Abstract
This systematic review evaluates the diagnostic accuracy of conventional oral examination (COE) versus incisional or excisional biopsy for the diagnosis of malignant and/or dysplastic lesions in patients with clinically evident lesions. Searches were conducted across five electronic databases from inception to January 2020. Meta‐analyses were undertaken, where appropriate. Among 18 included studies, 14 studies were included in the meta‐analysis, giving summary estimates for COE of 71% sensitivity and 85% specificity for the diagnosis of dysplastic and/or malignant lesions. The pooled diagnostic accuracy of identifying malignant‐only lesions was reported in seven studies, giving a pooled estimate of 88% sensitivity and 81% specificity. Diagnostic accuracy of different types of dental/medical professionals in identifying dysplastic or malignant lesions gave varying estimates of sensitivity and specificity across three studies. Further research is needed to improve the diagnostic accuracy of COE for early detection of dysplastic and malignant oral lesions.
Keywords: biopsy, conventional oral examination, diagnostic accuracy, oral cancer, systematic review
Abbreviations
- CI
confidence interval
- COE
conventional oral examination
- OC
oral cancer
- OCC
oral cavity cancer
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- QUADAS‐2
Quality Assessment of Diagnostic Accuracy Studies‐2
- ROC
receiver operating characteristic curve
1. INTRODUCTION
Oral cancer (OC), defined as cancers of the lips, tongue, cheeks, floor of the mouth, hard and soft palate, sinuses, and pharynx, has an incidence of more than 300 000 cases per year. 1 , 2 , 3 The prognosis of OC is improved when it is detected at an early stage, with a 5 year survival rate of 75% for stage I disease which is drastically reduced to 30% at stage IV of the disease. 2 , 4 This review focusses on cancer of the oral cavity, including the lips, the lining inside the cheeks and lips, the front two thirds of the tongue, the upper and lower gums, the floor of the mouth under the tongue, the bony roof of the mouth, and the small area behind the wisdom teeth.
The diagnostic pathway for identifying oral cavity cancers (OCC) and oral potentially malignant disorders (OPMD) in patients with a clinically evident lesion starts with a full clinical history, followed by conventional oral examination (COE), which includes a thorough head and neck examination, evaluation of oral mucosa by visual inspection under incandescent overhead light or halogen illumination available on the dental chair, and palpation. 5 , 6 , 7 Features of COE which may be indicative of OCC or oral dysplastic lesions include non‐homogenous appearance such as changes in surface texture, color and size; loss of surface integrity; alteration in the surface, for example, slightly raised lesions; nonhealing ulceration or tethering of the tissues (suggesting deeper invasion). 5 , 8 The suspicious lesion then undergoes histological examination using either incisional or excisional biopsy, the gold standard for diagnosing cancer or epithelial dysplasia. However, this diagnostic pathway approach has limitations in that the findings of COE are subjective and dependent on the experience and expertise of the clinician, while biopsy is invasive and can lead to morbidity. Although various aids and adjuncts to COE have been developed, 9 , 10 , 11 there is little consensus on which, if any, are most reliable, and standard care in many countries remains COE followed by biopsy, if needed.
This systematic review evaluates the diagnostic accuracy of COE (visual inspection) compared with incisional or excisional biopsy (gold standard) for the diagnosis of OCC and/or OPMDs in patients with a clinically evident oral lesion.
2. METHODS
A systematic review was undertaken in accordance with the general principles recommended by expert consensus guidelines for the conduct of diagnostic accuracy systematic reviews 12 , 13 and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 14 As this study met criteria for nonhuman subject research, approval by the University of Sheffield Research Ethics Committee was not required.
