Abstract
Purpose:
Lurbinectedin suppresses the oncogenic transcription factor EWS-FLI1 through relocalization to the nucleolus, and delays tumor growth in mice bearing Ewing sarcoma xenografts. On the basis of this rationale, lurbinectedin was evaluated in patients with relapsed Ewing sarcoma.
Patients and Methods:
This open-label, single-arm, Basket phase II trial included a cohort of 28 treated adult patients with confirmed Ewing sarcoma, measurable disease as per Response Evaluation Criteria In Solid Tumors (RECIST) v.1.1, Eastern Cooperative Oncology Group performance status ≤2, adequate organ function, no central nervous system metastasis, and pretreated with ≤2 chemotherapy lines for metastatic/recurrent disease. Patients received lurbinectedin 3.2 mg/m2 as a 1-hour infusion every 3 weeks. Primary endpoint was overall response rate (ORR) as per RECIST v.1.1. Secondary endpoints included time-to-event parameters and safety profile.
Results:
ORR was 14.3% [95% confidence interval (CI), 4.0%–32.7%], with median duration of response of 4.2 months (95% CI, 2.9–5.5 months). Median progression-free survival was 2.7 months (95% CI, 1.4–4.3 months), clinical benefit rate was 39.3%, and disease control rate was 57.1%. With 39% censoring, median overall survival was 12.0 months (95% CI, 8.5–18.5 months). Most common grade 3/4 adverse events were neutropenia (57%), anemia, thrombocytopenia, and treatment-related febrile neutropenia (14% each). No deaths or discontinuations were due to toxicity.
Conclusions:
Lurbinectedin was active in the treatment of relapsed Ewing sarcoma and had a manageable safety profile. Lurbinectedin could represent a valuable addition to therapies for Ewing sarcoma, and is currently being evaluated in combination with irinotecan in advanced Ewing sarcoma in a phase Ib/II trial.
Translational Relevance.
Novel therapeutic agents are needed for patients with relapsed/refractory Ewing sarcoma who have a dismal prognosis. Lurbinectedin blocks transcription and induces DNA double-strand breaks, leading to apoptosis. In preclinical models it was shown that lurbinectedin is effective in suppressing the activity of the oncogenic transcription factor EWS-FLI1 through relocalization to the nucleolus and delayed tumor growth in mice bearing Ewing sarcoma xenografts. This Basket clinical trial demonstrated clinical antitumor activity of lurbinectedin with an objective response rate of 14.3%, clinical benefit rate (response or disease stabilization for ≥4 months) of 39.3%, and disease control rate (response or disease stabilization of any duration) of 57.1% in a cohort of patients with relapsed Ewing sarcoma. Lurbinectedin could represent a valuable addition to relapsed Ewing sarcoma, which constitutes a highly unmet medical need.
Introduction
Ewing sarcoma, formerly referred to as the Ewing family of tumors (EFT), is an aggressive form of sarcoma that comprises malignancies such as classic Ewing sarcoma, peripheral neuroectodermic tumors (PNET), and Askin tumor. Ewing sarcoma is the second most common malignant bone tumor among children, adolescents, and young adults, striking them in the prime of their lives (1). Ewing sarcoma may also appear in soft tissue, with the most common sites being trunk and limbs (2). The average incidence of Ewing sarcoma is 2.93 cases per million per year (3), and most patients are under the age of 20 years.
The prognosis of Ewing sarcoma varies depending on primary tumor site, presence of metastases, and tumor size. First-line treatment with surgery, radiotherapy, and multi-agent chemotherapy has resulted in 5-year disease-free survival rates of 60% to 70% in patients with localized sarcoma, but less than 20% if metastases are present at diagnosis (4), with an inferior outcome being observed in patients younger than 18 years (5, 6). There is no established treatment for relapsed Ewing sarcoma. Management of relapsed disease mostly consists of different combinations of the same agents used as prior therapy (e.g., alkylators or topoisomerase inhibitors), radiotherapy of lung and bone, and surgical removal of metastases. However, failure on second-line therapy is very common, and the agents used are associated with short- and long-term toxicity (7, 8). Patients with relapsed Ewing sarcoma have a dismal prognosis, with a 5-year survival rate of 13% (9). Development of therapeutics in Ewing sarcoma is challenging due to its rarity and the absence of classic kinase targets (10). Therefore, there is an urgent need for new therapeutic agents with different mechanisms of action to manage this patient population.
Lurbinectedin (Zepzelca) is a synthetic tetrahydroisoquinoline alkaloid structurally related to trabectedin that inhibits oncogenic transcription primarily through binding to guanine-rich DNA sequences around gene promoters, thereby altering the 3D DNA structure and evicting oncogenic transcription factors from their binding sites (11–13). Lurbinectedin adducts may also inhibit mRNA synthesis and induce the ubiquitination and degradation of RNA polymerase II (14), and favor the production of DNA double-strand breaks and trigger apoptotic cell death (15). Lurbinectedin has received FDA accelerated approval for treatment of patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy (16). A previous phase II study had shown efficacy for trabectedin in pretreated patients with advanced Ewing sarcoma, including 3 partial responses (PR) and 7 disease stabilizations in a cohort of 20 patients; progression-free survival (PFS) rate at 6 months was 25% (17). Preclinical studies showed that lurbinectedin is more effective than trabectedin in suppressing the activity of the oncogenic transcription factor EWS-FLI1 in mice through relocalization to the nucleolus (18, 19). In vivo, administration of lurbinectedin delayed tumor growth in mice bearing Ewing sarcoma xenografts (19). Lurbinectedin also showed an improved therapeutic index relative to trabectedin, with suppression of EWS-FLI1 activity observed in mice at clinically achievable concentrations (19). Compared with trabectedin, lurbinectedin had a more favorable pharmacokinetic profile, as suggested by a higher recommended dose (RD) and greater exposure values at the RD when administered as single agent every 3 weeks (20, 21).
This study evaluated the monotherapy activity of lurbinectedin in terms of response rate, PFS, clinical benefit rate (CBR), and disease control rate (DCR) in a cohort of patients with relapsed Ewing sarcoma.
