Abstract
In order to explore the biochemical scope of ochratoxin A-producing penicillia, we screened 48 Penicillium verrucosum isolates for the production of secondary metabolites. Fungal metabolites were analyzed by high-pressure liquid or gas chromatography coupled to diode array detection or mass spectrometry. The following metabolites were identified: ochratoxins A and B, citrinin, verrucolones, verrucines, anacines, sclerotigenin, lumpidin, fumiquinazolines, alantrypinones, daldinin D, dipodazine, penigequinolines A and B, 2-pentanone, and 2-methyl-isoborneol. By use of average linking clustering based on binary (nonvolatile) metabolite data, the 48 isolates could be grouped into two large and clearly separated groups and a small outlying group of four non-ochratoxin-producing isolates. The largest group, containing 24 isolates, mainly originating from plant sources, included the type culture of P. verrucosum. These isolates produced ochratoxin A, verrucolones, citrinin, and verrucines and had a characteristic dark brown reverse color on yeast extract-sucrose agar medium. Almost all of a group of 20 isolates mainly originating from cheese and meat products had a pale cream reverse color on yeast extract-sucrose agar medium and produced ochratoxin A, verrucolones, anacines, and sclerotigenin. This group included the former type culture of P. nordicum. We also found that P. verrucosum isolates and three P. nordicum isolates incorporated phenylalanine into verrucine and lumpidin metabolites, a finding which could explain why those isolates produced relatively lower levels of ochratoxins than did most isolates of P. nordicum.
Ochratoxin A is an important nephrotoxic and nephrocarcinogenic mycotoxin which, despite having been reported from numerous species, is produced only by a relatively small number of species in the genera Aspergillus, Petromyces, Neopetromyces, and Penicillium (8). Black aspergilli, such as Aspergillus niger, A. carbonarius, and A. ochraceus, are important ochratoxin A (compound 1; Fig. 1)-producing species most frequently found in warmer, tropical regions of the world. More recently, the occurrence of ochratoxin A in wine and raisins due to the growth of fungi such as A. niger on grapes has gained public attention (19).
FIG. 1.
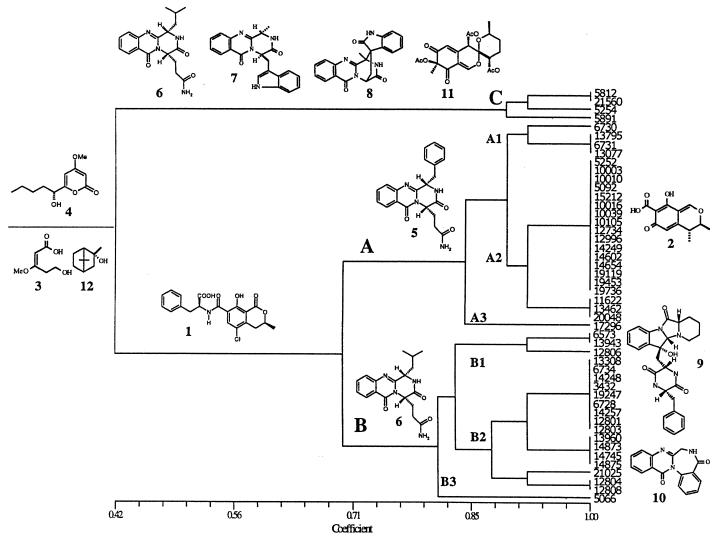
UPGMA cluster analysis based on qualitative metabolite data from the 48 isolates investigated. Included in the presentation are structures of known metabolites produced by isolates in the three clusters (A to C). Compounds: 1, ochratoxin A; 2, citrinin; 3, verrucolone; 4, PC-2; 5, verrucine A; 6, anacine; 7, fumiquinazoline F; 8, alantrypinone; 9, lumpidin; 10, sclerotigenin; 11, daldinin D; and 12, 2-methyl-isoborneol. The cluster analysis illustrates that isolates in all three clusters produced compounds 3, 4, and 12. In addition to those three metabolites, isolates in cluster A produced compounds 1, 2, and 5. Similarly, isolates in cluster B2 produced compounds 1, 6, and 10; isolates in cluster C produced compounds 6, 7, 8, and 11; and isolates in subgroup B1 produced compounds 1, 6, and 9.
Penicillium verrucosum is the most dominant ochratoxin A-producing contaminant of food and feeds in temperate regions, such as northern Europe and Canada (7). Major habitats of P. verrucosum are cereals and other plant sources; however, P. verrucosum also is found as a contaminant on food products with large amounts of proteins and fats, such as meat and cheese (9). Thus, ochratoxin A has been detected in a range of food and feeds, including cereals, bread, and beer (27, 28) and meat and cheese products (9).
