Abstract
Background
The overall survival rate of prostate cancer (PCa) has improved over the past decades. However, huge socioeconomic and racial disparities in overall and prostate cancer‐specific mortality exist. The neighborhood‐level factors including socioeconomic disadvantage and lack of access to care may contribute to disparities in cancer mortality. This study examines the impact of neighborhood deprivation on mortality among PCa survivors.
Methods
North Carolina–Louisiana Prostate Cancer Project (PCaP) data were used. A total of 2113 men, 1046 AA and 1067 EA, with PCa were included in the analysis. Neighborhood deprivation was measured by the Area Deprivation Index (ADI) at the census block group level using data from the US Census Bureau. Quintiles of ADI were created. Cox proportional hazards and competing risk models with mixed effects were performed to estimate the effect of neighborhood deprivation on all‐cause and PCa‐specific mortality adjusted for age, race, study site, insurance status, and comorbidities.
Results
Participants living in the most deprived neighborhoods had an increased risk for all‐cause mortality (quintiles 4 + 5: adjusted hazard ratio [aHR] = 1.51, 95% confidence interval [CI] = 1.16–1.96) compared to those in the least deprived (quintile 1) neighborhoods. The risk of prostate cancer‐specific mortality was also higher among those living in the deprived neighborhoods (quintiles 4 + 5: aHR = 1.90, 95% CI = 1.10–3.50) than those in the least deprived neighborhood.
Conclusions
The findings suggest neighborhood‐level resources or health interventions are essential to improve survival among men with PCa. Additional research should focus on the mechanisms of how the neighborhood environment affects mortality.
Keywords: ADI, mortality, neighborhood deprivation, North Carolina–Louisiana Prostate Cancer Project
1. INTRODUCTION
Among men in the United States, prostate cancer (PCa) is the second leading cause of cancer‐related death. 1 Individuals living in deprived neighborhoods are more likely to be diagnosed with aggressive forms of PCa and have poorer survival than those living in the least deprived neighborhoods. 2 , 3 , 4 The primary reasons for higher risk of and poor survival among men living in the deprived neighborhood may include lack of access to health care and utilization, neighborhood resources, social capital, and a poor built environment. In addition, deprived neighborhoods often have fewer medical facilities, lower quality education, poorer patient–provider relationships that may lead to less screening for PCa, increasing the risk of fatal cancer. 5 , 6 , 7 Many biological and behavioral risk factors have been studied in relation to PCa mortality. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 However, conflicting results on the association between PCa mortality and family history of PCa, socioeconomic status, smoking, obesity, and low social support have been reported. 10 , 15 , 16 , 18 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Furthermore, recent literature suggests that disparities in PCa mortality are associated with factors that present at both individual and societal levels. 3 , 4 In addition, systemic racial discrimination, inequality in education, income, and access to health care may contribute to the disparate cancer outcomes in the United States. 27 , 28 Disparities in PCa mortality may be explained through a complex interplay of patient factors, provider factors, and societal factors, including the built environment, access to health care, and its utilization. 29
An individual's health and well‐being are strongly influenced by the neighborhood in which they live. In addition to individual characteristics, neighborhood‐level factors that exist beyond the individual level influence many health outcomes, including cancer incidence and mortality. Socioeconomic, physical, and social characteristics of a neighborhood increase the risk of mortality. 3 , 4 Deprived neighborhoods are characterized not only by higher socioeconomic disadvantage, poor physical environment, and lack of access to health care, but also by higher levels of environmental pollutants, less walkability, less access to healthy food, and less greenery, all of which may increase the likelihood of cancer‐related mortality. 30 , 31 People living in the same neighborhood may share the same neighborhood socioeconomic environment or resources, lifestyles, social support, and access to health care resources. Therefore, it is likely that they experience similar health outcomes including increased cancer mortality rates. For instance, African Americans (AAs) are more likely to live in poorer neighborhoods and have less access to neighborhood‐level resources, which ultimately can lead to a greater risk of PCa‐specific mortality compared to European Americans (EAs), who may live in neighborhoods with greater resources. 32
More recently, studies that examined the effect of neighborhood deprivation on survival have reported an increased risk of mortality among cancer patients after adjusting for individual‐level demographics and socioeconomic factors. 3 , 4 , 33 , 34 Similarly, neighborhood characteristics such as racial distribution, and socioeconomic status are associated with availability and quality of health care resources. 35 Compared to least deprived neighborhoods, deprived neighborhoods often lack quality health care resources. Since AAs are more likely to live in deprived neighborhoods, the quality of health care obtained by AA men may differ from that received by EA men, which may have contributed to greater disparities in PCa mortality. 36 , 37 , 38 Furthermore, neighborhood social capital refers to the features of social structures including interpersonal trust and norms of reciprocity, 39 influences the availability, utilization of health care services, and attitude toward providers. 40
There is a growing body of literature exhibiting the role of neighborhood effects on cancer mortality. Studies have shown the adverse effects of neighborhood disadvantage on poor cancer outcomes, however, relatively few studies have examined the association between neighborhood deprivation and PCa‐specific mortality. The majority of studies examining neighborhood effects have also been limited to relatively few measures of neighborhood socioeconomic disadvantage such as median household income, percentage below, and unemployment rate that may not truly represent the neighborhood socioeconomic disadvantage. Previous studies have also used census tracts as proxies for neighborhood. However, block groups, statistical subdivisions of a census tract, are the smallest geographic areas that accurately represent neighborhood than those by census tracts or counties. This study aimed to examine the effect of neighborhood deprivation on all‐cause and PCa‐specific mortality among men diagnosed with PCa.