Patients and Methods
This single-arm, open-label, Basket phase II trial evaluated the efficacy and safety of lurbinectedin in 9 cohorts of patients with difficult-to-treat tumors. This report is focused on the cohort of patients with Ewing sarcoma (labelled as EFTs in the study protocol) treated at 11 sites in Belgium, France, Italy, Spain, and the U.S. The trial was conducted in compliance with ICH Good Clinical Practice guidelines. The protocol was approved by the centers’ Research Ethics Committees. Signed written informed consent was obtained for each patient before study-specific procedures. The trial is registered at https://www.clinicaltrials.gov as NCT02454972.
Eligibility criteria
Eligible patients were aged ≥18 years, with Ewing sarcoma previously treated with ≤2 chemotherapy lines in the metastatic/recurrent setting, measurable disease according to Response Evaluation Criteria In Solid Tumors (RECIST) v.1.1 (22) and documented disease progression, Eastern Cooperative Oncology Group (ECOG) performance status score ≤2, and adequate bone marrow, hepatic, renal, and metabolic function who had recovered from any previous toxicities.
Patients were excluded if they had been pretreated with lurbinectedin or trabectedin, had prior/concurrent malignant disease (unless in complete remission for >5 years), had impending need for radiotherapy, were pregnant or lactating women or women of childbearing potential who were not using effective contraceptives, or had central venous system involvement, relevant cardiac disease, severe dyspnea or daily intermittent oxygen requirement, active infection, unhealed wounds, external drainages, immunocompromise (including human immunodeficiency virus infection), or limited ability to comply with treatment or follow-up.
Study treatment
All patients were given lurbinectedin 3.2 mg/m2 as a 1-hour intravenous infusion once every 3 weeks. Treatment delays and dose reductions were allowed to manage toxicity at the investigator's discretion. Treatment was administered until disease progression, unacceptable toxicity, treatment delay >3 weeks (except if clear clinical benefit), requirement of >2 dose reductions, intercurrent illness precluding study continuation, and patient refusal and/or noncompliance with study requirements. Standard antiemetic prophylaxis was administered before each lurbinectedin infusion. Only secondary prophylaxis with granulocyte colony-stimulating factor (G-CSF) was allowed.
Study assessments
Antitumor activity was evaluated in patients who had at least one complete infusion of lurbinectedin, and who either had at least one tumor assessment (as per RECIST v.1.1) or were considered treatment failures (i.e., discontinued treatment due to toxicity/clinical disease progression or died due to the disease before the first tumor assessment). Radiologic tumor assessments (CT scans or MRI) were conducted every 6 weeks until Cycle 6, and every 9 weeks thereafter. Any patients showing a response had to have a confirmatory assessment using the same technique at least 4 weeks later.
Safety was evaluated in all patients who received at least one lurbinectedin infusion through the assessment of adverse events (AE), laboratory tests, physical examination, and vital signs. Laboratory tests were conducted weekly during Cycles 1 and 2, and on Day 1 of subsequent cycles. Safety was monitored throughout treatment and up to 30 days after the last lurbinectedin infusion, start of a new antitumor therapy, or death, whichever occurred first. Any lurbinectedin-related AE was followed until recovery. AEs and laboratory abnormalities were graded with the NCI Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.4 (23), and coded using the Medical Dictionary for Regulatory Activities v.21.0.
Endpoints
All study endpoints were assessed by the investigators. The primary endpoint was the antitumor activity of lurbinectedin in terms of overall response rate [ORR; percentage of patients with complete response (CR) or PR as per RECIST v.1.1]. Secondary endpoints were duration of response (DoR; time from the date of first response to the date of first disease progression or death from any cause in patients with response), CBR (percentage of patients with response or disease stabilization for ≥4 months), DCR (percentage of patients with response or disease stabilization of any duration), PFS (time from the date of first infusion to the date of disease progression, death from any cause, or last tumor evaluation), PFS at 4 and 6 months, overall survival (OS; time from the date of first infusion to the date of death or loss to follow-up), OS at 6 and 12 months, and pharmacogenomics and safety profile of lurbinectedin.
Statistical analysis
Up to 25 evaluable patients were to be enrolled to test the null hypothesis that 1% or fewer patients would achieve a response to lurbinectedin (P ≤ 0.01) versus the alternative hypothesis that 10% or more patients would achieve a response to lurbinectedin (P ≥ 0.10). The variance of the standardized test was based on the null hypothesis. The type I error (alpha) associated with this one-sided test was 0.025 and the type II error (beta) was 0.2; thus, statistical power was 80%. With these assumptions, the null hypothesis could be rejected if the number of patients who achieved a confirmed response was ≥2.
Frequency tables were prepared for categorical variables. Continuous variables were described using summary tables with the median, mean, standard deviation, minimum, and maximum for each variable. Noncontinuous variables were described using frequency tables with counts and percentages. Binomial exact estimates and 95% confidence intervals (CI) were used to evaluate the primary endpoint (ORR), CBR, and DCR. The Kaplan–Meier method was used to evaluate time-to-event endpoints. For DoR and PFS, patients who did not progress or die by data cutoff were censored at the date of their final tumor evaluation. For OS, patients who were still alive were censored at data cutoff. SAS v.9.4 was used for all statistical analyses.
Data sharing statement
Individual participant data are not publicly available because this requirement was not anticipated in the study protocol considering that this trial started patient enrollment in 2015. Clinical trial summary results were placed at ClinicalTrials.gov (https://www.clinicaltrials.gov).
Results
Characteristics of patients and treatment
A total of 29 patients with Ewing sarcoma were enrolled into the study between August 25, 2015 and November 16, 2020. Of these, 28 patients were treated with lurbinectedin and were evaluable for both safety and efficacy.