2.1. Data sources and searches
A comprehensive literature search was undertaken to identify potentially relevant studies. Searches were conducted in several databases including MEDLINE, EMBASE, CINAHL, and the Cochrane Library (including the CENTRAL Register of Controlled Trials and Cochrane Database of Systematic Reviews) from inception to January 2020. The search strategy used free text and thesaurus terms and combined synonyms relating to the condition (e.g., oral cancer, oral lesion, premalignant) with diagnostic testing terms (including a high‐precision filter developed by McMaster University). No date or language restrictions were applied. Searches were supplemented by examination of the reference lists of relevant studies including existing systematic reviews and contact with key experts in the field. Further details of the search strategy are provided in Appendix S1.
2.2. Study selection
All titles and abstracts were examined for inclusion by one reviewer. Any citations that clearly did not meet the inclusion criteria (e.g., nonhuman or unrelated to oral lesions) were excluded. A check of inclusion decisions was performed by a second reviewer for 10% of titles and abstracts with a very good agreement (Kappa = 0.81). All full text articles were then examined independently by two reviewers. Any disagreements in the selection process were resolved through discussion. In order to maintain relevance to current diagnostic approaches only studies published from January 1990 and studies from developed countries with comparable health system were included. Details of the selection criteria are provided in Appendix S2.
2.3. Data extraction
Data relating to study design, patient characteristics, diagnostic accuracy, and outcomes were extracted by one reviewer into a standardized data extraction form and independently checked for accuracy by a second reviewer. Any discrepancies were resolved through discussion to achieve agreement. Where multiple publications of the same study were identified, data were extracted and reported as a single study. For papers focusing primarily on adjunctive tests to clinical examination, subgroup results relating to COE only were extracted. If diagnostic accuracy of different dental and medical professionals performing the index test (COE) were evaluated within a single study, results were extracted separately for each professional group.
2.4. Clinical outcomes assessed
Diagnostic outcomes were extracted for two sets of data (where reported): (1) dysplastic lesions and malignant lesions together, and (2) malignant lesions alone. All the studies in this review included patients with clinically evident lesions, hence a negative test (negative lesion) refers to a lesion that has been detected but it is determined to be neither dysplastic nor malignant based on the clinical features. The authors' cut‐off for positive/negative result for OCC and/or epithelial dysplasia was accepted, and the algorithm used to generate the cut‐off was noted. For (1) that is, assessment of dysplastic and malignant lesions taken together, COE findings were considered positive where there was any level of concern for dysplasia or cancer, including atypical, abnormal, mild/moderate/severe dysplasia, carcinoma‐in‐situ, invasive cancer, or any other result that implied the presence of dysplasia/malignancy. Conversely, results such as inflammation or no dysplasia were considered negative, along with benign and normal results. Gold standard (incisional or excisional biopsy) results positive for cancer or any grade of dysplasia were also considered positive. For (2) that is, assessment of malignant lesions alone, results (for both COE and incision/excision) were considered positive if they indicated cancer/carcinoma, but negative if they indicated dysplasia or carcinoma‐in‐situ.
2.5. Quality assessment