2. METHODS
2.1. Study design and population
Data from the North Carolina–Louisiana Prostate Cancer Project (PCaP) were used in this study. 41 PCaP is a population‐based study investigating social, individual, and tumor‐level causes of racial differences in PCa. North Carolina and Louisiana men who were diagnosed with PCa between July 1, 2004, and August 31, 2009, and who met the following criteria: (1) 40–79 years old at the time of diagnosis; (2) able to complete the study interview in English; (3) not institutionalized; (4) not cognitively impaired; and (5) not under the influence of alcohol, severely medicated, or psychotic at the time of the interview were invited to participate. Eligible men must have self‐identified as AA or EA. A total of 2258 men (1227 from Louisiana and 1031 from North Carolina) were enrolled in the PCaP study. Additional information about the study design and participants can be found in a prior publication. 41
2.2. All‐cause and PCa‐specific mortality
The outcomes of interest were all‐cause and PCa‐specific mortality. The date and cause of death of PCaP participants were ascertained every year (2004–2020) by linkage with the Louisiana Tumor Registry (SEER) for Louisiana patients and the North Carolina Vital Records for North Carolina patients. Death due to PCa during the follow‐up period (2004–2020) was defined as an event for PCa‐specific mortality. Death from any cause was defined as an event for all‐cause mortality in survival analysis. Those who were still alive on March 31, 2020, or lost to follow‐up were censored in the analysis. Survival time was calculated from the date of diagnosis to whichever event occurred first: (1) date of death; (2) date of last contact; or (3) date at the end of the follow‐up period (March 31, 2020).
2.3. Neighborhood deprivation
Neighborhood deprivation was the primary exposure of interest. Neighborhood deprivation is measured using the Area Deprivation Index (ADI) calculated at the census block group level, the smallest geographical unit for which US Census Bureau publishes the data. The US Census 2000 was chosen to calculate the ADI because PCaP study participant enrollment began in 2004 and neighborhood deprivation before diagnosis was believed to be an etiologic relevant time frame. ADI is a robust and validated composite index of neighborhood socioeconomic measurement that takes into consideration of 17 US census indicators of income and poverty, education, housing, and employment. 42 , 43 A higher ADI score represents a higher level of neighborhood deprivation. The raw ADI scores were calculated and grouped into quintiles such that the most deprived neighborhoods were grouped as quintiles 4 and 5, and the least deprived neighborhoods were grouped as quintile 1. Because a threshold effect of neighborhood deprivation on all‐cause and PCa‐specific mortality was present and the risks of all‐cause and PCa‐specific mortality were similar in quintiles 4 and 5, these two quintiles were combined. Detailed methods on the calculation of ADI have been reported elsewhere 42 , 43 , 44 , 45 and are available in Table S1. The data set with ADI scores was then merged with the participant's individual‐level data using the Federal Information Processing Standard (FIPS) code. Participants' postal addresses were geocoded at the US Census block group level using Texas A&M web‐based geocoding services. 46
2.4. Covariates
Participant characteristics at the time of enrollment (2004–2009) were obtained from the PCaP baseline survey. 41 Trained PCaP nurses collected patients' demographic, health behavior, and clinical history. Diagnostic and treatment‐related information was obtained by medical record abstraction.
Age at diagnosis, race, education, insurance status, income, comorbidities, tumor stage, Gleason sum score, and PCa treatment was considered as covariates. Age at diagnosis was categorized as: (1) ≤50 years; (2) 51–60 years; (3) 61–70 years; and (4) more than 70 years. Education level was dichotomized as more than high school degree versus high school degree or less. Income was classified into five categories: (1) ≤$10,000; (2) $10,001–$40,000; (3) $40,001–$60,000; (4) $60,001–$80,000; and (5) more than $80,000 per year. Comorbidity was assessed using the Charlson Comorbidity Index (CCI), categorized into none (CCI = 0), mild (CCI < 2), moderate (CCI = 2–4), and severe (CCI > 4) morbidity. PCa treatment was dichotomized as received versus not received. Participants who received prostatectomy, radiation therapy, external beam radiation therapy, or brachytherapy were classified as received.
A total of 145 participants did not have a complete home address at diagnosis and were excluded from this study since their addresses could not be geocoded for ADI assignment. The final analytical data set included 2113 participants.
