Abstract
Background
Allergic rhinitis (AR) is one of the most common allergic diseases affecting children. Objective assessment of nasal obstruction is possible through active anterior rhinomanometry (AAR). Several factors, such as passive smoke exposure (PSE), are triggers for worsening nasal obstruction and chronic inflammation. PSE affects bacterial eubiosis in the upper respiratory tract. This study evaluates the influence of PSE and cotinine levels on both nasal obstruction and local microbiome composition in children with AR.
Methods
Fifty patients (aged between 6 and 16 years) with AR monosensitized grass pollen were enrolled. They underwent skin prick tests, a nasal swab to evaluate the microbial composition of the anterior nostrils, a basal AAR, a post‐decongestion AAR, and spirometry. Serum cotinine levels were assessed to evaluate PSE.
Results
A significantly lower percentage of mean nasal flow (mNF%) was observed before and after hydrazine administration in subjects exposed to passive smoke (Exp group) compared with the non‐exposed group. In contrast, higher cotinine levels were observed in the Exp group than in the controls. PSE has been associated with a decrease in biodiversity and a change in the nasal microbiome composition; instead, although to a different extent, the abundance of specific taxa resulted in being correlated to cotinine levels and nasal flow.
Conclusion
Children with AR exposed to passive smoke with positive serum cotinine could represent a risk factor for developing nasal obstruction and microbial dysbiosis, suggesting their possible role in pathophysiological processes.
Keywords: allergic rhinitis, children, nasal microbiome, passive smoke, serum cotinine
Key Message.
Children with allergic rhinitis exposed to passive smoke with positive serum cotinine increase their nasal obstruction with a reduced mean nasal flow also associated with a modification in their nasal microbiome.
1. INTRODUCTION
Allergic rhinitis (AR) is one of the most common allergic diseases, and its prevalence is continuously increasing in Western countries. 1 Common AR symptoms are nasal obstruction, itching, and rhinorrhea, often associated with asthma for the continuity between upper and lower airways. 2 The objective evaluation of the degree of nasal obstruction is possible through the anterior active rhinomanometry (AAR), a reliable and reproducible valuable method in pediatric age where nasal symptoms are often overestimated when subjectively assessed using questionnaires. 3
Various infectious and environmental factors, such as passive smoke exposure (PSE), are triggers for worsening nasal obstruction and chronic inflammation in AR, constituting risk factors for upper airway diseases. 4
Nevertheless, pediatric PSE is continuously increasing, reaching up to 40% of children. To date, few studies have reported in children the relationship between PSE and AR with conflicting results and without reaching a unique understanding of this relation. 5 , 6
It is widely recognized that both active smoking and passive smoking alter the microbiome affecting upper respiratory bacterial richness, with implications for smoking‐related diseases. 6
However, no studies have explored the impact of PSE and the related serum cotinine levels on both nasal obstruction and local microbiome composition in children with AR. To achieve this aim, in children suffering from AR, the role of these parameters was investigated. Furthermore, the influence of PSE and cotinine levels on nasal microbial composition was also evaluated.
2. MATERIALS AND METHODS
Materials and methods are available as supplementary material in the online repository.
3. RESULTS
A total of 50 patients (median (IQR) 9.1(7.2–9.9) years) were enrolled. Of these, 25 reported a PSE and entered the exposed group (Exp), while 25 negatives for this feature entered the non‐exposed group (NExp) and were taken as controls. No statistically significant differences were found between groups considering age, sex, and FEV1 (Table 1). A significantly lower mean nasal flow percentage (mNF%) was observed before and after hydrazine administration in Exp comparing the control group. Conversely, higher cotinine levels were observed in the Exp group compared with controls (Table 1). All 50 samples underwent 16s rRNA gene‐based microbiota analysis and were eligible for following downstream bioinformatics analysis. From a total of 2.286.119 sequences passing the filtering bioinformatic processes (median (IQR)), 45587.5(34164.5–56388.5), 391 OTUs were identified.