The methodological quality of each included study were assessed using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool 15 across the following key domains: patient selection (a consecutive or random sample of patients, avoidance of case–control study design and avoidance of inappropriate exclusions), index test (COE, interpreted without knowledge of reference standard and if prespecified threshold used), reference standard (validity of the reference standard and blinding of the pathologist to COE), flow and timing (time span between COE and histopathology, all patients received same reference standard, and missing data). Each domain was assessed in terms of risk of bias and the concern regarding applicability to the review (the latter for the first three domains only). The sub‐domains for each domain include a number of signaling questions to guide the overall judgment about whether a study is at high, low, or an unclear risk of bias. The studies were assessed by one reviewer and independently checked by another reviewer.
2.6. Data synthesis and analysis
Data were tabulated and discussed in a narrative synthesis. Meta‐analyses were undertaken, where appropriate to estimate a summary measure of effect on relevant outcomes using the random effects model to allow for inter‐study variability. Statistical analyses were performed using MetaDTA software, an interactive online application for conducting meta‐analysis of diagnostic test accuracy studies. 16 Pooled estimates of sensitivity and specificity are presented as point estimates and 95% confidence intervals (CIs) for COE versus biopsy. Results were recalculated from raw data presented in publications if alternative metrics of diagnostic accuracy were originally reported. In cases where articles used different cut‐offs for defining positive and negative test/biopsy results, the data were synthesized narratively and not included in the meta‐analysis.
3. RESULTS
3.1. Study flow
Figure 1 summarizes the process of identifying and selecting relevant literature. Of the 5750 citations identified, 18 studies met the inclusion criteria (14 studies were eligible for inclusion in the meta‐analysis). The majority of excluded studies either did not present data on OCC and/or OPMDs, did not use COE as a standalone approach to identify OCC and/or OPMDs, provided insufficient outcome data, or were conducted in nonrelevant countries.
FIGURE 1.
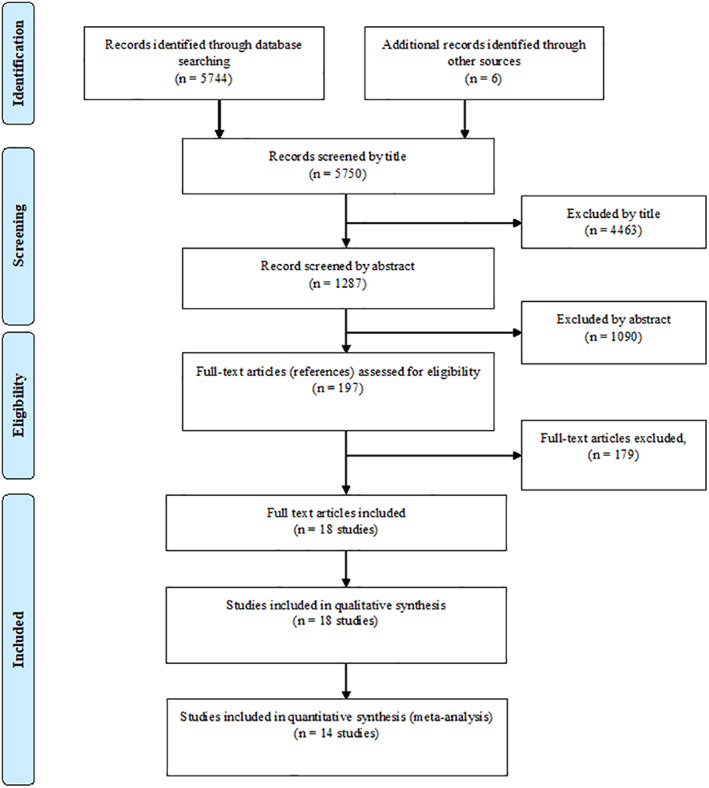
Study flowchart [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Study and patient characteristics
The design and patient characteristics of the 18 included studies are summarized in Table 1. All included studies investigated the diagnostic accuracy of COE compared with incisional biopsy apart from one study which compared COE versus excision of full lesions 17 and another study 18 which compared COE versus histologic confirmation without any further details. The studies were published between 1998 19 and 2016 17 and various methods were used for performing biopsy, including punch (n = 1), 20 scalpel (n = 5), 21 , 22 , 23 , 24 , 25 punch or scalpel (n = 1), 26 punch or wedge (n = 1), 27 surgical unspecified (n = 2), 19 , 28 scalpel or surgical (n = 1), 29 and in six studies the method used for biopsy was not reported. 18 , 30 , 31 , 32 , 33 , 34 The size of the studies varied considerably with the number of participants ranging from 10 19 to 3067 33 and the number of lesions ranging from 28 19 to 3127. 33 The proportion of male patients ranged from 33% 28 to 75% 27 and the mean age ranged from 45 25 , 30 to 65 years. 17 The comparability of study populations in terms of previous history of cancer was difficult to determine as this information was infrequently reported. Four studies 27 , 30 , 32 , 34 included some patients with a history of cancer, three studies 17 , 22 , 26 stated exclusion of participants with previous history of cancer and the remainder of the studies did not report this data. The studies were mainly conducted in either secondary or tertiary care settings apart from Brocklehurst et al. 18 and Patel et al. 33 in which the study assessed the diagnostic test accuracy of different members of the dental team in different care setting. The index test (COE) was performed by various dental or medical professionals including oral surgeons, 21 neck and head surgeons or dental oncologists, 19 oral medicine specialists, 22 otolaryngologists, 20 oral and maxillofacial surgeons 28 dental oncology specialists 23 , 32 primary care dentists, 18 , 33 dental hygienists and dental hygiene therapists. 18
