Abstract
Tumor outcome is determined not only by cancer cell–intrinsic features but also by the interaction between cancer cells and their microenvironment. There is great interest in tumor-infiltrating immune cells, yet mast cells have been less studied. Recent work has highlighted the impact of mast cells on the features and aggressiveness of cancer cells, but the eventual effect of mast cell infiltration is still controversial. Here, we review multifaceted findings regarding the role of mast cells in cancer, with a particular focus on breast cancer, which is further complicated because of its classification into subtypes characterized by different biological features, outcome, and therapeutic strategies.
Introduction
Mast cells (MC) belong to the innate arm of the immune system, they derive from CD34+ CD117+ pluripotent hematopoietic stem cells within the bone marrow (BM) and complete their differentiation in tissues (1). On the basis of their role, MCs are differentially located in human tissues although they are predominantly abundant in close proximity to vessels (2), epithelia, fibroblasts (3), and nerves (4). MCs store many small secretory granules, whose content allows the classification of MCs in two major types: MC(T) and MC(TC). The former are characterized by granules that are particularly rich in tryptase, they play mainly a role in the immune response and can be found near the external mucosa of the gastrointestinal and respiratory apparatuses (5, 6). Conversely, MC(TC) display secretory granules with tryptase together with chymase and carboxypeptidase, contribute to tissue repair and are sited in the submucosa and connective tissues in close proximity to blood and lymphatic vessels (6). The specific signals responsible for progenitor recruitment and the mechanisms underlying MC differentiation are still poorly understood. MC biology is often studied by employing mouse models, although MC features are not fully conserved between human and mice (7). Hence, in this review, we will indicate whether findings were obtained in mouse models.
MC function is well characterized in allergic reactions and parasite responses, but their role in cancer is less understood and it is still a matter of debate. MCs can be detected both at the margins or infiltrating the tumor and have been reported to be endowed with both protumor or antitumor (8) properties depending on their abundance and localization, the type of stimuli and tumor context. Upon activation, MCs release a wide range of soluble mediators with either proinflammatory (e.g., TNF) or anti-inflammatory (including IL10 and TGFβ) effects (9). Moreover, MCs can differentially promote an inflammatory or immunosuppressive tumor microenvironment (TME) by modulating the functions of diverse immune populations (10), such as CD8+ T cells (11). Moreover, in mouse models, MCs have been shown to regulate myeloid-derived suppressor cells (MDSC; ref. 12), tumor-associated macrophages (13), and regulatory T cells (Treg; ref. 14). MCs can sense environmental modifications and influence stromal (15) and immune components of the TME (16) in a bidirectional cross-talk, which enables them to finely tune the host responses in the presence of developing tumors, ultimately influencing their outcome (17).
The Ambiguous Role of MCs in Cancer
MC presence has been shown to be associated with poor prognosis and aggressive disease in diverse cancer types including Hodgkin lymphoma (18), pancreatic adenocarcinoma (19, 20), hepatocellular carcinoma (HCC, ref. 21), and cholangiocarcinoma (22). In patients with Hodgkin lymphoma, MC infiltration is predictive of worse relapse-free survival rates (18). The same effect has been reported in pancreatic cancers, where presence of tumor-infiltrating MCs is associated with higher grade and reduced survival. Notably, through in vitro experiments, it was shown that pancreatic tumor cells could promote MC infiltration, which, in turn, favors cancer cell growth and invasion, and in this manner worsens the outcome of the disease (20). MC number also increases along with carcinogenesis in HCC and intrahepatic cholangiocarcinoma (21). In these tumors, MCs could promote cancer fibrosis and immune response, negatively affecting patients' survival rate. In cholangiocarcinoma, a vicious circle has been described between cholangiocytes and MCs. By employing both in vitro and mouse models, it was proposed that MC-derived histamine promotes cholangiocyte proliferation, thus favoring cholangiocarcinoma progression and angiogenesis. Simultaneously, cholangiocytes secrete stem cell factor (SCF), which stimulates MCs via c-Kit (23).
In other types of tumors, MC role is less clear and could depend on their localization. This is the case of colorectal cancer where MC infiltration has been defined as a favorable independent prognostic factor (24), although the presence of a high number of MCs localized in the peritumoral is associated with poor prognosis (19, 25). Moreover, MCs were shown to be crucial for the development of preneoplastic polyps. In fact, it was reported that MCs are enriched in the polyp masses of patients and, at least in mouse models, are necessary to transform premalignant lesions in colon cancer (26). Also in prostate cancer preclinical models, MCs display a different effect depending on their tissue compartment localization (27). Intratumor MCs inhibit angiogenesis and tumor growth, whilst peritumor MCs promote the expansion of human prostate tumors. During the onset of castrate-resistant prostate tumors, MCs are mobilized to the peritumoral area where they contribute to tumor relapse (27). Hence, their inhibition could be exploited to enhance the effects of castration in this setting. In melanoma, MCs have been associated with better patients' survival (28), but also with poor prognosis (29) and resistance to immune therapy (30) as shown in patients and through mouse models. In breast carcinomas, the comprehension of the role of MCs is made even more complicated by the high intertumor and intratumor heterogeneity and by the diverse outcomes, which characterize the different breast carcinoma subtypes (31).
Relation between MC Density and Breast Carcinoma Subtypes
Breast carcinoma is currently the most common type of tumor in women with about 2 million new cases every year (32). Breast carcinoma is a highly heterogeneous disease in terms of phenotypical features and tumor aggressiveness, making patients’ outcome and response to therapy extremely variable (33). In the clinical practice, treatment decision is commonly based on histopathologic markers that is, expression of hormone receptors (HR), HER2, and Ki67, which define different breast carcinoma subtypes: luminal A and B, HER2-positive, and triple-negative (TN) breast carcinomas. Luminal breast carcinomas are usually characterized by better prognosis, while HER2-positive breast carcinomas and TNBCs show a more aggressive behavior and unfavorable prognosis (34). The efficacy of the treatment is not only influenced by the expression levels of these receptors, but also by the quality and quantity of immune infiltrate (35).
Recently, numerous studies have employed CIBERSORT (36) to infer the proportions of 22 immune cell subsets and evaluate the association between the abundance of the diverse immune subpopulations and the clinical outcome of solid tumors (37). CIBERSORT was employed to analyze the gene expression profiles of almost 11,000 tumors and verify whether differences in the immune infiltrate depend on the molecular subtype (38). This work allowed to evaluate the effect of innate immunity in cancer since, until then, the association between immune infiltration and clinical outcome was generally limited to adaptive immunity. Moreover, results confirmed the complexity between MC infiltration, cancer cell molecular profiles and clinical features.
