Abstract
Background
Hemostasis evaluation in chronic kidney disease (CKD) is critical for optimal management of thrombotic and bleeding events. Standard coagulation screens are inadequate for predicting coagulopathy in CKD.
Objective
To evaluate hemostasis parameters in patients with different stages of CKD using novel coagulation assays.
Patients/Methods
Cross‐sectional study of 30 healthy controls (HC) and 120 CKD patients (10 Stage 2, 20 Stage 3, 20 Stage 4, 20 Stage 5 not requiring renal replacement therapy, 20 transplant, 10 newly started on hemodialysis [HD], 20 established on HD). Standard laboratory tests were performed in addition to rotational thromboelastometry (ROTEM), multiple electrode aggregometry (MEA), thrombin generation assays, D‐dimer, and markers of thrombogenesis (thrombin‐antithrombin [TAT]), fibrinolysis, and endothelial activation (intercellular adhesion molecule‐1 [ICAM‐1]).
Results
D‐dimer, TAT, and ICAM‐1 concentrations were significantly higher in patients with CKD than HC (P < .01). ROTEM maximum clot firmness was significantly higher in patients than in HC (P < .01). In CKD Stage 5 patients (pre‐HD and started HD) adenosine diphosphate and thrombin receptor activating peptide MEA tests were significantly lower than HC indicating platelet aggregation defect (P < .05). Multivariate analysis confirmed the direct effect of estimated glomerular filtration rate (eGFR) in the variance of ROTEM and MEA tests. Endogenous thrombin potential and peak thrombin were not statistically different between groups, but Stage 5 CKD patients had prolonged lag time (7.91 vs. 6.33, P < .001) and time to thrombin peak (10.8 vs. 9.5, P < .05) compared to HC.
Conclusions
Patients with CKD exhibit features of concomitant hypercoagulability measured by ROTEM and platelet dysfunction measured with MEA. eGFR was an independent determinant of platelet dysfunction and hypercoagulability.
Keywords: chronic kidney disease, end‐stage kidney disease, hypercoagulability, platelet aggregation, thromboelastometry
Essentials.
Coagulopathy in chronic kidney disease (CKD) is challenging to detect with standard tests.
We assessed hemostasis using novel coagulation assays in 120 patients with varying stage CKD.
Patients with CKD exhibited features of concomitant hypercoagulability and platelet dysfunction.
Markers of both hypercoagulability and platelet dysfunction correlated with renal function.
1. INTRODUCTION
Chronic kidney disease (CKD) is associated with both bleeding and thrombotic tendencies. 1 The relationship between uremia and bleeding is likely to be multifactorial involving cellular (platelet function), 2 , 3 plasma (enzymatic coagulation cascade and fibrinolysis), 4 and endothelial abnormalities. 5 , 6 , 7 Anemia is also implicated because it is associated with reduced platelet‐vessel wall interaction. 8 Conversely, a prothrombotic state is proposed to be mediated by increased fibrinogen and von Willebrand factor (VWF) as part of the inflammatory state recognized by the pro‐inflammatory markers (C‐reactive protein [CRP], interleukin‐6) and endothelial dysfunction. 9 , 10 Most studies have focused on patients with end stage kidney disease (ESKD) and few have explored the relationship across the spectrum of CKD severity.
Clinical and laboratory assessment of bleeding and thrombotic risks in patients with CKD is critical for optimization prior to procedures and prevention of arterial and venous thromboembolic events. Strategies to reduce bleeding risk include commencing hemodialysis to reduce urea and/or the use of blood transfusion are based on clinical judgment to optimize risk prior to interventions. Both approaches are resource intensive and associated with additional complications; therefore, accurate individual risk prediction is required to reduce their need. However, standard coagulation screens provide limited information on hemostasis because they assess limited components of hemostasis. Enhanced approaches are desirable to enable a comprehensive risk evaluation of bleeding and thrombosis in patients with CKD.
Thromboelastometry (TEM) is a point‐of‐care test measuring viscoelastic clot formation in whole blood. It enables a global evaluation of coagulation 11 and its perioperative use has been demonstrated to reduce need for blood transfusion and improve outcomes during cardiac surgery. 12 Its utility in evaluating bleeding and thrombotic risk in patients with CKD is uncertain.
Multiple electrode aggregometry (MEA) assesses platelet aggregation in whole blood based on impedance aggregometry. 13 After activating platelets with specific agonists, this instrument detects the electrical resistance caused by their aggregation. 14 The Multiplate has been used in cardiac surgery to predict bleeding risk 15 and to reduce red cell transfusion requirement . 16 , 17 , 18 Specific defects in platelet function have been detected in small studies of pre‐dialysis patients but validation in larger cohorts has not been performed. 19 Thrombin generation assays (TGA) measure thrombin generated in plasma, initiated by the addition of recombinant tissue factor, and provide information about lag time, endogenous thrombin potential (ETP), peak thrombin, and time to thrombin peak (ttP). ETP has been demonstrated to be a predictor of both thrombotic 20 , 21 , 22 and hemorrhagic tendencies 23 , 24 in patients without renal disease, but TGA assessment in patients with renal disease is poorly understood.
The objectives of this study were to evaluate hemostasis parameters in patients with different stages of CKD using TEM, MEA, TGA, and markers of in vivo thrombin generation and fibrinolysis, and to explore the relationship between estimated glomerular filtration rate (eGFR), urea, dialysis, and endothelial activation with these assessments.
2. METHODS
2.1. Patient cohort and study design
A cross‐sectional study was conducted between September 2018 and January 2020 at King’s College Hospital with Research Ethics Committee approval (IRAS 245370). Patients with CKD including those starting and established on hemodialysis (HD) and with renal transplants and healthy controls (HC) were included. Exclusion criteria were inability to provide informed consent, antithrombotic medications (e.g., warfarin), known bleeding diathesis that can affect coagulation (e.g., liver disease), current malignancy (except skin cancers), paraproteinemia, thrombocytopenia (<100 × 109/L), active bleeding within 1 week, arterial and venous thromboembolism within the previous 3 months, and known thrombophilia (e.g., antiphospholipid syndrome). Sampling was by consecutive convenience, and all patients approached were confirmed to be eligible, provided data and samples, and completed the study.
For patients established on HD, samples were taken from fistulae or arterial line (with initial volume discarded) before injecting the anticoagulant prior to first session of the week and before first session in patients commencing HD. For HC and non‐dialysis CKD patients, samples were collected on a day of convenience. Serum samples were collected for urea and creatinine determination, full blood count was measured in ethylenediaminetetraacetic acid, and citrated (0.109 M) samples were collected for coagulation assays.
Citrated whole blood samples were centrifuged at 4750 g for 10 min, within 30 min of sample collection. Then, the top three‐quarters of supernatant was decanted into polypropylene tubes for TGA analysis. All the samples were then re‐centrifuged at 4750 g for 10 min. The resulting supernatant was transferred into 500 μl aliquots and stored at −80°C. Samples were analyzed in a batch at the end of the study. All samples were analyzed blinded to study group. Missing data were handled as missing; there was no imputation for missing data.