TABLE 1.
COE versus biopsy or excision: Study characteristics
| Author, year country study design | Population | History of oral cancer/lesions | Sample size (no. of lesions) | Mean age (years) | No. of males (%) | Index test | Reference standard | Dental professional performing index test | Prevalence: dysplasia or malignant lesions | Prevalence: malignant lesions |
|---|---|---|---|---|---|---|---|---|---|---|
|
Allegra 2009 20 Italy NR |
Oral mucosa lesions | NR | 32 (45) | 59 | 19/32 (59%) | COE | Punch biopsy | Experienced otolaryngologist | 30/45 (67%) | 7/45 (16%) |
|
Bhoopathi 2011 21 USA Retrospective a |
Atypical lesions or positive by brush biopsy | NR | 148 (148) | 55 | 80/148 (54%) | COE | Scalpel biopsy | Oral surgeons | 12/148 (8%) | NR |
|
Brocklehurst 2015 18 UK Retrospective |
Standardized clinical photographs of mouth cancer, PMDs and benign lesions of the oral mucosa | NR | 90 (90) | NR | NR | COE | Histological confirmation | Primary care dentists (N = 96) | 35/90 (39%) | NR |
| Hygienists/therapists (N = 63) | 35/90 (39%) | NR | ||||||||
| Hospital based dentists (N = 9) | 35/90 (39%) | NR | ||||||||
| Dental nurses (N = 24) | 35/90 (39%) | NR | ||||||||
|
Chainani‐Wu 2015 26 USA Cross‐sectional, consecutive |
Oral LP, ELP, or EP on clinical examination with no current history of oral cancer | No | 43 (77) | 61 | 23/43 (54%) | COE | Punch or scalpel biopsy | NR | 17/77 (22%) b | 6/77 (8%) |
|
Epstein 2003 27 USA Prospective |
Treated within past 2 years for upper aerodigestive tract or pulmonary carcinoma but no current treatment for oral cancer | Y (history of cancer) | 81 (96) | 61 | 61/81 (75%) | COE | Punch or wedge biopsy | NR | 30/96 (31%) c | NR |
|
Farah 2012 22 Australia Prospective |
Clinically suspicious oral mucosal white or mixed red/white lesion; no known oral epithelial dysplasia or SCC | No | 112 (118) | 59 | 46/112 (41%) | COE | Scalpel biopsy | Oral medicine specialists | 28/118 (24%) | NR |
|
Forman 2015 30 USA Retrospective |
Oral lesion with biopsy and pathology report with unequivocal clinical impression and histologic diagnosis | Y: 12.9% had a history of cancer | 1003 (1003) | 45 | 491/1003 (49%) | COE | Biopsy | Surgeons (25–30 years experience) or residents (< 5 years experience) | 74/1003 (7.4%) | NR |
|
Gillenwater 1998 19 USA NR |
Known or suspected premalignant or malignant oral cavity lesions and normal tissue from same patients | NR | 10 (28) | NR | NR | COE | Surgical biopsy | Experienced neck and head surgeon or dental oncologist | 17/28 (61%) | NR |
|
Hanken 2013 28 Germany Prospective |
Suspicious oral premalignant lesions but with no current advanced SCC | NR | 60 (60) | NR (range 38–82) | 20/60 (33%) | COE | Surgical biopsy | Experienced oral and maxillofacial surgeon | 54/60 (90%) | 3% (2/60) |
|
Jayaprakash 2013 (abst) 31 USA NR |
Potentially malignant white or white‐red oral mucosal lesions | NR | 146 (255) | NR | NR | COE | Biopsy | NR | 184/255 (72%) | NR |
|
Jayaprakash 2009 32 USA NR |
Clinically suspicious oral lesions or recently diagnosed untreated premalignant lesions or cancer, history of previously treated oral cancer but no evidence of cancer recurrence and no active malignancy treatment | Y: 47% previous head and neck cancer | 60 (249) | 60 | 41/60 (68%) | COE | Biopsy | Specialist dental oncologist | 170/249 (68%) | 15/249 (6%) d |
|
Kammerer 2015 29 Germany Prospective |
Potentially malignant oral disorders | NR | 44 (50) | 60 | 25/44 (57%) | COE | Scalpel/surgical biopsy | NR | 10/50 (20%) | 7/50 (14%) |
|
Koch 2011 23 Germany NR |
