotic administration.
Keywords: Allergy, Antibiotics, Beta-lactam, Clindamycin, Hypersensitivity, Surgical prophylaxis
1. Introduction
Surgical site infections (SSIs) are the most common surgical infections and occupy 20% of the estimated two million nosocomial infections in the U.S., responsible for the aggregate annual cost of $3.5-$10.1 billion.1 Because SSIs are associated with significant morbidities and mortalities, SSI prevention practice bundles are being used worldwide. SSI bundles include preoperative prepping and prophylactic antibiotic administration. Despite the institution of SSI bundles, SSIs remain a significant healthcare issue, and additional approaches are urgently needed.
Appropriate use of perioperative antibiotic is an important element to reduce the SSIs rate.2, 3 The selection of an appropriate surgical prophylactic antibiotic is based on; 1) the spectrum of activity against pathogens that commonly contaminate the surgical site, 2) the safety profile, 3) the adverse consequence for the microbial flora of the patient, or for the hospital, and 4) patient’s medication allergy profile.2 On that account, cefazolin, the first-generation cephalosporin, is generally used as a first-line antibiotic agent for prophylaxis. Nonetheless, penicillin allergy label is a major concern that hinders the use of cefazolin because of the concerns on the cross reactivity between penicillins and cepharosporins;4 and that clindamycin has become the most common alternative antibiotic chosen for surgical prophylaxis.5, 6 Despite 10% of all patients in the United States are labelled with penicillin allergy,7 only 1% of general population is truly allergic to penicillin.8 Over 95% of patients who were labeled as allergic to penicillin could tolerate the antibiotic well and were de-labelled after skin testing and oral challenge.9–11
Cefazolin have been successfully safely administered as a surgical prophylactic antibiotic in patients with penicillin allergy labels.5, 12, 13 It is still common to administer an alternative antibiotic for prophylaxis if patients are labeled to have penicillin allergy. However, clindamycin is associated with an increased incidence of SSIs.14–17
Implementing a structured allergy history for preoperative patients resulted in a dramatic reduction in alternative antibiotic prophylaxis with no serious adverse events, significant delays in antibiotic administration, or delays in operating times due to the interventions.13 De-labeling strategies after obtaining a detailed history of antibiotic allergy and appropriate allergy testing will maximize the number of patients who can successfully receive beta-lactam antibiotics for perioperative prophylaxis, leading into the reduction of SSIs. Currently there is a limited literature examining allergy profiles of patients who received clindamycin in the perioperative setting. Here we aimed to determine the characteristics of penicillin or cephalosporin allergic reactions in patients receiving clindamycin as surgical antibiotic prophylaxis. Stratification of patient’s risk could possibly indicate subsequent protocol management leading to de-labeling of penicillin or cephalosporin allergy. The ultimate goal is to mitigate the incidence of SSIs.
2. Material and methods
This study prospectively enrolled all consecutive patients who received clindamycin as a surgical antibiotic prophylaxis from September 2021 to March 2022 at Boston Children’s Hospital. The study protocol was approved by an Institutional Review Board, and informed consent was waived.
We recorded patients’ demographic data (age, gender), type of antibiotic allergy label, characteristics of allergic reactions, allergy testing, other drug / non-drug allergy, and type of surgery. All data were collected from electronic medical records and Anesthesia Information Management System™ (AIMS). Allergic reactions were characterized as 1) hypersensitivity reactions including non-severe (rash, hives, and flushing) and severe reactions (angioedema, airway involvement, bronchospasm, anaphylaxis, serum sickness, blisters / Steven-Johnson syndrome (SJS), and drug reaction eosinophilia and systemic symptoms (DRESS)). Low-risk history included drug side effects or intolerances, remote history of allergy, and family history of previous allergy.
Data were analyzed using Stata version 17.0 software (StataCorp, College Station, TX). Categorical and continuous variables were presented with number and percentages, and median and range, respectively.
3. Result
Over a 6 month of the study period, clindamycin was administered in 445 patients in a total of 451 operations. Of these patients, 53.0% (n = 236) were female with a median age of 15 years (range; 0.5–57.0 years) (Table 1). Approximately one-fourth of the study population were younger than 10 years old. Penicillin and cephalosporin allergies were labelled in 83.8% (n = 373) and 25.6% (n = 114), respectively; 11.4% (n = 51) of patients were allergic to both classes of antibiotics. Types of penicillins and cephalosporins that resulted in allergy labels were illustrated in Fig. 1. Nine patients (2%), who did not have previous history of beta-lactam antibiotic allergy, received clindamycin for surgical antibiotic prophylaxis without well-defined indications.
Table 1.