Rotational thromboelastometry (ROTEM) was measured with INTEM, EXTEM, and FIBTEM reagents, which evaluate the intrinsic pathway of coagulation, the extrinsic pathway, and fibrinogen contribution to clot, respectively. Whole blood samples were analyzed using the four‐channel ROTEM® delta, according to manufacturer’s instructions, within 4 h of sample collection. INTEM and EXTEM assays were performed by the addition of 20 μl CaCl2 0.2 mol/l (STAR‐TEM®) to 300 μl citrated whole blood with coagulation then activated by partial thromboplastin phospholipid derived from rabbit brain and ellagic acid (IN‐TEM®) or recombinant tissue factor and phospholipid (rEX‐TEM®), respectively. FIBTEM was performed by adding combined cytochalasin D (a thrombocyte inhibitor; FIB‐TEM®) and CaCl2 and then activating coagulation with 20 μl of rEX‐TEM® reagent. Parameters reported include those validated for clinical use: clotting time (CT; time taken for clot amplitude to reach 2 mm); clot firmness time (CFT; time for clot amplitude to increase from 2 mm to 20 mm); α‐angle (tangent from the point of CT to the slope of the curve); amplitude of the clot at 5, 10, and 20 min (A5, A10, and A20); maximum clot firmness (MCF; peak clot amplitude); lysis index at 30 min (LI30; percent of reduction in amplitude at 30 min after CT is detected); and maximum lysis (ML).
MEA was performed using a Multiplate® 5.0 analyzer, software V2.03.11 (Roche Diagnostics) according to manufacturer’s instructions. In Roche® Hirudin Tube or Sarstedt S‐Monovette® Hirudin test tubes, 1.6 ml whole blood was collected and analyzed within 30–120 min of blood collection after being allowed to rest for 30 min. In three measuring cells, 300 μl of blood were pipetted, mixed with 300μl of isotonic sodium chloride, and incubated at 37°C for 3 min. Twenty μl of adenosine diphosphate (ADP; 6.5 µM), arachidonic acid (0.5 mM), or thrombin receptor activating peptide (TRAP)‐6 (32 µM) were added for ADPtest, aspirin (ASPI) test, and TRAPtest, respectively. Maximum platelet aggregation was monitored over 6 min and expressed as aggregation unit (AU). Patients taking aspirin and/or P2Y12 inhibitors such as clopidogrel were excluded from analysis of ASPI and ADP tests, respectively.
TGAs were performed using platelet‐free plasma (PFP) after thawing the plasma at 37°C in a water bath. In a 96‐well microtiter plate, 20 µl of the PPP‐Low reagent (tissue factor 1 pM+ phospholipids 4 µM final concentration) were added to the thrombin generation wells. Twenty µl of thrombin calibrator were added to the calibration well. Then, 80 µl plasma were added to each well and incubated for 10 min at 37°C in the analyzer. After incubation, 20 µl Flu‐Ca (a mix of fluorinated substrate [amino‐methyl‐coumarin (AMC)] and fluorinated buffer [Hepes, calcium, and bovine serum albumin (BSA)]) were added automatically to each well. Fluoroskan Ascent‐ Thrombinoscope‐Hemker (CAT) (Serial No: 3743139) Software version Release 5.0.0.742 was used according to manufacturer’s instructions. The lag time, peak, ETP, and ttP were analyzed from the thrombin generation curve. Laboratory reference ranges using double spun normal plasma are derived from 60 healthy volunteers.
TAT, alpha 2‐antiplasmin, platelet activator inhibitor‐1 (PAI‐1), and intracellular adhesion molecule‐1 (ICAM‐1) were measured by enzyme‐linked immunosorbent assays using AssayPro LLP TAT (Universal Biologicals Ltd), Human Alpha 2‐antiplasmin ELISA Kit (Abbexa Ltd), and Quantikine PAI‐1 and ICAM‐1 ELISA kits (Biotechne, R & D Systems Europe) respectively. For TAT, alpha 2‐antiplasmin, PAI‐1, and ICAM‐1, the inter‐assay coefficient of variation was 8.8%, ≤10, 8.7%, and 7.8%, respectively; the intra‐assay coefficient of variation was 5.4%, ≤10%, 4.6%, and 3.6%, respectively.
D‐dimer measurement was performed with a latex enhanced immunoturbidimetric assay (Diazyme Laboratories, Inc.) on a Siemens Advia 1800 analyzer as per manufacturer’s instructions. The inter‐assay coefficient of variation was 6.2%; the intra‐assay coefficient of variation was 5%.
2.2. Sample size calculation and statistical analysis
A sample size of 20 patients and 4 healthy controls would enable an effect size of 1.05 estimated from differences in R time between cases and controls in previous work with 80% power with significance level .05. 25 Recruitment of 120 patients and 30 healthy controls was decided upon to enable subgroup analysis to be sufficiently powered.
Data analysis was performed with IBM SPSS® Statistics version 23.0 software. Normal distribution was assessed by Shapiro‐Wilk test. Data were expressed as median and interquartile range for non‐parametric data and as mean and standard deviation for parametric data. For continuous variables, comparison between healthy controls and patients were performed using Student’s t‐test and Mann–Whitney test for parametric and non‐parametric data, respectively.
To compare different patient groups, analysis of variance (ANOVA) and Kruskal–Wallis tests were used for parametric and non‐parametric data, respectively. For categorical data, comparisons between groups were performed using Chi‐square and Monte Carlo tests. For correlations, Spearman correlation coefficient was used. A value of P < .05 was considered significant. In a second step, multivariate analysis was performed to determine contribution of kidney disease severity to the variance of the novel assays when controlling for other predictor variables. The analysis performed was linear regression using each of the hemostasis parameters as continuous dependent variables in separate regression analysis. The hemostasis parameters included in the regression analysis were selected a priori due to potential contribution to the variance of the global assessments. Demographic characteristics of participants (age, sex, ethnicity, and body mass index [BMI]) were introduced in all analyses. In ROTEM assays, Hb, platelet concentration, urea, eGFR, and PAI‐1 were introduced as predictor variables. MEA analysis included Hb, platelet, urea, eGFR, and exposure to aspirin or clopidogrel. In TGAs, eGFR and presence of diabetes were introduced as predictor variables, in addition to demographic variables. Ethnicity was categorized (Caucasian, Black, Asian, and other) into a binary variable and introduced as single dichotomous predictor variables. Tolerance and variance inflation factor (VIF) were calculated in all analyses to exclude structural multicollinearity (thresholds defined for multicollinearity was a tolerance of less than 0.1 and VIF of higher than 10). Adjustment for multiple testing was not undertaken due to the exploratory nature of the work, relatively small sample size, and the need to identify potential contributory pathways to validate in a larger cohort.
3. RESULTS
One hundred and fifty participants included 30 HC and 120 CKD patients (10 Stage 2, 20 Stage 3, 20 Stage 4, 20 Stage 5 not yet requiring renal replacement therapy, 10 newly started on HD, and 20 patients established on HD). Demographics and baseline characteristics of participants are presented in Table 1. Minimal data was missing (see Table S1 in supporting information). There were significant differences in age, sex, and ethnicity between patients and controls (P < .05).
