Abstract
Objective
Using data from the SENSCIS trial, these analyses were undertaken to assess the effects of nintedanib versus placebo in subgroups of patients with systemic sclerosis–associated interstitial lung disease (SSc‐ILD), based on characteristics previously identified as being associated with the progression of SSc‐ILD.
Methods
Patients with SSc‐ILD were randomized to receive either nintedanib or placebo, stratified by anti–topoisomerase I antibody (ATA) status. We assessed the rate of decline in forced vital capacity (FVC) (expressed in ml/year) over 52 weeks in subgroups based on baseline ATA status, modified Rodnan skin thickness score (MRSS) (<18 versus ≥18), and SSc subtype (limited cutaneous SSc [lcSSc] versus diffuse cutaneous SSc [dcSSc]).
Results
At baseline, 60.8% of 576 patients who received treatment with either nintedanib or placebo were positive for ATA, 51.9% had dcSSc, and 77.5% of 574 patients with MRSS data available had an MRSS of <18. The effect of nintedanib versus placebo on reducing the rate of decline in FVC (ml/year) was numerically more pronounced in ATA‐negative patients compared to ATA‐positive patients (adjusted difference in the rate of FVC decline, 57.2 ml/year [95% confidence interval (95% CI) –3.5, 118.0] versus 29.9 ml/year [95% CI –19.1, 78.8]), in patients with a baseline MRSS ≥18 compared to those with a baseline MRSS of <18 (adjusted difference in the rate of FVC decline, 88.7 ml/year [95% CI 7.7, 169.8] versus 26.4 ml/year [95% CI –16.8, 69.6]), and in patients with dcSSc compared to those with lcSSc (adjusted difference in the rate of FVC decline, 56.6 ml/year [95% CI 3.2, 110.0] versus 25.3 ml/year [95% CI –28.9, 79.6]). However, all exploratory interaction P values were nonsignificant (all P > 0.05), indicating that there was no heterogeneity in the effect of nintedanib versus placebo between these subgroups of patients.
Conclusion
In patients with SSc‐ILD, reduction in the annual rate of decline in FVC among patients receiving nintedanib compared to those receiving placebo was not found to be heterogenous across subgroups based on ATA status, MRSS, or SSc subtype.
Short abstract
INTRODUCTION
Systemic sclerosis (SSc) is a complex autoimmune disease characterized by progressive fibrosis of the skin and internal organs (1). Interstitial lung disease (ILD) is a common manifestation of SSc and the leading cause of death in patients with SSc (2). Progressive SSc‐ILD is associated with poor outcomes, and SSc patients who have progressive ILD need to be identified in clinical practice so that the disease can be managed appropriately (3, 4, 5).
In clinical practice, patients with SSc are classified into 2 subtypes based on the extent of skin involvement: limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) (6). The dcSSc subtype is associated with earlier onset of non–Raynaud's phenomenon symptoms (7), higher mortality (8), and a greater risk of developing ILD (7), but ILD is also a common cause of death in patients with lcSSc (9). The course of skin fibrosis in patients with dcSSc typically involves worsening early in the course of the disease, followed by gradual improvement (10). Among patients with dcSSc in the European Scleroderma Trials and Research (EUSTAR) database, a high modified Rodnan skin thickness score (MRSS) at baseline was a predictor of improvement in the MRSS over the next 12 months, independent of disease duration, and an upper MRSS threshold of 18–25 was proposed to be an effective cutoff for identifying a cohort of SSc patients enriched for the phenotype of progressive skin fibrosis (11).
Specific autoantibody profiles have been associated with organ involvement and mortality in patients with SSc (8, 12, 13, 14, 15). Patients who are positive for anti–topoisomerase I antibody (ATA) have been reported to have a greater risk of developing clinically significant ILD (8, 15). In the Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohort of 266 patients with early SSc, ATA positivity was associated with a greater rate of decline in forced vital capacity (FVC) over 3 years (16). In a single‐center analysis, among 505 patients who developed SSc‐ILD, ATA positivity was predictive of developing an FVC <70% predicted within 5 years of the onset of SSc (17).
Nintedanib is an intracellular inhibitor of tyrosine kinases that inhibits processes involved in the progression of pulmonary fibrosis (18). In patients with SSc‐ILD in the SENSCIS trial, nintedanib was associated with a significant reduction in the rate of decline in FVC (expressed in ml/year) over 52 weeks compared to placebo, while there was no significant difference in the change from baseline in the MRSS (19). In addition, numerically lower proportions of patients treated with nintedanib showed a decline in FVC of >5% to ≤10% predicted or a decline in FVC of >10% over 52 weeks (20) compared to patients who received placebo. We used data from the SENSCIS trial to assess the progression of ILD, the progression of skin fibrosis, and the effects of nintedanib in subgroups based on baseline ATA status, MRSS, and SSc subtype.