Other studies aimed at determining whether specific molecular profiles of breast carcinomas are characterized by a different density of infiltrating MCs (39–42). MC abundance has been compared between highly HR-positive (>50%) cancers versus tumors with low expression of HRs (<5%; ref. 41) finding an increased number of MCs (detected by Giemsa and Alcian blue staining) in the former group, mainly in the peritumoral zone. Glajcar and coworkers investigated the density of tryptase- and chymase-expressing MCs in different molecular subtypes of breast carcinoma, according to the molecular classification of St Gallen 2013 International Expert Consensus (43), to evaluate their association with standard prognostic markers (39). This study showed that low- and intermediate-grade breast carcinomas are characterized by high density of MCs both infiltrating the tumor and at the invasive margins. A statistically significant higher presence of both chymase- and tryptase-positive MCs was observed in luminal (estrogen or progesterone receptor positive; ER+ or PR+) compared with non-luminal (ER− and PR−) tumors (Fig. 1). The in silico analysis of gene expression profiles of the molecular taxonomy of breast carcinoma international consortium (METABRIC) database through CIBERSORT algorithm (36) confirmed that there is a significantly higher infiltration of MCs in luminal tumors, particularly luminal A, compared with more aggressive HER2-positive and TNBC subtypes (40).
Figure 1.
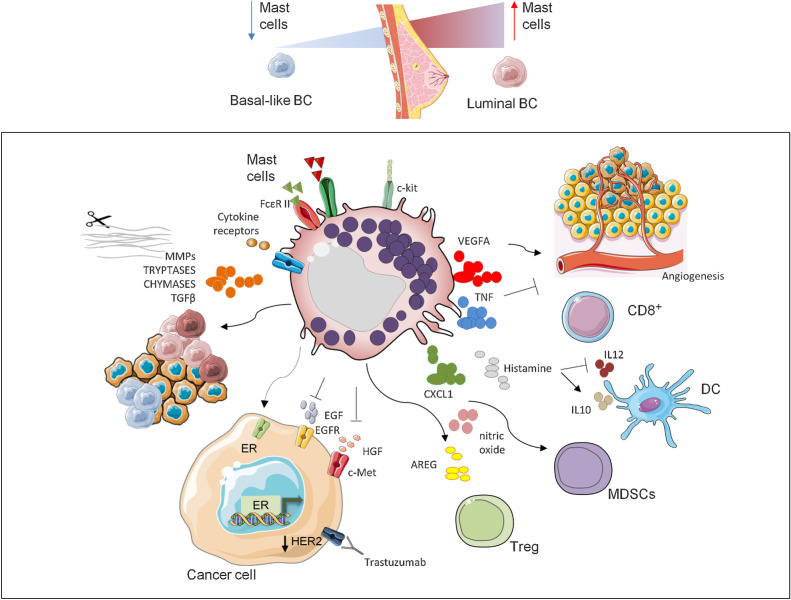
Multiple roles of MCs in breast tumors. Diverse breast carcinoma subtypes are differently infiltrated by MCs with a higher presence in luminal compared with HER2-positive tumors and TNBCs. The release of diverse factors for example, VEGFA, TNF, CXCL1, histamine, nitric oxide, and AREG together with the interaction with various populations of the immune system, including CD8+, DC, MDSC, and Tregs, lead MCs to mold the TME in a different manner and play both protumor and antitumor roles according to the tumor context. Because of their capability to shape the TME with tryptases, chymases, and through the release of TGFβ and MMPs, MCs influence tumor aggressiveness. Finally, MCs also affect the therapeutic response by limiting the efficacy of anti-HER2 treatment through the stimulation of the survival escape route favored by ER activity and reducing the activity of the basal markers EGFR (45) and cMET (40, 46). BC, breast carcinoma.
Different Breast Carcinoma Molecular Subtypes, Diverse Prognostic/Predictive Value of MCs?
Luminal/HR-positive
Immune infiltrate is differently associated with survival probability (44) based on the expression of HRs. Because MCs are more abundant in HR-positive breast carcinoma tumors, which are characterized by a better prognosis, it has been hypothesized that MCs could be endowed with a favorable prognostic value (Table 1). Accordingly, MCs are negatively associated with the proliferation rate, identified by a lower expression of Ki67 (39). The positive correlation between MC presence and expression of ER, or the ER-target gene BCL2, was confirmed also showing a negative correlation of MCs with the TNBC marker EGFR (45). In accordance to this, we found that in vitro MCs reduce the activation of EGFR and cMET receptors (40), which are both regulators of the basal program and are highly expressed in TNBCs. MCs were shown to inhibit cMET also by cleaving its ligand, HGF, into an NK4-like inhibitory molecule (46). Altogether these findings support the hypothesis that MCs could not only be recruited more efficiently by ER-positive breast carcinoma cells, but could also promote their luminal phenotype by favoring the expression and activity of ER, while simultaneously inhibiting the function of basal receptors such as cMET and EGFR (Fig. 1). In preclinical settings, MC presence has been associated to increased aggressiveness in luminal B models. In fact, breast carcinoma growth and metastasis formation were increased in a C57BL/6 MMTV-PyMT mouse model, when compared with C57BL/6-KitW-sh/W-sh (Wsh) mice, which lack MCs due to an inversion of the c-Kit promoter (47).
Table 1.
Role of MCs in the outcome of breast carcinoma.