TABLE 1.
Demographic and clinical characteristics of patients with CKD and healthy controls
| Patients with CKD (n = 120) | Healthy controls (n = 30) | P | |
|---|---|---|---|
| Age (years) | 55±14 | 48±13 | .013 |
| Male n (%) | 74 (61%) | 8 (26%) | .001 |
| Race n (%) | |||
| Caucasian | 68 (56%) | 13 (43%) | .010 |
| Asian | 9 (30%) | 9 (7%) | |
| Black | 38 (31%) | 7 (23%) | |
| Others | 1 (3%) | 5 (4%) | |
| BMI (kg/m2) | 28.76 ± 5.4 | 26.07 ± 3.7 | 0.013 |
| Cause of CKD n (%) | |||
| Diabetes mellitus | 35 (29%) | ||
| Hypertension | 25 (21%) | ||
| Cystic kidney disease | 21 (17%) | ||
| Obstructive uropathy | 25 (21%) | ||
| Miscellaneous | 10 (8%) | ||
| Unknown | 2 (2%) | ||
| Medications n (%) | |||
| Aspirin | 46 (38%) | 0 (0%) | |
| Clopidogrel | 17 (14%) | 0 (0%) | |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease.
Standard laboratory tests including urea, eGFR, hemoglobin (HGB), packed cell volume (PCV), platelets (PLT), international normalized ratio (INR), and activated partial thromboplastin time ratio (APTR) are summarized in Table 2. Median HGB, PCV, and PLT were lower in patients with CKD compared to HC but there were no differences in INR or APTR.
TABLE 2.
Laboratory values: renal function, markers of hemostasis, thrombogenesis, fibrinolysis, and endothelial activation. Data are expressed as median (IQR)
| Patients with CKD (n = 120) | Healthy Controls (n = 30) | p | CKD stage 2 (n = 10) | CKD stage 3 (n = 20) | CKD stage 4 (n = 20) | CKD stage 5 (n = 20) | HD new starters (n = 10) | Established HD (n = 20) | Transplant (n = 20) | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea mmol/L | 15 (8,24) | 4.6 (4–5) | <.001 | 5 (5, 8) | 10 (7, 11) | 16 (14, 21) | 24 (18, 31) | 32 (30, 36) | 20 (16, 26) | 7 (6, 12) | <.001 |
| eGFR ml/min*/1.73 m2 | 18 (9,46) | 90 (80,90) | <.001 | 66 (64, 68) | 45 (35, 49) | 18 (18, 25) | 11 (9, 13) | 5 (4, 7) | 5 (4, 6) | 56 (40, 63) | <.001 |
| Hemoglobin g/L | 118 (105,131) | 138 (129, 142) | <.001 | 145 (136, 158) | 129 (123, 131) | 112 (103, 120) | 114 (105, 124) | 93 (86, 99) | 111 (104, 117) | 133 (121, 143) | <.001 |
| PCV | 0.38 (0.34, 0.42) | 0.42 (0.4, 0.44) | <.001 | 0.45 (0.43, 0.47) | 0.40 (0.38, 0.42) | 0.36 (0.34, 0.38) | 0.36 (0.33, 0.38) | 0.30 (0.28, 0.31) | 0.36 (0.34, 0.38) | 0.42 (0.38, 0.44) | <.001 |
| Platelets × 109/L | 233 (196, 275) | 263 (221, 286) | .044 | 248 (233, 289) | 240 (213, 269) | 246 (204, 290) | 200 (163, 269) | 293 (217, 369) | 207 (171, 236) | 234 (196, 262) | .018 |
| INR | 1.03 (0.99, 1.08) | 1.01 (0.95, 1.04) | N.S. | 0.98 (0.98, 1.05) | 1.0 (0.99, 1.06) | 1.02 (0.98, 1.04) | 1.03 (0.98, 1.08) | 1.10 (1.08, 1.12) | 1.05 (1.01, 1.19) | 1.03 (0.99, 1.07) | .002 |
| APTR | 1.04 (1.0, 1.11) | 1 (0.98, 1.08) | N.S. | 1.04 (0.98, 1.05) | 1.02 (1.0, 1.10) | 1.03 (0.96, 1.07) | 1.03 (0.96, 1.09) | 1.12 (1.02, 1.22) | 1.08 (1.03, 1.23) | 1.06 (0.98, 1.11) | N.S. |
| α‐2 antiplasmin μg/L | 2142 (452, 6148) | 2173 (993, 3640) | N.S. | 956 (255, 1795) | 2616 (471, 5734) | 2787 (576, 8000) | 3712 (594, 6738) | 1743 (800, 3035) | 702 (365, 19588) | 648 (291, 2690) | N.S. |
| PAI‐1 μg/L | 1.5 (1.1, 2.7) | 1.7 (1.0, 2.5) | N.S. | 1.4 (1.3, 2.2) | 1.5 (0.9, 2.5) | 1.4 (1.1, 2.4) | 1.4 (1.1, 1.5) | 0.8 (0.7, 1.0) | 2.0 (1.2, 3.4) | 3.1 (1.9, 4.6) | <.001 |
| TAT μg/L | 2.5 (1.8, 3.5) | 1.7 (1.0, 2.5) | .001 | 2.3 (1.8, 2.8) | 3.0 (2.3, 3.6) | 2.6 (2.2, 3.4) | 2.2 (1.8, 3.7) | 1.9 (1.5, 3.1) | 2.5 (1.5, 4.5) | 2.7 (2.0, 3.5) | N.S. |
| D‐dimer μg/L | 0.5 (0.3, 1.3) | 0. 2 (0.1, 0.3) | <.001 | 0.2 (0.1, 0.3) | 0.4 (0.3, 0.6) | 0.7 (0.4, 1.4) | 0.5 (0.3, 1.4) | 1.0 (0.6, 2.5) | 1.0 (0.4, 1.5) | 0.3 (0.2, 0.7) | .001 |
| ICAM‐1 μg/L | 281 (242, 325) | 222 (191, 248) | <.001 | 231 (182, 262) | 292 (258, 338) | 305 (268, 334) | 303 (262, 342) | 270 (219, 313) | 259 (240, 321) | 252 (208, 292) | .004 |
Abbreviations: APTR, activated partial thromboplastin ratio; eGFR, estimated glomerular filtration rate; ICAM‐1, intercellular adhesion molecule‐1; INR, international normalized ratio; N.S., non‐significant; PAI‐1, platelet activator inhibitor‐1; PCV, packed cell volume; TAT, thrombin antithrombin.
3.1. Markers of hemostasis and endothelial dysfunction
Both markers of fibrinolysis, alpha 2‐antiplasmin and PAI‐1, were comparable between patients with CKD and HC. In contrast, D‐dimer, TAT, and ICAM‐1 were significantly higher in patients with CKD than HC (Table 2).
