Abstract
Objective
The purpose of this study was to propose a definition of “wearing off” at the individual patient‐level and determine the percentage of patients with migraine who experience “wearing off” of efficacy of galcanezumab at the end of a treatment cycle using this predefined threshold.
Background
Anecdotal reports suggest that some patients may experience “wearing off” of efficacy during the last week of their calcitonin gene‐related peptide monoclonal antibody treatment cycle. A previous post hoc analysis of galcanezumab demonstrated consistent efficacy at each week throughout all monthly dosing intervals at the population‐level, but “wearing off” has not been assessed at the individual patient‐level.
Methods
Post hoc analyses of clinical trial data from four galcanezumab phase III, randomized, placebo‐controlled studies in a total of 2680 patients with high‐frequency episodic migraine (EVOLVE‐1, EVOLVE‐2, and CONQUER studies) or chronic migraine (CM; REGAIN and CONQUER studies) were conducted. “Wearing off” was defined as an increase of greater than or equal to 2 weekly migraine headache days in the last week of the treatment cycle compared to the second week for at least 2 months. The analyses were conducted (1) in all patients and (2) in patients with a clinically meaningful response to treatment.
Results
The percentage of patients meeting the threshold of “wearing off” was not statistically significantly different among the placebo, galcanezumab 120 mg, and galcanezumab 240 mg treatment groups, both in the total population and in patients with a clinically meaningful response (all ≤9.0%). Although the frequency of “wearing off” in patients with CM and prior preventive failures was numerically greater in the galcanezumab groups (8/89 or 9.0%) compared to placebo (3/95 or 3.2%), these differences were not statistically significant.
Conclusions
Consistent with previous analyses at the population‐level that showed no evidence of decreased efficacy at the end of a treatment cycle, rates of individual patients meeting the threshold of “wearing off” in this analysis were low and similar among placebo, galcanezumab 120 mg, and galcanezumab 240 mg treatment groups.
Keywords: calcitonin gene‐related peptide, galcanezumab, migraine, migraine prevention, monoclonal antibody, wear off
Abbreviations
- ANOVA
analysis of variance
- CGRP
calcitonin gene‐related peptide
- CM
chronic migraine
- EM
episodic migraine
- HFEM
high‐frequency episodic migraine
- K D
binding affinity
- k off
dissociation rate
- k on
association rate
- LFEM
low‐frequency episodic migraine
- mAb
monoclonal antibody
INTRODUCTION
Migraine is a neurologic disease that is characterized by recurring episodes of head pain with associated symptoms that can be disabling. 1 Galcanezumab is a humanized monoclonal antibody (mAb) that binds to the calcitonin gene‐related peptide (CGRP) ligand and is approved for the preventive treatment of migraine in adults. 2 Previous clinical studies have demonstrated its safety/tolerability and efficacy in reducing monthly migraine headache days in patients with episodic migraine (EM; defined as 4–14 monthly migraine headache days) and chronic migraine (CM; defined as 15 or more monthly headache days of which at least 8 days have features of migraine headache). 3 , 4 , 5 , 6
Anecdotal reports suggest that some patients may experience “wearing off” of efficacy, or increase in the number of migraine headache days, during the last week of the treatment cycle prior to receiving their next CGRP mAb dose. Existing literature on this topic is limited with varying conclusions. Retrospective chart reviews and small observational studies suggest that 8.9% to 39.1% of patients experience “wearing off” with CGRP mAbs. 7 , 8 , 9 On the other hand, a post hoc analysis of a fremanezumab clinical trial showed no evidence of “wearing off” at the population‐level for patients with EM or CM on monthly or quarterly dosing regimens. 10 “Wearing off” has also been described with onabotulinumtoxinA in 23.3% to 62.9% of patients, most commonly 2 to 4 weeks before the next injection. 11 , 12 , 13 , 14
A prior post hoc analysis of galcanezumab 120 mg monthly (with a 240 mg loading dose) demonstrated consistent efficacy at each week throughout all monthly dosing intervals for patients with EM and CM. 15 There was a statistically significant mean reduction from baseline in migraine headache days for galcanezumab relative to placebo at each week of every month. 15 Although there was no evidence of decreased efficacy toward the end of the treatment cycle at the population‐level, there may still be individual patients who experience “wearing off”, and this possibility has not been previously assessed. Because CGRP mAbs are relatively new, there is no established definition of what changes in patient response constitute “wearing off”. Defining “wearing off” is further complicated by the natural variability of migraine headache days over time. 16 The objective of this study is to propose a definition of “wearing off” at the individual patient‐level and use this threshold to determine the percentage of patients with migraine who experience “wearing off” of efficacy of galcanezumab at the end of a treatment cycle. Based on the consistent efficacy observed with galcanezumab at the population‐level, we hypothesized that “wearing off” of galcanezumab at the end of the dosing interval would be observed in only a small percentage (<10%) of individual patients.