The taxonomic status of ochratoxin-producing penicillia has changed often during the past century, and for years such penicillia were classified as P. viridicatum (Table 1). Ciegler et al. (2) were the first authors to describe subgroups (I to III) for P. viridicatum Westling (Table 1), using the production of ochratoxin A and other secondary metabolites, color, growth rate, and isolation source as characters for 52 isolates. Later, Frisvad and Filtenborg (5, 6) and Pitt (20, 21) also considered chemotypes within P. viridicatum or P. verrucosum, and these authors agreed that P. verrucosum is the only ochratoxin-producing species in the genus Penicillium. However, both Pitt (21) and Frisvad and Filtenborg (6) retained two chemotypes within the species, chemotype II being the only type capable of producing citrinin (compound 2; Fig. 1).
TABLE 1.
Various species concepts for P. viridicatum, P. verrucosum, P. nordicum, P. mediolanense, and P. casei
| Original species named by: | Original name for:
|
|||||
|---|---|---|---|---|---|---|
| P. viridicatum | P. verrucosum chemotype citrinin | P. verrucosum | P. nordicum | P. mediolanense | P. casei | |
| Raper and Thom (23) | P. viridicatum | P. verrucosum | P. verrucosum | |||
| Ciegler et al. (2) | P. viridicatum I | P. viridicatum II | P. viridicatum III | |||
| Pitt (20) | P. viridicatum I | P. viridicatum II | P. verrucosum | |||
| Frisvad and Filtenborg (5) | P. viridicatum I | P. viridicatum II | P. viridicatum III | P. viridicatum II | P. viridicatum III | P. viridicatum III |
| Pitt (21) | P. viridicatum I | P. verrucosum chemotype citrinin | P. verrucosum | |||
| Frisvad and Filtenborg (6) | P. viridicatum | P. verrucosum chemotype II | P. verrucosum chemotype I | P. verrucosum chemotype I | P. verrucosum chemotype I | P. verrucosum chemotype I |
| This study | P. viridicatum | P. verrucosum | P. nordicum | P. nordicum | P. nordicum | P. verrucosum |
A number of P. verrucosum metabolites, such as verrucolone (compound 3; Fig. 1) and related α-pyrones, e.g., PC-2 (compound 4; Fig. 1), have been isolated (15; L. Rahbæk, S. Sperry, J. C. Frisvad, and T. O. Larsen, submitted for publication) from both chemotypes. A major metabolite with a retention index and a UV spectrum very similar to those of anacine, a benzodiazepine metabolite first reported for P. aurantiogriseum (1), was found in P. verrucosum extracts. However, the UV spectra of the two compounds were slightly different, and recently the structures of verrucines A (compound 5; Fig. 1) and B and a revised structure of anacine (compound 6; Fig. 1) were described. All three metabolites are quinazolines rather than benzodiazepines (12). Some additional oxygenated analogs of the verrucines have also been isolated from P. verrucosum (T. O. Larsen, unpublished results). Furthermore, two other quinazolines, fumiquinazoline F (compound 7; Fig. 1) and the spirocyclic metabolite alantrypinone (compound 8; Fig. 1), have been isolated from a morphologically atypical isolate originally identified as P. verrucosum (17).
During UV-guided screening for novel diketopiperazine-producing penicillia, Larsen et al. (T. O. Larsen, B. O. Petersen, and J. Ø. Duus, submitted for publication) discovered that some isolates of P. verrucosum produced the metabolite lumpidin (compound 9; Fig. 1), with a UV spectrum very similar to that of verrucofortine (10). Furthermore, some isolates identified as P. verrucosum also produced the benzodiazepine sclerotigenin (compound 10; Fig. 1) (16). Since none of the sclerotigenin-producing isolates produced citrinin, the study suggested a sharper separation of the two chemotypes; however, only eight isolates were studied.
The objective of this study was to determine if any systematic differences in metabolite production could be established when a relatively large number of possible ochratoxin-producing Penicillium isolates were examined simultaneously.
MATERIALS AND METHODS
Fungal isolates (Table 2) were obtained from the IBT Culture Collection, BioCentrum-DTU, Technical University of Denmark. Isolates were inoculated in triplicate on CYA (20) and YES (5) and incubated at 25°C for 7 days in the dark.
TABLE 2.