TABLE 1.
Characteristics of the study population
| Variables |
Not exposed No. 25 |
Exposed No. 25 |
p‐value |
|---|---|---|---|
| Age | 9.3 (7.5–9.6) | 9.1 (7.2–9.9) | .946 |
| Sex (male) | 17 (68%) | 12 (48%) | .252 |
| FEV1 | 99 (90–103) | 99 (90–100) | .923 |
| mNF (%) pre‐hydrazine | 79 (73–89) | 67 (58–69) | <.0001 |
| mNF (%) post‐hydrazine | 91 (79–99) | 70 (68–72) | <.0001 |
| Cotinine | 2.4 (1.8–3.2) | 23.2 (19.5–27.5) | <.0001 |
Continuous variables are expressed as median and interquartile range (IQR), while discrete variables are reported as number and percentage.
Significantly, lower values of the Simpson index have been determined for the group of children exposed to secondhand smoke compared with the control group. Interestingly, we observed that patients exposed to secondhand smoke tended to show simultaneously lower biodiversity values and a wider variability as determined by the more comprehensive interquartile range (IQR) in Figure 1B. Regarding β‐diversity, the Principal Coordinates Analysis (PCoA) in Figure 1C,D determined statistically significant separations for Bray‐Curtis's dissimilarity and weighted UniFrac distance between the two phenotypes (Bray‐Curtis, p = .001; weighted UniFrac, p = .001).
FIGURE 1.
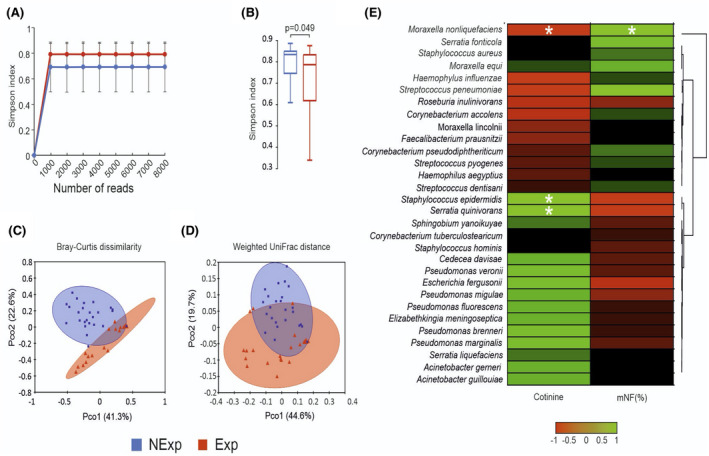
Bioinformatic analysis of 16S metagenomic reads. A, Color‐coded rarefaction curves. For each group, the average values of α‐diversity indexes with 95% confidence intervals were reported at different sequencing depths. B, Box plots showing the Simpson index α‐diversity estimator, measured for each group. C,D, PCoA plot of bacterial β‐diversity based on Bray‐Curtis dissimilarity and weighted UniFrac distance according to exposure to secondhand smoke. For each group, the 95% confidence interval has been drawn. Numbers between parentheses represent the percentage of the total variance explained by the principal coordinates. Values are expressed as mean ±SD. E, Cross‐correlation heatmaps based on Spearman's correlation coefficients computed between the relative abundance of taxa ≥0.05% in at least one group and the values observed for mNF% and cotinine across the whole population of studied subjects. The color scale represents values assumed by Spearman's correlation coefficient (ρ) with green and red for positive and negative correlations. According to hierarchical simple‐linkage clustering, taxa were ordered based on Spearman's coefficients computed on relative abundances (dendrogram on the left). A white asterisk indicates a significant correlation at α level 0.05 after FDR correction for multiple comparisons
A general positive correlation was observed between the cotinine levels and those taxa significantly more abundant in the Exp group; however, significant results were obtained only for S. epidermidis and S. quinivorans. On the other hand, a significant negative correlation was found between serum cotinine levels and M. nonliquefacients. The opposite scenario could be observed between the mNF% and the relative abundance of taxa, although the only statistically significant result was the positive correlation with M. nonliquefaciens (Figure 1E ).