2.5. Statistical analysis
Descriptive statistics were computed including frequencies and proportions for categorical variables and mean (SD) or median (IQR) for continuous variables. Stage stratified Cox proportional hazards models with mixed effects were performed to estimate the effect of neighborhood deprivation on all‐cause and PCa‐specific mortality. Because the traditional Cox proportional hazards model does not account for within block group homogeneity, extended Cox models were used to incorporate random effect terms. 47 The distribution of the random effect was modeled as a gamma distribution. The cause‐specific model of competing risks was used to estimate the hazard of PCa‐specific mortality. 48 , 49 , 50
The multivariable models were adjusted for participants' baseline characteristics including age at diagnosis, race, study site, insurance status, and CCI. Nelson‐Aalen cumulative hazard function graph was plotted for all‐cause and PCa‐specific mortality by ADI quintiles. Confounding variables were selected based on literature review and Directed Acyclic Graphs (DAGs). Stage stratified (I/II vs. III/IV stage) models were used to allow the baseline hazards to vary by stage; however, stage effect cannot be estimated. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc.) at statistical significance level of 0.05.
3. RESULTS
3.1. Distribution of participants' baseline characteristics
Out of the 2113 participants, 701 (33%) died during the study period, of whom 144 died of PCa (Table 1). The median (IQR) ADI score for all participants was 101.69 (23.54) and the ADI ranged from −111.90 to 136.10. Participants who died during the follow‐up period, due to all‐cause mortality or PCa‐specific mortality, had a higher median of neighborhood deprivation at baseline than overall participants. Those who died of PCa or any other cause had almost the same median ADI score. When participants' neighborhoods were grouped by quintile, the highest proportion of mortality was observed among those grouped in quintile 4 for both all‐cause and PCa‐specific deaths (Table 1).
Table 1.
Baseline characteristics of participants by all‐cause and PCa‐specific mortality
| Characteristics | All participants (N = 2113) | All‐cause mortality (N = 701) | PCa‐specific mortality (N = 144) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| ADI quintiles | |||
| Quintile 1 | 422 (20) | 107 (15.26) | 18 (12.50) |
| (ADI range = −111.90 to 81.80) | |||
| Quintile 2 | 423 (20) | 124 (17.69) | 30 (20.83) |
| (ADI range = 81.81–96.85) | |||
| Quintile 3 | 423 (20) | 135 (19.26) | 27 (18.75) |
| (ADI range = 96.86–104.99) | |||
| Quintile 4 | 423 (20) | 173 (24.68) | 39 (27.08) |
| (ADI range = 105.01–111.71) | |||
| Quintile 5 | 422 (20) | 162 (23.11) | 30 (20.83) |
| (ADI range = 111.72–136.10) | |||
| ADI, median (IQR) | 101.7 (23.54) | 104.30 (20.08) | 104.23 (20.40) |
| Age at diagnosis (year) | |||
| ≤50 | 120 (5.68) | 15 (2.14) | 6 (4.17) |
| 51–60 | 691 (32.70) | 134 (19.12) | 42 (29.17) |
| 61–70 | 884 (41.84) | 306 (43.65) | 54 (37.50) |
| More than 70 | 418 (19.78) | 246 (35.09) | 42 (29.17) |
| Age, mean (SD) | 63 (8) | 678 | 65 ± 8 |
| Site | |||
| Louisiana | 1141 (54) | 387 (55.21) | 65 (45.14) |
| North Carolina | 972 (46) | 314 (44.79) | 79 (54.86) |
| Race | |||
| African American | 1046 (49.5) | 364 (51.93) | 86 (59.72) |
| European American | 1067 (50.5) | 337 (48.07) | 58 (40.28) |
| Education | |||
| High school or less | 956 (45.24) | 402 (57.35) | 83 (57.64) |
| More than high school | 1157 (54.76) | 299 (42.65) | 61 (42.36) |
| Income | |||
| ≤$10,000 | 180 (9.4) | 101 (16.03) | 30 (23.08) |
| $10,001–$40,000 | 731 (38.19) | 307 (48.73) | 60 (46.15) |
| $40,001–$60,000 | 332 (17.35) | 94 (14.92) | 18 (13.85) |
| $60,001–$80,000 | 224 (11.70) | 48 (7.62) | 10 (7.69) |
| $80,000+ | 447 (23.35) | 80 (12.70) | 12 (9.23) |
| Insurance status | |||
| No | 206 (9.82) | 85 (12.23) | 29 (20.42) |
| Yes | 1892 (90.18) | 610 (87.77) | 113 (79.58) |
| CCI | |||
| None | 1058 (50.29) | 248 (35.68) | 61 (42.36) |
| Mild | 514 (24.43) | 171 (24.60) | 41 (28.47) |
| Moderate | 464 (22.05) | 231 (33.24) | 35 (24.31) |
| Severe | 68 (3.23) | 45 (6.47) | 7 (4.86) |
| CCI, mean (SD) | 1.01 (1.45) | 1.52 (1.74) | 1.27 (1.70) |
| PCa treatment | |||
| Received | 1399 (66.21) | 411 (58.63) | 87 (60.42) |
| Not received | 714 (33.79) | 290 (41.37) | 57 (39.58) |
| Clinical stage at diagnosis | |||
| I/II | 2023 (98.30) | 653 (96.31) | 120 (89.55) |
| III/IV | 35 (1.70) | 25 (3.69) | 14 (10.45) |
| Gleason sum score | |||
| <8 | 1859 (88.31) | 559 (80.00) | 120 (55.24) |
| ≥8 | 246 (11.69) | 140 (20.00) | 14 (44.76) |
Note: Missing values: Income = 199; Insurance status = 15; CCI = 9; Clinical stage = 55; Gleason sum score = 8.