PATIENTS AND METHODS
Trial design and patients
The SENSCIS trial (ClinicalTrials.gov identifier: NCT02597933) was a randomized, placebo‐controlled trial conducted in 32 countries (19). The trial was conducted in accordance with the trial protocol, the principles of the Declaration of Helsinki, and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation and was approved by local authorities. Written informed consent was obtained from all patients before study entry.
The design of the SENSCIS trial has been published, together with the trial protocol and statistical analysis plan (19). In brief, patients enrolled in the study had SSc with onset of first non–Raynaud's phenomenon symptom ≤7 years before screening, had fibrotic ILD extending over ≥10% of the lung on a high‐resolution computed tomography (HRCT) scan based on assessment of the whole lung, FVC ≥40% predicted, and diffusing capacity for carbon monoxide 30–89% predicted. Patients receiving prednisone ≤10 mg/day and/or stable therapy with mycophenolate or methotrexate for ≥6 months prior to randomization were allowed to participate. At screening, patients were classified as having lcSSc or dcSSc by the investigators. Patients were randomized 1:1 to receive nintedanib 150 mg twice a day or placebo, stratified by the presence of ATA. The participants' ATA status was determined based on review of historical information (local laboratory data recorded for each patient) or, if historical information was not available, based on detection of ATA in the patient's blood, using a BioPlex 2200 System bead assay (obtained at a central laboratory).
Patients received their randomized treatment in a blinded manner until the last patient had reached week 52 but for ≤100 weeks. Patients who discontinued trial medication were asked to attend all scheduled visits and undergo examinations as originally planned. Spirometry was performed in accordance with international guidelines (21) at baseline and at weeks 2, 4, 6, 12, 24, 36, and 52. The MRSS was measured at baseline and at weeks 12, 24, 36, and 52. The MRSS evaluates a patient's skin thickness through palpation of 17 areas using a scale of 0 to 3 to give a maximum score of 51 (22, 23).
End points
Analyses conducted using the overall population of the SENSCIS trial have been described (19). Here we report the results of analyses in subgroups based on baseline ATA status (based on historical [local laboratory] information, as reported in the case report form, or on central laboratory data if historical information was not available), baseline MRSS (MRSS <18 versus MRSS ≥18 or MRSS ≤10 versus MRSS >10 to <22 versus MRSS ≥22), and SSc subtype (lcSSc versus dcSSc). In these subgroups, we assessed the annual rate of decline in FVC (expressed in ml/year) over 52 weeks. The following were assessed at week 52 in subgroups based on baseline ATA status, MRSS (<18 versus ≥18), and SSc subtype (lcSSc versus dcSSc): the proportions of patients who met proposed thresholds for minimum clinically important differences (MCIDs) for stable or improved FVC (increase in FVC or absolute decrease <3.3% predicted) and worsened FVC (absolute decrease ≥3.3% predicted), based on estimates derived from Scleroderma Lung Studies I and II, anchored to the health transition question from the Medical Outcomes Study Short Form 36 (24); and the change from baseline in the MRSS. In the overall population, we assessed the correlations between FVC (in ml) at baseline and change from baseline in MRSS at week 52, MRSS at baseline and change from baseline in FVC (in ml) at week 52, and changes from baseline in MRSS and FVC (in ml) at week 52. Finally, we assessed the rate of decline in FVC (expressed in ml/year) considering MRSS at baseline as a continuous variable.
Statistical analysis
All analyses were conducted in patients who received ≥1 dose of trial medication. A random coefficient regression model (with random slopes and intercepts) was used to analyze the annual rate of decline in FVC (expressed in ml/year) in the subgroups, with ATA status (ATA‐positive, ATA‐negative) and sex as fixed categorical effects, baseline FVC (in ml), age, and height as fixed continuous effects, and with baseline‐by‐time, treatment‐by‐subgroup, and treatment‐by‐subgroup‐by‐time interaction included as interaction terms. The analysis was based on all measurements obtained within the first 52 weeks, including those from patients who discontinued trial medication. The proportions of patients who met proposed thresholds for stable or improved FVC and worsened FVC at week 52 were compared between treatment groups using a logistic regression model, including treatment, ATA status, subgroup, and treatment‐by‐subgroup interaction as terms. Odds ratios were estimated for the independent effect of treatment within each subgroup. Missing values were imputed using a worst value carried forward approach. Subgroup analyses of change from baseline in the MRSS at week 52 were based on a mixed model for repeated measures (MMRM), using ATA status and treatment‐by‐subgroup‐by‐visit interaction as fixed categorical effects, and baseline MRSS‐by‐visit interaction as a fixed continuous covariate.
For every subgroup analysis, exploratory interaction P values were calculated (using an F test in random coefficient regression or MMRM analyses, or Wald's test in logistic regression analyses) to evaluate potential heterogeneity in the treatment effect of nintedanib versus placebo across the subgroups, with no adjustment for multiple testing. Spearman's correlation coefficients were calculated to analyze the correlations between FVC and MRSS described above. The rate of decline in FVC (expressed in ml/year) considering MRSS at baseline as a continuous variable was analyzed using a random coefficient regression model with treatment, ATA status, and sex as fixed categorical effects, baseline FVC (ml), age, and height as fixed continuous effects, and including baseline FVC‐by‐time, treatment‐by‐time, treatment‐by‐baseline MRSS, and treatment‐by‐baseline‐MRSS‐by‐time as interaction terms.