3.2. Rotational thromboelastometry
INTEM and EXTEM CFT were significantly shorter in patients with CKD than HC (49 s vs. 55 s, P < .01 and 58 s vs. 68 s, P < .01 respectively). However, only a minority of patients with CKD had CFT below the lower limit of the published reference range (INTEM: 22/120; EXTEM: 28/120). CFT was directly correlated with eGFR for both INTEM (r = .36, P < .001) and EXTEM (r = .40, P < .001), HGB and PCV, and urea, and inversely with PLT.
α‐angle, A5, A10, A20, and MCF for INTEM and EXTEM were significantly increased in patients with CKD compared to HC (P < .01). These parameters directly correlated with eGFR and PLT and inversely with HGB and PCV (P < .001). Similarly, MCF of FIBTEM was higher in CKD patients than controls (24 mm vs. 18 mm, P < .001) and inversely correlated with eGFR (r = −.45, P < .001), HGB (r = −.61, P < .001) and PCV (r = −.62, P < .001). CT INTEM correlated with APTR (r = .31, P <.001) but there was no association between CT EXTEM and INR. There were no differences in LI30 between CKD patients and HC. Although ML showed a significant difference between CKD patients and HC, the values remained within normal range indicating that no hyperfibrinolysis was detected in CKD patients. INTEM LI30 correlated with PAI‐1 (r = .33, P < .001). Figure 1 shows the progression of CFT, MCF, and ML of EXTEM and MCF of FIBTEM with progressive CKD.
FIGURE 1.
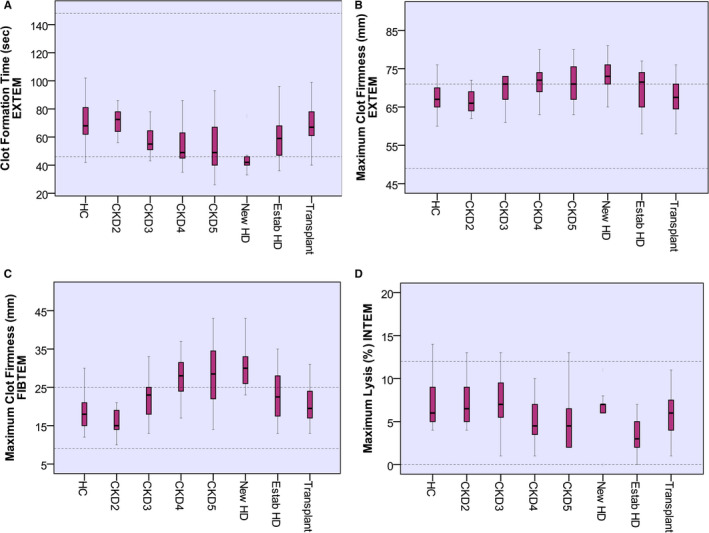
Results of ROTEM parameters in different studied groups. Data are given as median and interquartile ranges. Clot formation time EXTEM (A) was shorter with progression of CKD, P < .01. Maximum clot firmness EXTEM (B) and Maximum clot firmness FIBTEM (C) were increasing with worsening of eGFR, P < .01. Maximum lysis INTEM (D) is lower with progression of CKD, P < .01. Dotted lines indicate normal range. CKD, chronic kidney disease; HC, healthy control; HD, hemodialysis; ROTEM, rotational thromboelastometry
Linear regression analysis confirms the relationship between CKD severity and variance of TEM assays. eGFR has a significant explanatory effect in the variance of MCF and ML of INTEM parameters, CFT, α‐angle, and MCF of EXTEM parameters, holding all other predictor variables constant (Table 3).
TABLE 3.
Linear regression analysis of ROTEM assays: unstandardised coefficients
| INTEM | EXTEM | FIBTEM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CT | CFT | α‐angle | MCF | ML | CFT | α‐angle | MCF | MCF | |
| Age | −0.04 | 0.04 | −0.01 | 0 | −0.03 | 0.05 | −0.02 | 0.01 | 0.05 |
| Sex (Male = 1) | 2.03 | −4.04 | 0.44 | 0.02 | −0.47 | 2.66 | −0.32 | −1.06 | −1.71 |
| Caucasian | −8.94 | 22.94 | −2.96+ | −3.99+ | 0.14 | 6.46 | −1.31 | −3.04+ | −2.35 |
| Black | 3.09 | 8.35 | −1.27 | −2.88 | 0.6 | 4.04 | −0.75 | −3.02+ | −3.68 |
| Asian | 0.61 | 14.85 | −1.77 | −2.4 | −0.45 | 1.83 | −0.55 | −1.73 | −1.37 |
| BMI | 0.27 | 0.41 | −0.03 | 0.02 | 0.01 | 0.02 | 0 | 0 | 0.17+ |
| HGB | 0.21 | −0.49* | 0.04 | −0.01 | n/a | 0.34** | −0.07** | −0.07** | −0.15** |
| Platelets | n/a | −0.22** | 0.03** | 0.04** | n/a | −0.11** | 0.02** | 0.04** | n/a |
| Urea | −0.89** | −0.38 | 0.05 | −0.03 | −0.06 | 0.07 | −0.03 | −0.05 | 0.04 |
| eGFR | −0.19+ | 0.27 | −0.04+ | −0.09** | 0.04* | 0.16* | −0.04* | −0.06** | −0.04 |
| PAI‐1 | n/a | n/a | n/a | n/a | −0.22+ | n/a | n/a | n/a | n/a |
| Intercept | 170.42** | 136.32** | 70.07** | 64.49** | 7.25** | 30.78+ | 86.62** | 74.92** | 38.33** |
| Adjusted R 2 | 0.1 | 0.11 | 0.18 | 0.26 | 0.22 | 0.36 | 0.35 | 0.4 | 0.34 |
| Significance | 0.01 | 0.01 | <.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Abbreviations: BMI, body mass index; CFT, clot formation time; CT, clotting time; eGFR, estimated glomerular filtration rate; HGB, hemoglobin; MCF, maximum clot firmness; ML, maximum lysis; PAI‐1, platelet activator inhibitor‐1.
Notes: Significance level: **P ≤ .01; *P ≤ .05; +P ≤ .10. CT and ML were not included for EXTEM, as regression model was not statistically significant.
3.3. Multiple electrode aggregometry
There were no statistical differences between patients with CKD and HC in ADP, ASPI, and TRAP tests (Table 4). However, when ESKD (pre‐dialysis CKD stage 5 and HD patients) were compared to HC, ADP and TRAP tests were significantly lower in ESKD (37 AU vs. 61 AU, P < .01 and 78 AU vs. 112 AU, P < .05, respectively). Analysis for ASPI and ADP tests excluded 46 patients taking aspirin and 18 taking clopidogrel, respectively. Higher proportions of patients with CKD tended to have lower than normal reference range MEA tests than HC but this was not statistically significant: ADP <57 AU: 59/103 (57%) versus 13/29 (44%; P = .34); ASPI <71 AU: 30/74 (40%) versus 12/30 (40%; P = .32); TRAP <84 AU: 49/120 (40%) versus 7/30 (23%; P = .12). Multivariate analysis confirmed the direct effect of urea and eGFR in the variance of both ADP and TRAP tests, holding all other variables constant (demographic variables, HGB, PLT, and presence of clopidogrel for ADP test; Table 5).