METHODS
Study design
Data for these analyses were drawn from four galcanezumab phase III, randomized, double‐blind, placebo‐controlled studies in patients with EM (EVOLVE‐1, EVOLVE‐2, and CONQUER studies) or CM (REGAIN and CONQUER studies). 3 , 4 , 5 , 6 Patients were randomized to receive monthly subcutaneous injections of galcanezumab or placebo. The double‐blind treatment periods for EVOLVE‐1 and ‐2 were 6 months in duration whereas REGAIN and CONQUER were 3 months. Detailed descriptions of the study designs were previously published. 3 , 4 , 5 , 6 Key similarities/differences among the four studies are outlined in Table 1. The study protocols were reviewed and approved by the institutional review board, medical ethics committee, or medical research and ethics committee of the participating study sites. All patients provided written informed consent before study participation. All studies were conducted in accordance with the ethical principles of the Declaration of Helsinki guidelines. 17
TABLE 1.
| Study name | EVOLVE‐1 | EVOLVE‐2 | REGAIN | CONQUER |
|---|---|---|---|---|
| NCT number | NCT02614183 | NCT02614196 | NCT02614261 | NCT03559257 |
| Treatment groups and randomization | 2:1:1: PBO:GMB 120 mg a :GMB 240 mg | 2:1:1: PBO:GMB 120 mg a :GMB 240 mg | 2:1:1: PBO:GMB 120 mg a :GMB 240 mg | 1:1: PBO:GMB 120 mg a |
| Number of patients randomized and treated (ITT) | Total: 858 | Total: 915 | Total: 1113 | Total: 462 |
| LFEM: 294 | LFEM: 303 | CM: 1113 | LFEM: 71 | |
| HFEM: 564 | HFEM: 612 | HFEM: 198 | ||
| CM: 193 | ||||
| Baseline period | 30–40 days | 30–40 days | 30–40 days | 1 month |
| Double‐blind treatment period | 6 months | 6 months | 3 months | 3 months |
| Open‐label extension period | None | None | 9 months | 3 months |
| Post‐treatment follow‐up period | 4 months | 4 months | 4 months | None |
| Patient age | 18–65 years | 18–65 years | 18–65 years | 18–75 years |
| Key inclusion criteria | 4–14 monthly migraine headache days | 4–14 monthly migraine headache days | ≥15 headache days per month, of which ≥8 were migraine headache days | ≥4 monthly migraine headache days. History of 2–4 prior migraine preventive medication category b failures in the past 10 years |
| Primary endpoint | Overall mean change from baseline in the number of monthly migraine headache days during the double‐blind treatment period | |||
| Study sites | North America | Global | Global | Global |
| Additional migraine preventive medications | Not permitted | Not permitted | Stable doses of topiramate or propranolol were allowed and taken by ~15% of population | Not permitted |
Abbreviations: CM, chronic migraine (≥15 monthly headache days of which ≥8 are migraine headache days); GMB, galcanezumab; HFEM, high‐frequency episodic migraine (8–14 monthly migraine headache days and <15 monthly headache days); ITT, intent‐to‐treat population; LFEM, low‐frequency episodic migraine (4–7 monthly migraine headache days); NCT, National Clinical Trial; PBO, placebo.
With a loading dose of 240 mg.
Medication categories included propranolol or metoprolol, topiramate, valproate or divalproex, amitriptyline, flunarizine, candesartan, and botulinum toxin A or B (if taken for CM).
Patient population
The EVOLVE‐1 and ‐2, REGAIN, and CONQUER studies included adults ages 18 to 65 years (18 to 75 years in the CONQUER study) with a diagnosis of migraine. The post hoc analyses presented here assessed patients with high‐frequency episodic migraine (HFEM; 8 to 14 monthly migraine headache days and <15 monthly headache days) 18 or CM (≥15 monthly headache days of which ≥8 were migraine headache days). The decision to include these subgroups was based on a query of 40 board‐certified headache specialists with experience prescribing CGRP mAbs, some of whom served as investigators for CGRP mAb trials. Per their expert opinion, patients with HFEM and CM were more likely to report “wearing off”. Patients with low‐frequency episodic migraine (LFEM; 4 to 7 monthly migraine headache days) 18 were excluded from this analysis because it is likely difficult for patients to perceive “wearing off” at such a low frequency.
Definition of “wearing off” for an individual patient
For an individual patient, “wearing off” was defined as an increase of greater than or equal to two migraine headache days per week from week 2 (8 to 14 days post‐treatment) to week 4 (defined as the final 7 days before the next dose) in at least 2 of the treatment months considered in this analysis (Figure 1). The threshold of greater than or equal to two migraine headache days was chosen based on an intrapatient variability analysis in weekly migraine headache days and to minimize the influence of fluctuations due to the natural variability of migraine, as detailed in the Statistical analysis section. Week 2, rather than week 1, was chosen for comparison to allow time for galcanezumab concentration to reach median peak levels, which occurs ~ 5 days after dosing. 19
FIGURE 1.