Clinically suspicious epithelial lesions or diagnosed oral mucosal lesion as SCC | NR | 78 (78) | 62 | 46/78 (59%) | COE | Scalpel biopsy | Specialist dental oncologist | 33/78 (42%) | 30/78 (38%) |
|
Marzouki 2012 34 Canada Prospective |
Strong history of smoking, alcohol and suspicious lesion referred by GP, or previous history of cancer but cancer free and having regular follow‐ups | Y: 68% previous head and neck cancer | 33 (33) | 62 | 49/85 (58%) | COE | Biopsy | Head and neck oncology staff person | 13/33 (40%) | NR |
|
Australia NR |
Clinically suspicious oral mucosal white lesion | NR | 50 (50) | 57 | 23/50 (46%) | COE | Scalpel biopsy | NR | 9/50 (18%) | NR |
|
McNamara 2012 25 USA NR |
Undergoing initial oral evaluation and routine dental care | NR | NR (34) | 45 | 67/130 (52%) | COE | Scalpel biopsy | Resident, oral and maxillofacial pathology | 3/34 (9%) | NR |
|
Patel 2011 33 New Zealand Retrospective |
All lesions involving soft tissues of mouth: tongue, gingiva, unattached mucosa, and the lips to the vermillion‐skin junction | NR | 3067 (3127) | 49 | 1308/3067 (43%) | COE | Biopsy | All clinicians combined | 391/2517 (16%) | 66/2517 (2.6%) |
| General dental practitioner | 32/404 (8%) | 3/404 (0.7%) | ||||||||
| Specialist dentist with postgraduate qualifications | 350/2079 (17%) | 58/2079 (2.8%) | ||||||||
|
Piazza 2016 17 Italy Prospective |
Not treated for OC/OP with LPs and EPs and not been biopsied | No | 128 (128) | 65 | 54/128 (42%) | COE | Excisional biopsy f | NR | 87/128 (68%) | NR |
Abbreviations: Abst, conference abstract; CIS, carcinoma in situ; COE, conventional oral examination; ELP, erythroleukoplakia; EPs, erythroplakias; LPs, leukoplakias; NR, not reported; OC, oral cavity; OP, oropharyngeal; PMDs, premalignant diseases; SCC, squamous cell carcinoma.
Consecutive cohort but retrospective analyses.
For severe dysplasia and cancer only.
For cancer or CIS only, not dysplasia.
Calculated for invasive SCC and other carcinomas (one salivary gland carcinoma and two verrucous carcinomas with SSC component).
Data reported for Microlux/DL examination but was same as clinical provisional diagnosis.
Excisional biopsy of the entire lesion under local or general anesthesia regardless of the appearance at COE.
The classification and cut‐off used to define positive/negative tests for dysplastic or malignant lesions in the included studies was broadly similar apart from two studies 26 , 27 which were excluded from the meta‐analysis. Chainani‐Wu et al. 26 categorized a positive test as severe dysplasia, carcinoma in situ or carcinoma, while a negative test included mild or moderate dysplasia and hyperkeratosis. Epstein et al. 27 considered carcinoma or carcinoma in situ as a positive test and dysplasia, keratosis, hyperkeratosis, and hyperplasia as negative test. The majority of the included studies defined a positive result as a lesion with any degree of dysplasia or malignancy, while a negative result was defined as having no dysplasia or being benign. Further details of definitions used by each of the included studies are described in Table 3 for dysplastic or malignant lesions and in Table 4 for malignant lesions. Prevalence of dysplastic or malignant lesions varied widely in the 18 included studies, ranging from 7.4% 30 to 90%. 28 The prevalence of malignant‐only lesions was reported in seven studies and ranged from 0.7% 33 to 38%. 23
TABLE 3.
COE versus incisional or excisional biopsy: Sensitivity and specificity for dysplastic/malignant lesions
| Author, Year | Description of positive/negative case definition by reference standard test as reported | Dental professional performing index test | Prevalence: Dysplasia or malignant lesions (positive test) | TP | FN | FP | TN | Sens | Spec | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies included in meta‐analysis | |||||||||||
| Allegra 2009 20 |
Pos: Dysplasia or malignant Neg: Benign |
Experienced otolaryngologist | 30/45 (67%) |
16 [C: 6 CiS: 3 SiD: 3 MoD:2 MiD: 2] |
14 [C: 1 CiS: 1 SiD: 3 MoD:3 MiD: 6] |
3 | 12 | 53% | 80% | 84% | 46% |
| Bhoopathi 2011 21 |