Baseline characteristics of these 28 treated patients are summarized in Table 1. Most patients (57%) were male, with median age 33 years (range, 18–74 years). Ewing sarcoma were mostly extraosseous (58%; PNET in 50%); the other 42% were osseous. Ten patients (36%) had ≥3 metastatic sites, with the most common sites being lung, bone, and pleura. All patients had received previous systemic therapy, with a median of 2 lines (range, 1–5 lines) each. The most common prior anticancer agents were vincristine (93%), doxorubicin, ifosfamide (89% each), cyclophosphamide (79%), etoposide (71%), irinotecan (61%), and temozolomide (50%).
Table 1.
Baseline characteristics of patients with Ewing sarcoma.
| All treated patients | |
|---|---|
| (n = 28) | |
| Gender | |
| Male | 16 (57%) |
| Female | 12 (43%) |
| Median age, years (range) | 33 (18–74) |
| ECOG performance status | |
| 0 | 11 (39%) |
| 1 | 16 (57%) |
| 2 | 1 (4%) |
| Median BSA, m2 (range) | 1.9 (1.6–2.4) |
| Abnormal LDH (>ULN) | 10 (36%) |
| Disease stage at diagnosis | |
| Early | 14 (50%) |
| Locally advanced | 5 (18%) |
| Metastatic | 9 (32%) |
| Ewing sarcoma anatomical subtypea | |
| Extraosseous | 15 (58%) |
| Osseous | 11 (42%) |
| Median number of tumor sites at baseline (range) | 2 (1–6) |
| ≥3 sites | 10 (36%) |
| Most common sites of disease at baseline | |
| Lung | 18 (64%) |
| Bone | 16 (57%) |
| Pleura | 9 (32%) |
| Lymph nodes | 6 (21%) |
| Skin | 5 (18%) |
| Prior surgery | 20 (71%) |
| Prior radiotherapy | 20 (71%) |
| Median number of prior systemic therapy lines (range)b | 2 (1–5) |
| Setting of prior systemic therapy | |
| Neoadjuvant | 9 (32%) |
| Adjuvant | 10 (36%) |
| Neoadjuvant + adjuvant | 2 (7%) |
| Advanced | 23 (82%) |
| Prior anticancer agents | |
| Vincristine | 26 (93%) |
| Doxorubicin | 25 (89%) |
| Ifosfamide | 25 (89%) |
| Cyclophosphamide | 22 (79%) |
| Etoposide | 20 (71%) |
| Irinotecan | 17 (61%) |
| Temozolomide | 14 (50%) |
Note: Data are n (%) of patients or median (range).
Abbreviations: BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN; upper limit of normal.
aMissing data for 2 patients.
bAll but one of these lines were chemotherapy-containing lines.
A total of 135 treatment cycles were administered, for a median of 4 cycles (range, 1–14 cycles) per patient. Eleven patients (39.3%) received ≥6 cycles each. Median relative dose intensity was 97.7% (range, 69.7%–104.5%). Treatment-related AEs resulted in dose administration delays in 7 patients (29%) and dose reduction in 6 patients (25%); all delays and reductions were due to hematologic toxicity (mostly afebrile neutropenia).
Efficacy
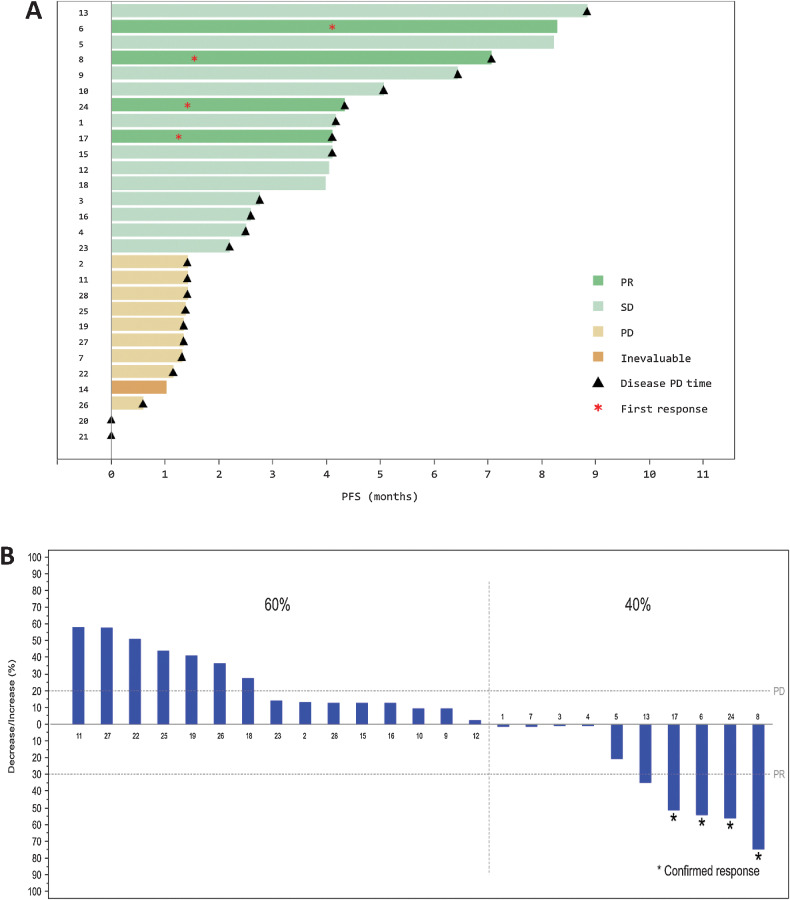
Median follow-up was 8.3 months (95% CI, 4.0 months—upper limit not reached). PR was observed in 4 patients with extraosseous (n = 2) and osseous (n = 2) Ewing sarcoma (ORR = 14.3%; 95% CI, 4.0%–32.7%; Table 2 and Table 3). Median DoR was 4.2 months (95% CI, 2.9–5.5 months). Disease stabilization was observed in 12 patients (43%), which lasted ≥4 months in 7 of them (25%; Table 3). Hence, CBR was 39.3% (95% CI, 21.5%–59.4%) and DCR was 57.1% (95% CI, 37.2%–75.5%). Median PFS was 2.7 months (95% CI, 1.4–4.3 months; Fig. 1). With a censoring of 39% (11 of 28 patients alive), median OS was 12.0 months (95% CI, 8.5–18.5 months; Table 2).