Characteristics of cohort patients.
| N = 445 | |
|---|---|
| Gender; female, n (%) | 236 (53%) |
| Age group (yr), n (%) | |
| <5 | 32 (7.2) |
| 5–10 | 73 (16.4) |
| 11–20 | 266 (59.8) |
| 21–30 | 57 (12.8) |
| ≥31 | 17 (3.8) |
| Types of surgery, n (%) | |
| Orthopedic | 204 (45.8) |
| Plastic | 60 (13.5) |
| Neurologic | 41 (9.2) |
| Urologic | 35 (7.9) |
| Gastrointestinal | 31 (7.0) |
| Otolaryngologic | 29 (6.5) |
| Cardiac catheterization | 21 (4.7) |
| Dental | 11 (2.5) |
| Interventional radiology | 6 (1.3) |
| Gynecologic | 3 (0.7) |
| Gastrointestinal endoscopy | 2 (0.4) |
| Cardiothoracic | 2 (0.4) |
| Types of beta-lactam allergy, n (%) | |
| Penicillins class | 373 (83.8) |
| Cephalosporins class | 114 (25.6) |
| Isolated PCN allergy, n (%) | 322 (72.4) |
| Isolated cephalosporin allergy, n (%) | 63 (14.2) |
| Combined beta-lactams allergies, n (%) | 51 (11.5) |
| Total numbers of PCN/cephalosporin allergy, n (%) | |
| 0 | 9 (2.0) |
| 1 | 331 (74.4) |
| 2 | 88 (19.8) |
| ≥3 | 17 (3.8) |
| Total numbers of other drug allergies, n (%) | |
| 1 | 80 (18.0) |
| 2 | 21 (4.7) |
| ≥3 | 15 (3.4) |
| Total numbers of other non-drug allergies, n (%) | |
| 1 | 80 (18.0) |
| 2 | 28 (6.3) |
| ≥3 | 34 (7.6) |
Fig. 1.
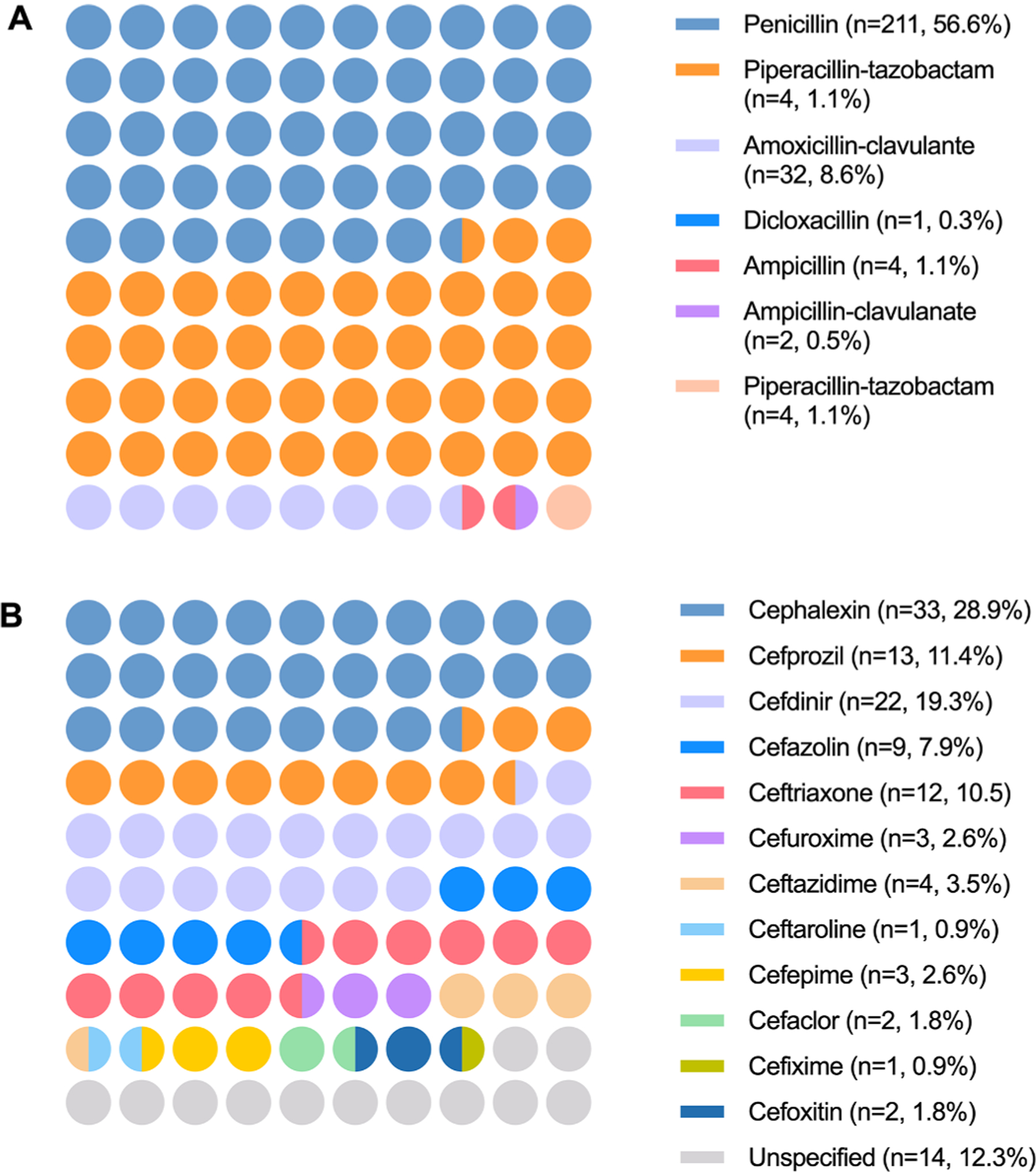
Types of penicillin (A) and cephalosporin (B) allergy labels.