TABLE 4.
Multiple electrode aggregometry analysis according to study group and healthy controls and patients with end‐stage kidney disease and healthy controls after excluding the patients on antiplatelets medications. Data are expressed as median (IQR). Normal ranges for each test are presented in the left‐hand column
| Study group (n = 103) | Healthy Controls (n = 29) | P | ESKD (n = 28) | Healthy Controls (n = 29) | P | |
|---|---|---|---|---|---|---|
| ADP test 57–113 AU | 50 (28, 70) | 61 (41, 78) | .13 | 37 (20, 61) | 61 (41, 78) | .005 |
| Study group (n = 74) | Healthy controls (n = 30) | P | ESKD (n = 41) | Healthy controls (n = 30) | P | |
|---|---|---|---|---|---|---|
| ASPI test 71–115 AU | 87 (58, 103) | 75 (56, 89) | .07 | 64 (48, 100) | 75 (56, 89) | .976 |
| Study group (n = 120) | Healthy controls (n = 30) | P | ESKD (n = 31) | Healthy controls (n = 30) | P | |
|---|---|---|---|---|---|---|
| TRAP test 84–128 AU | 92 (67, 120) | 112 (84, 121) | .16 | 78 (55, 115) | 112 (84, 121) | .011 |
Abbreviations: ADP, adenosine diphosphate; ASPI, aspirin; ESKD, end‐stage kidney disease; TRAP, thrombin receptor activating peptide.
TABLE 5.
Linear regression analysis of MEA and TGA assays: unstandardized coefficients
| Multiple electrode aggregometry | Thrombin generation assays | ||||||
|---|---|---|---|---|---|---|---|
| ASPI test | ADP test | TRAP test | Lag Time | ETP | Time to peak | Peak thrombin | |
| Age | −0.16 | 0.12 | 0.14 | 0.02 | −0.28 | 0.02 | 0.59 |
| Sex (Male = 1) | −10.10+ | −3.68 | −9.91 | 0.62+ | −66.51 | 0.48 | 1.97 |
| Caucasian | 1.28 | −8.74 | 1.12 | 0.59 | −55.16 | 1.3 | −70.26* |
| Black | −6.04 | −20.99+ | −24.41 | 0.41 | −126.35 | 1.34 | −96.78** |
| Asian | −24.33 | −13.21 | −12.35 | 0.27 | 51.49 | 1.11 | −51.81 |
| BMI | 0.45 | 0.24 | −0.05 | −0.04 | 14.17** | −0.06+ | 4.17** |
| HGB | 0.19 | 0.05 | −0.32 | n/a | n/a | n/a | n/a |
| Platelets | 0.19** | 0.25** | n/a | n/a | n/a | n/a | n/a |
| Urea | 1.07** | 1.29** | 1.28** | n/a | n/a | n/a | n/a |
| eGFR | 0.03 | 0.31* | 0.59** | −0.02** | 0.57 | −0.01* | −0.34 |
| Aspirin (1 = Yes) | −57.18** | n/a | n/a | n/a | n/a | n/a | n/a |
| Clopidogrel (1 = Yes) | n/a | −32.64** | n/a | n/a | n/a | n/a | n/a |
| Diabetes (1 = Yes) | n/a | n/a | n/a | n/a | n/a | n/a | −21.04 |
| Intercept | −6.29** | −42.76** | 99.29** | 7.58** | 771.61** | 10.69** | 151.92** |
| Adjusted R 2 | 0.54 | 0.39 | 0.11 | 0.12 | 0.07 | 0.04 | 0.11 |
| Significance | 0 | 0 | 0.006 | 0.002 | 0.025 | 0.11 | 0.004 |
Abbreviations: ADP, adenosine diphosphate; ASPI, aspirin; BMI, body mass index; eGFR, estimated glomerular filtration rate; ETP, endogenous thrombin potential; HGB, hemoglobin; TRAP, thrombin receptor activating peptide.
Notes: Significance level: ** P ≤ .01; * P ≤ .05; + P ≤ .10. Time to peak was not included as regression model was not statistically significant.
3.4. Thrombin generation assays
Lag time and ttP were longer in patients with CKD than HC (7.58 min vs. 6.33 min, P < .001) and (10.6 min vs. 9.5 min, P < .01), respectively. ETP and peak thrombin were not statistically different between groups (Figure 2). Comparing ESKD patients to HC, the former had a prolonged lag time (7.91 vs. 6.33, P < .001) and ttP (10.8 vs. 9.5, P < .05). Figure 3 shows the progression of lag time, ETP, peak thrombin, and ttP with progressive CKD. Lag time and ttP correlated directly with urea (r = .39, P < .001 and r = .25, P < .01, respectively) and inversely with eGFR (r = −.36, P < .001 and r = −.23, P < .01, respectively). ETP and peak thrombin inversely correlated with CT INTEM (r = −.20, P = .02, r = −.34, P < .001), respectively. Lag time inversely correlated with CT EXTEM (r = −.29, P < .001) and CFT EXTEM (r = −.32, P < .001). On regression analysis, eGFR was a significant contributor to lag time, with BMI representing the main determinant of ETP and peak thrombin.
FIGURE 2.
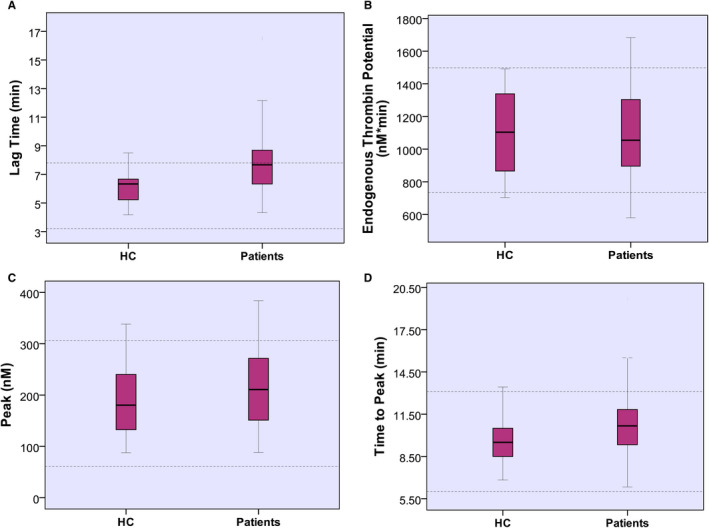
Results of thrombin generation assays in healthy controls and chronic kidney disease patients. Lag time (A) was longer in patients than healthy controls, p < .001. Endogenous thrombin potential (B) and peak thrombin (C) are comparable between healthy controls and patients. Time to peak (D) is longer in patients than healthy controls, P < .01. Dotted lines indicate normal range
FIGURE 3.