Table 2.
Overall efficacy of lurbinectedin treatment in patients with Ewing sarcoma.
| All treated patients | ||
|---|---|---|
| (n = 28) | ||
| Response by RECIST | ||
| CR | — | |
| PR | 4 (14%) | |
| SD | 12 (43%) | |
| ≥4 monthsa | 7 (25%) | |
| <4 months | 5 (18%) | |
| PD | 9 (32%) | |
| Not evaluableb | 3 (11%) | |
| ORR (%) (95% CI) | 14.3% (4.0%–32.7%) | |
| CBR (%) (95% CI)c | 39.3% (21.5%–59.4%) | |
| DCR (%) (95% CI)d | 57.1% (37.2%–75.5%) | |
| DoR | ||
| Events, n/N (%) | 3/4 (75%)e | |
| Median DoR, months (95% CI) | 4.2 (2.9–5.5) | |
| Patients still responding at 4 months (95% CI) | 50.0% (1.0%–99.0%) | |
| PFS | ||
| Events, n/N (%) | 22/28 (79%) | |
| Median PFS, months (95% CI) | 2.7 (1.4–4.3) | |
| 4-month PFS (95% CI) | 46.2% (27.0%–65.3%) | |
| 6-month PFS (95% CI) | 23.1% (5.9%–40.3%) | |
| Overall survival | ||
| Events, n/N (%) | 17/28 (61%) | |
| Median OS, months (95% CI) | 12.0 (8.5–18.5) | |
| 6-month OS (95% CI) | 88.2% (75.7%–100.8%) | |
| 12-month OS (95% CI) | 48.5% (27.8%–69.2%) | |
Abbreviations: CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; DoR, duration of response; ORR, overall response rate; OS, overall survival; PD, disease progression; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease.
aIncludes 1 patient who had an unconfirmed PR.
bThree patients were not evaluable because they had no radiologic assessments during treatment, either due to symptomatic deterioration caused by disease progression (n = 2) or early death from malignant disease (n = 1).
cPR or stable disease for ≥4 months.
dPR or stable disease.
eOne patient with confirmed PR discontinued treatment after showing clinical deterioration following an episode of disease-related cognitive disorder; this was a decision by the Investigator. No radiologic disease progression was observed at the time of discontinuation, and hence the patient was censored for DoR assessment.
Table 3.
Characteristics of patients with clinical benefit (confirmed response or disease stabilization for ≥4 months).
| Baseline characteristics | Study treatment characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years)/gender/ECOG PS | Location sites | Disease subtype | No. of prior lines | Last therapy/Best response | TTP to last prior therapy (mo) | Cycles received | Sum of target lesions at baseline (mm) | Best response | DoR (mo) | PFS (mo) | OS (mo) |
| 30/M/0 | Lung Pleura |
Extra-osseous | 2 | Vincristine, dactinomycin, cyclophosphamide, ifosfamide, and etoposide/SD | 16.6 | 6 | 43 | SD≥4 | — | 4.2 | 38.1+ |
| 58/M/0 | Lung Pleura Bone |
Extra-osseous | 3 | Irinotecan, temozolomide/UK | 7.8 | 14 | 29 | SD≥4 | — | 8.2+ | 9.9+ |
| 37/M/0 | Lung Lymph nodes Bone |
Extra-osseous | 2 | Vincristine, irinotecan, temozolomide/UK | 3.2 | 14 | 35 | PR (54% reduction) | 4.2+ | 8.3+ | 9.8+ |
| 22/M/1 | Skin Subcutaneous tissue Bone |
Osseous | 2 | Vincristine, irinotecan, temozolomide/UK | 6.4 | 9 | 71 | PR (75% reduction) | 5.5 | 7.1 | 13.4 |
| 24/F/0 | Skin Subcutaneous tissue | Osseous | 2 | Vincristine, irinotecan, temozolomide/NA | 22.2 | 9 | 32 | SD≥4 | — | 6.4 | 26.7+ |
| 30/F/0 | Lung Bone |
Extra-osseous | 2 | Vincristine, irinotecan/SD | 4.8 | 6 | 53 | SD≥4 | — | 5.1 | 14.9 |
| 74/M/1 | Lung Lymph nodes Pleura |
Extra-osseous | 2 | Vincristine, irinotecan, temozolomide/SD | 2.6 | 5 | 82 | SD≥4 | — | 4.0+ | 18.5 |
| 37/F/0 | Bone | Osseous | 2 | Cyclophosphamide, topotecan/CR | 16.7 | 12 | 43 | SD≥4 | — | 8.8 | 20.1+ |
| 30/F/1 | Lymph nodes | Extra-osseous | 2 | Irinotecan, temozolomide/SD | 24.7 | 6 | 32 | SD≥4 | — | 4.1 | 9.3 |
| 49/M/1 | Lymph nodes Pleura Pericardial effusion |
Extra-osseous | 2 | Irinotecan, temozolomide/PD | 2.4 | 6 | 85 | PR (52% reduction) | 2.9 | 4.1 | 12.0 |
| 54/M/1 | Lung Lymph nodes Pleura |
Osseous | 1 | Vincristine, dactinomycin, ifosfamide/SD | 8.4 | 6 | 76 | PR (57% reduction) | 2.9 | 4.3 | 19.1 |
Abbreviations: CR, complete response; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; F, female; M, male; mo, months; NA, not available; OS, overall survival; PD, disease progression; PFS, progression-free survival; PR, partial response; PS, performance status; SD; stable disease; TTP, time to progression; UK, unknown.
Figure 1.
A, PFS. Each numbered bar represents a patient with Ewing sarcoma treated with lurbinectedin (n = 28). The times when each patient experienced disease progression as per RECIST, and response to treatment, are shown with triangles and asterisks, respectively. B, Maximum variation of target lesions in patients with measurable disease and at least one radiologic tumor assessment. Each patient is identified using the same number as in A. PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease.