| Patients’ tumor type | Prognosis | Detection | Localization | Mechanism proposed |
|---|---|---|---|---|
| Basal-like | Negative | CIBERSORT | NA | MC associated with CAF-derived high-risk score (63) |
| HER2-negative (TNBC and luminal) | Negative | 5 genes MC signature (95) | NA | MC associated with no-pCR in durvalumab with olaparib and paclitaxel patients (62) |
| HER2-positive | Negative | CIBERSORT | NA | Activated MCs are reduced in TRAR-low patients of the NeoALTTO trial (55) |
| Luminal B, HER2-enriched and basal-like BC | Negative | CIBERSORT | NA | Activated MCs are included in a immunorisk score (IRS) signature, which correlates with reduced OS (44) |
| TNBC | Negative | CIBERSORT | NA | ANXA1 is associated with activated MC and is predictive of reduced OS (60) |
| Inflammatory breast cancer | Negative | Tryptase | Intratumoral | MC infiltration associated with poor response (pCR) to neoadjuvant chemotherapy (11) |
| pan-BC | Negative | Tryptase | Intratumoral/peritumoral | MC distribution depends on HR and HER2 expression; intratumor MC is associated with worse prognosis (97) |
| Ductal invasive carcinomas | Negative | Tryptase | Interstitial/periglandular | Periglandular MC position are more numerous in G3 compared with G1/G2 tumors and control samples (102) |
| pan-BC | Negative/Positive | CIBERSORT | NA | Activated MCs are associated with worse DFS and OS in HER2-positive BC, while MC are linked with pCR in TNBC (54) |
| pan-BC | Negative | MC-dependent genes signature | NA | A MC-dependent genes signature predicts recurrence-free survival (98) |
| Nonspecified | Negative | Tryptase | Metastases | MC-rich primary tumors are more prone to form metastases (71) |
| pan-BC | Positive | Tryptase- and chymase | Intratumoral/invasive margins | MC density is associated with lower tumor grade, higher ER and PR expression, lower proliferation (39) |
| pan-BC | Positive | Toluidine blue | NA | MCs correlate with HR positivity and reduced Ki67 (42) |
| pan-BC | Negative | MCT | NA | MCs interact with HLA-G+ BC cells favoring invasion and metastasis (108) |
| pan-BC | Negative | Tryptase | NA | Role of MC tryptase in angiogenesis (103) |
| pan-BC | Negative | Tryptase | Intratumoral/peritumoral | MCs interact with lymphatic vessels favoring lymphovascular invasion in a subtype-specific manner (101) |
| pan-BC | Negative | Tryptase/Toluidine blue | Intratumoral/peritumoral | MCs contribute to stromal remodeling during BC progression (15) |
| pan-BC | Positive | c-Kit | NA | MC infiltration is an independent good prognostic marker (45) |
| High and low ER | Positive | Alcian blue/Giemsa | Peritumoral | Mast cells exhibit cytolytic activity against tumor cells (41) |
| pan-BC | Negative | Tryptase | Peritumoral | MC tryptase in the peritumoral tissue may promote breast cancer invasion (72) |
| pan-BC | Negative | Toluidine blue | Peritumoral | MCs could promote angiogenesis (104) |
| pan-BC | Positive | Tryptase | Peritumoral | MC heparin inhibits primary and metastatic tumors (8) |
| Preclinical tumor model | Effect on tumor | Detection | Localization | Mechanism proposed |
|---|---|---|---|---|
| Luminal A, B, and basal | Negative | Toluidine blue | Stromal | MC degranulation responsible for antibiotic-dependent increased growth (109) |
| Luminal B | Negative | Toluidine blue/Real time on MC genes | Peritumoral | MCs favor primary tumor growth and mets formation in MMTV-PyMT mice and tumor engraftment in NeuT-derived BC cell line (40) |
| Normal mammary tissue | NA | Tryptase | Ducts and lymph nodes | MC infiltration increased in C57BL/6 mammary tissue upon exposure to cigarette smoke (105) |
| Luminal B | Negative | Toluidine blue | Peritumoral | MMTV-PyMT mammary growth and metastasis are increased in the presence of MCs (47) |
| Luminal B and TNBC | Negative | Tryptase/Toluidine blue | NA | MCs remodel TME and metastatic niche to promote mets through SCF/cKit interaction in BC with arthritis (113) |
| Basal and luminal | Negative | Toluidine blue | Peritumoral, tumor-stroma interface | MCs secrete IL6-activating fibroblasts and promoting tumor progression (16) |
| Basal | Negative | Tryptase | NA | MC tryptase protects tumors from blood clotting and hypoxia (111) |
Note: Both clinical and preclinical studies are listed together with the subtype/model investigated, the effect, the detection method and localization of MC, and a brief description of the mechanism/effect observed. Papers are listed in reverse chronological order.
Abbreviations: BC, breast carcinoma; CAF, cancer-associated fibroblast; NA, information not available/not specified.
HER2-positive
In HR-positive luminal tumors, the expression of ER is obviously a positive predictive marker of responsiveness to hormonal-based interventions, but recent data from neoadiuvant studies suggest that ER status displays an opposite effect in patients with HER2-positive breast carcinoma treated with anti-HER2 therapy (48, 49). Trastuzumab, the standard of care for the treatment of HER2-positive breast carcinomas (34), is often effective in combination with chemotherapy, but some patients do not respond and eventually progress (50). To overcome resistance to trastuzumab, several novel HER2-targeting agents have been developed, including the tyrosine kinase inhibitor (TKI) lapatinib, and the mAb pertuzumab (51). However, ER activation could represent an escape pathway that promotes cell survival and resistance to therapy (52, 53). Because ER expression hampers the efficacy of anti–HER2-based therapy and given the capacity of MCs to increase ER level and activity (40), MCs may negatively affect the response to anti-HER2 therapy also via ER stimulation. The analysis of breast carcinoma transcriptomes confirmed that the presence of a high fraction of MCs correlates with worse disease-free survival (DFS) (54) and overall survival (OS). These observations have been confirmed by independent works that highlighted a negative effect of MCs in HER2-positive breast carcinoma patients’ outcome (38, 54, 55). Patients treated with trastuzumab and displaying a high risk of relapse [trastuzumab risk model (TRAR)-high] (56) express higher levels of genes associated to MCs. Furthermore, the MC-related gene carboxypeptidase A (CPA3) correlates with ER expression (ESR1) and ER-activated genes, for example, PGR, BCL2, and SCUBE2, unveiling a novel possible link between MCs and clinical resistance to trastuzumab therapy (40).
TNBC/basal-like
In contrast with data obtained from HER2-positive tumors, Bense and colleagues reported that activated MCs are associated with a higher pathologic complete response (pCR) rate in TNBC patients, thus supporting the role of MCs as favorable predictive markers in this breast carcinoma histotype (54). It has been shown that MCs are recruited by tumors, and TNBC cells then stimulate their activation and degranulation (57). In this scenario, activated MCs appear to mold a tumor stroma characterized by antitumor rather than supportive features (57). In accordance to the potential positive prognostic value of MCs in breast carcinomas, other studies depict the possible association between MC infiltrates and low tumor grade (41, 45, 58). The presence of MCs in the peritumoral stroma improves the prognosis of breast carcinomas with long-term follow-up, particularly in the node-negative subset, supporting an important biological role for MCs in mammary tumors (58). Moreover, the value of MC presence as an independent prognostic factor of good prognosis in invasive breast carcinomas was validated in a large tissue microarray study of 4,444 cases (45). Herein, Kaplan–Meier survival curves showed that MC presence is a favorable prognostic marker in the entire set of analyzed invasive breast carcinomas. Conversely, Okano and colleagues described an association between Annexin A1, a protein that has been reported in various studies to be predictive of a significantly shorter OS in patients with TNBC (59), with MCs. Here, MCs have also been linked with various aggressive phenotype features of TNBC, such as epithelial-to-mesenchymal transition (EMT) and angiogenesis (60, 61). MCs were shown to negatively correlate with therapy efficacy in inflammatory (I) breast carcinoma (11) and to be associated with no-pCR in a durvalumab/olaparib/paclitaxel trial enrolling patients with HER2-negative breast carcinoma, both HR-positive and TN (62). Moreover, by identifying a cancer-associated fibroblast signature predictive of OS, it was shown that a high-risk score correlated to increased MC infiltration in basal-like breast carcinomas (63).