Collection numbers, origin, color, and new identity of isolates studied
| IBT | Other designationc | Origin | Product | Color of reverse on YES agar | Identification in this study |
|---|---|---|---|---|---|
| 3432 | Salami | Pale cream | P. nordicum | ||
| 5066 | IJFM 7812; former type culture of P. mediolanense | Pale cream | P. nordicum | ||
| 5092 | NRRL 5571 | Light brown | P. verrucosum | ||
| 5252ab | CBS 223.71 | Canada | White bean | Violet-brown | P. verrucosum |
| 5254ab | Sudan | Sorghum | Strong yellow | Penicillium sp. | |
| 5812ab | Strong yellow | Penicillium sp. | |||
| 5891ab | Southern Europe | Thyme | Orange-yellow | Penicillium sp. | |
| 6573ab | Denmark | Lumpfish roe | Yellow-orange | P. nordicum | |
| 6728 | SP.991 | Pale yellow | P. nordicum | ||
| 6730 | NRRL 965; type culture of P. verrucosum | Belgium | Brown | P. verrucosum | |
| 6731 | CBS 302.48; former type culture of P. casei | United States | Swiss cheese | Brown | P. verrucosum |
| 6734ab | Crete | Cheese | Pale cream | P. nordicum | |
| 10003 | Denmark | Wheat | Brown | P. verrucosum | |
| 10010 | Denmark | Peanut | Brown | P. verrucosum | |
| 10016 | Denmark | Almond | Brown | P. verrucosum | |
| 10039b | Denmark | Hazelnut | Brown | P. verrucosum | |
| 10105 | Canada | Rice | Light brown | P. verrucosum | |
| 11622b | Canada | Wheat | Cream-green | P. verrucosum | |
| 12734b | Sweden | Brown | P. verrucosum | ||
| 12801 | NRRL 3711 | Canada | Ham | Pale cream | P. nordicum |
| 12803b | NRRL 6062 | Italy | Sausage | Pale cream | P. nordicum |
| 12804 | NRRL 6063 | Italy | Sausage | Pale cream | P. nordicum |
| 12806b | NRRL 1161 | Canada | Air sample from meat-packing plant | Yellow-orange | P. nordicum |
| 12808 | NRRL 5574 | Italy | Sausage | Pale cream | P. nordicum |
| 12996 | Denmark | Wheat flour | Orange-brown | P. verrucosum | |
| 13077 | Denmark | Cheese | Red-brown | P. verrucosum | |
| 13308 | ATCC 44219; former type culture of P. nordicum | Italy | Sausage | Pale cream | P. nordicum |
| 13462 | Denmark | Barley | Red-brown | P. verrucosum | |
| 13795 | Denmark | Air sample from cheese factory | Brown | P. verrucosum | |
| 13943b | Holland | Serrano ham | Yellow | P. nordicum | |
| 14248b | IMI 351304 | Pale cream | P. nordicum | ||
| 14249 | Peanut (Arachis hypogeae) | Light brown | P. verrucosum | ||
| 14257b | CBS 226.71G | Canada | Onion | Cream-green | P. nordicum |
| 14602 | Hay | Light brown | P. verrucosum | ||
| 14654 | Denmark | Potato tuber | Cream-yellow | P. verrucosum | |
| 14745 | Spain | Cheese | Cream-orange | P. nordicum | |
| 14873 | Denmark | Manchego cheese | Cream | P. nordicum | |
| 14875 | Spain | Manchego cheese | Cream-orange | P. nordicum | |
| 15212 | Denmark | Rye | Red-brown | P. verrucosum | |
| 17296 | Denmark | Air sample from industrial bakery | Red-brown | P. verrucosum | |
| 19119 | Denmark | Layer cake | Light brown | P. verrucosum | |
| 19247 | Pale cream | P. nordicum | |||
| 19453 | Denmark | Orange-brown | P. verrucosum | ||
| 19736 | Denmark | Air sample from industrial bakery | Brown | P. verrucosum | |
| 20048 | Denmark | Onions (Rocambo) | Orange-brown | P. verrucosum | |
| 21025 | Pale cream | P. nordicum | |||
| 21560 | Yellow | Penicillium sp. |
Studied by LC-MS.
Studied by GC-MS.
Sources of microorganisms are as follows: IJFM, Instituto Jaime Ferran de Microbiologia, Madrid, Spain; NRRL, Northern Regional Research Laboratory, Peoria, Ill.; CBS, Centaalbureau voor Schimmelcultures, Utrecht, The Netherlands; ATCC, American Type Culture Collection, Manassas, Va.; IMI, Commonwealth Agricultural Bureau International Mycological Institute, Kew, Great Britain; SP.991 was from Bundesanstalt für Fleichforchung, Kulmbach, Federal Republic of Germany.