There were 191 (51.2%) and 73 (64.0%) possible hypersensitivity reactions in penicillin and cephalosporin groups, respectively. Characteristics of allergic reactions; classified by non-severe hypersensitivity reaction, severe hypersensitivity reaction, low-risk history, and unknown reaction, were demonstrated in Table 2 and Fig. 2. Of all hypersensitivity reactions reported, 80–90% of the reactions were considered non-severe reactions. These included rashes (penicillin: n = 99, 26.5%; cephalosporin: n = 35, 30.7%), hives (penicillin: n = 71, 19.0%; cephalosporin: n = 24, 21.1%) and flushing (penicillin: n = 1, 0.3%). Severe hypersensitivity reactions that potentially preclude the use of beta-lactam antibiotic were angioedema (penicillin: n = 7, 1.9%; cephalosporin: n = 5, 4.4%), anaphylaxis (penicillin: n = 8, 2.1%; cephalosporin: n = 7, 6.1%), bronchospasm (cephalosporin: n = 1, 0.9%), airway involvement (penicillin: n = 1, 0.3%; cephalosporin: n = 1, 0.9%), serum sickness (penicillin: n = 1, 0.3%), blisters (penicillin: n = 1, 0.3%), and drug reaction with eosinophilia and systemic symptoms (DRESS) (penicillin: n = 1, 0.3%).
Table 2.
Characteristics of allergic history/reaction.
| Penicillins (n = 373) | Cephalosporins (n = 114) | |
|---|---|---|
| Non-severe HSRs, n (%) | ||
| Rash | 99 (26.5%) | 35 (30.7) |
| Hives | 71 (19.0) | 24 (21.1) |
| Flushing | 1 (0.3) | 0 (0.0) |
| Severe HSRs, n (%) | ||
| Angioedema | 7 (1.9) | 5 (4.4) |
| Airway involvement | 1 (0.3) | 1 (0.9) |
| Wheezing | 0 (0.0) | 1 (0.9) |
| Anaphylaxis | 8 (2.1) | 7 (6.1) |
| Blisters | 1 (0.3) | 0 (0.0) |
| Serum sickness | 1 (0.3) | 0 (0.0) |
| DRESS | 1 (0.3) | 0 (0.0) |
| Low-risk allergic history, n (%) | ||
| GI side effects | 9 (2.4) | 3 (2.6) |
| Family history | 7 (1.9) | 1 (0.9) |
| Remote history | 2 (0.5) | 0 (0.0) |
| Unknown, n (%) | 201 (53.9) | 53 (46.5) |
DRESS, drug reaction with eosinophilia and systemic symptoms; GI, gastrointestinal; HSR, hypersensitivity reaction.
Fig. 2.
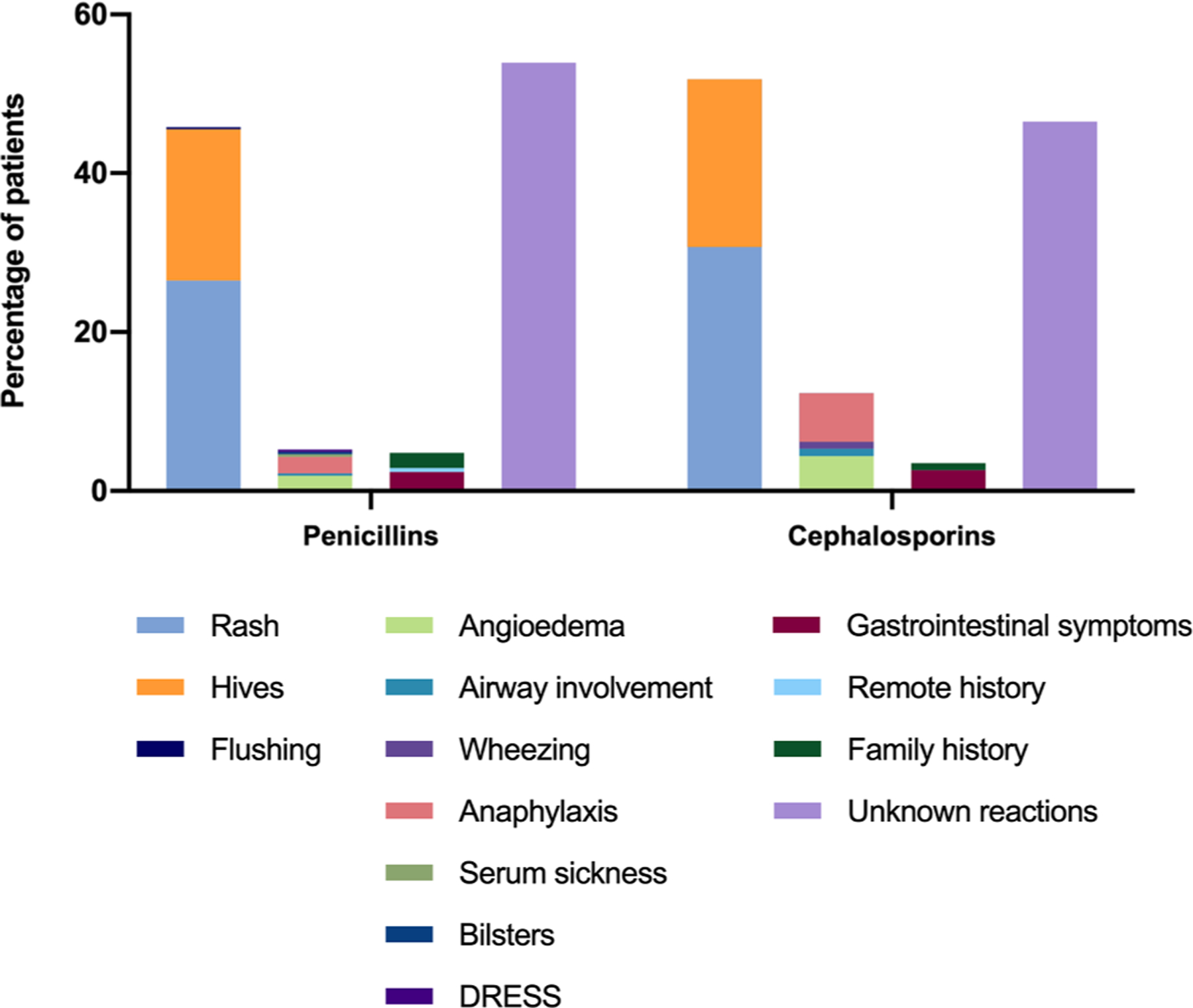
Characteristics of allergic reaction/history classified into 1) non-severe hypersensitivity reactions (rash, hives, flushing), 2) severe hypersensitivity reaction (angioedema, airway involvement, wheezing, anaphylaxis, serum sickness, blisters, drug reaction with eosinophilia and systemic symptoms (DRESS)), 3) low-risk history (gastrointestinal symptoms, remote history, family history), and 4) unknown reaction.
In the overall cohort, 7 patients were labelled with beta-lactam allergies (7 penicillin labels, 1 cephalosporin label), without previous exposures to the antibiotics, because their parents were concerned of familial inherited allergy. There were 201 (53.9%) and 53 (46.5%) unknown reactions to beta-lactam allergy in PCN and cephalosporin groups, respectively. Skin testing was performed in 3 patients (penicillin: n = 2, 0.5%; cephalosporin: n = 2, 1.8%) of the study population.