Pos: Dysplasia or malignant Neg: No dysplasia |
Oral surgeons | 12/148 (8%) | 12 | 0 | 104 | 32 | 100% | 24% | 10% | 100% |
| Farah 2012 22 |
Pos: Dysplasia Neg: No dysplasia |
Oral medicine specialists | 28/118 (24%) | 7 | 21 | 16 | 74 | 25% | 82% | 30% | 78% |
| Forman 2015 30 |
Pos: Dysplasia, moderate to severe cellular atypia or malignant Neg: Benign |
Residents (<5 years' experience) or surgeons (25–30 years' experience) | 74/1003 (7.4%) | 36 | 38 | 18 | 911 | 49% | 98% | 67% | 96% |
| Gillenwater 1998 19 |
Pos: Dysplasia or malignant Neg: Normal, no dysplasia |
Experienced neck and head surgeon or dental oncologist | 17/28 (61%) | 13 | 4 | 0 | 11 | 76% | 100% | 100% | 73% |
| Hanken 2013 28 |
Pos: Dysplasia or premalignant Neg: No lesions |
Experienced Oral and Maxillofacial surgeon | 54/60 (90%) | 41 |
13 [C: 0 Grading of dysplasia NR] |
4 | 2 | 76% | 33% | 91% | 13% |
| Jayaprakash 2013 31 (abst) |
Pos: Dysplasia, carcinoma in situ or malignant Neg: Normal/Benign |
NR | 184/255 (72%) | 99 |
85 |
38 | 33 | 54% | 46% | 72% | 28% |
| Jayaprakash 2009 32 |
Pos: Dysplasia or malignant Neg: Normal/Benign |
Specialist dental oncologist | 170/249 (68%) |
89 [C: 12 CiS/microinvasive SCC: 5 SeD: 6 MoD: 13 MiD/PA:53] |
81 [C: 3 CiS/microinvasive SCC: 5 MoD: 5 MiD/PA:68] |
24 | 55 | 52% | 70% | 79% | 40% |
| Kammerer 2015 29 |
Pos: Moderate dysplasia (squamous intraepithelial neoplasia >1), malignant Neg: Normal or with inflammatory alterations |
NR | 10/50 (20%) | 9 |
1 [SCC:0 MoD:1] |
0 | 40 | 90% | 100% | 100% | 98% |
| Koch 2011 23 |
Pos: Dysplasia or malignant (suspicious for premalignant or malignant lesions) Neg: Benign or no dysplasia (abnormal but innocuous) |
Specialist dental oncologist | 33/78 (42%) | 31 |
2 [SCC:1 Grading of dysplasia NR] |
1 | 44 | 94% | 98% | 97% | 96% |
| Marzouki 2012 34 |
Pos: Dysplasia or malignant Neg: Non suspicious |
Head and neck oncology staff person | 13/33 (40%) | 8 | 5 | 9 | 11 | 62% | 55% | 47% | 69% |
| McIntosh 2009 24 |
Pos: Dysplasia or malignant Neg: Benign |
NR | 9/50 (18%) | 7 | 2 | 12 | 29 | 78% | 71% | 37% | 94% |
| McNamara 2012 25 |
Pos: Premalignant, carcinoma in situ or malignant Neg: Benign |
NR | 3/34 (9%) |
2 [C:1 SeD: 1] |
1 [SeD] | 1 | 30 | 67% | 97% | 67% | 97% |
| Patel 2011 33 |
Pos: Premalignant or malignant Neg: Benign |
Specialists dentists with postgrad qualifications | 350/2079 (17%) | 292 | 58 | 186 | 1543 | 83% | 89% | 61% | 96% |
| General dental practitioners | 32/404 (8%) | 28 | 4 | 9 | 363 | 88% | 98% | 76% | 99% | ||
| All clinicians combined | 391/2517 (16%) | 327 | 64 | 196 | 1930 | 84% | 91% | 63% | 97% | ||
| Studies not included in meta‐analysis | |||||||||||
| Brocklehurst 2015 18 , a |
Pos: Potentially malignant disorders or malignant Neg: Benign |
Primary care dentists | 35/90 (39%) | NR | NR | NR | NR | Median 81% (32–100) | Median 73% (32–97) | NR | NR |
| Hygienists/therapists | 35/90 (39%) | NR | NR | NR | NR | Median 77% (35–100) | Median 69% (42–90) | NR | NR | ||
| Hospital based dentists | 35/90 (39%) | NR | NR | NR | NR | Median 90% (81–100) | Median 76% (68–88) | NR | NR | ||
| Dental nurses | 35/90 (39%) | NR | NR | NR | NR | Median 68% (48–87) | Median 59% (41–92) | NR | NR | ||
| Chainani‐Wu 2015 26 , b |
Pos: Severe dysplasia, carcinoma in situ or carcinoma Neg: Hyperkeratosis, mild or moderate dysplasia |
NR | 17/77 (22%) b |
14 [C: 5 CiS: 9] |
3 [C: 1 CiS: 2] |
29 | 31 | 82% | 52% | 33% | 91% |
| Epstein 2003 27 , c |
Pos: Carcinoma or carcinoma in situ Neg: Benign (keratosis, hyperkeratosis, hyperplasia) or dysplasia |
NR | 30/96 (31%) c | 12 | 18 | 45 | 21 | 40% | 32% | 36% | 54% |
| Piazza 2016 17 , d |
Pos: Dysplasia or carcinoma Neg: Chronic mucositis, lichen planus without atypia, and keratosis without atypia |
NR | 87/128 (68%) | 44 | 43 | 13 | 28 | 51% | 68% | 77% | 39% |
Abbreviations: Abst, conference abstract; C, carcinoma; CiS, carcinoma in situ; FN, false negative; FP, false positive; MoD, moderate dysplasia; MiD, mild dysplasia; NPV, negative predictive value; PA, parakeratosis with atypia PPV, positive predictive value; SCC, squamous cell carcinoma; Sens, sensitivity; Spec, specificity; SeD, severe dysplasia; TN, true negative; TP, true positive.