Procedures for microextraction of fungal secondary metabolites and analytical high-pressure liquid chromatography conditions were similar to those described by Smedsgaard (25). Retention indices (RI) for fungal metabolites were calculated as described by Frisvad (4).
Liquid chromatography (LC)-mass spectrometry (MS) analyses (Table 2) were performed with an HP-1100 system, an HP-1100 diode array detector, and an HP-1100 LC/mass selective detector coupled in series using atmospheric pressure chemical ionization. A Hypersil BDS C18 column (4 by 100 mm) with 3-μm particles was used for separation. A linear gradient of H2O-CH3CN (both containing 500 μl of formic acid/liter) was used; the gradient was changed from 10% CH3CN to 100% over 30 min and then was maintained at 100% CH3CN for 5 min before a return to starting conditions. The flow rate was 0.5 ml/min.
Volatile metabolites from 14 isolates (Table 2) were collected by diffusive sampling and analyzed by gas chromatography (GC)-MS as described by Larsen (11).
Cluster analysis of qualitative metabolite data (0/1 data) was performed using the software package NTSYS version 2.0 (Exeter Software, Setauket, N.Y.). The input data consisted of a 48-object (fungal isolates) and a 13-variable (biosynthetic groups of secondary metabolites) (Table 3) matrix. These data were analyzed statistically by using the simple matching distance coefficient and by using average linking clustering (UPGMA) (Exeter Software). The complete data matrix can be obtained from the authors.
TABLE 3.
Biosynthetic groups for the 22 different metabolites detected in this studya
| Biosynthetic group | Secondary metabolite(s) (RI) |
|---|---|
| Anacines | Anacine (790), anacinol (840) |
| Alantrypinones | Serantrypinone (637), alantrypinone (699) |
| Verrucines | Verrucine C (782), verrucine A (790), verrucine E (840), verrucine B (862), verrucine D (876) |
| Verrucolones | Verrucolone (614), PC-2 (828) |
| Ochratoxins | Ochratoxin A (1023), ochratoxin B (940) |
| Penigequinolones | Penigequinolones A and B (both 1174) |
| Citrinin | Citrinin (854) |
| Daldinin | Daldinin D (1016) |
| Dipodazine | Dipodazine (658) |
| Fumiquinazoline | Fumiquinazoline F (897) |
| Sclerotigenin | Sclerotigenin (705) |
| Metabolite I | Metabolite I (1313) |
| Lumpidin | Lumpidin (902) |
Metabolites in each group have very similar UV spectra, indicating a close biosynthetic relationship. RI were measured as described by Frisvad (4).
RESULTS
In total, 20 known and 2 major distinct, but unknown, metabolites were detected by high-pressure liquid chromatography with a diode array detector and LC-MS analyses of fungal extracts. These metabolites could be arranged into 13 biosynthetic groups; metabolites in each group had similar UV spectra, indicating a close relationship (Table 3). All 48 isolates produced one or more verrucolones; 38 isolates produced unknown metabolite I; 36 isolates produced ochratoxin A; 23 isolates produced either all or some of the verrucines; 24 isolates produced anacines; 11 isolates produced sclerotigenin; 4 isolates all produced alantrypinone, serantrypinone (M. R. Ariza, B. O. Petersen, J. Ø. Duus, A. F. Barrero, C. Christophersen, and T. O. Larsen, submitted for publication), fumiquinazoline F, and dipodazine (26); 3 isolates produced daldinin D (compound 11; Fig. 1) (Ariza et al., submitted); 3 isolates produced lumpidin; and 1 isolate produced penigequinolones (18).