Effects of MC-Released Soluble Factors on Breast Carcinoma Outcome
MCs contain a large cargo of soluble factors whose release and composition could determine the activity of MCs in tumors. For instance, MC-related antitumor effect could be a consequence of ROS induction (64) or caused by release in the TME of cytotoxic mediators, as TNF (65). The expression of TNF is lower in cancer compared with non-cancer tissues, indicating that tumor cells could negatively affect MCs by reducing TNF and hence hindering their antitumor activities (66). The importance of MC-derived TNF is also supported by the observation that TNF levels are increased in responder or stable patients with lung cancer, compared with patients with progressive disease (66). In contrast, MC-derived proangiogenic factor VEGFA, whose abundance is positively correlated with microvessel density (17, 29), is linked to the protumoral effect of MCs (67). The expression of TNF and VEGFA in MCs is mutually exclusive and their levels vary across different cancer types. This observation could contribute to explain why MCs act in a different manner according to the type of cancer cells (66). MCs expressing a high VEGFA:TNF ratio appear to display a dominant proangiogenic effect in agreement with the observed correlation between MC abundance and vessel formation (68). MCs release numerous other angiogenic factors, including endothelin-1, GMCSF, CXCL8, and CCL2 (69). Barkaway and colleagues have recently described a new link between MCs and aged vasculature in mouse models (70). They found that MC quantity increases in aged organs and promotes tissue damage through the production of high levels of the inflammatory chemoattractant CXCL1, which stimulates the reverse transendothelial migration of neutrophils. This effect still needs to be investigated in the context of tumors, but could potentially impact cancer outcome by favoring the accumulation of MDSC.
The protumoral effect of MCs could also be mediated by the release of other factors such as TGFβ, which stimulates EMT, several proteases including matrix metalloproteinases (MMP; refs. 71, 72), for example, MMP9, which contribute to MC-mediated shaping of the extracellular matrix (ECM), as well as chymase and tryptase that modify pro-MMPs to their active forms (73). Furthermore, MCs are the major source of histamine, whose activity has been largely investigated in allergic reactions (74). However, there is increasing evidence showing that histamine is endowed also with immune modulating activities ultimately affecting cancer outcome. Upon MC activation, the endogenous production of histamine was shown to suppress the immune response and to contribute to breast carcinoma growth in mouse models (75). Moreover, the in vitro binding of histamine to H2 histamine receptors stimulated human monocyte-derived dendritic cells (DC) to synthesize IL10 (76) and, in patient-derived xenograft tumor models, prevented the production of IL12, which is responsible for Th1 expansion (77). This event causes DC-driven polarization of CD4+ T cells toward a Th2 phenotype (78). The protumoral effect of histamine has also been reported in patients with cholangiocarcinoma where it contributes to tumor growth (79). In this scenario, MC-mediated release of histamine increases cancer progression and angiogenesis by enhancing the expression of VEGF (23).
In breast carcinomas, MC-released IL4 has an ambiguous effect because of its dual role both in promoting cancer cell dissemination or induction of apoptosis of breast carcinoma cells. Specifically, an increase of IL4, which depends on HR status, and its positive correlation with resistance to apoptosis have been described supporting the idea that IL4 is a negative prognostic factor in patients with breast carcinoma (80). Conversely, it has been reported that IL4 inhibits growth and induces apoptosis of human breast carcinoma cell lines, such as MCF7 and MDA-MB-231 (81).
Cross-talk between MCs and Other Immune Populations in Breast Carcinoma and Beyond
Several studies reported the capability of tumor-infiltrating MCs to shape the immune landscape (Fig. 1) either towards an antitumor or a protumor microenvironment (82), thus modulating the response to diverse therapeutic treatments. Because the composition of infiltrating immune cells differs among breast carcinoma subtypes, this may contribute to explain why MCs have a diverse impact in different breast carcinoma types. In particular, Dudeck and colleagues described the effect of MC TNF on T-cell priming. This mechanism could eventually influence their antitumor activity in the context of cancer. This work highlighted the potential of MC-derived TNF to amplify CD8+ DC functionality and linked MCs with T-cell and DC modulation (65). TNF is known to be beneficial for tumor shrinkage (83), to sensitize breast carcinoma cells in vitro and in vivo to chemotherapy and radiotherapy (84), and to play a critical role in mouse DC functionality and T-cell priming (65). These observations indicate that MCs promote a proinflammatory microenvironment through the release of TNF, affecting the efficacy of immunotherapy and vaccination strategies, at least in mouse models (65).
Collectively, these observations indicate that MCs support CD8+ T-cell activity, in disagreement with what suggested in inflammatory (I) breast carcinomas (11). In this context, MCs were shown to prevent treatment efficacy through their interaction with other immune subpopulations. More specifically, MCs, identified by tryptase staining, resulted more abundant in nonresponders where they were found in close proximity with CD8+ T cells, CD163+ macrophages, and tumor cells (11).
Several mouse studies described the engagement of MCs in the regulation of T-cell activities, including their recruitment and activation (85), as well as the impact on Tregs (86), which is bidirectional (87, 88). Nonetheless, the influence of MCs on T-cell functions is still controversial. In some studies, MCs have been shown to hinder the immune evasion mediated by Tregs in favor of the development of an effective antitumor immunity (14). In particular, it has been demonstrated that MCs are involved in the pathogenesis of diverse inflammatory conditions, for example, airway hyperreactivity and autoimmune encephalomyelitis, because they are able to counteract the immune suppression mediated by Tregs through the release of IL6, thus enabling their switch toward Th17 differentiation (14). In other works, it was described an opposite effect and MCs were shown to favor the immunosuppressive action of Tregs (86, 89). It has been reported that the stimulation with EGF-like growth factor amphiregulin (AREG) markedly enhanced the functions and efficiency of FOXP3+ Tregs, which express EGFR under inflammatory conditions (89). Notably, AREG is a predictive marker of poor therapy efficacy, particularly in patients with colorectal cancer bearing unmutated K-Ras (89–91), and it is highly upregulated upon MC activation (92). Therefore, MC-produced AREG may have a critical role for Treg functions and its release may represent a possible mechanism linking MCs and Tregs at the site of inflammation.