Brocklehurst 2015—Sen and spec reported as median.
Chainani‐Wu 2015—Data reported for severe dysplasia and cancer only.
Epstein 2003—Data reported for cancer or CIS only, not dysplasia.
Piazza 2016—Data reported for COE versus excisional biopsy of the entire lesion under local or general anesthesia regardless of the appearance at COE.
TABLE 4.
COE versus incisional or excisional biopsy: Sensitivity and specificity for malignant lesions only
| Author, year | Description of positive/negative case definition by reference standard test as reported | Dental professional performing index test | Prevalence of oral invasive cancer | TP | FN | FP | TN | Sens | Spec | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allegra 2009 20 |
Pos: Carcinoma Neg: Non‐carcinoma |
Experienced otolaryngologist | 7/45 (16%) | 6 | 1 | 13 | 25 | 86% | 66% | 32% | 96% |
| Chainani‐Wu 2015 26 |
Pos: Carcinoma Neg: Non‐carcinoma |
NR | 6/77 (8%) | 5 | 1 | 38 | 33 | 83% | 46% | 12% | 97% |
| Hanken 2013 28 |
Pos: Carcinoma Neg: Non‐carcinoma |
Experienced oral and maxillofacial surgeon | 2/60 (3%) | 2 | 0 | 43 | 15 | 100% | 26% | 4% | 100% |
| Jayaprakash 2009 32 , a |
Pos: Invasive SCC and other carcinomas Neg: Non‐carcinoma |
Specialist dental oncologist | 15/249 (6%) | 12 | 3 | 101 | 133 | 80% | 57% | 11% | 98% |
| Kammerer 2015 29 |
Pos: OSCC Neg: Non‐carcinoma |
NR | 7/50 (14%) | 7 | 0 | 2 | 41 | 100% | 95% | 78% | 100% |
| Koch 2011 23 |
Pos: SCC Neg: Non‐SCC |
Specialist dental oncologist | 30/78 (38%) | 29 | 1 | 2 | 48 | 97% | 96% | 94% | 98% |
| Patel 2011 33 |
Pos: Malignant Neg: Nonmalignant |
Specialists dentist with registered postgraduate qualifications | 58/2079 (2.8%) | 42 | 16 | 30 | 1991 | 72% | 99% | 58.% | 99% |
| General dental practitioners | 3/404 (0.7%) | 2 | 1 | 5 | 396 | 67% | 99% | 29% | 100% | ||
| All clinicians combined | 66/2517 (2.6%) | 47 | 19 | 36 | 2415 | 71% | 99% | 57% | 99% |
Abbreviations: FN, false negative; FP, false positive; NPV, negative predictive value; OSCC, oral squamous cell carcinoma; PPV, positive predictive value; SCC, squamous cell carcinoma; Sens, sensitivity; Spec, specificity; TN, true negative; TP, true positive.
Data calculated for invasive SCC and other carcinomas (one salivary gland carcinoma + two verrucous carcinomas with SSC component).
3.3. Quality assessment
The overall methodological quality of the included studies is summarized in Table 2. Generally, the ratings indicated either low or unclear risk of bias in the majority of studies. The domain with the highest risk of bias concerned “flow and timing” in four studies, 19 , 21 , 26 , 27 primarily due to the lack of inclusion of all patients in the analyses, for example, participants missing or excluded from analysis and no explanation given. This was followed by patient selection which was rated as high risk of bias in two studies. 21 , 27 Bhoopathi et al. 21 only included patients with lesions that were atypical or positive by brush biopsy and Epstein et al. 27 included patients who were treated within past 2 years for upper aerodigestive tract or pulmonary carcinoma. None of the studies specified the time interval between the index test (COE) and the reference standard (biopsy) and were therefore judged as unclear on this aspect. Furthermore, nine studies 18 , 21 , 23 , 25 , 28 , 30 , 31 , 33 , 34 did not report on whether the pathologist was blinded to the index test when interpreting the histopathologic findings of the biopsy. However, in all the studies the index test (COE) was interpreted without the knowledge of the reference standard. With respect to applicability, all the studies except two 21 , 27 had a low risk of bias. These two studies 21 , 27 only included selected patients.
TABLE 2.
Risk of bias summary: Judgments of risk of bias for each included study
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Allegra 2009 20 | ? |