Cluster analysis of qualitative secondary metabolite production (0/1 data) for the 13 biosynthetic groups showed that the 48 isolates could be grouped into two major clusters, A and B, and one minor cluster, C (Fig. 1). The cophenetic correlation was 0.945, representing a good correlation between the dendrogram and the first similarity matrix. The 24 isolates in cluster A included the type culture (IBT 6730) of P. verrucosum and were grouped on the basis of the production of the following metabolite(s) (number of isolates in parentheses): ochratoxin A (19), ochratoxin B (9), citrinin (20), verrucolones (24), and verrucines (23). Sixteen isolates (A2; Fig. 1), including IBT 5092 previously studied by Ciegler et al. (2), Pitt (21), and Frisvad and Filtenborg (6), all produced metabolites from the five biosynthetic groups characteristic for the P. verrucosum group (Fig. 1). The type culture did not produce ochratoxin A or citrinin and was grouped (A1; Fig. 1) with IBT 13795, IBT 6731 (former type culture of P. casei), and IBT 13077. The two latter isolates were the only P. verrucosum isolates originating from cheese. IBT 17296 was separated (A3; Fig. 1) from the rest of the isolates in cluster A as the only isolate not producing verrucines. Finally, 22 out of 24 isolates in cluster A had a characteristic dark brown color on the reverse on YES agar plates (Table 2). The two nonbrown isolates, IBT 11622 and IBT 14654, both produced citrinin as well as verrucolones and verrucines. IBT 14654 also produced ochratoxin A.
The 20 isolates in cluster B (Fig. 1) were grouped on the basis of the production of several of the following metabolites (number of isolates in parentheses): ochratoxin A (17), verrucolones (19), anacines (19), metabolite I (16), sclerotigenin (11), and lumpidin (3). Included in subgroup B2 (Fig. 1) were IBT 12801 (=NRRL 3711) studied by Ciegler et al. (2); IBT 12808 (=NRRL 5574) studied by Ciegler et al. (2), Pitt (21), and Frisvad and Filtenborg (6); and the former type culture of P. nordicum (IBT 13308). The UV data indicated at least four metabolites in the anacine series. LC-MS indicated two protonated anacine isomers (m/z 343) and two possible protonated hydroxylated isomers (m/z 359), as seen for the verrucines (Larsen, unpublished results).
A subgroup (B1; Fig. 1) of three isolates (IBT 6573, IBT 12806 [=NRRL 1161], and IBT 13943) can be seen in cluster B. These isolates were grouped together since they were the only isolates producing lumpidin as a major component. The former type culture of P. mediolanense (IBT 5066) was separated (B3; Fig. 1) from the rest of the isolates in cluster B as the only isolate not producing ochratoxins and metabolite I. All isolates in subgroup B2 (Fig. 1) were light cream or dull yellow on the reverse on YES agar plates.
A small group of 4 isolates (cluster C; Fig. 1) clearly appeared as an outgroup within our set of 48 isolates. The major metabolites produced by these isolates were fumiquinazoline F and alantrypinones, together with verrucolones, anacines, and dipodazine. Three out of the four isolates produced daldinin D, while penigequinolones A and B were detected only from one isolate (IBT 5891). These four isolates had the most orange-yellow reverse on YES agar plates of all the isolates (Table 2). None of the four isolates produced ochratoxins or citrinin, as did cluster A and B isolates.
A large similarity in patterns of volatile metabolites could be seen from the GC-MS analyses (results not shown). The major volatile compounds of all the isolates were 2-pentanone and small alcohols, such as isobutanol and isopentanol. In addition, all of the isolates produced the moldy odorous monoterpene 2-methyl-isoborneol (compound 12; Fig. 1).
DISCUSSION
Ochratoxin A- and citrinin-producing isolates.
The large group of 24 isolates that clustered with the type culture of P. verrucosum (cluster A; Fig. 1) fits very well with group II isolates of Ciegler et al. (2), Pitt (21), and Frisvad and Filtenborg (6). One isolate, IBT 5092 (=NRRL 5571), was included in group II in all three studies. We found that 79% of the isolates produced ochratoxin A and 83% produced citrinin, results which parallel the findings of Ciegler et al. (2). However, we did not detect ochratoxin A or citrinin production by the P. verrucosum type culture, probably because it has been in cultures for many years. Variation from normal is to be expected, as concluded by Pitt (21), who found that the type culture did not produce citrinin. It is likely that this is the reason why Frisvad and Filtenborg (6) placed the type culture in their chemotype I rather than in their chemotype II. We found that the type culture of P. verrucosum produces verrucines, like 23 out of 24 isolates in cluster A, and verrucolones (Fig. 1), but never anacines or sclerotigenin, which are typical of the isolates grouping together with the former type culture of P. nordicum in cluster B (Fig. 1). Therefore, our results clearly place the type culture of P. verrucosum in cluster A.
Ochratoxin A- but non-citrinin-producing isolates.
The large group of 20 isolates in cluster B corresponds to the group III isolates described by Ciegler et al. (2) and Pitt (21) and to chemotype I of Frisvad and Filtenborg (6) and includes IBT 12808, which was investigated in all three studies. None of these isolates produced citrinin or verrucines unique to cluster A isolates; instead, all 20 isolates produced anacines, 11 isolates produced sclerotigenin, and 3 isolates produced lumpidin, metabolites not produced by any of the isolates in cluster A.