4. DISCUSSION
Nasal obstruction is one of the most common and troublesome symptoms in children with AR evaluated both subjectively by questionnaires and objectively by AAR; this symptom is due to the presence of a persistent minimal chronic inflammation of nasal mucosa where environmental triggers, such as passive smoke, increase local inflammation worsening the obstruction itself. Serum cotinine is one of the major nicotine metabolites detected in the blood and represents a specific indicator of PSE. 4
Conflicting results emerge from the scientific literature regarding the relationship between PSE and AR. Shargorodsky et al., in a broad pediatric cohort, found a significantly high prevalence of AR symptoms in children with PSE independently from their sensitization, proving the need for PSE reduction among children. 5 Also, the systematic review and meta‐analysis conducted by Saulyte et al. examined the association between PSE and allergic conditions, founding an association between PSE and an increased risk for allergic diseases. 6 Additionally, Montano‐Velazquez et al. objectively evaluated nasal obstruction performing a basal AAR in AR patients with and without PSE. The authors found that patients with PSE, evaluated through urine cotinine concentration (cotinine⁄creatinine ratio), presented an increased nasal resistance compared with those not exposed to passive smoke. 7 Conversely, Fenton et al. reported that although nasal symptoms were significantly worse in children with AR, passive smoke enhanced nasal obstruction mostly in children without AR. However, these results were not objectively assessed through an AAR but using only a visual analog scale. 4
Our results show that children with AR and positivity for serum cotinine related to PSE presented nasal obstruction objectively assessed through AAR regarding those not exposed. There were statistically significant differences in mNF values between the EXP and not EXP group, evaluated both with a basal and a post‐decongestion AAR. When a post‐decongestion AAR was performed, the vasoconstrictor effect of hydrazine reduced the local congestion and the relative nasal obstruction in the EXP group, but to a lesser extent regarding the not EXP group, remaining statistically significant differences between the two groups.
This finding is in line with the fact that PSE enhances local congestion and even more in patients with AR where there is minimal chronic inflammation. 7
The objective evaluation of nasal obstruction, often challenging to be realized in children, is the main strength of this study, and it was possible thanks to the use of a basal and post‐decongestion AAR.
Moreover, we assayed the influence of passive smoke on the nasal microbial composition and the correlations with cotinine levels and mNF% values. Our findings evidence that PSE is associated with a decrease in biodiversity and shift in the microbiota composition, as shown by α‐diversity and β‐diversity. It has been recognized that dysbiosis promotes the acquisition of new specific bacterial taxa and the overgrowth of selectively advantaged bacteria. 8 The changes in the microbial composition may also be related to the nature of tobacco that suppresses the Th1 and Th17 reactions, but not the Th2 response, promoting colonization and persistence of specific bacterial taxa. 9 The cross‐correlation analysis revealed that M. nonliquefaciens, in agreement with determined cotinine levels, was negatively correlated with passive smoke exposure and positively correlated with the nasal flow. Such findings strengthen the idea that M. nonliquefaciens could be a microbial component associated with a healthy nasal microbiota in children, as reported in our previous study. 8 Furthermore, cotinine levels were positively correlated with S. epidermidis and S. quinivorans, suggesting a higher capability of these bacterial species to adapt to smoke exposure–associated environmental changes. In this context, PSE could represent an additional factor affecting the eubiosis in the nasal environment and exacerbating the clinical manifestation of AR.
4.1. Limitations
The main limitations of this pilot study are the small sample size and the cross‐sectional design that do not provide a causal relationship between PSE and nasal obstruction in AR. Moreover, this study was performed as a monocentric evaluation. Thus, further studies are needed in the future with more extensive sample size and a multicentric investigation to assess PSE and AR's relationship better.