RESULTS
Patients
The baseline characteristics of the patients in the SENSCIS trial have been described previously (19). At baseline, 173 patients (60.1%) in the nintedanib group and 177 patients (61.5%) in the placebo group were ATA‐positive. ATA status based on historical information was generally consistent with that based on central laboratory data (see Supplementary Table 1, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965). Compared to ATA‐negative patients, the subgroup of ATA‐positive patients had a lower proportion of male patients, a greater proportion of patients with dcSSc, and a higher mean MRSS at baseline (Supplementary Table 2, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965). Similar proportions of ATA‐positive and ATA‐negative patients were receiving treatment with mycophenolate at baseline (49.1% and 47.3%, respectively).
Two patients in the placebo group did not have information on MRSS at baseline. Of the patients who had information on MRSS at baseline, 219 (76.0%) of 288 in the nintedanib group and 226 (79.0%) of 286 in the placebo group had an MRSS <18. All patients with an MRSS ≥18 and 37.8% of patients with an MRSS <18 were classified as having dcSSc. Compared to patients with an MRSS ≥18, patients with an MRSS <18 had a greater mean baseline FVC % predicted, and the MRSS <18 group had a higher proportion of male patients and a higher proportion of patients who were negative for ATA (Supplementary Table 3, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965). A smaller proportion of patients with an MRSS <18 were receiving treatment with mycophenolate at baseline compared to patients who had an MRSS ≥18 (45.6% versus 58.1%, respectively). In the nintedanib and placebo groups, 153 (53.1%) of 288 patients and 146 (50.7%) of 288 patients, respectively, were classified as having dcSSc; their baseline characteristics are shown in Supplementary Table 4 (available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965).
Outcomes in subgroups by ATA status
In the placebo group, the adjusted annual rate of decline in FVC was consistent between patients who were ATA‐positive and those who were ATA‐negative at baseline (adjusted annual rate of decline in FVC, ± SE –93.5 ± 17.3 ml/year versus –93.1 ± 21.9 ml/year) (Table 1 and Figure 1A). In analyses of the adjusted annual rate of decline in FVC that also adjusted for use of mycophenolate at baseline, the rate of FVC decline among patients in the placebo group was similar between ATA‐positive and ATA‐negative patients (adjusted annual rate of decline in FVC ± SE –93.4 ± 17.3 ml/year versus –93.2 ± 21.9 ml/year). With regard to nintedanib, the effect of nintedanib on reducing the annual rate of decline in FVC compared with placebo was numerically more pronounced in ATA‐negative patients compared to ATA‐positive patients (adjusted difference in annual rate of decline in FVC, 57.2 ml/year [95% confidence interval (95% CI) –3.5, 118.0] versus 29.9 ml/year [95% CI –19.1, 78.8]), but the exploratory interaction P value did not indicate heterogeneity in the treatment effect of nintedanib versus placebo between the subgroups classified according to ATA status (P = 0.49) (Figure 1A).
Table 1.
Annual rate of decline in FVC, proportions of patients with worsening of FVC and stable or improved FVC, and changes in the MRSS from baseline to week 52 in patients with systemic sclerosis–associated interstitial lung disease in each treatment group in the SENSCIS trial, according to baseline ATA status
| ATA‐positive | ATA‐negative | ||||
|---|---|---|---|---|---|
| Variable | Nintedanib (n = 173) | Placebo (n = 177) | Nintedanib (n = 115) | Placebo (n = 111) | P for interaction* |
| Annual rate of decline in FVC (ml/year)† | |||||
| Adjusted rate of decline in FVC over 52 weeks, ± SE, ml/year | –63.6 ± 18.0 | –93.5 ± 17.3 | –35.9 ± 21.8 | –93.1 ± 21.9 | |
| Adjusted difference (95% CI) vs. placebo, ml/year‡ | 29.9 (–19.1, 78.8) | 57.2 (–3.5, 118.0) | 0.49 | ||
| Proportion of patients meeting proposed MCID thresholds for worsening of FVC and stable or improved FVC at week 52†§ | |||||
| Decrease in FVC ≥3.3% predicted, no. (%) | 62 (35.8) | 81 (45.8) | 37 (32.5) | 45 (40.5) | |
| Odds ratio (95% CI) vs. placebo‡ | 0.66 (0.43, 1.02) | 0.70 (0.41, 1.22) | 0.86 | ||
| Increase in FVC or decrease in FVC <3.3% predicted, no. (%) | 111 (64.2) | 96 (54.2) | 77 (67.5) | 66 (59.5) | |
| Odds ratio (95% CI) vs. placebo‡ | 1.51 (0.98, 2.32) | 1.42 (0.82, 2.45) | 0.86 | ||
| Change from baseline in MRSS at week 52¶ | |||||
| Adjusted change in MRSS at week 52, mean ± SE | –1.5 ± 0.3 | –1.7 ± 0.3 | –3.2 ± 0.4 | –2.4 ± 0.4 | |
| Adjusted difference (95% CI) vs. placebo‡ | 0.2 (–0.7, 1.2) | –0.8 (–2.0, 0.4) | 0.18 | ||
P values evaluated heterogeneity in the treatment effect of nintedanib versus placebo between the subgroups; annual rate of decline in forced vital capacity (FVC), P for treatment‐by‐time‐by‐subgroup interaction; proportions of patients meeting proposed minimum clinically important difference (MCID) thresholds for worsening of FVC and stable or improved FVC at week 52, P for treatment‐by‐subgroup interaction; change from baseline in the modified Rodnan skin thickness score (MRSS), P for treatment‐by‐visit‐by‐subgroup interaction.