Other findings describing the cross-talk between MCs and Tregs support an effect of MCs on the response to anti-PD1 immune checkpoint inhibitor in murine melanoma models (30). A higher presence of MCs colocalizing with FOXP3+ Tregs was found in tumor tissue sections upon anti-PD-1 administration. By using the multi-targeted receptor TKI sunitinib, able to deplete MCs and Tregs, authors assessed a complete regression of tumors in combination with anti-PD-1 therapy. MC infiltration in tumors was also confirmed by CIBERSORT analysis of three independent RNA sequencing datasets of patients with melanoma treated with anti-PD-1 or immune checkpoint therapies. In another trial (93), MC presence was increased in anti-PD-1 nonresponder patients (30). These results show that MC infiltration is associated with the presence of FOXP3+ Tregs, along with the downmodulation of HLA-class I on tumor cells, lack of CD8+ T cells and subsequent ineffectiveness of the anti-PD-1 treatment.
Another mechanism by which MCs could promote immune evasion is constituted by their capability to interact with MDSC. MDSC are the key regulators of immunesurveillance escape given their capability to suppress T-cell responses (94). In colon carcinoma, MC presence increases the recruitment and activity of MDSC, supported by increased release of nitric oxide, which results in a proportional inhibition of T-cell proliferation and consequent tumor-induced tolerance (94). Concerning this aspect, it has been demonstrated that MC and MDSC cross-talk is mediated by CD40L-CD40 and this axis results in the suppression of tumor-specific T-cell response in prostate cancer models (12).
Evaluating MC Infiltration in Human Tumors
Tissue MCs can be detected in situ by metachromatic staining, such as toluidine blue, or by specific IHC to detect c-Kit (CD117) and tryptases (7). Alternatively, MC density can be inferred by gene expressing profile of whole tumors (95) exploiting deconvolution software as CIBERSORT (36). In situ detection of MCs displays the advantage that also the localization and the shape of MCs can be appreciated providing crucial information on their effect (96). In fact, several reports agree that MC predictive/prognostic value differs according to their localization: around tumor margins (41) or infiltrating the tumor mass (97). Moreover, the distance from other immune cell populations also affects the local immune microenvironment, possibly impacting on breast carcinoma response to therapy (11). MCs are endowed with immunomodulator activities in breast carcinoma and other solid tumors modifying the TME by either direct contact or secreting soluble factors. Therefore, the use of IHC or metachromatic staining allows to evaluate the spatial distribution of MCs, pending some limitations. In theory, the whole tumor should be analyzed because the distribution of MCs within the tumor is heterogeneous, more than one marker needs to be employed because no truly specific marker for MCs (e.g., c-Kit) is available and, finally, degranulated MCs are often difficult to detect.
On the other hand, MC signatures are increasingly used to quantify the presence of MCs in tumor tissues (98) and their activation state. CIBERSORT represents the most employed signature to estimate tumor-infiltrating immune populations and includes about 50 genes suitable to quantify the level of MC infiltration, also discriminating between resting and activated MCs (36). Resting MCs are generally less abundant in breast tumors compared with normal tissues, vice versa activated MCs are increased in breast carcinoma tissues. Nevertheless, these two MC statuses are associated with different cancer outcomes (38), sometimes even opposite, suggesting that the presence of MCs may not have a negative impact on tumors per se, but their activation is somehow linked to a more aggressive phenotype. This was first described in a pan-cancer analysis (37) and then applied specifically to breast carcinoma. Notably, the negative impact on OS and DFS of activated MCs is not observed in every breast carcinoma subtype, but it is significant in HER2-positive tumors (54). The same work supports the idea that activated MCs display even a positive effect on pCR in TNBC. As already mentioned, many genes, which are commonly exploited to identify MCs, are not really MC-specific and differently localized MCs are characterized by a diverse gene expression profile. Hence, a number of works tried to identify MC-specific genes also able to identify their particular activation states/subsets (99). As already mentioned, MC VEGFA:TNF ratio was shown to be prognostic (66). MCs represent the only cell type able to store preformed TNF in their granules (100), and hence display antitumor activities, while being capable of protumor effects via angiogenesis promotion (67, 101–105). Accordingly, different groups described the prognostic values of MC-related signatures (106) in diverse tumor types (12, 28, 107).
Pharmacologic Approaches to Target MCs in Preclinical Models
Because MCs represent key components of immune tumor infiltrate and have a crucial role in inflammation and angiogenesis (1), their targeting represents a possible strategy for therapeutic purposes (108). Several compounds for example, cromolyn sodium (109), nedocromil and lodoxamide are able to stabilize MCs hereby preventing their degranulation as well as the release of their mediators (110). It has been reported that the administration of cromolyn in TRAMP mice, a murine model of prostate cancer, increased the development of aggressive neuroendocrine areas, suggesting a protective effect of MCs in this tumor type (17). In mouse models of breast carcinoma, treatment with cromolyn induced an increase of blood clotting and hypoxia in subcutaneous 4T1 mammary adenocarcinoma cell line tumors supporting a role of MCs in the inhibition of hypoxia and blood clotting, which likely occurs via release of heparin, chymase, and tryptase (111). The inhibition of MCs by cromolyn sodium in cholangiocarcinoma determines the block of histamine release and, consequently, results in the reduction of tumor proliferation, angiogenesis and expression of mesenchymal markers (23).