Abbreviations: ADI, Area Deprivation Index; CCI, Charlson Comorbidity Index; PCa, prostate cancer.
Table 2 shows the median (IQR) ADI scores for overall participants, all‐cause, and PCa‐specific mortality by race. The median (IQR) ADI scores were substantially different between AAs and EAs in all groups. The overall median (IQR) ADI for all participants was higher among AA (107.00 [16.21]) compared to EA (93.92 [28.30]). Similar differences in median ADI among AA and EA were observed in both all‐cause and PCa deaths. Among those who died from all causes, AAs had a higher median ADI (109.00 [12.34]) compared to EAs (97.48 [24.66]). Likewise, among those who died of PCa, AAs had a higher median ADI (108.54 [12.70]) than those EAs (95.41 [23.40]).
Table 2.
Median (IQR) ADI score among African American and European American men diagnosed with PCa
| Race | ADI: Median (IQR) | |||
|---|---|---|---|---|
| N (%) | All participants (N = 2113) | All‐cause mortality (N = 701) | PCa‐specific mortality (N = 144) | |
| African American | 1046 (49.5) | 107.00 (16.21) | 109.00 (12.34) | 108.54 (12.70) |
| European American | 1067 (50.5) | 93.92 (28.30) | 97.48 (24.66) | 95.41 (23.40) |
| p | <0.001* | <0.001* | <0.001* | |
Abbreviations: ADI, Area Deprivation Index; Pca, prostate cancer.
p value indicates the statistically significant difference in median ADI score between African American and European American.
3.2. All‐cause mortality and neighborhood deprivation
Figure 1 shows Nelson‐Aalen estimates of the cumulative hazard of all‐cause mortality among participants by ADI quintiles. Figure 1 indicates that patients living in the deprived neighborhoods had an increased risk of all‐cause mortality compared to those living in the least deprived neighborhoods (log‐rank test, p < 0.001).
Figure 1.
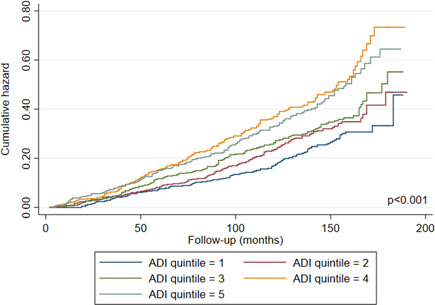
Cumulative hazard of all‐cause mortality among participants according to ADI quintiles. ADI, Area Deprivation Index [Color figure can be viewed at wileyonlinelibrary.com]
Table 3 shows hazard ratios (HRs) and corresponding 95% confidence interval of all‐cause mortality. The crude model assessing the relationship between all‐cause mortality and neighborhood deprivation without adjusting for confounding factors suggested a 86% increased risk of all‐cause mortality among participants living in the most deprived neighborhood (quintiles 4 + 5) compared to those living in the least deprived neighborhood (quintile 1; HR = 1.86, 95% CI = 1.49–2.33). In the model adjusted only for age (Table 3, Model 1), the risk of all‐cause mortality among those living in the most deprived neighborhoods did not change substantially from the crude model (HR = 1.84, 95% CI = 1.47–2.30). The association between neighborhood deprivation and all‐cause mortality remained statistically significant after adjusting for age, race, study site, and insurance status (Table 3, Model 2), those living in the most deprived neighborhood had a 65% increased risk of all‐cause mortality than those living in the least deprived neighborhood (HR = 1.65, 95% CI = 1.30–2.12). The association between neighborhood deprivation and all‐cause mortality was further attenuated with the addition of CCI into Model 2 (Table 3, Model 3). Compared to those residing in the least deprived neighborhoods, the risk of all‐cause mortality was 51% higher among those in the most deprived neighborhood (adjusted hazard ratio [aHR] = 1.51, 95% CI = 1.16–1.96; Table 3, Model 3).
Table 3.
Crude and adjusted models estimating the effect of neighborhood deprivation on all‐cause mortality
| Neighborhood deprivation | Crude Model | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| HR | aHR | aHR | aHR | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| ADI quintiles | ||||
| Quintile 1 (least deprived) | Reference | Reference | Reference | Reference |
| Quintile 2 | 1.22 | 1.27 | 1.22 | 1.18 |
| (0.93–1.60) | (0.98–1.66) | (0.93–1.60) | (0.88–1.57) | |
| Quintile 3 | 1.38 | 1.50 | 1.43 | 1.36 |
| (1.06–1.80)* | (1.15–1.94)* | (1.09–1.88)* | (1.02–1.81) | |
| Quintiles 4 + 5 (most deprived) | 1.86 | 1.84 | 1.65 | 1.51 |
| (1.49–2.33)* | (1.47–2.30)* | (1.30–2.12)* | (1.16–1.96)* |
Note: Model 1: Adjusted for age. Model 2: Adjusted for age, race, study site, and insurance status. Model 3: Adjusted for age, race, study site, insurance status, and CCI.