Study design. The duration of the double‐blind treatment period was 6 months for EVOLVE‐1 and ‐2 and 3 months for REGAIN and CONQUER. Months 1 to 3 of EVOLVE‐1 and ‐2 and month 1 of REGAIN and CONQUER were used to determine whether the patient had a clinically meaningful response. “Wearing off” was calculated using data from months 4 to 6 in EVOLVE‐1 and ‐2 and months 2 and 3 in REGAIN and CONQUER. Month 1 was not included in the analysis because a loading dose of 240 mg was administered
Outcomes
The post hoc analyses assessed the percentage of individual patients with HFEM and CM who demonstrated “wearing off” as defined earlier. Whereas EVOLVE‐1 and ‐2 consisted of 6‐month double‐blind treatment periods, this analysis used data from months 4 to 6 because the majority of queried headache specialists reported “wearing off” typically occurred after several months of use. Further, the American Headache Society consensus statement recommends trialing once‐monthly administered CGRP mAbs for 3 months before determining a patient’s response. 20 Because REGAIN and CONQUER consisted of 3‐month double‐blind treatment periods, analyses were conducted for months 2 and 3. Month 1 was excluded from analyses of all the trials because patients received a 240 mg loading dose during that month. “Wearing off” was evaluated for 2 or 3 months of months 4 to 6 in EVOLVE‐1 and ‐2 and for months 2 and 3 in REGAIN and CONQUER (Figure 1).
The analyses were conducted in the following populations: (1) all patients with HFEM or CM from the aforementioned trials and (2) patients with HFEM or CM with a clinically meaningful response to treatment. A clinically meaningful response was defined as patients with HFEM who had a greater than or equal to 50% reduction from baseline in monthly migraine headache days across months 1 to 3 for EVOLVE‐1 and ‐2 and in month 1 for CONQUER. This also included patients with CM who had a greater than or equal to 30% reduction from baseline in monthly migraine headache days in month 1 for REGAIN and CONQUER. The threshold of greater than or equal to 50% for HFEM and greater than or equal to 30% for CM were chosen because these are common outcome measures used in clinical trials for migraine prevention and are considered clinically meaningful. 21 , 22 , 23
Statistical analysis
Patient demographics and baseline characteristics were summarized using mean and standard deviation or frequency and percentages as appropriate, and compared between treatment groups using Fisher’s exact test, two‐way analysis of variance (ANOVA) with treatment group and study for EVOLVE‐1 and ‐2, one‐way ANOVA with treatment group for REGAIN and CONQUER, and the Cochran‐Mantel‐Haenszel test. To determine the threshold used to define “wearing off”, estimates of intrapatient variability in weekly migraine headache days were calculated using the standard deviation in weekly migraine headache days from months 4 to 6 for EVOLVE‐1 and ‐2 and months 2 and 3 for REGAIN and CONQUER (Table S1). Intrapatient variability was calculated within each patient and averaged across all patients included in the analyses. Standard deviations ranged from 0.72 to 1.33 among patients with HFEM in EVOLVE‐1 and ‐2 and CONQUER, and 1.21 to 1.56 among patients with CM in REGAIN and CONQUER. To minimize erroneously identifying patients with natural variability in migraine headache days as patients with “wearing off”, a threshold increase of two migraine headache days per week within an individual patient was chosen because it was approximately two times the estimated standard deviation of weekly migraine headache days among patients with HFEM. This threshold was retained for patients with CM because the estimate of coefficient of variation (standard deviation relative to the mean) was relatively lower for patients with CM compared with patients with HFEM, suggesting a threshold of two migraine headache days per week may be appropriate for patients with CM as well.
For each treatment group, the percentage of patients demonstrating an increase of greater than or equal to 2 weekly migraine headache days from week 2 to week 4 in 2 or 3 months of months 4 to 6 in EVOLVE‐1 and ‐2 and months 2 and 3 in REGAIN and CONQUER were summarized and compared between treatment groups using Fisher’s exact test. This comparison was assessed for the total population and for patients with a clinically meaningful response. All analyses should be considered post hoc and no adjustments for multiple testing or multiplicity were done. The sample sizes used in these comparisons were dictated by the size of the original studies and not necessarily powered to detect a statistically significant difference in “wearing off” between groups. Therefore, although p values for these comparisons are shown for informational purposes, inferences regarding differential rates of “wearing off” between groups should be interpreted with caution. All statistical analyses were conducted using SAS Enterprise Guide version 7.1 for Windows. All tests were two‐sided and p values less than 0.05 were considered statistically significant.
RESULTS
Patient demographics and baseline disease characteristics
These post hoc analyses included 2680 patients from the EVOLVE‐1 and ‐2, REGAIN, and CONQUER studies. Of these patients, 1374 had HFEM and 1306 had CM. Most patients were women, White, and ~ 41 to 46 years old. Many had a diagnosis of migraine for over 20 years and were severely disabled by their disease. Additional demographic and disease characteristics are presented in Table 2. Although there were some statistically significant differences in baseline characteristics between treatment groups with respect to sex distribution, age, time since migraine diagnosis, number of monthly migraine headache days with acute medication use, and number of patients with greater than or equal to two prior treatment failures, these differences were not considered clinically relevant.
TABLE 2.