Post–baseline FVC data were not available for 1 anti–topoisomerase I antibody (ATA)–negative patient in the nintedanib group; this patient was excluded from the analysis.
95% CI = 95% confidence interval.
The proposed MCID thresholds for worsening of FVC and stable or improved FVC were based on estimates derived from the Scleroderma Lung Studies I and II, anchored to the health transition question from the Medical Outcomes Short Form 36 (24).
Baseline MRSS data were not available for 2 ATA‐positive patients in the placebo group; these patients were excluded from the analysis.
Figure 1.
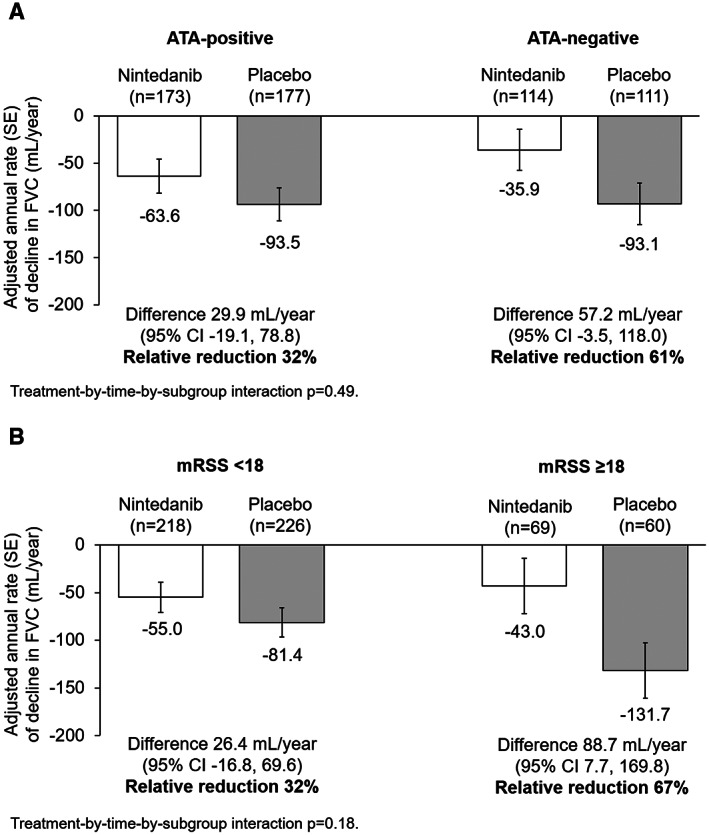
Adjusted annual rate of decline in forced vital capacity (FVC) (ml/year) in subgroups of patients with systemic sclerosis–associated interstitial lung disease based on anti–topoisomerase I antibody (ATA) status at baseline (A) and modified Rodnan skin thickness score (MRSS) at baseline (B) in the SENSCIS trial. The adjusted annual rate of decline in FVC ± SE is shown. The difference between treatment groups is shown with 95% confidence interval (95% CI) and relative reduction.
The proportion of patients with an absolute decrease in FVC of ≥3.3% predicted at week 52 was lower in the nintedanib group versus the placebo group among ATA‐positive patients (35.8% versus 45.8%) as well as among ATA‐negative patients (32.5% versus 40.5%); the exploratory interaction P value did not indicate heterogeneity in the treatment effect of nintedanib versus placebo between the subgroups based on ATA status (P = 0.86). The proportion of patients with an increase or absolute decrease in FVC of <3.3% predicted was higher in the nintedanib group versus the placebo group among ATA‐positive patients (64.2% versus 54.2%) as well as among ATA‐negative patients (67.5% versus 59.5%) (exploratory interaction P = 0.86) (Table 1).
Small reductions (improvements) in the MRSS were observed in patients who were ATA‐positive and in patients who were ATA‐negative. Reductions in the MRSS were similar between the nintedanib and placebo groups, with no heterogeneity in the treatment effect detected between subgroups classified by ATA status (Table 1).