Abbreviations: ADI, Area Deprivation Index; CI, confidence interval; aHR, adjusted hazard ratio; HR, hazard ratio.
Significant association with all‐cause mortality at Type I error = 0.05.
3.3. PCa‐specific mortality and neighborhood deprivation
Figure 2 shows Nelson‐Aalen estimates of the cumulative hazard of PCa‐specific mortality among participants by ADI quintiles. Figure 2 indicates that patients living in deprived neighborhoods had an increased risk of PCa‐specific mortality compared to those living in least deprived neighborhoods (log‐rank test, p = 0.04).
Figure 2.
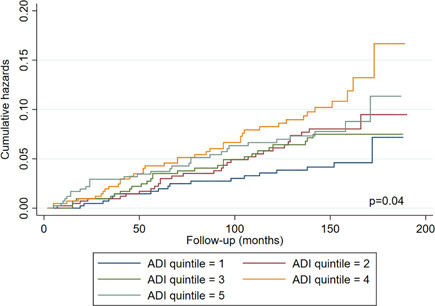
Cumulative hazard of PCa‐specific mortality among participants according to ADI quintiles. ADI, Area Deprivation Index; PCa, prostate cancer [Color figure can be viewed at wileyonlinelibrary.com]
Table 4 shows hazard ratios (HRs) and corresponding 95% confidence interval of PCa‐specific mortality. In the crude model, more than twice the risk of PCa‐specific mortality was observed among individuals living in the deprived neighborhoods compared to those living in the least deprived neighborhoods (quintile 1). According to the crude model, residents of the most deprived neighborhoods had a 130% increased risk of PCa‐mortality compared to residents of the least deprived neighborhood (HR = 2.30, 95% CI = 1.33–3.99). The hazard ratio of PCa‐specific mortality adjusted for age (Table 4, Model 1) remained almost the same as the crude model (quintiles 4 + 5: aHR = 2.27, 95% CI = 1.31–3.92; Table 4, Model 1). With additional adjustment for race, study site, and insurance status in model 2, the association between neighborhood deprivation and PCa‐specific mortality was slightly attenuated (Table 4, Model 2; quintiles 4 + 5: aHR = 1.97, 95% CI = 1.07–3.62; Table 4, Model 2). Model 3 adds CCI into Model 2 showed that the participants in the most deprived neighborhood had a 90% increased risk of PCa‐specific mortality compared to those in quintile 1 (aHR = 1.90, 95% CI = 1.10–3.50; Table 4, Model 3).
Table 4.
Crude and adjusted models estimating the effect of neighborhood deprivation on PCa‐specific mortality
| Neighborhood deprivation | Crude Model | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| HR | aHR | aHR | aHR | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| ADI quintiles | ||||
| Quintile 1 (least deprived) | Reference | Reference | Reference | Reference |
| Quintile 2 | 1.71 | 1.70 | 1.60 | 1.59 |
| (0.92–3.17) | (0.92–3.16) | (0.84–3.05) | (0.83–3.02) | |
| Quintile 3 | 1.73 | 1.75 | 1.63 | 1.60 |
| (0.93–3.23) | (0.94–3.27) | (0.85–3.13) | (0.83–3.08) | |
| Quintiles 4 + 5 (most deprived) | 2.30 | 2.27 | 1.97 | 1.90 |
| (1.33–3.99)* | (1.31–3.92)* | (1.07–3.62)* | (1.10–3.50)* |
Note: Model 1: Adjusted for age. Model 2: Adjusted for age, race, study site, and insurance status. Model 3: Adjusted for age, race, study site, insurance status, and CCI.
Abbreviations: ADI, Area Deprivation Index; CI, confidence interval; aHR, adjusted hazard ratio; HR, hazard ratio; PCa, prostate cancer.
Significant association with PCa‐specific mortality at Type I error = 0.05.
4. DISCUSSION
Living in a deprived neighborhood was associated with an increased risk of both all‐cause and PCa‐specific mortality. The greatest risk of all‐cause and PCa‐specific mortality was observed among participants living in the deprived neighborhoods (quintiles 4 + 5) and associations remained even after adjustment for age, race, study site, insurance status, and CCI.
Mechanisms of neighborhood effects on adverse health outcomes, including cancer mortality, are complex. 51 , 52 , 53 Some have suggested that neighborhood characteristics such as lack of access to health care, income inequality, overcrowding, lack of access to healthy food, and poor physical environment may explain the increased mortality risk. People in deprived neighborhoods are also more likely to be stressed and socially isolated, which may affect their survival. 54 , 55 , 56 The impact of living in deprived neighborhoods among cancer survivors may be even greater than among those without cancer, which may also increase the risk of death. For example, men diagnosed with PCa and living in deprived neighborhoods, regardless of individual household income, are less likely to receive definitive treatment. 57 , 58 Such contextual factors play a significant role in health‐seeking behavior, which is linked to survival outcomes.