The present work is thus the first to demonstrate that non-citrinin-producing isolates of P. verrucosum produce metabolites not produced by citrinin-producing isolates (Fig. 1) and hence may be clearly distinguishable. Cluster B includes the former type culture of P. nordicum (IBT 13308), together with isolates originating from meat and cheese. P. nordicum was first described by Dragoni and Cantoni (3), with the Latin diagnosis required to validate the name given by Ramiréz (22).
The three isolates (IBT 6537, IBT 12806, and IBT 13943) in subgroup B1 (Fig. 1) are the only isolates producing lumpidin. In addition to lumpidin, they also produce metabolites typical of subgroup B2 isolates (verrucolones, anacines, and ochratoxin A but not sclerotigenin). These three isolates did not have the typical pale cream reverse color of P. nordicum on YES agar but instead were more yellow (Table 2). Ciegler et al. (2) also included IBT 12806 (as NRRL 1161) among their P. viridicatum III isolates, with the remarks that the isolate also had features in common with their type II isolates. Further studies are needed to establish whether lumpidin-producing isolates belong to a separate species or should remain as a chemotype within cluster B isolates.
Non-ochratoxin A-producing isolates.
The distinct cluster C (Fig. 1) represents a group of four biochemically unique isolates that produce alantrypinones and fumiquinazoline F as the major metabolites. These four isolates also could be separated from the rest of the isolates due to their pronounced orange-yellow reverse color on YES agar (Table 2). However, these isolates also produce metabolites such as the verrucolones, anacines, 2-pentanone, and 2-methyl-isoborneol, as do the other studied isolates, indicating a close relationship between cluster C isolates and cluster A and B isolates. As for subgroup B1 (Fig. 1) isolates, further studies are needed to correctly identify and classify these four cluster C isolates and to determine whether they are capable of producing ochratoxins under growth conditions other than those used here.
Production of volatile metabolites.
The very similar profiles of volatile metabolites produced by representative isolates (Table 2) of clusters A to C suggest that they are closely related, despite the differences in the production of nonvolatile metabolites. These results agree to some extent with those of Ciegler et al. (2), who also found 2-pentanone to be the major volatile compound of isolates of P. viridicatum II. Our findings thus are concordant with the results found for the series Viridicata, where closely related species such as P. aurantiogriseum, P. polonicum, P. tricolor, and P. cyclopium have been found to produce almost identical profiles of volatile sesquiterpenes (13, 14).
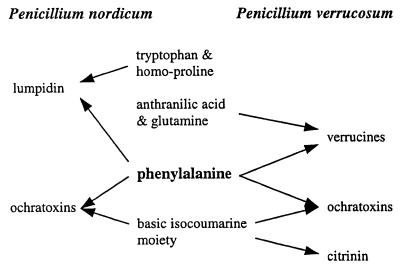
Competitive biosynthesis involving phenylalanine.
In general, isolates in the P. nordicum group produced much larger amounts of ochratoxins than did isolates in the P. verrucosum group. One reason for this difference could be that phenylalanine is used in the biosynthesis of both ochratoxins and verrucines by isolates in the P. verrucosum group but in the biosynthesis of only ochratoxins by the P. nordicum group of isolates (Fig. 2). Instead of verrucines, isolates in the P. nordicum group produce anacines, which are very similar quinazoline metabolites that incorporate leucine instead of phenylalanine.
FIG. 2.
Biosynthetic pathways leading to phenylalanine-derived metabolites. Phenylalanine can be incorporated into verrucines and ochratoxins produced by isolates that group together with the type culture of P. verrucosum (cluster A; Fig. 1) and into lumpidin produced by the three isolates in subgroup B1 (Fig. 1). The same basic isocoumarin moiety is incorporated into both ochratoxins and citrinin produced by the P. verrucosum group of isolates.
This hypothesis, competitive biosynthesis between different pathways that use phenylalanine, is supported by the fact that the three isolates that produce lumpidin (B1; Fig. 1) also are relatively poor ochratoxin producers. Another likely reason for lower ochratoxin A production by isolates in the P. verrucosum group is the incorporation of the similar basic isocoumarin moiety into both ochratoxin A and citrinin (Fig. 2).
Chemosystematic and ecological significance.