Outcomes in subgroups by MRSS at baseline
In the placebo group, the adjusted annual rate of decline in FVC was greater in patients with an MRSS ≥18 compared to those with an MRSS <18 (adjusted annual rate of decline in FVC ± SE –131.7 ± 29.2 ml/year versus –81.4 ± 15.4 ml/year) (Table 2 and Figure 1B). The effect of nintedanib on reducing the annual rate of decline in FVC compared with placebo was numerically more pronounced in patients with a baseline MRSS ≥18 compared to those with a baseline MRSS <18 (adjusted difference in annual rate of decline in FVC, 88.7 ml/year [95% CI 7.7, 169.8] versus 26.4 ml/year [95% CI –16.8, 69.6]). However, the exploratory interaction P value did not indicate heterogeneity in the treatment effect of nintedanib versus placebo across the subgroups classified according to baseline MRSS (P = 0.18) (Figure 1B). Similarly, in analyses of subgroups based on different cutoffs for the baseline MRSS (MRSS ≤10 [n = 315] versus MRSS >10 to MRSS <22 [n = 182] versus MRSS ≥22 [n = 76]), the exploratory interaction P value did not indicate heterogeneity in the treatment effect of nintedanib compared to placebo across these MRSS subgroups (P = 0.07) (Supplementary Table 5, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965).
Table 2.
Annual rate of decline in FVC, proportions of patients with worsening of FVC and stable or improved FVC, and changes in the MRSS from baseline to week 52 in patients with systemic sclerosis–associated interstitial lung disease in each treatment group in the SENSCIS trial, according to baseline MRSS <18 and MRSS ≥18*
| MRSS <18 | MRSS ≥18 | ||||
|---|---|---|---|---|---|
| Variable | Nintedanib (n = 219) | Placebo (n = 226) | Nintedanib (n = 69) | Placebo (n = 60) | P for interaction† |
| Annual rate of decline in FVC, ml/year‡ | |||||
| Adjusted rate of decline in FVC over 52 weeks, ± SE, ml/year | –55.0 ± 15.7 | –81.4 ± 15.4 | –43.0 ± 29.2 | –131.7 ± 29.2 | |
| Adjusted difference (95% CI) vs. placebo, ml/year§ | 26.4 (–16.8, 69.6) | 88.7 (7.7, 169.8) | 0.18 | ||
| Proportions of patients meeting proposed MCID thresholdsfor worsening of FVC and stable or improved FVC at week 52‡, ¶ | |||||
| Decrease in FVC ≥3.3% predicted, no. (%) | 70 (32.1) | 92 (40.7) | 29 (42.0) | 32 (53.3) | |
| Odds ratio (95% CI) vs. placebo§ | 0.69 (0.47, 1.02) | 0.62 (0.31, 1.25) | 0.79 | ||
| Increase in FVC or decrease in FVC <3.3% predicted, no. (%) | 148 (67.9) | 134 (59.3) | 40 (58.0) | 28 (46.7) | |
| Odds ratio (95% CI) vs. placebo§ | 1.44 (0.98, 2.13) | 1.61 (0.80, 3.24) | 0.79 | ||
| Change from baseline in MRSS at week 52 | |||||
| Adjusted change in MRSS at week 52, mean ± SE | –2.2 ± 0.3 | –2.1 ± 0.3 | –2.1 ± 0.7 | –1.6 ± 0.7 | |
| Adjusted difference (95% CI) vs. placebo§ | –0.1 (–1.0, 0.7) | –0.6 (–2.1, 1.0) | 0.62 | ||
Baseline modified Rodnan skin thickness score (MRSS) data were not available for 2 patients in the placebo group; these patients were excluded from all analyses shown.
P values evaluated heterogeneity in the treatment effect of nintedanib versus placebo between the subgroups: annual rate of decline in forced vital capacity (FVC), P for treatment‐by‐time‐by‐subgroup interaction; proportions of patients meeting proposed minimum clinically important difference (MCID) thresholds for worsening of FVC and stable or improved FVC at week 52, P for treatment‐by‐subgroup interaction; change from baseline in the MRSS, P for treatment‐by‐visit‐by‐subgroup interaction.
Post–baseline FVC data were not available for 1 patient with MRSS <18 at baseline in the nintedanib group; this patient was excluded from the analysis.
95% CI = 95% confidence interval.
The proposed MCID thresholds for worsening of FVC and stable or improved FVC were based on estimates derived from the Scleroderma Lung Studies I and II, anchored to the health transition question from the Medical Outcomes Short Form 36 (24).
The proportion of patients with an absolute decrease in FVC of ≥3.3% predicted was lower in the nintedanib group compared to the placebo group both among patients with a baseline MRSS ≥18 (42.0% versus 53.3%) and among those with a baseline MRSS <18 (32.1% versus 40.7%). The proportion of patients with an increase or absolute decrease in FVC <3.3% predicted was higher in the nintedanib group than in the placebo group both among patients with a baseline MRSS ≥18 (58.0% versus 46.7%) and among those with a baseline MRSS <18 (67.9% versus 59.3%). Exploratory interaction P values did not indicate heterogeneity in the treatment effect of nintedanib versus placebo between the subgroups classified by baseline MRSS (Table 2).