Demographics and baseline disease characteristics in patients with HFEM or CM from the EVOLVE‐1 and ‐2, REGAIN, and CONQUER studies
| Characteristic | HFEM populations | CM populations | ||
|---|---|---|---|---|
| EVOLVE‐1/‐2 (N = 1176) a | CONQUER (N = 198) b | REGAIN (N = 1113) c | CONQUER (N = 193) d | |
| Age, years, mean (SD) | 41.0 (11.4) | 45.5 (11.4) | 41.0 (12.1) | 45.3 (12.4) |
| Female, n (%) | 1018 (86.6%) | 170 (85.9%) | 946 (85.0%) | 168 (87.1%) |
| Race, n (%) | ||||
| Asian | 74 (6.3%) | 18 (9.3%) | 53 (4.8%) | 46 (25.0%) |
| Black or African American | 106 (9.0%) | 1 (0.5%) | 72 (6.5%) | 4 (2.2%) |
| White | 889 (75.6%) | 172 (89.1%) | 879 (79.1%) | 132 (71.7%) |
| Body mass index, kg/m2, mean (SD) | 27.8 (5.6) | 25.5 (5.0) | 26.7 (5.5) | 25.8 (5.9) |
| Time since migraine diagnosis, years, mean (SD) | 20.2 (12.2) | 22.3 (12.7) | 21.1 (12.8) | 24.6 (14.4) |
| Number of monthly headache days, mean (SD) | 12.2 (2.9) | 11.6 (1.9) | 21.4 (4.1) | 20.9 (4.4) |
| Number of monthly migraine headache days, mean (SD) | 10.8 (2.0) | 10.7 (1.9) | 19.4 (4.5) | 18.7 (4.7) |
| Number of monthly migraine headache days with acute medication use, mean (SD) | 8.8 (3.3) | 10.3 (3.4) | 15.2 (6.4) | 16.2 (6.4) |
| MIDAS total score, mean (SD) | 36.6 (30.7) | 42.4 (29.9) | 67.2 (57.3) | 67.2 (57.0) |
| MSQ‐RFR score, mean (SD) | 49.5 (15.4) | 46.4 (16.4) | 38.7 (17.2) | 41.2 (18.4) |
| Number of comorbid conditions, mean (SD) | 3.6 (3.6) | 3.9 (3.7) | 4.3 (3.5) | 4.4 (3.6) |
| Patients with ≥2 prior preventive treatment failures, n (%) | 125 (10.6%) | 198 (100%) | 328 (29.5%) | 192 (99.5%) |
Differences between treatment groups were not statistically significant except for the following: sex distribution in the HFEM population of CONQUER differed between PBO and GMB 120 mg (p = 0.026 per Fisher’s exact test), age in REGAIN differed between PBO and GMB 120 mg (p = 0.027 per ANOVA), time since migraine diagnosis (p = 0.046 per ANOVA) and number of monthly migraine headache days with acute medication use (p = 0.031 per ANOVA) in REGAIN differed between PBO and GMB 240 mg, greater than or equal to two prior treatment failures in REGAIN differed between GMB 120 mg and GMB 240 mg (p = 0.007 per Fisher’s exact test).
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; CM, chronic migraine (≥15 monthly headache days of which ≥8 are migraine headache days); GMB, galcanezumab; HFEM, high‐frequency episodic migraine (8–14 monthly migraine headache days and <15 monthly headache days); LS, least‐squares; MIDAS, Migraine Disability Assessment; MSQ‐RFR, Migraine‐Specific Quality of Life Questionnaire Role Function‐Restrictive; N, number of patients in each population; n, number of patients within each specific category; PBO, placebo; SD, standard deviation.
Number of patients with HFEM in EVOLVE‐1 and ‐2 with available MSQ and MIDAS data were 1166 patients.
Number of patients in the HFEM group of CONQUER with available race information was 193 and number of comorbid conditions was available for 147 patients.
Number of patients in REGAIN with available information on race was 1112, BMI was available for 1111, number of comorbid conditions was available for 937, and MSQ and MIDAS scores were collected for 1090 patients.
Number of patients in the CM group of CONQUER with available race information was 184, BMI was available for 192, and number of comorbid conditions was available for 176 patients.
Percentage of total patients who experienced “wearing off”
Of the 2680 patients, only those who had data for week 2 and week 4 during months 4 to 6 in EVOLVE‐1 and ‐2 and months 2 and 3 in REGAIN and CONQUER were included in this analysis, yielding 2409 patients. The percentage of patients with HFEM or CM who met the threshold of “wearing off” was not statistically significantly different among the placebo, galcanezumab 120 mg, and galcanezumab 240 mg treatment groups (Figure 2, Table S2). In patients with HFEM from EVOLVE‐1 and ‐2 and CONQUER, 4.3% to 7.0% of patients on placebo and 5.0% to 5.8% of patients on galcanezumab met the threshold of “wearing off” for 2 months. In patients with CM from REGAIN and CONQUER, 3.2% of patients on placebo and 5.4% to 9.0% of patients on galcanezumab met the threshold of “wearing off” for 2 months. Fewer than 1% of patients with HFEM from EVOLVE‐1 and ‐2 met the threshold of “wearing off” for 3 months. Although the frequency of “wearing off” was numerically greater in the galcanezumab groups compared to placebo in patients with CM (REGAIN and CONQUER) and patients with HFEM who did not benefit from multiple prior preventive treatments (CONQUER), these differences were not statistically significant (Table S2).