Ten patients (40%) showed objective tumor shrinkage in target lesions: 7 patients with extraosseous Ewing sarcoma, and 3 with osseous Ewing sarcoma (Fig. 1 and Fig. 2).
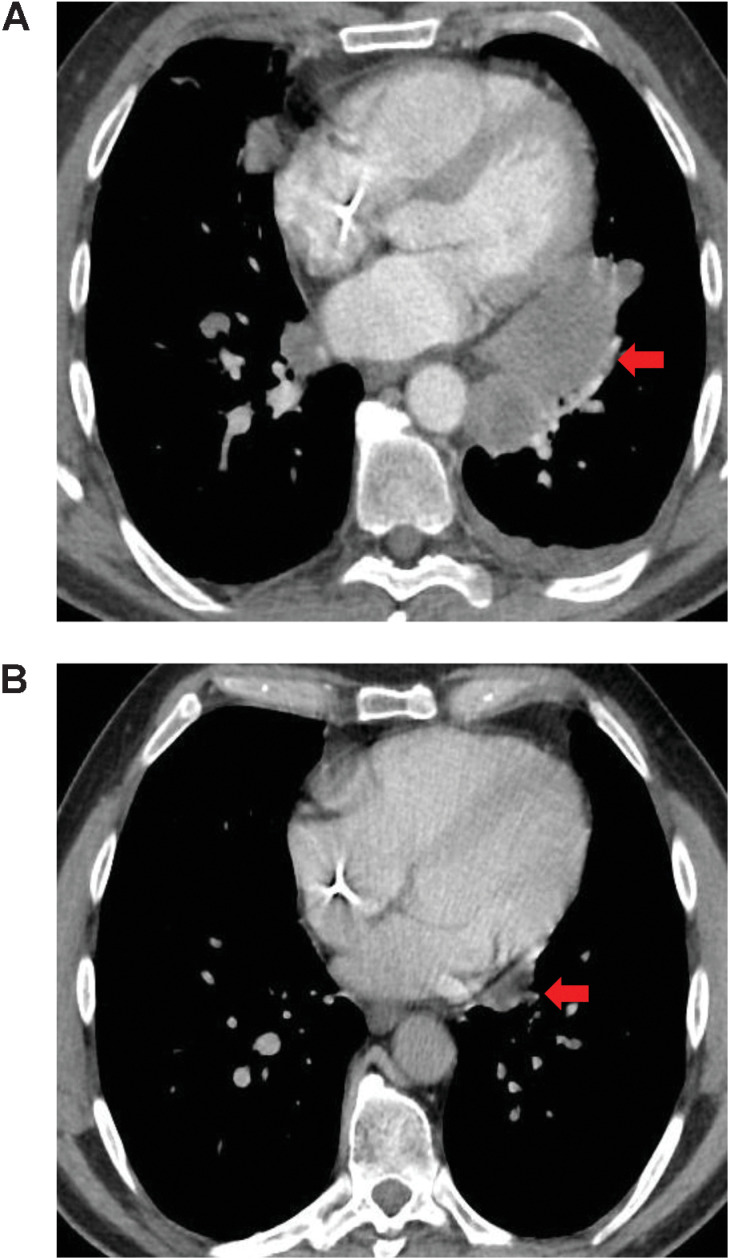
Figure 2.
A, Tumor shrinkage observed in a 54-year-old male with osseous Ewing sarcoma who achieved PR with lurbinectedin. The patient had been pretreated with two lines of chemotherapy for advanced disease. Three target lesions were present at baseline: two in the lung and one in a right hilar lymph node. B, After two cycles of treatment with lurbinectedin all target lesions were smaller, with the sum of longest diameters decreasing from 76 to 35 mm (i.e., a tumor shrinkage of 53.9%). In both images, the red arrow shows the location of the lesions. PR, partial response.
After discontinuing lurbinectedin, 19 patients (67.9%) received further antitumor therapy (the most common drugs received were cyclophosphamide, gemcitabine, and ifosfamide). Response to first subsequent therapy was observed in 2 patients (10.5%), neither of whom had shown response to lurbinectedin.
Safety
All 28 treated patients were evaluable for safety (Table 4). Most treatment-related AEs and laboratory abnormalities regardless of relationship were grade 1 or 2. The most common grade 3/4 AEs and abnormalities were hematologic disorders, including neutropenia (57% of patients; grade 4 in 43%), leukopenia (46%; grade 4 in 11%), thrombocytopenia (14%; grade 4 in 4%), grade 3 anemia (14%), and treatment-related febrile neutropenia (14%; grade 4 in 4%). Eleven patients (39.3%) required G-CSF support as secondary prophylaxis or treatment for neutropenia, 3 patients (10.7%) received red blood cell transfusions, and 1 patient (3.6%) received platelet transfusions. No treatment discontinuations or deaths were due to toxicity. Twenty-three patients (82%) discontinued lurbinectedin treatment due to disease progression as per RECIST v.1.1; the other five treatment discontinuations were due to the patient's decision to start another therapy (n = 2), multiple cycle delays caused by the study disease or other illnesses (n = 1), clinical decline unrelated to treatment (n = 1), or death caused by disease progression (n = 1).
Table 4.