Small reductions (improvements) in the MRSS were observed in patients with a baseline MRSS of ≥18 and in patients with a baseline MRSS of <18. Reductions in the MRSS were similar in the nintedanib and placebo groups, with no heterogeneity in treatment effect detected between the subgroups (Table 2). Similarly, there was no heterogeneity in the treatment effect of nintedanib across subgroups classified by a baseline MRSS ≤10 versus baseline MRSS >10 to <22 versus baseline MRSS ≥22 (Supplementary Table 5, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965).
Relationships between FVC and MRSS
In the overall population, no meaningful correlations were observed between the FVC (in ml) at baseline and change in the MRSS from baseline to week 52, between the MRSS at baseline and change in the FVC (in ml) from baseline to week 52, or between change in the MRSS and change in the FVC (in ml) from baseline to week 52 (Supplementary Table 6, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965). The analysis that considered MRSS at baseline as a continuous variable showed no significant interaction between baseline MRSS and the rate of decline in FVC (in ml/year) (P = 0.12).
Outcomes in subgroups with lcSSc or dcSSc
In the placebo group, the adjusted annual rate of decline in FVC was greater in patients with dcSSc compared to those with lcSSc (adjusted annual rate of decline ± SE –112.0 ± 19.1 ml/year versus –74.5 ± 19.2 ml/year). The effect of nintedanib on reducing the annual rate of decline in FVC compared with placebo was numerically more pronounced in patients with dcSSc compared to those with lcSSc (adjusted difference in annual rate of decline in FVC, 56.6 ml/year [95% CI 3.2, 110.0] versus 25.3 ml/year [95% CI –28.9, 79.6]). However, the exploratory interaction P value did not indicate heterogeneity in the treatment effect of nintedanib versus placebo between these subgroups of patients with lcSSc or dcSSc (P = 0.42). Small reductions (improvements) in the MRSS were observed in patients with lcSSc and in those with dcSSc. Reductions in the MRSS were similar in the nintedanib and placebo groups, with no heterogeneity in the between‐group difference detected across subgroups based on SSc subtype (Supplementary Table 7, available on the Arthritis & Rheumatology website at https://onlinelibrary.wiley.com/doi/10.1002/art.41965).
DISCUSSION
We used data from the SENSCIS trial to assess the progression of ILD and skin fibrosis, and the effects of nintedanib versus placebo, in subgroups of patients with SSc‐ILD based on baseline characteristics that have previously been associated with disease progression. In the placebo group, the rate of decline in FVC over 52 weeks was similar between ATA‐positive patients and ATA‐negative patients, greater in patients with a baseline MRSS ≥18 compared to those with a baseline MRSS <18, and greater in patients with dcSSc compared to those with lcSSc, as reported by the site investigator. Across these subgroups, a lower annual rate of decline in FVC was observed in patients who received nintedanib compared to those who received placebo, with no heterogeneity detected in the treatment effect of nintedanib versus placebo in any of the subgroups studied. These findings add to previous analyses of data from the SENSCIS trial showing that the effect of nintedanib on the annual rate of FVC decline was consistent across subgroups based on ATA status, SSc subtype, age, sex, race, and use of mycophenolate at baseline (19, 25). MCIDs for improvement and worsening in FVC in patients with SSc‐ILD have been proposed based on data from Scleroderma Lung Studies I and II, anchored to the health transition question from the Medical Outcomes Study Short Form 36 (24). Over 52 weeks, the proportion of patients who met the proposed threshold for MCID for improved or stable FVC was numerically greater, and the proportion of patients who met the proposed threshold for MCID for worsening of FVC was numerically lower, in patients who received nintedanib compared to those who received placebo across the subgroups based on ATA status, baseline MRSS, and SSc subtype, with no evidence of heterogeneity detected across the subgroups. These findings support a clinically meaningful benefit of nintedanib in reducing the rate of progression of ILD across a broad population of patients with SSc‐ILD.
In the placebo group, we observed a numerically greater rate of decline in FVC over 52 weeks in patients with dcSSc compared to those with lcSSc. A single‐center study of 105 patients with early SSc found that dcSSc was a predictor of decline in FVC of ≥10% predicted over a mean follow‐up of 6 years (26). However, in the GENISOS cohort of 266 patients with early SSc, decline in FVC % predicted over a mean follow‐up of 3.8 years was similar between patients with lcSSc and those with dcSSc (16). A recent analysis of data from >12,000 patients in the EUSTAR database also found that changes in FVC % predicted over 1, 2, and 3 years were similar between patients with lcSSc and those with dcSSc (27). In our analyses, 51% of patients classified as having lcSSc were ATA‐positive at baseline. This is a much higher proportion than has been shown in data from large registries of patients with SSc (11–23%) (28, 29, 30). This may reflect either misclassification of some patients who had dcSSc and whose skin fibrosis had regressed prior to screening, or selection bias in the SENSCIS trial for patients with lcSSc who had more progressive lung disease. These findings highlight the limitations of using the dcSSc versus lcSSc classification in large multicenter trials. While data from the GENISOS cohort suggested that ATA positivity was associated with an increased rate of decline in FVC in patients with early SSc (16), ATA status did not seem to affect the rate of progression of ILD in the SENSCIS trial. These different findings across studies may reflect patient populations at different stages of disease or confounders such as comedication.