FIGURE 2.
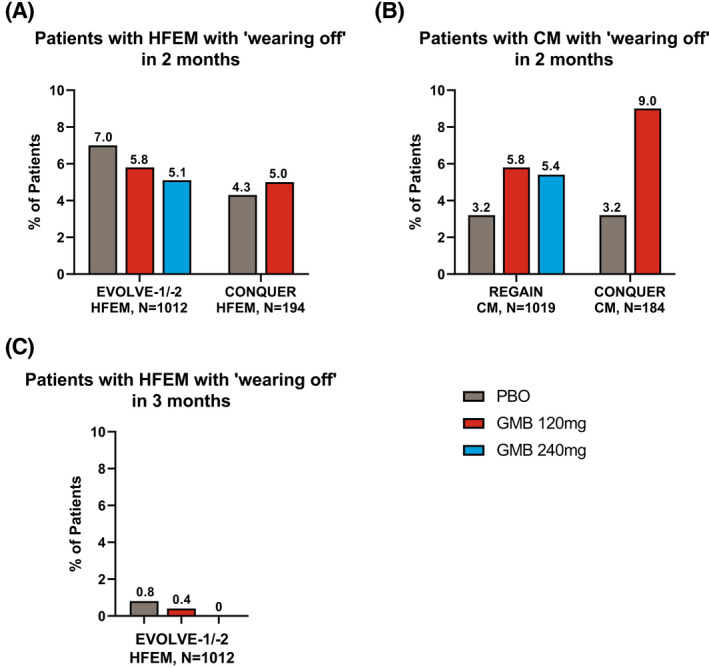
Percentage of total patients who experienced “wearing off”. Percentage of patients with HFEM (A) and CM (B) who had “wearing off” for 2 months, and percentage of patients with HFEM who had “wearing off” for 3 months (C). “Wearing off” was defined as an increase of greater than or equal to 2 migraine headache days per week from week 2 (8 to 14 days post‐treatment) to week 4 (7 days prior to next dose) during 2 or 3 months of months 4 to 6 in EVOLVE‐1 and ‐2 or in both months 2 and 3 in REGAIN and CONQUER. Two‐sided Fisher’s exact test was used to compare rates of “wearing off” between treatment groups. The p values were greater than 0.05 for all comparisons between treatment groups. Refer to Table S2 for exact p values. CM, chronic migraine (≥15 monthly headache days of which ≥8 are migraine headache days); GMB, galcanezumab; HFEM, high‐frequency episodic migraine (8–14 monthly migraine headache days and <15 monthly headache days); N, number of patients in each subgroup; PBO, placebo
Percentage of patients with a clinically meaningful response who experienced “wearing off”
Among the 2680 total patients, 903 had a clinically meaningful response early in their course of treatment and had data for week 2 and week 4 in the latter months used in this analysis. A clinically meaningful response was defined as a greater than or equal to 50% reduction from baseline in monthly migraine headache days across months 1 to 3 for EVOLVE‐1 and ‐2 and in month 1 for CONQUER for patients with HFEM, and a greater than or equal to 30% reduction from baseline in monthly migraine headache days in month 1 for REGAIN and CONQUER for patients with CM. The percentage of patients with HFEM or CM with a clinically meaningful response who met the threshold of “wearing off” was not statistically significantly different among the placebo, galcanezumab 120 mg, and galcanezumab 240 mg treatment groups (Figure 3, Table S3).
FIGURE 3.

Percentage of patients with a clinically meaningful response who experienced “wearing off”. 1A clinically meaningful response was defined as patients with HFEM who had a ≥50% reduction from baseline in monthly migraine headache days across months 1 to 3 in EVOLVE‐1 and ‐2 and in month 1 in CONQUER, and patients with CM who had a greater than or equal to 30% reduction from baseline in monthly migraine headache days in month 1 in REGAIN and CONQUER. 2“Wearing off” was defined as an increase of greater than or equal to 2 migraine headache days per week from week 2 (8 to 14 days post‐treatment) to week 4 (7 days prior to next dose) during 2 or 3 months of months 4 to 6 in EVOLVE‐1 and ‐2 or in both months 2 and 3 in REGAIN and CONQUER. Two‐sided Fisher’s exact test was used to compare rates of “wearing off” between treatment groups. The p values were greater than 0.05 for all comparisons between treatment groups. Refer to Table S3 for exact p values. CM, chronic migraine (≥15 monthly headache days of which ≥8 are migraine headache days); GMB, galcanezumab; HFEM, high‐frequency episodic migraine (8–14 monthly migraine headache days and <15 monthly headache days); N, number of patients in each subgroup; PBO, placebo
Of patients with HFEM from EVOLVE‐1 and ‐2 and CONQUER, 11.5% to 24.5% of patients on placebo and 39.2% to 50.0% of patients on galcanezumab had a clinically meaningful response. Among them, 0% to 1.4% of patients on placebo and 0% to 4.0% of patients on galcanezumab met the threshold of “wearing off” for 2 months. Of patients with CM from REGAIN and CONQUER, 20.4% to 23.7% of patients on placebo and 41.4% to 51.6% of patients on galcanezumab had a clinically meaningful response. Among them, 0% to 2.3% of patients on placebo and 5.2% to 8.2% of patients on galcanezumab met the threshold of “wearing off” for 2 months. Although the frequency of “wearing off” was numerically greater in the galcanezumab groups compared to placebo, these differences were not statistically significant (Table S3).