4. Discussion
We reported remarkably high proportions of patients, with unknown reaction, low-risk history, or non-severe hypersensitivity reactions to penicillin and cephalosporin allergies, whom received clindamycin as an alternative antibiotic prophylaxis. The majority of hypersensitivity reactions were rashes and hives. Of all 33 severe hypersensitivity reactions, only 3 reactions (serum sickness, blisters, DRESS) were truly contraindicated for cefazolin.18 Moreover, 1.5% of our study population have never been exposed to penicillins or cephalosporins but were inappropriately labelled as antibiotic allergy due to previous family history of allergy.
These uncritical notions of allergic reaction to drug-associated side effects, non-specific rash, or unfavorable past experience have created much of the uncertainty regarding cephalosporin use in “penicillin-allergic” individuals. The same is true for “cephalosporin-allergic” individuals. The unsubstantiated allergic labels have been increasingly acknowledged as a significant health problem that proclaims the use of cefazolin for surgical prophylaxis.
The primary reason that most physicians elect to omit the use of cefazolin in the presence of penicillin or cephalosporin allergy labels is largely due to a misunderstanding of cross-reactivity pathophysiology; and also, to avoid litigation that cefazolin administration would result in an anaphylactic reaction. However, an outdated 10% cross-reactivity dogma19 between the two drug classes could be overestimated.20 This overestimation was attributable to 1) the contamination of small amount of penicillin to the first-generation cephalosporins manufactured before 1980,21 and 2) the poor methodology of the researches cited as references.22 Contemporary studies reported only 2–3% at most of cross-reaction in patients with positive skin test for penicillin and cephalosporin.23, 24 A recent systematic review and meta-analysis revealed the cross-reactivity rate of less 1% in patients with unconfirmed penicillin allergy.24
Regarding basic chemical structures, penicillins have one side chain (R1) and cephalosporins have two side chains (R1 and R2) (Fig. 3). The cross-reactivity is primarily based on the immunogenicity of R1 side chain, not the shared beta-lactam ring or R2 side chain.8, 25, 26 In contrast to other cephalosporins, cefazolin does not share an R1 side chain with any of the current FDA-approved beta-lactams (Fig. 4).25, 26 This property should make cefazolin safe to be administered in patients with a penicillin or even cephalosporin allergy, which is consistent with other clinical studies.12, 13, 24 Table 3 demonstrated penicillins and cephalosporins with similar R1 side chains and possible cross-reaction.26
Fig. 3.