Consistent with findings in the overall SENSCIS population (19), nintedanib was not found to have an effect on the change in MRSS in any of the subgroups analyzed. The MRSS improved in both the nintedanib group and the placebo group, reflecting the natural history of skin fibrosis in patients with SSc (10). In our analysis of subgroups based on baseline MRSS using a threshold of 18 (based on data suggesting an upper MRSS threshold of 18–25 to enrich a cohort of patients with dcSSc for patients with the phenotype of progressive skin fibrosis [11]), change in MRSS at week 52 was similar between patients with a baseline MRSS ≥18 and patients with a baseline MRSS <18.
Among all of the subgroups we analyzed, the rate of decline in FVC over 52 weeks was highest in patients in the placebo group who had a baseline MRSS ≥18; however, we found no meaningful correlation between MRSS at baseline and decline in FVC over 52 weeks. We observed no meaningful correlation between progression of skin fibrosis over 52 weeks and progression of SSc‐ILD over the same period. The relationship between progression of skin fibrosis and later decline in FVC observed over several years of follow‐up in patients with dcSSc in the EUSTAR database (31) could not be investigated using data from the SENSCIS trial due to the limited follow‐up period.
A limitation of the subgroup analyses of data from the SENSCIS trial is that they were not powered for formal statistical testing of the individual subgroups, and the interaction P values should be regarded as exploratory. The results of these subgroup analyses should be interpreted with caution, particularly those in the relatively small subgroups. A further limitation was that progression of ILD was assessed solely by looking at changes in FVC and did not consider other metrics for ILD progression, such as changes in the extent of fibrosis on HRCT.
In conclusion, these analyses of data from the SENSCIS trial suggest that while the course of FVC decline in patients with SSc‐ILD remains difficult to predict, nintedanib is effective at reducing the annual rate of progression of ILD across subgroups of patients based on ATA status, SSc subtype, and MRSS at baseline.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Kuwana had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kuwana, Mayes, Gahlemann, Alves, O. Distler.
Acquisition of data
Kuwana, Allanore, Denton, J. Distler, Steen, Khanna, Mayes, O. Distler.
Analysis and interpretation of data
Kuwana, Allanore, Denton, J. Distler, Steen, Khanna, Matucci‐Cerinic, Mayes, Volkmann, Miede, Gahlemann, Quaresma, Alves, O. Distler.
ROLE OF THE STUDY SPONSOR
The SENSCIS trial was supported by Boehringer Ingelheim International GmbH. Boehringer Ingelheim participated in the study design, data collection, statistical analyses, data interpretation, and the writing of the report. Writing assistance was provided by Elizabeth Ng, BSc and Wendy Morris, MSc of FleishmanHillard, London, UK, which was contracted and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Dr. Kuwana had full access to all data and had final responsibility for the decision to submit for publication.
ADDITIONAL DISCLOSURES
Author Miede is an employee of mainanalytics.
Supporting information
Disclosureform
Appendix S1: Supporting information
ACKNOWLEDGMENTS
We thank the patients who participated in the SENSCIS trial.
A video abstract of this article can be found at https://players.brightcove.net/3806881048001/default_default/index.html?videoId=6295470032001.