DISCUSSION
The question of whether “wearing off” of efficacy takes place at the end of a dosing interval was addressed using patient‐level data from post hoc analyses of four phase III galcanezumab clinical trials. There are anecdotal reports of “wearing off” in the literature with CGRP mAbs, 7 , 8 , 9 but this term has not been well‐defined. Based on input from headache experts, a threshold was set for “wearing off”, which consisted of an increase of greater than or equal to 2 migraine headache days per week from week 2 to week 4 in a treatment month among patients with HFEM and CM. The results showed that a small percentage of patients (≤7.0% on placebo and ≤9.0% on galcanezumab) met the threshold of “wearing off” for 2 months and fewer than 1% of patients met the threshold for 3 months. These numbers were similar in patients who had a clinically meaningful response to treatment in earlier months. Interestingly, the frequency of “wearing off” was not statistically significantly different between the placebo and galcanezumab treatment groups.
The similar rates of “wearing off” among placebo, galcanezumab 120 mg, and galcanezumab 240 mg treatment groups suggest that this observed decrease in effectiveness is likely not based on pharmacokinetics. Galcanezumab has a half‐life of 27 days 2 and a binding affinity (KD ) of 31 pM. 24 The KD is calculated by dividing the dissociation rate (k off) by the association rate (k on). Galcanezumab has a k off of 2.2 × 10−4 s−1 and a k on of 7.4 × 106 M−1 s−1 yielding a low KD of 31 pM, which is indicative of a high affinity and specificity to the CGRP ligand. 24 Thus, the fast association rate allows galcanezumab to bind CGRP before it can bind to the receptor, the slow dissociation rate ensures that CGRP is not released, and the long half‐life allows for a long duration of effect with a single dose. 24
The observed “wearing off” may be attributable to a variety of reasons. Patients may anticipate or pay more attention to their migraine headache days prior to receiving their next injection or visiting their clinician, resulting in higher levels of reporting during this time. Previous chart reviews and observational studies indicate that reports of “wearing off” with CGRP mAbs were higher if patients were queried about it. 7 , 8 , 9 Clinicians may also counsel their patients on the possibility of “wearing off”, which could influence patients’ expectations and reporting of this occurrence. 14 This anticipation of a negative outcome is known as the ‘nocebo’ effect. It is also natural for migraine headache day frequency to vary throughout the course of a month, 16 which could contribute to the appearance of “wearing off” in some patients. There may be patients who are more likely to experience a decrease in efficacy toward the end of their dosing interval. In fact, the results of these post hoc analyses showed that “wearing off” was more common in patients with CM from the CONQUER trial, which enrolled patients who previously did not benefit from two to four migraine preventive medication categories. This subgroup had the largest numerical difference in “wearing off” rates between galcanezumab and placebo treatment groups (although not statistically significant), suggesting that patients with CM who have not benefited from prior preventive treatments may be more susceptible to “wearing off”.
Further, “wearing off” may be more common in the real‐world setting compared to clinical trials due to lower rates of medication adherence. 25 In the galcanezumab clinical trials, patients were encouraged to take their next dose 28 to 32 days after their prior dose. In the real‐world, patients may not follow the dosing instructions and wait longer than 1 month before filling their next dose. In an IBM Marketscan administrative claims study, wherein 7630 patients with commercial insurance and at least two galcanezumab claims were followed for 6 months post‐initiation of galcanezumab, 73.6% of patients had no gap in treatment between their first and second galcanezumab claims, 9.0% had 1 to 7 gap days, 4.4% had 8 to 14 gap days, 6.7% had 15 to 30 gap days, and 6.3% had greater than 30 gap days (data on file). This extended time between doses could contribute to higher reports of “wearing off” in clinical practice.
Strategies to mitigate or prevent “wearing off” remain uncertain. Increasing the dose or frequency of treatment, as in the case with onabotulinumtoxinA or erenumab, have been reported with some success. 8 , 11 , 14 , 26 An individual patient who experiences “wearing off” on galcanezumab 120 mg may or may not experience “wearing off” with a higher dose. The clinical trial design prohibited this evaluation because patients remained on their assigned dose of galcanezumab during the double‐blind treatment period. Further, galcanezumab 240 mg monthly is not an approved dose. However, the lack of difference in rates of “wearing off” between the galcanezumab 120 and 240 mg groups suggests that, on average, patients on higher doses do not have lower rates of “wearing off”. Another potential approach to counter “wearing off” is to use additional acute treatments toward the end of the dosing interval. 12 , 13 Further, encouraging patients to take their next dose of CGRP mAb within 30 days of the previous dose may also help diminish the likelihood of “wearing off”.