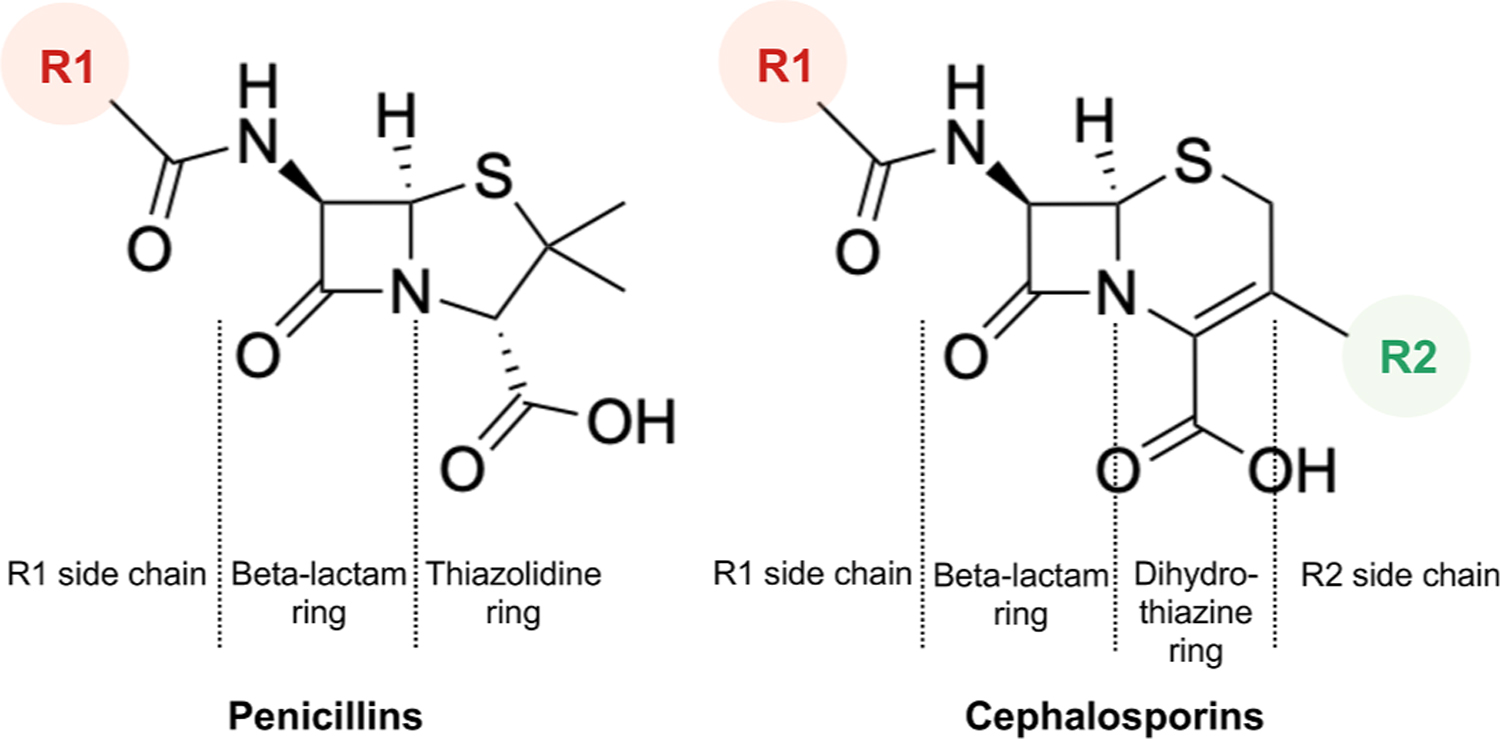
Basic chemical structures of penicillins and cephalosporins.
Fig. 4.