In an attempt to inhibit the activity of MCs in angiogenesis, VEGFA was blocked through the employment of FDA-approved anti-angiogenic drugs that either target VEGF or its receptors (112). MC presence negatively affects the efficacy of antiangiogenic therapy (AAT) through the release of matrix-degrading granzyme B. By using a pancreatic tumor model, authors found that the absence of MC increased the antitumor efficacy of anti-VEGF-R2 antibody DC101, hence indicating that MCs hinder the sensitivity of tumors toward AAT. The mechanisms involved in this event are related to the expression of ECM-degrading proteases, specifically granzyme B, which is responsible for the release of proangiogenic factors, such as FGF-1 and GMCSF from the ECM (112). Another potential therapeutic approach to inhibit MCs in cancer is achievable through the pharmacologic targeting of the c-Kit receptor tyrosine kinase (113), which is essential for MC homeostasis, by the employment of TKI compounds including imatinib, dasatinib, and sunitinib, which inhibit the catalytic activity of both wild type and mutated, for example, D816V (114), c-Kit. The targeting of c-Kit/SCF interaction is also promising for the treatment of patients affected by cholangiocarcinoma because it results in the disruption of the cross-talk between MCs and cholangiocarcinoma cells with consequent reduction of tumor progression (23).
Conclusions
Initially neglected, MCs are progressively becoming crucial players in cancer, because increasing evidence supports their capability to affect outcome and therapy efficacy. Nonetheless, despite the many works that have been published in the last year, the ultimate role of MCs in tumors is far from being understood. Findings are characterized by apparently contradictory data, which actually are consequent of the plastic nature of MCs that are extremely sensitive to microenvironmental cues to which they suddenly respond. Hence, the effect of MCs cannot be limited to the dichotomy presence/absence, but it is caused, at least in breast carcinoma, by their activation and degranulation state, localization, secretion of cytokines and/or proteases, density, proximity to other immune and cancer cells. MCs could so represent an important tool to manipulate and predict cancer outcome, but, before they can be employed as prognostic/predictive markers or even as targets for novel therapeutic approaches, a deeper characterization of their biology and the identification of specific profiles associated to their activation and localization are still necessary.
Authors' Disclosures
No disclosures were reported.
Acknowledgments
D. Lecis and M.P. Colombo are supported by Associazione Italiana per la Ricerca sul Cancro (IG 2020 ID 24309 and IG 2020 IG 24363, respectively). M.T. Majorini is supported by a Fondazione Pezcoller-SIC fellowship.
References
- 1. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are mast cells MASTers in cancer? Front Immunol 2017;8:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Komi DEA, Redegeld FA. Role of mast cells in shaping the tumor microenvironment. Clin Rev Allergy Immunol 2020;58:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Artuc M, Steckelings UM, Henz BM. Mast cell-fibroblast interactions: human mast cells as source and inducers of fibroblast and epithelial growth factors. J Invest Dermatol 2002;118:391–5. [DOI] [PubMed] [Google Scholar]
- 4. Kleij HP, Bienenstock J. Significance of conversation between mast cells and nerves. Allergy Asthma Clin Immunol 2005;1:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol 2010;40:1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fong M, Crane JS. Histology, mast cells. In: StatPearls. Treasure Island, FL): StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 7. Aponte-Lopez A, Fuentes-Panana EM, Cortes-Munoz D, Munoz-Cruz S. Mast cell, the neglected member of the tumor microenvironment: role in breast cancer. J Immunol Res 2018;2018:2584243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samoszuk M, Kanakubo E, Chan JK. Degranulating mast cells in fibrotic regions of human tumors and evidence that mast cell heparin interferes with the growth of tumor cells through a mechanism involving fibroblasts. BMC Cancer 2005;5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maciel TT, Moura IC, Hermine O. The role of mast cells in cancers. F1000Prime Rep 2015;7:09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lichterman JN, Reddy SM. Mast cells: a new frontier for cancer immunotherapy. Cells 2021;10:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reddy SM, Reuben A, Barua S, Jiang H, Zhang S, Wang L, et al. Poor response to neoadjuvant chemotherapy correlates with mast cell infiltration in inflammatory breast cancer. Cancer Immunol Res 2019;7:1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jachetti E, Cancila V, Rigoni A, Bongiovanni L, Cappetti B, Belmonte B, et al. Cross-talk between myeloid-derived suppressor cells and mast cells mediates tumor-specific immunosuppression in prostate cancer. Cancer Immunol Res 2018;6:552–65. [DOI] [PubMed] [Google Scholar]
- 13. Eissmann MF, Dijkstra C, Jarnicki A, Phesse T, Brunnberg J, Poh AR, et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun 2019;10:2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009;114:2639–48. [DOI] [PubMed] [Google Scholar]
- 15. Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, et al. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology 2011;58:1096–106. [DOI] [PubMed] [Google Scholar]
- 16. Hugo HJ, Lebret S, Tomaskovic-Crook E, Ahmed N, Blick T, Newgreen DF, et al. Contribution of fibroblast and mast cell (afferent) and tumor (efferent) IL-6 effects within the tumor microenvironment. Cancer Microenviron 2012;5:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, Rigoni A, et al. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res 2011;71:5987–97. [DOI] [PubMed] [Google Scholar]
- 18. Molin D, Edstrom A, Glimelius I, Glimelius B, Nilsson G, Sundstrom C, et al. Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol 2002;119:122–4. [DOI] [PubMed] [Google Scholar]
- 19. Molderings GJ, Zienkiewicz T, Homann J, Menzen M, Afrin LB. Risk of solid cancer in patients with mast cell activation syndrome: results from Germany and USA. F1000Res 2017;6:1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res 2010;16:2257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol 2000;33:961–6. [DOI] [PubMed] [Google Scholar]
- 22. Pham L, Kennedy L, Baiocchi L, Meadows V, Ekser B, Kundu D, et al. Mast cells in liver disease progression: an update on current studies and implications. Hepatology 2022;75:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson C, Huynh V, Hargrove L, Kennedy L, Graf-Eaton A, Owens J, et al. Inhibition of mast cell-derived histamine decreases human cholangiocarcinoma growth and differentiation via c-kit/stem cell factor-dependent signaling. Am J Pathol 2016;186:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol 2009;24:1265–75. [DOI] [PubMed] [Google Scholar]
- 25. Fisher ER, Paik SM, Rockette H, Jones J, Caplan R, Fisher B. Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the national surgical adjuvant breast and bowel project (protocol R-01). Hum Pathol 1989;20:159–63. [DOI] [PubMed] [Google Scholar]