Several limitations of the post hoc analyses should be considered. Patients with LFEM (4 to 7 monthly migraine headache days) were not included because the group of queried headache experts reported “wearing off” to be rare in this subgroup as it is likely difficult to perceive at such a low frequency. The duration of the CONQUER and REGAIN double‐blind treatment periods was 3 months, so it is unclear what the rate of “wearing off” would be with long‐term use of galcanezumab. Because this analysis required patients to complete 3 months of treatment in REGAIN and CONQUER, and 6 months of treatment in EVOLVE‐1 and ‐2, this could introduce bias, as patients who completed the trial may be those who experienced better efficacy and less “wearing off”, although greater than or equal to 81% of patients completed the double‐blind treatment periods of these trials. 3 , 4 , 5 , 6 Additionally, this post hoc analysis may not be sufficiently powered to detect statistically significant differences, so lack of statistical significance does not necessarily indicate lack of a clinically meaningful difference. Missing data may also limit full interpretation of the results and inferences made. The number of patients enrolled in CONQUER was low, thus making it difficult to interpret the low rate of “wearing off” in patients with HFEM with a clinically meaningful response in that study. Further, the threshold of “wearing off” was defined by a panel of 40 headache experts based on clinically meaningful differences and supported using statistical measures, such as coefficient of variation, but its reliability has not been verified. This definition only incorporated frequency of migraine headache days but not severity or attack duration. Regardless, the analyses reported here are the first to examine a decrease in efficacy toward the end of a dosing interval at the individual patient‐level rather than the population‐level, using data from more than 2500 phase III clinical trial patients, thereby providing new insights into the issue of “wearing off” of efficacy of CGRP mAbs.
CONCLUSIONS
Consistent with previous population‐level analyses that showed no evidence of decreased efficacy of galcanezumab at the end of a treatment cycle, rates of individual patients meeting a predefined threshold of “wearing off” in this analysis were low and similar among placebo, galcanezumab 120 mg, and galcanezumab 240 mg treatment groups.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The studies were conducted in concordance with the ethical principles that have their origin in the Declaration of Helsinki guidelines. All patients provided written informed consent before study participation. The study protocols were reviewed and approved by the institutional review board, medical ethics committee, or medical research and ethics committee of the participating study sites.
CONFLICT OF INTEREST
J.A. has received honoraria for consulting from Amgen, Allergan/Abbvie, Aeon, Biohaven, Eli Lilly and Company, GlaxoSmithKline, Lundbeck, Teva, Impel, Satsuma, Nesos, Theranica, Axsome, and Medscape. She received honoraria for speaking from Allergan, Amgen, Biohaven, Eli Lilly and Company, Lundbeck, and Teva. She also received honoraria for editorial services from Current Pain and Headache Reports, Section editor, Unusual Headache Syndromes, NeurologyLive, and SELF. Clinical trial grants have been paid to J.A.’s institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly and Company, Satsuma, and Zosano. DKK, MR, TO, KS, LW, and MP are employees and minor stockholders of Eli Lilly and Company. A.M.B. has received honoraria for serving on advisory boards of Allergan, Aeon, Alder, Amgen, Biohaven, Eli Lilly and Company, Novartis, Revance, Teva, Theranica, and Zosano. He has received fees for speaking from Allergan, Amgen, Eli Lilly and Company, and Teva and as a consultant for Allergan, Alder, Amgen, Biohaven, Eli Lilly and Company, Novartis, Teva, Theranica, and Zosano. He has been a contributing author in partnership with Novartis, Teva, Allergan, and Biohaven. He has received grant support from Allergan and Amgen.
AUTHOR CONTRIBUTIONS
Study concept and design: Dulanji K. Kuruppu, Mallikarjuna Rettiganti, Tina Oakes. Acquisition of data: Mallikarjuna Rettiganti. Analysis and interpretation of data: Jessica Ailani, Dulanji K. Kuruppu, Mallikarjuna Rettiganti, Tina Oakes, Krista Schroeder, Linda Wietecha, Martha Port, Andrew M. Blumenfeld. Drafting of the manuscript: Dulanji K. Kuruppu, Mallikarjuna Rettiganti, Martha Port. Revising it for intellectual content: Jessica Ailani, Dulanji K. Kuruppu, Mallikarjuna Rettiganti, Tina Oakes, Krista Schroeder, Linda Wietecha, Martha Port, Andrew M. Blumenfeld. Final approval of the completed manuscript: Jessica Ailani, Dulanji K. Kuruppu, Mallikarjuna Rettiganti, Tina Oakes, Krista Schroeder, Linda Wietecha, Martha Port, Andrew M. Blumenfeld.