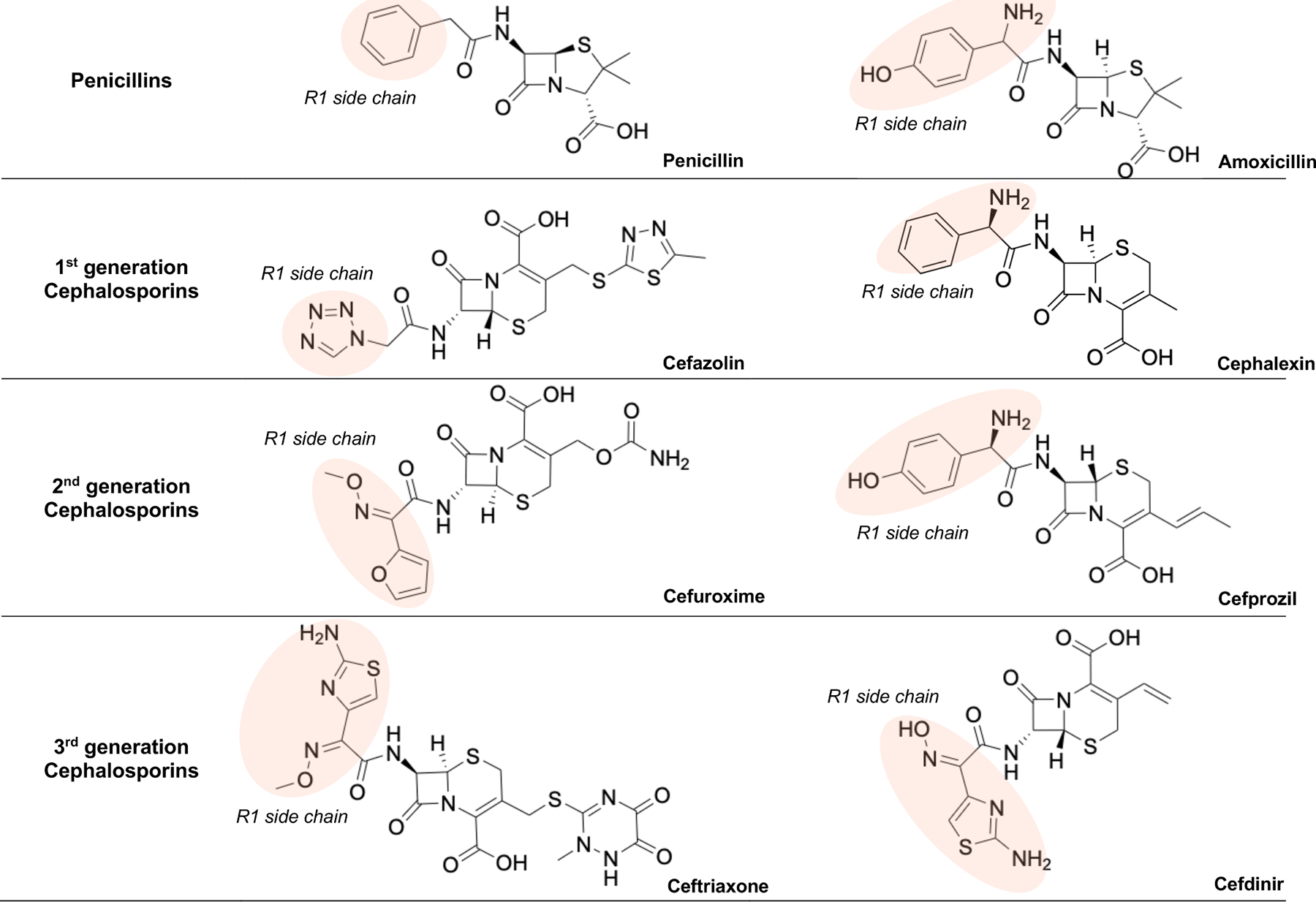
Chemical structures of commonly used penicillins and cephalosporins showing the similarities of R1 side chains (highlighted in red).
Table 3.
Commonly used penicillins and cephalosporins that share similar R1 side chain.
| Penicillin | Amoxicillin | Ampicillin | Cephalexin | Cefprozil | Cefazolin | Cefuroxime | Cefoxitin | Ceftriaxone | Cefotaxime | Ceftazidime | Cefepime | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin | ++ | ++ | ++ | |||||||||
| Amoxicillin | ++ | ++ | ++ | + | ||||||||
| Ampicillin | ++ | ++ | + | |||||||||
| Cephalexin | ++ | ++ | + | |||||||||
| Cefprozil | + | |||||||||||
| Cefazolin | ||||||||||||
| Cefuroxime | + | + | + | + | ||||||||
| Cefoxitin | + | |||||||||||
| Ceftriaxone | + | + | + | |||||||||
| Cefotaxime | + | + | ||||||||||
| Ceftazidime | ||||||||||||
| Cefepime | + | + | + |
Beta-lactam antibiotics that are known to be cross-reactive.
Beta-lactam antibiotics that share identical R1 side chain.
The use of clindamycin has been associated with a higher incidence of SSI in several procedures including pediatric spinal fusion surgeries,16 ventriculoperitoneal shunt for pediatric hydrocephalus,17 and head, neck osteomyocutaneous free flap surgery.15 There was a 50% increasedodds of SSIs among patients who were labelled with penicillin allergy.6 The increased SSI risk is potentially reducible if the patients could receive first-line beta-lactam antibiotic prophylaxis after a negative penicillin allergy evaluation.6 Even though clindamycin retains activity against many Gram-positive cocci and anaerobes,27, 28 its efficacy could be inferior to cefazolin for several reasons. The antimicrobial steward-ship guideline proposed clindamycin as a second-line agent against organisms for SSIs due to its overly broad spectrum, toxicity, and paucity of direct evidence of effectiveness.27 The comparison of antibacterial spectra between cefazolin and clindamycin was shown in Table 4.27 Additionally, clindamycin is predominantly metabolized by the liver CYP450, 3A4 enzyme; the concurrent use of CYP3A4 inducers can potentially reduce plasma clindamycin concentration including its bacteriostatic efficacy. For example, St. John’s wort, commonly consumed over the counter herbal medication, is one of CYP34A inducers. A recent study also demonstrated a significant increase in clindamycin-resistant S. aureus isolated from patients with SSIs.29
Table 4.