5. CONCLUSION
Children with AR exposed to passive smoke and positivity for serum cotinine levels present an increase in their nasal obstruction linked to microbial modifications that could play a contributive or causative role in the pathophysiological processes. PSE could play a role in the worsening of AR symptoms and an additional risk factor for dysbiosis in the nasal environment.
CONFLICT OF INTERESTS
The authors declared they have no conflict of interests.
AUTHOR CONTRIBUTIONS
Giulia Brindisi: Writing—review & editing (equal). Massimiliano Marazzato: Writing—review & editing (equal). Francesca Brunetti: Conceptualization (equal), methodology (equal), and writing—review & editing (equal). Giovanna De Castro: Conceptualization (equal), methodology (equal), and writing—review & editing (equal). Lorenzo Loffredo: Conceptualization (equal), methodology (equal), and writing—review & editing (equal). Roberto Carnevale: Conceptualization (equal), methodology (equal), and writing—review & editing (equal). Bianca Cinicola: Conceptualization (equal), methodology (equal), and writing—review & editing (equal). Anna Teresa Palamara: Conceptualization (equal), methodology (equal), and writing—review & editing (equal). Maria Pia Conte: Conceptualization (equal), methodology (equal), supervision (lead), and writing—review & editing (equal). Anna Maria Zicari: Conceptualization (equal), methodology (equal), supervision (lead), and writing—review & editing (equal).
ETHICAL STATEMENT
The study was approved by the medical ethics review board of the Sapienza University of Rome, Policlinico Umberto I.
ACKNOWLEDGEMENTS
Open Access funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement. [Correction added on 11‐May‐2022, after first online publication: CRUI‐CARE funding statement has been added.]
Brindisi G, Marazzato M, Brunetti F, et al. Allergic rhinitis, microbiota and passive smoke in children: A pilot study. Pediatr Allergy Immunol. 2022;33 (Suppl. 27):22–26. 10.1111/pai.13621
Giulia Brindisi and Massimiliano Marazzato contributed equally to this work as first name.
Maria Pia Conte and Anna Maria Zicari contributed equally to this work as the last name.
REFERENCES
- 1. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines‐2016 revision. J Allergy ClinImmunol. 2017;140:950‐958. [DOI] [PubMed] [Google Scholar]
- 2. De Vittori V, Pacilio A, Indinnimeo L, et al. When asthma and rhinitis coexist, could rhinitis reduce asthma control in children? Allergy Asthma Proc. 2019;40:e8‐e13. [DOI] [PubMed] [Google Scholar]
- 3. Cilluffo G, Zicari AM, Ferrante G, et al. Assessing repeatability and reproducibility of Anterior Active Rhinomanometry (AAR) in children. BMC Med Res Methodol. 2020;20:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De S, Fenton JE, Jones AS, et al. Passive smoking, allergic rhinitis and nasal obstruction in children. J Laryngol Otol. 2005;119:955‐957. [DOI] [PubMed] [Google Scholar]
- 5. Shargorodsky J, Garcia‐Esquinas E, Navas‐Acien A, et al. Allergic sensitization, rhinitis, and tobacco smoke exposure in U.S. children and adolescents. Int Forum AllergyRhinol. 2015;5:471‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saulyte J, Regueira C, Montes‐Martinez A, et al. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta‐analysis. PLoSMed. 2014;11:e1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zapletal A, Chalupová J. Nasal airflow and resistance measured by active anterior rhinomanometry in healthy children and adolescents. Pediatr Pulmonol. 2002;33:174‐180. [DOI] [PubMed] [Google Scholar]
- 8. Montaño‐Velázquez BB, Navarrete RC, Mogica Martínez MD, et al. Rhinomanometry in young patients with perennial allergic rhinitis with/without recent exposure to tobacco smoke. Clin Otolaryngol. 2011;36:320‐324. [DOI] [PubMed] [Google Scholar]
- 9. Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation, and autoimmunity. J Autoimmun. 2010;34:J258‐265. [DOI] [PubMed] [Google Scholar]