CLINICAL TRIALS REGISTRATION NUMBERS
ClinicalTrials.gov EVOLVE‐1, NCT02614183; EVOLVE‐2, NCT02614196; REGAIN, NCT02614261; and CONQUER, NCT03559257.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
Rohit Bhandari, a former employee of Eli Lilly Services India Private Limited, provided writing support. The authors thank all the study participants, site investigators, and personnel involved in the studies.
Ailani J, Kuruppu DK, Rettiganti M, et al. Does “wearing off” of efficacy occur in galcanezumab‐treated patients at the end of the monthly treatment cycle? Post hoc analyses of four phase III randomized trials. Headache. 2022;62:198–207. doi: 10.1111/head.14257
DATA AVAILABILITY STATEMENT
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
REFERENCES
- 1. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 2. Emgality (galcanezumab‑gnlm) [package insert]. Eli Lilly and Company; 2019. https://pi.lilly.com/us/emgality‐uspi.pdf. Accessed June 24, 2021. [Google Scholar]
- 3. Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE‐1 randomized clinical trial. JAMA Neurol. 2018;75:1080‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE‐2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442‐1454. [DOI] [PubMed] [Google Scholar]
- 5. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double‐blind, placebo‐controlled REGAIN study. Neurology. 2018;91:e2211‐e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulleners WM, Kim B‐K, Láinez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet Neurol. 2020;19:814‐825. [DOI] [PubMed] [Google Scholar]
- 7. Feldman D, Reed D, George N. The Aimovig “wear‐off”: a retrospective review. Headache. 2019;59:1‐208. [Google Scholar]
- 8. Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real‐world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60:2014‐2025. [DOI] [PubMed] [Google Scholar]
- 9. Restrepo S. Wearing off in CGRP fremanezumab‐vfrm and galcanezumab‐gnlm. Headache. 2020;60:1‐156.32533851 [Google Scholar]
- 10. Blumenfeld AM, Stevanovic DM, Ortega M, et al. No “wearing‐off effect” seen in quarterly or monthly dosing of fremanezumab: subanalysis of a randomized long‐term study. Headache. 2020;60:2431‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quintas S, Garcia‐Azorin D, Heredia P, Talavera B, Gago‐Veiga AB, Guerrero AL. Wearing off response to onabotulinumtoxinA in chronic migraine: analysis in a series of 193 patients. Pain Med. 2019;20:1815‐1821. [DOI] [PubMed] [Google Scholar]
- 12. Khan FA, Mohammed AE, Poongkunran M, Chimakurthy A, Pepper M. Wearing off effect of onabotulinumtoxinA near the end of treatment cycle for chronic migraine: a 4‐year clinical experience. Headache. 2020;60:430‐440. [DOI] [PubMed] [Google Scholar]
- 13. Ruscheweyh R, Athwal B, Gryglas‐Dworak A, et al. Wear‐off of onabotulinumtoxinA effect over the treatment interval in chronic migraine: a retrospective chart review with analysis of headache diaries. Headache. 2020;60:1673‐1682. [DOI] [PubMed] [Google Scholar]
- 14. Masters‐Israilov A, Robbins MS. OnabotulinumtoxinA wear‐off phenomenon in the treatment of chronic migraine. Headache. 2019;59:1753‐1761. [DOI] [PubMed] [Google Scholar]
- 15. Pozo‐Rosich P, Samaan KH, Schwedt TJ, Nicholson RA, Rettiganti M, Pearlman EM. Galcanezumab provides consistent efficacy throughout the dosing interval among patients with episodic and chronic migraine: a post hoc analysis. Adv Ther. 2021;38:3154‐3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Medical Association . World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 18. Silberstein SD, Stauffer VL, Day KA, Lipsius S, Wilson MC. Galcanezumab in episodic migraine: subgroup analyses of efficacy by high versus low frequency of migraine headaches in phase 3 studies (EVOLVE‐1 & EVOLVE‐2). J Headache Pain. 2019;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kielbasa W, Quinlan T. Population pharmacokinetics of galcanezumab, an anti‐CGRP antibody, following subcutaneous dosing to healthy individuals and patients with migraine. J Clin Pharmacol. 2020;60:229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Headache Society . The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021‐1039. [DOI] [PubMed] [Google Scholar]
- 21. Tfelt‐Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32:6‐38. [DOI] [PubMed] [Google Scholar]
- 22. Tassorelli C, Diener H‐C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815‐832. [DOI] [PubMed] [Google Scholar]
- 23. Diener H‐C, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020;40:1026‐1044. [DOI] [PubMed] [Google Scholar]
- 24. Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene‐related peptide for the treatment of osteoarthritis‐related pain. Osteoarthritis Cartilage. 2014;22:578‐585. [DOI] [PubMed] [Google Scholar]
- 25. Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008;1:211‐217. [DOI] [PubMed] [Google Scholar]
- 26. George N, Feldman D, Reed D. The Aimovig “wear‐off”: a retrospective case series of response to 14 day dosing. Headache. 2019;59:1‐208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.