- 26. Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A 2007;104:19977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johansson A, Rudolfsson S, Hammarsten P, Halin S, Pietras K, Jones J, et al. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am J Pathol 2010;177:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaesler S, Wolbing F, Kempf WE, Skabytska Y, Koberle M, Volz T, et al. Targeting tumor-resident mast cells for effective anti-melanoma immune responses. JCI Insight 2019;4:e125057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, et al. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest 2003;33:420–5. [DOI] [PubMed] [Google Scholar]
- 30. Somasundaram R, Connelly T, Choi R, Choi H, Samarkina A, Li L, et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat Commun 2021;12:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polyak K. Heterogeneity in breast cancer. J Clin Invest 2011;121:3786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 33. Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med 2017;4:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loibl S, Gianni L. HER2-positive breast cancer. Lancet 2017;389:2415–29. [DOI] [PubMed] [Google Scholar]
- 35. Bates JP, Derakhshandeh R, Jones L, Webb TJ. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018;18:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ali HR, Chlon L, Pharoah PD, Markowetz F, Caldas C. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med 2016;13:e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glajcar A, Szpor J, Pacek A, Tyrak KE, Chan F, Streb J, et al. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch 2017;470:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Majorini MT, Cancila V, Rigoni A, Botti L, Dugo M, Triulzi T, et al. Infiltrating mast cell-mediated stimulation of estrogen receptor activity in breast cancer cells promotes the luminal phenotype. Cancer Res 2020;80:2311–24. [DOI] [PubMed] [Google Scholar]
- 41. della Rovere, F, Granata A, Familiari D, D'Arrigo G, Mondello B, Basile G. Mast cells in invasive ductal breast cancer: different behavior in high and minimum hormone-receptive cancers. Anticancer Res 2007;27:2465–71. [PubMed] [Google Scholar]
- 42. Sang J, Yi D, Tang X, Zhang Y, Huang T. The associations between mast cell infiltration, clinical features and molecular types of invasive breast cancer. Oncotarget 2016;7:81661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013;24:2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang J, Pan W, Xu Y, Ni C, Xue D, Chen Z, et al. Tumour-infiltrating immune cell-based subtyping and signature gene analysis in breast cancer based on gene expression profiles. J Cancer 2020;11:1568–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rajput AB, Turbin DA, Cheang MC, Voduc DK, Leung S, Gelmon KA, et al. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: a study of 4,444 cases. Breast Cancer Res Treat 2008;107:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raymond WW, Cruz AC, Caughey GH. Mast cell and neutrophil peptidases attack an inactivation segment in hepatocyte growth factor to generate NK4-like antagonists. J Biol Chem 2006;281:1489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He L, Zhu Z, Chen S, Wang Y, Gu H. Mammary tumor growth and metastasis are reduced in c-kit mutant sash mice. Cancer Med 2016;5:1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montemurro F, Di Cosimo S, Arpino G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann Oncol 2013;24:2715–24. [DOI] [PubMed] [Google Scholar]
- 49. Loi S, Dafni U, Karlis D, Polydoropoulou V, Young BM, Willis S, et al. Effects of estrogen receptor and human epidermal growth factor receptor-2 levels on the efficacy of trastuzumab: a secondary analysis of the HERA trial. JAMA Oncol 2016;2:1040–7. [DOI] [PubMed] [Google Scholar]
- 50. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019;11:1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A 2006;103:7795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers–role of estrogen receptor and HER2 reactivation. Breast Cancer Res 2011;13:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bense RD, Sotiriou C, Piccart-Gebhart MJ, Haanen JB, van Vugt MA, de Vries EG, et al. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst 2016;109:djw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pizzamiglio S, Ciniselli CM, Triulzi T, Gargiuli C, De Cecco L, de Azambuja E, et al. Integrated molecular and immune phenotype of HER2-positive breast cancer and response to neoadjuvant therapy: a NeoALTTO exploratory analysis. Clin Cancer Res 2021;27:6307–13. [DOI] [PubMed] [Google Scholar]
- 56. Triulzi T, De Cecco L, Sandri M, Prat A, Giussani M, Paolini B, et al. Whole-transcriptome analysis links trastuzumab sensitivity of breast tumors to both HER2 dependence and immune cell infiltration. Oncotarget 2015;6:28173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aponte-Lopez A, Enciso J, Munoz-Cruz S, Fuentes-Panana EM. An in vitro model of mast cell recruitment and activation by breast cancer cells supports anti-tumoral responses. Int J Mol Sci 2020;21:5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod Pathol 2004;17:690–5. [DOI] [PubMed] [Google Scholar]
- 59. Okano M, Kumamoto K, Saito M, Onozawa H, Saito K, Abe N, et al. Upregulated Annexin A1 promotes cellular invasion in triple-negative breast cancer. Oncol Rep 2015;33:1064–70. [DOI] [PubMed] [Google Scholar]
- 60. Okano M, Oshi M, Butash AL, Katsuta E, Tachibana K, Saito K, et al. Triple-negative breast cancer with high levels of Annexin A1 expression is associated with mast cell infiltration, inflammation, and angiogenesis. Int J Mol Sci 2019;20:4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bhardwaj A, Ganesan N, Tachibana K, Rajapakshe K, Albarracin CT, Gunaratne PH, et al. Annexin A1 preferentially predicts poor prognosis of basal-like breast cancer patients by activating mTOR-S6 signaling. PLoS One 2015;10:e0127678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021;39:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun C, Wang S, Zhang Y, Yang F, Zeng T, Meng F, et al. Risk signature of cancer-associated fibroblast-secreted cytokines associates with clinical outcomes of breast cancer. Front Oncol 2021;11:628677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Swindle EJ, Coleman JW, DeLeo FR, Metcalfe DD. FcepsilonRI- and fcgamma receptor-mediated production of reactive oxygen species by mast cells is lipoxygenase- and cyclooxygenase-dependent and NADPH oxidase-independent. J Immunol 2007;179:7059–71. [DOI] [PubMed] [Google Scholar]
- 65. Dudeck J, Ghouse SM, Lehmann CH, Hoppe A, Schubert N, Nedospasov SA, et al. Mast-cell-derived TNF amplifies CD8(+) dendritic cell functionality and CD8(+) T cell priming. Cell Rep 2015;13:399–411. [DOI] [PubMed] [Google Scholar]
- 66. Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 2021;184:792–809. [DOI] [PubMed] [Google Scholar]
- 67. Marone G, Varricchi G, Loffredo S, Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol 2016;778:146–51. [DOI] [PubMed] [Google Scholar]
- 68. Hiromatsu Y, Toda S. Mast cells and angiogenesis. Microsc Res Tech 2003;60:64–9. [DOI] [PubMed] [Google Scholar]