In conclusion, 44 of the 48 isolates that we examined can be separated into two large groups (A and B) of ochratoxin A-producing isolates (Fig. 1) by cluster analysis based on metabolite production. Our results are consistent with those of Ciegler et al. (2). However, they did not describe their P. viridicatum II and III as new subspecies or varieties of P. viridicatum due to the lack of distinctive morphological characteristics.
We suggest that the two large groups represent the species P. verrucosum and P. nordicum and that both species belong to the series Verrucosa, subgenus Penicillium. Despite the facts that both P. verrucosum and P. nordicum are slowly growing species, with very similar colony diameters on many media, and that both have rough stipes, they can be clearly distinguished from each other by use of the following criteria: (i) these species produce different secondary metabolite profiles (Fig. 1); (ii) under many laboratory conditions, P. nordicum isolates produce more ochratoxin than P. verrucosum isolates; (iii) P. verrucosum cultures commonly have a diagnostic dark brown reverse color on YES agar, whereas P. nordicum cultures have a pale, creamy, or dull yellow reverse color on YES agar (in the present study, two nonbrown P. verrucosum isolates both produced citrinin, a metabolite that can be easily detected by thin-layer chromatography [24]); and (iv) all meat-derived ochratoxin A-producing isolates that we examined were P. nordicum, whereas all isolates originating from plant-derived material were P. verrucosum (Table 2), suggesting that the two species usually occupy different ecological niches.
Assigning ochratoxin-producing penicillia to either P. verrucosum or P. nordicum is very important because it emphasizes that despite sharing the ability to produce ochratoxins, these groups of fungi are biosynthetically and ecologically very different. Future work should result in more detailed insight into the biotic and abiotic factors that influence and regulate the biosynthesis of ochratoxins and other secondary metabolites produced by P. verrucosum and P. nordicum. Such studies might also lead to a better understanding of the nature of the apparently different ecological origins of P. verrucosum and P. nordicum.
ACKNOWLEDGMENTS
This research was supported in part by the Advanced Center for Food Research, the Danish Dairy Foundation (Danish Dairy Board), the Danish Research and Development Program for Food Technology, and the Danish Research Councils (grant 9800555).
We thank Agilent Technologies, Birkerød, Denmark, for the use of the HP-1100 LC/mass selective detector, Hanne Jakobsen for performing the LC-MS analyses, Amira Azar and Trine Camilla Rasted for processing the cultures, and Jens C. Frisvad and Ulf Thrane for fruitful discussions.
REFERENCES
- 1.Boyes-Korkis J M, Guerney K A, Penn J, Mantle P G, Bilton J N, Sheppard R N. Anacine, a new benzodiazepine metabolite of Penicillium aurantiogriseum produced with other alkaloids in submerged fermentation. J Nat Prod. 1993;56:1707–1717. [Google Scholar]
- 2.Ciegler A, Fennell D I, Sansing G A, Detroy R W, Bennet G A. Mycotoxin-producing strains of Penicillium viridicatum: classification into subgroups. Appl Microbiol. 1973;26:271–278. doi: 10.1128/am.26.3.271-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragoni I, Cantoni C. Le muffe negli insaccati crudi stagionati. Ind Aliment. 1979;19:281–284. [Google Scholar]
- 4.Frisvad J C. High-performance liquid chromatography determination of profiles of mycotoxins and other secondary metabolites. J Chromatogr. 1987;392:333–347. doi: 10.1016/s0021-9673(01)94277-3. [DOI] [PubMed] [Google Scholar]
- 5.Frisvad J C, Filtenborg O. Classification of terverticillate penicillia based on profiles of mycotoxins and other secondary metabolites. Appl Environ Microbiol. 1983;46:1301–1310. doi: 10.1128/aem.46.6.1301-1310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisvad J C, Filtenborg O. Terverticillate penicillia: chemotaxonomy and mycotoxin production. Mycologia. 1989;81:837–861. [Google Scholar]