Cefazolin and clindamycin antibacterial spectra.
| Cefazolin | Clindamycin | |
|---|---|---|
| Aerobic, gram-positive cocci | ||
| S. aureus MSSA | ++ | + |
| S. aureus MRSA | 0 | ± |
| Staph coag-neg (S) | ++ | + |
| Staph coag-neg (R) | 0 | ± |
| S. epidermidis (S) | ++ | + |
| S. epidermidis (R) | 0 | ± |
| S. lugdunensis | ++ | + |
| S. sapophyticus | ++ | + |
| Strep. Anginosus gp | + | + |
| Strep. pyogenes (A) | + | + |
| Strep. agalactiae (B) | + | + |
| Strep. Pneumoniae | + | + |
| Viridans Strep. | + | ± |
| Aerobic, gram-negative bacilli, Enterobacter | ||
| E. coli (S) | + | 0 |
| Klebsiella sp. (S) | + | 0 |
| P. mirabilis | + | 0 |
| Aerobic, gram-negative bacilli, non-enterobacter | ||
| Capnocytophaga sp. | 0 | ++ |
| Kingella sp. | + | 0 |
| Anaerobic, gram-negative bacilli | ||
| B. fragilis | 0 | 0 * |
| F. necrophorum | 0 | + |
| Prevotella sp. | 0 | + |
| Anaerobic, gram positive | ||
| Actinomyces sp. | 0 | ++ |
| Clostridium sp. | 0 | + |
| P. acnes | + | ± |
| Peptostreptococci | + | + |
(Modified from source: The Sanford Guide to Antimicrobial Therapy 2021).
Recommend: Agent is a first line therapy: reliably active in vitro, clinically effective, guideline recommended, recommended as a first-line agent or acceptable alternative agent in the Sanford Guide.
Active: Agent is a potential alternative agent: active in vitro, possesses class activity comparable to known effective agents or a therapeutically interchangeable agents and hence likely to be clinically effective, but second line due to overly broad spectrum, toxicity, limited clinical experience, or paucity of direct evidence of effectiveness.
Variable: Variable activity such that the agent is not reliable effective in some infections or should be used in combination with other agent, and/or its efficacy is limited by resistance which has been associated with treatment failure.
Not Recommended.
Clindamycin activity against B. fragilis has been declining and rates of resistance world-wide approach 60%.
It is worth noted that multiple allergies could have biased the labeling of penicillin or cephalosporin allergy. We identified a remarkable number of patients who were labelled with combined penicillin and cephalosporin allergies although the dual allergy rate is supposed to be relatively low. Other coexisting non-beta-lactam or other non-drug allergies were also common in our study. In addition, previous study reported that penicillin-allergic individuals were more likely to have a new cephalosporin allergy report after a treatment with cephalosporin.30
Penicillin or cephalosporin allergy is not uncommon among young age group. The non-urticarial rash that occurred after the treatment of common pediatric infections with oral penicillin could be mislabeled as drug allergy, rather it could have been drug-induced delayed rashes, viral exanthem, or drug intolerance. Once the antibiotic allergy is labelled to the pediatric individual, it is likely to be carried forward into adulthood. This could result in a lifelong avoidance of beta-lactam class of antibiotic and adverse outcomes as previously mentioned. Most children with a history limited to rash can tolerate penicillin well after subsequent challenge. Targeting de-labeling of unsubstantiated penicillin or cephalosporin allergy in pediatric population will positively improve the rate of appropriate antibiotic use.
This study also has several limitations. Although our study was conducted at a single institution, many of our patients have carried antibiotic allergy labels from different hospitals. Many of the allergic reactions in our electronic medical record were depended on parental recall or self-reported. This potentially led to missing or incomplete history, and we were unable to classify allergic reactions based on the existing data. However, we believed that the significant allergic reactions would not have been missed on our database.
5. Conclusions
This study identified a large proportion of patients with non-severe hypersensitivity reactions, low-risk history, or unknown reactions of penicillin or cephalosporin allergy; whom cefazolin could possibly have been administered safely. Early de-labeling of unsubstantiated antibiotic allergy can prevent allergy labels carrying into adulthood. Obtaining a detailed history of antibiotic allergy, allergy testing and/or direct oral challenge can de-label penicillin or cephalosporin allergy; and ultimately reduce the incidence of SSIs by optimizing the rate of more effective antibiotics administration.
Acknowledgements
We would like to thank Grace Yuki for helping with data collection.
Funding
This study was in part supported by NIH R01GM127600 (K.Y.) and Data Quality and Outcomes Research (D-QORE) grant (Departmental) (W.M.)
Footnotes
Declaration of Competing Interest
None.