Laboratory abnormalities and treatment-related AEs in patients with Ewing sarcoma treated with lurbinectedin.
| All treated patients | ||||
|---|---|---|---|---|
| (n = 28) | ||||
| NCI-CTCAE grade | 1–2 | 3 | 4 | Total |
| Hematologic laboratory abnormalities | ||||
| Anemia | 20 (71%) | 4 (14%) | — | 24 (86%) |
| Leukopenia | 11 (39%) | 10 (36%) | 3 (11%) | 24 (86%) |
| Neutropenia | 3 (11%) | 4 (14%) | 12 (43%) | 19 (68%) |
| Thrombocytopenia | 12 (43%) | 3 (11%) | 1 (4%) | 16 (57%) |
| Biochemical laboratory abnormalities | ||||
| Creatinine increased | 23 (82%) | 1 (4%) | — | 24 (86%) |
| ALT increased | 19 (68%) | 2 (7%) | — | 21 (75%) |
| GGT increased | 17 (61%) | — | — | 17 (61%) |
| AST increased | 15 (54%) | — | — | 15 (54%) |
| AP increased | 14 (50%) | — | — | 14 (50%) |
| Bilirubin increased | 3 (11%) | — | — | 3 (11%) |
| CPK increased | 2 (7%) | 1 (4%) | — | 3 (11%) |
| Treatment-related AEs | ||||
| Fatigue | 11 (39%) | — | — | 11 (39%) |
| Nausea | 8 (29%) | — | — | 8 (29%) |
| Decreased appetite | 4 (14%) | — | — | 4 (14%) |
| Febrile neutropenia | — | 3 (11%) | 1 (4%) | 4 (14%) |
| Diarrhea | 3 (11%) | — | — | 3 (11%) |
| Headache | 3 (11%) | — | — | 3 (11%) |
| Peripheral neuropathy | 3 (11%) | — | — | 3 (11%) |
| Gastroesophageal reflux disease | 2 (7%) | — | — | 2 (7%) |
| Arthralgia | 1 (4%) | — | — | 1 (4%) |
| Constipation | 1 (4%) | — | — | 1 (4%) |
| Pyrexia | 1 (4%) | — | — | 1 (4%) |
| Upper respiratory tract infection | 1 (4%) | — | — | 1 (4%) |
| Vomiting | 1 (4%) | — | — | 1 (4%) |
Note: Data are n (%) of patients. Hematologic and biochemical abnormalities are shown regardless of relationship to treatment.
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; GGT, gamma-glutamyltransferase; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Discussion
A total of 28 patients with Ewing sarcoma pretreated with a median of 2 lines of systemic therapy each were treated with lurbinectedin in this Basket phase II trial. Confirmed response assessed by Investigators was observed in 4 patients (ORR = 14.3%), with a median DoR of 4.2 months. Furthermore, 39.3% of patients had clinical benefit (response or disease stabilization for ≥4 months) and 57.1% showed disease control (response or disease stabilization of any duration). Of note, 5 patients (18%) had disease stabilization for ≥6 months, including 2 patients with ongoing stabilization at 8.2+ and 8.3+ months at the time of study termination. The number of patients with confirmed response assessed by Investigators was higher than the statistical boundary of ≥2 responses defined per protocol. Therefore, lurbinectedin at a dose of 3.2 mg/m2 given as a 1-hour intravenous infusion every 3 weeks was active in relapsed Ewing sarcoma.
Management of patients with metastatic or treatment refractory Ewing sarcoma is far from established, because robust evidence is lacking. The different polychemotherapy regimens currently used are based on small studies, and are largely dependent on institutional preferences. Patients with relapsed Ewing sarcoma are usually treated with high-dose chemotherapy combinations such as cyclophosphamide and topotecan, or irinotecan and temozolomide with or without vincristine (24–29). Agents used in these regimens have shown little activity against relapsed Ewing sarcoma when given as monotherapies, but synergistic activity when given as combination therapies. For instance, the response rate reported for topotecan in recurrent and refractory Ewing sarcoma increased from 7% as single agent (24, 30) to 32%–35% when combined with cyclophosphamide (24, 26). The current study had the limitation of not including patients aged <18 years, a population with a high incidence of Ewing sarcoma. Nevertheless, the response rate of 14.3% observed herein for single-agent lurbinectedin warrants further development of the drug in the treatment of relapsed Ewing sarcoma. Combination of lurbinectedin with irinotecan or temozolomide might improve the antitumor activity of single-agent lurbinectedin in relapsed Ewing sarcoma. An ongoing phase Ib/II trial (NCT02611024) is currently evaluating lurbinectedin in combination with irinotecan in advanced solid tumors, including Ewing sarcoma (31).
The safety profile of single-agent lurbinectedin was manageable. Reversible myelosuppression was the most common toxicity, and was managed with cycle delays, dose reductions, G-CSF support, and transfusions. Severe hematologic abnormalities were more frequent in this cohort of patients with Ewing sarcoma than in a cohort of patients with second-line SCLC in this same Basket study (32), and among patients with platinum-resistant ovarian cancer in a randomized phase III trial (33). Thus, higher incidences were observed in patients with Ewing sarcoma for grade 3/4 neutropenia (57% vs. 46% and 32% respectively), grade 3/4 thrombocytopenia (14% vs. 7% and 9%), and treatment-related febrile neutropenia (14% vs. 5% and 5.5%). This is likely due to a heavier pretreatment with chemotherapy in the cohort of patients with Ewing sarcoma compared to the other two populations, taking into account that current management of primary Ewing sarcoma consists of high-dose induction chemotherapy to reduce the primary tumor and target microscopic disease, followed by consolidation chemotherapy to remove any residual cells (34). Patients with relapsed Ewing sarcoma in this trial received a median of 2 prior chemotherapy-containing regimens. Overall, these results suggest that primary G-CSF prophylaxis should be given to patients with relapsed Ewing sarcoma while on treatment with lurbinectedin.
In conclusion, this single-arm phase II study showed signs of antitumor activity with lurbinectedin used as monotherapy at 3.2 mg/m2 every 3 weeks in patients with relapsed Ewing sarcoma, with a manageable safety profile. Lurbinectedin could represent a valuable addition to therapies currently used in the management of these complex diseases, which constitute a highly unmet medical need.
Acknowledgments
Authors thank all the motivated patients, the caregivers who enrolled on the trial, and all the clinical trial support staff in all the enrolling sites.