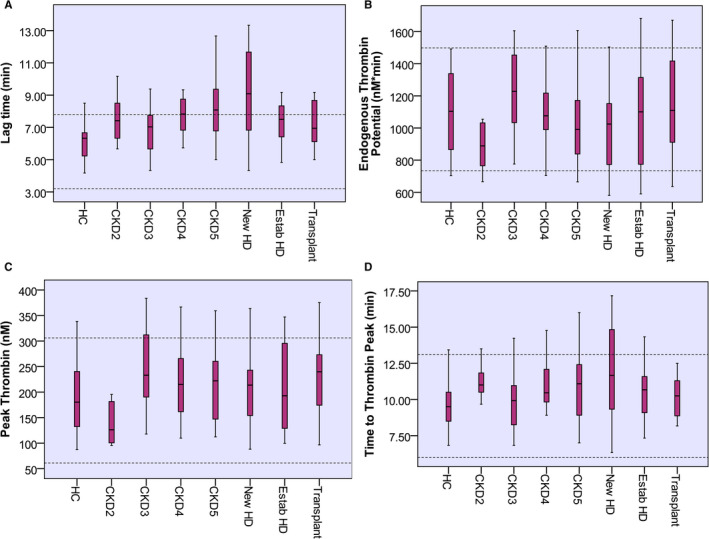
Results of thrombin generation assays in different studied groups. Data are presented as median and interquartile ranges. Lag time (A), peak thrombin (C), and time to peak (D) were comparable between subgroups. Endogenous thrombin potential (B) was significantly different between patient subgroups P = .036. Dotted lines indicate normal range. CKD, chronic kidney disease; HC, healthy control; HD, hemodialysis
4. DISCUSSION
The findings of this study confirm that patients with CKD have changes of both hyper‐ and hypo‐coagulability identified with novel global assessments of hemostasis. Analyzing the viscoelastic properties of clot formation with ROTEM demonstrated a prothrombotic state (with reduced CFT and increased MCF) while measures of fibrinolysis showed hypofibrinolysis. MEA detected a platelet aggregation defect in ESKD patients.
As a rapid, point‐of‐care device, ROTEM can be used to detect early coagulopathy and inform treatment in the surgical setting. 26 For example, hemostatic monitoring with ROTEM is used to ensure appropriate blood product transfusion in bleeding during liver transplantation surgery, 27 trauma, 28 cardiac surgery, 12 and postpartum hemorrhage. 29 When optimizing patients with CKD for procedures, ROTEM parameters are unlikely to be useful in predicting bleeding as no features of hypocoagulability were seen. Of note, the ROTEM assays we utilized in this study are insensitive to platelet dysfunction; further study with ROTEM in this patient group should investigate the role (if any) of platelet mapping.
Poor correlations were found between INR and APTT and EXTEM (extrinsic pathway) and INTEM (intrinsic pathway) parameters, respectively, which has also been described by others 30 and may be due to ROTEM using whole blood rather than plasma. However, strong correlations between INTEM and APTT and EXTEM and PT have been reported in different patient groups, which may be attributed to the presence of coagulopathies, for example, patients with liver cirrhosis who have defective liver synthetic function, 31 whereas, in our study standard coagulation tests and ROTEM parameters were within the normal range.
In our study, ROTEM detected a prothrombotic and hypofibrinolytic state in CKD patients. This finding has also been described by Gäckler et al., 32 who performed ROTEM in 40 ESKD patients and reported raised MCF FIBTEM, which has also been described in smaller cohorts, 25 and in other hypercoagulable conditions including severe COVID‐19 pneumonia 33 and malignancy. 34 Similarly, Darlington et al. 35 assessed 70 ESKD patients with thromboelastography (TEG) and reported 41.4% of ESKD patients had hypercoagulable features; however, hypocoagulable features were also identified in 42.9% of patients and 15.7% had both abnormalities, but regression analysis was not undertaken by the authors to identify which components of the coagulation pathway were contributory.
Regression analysis confirmed ROTEM parameters were strongly correlated with hemoglobin (CFT INTEM, CFT EXTEM, MCF EXTEM, and FIBTEM, inversely) and platelet count (MCF INTEM and EXTEM, directly; CFT INTEM and EXTEM, inversely), as MCF and CFT depend on platelet count. This has also been described in bleeding trauma patients 28 and orthotopic liver transplantation 36 studies, which concluded ROTEM parameters can be used as a surrogate for thrombocytopenia and to guide the need for platelet transfusion. However, no other studies have demonstrated the independent association between eGFR and ROTEM parameters and notably urea was only a predictor of INTEM CT. A proposed mechanism for the prothrombotic state in CKD is that it is secondary to the inflammatory state that occurs due to reduced clearance and accumulation of pro‐inflammatory substances such as advanced glycation end‐products (AGE), reduced defence mechanisms against oxidative stress, and dialysis‐related problems (for example, vascular access infections or dialysate back‐leak). These factors will contribute to endothelial and platelet activation and increased production of coagulation factors by the liver and thus generate a prothrombotic state. 37 This is supported by the relationship between MCF FIBTEM and ICAM‐1 identified in this study.
In our study, MEA showed significant reduction in platelet aggregation using the ADP and TRAP tests in ESKD patients compared to controls and regression analysis confirmed a direct effect of platelet count, urea, and eGFR, with urea being the strongest predictor. Platelet dysfunction on evaluation with either MEA or an alternative platelet function analyzer, PFA‐100®, has been reported in three previous small cohorts of patients with ESKD but without further evaluation of independent determinants. 25 , 31 , 38
As platelets are pivotal in primary hemostasis, platelet dysfunction in CKD is likely to contribute to recognized bleeding risk. MEA offers the advantage of rapid evaluation of platelet function before interventional procedures such as renal biopsies and vascular line insertions and may be a useful predictor of bleeding risk. 39 It could also be used to guide periprocedural safety of antiplatelet medications (for example, aspirin and clopidogrel), which are widely prescribed in uremic CKD patients. 40 However, others have suggested that MEA is an assessment of platelet aggregation only and that increased levels of platelet adhesive protein VWF in CKD may compensate for this defect. 41 Prospective study of the predictive performance of MEA to assess bleeding risk in patients with CKD is needed.
In this study, TGA did not show evidence of increased thrombin generation in CKD, but lag time and time to peak were prolonged suggesting delayed thrombin generation. Gäckler et al. also reported a prolonged lag time with lower ETP, peak, and velocity index in 10 HD patients compared to HC. 32 Brophy et al. 42 investigated thrombin generation time in 10 CKD stage 3–5 patients and 10 ESKD on maintenance HD but found no differences compared to HC, which may reflect the small sample size. However, others 24 have reported that ETP in 58 stable HD patients with fistulae was significantly lower compared to HC, with no significant differences found in lag time, peak thrombin, or ttP. Unsurprisingly, 11 HD patients with acute vascular access thrombosis had increased ETP compared to those without thrombosis. In our study, we found BMI to be a significant determinant of ETP and peak thrombin in keeping with Campello et al. 43 Earlier studies did not report BMI, but it is possible that the increased BMI along with other comorbid disease in our cohort contributes to the differences in reported findings. Additional contributors may be variation in pre‐analytic variables, such as sample collection. The lack of inter‐laboratory standardization is a recognized limitation of TGA, which has limited its integration into routine clinical care. 44
Markers of fibrinolysis, α‐2 anti‐plasmin, and PAI‐1 did not demonstrate hyperfibrinolysis in patients with CKD, indeed they suggested hypofibrinolysis, in keeping with historic studies of patients with ESKD. 45 , 46 Moreover, Lottermoser et al. 46 suggested that decreased availability of tissue plasminogen activator (t‐PA) in 22 ESKD patients may contribute to increased thrombotic risk.