Reference
- 1.Shepard J, Ward W, Milstone A, et al. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148(10):907–914. 10.1001/jamasurg.2013.2246. Oct. [DOI] [PubMed] [Google Scholar]
- 2.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14(1):73–156. [DOI] [PubMed] [Google Scholar]
- 3.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, Committee HICPA. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20(4):247–280. [DOI] [PubMed] [Google Scholar]
- 4.Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med 2012;42(5):612–620. [DOI] [PubMed] [Google Scholar]
- 5.Beltran RJ, Kako H, Chovanec T, Ramesh A, Bissonnette B, Tobias JD. Penicillin allergy and surgical prophylaxis: cephalosporin cross-reactivity risk in a pediatric tertiary care center. J Pediatr Surg. 2015;50(5):856–859. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Arch Clin Infect Dis. 2018;66(3): 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790–796. [DOI] [PubMed] [Google Scholar]
- 8.Trubiano JA, Adkinson NF, Phillips EJ. Penicillin allergy is not necessarily forever. JAMA. 2017;318(1):82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savic LC, Khan DA, Kopac P, et al. Management of a surgical patient with a label of penicillin allergy: narrative review and consensus recommendations. Br J Anaesth. Jul 2019;123(1):e82–e94. 10.1016/j.bja.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258–263. [DOI] [PubMed] [Google Scholar]
- 11.Sacco K, Bates A, Brigham T, Imam J, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72(9):1288–1296. [DOI] [PubMed] [Google Scholar]
- 12.Grant JM, Song WH, Shajari S, et al. Safety of administering cefazolin versus other antibiotics in penicillin-allergic patients for surgical prophylaxis at a major Canadian teaching hospital. Surgery. 2021;170(3):783–789. [DOI] [PubMed] [Google Scholar]
- 13.Isserman RS, Cheung J, Varallo D, et al. Increasing cefazolin use for perioperative antibiotic prophylaxis in penicillin-allergic children. Pediatrics. 2022;149(3). [DOI] [PubMed] [Google Scholar]
- 14.Venkatesh KK, Hughes BL, Grotegut CA, et al. Preoperative cefazolin rather than clindamycin or metronidazole is associated with lower postpartum infection among women with chorioamnionitis delivering by cesarean delivery. Am J Obstet Gynecol MFM. 2020;2(1), 100074. [DOI] [PubMed] [Google Scholar]
- 15.Murphy J, Isaiah A, Dyalram D, Lubek JE. Surgical site infections in patients receiving osteomyocutaneous free flaps to the head and neck. Does choice of antibiotic prophylaxis matter? J Oral Maxillofac Surg. 2017;75(10):2223–2229. [DOI] [PubMed] [Google Scholar]
- 16.Linam WM, Margolis PA, Staat MA, et al. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol. 2009;30(2):109–116. [DOI] [PubMed] [Google Scholar]
- 17.Shibamura-Fujiogi M, Ormsby J, Breibart M, et al. Risk factors for pediatric surgical site infection following neurosurgical procedures for hydrocephalus: a retrospective single-center cohort study. BMC Anesthesiol. 2021;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019; 393(10167):183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis. 1978;137(Supplement):S74–S79. [DOI] [PubMed] [Google Scholar]
- 20.Pichichero ME A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115(4):1048–1057. [DOI] [PubMed] [Google Scholar]
- 21.SAXON A, BEALL GN, ROHR AS, ADELMAN DC Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med. 1987;107(2):204–215. [DOI] [PubMed] [Google Scholar]
- 22.Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngol Head Neck Surg. 2007;136(3):340–347. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Allergy A. American college of allergy A, joint council of allergy A, parameters JTFoP. drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. [DOI] [PubMed] [Google Scholar]
- 24.Sousa-Pinto B, Blumenthal KG, Courtney L, Mancini CM, Jeffres MN. Assessment of the frequency of dual allergy to penicillins and cefazolin: a systematic review and meta-analysis. JAMA Surg. 2021;156(4). e210021–e210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry SB, Veve MP, Wagner JL. Cephalosporins: a focus on side chains and β-lactam cross-reactivity. Pharmacy. 2019;7(3):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trubiano JA, Stone CA, Grayson ML, et al. The 3 Cs of antibiotic allergy—classification, cross-reactivity, and collaboration. J Allergy Clin Immunol Pract. 2017;5(6):1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert DN, Chambers HF, Saag MS, et al. The Sanford guide to Antimicrobial Therapy 2021. Sperryville, VA, USA: Antimicrobial Therapy, Inc.; 2021. [Google Scholar]
- 28.Smieja M Current indications for the use of clindamycin: a critical review. Can J Infect Dis. 1998;9(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khamash DF, Milstone AM, Carroll KC, et al. Changing antibiotic resistance patterns for Staphylococcus aureus surgical site infections. Infect Control Hospital Epidemiol. 2019;40(4):486–487. [DOI] [PubMed] [Google Scholar]
- 30.Macy E Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep. 2014;14(11):1–7. [DOI] [PubMed] [Google Scholar]