- 69. McHale C, Mohammed Z, Gomez G. Human skin-derived mast cells spontaneously secrete several angiogenesis-related factors. Front Immunol 2019;10:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barkaway A, Rolas L, Joulia R, Bodkin J, Lenn T, Owen-Woods C, et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 2021;54:1494–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roy A, Libard S, Weishaupt H, Gustavsson I, Uhrbom L, Hesselager G, et al. Mast cell infiltration in human brain metastases modulates the microenvironment and contributes to the metastatic potential. Front Oncol 2017;7:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep 2010;23:615–9. [DOI] [PubMed] [Google Scholar]
- 73. Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 2007;217:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol 2018;9:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hegyesi H, Colombo L, Pallinger E, Toth S, Boer K, Molnar V, et al. Impact of systemic histamine deficiency on the crosstalk between mammary adenocarcinoma and T cells. J Pharmacol Sci 2007;105:66–73. [DOI] [PubMed] [Google Scholar]
- 76. Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest 2001;108:1865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hewitt SL, Bailey D, Zielinski J, Apte A, Musenge F, Karp R, et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin Cancer Res 2020;26:6284–98. [DOI] [PubMed] [Google Scholar]
- 78. Angeles JMM, Mercado VJP, Rivera PT. Behind enemy lines: immunomodulatory armamentarium of the schistosome parasite. Front Immunol 2020;11:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut 2012;61:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Konig A, Vilsmaier T, Rack B, Friese K, Janni W, Jeschke U, et al. Determination of interleukin-4, -5, -6, -8 and -13 in serum of patients with breast cancer before treatment and its correlation to circulating tumor cells. Anticancer Res 2016;36:3123–30. [PubMed] [Google Scholar]
- 81. Gooch JL, Lee AV, Yee D. Interleukin 4 inhibits growth and induces apoptosis in human breast cancer cells. Cancer Res 1998;58:4199–205. [PubMed] [Google Scholar]
- 82. Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 2008;8:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 2006;11:397–408. [DOI] [PubMed] [Google Scholar]
- 84. Wu X, Wu MY, Jiang M, Zhi Q, Bian X, Xu MD, et al. TNF-alpha sensitizes chemotherapy and radiotherapy against breast cancer cells. Cancer Cell Int 2017;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Orinska Z, Bulanova E, Budagian V, Metz M, Maurer M, Bulfone-Paus S. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 2005;106:978–87. [DOI] [PubMed] [Google Scholar]
- 86. Bulfone-Paus S, Bahri R. Mast cells as regulators of T cell responses. Front Immunol 2015;6:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res 2009;69:5490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008;29:771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013;38:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jacobs B, De Roock W, Piessevaux H, Van Oirbeek R, Biesmans B, De Schutter J, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2009;27:5068–74. [DOI] [PubMed] [Google Scholar]
- 91. Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007;25:3230–7. [DOI] [PubMed] [Google Scholar]
- 92. Wang SW, Oh CK, Cho SH, Hu G, Martin R, Demissie-Sanders S, et al. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J Allergy Clin Immunol 2005;115:287–94. [DOI] [PubMed] [Google Scholar]
- 93. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Danelli L, Frossi B, Gri G, Mion F, Guarnotta C, Bongiovanni L, et al. Mast cells boost myeloid-derived suppressor cell activity and contribute to the development of tumor-favoring microenvironment. Cancer Immunol Res 2015;3:85–95. [DOI] [PubMed] [Google Scholar]
- 95. Danaher P, Warren S, Dennis L, D'Amico L, White A, Disis ML, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer 2017;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ribatti D. The staining of mast cells: a historical overview. Int Arch Allergy Immunol 2018;176:55–60. [DOI] [PubMed] [Google Scholar]
- 97. Carpenco E, Ceausu RA, Cimpean AM, Gaje PN, Saptefrati L, Fulga V, et al. Mast cells as an indicator and prognostic marker in molecular subtypes of breast cancer. In Vivo 2019;33:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ko EA, Sanders KM, Zhou T. A transcriptomic insight into the impacts of mast cells in lung, breast, and colon cancers. Oncoimmunology 2017;6:e1360457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014;123:e58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol 2007;178:5701–9. [DOI] [PubMed] [Google Scholar]
- 101. Raica M, Cimpean AM, Ceausu R, Ribatti D, Gaje P. Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer Res 2013;33:957–63. [PubMed] [Google Scholar]
- 102. Tamma R, Ruggieri S, Annese T, Simone G, Mangia A, Rega S, et al. Bcl6/p53 expression, macrophages/mast cells infiltration and microvascular density in invasive breast carcinoma. Oncotarget 2018;9:22727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Marech I, Ammendola M, Sacco R, Capriuolo GS, Patruno R, Rubini R, et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: possible translational significance. BMC Cancer 2014;14:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kwon GY, Lee SD, Park ES. Mast cell and macrophage counts and microvessel density in invasive breast carcinoma-comparison analysis with clinicopathological parameters. Cancer Res Treat 2005;37:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kispert S, Crawford S, Kolar G, McHowat J. In vivo effects of long-term cigarette smoke exposure on mammary tissue in mice. Am J Pathol 2017;187:1238–44. [DOI] [PubMed] [Google Scholar]
- 106. Dwyer DF, Barrett NA, Austen KF, Immunological Genome Project Consortium. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol 2016;17:878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bao X, Shi R, Zhao T, Wang Y. Mast cell-based molecular subtypes and signature associated with clinical outcome in early-stage lung adenocarcinoma. Mol Oncol 2020;14:917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ueshima C, Kataoka TR, Hirata M, Furuhata A, Suzuki E, Toi M, et al. The killer cell ig-like receptor 2DL4 expression in human mast cells and its potential role in breast cancer invasion. Cancer Immunol Res 2015;3:871–80. [DOI] [PubMed] [Google Scholar]
- 109. McKee AM, Kirkup BM, Madgwick M, Fowler WJ, Price CA, Dreger SA, et al. Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth. iScience 2021;24:103012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ribatti D. Mast cells as therapeutic target in cancer. Eur J Pharmacol 2016;778:152–7. [DOI] [PubMed] [Google Scholar]
- 111. Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer 2003;107:159–63. [DOI] [PubMed] [Google Scholar]
- 112. Wroblewski M, Bauer R, Cubas Cordova M, Udonta F, Ben-Batalla I, Legler K, et al. Mast cells decrease efficacy of anti-angiogenic therapy by secreting matrix-degrading granzyme B. Nat Commun 2017;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Das Roy, L, Curry JM, Sahraei M, Besmer DM, Kidiyoor A, Gruber HE, et al. Arthritis augments breast cancer metastasis: role of mast cells and SCF/c-kit signaling. Breast Cancer Res 2013;15:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol 2008;154:1572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]