The study was funded by PharmaMar S.A, including grants from the Centro para el Desarrollo Tecnológico Industrial (CDTI) during the conduct of the study. V. Subbiah is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. V. Subbiah acknowledges support of The Jacquelyn A. Brady Fund. V. Subbiah is supported by NIH grant R01CA242845. MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (1U01 CA180964), NCATS Grant UL1 TR000371 (Center for Clinical and Translational Sciences), and the MD Anderson Cancer Center Support Grant (P30 CA016672).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Authors' Disclosures
V. Subbiah reports grants from PharmaMar during the conduct of the study. V. Subbiah also reports grants from PharmaMar, Eli Lilly/LOXO Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, Boston Pharmaceuticals, and Helsinn Pharmaceuticals; clinical trial research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, Fujifilm, D3, Pfizer, MultiVir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, PharmaMar, and Medimmune; advisory board/consultant position with Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Relay Therapeutics, Roche, and Medimmune; travel funds from PharmaMar, Incyte, ASCO, and ESMO; and other support from Medscape outside the submitted work. I. Braña reports grants from PharmaMar during the conduct of the study. I. Braña also reports grants from Sanofi, La Caixa Foundation, Cellex Foundation, AstraZeneca, Bicycle Therapeutics, Celgene, Dragonfly, GlaxoSmithKline, Gliknik, Immutep, ISA Pharmaceuticals, Janssen Oncology, Kura, Nanobiotix, Novartis, Northern Biologics, Odonate Therapeutics, Regeneron, and Pfizer; grants and personal fees from Merck Sharp & Dohme, Bristol Myers Squibb, Merck Serono, and Boehringer Ingelheim; and personal fees from Achilles Therapeutics, Cancer Expert Now, eTheRNA immunotherapies, and PCI Biotech outside the submitted work. V. Boni reports other support from AbbVie, ACEO, Adaptimmune, Amcure, Amgen, AstraZeneca, Bristol Myers Squibb, Cytomx, GSK, Genentech/Roche, H3, Incyte, Janssen, Kura, Lilly, Loxo, Nektar, MacroGenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, INOVIO, Merck Sharp & Dohme, PsiOxus, Seattle Genetics, Mersana, Daiichi-Sankyo, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Boehringer Ingelheim, Regeneron, Millennium, Synthon, Spectrum, Rigontec, and Zenith; consultant or advisory role from Puma Biotechnology, Ideaya Biosciences, Loxo Therapeutics, CytomX Therapeutics, Guidepoint, and Oncoart; speaking honoraria from Eli Lilly and Merck Sharp & Dohme; and travel/inscription/accommodation from Bayer outside the submitted work. J.-P. Delord reports grants from Genentech, Roche, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, and Transgene outside the submitted work. A. Awada reports personal fees from Roche, Lilly, Amgen, Eisai, Bristol Myers Squibb, Pfizer, Novartis, Merck Sharp & Dohme, Genomic Health, Ipsen, AstraZeneca, Bayer, Leo Pharma, Merck, Daiichi-Sankyo, Seattle Genetics, and Pierre Fabre outside the submitted work. P. Boudou-Rouquette reports other support from PharmaMar and Pfizer, as well as personal fees from Ipsen outside the submitted work. G.I. Shapiro reports other support from PharmaMar during the conduct of the study. G.I. Shapiro also reports grants and personal fees from Eli Lilly, Merck KGaA/EMD-Serono, Sierra Oncology, and Pfizer; grants from Merck & Co.; and personal fees from G1 Therapeutics, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Bayer, Boehringer Ingelheim, ImmunoMet Therapeutics, Asana, Artios, Atrin, Concarlo Holdings, Syros, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera Therapeutics, Janssen, and Xinthera outside the submitted work. In addition, G.I. Shapiro has a patent for Dosage regimen for sapacitabine and seliciclib issued to Cyclacel Pharmaceuticals and Geoffrey I. Shapiro, as well as a patent for Compositions and methods for predicting response and resistance to CDK4/6 inhibition pending to Liam Cornell and Geoffrey I. Shapiro. R. Ratan reports other support from Oncternal Therapeutics outside the submitted work. C. Fernandez reports other support from PharmaMar during the conduct of the study, as well as other support from PharmaMar outside the submitted work. C. Kahatt reports other support from PharmaMar during the conduct of the study, as well as other support from PharmaMar outside the submitted work. M. Cullell-Young reports personal fees from PharmaMar during the conduct of the study, as well as personal fees from PharmaMar outside the submitted work. M. Siguero reports personal fees from PharmaMar during the conduct of the study, as well as personal fees from PharmaMar outside the submitted work. A. Zeaiter reports other support from PharmaMar outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
V. Subbiah: Conceptualization, resources, investigation, writing–original draft, writing–review and editing. I. Braña: Resources, investigation, writing–review and editing. A. Longhi: Resources, investigation, writing–review and editing. V. Boni: Resources, investigation, writing–review and editing. J.-P. Delord: Resources, investigation, writing–review and editing. A. Awada: Resources, investigation, writing–review and editing. P. Boudou-Rouquette: Resources, investigation, writing–review and editing. J. Sarantopoulos: Resources, investigation, writing–review and editing. G.I. Shapiro: Resources, investigation, writing–review and editing. A. Elias: Resources, investigation, writing–review and editing. R. Ratan: Resources, investigation, writing–review and editing. C. Fernandez: Formal analysis, methodology, writing–review and editing. C. Kahatt: Conceptualization, supervision, methodology, writing–review and editing. M. Cullell-Young: Methodology, writing–original draft, writing–review and editing. M. Siguero: Formal analysis, methodology, writing–review and editing. A. Zeaiter: Supervision, methodology, writing–review and editing. S.P. Chawla: Resources, investigation, writing–review and editing.