The results of this study are consistent with other studies of neighborhood socioeconomic disadvantage and all‐cause and cancer‐specific mortality. 59 , 60 Only a few studies examined neighborhood effects on PCa‐specific and all‐cause mortality among men diagnosed with PCa. Li et al. 4 assessed the impact of neighborhood deprivation and PCa mortality and reported a 25% increased risk of all‐cause mortality and about 20% increased risk of PCa‐specific mortality among residents of deprived neighborhoods. Compared to the findings of this study, the effect of neighborhood deprivation reported by Li et al. 4 in Sweden is relatively smaller. The difference in findings is likely due to methodological differences in defining neighborhood deprivation and study population. Unlike a composite and validated measure consisting of 17 neighborhood‐level used in this study, Li et al. 4 used only four neighborhood measures of neighborhood‐level deprivation including educational status, income, unemployment rate, and percent population on social welfare assistance. Furthermore, access to universal health coverage for Swedish population might have contributed to the differences in the findings. The current study examines the impact of individual and neighborhood factors on PCa survival among men residing in the Southern United States. 61
Neighborhood environment has been recognized as an important risk factor for many health outcomes. However, a number of weaknesses exist throughout the prior literature. Neighborhood measures used by previous studies are inconsistent. While some studies have used only income, education, and employment measures, a few studies have used a broader range of neighborhood characteristics such as income, education, employment, house values, rent values, and poverty. Definitions of neighborhoods are also inconsistent. Most neighborhood studies are conducted at the census tract level which is a much bigger geographical approximation of neighborhood than block group. Census tract may not be an appropriate proxy for neighborhood which may also impact the study's validity. Therefore, this study uses a validated measure of neighborhood socioeconomic disadvantage that includes a wide range of neighborhood indicators and conducted at block group.
There are important limitations in this study. First, participants without complete home address data at the time of diagnosis were excluded from this study. These participants were diagnosed with higher stage cancer at baseline than those included in the study which may have introduced selection bias in the study. However, the number of excluded participants was relatively low, therefore, it is not likely to substantially bias the results. Second, direct measures of healthcare access, patient–provider relationship, health care facilities in the neighborhoods, service utilization, and access to healthy food were not measured in this study. Another limitation of this study is that the stage stratified HR was not calculated because of small sample size in stage categories III and IV. Finally, the study only included men who identified themselves as AA or EA from North Carolina and Louisiana. Therefore, the findings may not be generalizable to other races in other geographic areas.
This study has several strengths. First, the study uses the data from one of the largest population‐based studies of newly diagnosed prostate cancer in AA and EA men ever conducted, with roughly equal numbers of AA and EA participants. Second, the study uses a validated measure of neighborhood socioeconomic disadvantage that captures 17 US census indicators that are highly relevant social determinants of health. Third, census block group, considered more appropriate than larger aggregations, is used as a proxy for neighborhood. Finally, this study is one of the first multi‐level long‐term follow‐up studies that examine the association between neighborhood deprivation and all‐cause and PCa‐specific mortality.
5. CONCLUSION
In summary, neighborhood deprivation is associated with an increased risk of all‐cause and PCa‐specific mortality among a racially diverse population even after adjustment for potential confounders such as insurance status and comorbidities. These findings highlight the importance of social determinants of health such as neighborhood factors in affecting mortality in men after a PCa diagnosis.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The North Carolina–Louisiana Prostate Cancer Project (PCaP) is carried out as a collaborative study supported by the Department of Defense contract DAMD 17‐03‐2‐0052 and W81XWH‐17‐1‐0119. The authors thank the staff, advisory committees, and research subjects participating in the PCaP study for their important contributions.