Supported by Boehringer Ingelheim.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fart.41965&file=art41965‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir‐Gurman A, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. [DOI] [PubMed] [Google Scholar]
- 3. Goh NS, Hoyles RK, Denton CP, Hansell DM, Renzoni EA, Maher TM, et al. Short‐term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017;69:1670–8. [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann‐Vold AM, Fretheim H, Halse AK, Seip M, Bitter H, Wallenius M, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019;200:1258–66. [DOI] [PubMed] [Google Scholar]
- 5. Volkmann ER. Natural history of systemic sclerosis–related interstitial lung disease: how to identify a progressive fibrosing phenotype. J Scleroderma Relat Disord 2020;5 Suppl:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 7. Walker UA, Tyndall A, Czirják L, Denton C, Farge‐Bancel D, Kowal‐Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long‐term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 9. Sánchez‐Cano D, Ortego‐Centeno N, Callejas JL, Fonollosa Plá V, Ríos‐Fernández R, Tolosa‐Vilella C, et al. Interstitial lung disease in systemic sclerosis: data from the Spanish scleroderma study group. Rheumatol Int 2018;38:363–74. [DOI] [PubMed] [Google Scholar]
- 10. Wirz EG, Jaeger VK, Allanore Y, Riemekasten G, Hachulla E, Distler O, et al. Incidence and predictors of cutaneous manifestations during the early course of systemic sclerosis: a 10‐year longitudinal study from the EUSTAR database. Ann Rheum Dis 2016;75:1285–92. [DOI] [PubMed] [Google Scholar]
- 11. Dobrota R, Maurer B, Graf N, Jordan S, Mihai C, Kowal‐Bielecka O, et al. Prediction of improvement in skin fibrosis in diffuse cutaneous systemic sclerosis: a EUSTAR analysis. Ann Rheum Dis 2016;75:1743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 2005;35:35–42. [DOI] [PubMed] [Google Scholar]
- 13. Fertig N, Domsic RT, Rodriguez‐Reyna T, Kuwana M, Lucas M, Medsger TA Jr, et al. Anti‐U11/U12 RNP antibodies in systemic sclerosis: a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum 2009;61:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuwana M. Circulating anti‐nuclear antibodies in systemic sclerosis: utility in diagnosis and disease subsetting [review]. J Nippon Med Sch 2017;84:56–63. [DOI] [PubMed] [Google Scholar]
- 15. Nihtyanova SI, Sari A, Harvey JC, Leslie A, Derrett‐Smith EC, Fonseca C, et al. Using autoantibodies and cutaneous subset to develop outcome‐based disease classification in systemic sclerosis. Arthritis Rheumatol 2020;72:465–76. [DOI] [PubMed] [Google Scholar]
- 16. Assassi S, Sharif R, Lasky RE, McNearney TA, Estrada‐Y‐Martin RM, Draeger H, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010;12:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nihtyanova SI, Derrett‐Smith E, Fonseca C, Ong V, Denton C. Frequency and predictors of meaningful decline in forced vital capacity during follow up оf a large cohort of systemic sclerosis associated pulmonary fibrosis patients [abstract]. Arthritis Rheumatol 2019;71 Suppl 10. URL:https://acrabstracts.org/abstract/frequency‐and‐predictors‐of‐meaningful‐decline‐in‐forced‐vital‐capacity‐during‐follow‐up‐%d0%bef‐a‐large‐cohort‐of‐systemic‐sclerosis‐associated‐pulmonary‐fibrosis‐patients/. [Google Scholar]
- 18. Wollin L, Distler JH, Denton CP, Gahlemann M. Rationale for the evaluation of nintedanib as a treatment for systemic sclerosis‐associated interstitial lung disease. J Scleroderma Relat Disord 2019;4:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis‐associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 20. Maher TM, Mayes MD, Kreuter M, Volkmann ER, Aringer M, Castellvi I, et al. Effect of nintedanib on lung function in patients with systemic sclerosis–associated interstitial lung disease: further analyses of the SENSCIS trial. Arthritis Rheumatol 2021;73:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 22. Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high‐dose versus low‐dose penicillamine trial. Arthritis Rheum 2000;43:2445–54. [DOI] [PubMed] [Google Scholar]
- 23. Khanna D, Furst DE, Clements PJ, Allanore Y, Baron M, Czirjak L, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Dis 2017;2:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kafaja S, Clements PJ, Wilhalme H, Tseng CH, Furst DE, Kim GH, et al. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS‐I and SLS‐II). Am J Respir Crit Care Med 2018;197:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Highland KB, Distler O, Kuwana M, Allanore Y, Assassi S, Azuma A, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis‐associated interstitial lung disease treated with mycophenolate: subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021;9:96–106. [DOI] [PubMed] [Google Scholar]
- 26. Gilson M, Zerkak D, Wipff J, Dusser D, Dinh‐Xuan AT, Abitbol V, et al. Prognostic factors for lung function in systemic sclerosis: prospective study of 105 cases. Eur Respir J 2010;35:112–7. [DOI] [PubMed] [Google Scholar]
- 27. Frantz C, Huscher D, Avouac J, Hachulla E, Balbir‐Gurman A, Riemekasten G, et al. Outcomes of limited cutaneous systemic sclerosis patients: results on more than 12,000 patients from the EUSTAR database. Autoimmun Rev 2020;19:102452. [DOI] [PubMed] [Google Scholar]
- 28. Meier FM, Frommer KW, Dinser R, Walker UA, Czirjak L, Denton CP, et al. Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2012;71:1355–60. [DOI] [PubMed] [Google Scholar]
- 29. Srivastava N, Hudson M, Tatibouet S, Wang M, Baron M, Fritzler MJ, et al. Thinking outside the box: the associations with cutaneous involvement and autoantibody status in systemic sclerosis are not always what we expect. Semin Arthritis Rheum 2015;45:184–9. [DOI] [PubMed] [Google Scholar]
- 30. Dougherty DH, Kwakkenbos L, Carrier ME, Salazar G, Assassi S, Baron M, et al. The Scleroderma Patient‐Centered Intervention Network cohort: baseline clinical features and comparison with other large scleroderma cohorts. Rheumatology (Oxford) 2018;57:1623–31. [DOI] [PubMed] [Google Scholar]
- 31. Wu W, Jordan S, Graf N, de Oliveira Pena J, Curram J, Allanore Y, et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann Rheum Dis 2019;78:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosureform
Appendix S1: Supporting information