Raised markers of endothelial activation including ICAM‐1 have been described in other cohorts of ESKD patients 47 including in children and young adults 48 and in both pre‐dialysis and ESKD patients receiving HD. Impaired ICAM‐1 clearance and enhanced synthesis, related to malnutrition and inflammation, were reported to be associated with elevated ICAM‐1 concentrations in 88 pre‐dialysis CKD and HD patients, 49 and endothelial activation due to accumulation of AGE leading to oxidative stress that results in decreased release of nitric oxide was proposed. 50 Others have also reported elevated D‐dimer 51 in patients with renal insufficiency, consistent with our study findings. Vaziri et al. 2 found that D‐dimer was elevated in ESKD patients compared to HC. Catena et al. 51 found that D‐dimer was elevated in patients with hypertension and mild to moderate renal impairment independent of age, blood pressure, duration of hypertension, triglyceride level, urinary protein excretion, and erythrocyte sedimentation rate, due to increased production of thrombin that is related to severity of renal impairment.
There are limitations to this study including the generalizability of a single‐center study and small numbers potentially precluding adequate power and the lack of exploration of clinical thrombotic or bleeding events. Only platelet aggregation was tested, and platelet secretion and adhesions assessments were not performed. The impact of serial dialysis on platelet defects needs studying but was too challenging to undertake in this study due to timing of study sample collection in those requiring urgent dialysis.
In conclusion, this comprehensive assessment of hemostasis in a spectrum of patients with CKD showed platelet dysfunction and features of hypercoagulability on ROTEM. eGFR was a significant determinant of TEM parameters (CFT and MCF), with urea and eGFR significantly contributing to platelet aggregation on MEA ADP and TRAP tests. We suggest that ROTEM is unlikely to be useful for assessing bleeding risk but its role in predicting arterial and venous thromboembolic events needs to be explored. MEA assessment of platelet function prior to interventional procedures may extend the diagnostic spectrum beyond that offered by the standard coagulation tests for patients with CKD and warrants further study to assess its ability to predict bleeding risk in CKD.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
A. Abdelmaguid carried out experiments, undertook data analysis and interpretation, and made the figures and drafted the manuscript. L. N. Roberts carried out experiments and revised the manuscript. L. Tugores undertook data analysis and interpretation. J. R. Joslin undertook data analysis and interpretation and revised the manuscript. B. J. Hunt carried out experiments and revised the manuscript. K. Parmar carried out experiments. D. Nebres carried out experiments. S. S. Naga made the figures and drafted the manuscript. E. S. Khalil made the figures and drafted the manuscript. K. Bramham designed the study and revised the manuscript. All authors approved the final version of the manuscript.
Supporting information
Table S1
ACKNOWLEDGMENTS
This work was supported by grants from Kidney Research UK; the British Society of Haematology; the Missions Sector‐Ministry of Higher Education, Egypt; and the Newton‐Mosharafa program. The authors thank Erika Manolo for her excellent assistance with data and sample collection and for performing ROTEM.
Abdelmaguid A, Roberts LN, Tugores L, et al. Evaluation of novel coagulation and platelet function assays in patients with chronic kidney disease. J Thromb Haemost. 2022;20:845–856. doi: 10.1111/jth.15653
Manuscript handled by: Matthew T. Rondina
Final decision: Matthew T. Rondina, 18 January 2022
REFERENCES
- 1. Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 2. Vaziri ND, Gonzales EC, Wang J, Said S. Blood coagulation, fibrinolytic, and inhibitory proteins in end‐stage renal disease: effect of hemodialysis. Am J Kidney Dis. 1994;23(6):828‐835. [DOI] [PubMed] [Google Scholar]
- 3. Ho SJ, Gemmell R, Brighton TA. Platelet function testing in uraemic patients. Hematology. 2008;13(1):49‐58. [DOI] [PubMed] [Google Scholar]
- 4. Molino D, De Lucia D, Gaspare De Santo N. Coagulation disorders in uremia. Semin Nephrol. 2006;26(1):46‐51. [DOI] [PubMed] [Google Scholar]
- 5. Di Minno G, Martinez J, McKean ML, De La Rosa J, Burke JF, Murphy S. Platelet dysfunction in uremia. Multifaceted defect partially corrected by dialysis. Am J Med. 1985;79(5):552‐559. [DOI] [PubMed] [Google Scholar]
- 6. Evans EP, Branch RA, Bloom AL. A clinical and experimental study of platelet function in chronic renal failure. J Clin Pathol. 1972;25(9):745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballard HS, Marcus AJ. Primary and secondary platelet aggregation in uraemia. Scand J Haematol. 1972;9(3):198‐203. [DOI] [PubMed] [Google Scholar]
- 8. Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011;25(6):271‐278. [DOI] [PubMed] [Google Scholar]
- 9. Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 10. Stam F, van Guldener C, Schalkwijk CG, ter Wee PM, Donker AJ, Stehouwer CD. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18(5):892‐898. [DOI] [PubMed] [Google Scholar]
- 11. Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point‐of‐care coagulation devices. Anesth Analg. 2008;106(5):1366‐1375. [DOI] [PubMed] [Google Scholar]
- 12. Raphael J, Mazer CD, Subramani S, et al. Society of cardiovascular anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth Analg. 2019;129(5):1209‐1221. [DOI] [PubMed] [Google Scholar]
- 13. Cardinal DC, Flower RJ. The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods. 1980;3(2):135‐158. [DOI] [PubMed] [Google Scholar]
- 14. Grove EL, Storey RF, Wurtz M. Platelet function testing in atherothrombotic disease. Curr Pharm Des. 2012;18(33):5379‐5391. [DOI] [PubMed] [Google Scholar]
- 15. Gorlinger K, Dirkmann D, Hanke AA, et al. First‐line therapy with coagulation factor concentrates combined with point‐of‐care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single‐center cohort study. Anesthesiology. 2011;115(6):1179‐1191. [DOI] [PubMed] [Google Scholar]
- 16. Agarwal S, Johnson RI, Shaw M. Preoperative point‐of‐care platelet function testing in cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29(2):333‐341. [DOI] [PubMed] [Google Scholar]
- 17. Petricevic M, Konosic S, Biocina B, et al. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM((R)) platelet and Multiplate((R)) impedance aggregometry. Anaesthesia. 2016;71(6):636‐647. [DOI] [PubMed] [Google Scholar]