KC M, Oral E, Rung AL, et al. Neighborhood deprivation and risk of mortality among men with prostate cancer: findings from a long‐term follow‐up study. The Prostate. 2022;82:783‐792. 10.1002/pros.24320
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from PCaP management team. Restrictions apply to the availability of these data.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Lynch SM, Mitra N, Ross M, et al. A Neighborhood‐Wide Association Study (NWAS): example of prostate cancer aggressiveness. PLOS One. 2017;12(3):e0174548. 10.1371/journal.pone.0174548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeRouen MC, Schupp CW, Koo J, et al. Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol. 2018;53:1‐11. 10.1016/j.canep.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X, Sundquist K, Sundquist J. Neighborhood deprivation and prostate cancer mortality: a multilevel analysis from Sweden. Prostate Cancer Prostatic Dis. 2012;15(2):128‐134. 10.1038/pcan.2011.46 [DOI] [PubMed] [Google Scholar]
- 5. Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666‐668. [PMC free article] [PubMed] [Google Scholar]
- 6. Landrine H, Corral I. Separate and unequal: residential segregation and black health disparities. Ethn Dis. 2009;19(2):179‐184. [PubMed] [Google Scholar]
- 7. Freeman Anderson K. Racial residential segregation and the distribution of health‐related organizations in urban neighborhoods. Soc Probl. 2017;64(2):256‐276. [Google Scholar]
- 8. Giovannucci E, Rimm EB, Ascherio A, et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol Biomarkers Prev. 1999;8(4 Pt 1):277‐282. [PubMed] [Google Scholar]
- 9. Nguyen PL, Chen MH, Catalona WJ, Moul JW, Sun L, D'Amico AV. Predicting prostate cancer mortality among men with intermediate to high‐risk disease and multiple unfavorable risk factors. Int J Radiat Oncol. 2009;73(3):659‐664. 10.1016/j.ijrobp.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 10. Jan M, Bonn SE, Sjölander A, et al. The roles of stress and social support in prostate cancer mortality. Scand J Urol. 2016;50(1):47‐55. 10.3109/21681805.2015.1079796 [DOI] [PubMed] [Google Scholar]
- 11. Cheng I, Witte JS, McClure LA, et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Cause Control. 2009;20(8):1431‐1440. 10.1007/s10552-009-9369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomic K, Ventimiglia E, Robinson D, Haggstrom C, Lambe M, Stattin P. Socioeconomic status and diagnosis, treatment, and mortality in men with prostate cancer. Nationwide population‐based study. Int J Cancer. 2018;142(12):2478‐2484. 10.1002/ijc.31272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hickey K, Do KA, Green A. Smoking and prostate cancer. Epidemiol Rev. 2001;23(1):115‐125. 10.1093/oxfordjournals.epirev.a000776 [DOI] [PubMed] [Google Scholar]
- 14. Adami HO, Bergström R, Engholm G, et al. A prospective study of smoking and risk of prostate cancer. Int J Cancer. 1996;67(6):764‐768. [DOI] [PubMed] [Google Scholar]
- 15. Islami F, Moreira DM, Boffetta P, Freedland SJ. A systematic review and meta‐analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur Urol. 2014;66(6):1054‐1064. 10.1016/j.eururo.2014.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta‐analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305(24):2548‐2555. 10.1001/jama.2011.879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ang M, Borg M, O'Callaghan ME, South Australian Prostate Cancer Clinical Outcomes Collaborative (SA‐PCCOC) . Survival outcomes in men with a positive family history of prostate cancer: a registry based study. BMC Cancer. 2020;20(1):894. 10.1186/s12885-020-07174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodríguez C, Calle EE, Miracle‐McMahill HL, et al. Family history and risk of fatal prostate cancer. Epidemiology. 1997;8(6):653‐657. 10.1097/00001648-199710000-00007 [DOI] [PubMed] [Google Scholar]
- 20. Liss MA, Chen H, Hemal S, et al. Impact of family history on prostate cancer mortality in white men undergoing prostate specific antigen based screening. J Urol. 2015;193(1):75‐79. 10.1016/j.juro.2014.07.085 [DOI] [PubMed] [Google Scholar]
- 21. Fowke JH, McLerran DF, Gupta PC, et al. Associations of body mass index, smoking, and alcohol consumption with prostate cancer mortality in the Asia Cohort Consortium. Am J Epidemiol. 2015;182(5):381‐389. 10.1093/aje/kwv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdel‐Rahman O. Prostate cancer incidence and mortality in relationship to family history of prostate cancer; findings from the PLCO Trial. Clin Genitourin Cancer. 2019;17(4):e837‐e844. 10.1016/j.clgc.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 23. Tomic K, Ventimiglia E, Robinson D, Häggström C, Lambe M, Stattin P. Socioeconomic status and diagnosis, treatment, and mortality in men with prostate cancer. Nationwide population‐based study. Int J Cancer. 2018;142(12):2478‐2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chalfin HJ, Lee SB, Jeong BC, et al. Obesity and long‐term survival after radical prostatectomy. J Urol. 2014;192(4):1100‐1104. 10.1016/j.juro.2014.04.086 [DOI] [PubMed] [Google Scholar]
- 25. Haque R, Van Den Eeden SK, Wallner LP, et al. Association of body mass index and prostate cancer mortality. Obes Res Clin Pract. 2014;8(4):e374‐e381. 10.1016/j.orcp.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow‐up in the Whitehall study. Cancer Causes Control. 2011;22(2):311‐318. 10.1007/s10552-010-9691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wild CP, Weiderpass E, Stewart BW, eds. International Agency for Research on Cancer (IARC) . World Cancer Report: Cancer Research for Cancer Prevention. 2020. [Google Scholar]
- 28.National Cancer Institute. Cancer Disparities. 2020.