References
- 1. Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing's sarcoma: standard and experimental treatment options. Curr Treat Options Oncol 2009;10:126–40. [DOI] [PubMed] [Google Scholar]
- 2. Raney RB, Asmar L, Newton WA Jr, Bagwell C, Breneman JC, Crist W, et al. Ewing's sarcoma of soft tissues in childhood: a report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J Clin Oncol 1997;15:574–82. [DOI] [PubMed] [Google Scholar]
- 3. Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 2008;30:425–30. [DOI] [PubMed] [Google Scholar]
- 4. Subbiah V, Anderson P. Targeted therapy of Ewing's sarcoma. Sarcoma 2011;2011:686985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol 2012;30:4148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol 2009;27:2536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs B, Valenzuela RG, Inwards C, Sim FH, Rock MG. Complications in long-term survivors of Ewing sarcoma. Cancer 2003;98:2687–92. [DOI] [PubMed] [Google Scholar]
- 8. Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, et al. Ewing's sarcoma family of tumors: current management. Oncologist 2006;11:503–19. [DOI] [PubMed] [Google Scholar]
- 9. Leavey P, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence. J Clin Oncol 2007;25:Abstract 10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subbiah V, Kurzrock R. Ewing's sarcoma: overcoming the therapeutic plateau. Discov Med 2012;13:405–15. [PMC free article] [PubMed] [Google Scholar]
- 11. Cuevas C, Perez M, Martin MJ, Chicharro JL, Fernandez-Rivas C, Flores M, et al. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org Lett 2000;2:2545–8. [DOI] [PubMed] [Google Scholar]
- 12. Bueren-Calabuig JA, Giraudon C, Galmarini CM, Egly JM, Gago F. Temperature-induced melting of double-stranded DNA in the absence and presence of covalently bonded antitumor drugs: insight from molecular dynamics simulations. Nucleic Acids Res 2011;39:8248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harlow ML, Maloney N, Roland J, Guillen Navarro MJ, Easton MK, Kitchen-Goosen SM, et al. Lurbinectedin inactivates the Ewing sarcoma oncoprotein EWS-FLI1 by redistributing it within the nucleus. Cancer Res 2016;76:6657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santamaria Nunez G, Robles CM, Giraudon C, Martinez-Leal JF, Compe E, Coin F, et al. Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol Cancer Ther 2016;15:1–14. [DOI] [PubMed] [Google Scholar]
- 15. Leal JF, Martinez-Diez M, Garcia-Hernandez V, Moneo V, Domingo A, Bueren-Calabuig JA, et al. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo antitumor activity. Br J Pharmacol 2010;161:1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh S, Jaigirdar AA, Mulkey F, Cheng J, Hamed SS, Li Y, et al. FDA approval summary: lurbinectedin for the treatment of metastatic small cell lung cancer. Clin Cancer Res 2021;27:2378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dileo P, Grosso F, Casanova M, Jimeno J, Marsoni S, Sanfilippo R, et al. Trabectedin (T) in metastatic Ewing's family tumors (EFT) patients (pts) progressing after standard chemotherapy. J Clin Oncol 2007;25:Abstract 10040. [Google Scholar]
- 18. Harlow M, Maloney N, Segars L, Woldemichael G, Helman LJ, Grohar PJ. Identification of ET-743 analogs with improved selectivity and potency for EWS-FLI1 and Ewing sarcoma cells. Cancer Res 2013;73:Abstract 2760. [Google Scholar]
- 19. Harlow M, Maloney N, Guillen Navarro MJ, D'Incalci M, Galmarini C, Aviles Marin PM, et al. PM01183 shows an improved therapeutic index relative to trabectedin and suppresses EWS/FLI1 activity at clinically achievable concentrations. Cancer Res 2014;74:Abstract 3962. [Google Scholar]
- 20. Taamma A, Misset JL, Riofrio M, Guzman C, Brain E, Lopez Lazaro L, et al. Phase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-hour continuous infusion in patients with solid tumors. J Clin Oncol 2001;19:1256–65. [DOI] [PubMed] [Google Scholar]
- 21. Elez ME, Tabernero J, Geary D, Macarulla T, Kang SP, Kahatt C, et al. First-in-human phase I study of Lurbinectedin (PM01183) in patients with advanced solid tumors. Clin Cancer Res 2014;20:2205–14. [DOI] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 23. National Cancer Institute. ( 2009) Common Terminology Criteria for Adverse Events v.4.0 (CTCAE).
- 24. Saylors RL 3rd, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 2001;19:3463–9. [DOI] [PubMed] [Google Scholar]
- 25. McTiernan A, Driver D, Michelagnoli MP, Kilby AM, Whelan JS. High-dose chemotherapy with bone marrow or peripheral stem cell rescue is an effective treatment option for patients with relapsed or progressive Ewing's sarcoma family of tumors. Ann Oncol 2006;17:1301–5. [DOI] [PubMed] [Google Scholar]
- 26. Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jurgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer 2006;47:795–800. [DOI] [PubMed] [Google Scholar]
- 27. Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer 2007;48:132–9. [DOI] [PubMed] [Google Scholar]
- 28. Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan Kettering experience. Pediatr Blood Cancer 2009;53:1029–34. [DOI] [PubMed] [Google Scholar]
- 29. Raciborska A, Bilska K, Drabko K, Chaber R, Pogorzala M, Wyrobek E, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer 2013;60:1621–5. [DOI] [PubMed] [Google Scholar]
- 30. Nitschke R, Parkhurst J, Sullivan J, Harris MB, Bernstein M, Pratt C. Topotecan in pediatric patients with recurrent and progressive solid tumors: a Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol 1998;20:315–8. [DOI] [PubMed] [Google Scholar]
- 31. Cote GM, Ponce S, Falcon A, Sanchez-Simon I, Jimenez Aguilar E, Nuñez R, et al. Lurbinectedin in combination with irinotecan in patients (PTS) with soft tissue sarcomas (STS). CTOS 2020 Virtual Annual Meeting2020; 2020. p. Poster 164.
- 32. Trigo J, Subbiah V, Besse B, Moreno V, Lopez R, Sala MA, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase II basket trial. Lancet Oncol 2020;21:645–54. [DOI] [PubMed] [Google Scholar]
- 33. Gaillard S, Oaknin A, Ray-Coquard I, Vergote I, Scambia G, Colombo N, et al. Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: a multicenter, randomized, controlled, open-label phase III study (CORAIL). Gynecol Oncol 2021;163:237–45. [DOI] [PubMed] [Google Scholar]
- 34. Riggi N, Suva ML, Stamenkovic I. Ewing's sarcoma. N Engl J Med 2021;384:154–64. [DOI] [PubMed] [Google Scholar]