- 18. Zeck J, Schallheim J, Lew SQ, DePalma L. Whole blood platelet aggregation and release reaction testing in uremic patients. Biomed Res Int. 2013;2013:486290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood. 2005;106(7):2363‐2365. [DOI] [PubMed] [Google Scholar]
- 20. Brandts A, van Hylckama VA, Rosing J, Baglin TP, Rosendaal FR. The risk of venous thrombosis associated with a high endogenous thrombin potential in the absence and presence of activated protein C. J Thromb Haemost. 2007;5(2):416‐418. [DOI] [PubMed] [Google Scholar]
- 21. Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/‐ thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost. 2006;96(5):562‐567. [PubMed] [Google Scholar]
- 22. Castoldi E, Govers‐Riemslag JW, Pinotti M, et al. Coinheritance of Factor V (FV) Leiden enhances thrombin formation and is associated with a mild bleeding phenotype in patients homozygous for the FVII 9726+5G>A (FVII Lazio) mutation. Blood. 2003;102(12):4014‐4020. [DOI] [PubMed] [Google Scholar]
- 23. Dargaud Y, Beguin S, Lienhart A, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93(3):475‐480. [DOI] [PubMed] [Google Scholar]
- 24. Jeong JC, Kim JE, Ryu JW, Joo KW, Kim HK. Plasma haemostatic potential of haemodialysis patients assessed by thrombin generation assay: hypercoagulability in patients with vascular access thrombosis. Thromb Res. 2013;132(5):604‐609. [DOI] [PubMed] [Google Scholar]
- 25. Pluta J, Nicinska B, Grzeszczyk M, et al. Assessment of the hemostatic parameters and platelet function on thromboelastometry and impedance aggregometry in hemodialysis patients qualified for kidney transplantation: preliminary report. Transplant Proc. 2016;48(5):1431‐1434. [DOI] [PubMed] [Google Scholar]
- 26. Akay OM. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a global assessment by Rotational Thromboelastometry (ROTEM). Clin Appl Thromb Hemost. 2018;24(6):850‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fayed N, Mourad W, Yassen K, Gorlinger K. Preoperative thromboelastometry as a predictor of transfusion requirements during adult living donor liver transplantation. Transfus Med Hemother. 2015;42(2):99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5(2):289‐295. [DOI] [PubMed] [Google Scholar]
- 29. Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG. 2009;116(8):1097‐1102. [DOI] [PubMed] [Google Scholar]
- 30. Haas T, Spielmann N, Mauch J, et al. Comparison of thromboelastometry (ROTEM(R)) with standard plasmatic coagulation testing in paediatric surgery. Br J Anaesth. 2012;108(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 31. Tripodi A, Primignani M, Chantarangkul V, et al. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb Res. 2009;124(1):132‐136. [DOI] [PubMed] [Google Scholar]
- 32. Gackler A, Rohn H, Lisman T, et al. Evaluation of hemostasis in patients with end‐stage renal disease. PLoS One. 2019;14(2):e0212237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID‐19 pneumonia. J Thromb Thrombolysis. 2020;50(2):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol. 2009;26(3):358‐364. [DOI] [PubMed] [Google Scholar]
- 35. Darlington A, Ferreiro JL, Ueno M, et al. Haemostatic profiles assessed by thromboelastography in patients with end‐stage renal disease. Thromb Haemost. 2011;106(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 36. Roullet S, Pillot J, Freyburger G, et al. Rotation thromboelastometry detects thrombocytopenia and hypofibrinogenaemia during orthotopic liver transplantation. Br J Anaesth. 2010;104(4):422‐428. [DOI] [PubMed] [Google Scholar]
- 37. Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12(7):1549‐1557. [DOI] [PubMed] [Google Scholar]
- 38. van Bladel ER, de Jager RL, Walter D, et al. Platelets of patients with chronic kidney disease demonstrate deficient platelet reactivity in vitro. BMC Nephrol. 2012;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wurtz M, Hvas AM, Christensen KH, Rubak P, Kristensen SD, Grove EL. Rapid evaluation of platelet function using the Multiplate(R) Analyzer. Platelets. 2014;25(8):628‐633. [DOI] [PubMed] [Google Scholar]
- 40. Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on‐treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261‐2273. [DOI] [PubMed] [Google Scholar]
- 41. Zwaginga JJ, Ijsseldijk MJ, Beeser‐Visser N, de Groot PG, Vos J, Sixma JJ. High von Willebrand factor concentration compensates a relative adhesion defect in uremic blood. Blood. 1990;75(7):1498‐1508. [PubMed] [Google Scholar]
- 42. Brophy DF, Martin EJ, Gehr TW, Carr ME Jr. Enhanced anticoagulant activity of enoxaparin in patients with ESRD as measured by thrombin generation time. Am J Kidney Dis. 2004;44(2):270‐277. [DOI] [PubMed] [Google Scholar]
- 43. Campello E, Zabeo E, Radu CM, et al. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb Haemost. 2015;113(1):85‐96. [DOI] [PubMed] [Google Scholar]
- 44. de Laat‐Kremers RMW, Ninivaggi M, Devreese KMJ, de Laat B. Towards standardization of thrombin generation assays: Inventory of thrombin generation methods based on results of an International Society of Thrombosis and Haemostasis Scientific Standardization Committee survey. J Thromb Haemost. 2020;18(8):1893‐1899. [DOI] [PubMed] [Google Scholar]
- 45. Vaziri ND, Gonzales E, Barton CH, Chen HT, Nguyen Q, Arquilla M. Factor XIII and its substrates, fibronectin, fibrinogen, and alpha 2‐antiplasmin, in plasma and urine of patients with nephrosis. J Lab Clin Med. 1991;117(2):152‐156. [PubMed] [Google Scholar]
- 46. Lottermoser K, Petras S, Poge U, et al. The fibrinolytic system in chronic renal failure. Eur J Med Res. 2001;6(9):372‐376. [PubMed] [Google Scholar]
- 47. Bonomini M, Reale M, Santarelli P, Stuard S, Settefrati N, Albertazzi A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79(4):399‐407. [DOI] [PubMed] [Google Scholar]
- 48. Musial K, Zwolinska D, Polak‐Jonkisz D, Berny U, Szprynger K, Szczepanska M. Serum VCAM‐1, ICAM‐1, and L‐selectin levels in children and young adults with chronic renal failure. Pediatr Nephrol. 2005;20(1):52‐55. [DOI] [PubMed] [Google Scholar]
- 49. Stenvinkel P, Lindholm B, Heimburger M, Heimburger O. Elevated serum levels of soluble adhesion molecules predict death in pre‐dialysis patients: association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant. 2000;15(10):1624‐1630. [DOI] [PubMed] [Google Scholar]
- 50. Weiss MF, Erhard P, Kader‐Attia FA, et al. Mechanisms for the formation of glycoxidation products in end‐stage renal disease. Kidney Int. 2000;57(6):2571‐2585. [DOI] [PubMed] [Google Scholar]
- 51. Catena C, Zingaro L, Casaccio D, Sechi LA. Abnormalities of coagulation in hypertensive patients with reduced creatinine clearance. Am J Med. 2000;109(7):556‐561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