- 29. Morris AM, Rhoads KF, Stain SC, Birkmeyer JD. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211(1):105‐113. 10.1016/j.jamcollsurg.2010.02.051 [DOI] [PubMed] [Google Scholar]
- 30. Keralis JM, Javanmardi M, Khanna S, et al. Health and the built environment in United States cities: measuring associations using Google Street View‐derived indicators of the built environment. BMC Public Health. 2020;20(1):215. 10.1186/s12889-020-8300-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Renalds A, Smith TH, Hale PJ. A systematic review of built environment and health. Fam Community Health. 2010;33(1):68‐78. 10.1097/FCH.0b013e3181c4e2e5 [DOI] [PubMed] [Google Scholar]
- 32. Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci USA. 2016;113(47):13372‐13377. 10.1073/pnas.1607220113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh GK, Jemal A. Socioeconomic Inequalities in Cancer Incidence and Mortality. The American Cancer Society's Principles of Oncology; 2018:23‐32. [Google Scholar]
- 34. Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020;8(2):49‐54. 10.1016/j.prnil.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussein M, Diez Roux AV, Field RI. Neighborhood socioeconomic status and primary health care: usual points of access and temporal trends in a major US urban area. J Urban Health. 2016;93(6):1027‐1045. 10.1007/s11524-016-0085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jayadevappa R, Chhatre S, Johnson JC, Malkowicz SB. Association between ethnicity and prostate cancer outcomes across hospital and surgeon volume groups. Health Policy. 2011;99(2):97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health. 2016;37:375‐394. 10.1146/annurev-publhealth-032315-021439 [DOI] [PubMed] [Google Scholar]
- 38. McBean AM, Gornick M. Differences by race in the rates of procedures performed in hospitals for Medicare beneficiaries. Health Care Financ Rev. 1994;15(4):77‐90. [PMC free article] [PubMed] [Google Scholar]
- 39. Berkman LF, Kawachi I, Glymour MM. Social Epidemiology. Oxford University Press; 2014. [Google Scholar]
- 40. Derose KP, Varda DM. Social capital and health care access: a systematic review. Med Care Res Rev. 2009;66(3):272‐306. 10.1177/1077558708330428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schroeder JC, Bensen JT, Su LJ, et al. The North Carolina‐Louisiana Prostate Cancer Project (PCaP): methods and design of a multidisciplinary population‐based cohort study of racial differences in prostate cancer outcomes. Prostate. 2006;66(11):1162‐1176. 10.1002/pros.20449 [DOI] [PubMed] [Google Scholar]
- 42. Singh GK. Area deprivation and widening inequalities in US mortality, 1969‐1998. Am J Public Health. 2003;93(7):1137‐1143. 10.2105/ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30‐day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765‐774. 10.7326/M13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS. 2016;4(3):1238. 10.13063/2327-9214.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. KC M, Oral E, Straif‐Bourgeois S, Rung AL, Peters ES. The effect of area deprivation on COVID‐19 risk in Louisiana. PLOS One. 2020;15(12):e0243028. 10.1371/journal.pone.0243028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Texas A&M: Texas A&M GeoServices; 2020. Accessed June 2020. https://geoservices.tamu.edu/(2020).
- 47. Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185‐203. 10.1111/insr.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo C, So Y. Cause‐specific analysis of competing risks using the PHREG procedure. SAS Global Forum. 2018:8‐11. [Google Scholar]
- 49. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601‐609. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244‐256. 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783‐1789. 10.2105/Ajph.91.11.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Galster GC. How Neighborhoods Affect Health, Well‐being, and Young People's Futures. McArthur Foundation; 2014. [Google Scholar]
- 53. Galster GC. The mechanism(s) of neighbourhood effects: theory, evidence, and policy implications. In: van Ham M, Manley D, Bailey N, Simpson L, Maclennan D, eds. Neighbourhood Effects Research: New Perspectives. Springer Netherlands; 2012:23‐56. [Google Scholar]
- 54. Marcus AF, Illescas AH, Hohl BC, Llanos AAM. Relationships between social isolation, neighborhood poverty, and cancer mortality in a population‐based study of US adults. PLOS One. 2017;12(3):e0173370. 10.1371/journal.pone.0173370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Remes O, Lafortune L, Wainwright N, Surtees P, Khaw KT, Brayne C. Association between area deprivation and major depressive disorder in British men and women: a cohort study. BMJ Open. 2019;9(11):e027530. 10.1136/bmjopen-2018-027530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hamano T, Li X, Sundquist J, Sundquist K. Neighborhood social capital and incidence and mortality of prostate cancer: a Swedish cohort study. Aging Clin Exp Res. 2021;33:3333‐3342. 10.1007/s40520-021-01852-9 [DOI] [PubMed] [Google Scholar]
- 57. Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005;46(1):15‐31. 10.1177/002214650504600103 [DOI] [PubMed] [Google Scholar]
- 58. Watson M, Grande D, Radhakrishnan A, Mitra N, Ward KR, Pollack CE. Racial differences in prostate cancer treatment: the role of socioeconomic status. Ethn Dis. 2017;27(3):201‐208. 10.18865/ed.27.3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Major JM, Doubeni CA, Freedman ND, et al. Neighborhood socioeconomic deprivation and mortality: NIH‐AARP diet and health study. PLOS One. 2010;5(11):e15538. 10.1371/journal.pone.0015538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Warren Andersen S, Blot WJ, Shu XO, et al. Associations between neighborhood environment, health behaviors, and mortality. Am J Prev Med. 2018;54(1):87‐95. 10.1016/j.amepre.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wild CP, Weiderpass E, BWS, eds. World Cancer Report: Cancer Research for Cancer Prevention. International Agency for Research on Cancer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from PCaP management team. Restrictions apply to the availability of these data.