- 7.Frisvad J C, Filtenborg O, Lund F, Samson R A. The homogeneous species and series in subgenus Penicillium are related to mammal nutrition and excretion. In: Samson R A, Pitt J I, editors. Integration of modern taxonomic methods for Aspergillus and Penicillium classification. Reading, United Kingdom: Harwood Academic Publishers; 1999. pp. 259–277. [Google Scholar]
- 8.Frisvad J C, Samson R A. Neopetromyces gen. nov. and an overview of teleomorphs of Aspergillus subgenus Circumdati. Stud Mycol (Baarn) 2000;45:201–207. [Google Scholar]
- 9.Gareis M, Scheuer R. Ochratoxin A in meat and meat products. Arch Lebensmittelhyg. 2000;51:102–103. [Google Scholar]
- 10.Hodge R P, Harris C M, Harris T M. Verrucofortine, a major metabolite of Penicillium verrucosum var. cyclopium. J Nat Prod. 1988;55:66–73. doi: 10.1021/np50055a008. [DOI] [PubMed] [Google Scholar]
- 11.Larsen T O. Volatile flavor production by Penicillium caseifulvum. Int Dairy J. 1998;8:883–887. [Google Scholar]
- 12.Larsen T O, Franzyk H, Jensen S R. UV-guided isolation of verrucines A and B, novel quinazolines from Penicillium verrucosum structurally related to anacine from Penicillium aurantiogriseum. J Nat Prod. 1999;62:1578–1580. doi: 10.1021/np990251p. [DOI] [PubMed] [Google Scholar]
- 13.Larsen T O, Frisvad J C. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol Res. 1995;99:1153–1166. [Google Scholar]
- 14.Larsen T O, Frisvad J C. Chemosystematics of Penicillium based on profiles of volatile metabolites. Mycol Res. 1995;99:1167–1174. [Google Scholar]
- 15.Larsen T O, Frisvad J C, Christophersen C. Arabenoic acid (verrucolone), a major chemical indicator of Penicillium verrucosum. Biochem Syst Ecol. 1998;26:463–465. [Google Scholar]
- 16.Larsen T O, Frydenvang K, Frisvad J C. UV-guided screening for benzodiazepine producing species in Penicillium. Biochem Syst Ecol. 2000;28:881–886. doi: 10.1016/s0305-1978(99)00126-x. [DOI] [PubMed] [Google Scholar]
- 17.Larsen T O, Frydenvang K, Frisvad J C, Christophersen C. UV-guided isolation of alantrypinone, a novel Penicillium alkaloid. J Nat Prod. 1998;61:1154–1157. doi: 10.1021/np980056v. [DOI] [PubMed] [Google Scholar]
- 18.Larsen T O, Smedsgaard J, Frisvad J C, Anthoni U, Christophersen C. Consistent production of penigequinolone A and B by Penicillium scabrosum. Biochem Syst Ecol. 1999;27:329–332. [Google Scholar]
- 19.Majerus P, Bresch H, Otteneder H. Ochratoxin A in wines, fruit juices and seasonings. Arch Lebensmittelhyg. 2000;51:95–97. [Google Scholar]
- 20.Pitt J I. The genus Penicillium and its teleomorphic states Eupenicillium and Taleromyces. London, United Kingdom: Academic Press, Inc.; 1979. [Google Scholar]
- 21.Pitt J I. Penicillium viridicatum, Penicillium verrucosum, and production of ochratoxin A. Appl Environ Microbiol. 1987;53:266–269. doi: 10.1128/aem.53.2.266-269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramírez C. Revision of recently described Penicillium taxa. In: Samson R A, Pitt J I, editors. Advances in Penicillium and Aspergillus systematics. New York, N.Y: Plenum Press; 1985. pp. 135–142. [Google Scholar]
- 23.Raper K B, Thom C. A manual of the penicillia. Baltimore, Md: Williams & Wilkins; 1949. [Google Scholar]
- 24.Samson R A, Hoekstra E S, Lund F, Filtenborg O, Frisvad J C. Methods for the detection, isolation and characterisation of food-borne fungi. In: Samson R A, Hoekstra E S, Frisvad J C, Filtenborg O, editors. Introduction to food- and airborne fungi. Utrecht, The Netherlands: Centraalbureau voor Schimmelcultures; 2000. pp. 283–297. [Google Scholar]
- 25.Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J Chromatogr A. 1997;760:264–270. doi: 10.1016/s0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen D, Larsen T O, Christophersen C, Nielsen P H, Anthoni U. Dipodazine, a diketopiperazine from Penicillium dipodomyis. Phytochemistry. 1999;51:1181–1183. [Google Scholar]
- 27.Visconti A, Pascale M, Centonze G. Determination of ochratoxin A in domestic and imported beers in Italy by immunoaffinity clean-up and liquid chromatography. J Chromatogr A. 2000;888:321–326. doi: 10.1016/s0021-9673(00)00549-5. [DOI] [PubMed] [Google Scholar]
- 28.Wolff J. Ochratoxin A in cereals and cereal products. Arch Lebensmittelhyg. 2000;51:85–88. [Google Scholar]