|

|
? |

|

|

|
| Bhoopathi 2011 21 |

|

|
? |

|

|

|

|
| Brocklehurst 2015 18 | ? |

|
? | ? |

|

|

|
| Chainani‐Wu 2015 26 |

|

|

|

|

|

|

|
| Epstein 2003 27 |

|

|

|

|

|

|

|
| Farah 2012 22 | ? |

|

|
? |

|

|

|
| Forman 2015 30 | ? |

|
? | ? |

|

|

|
| Gillenwater 1998 19 | ? |

|

|

|

|

|

|
| Hanken 2013 28 | ? |

|
? | ? |

|

|

|
| Jayaprakash 2013 (abst) 31 | ? |

|
? | ? |

|

|

|
| Jayaprakash 2009 32 | ? |

|

|
? |

|

|

|
| Kammerer 2015 29 | ? |

|

|
? |

|

|

|
| Koch 2011 23 | ? |

|
? | ? |

|

|

|
| Marzouki 2012 34 | ? |

|
? | ? |

|

|

|
| McIntosh 24 2009 | ? |

|

|
? |

|

|

|
| McNamara 2012 25 |

|

|
? | ? |

|

|

|
| Patel 2011 33 | ? |

|
? | ? |

|

|

|
| Piazza 2016 17 |

|

|

|
? |

|

|

|
Note: ( ) Low risk; (
) Low risk; ( ) high risk; (?) unclear risk.
) high risk; (?) unclear risk.
3.4. Diagnostic performance of COE (summary of results)
Despite wide variation in the types of dental and medical professionals performing the COE and the methodology used for undertaking biopsy across the included studies, meta‐analyses were conducted, where possible, for all relevant outcomes.
3.4.1. Sensitivity and specificity for dysplastic or malignant lesions
The diagnostic performance of COE versus incisional or excisional biopsy for identification of dysplastic or malignant lesions was reported in 18 studies and is summarized in Table 3. The sensitivity varied widely from 25% 22 to 100%, 21 as did the specificity from 24% 21 to 100%. 19 , 29
Fourteen 19 , 20 , 21 , 22 , 23 , 24 , 25 , 28 , 29 , 30 , 31 , 32 , 33 , 34 studies were included in the meta‐analysis of COE versus incisional biopsy which considered all dysplasia and carcinoma as test‐positive. Four studies were not eligible for meta‐analysis. 17 , 18 , 26 , 27 Chainani‐Wu et al. 26 and Epstein et al. 27 did not consider all grades of dysplasia as test positive, while Piazza et al. 17 compared COE versus excisional biopsy, and Brocklehurst et al. 18 did not report appropriate diagnostic performance data. The summary estimate for sensitivity of COE was 71% (95% CI: 57%–81%), the specificity was slightly better at 85% (95% CI: 68%–94%). The false positive rate was 15% (95% CI: 6%–32%) and the false negative rate was 29% (95% CI: 19%–43%). The summary receiver operating characteristic (ROC) curve using a random effects model is presented in Figure 2.
FIGURE 2.
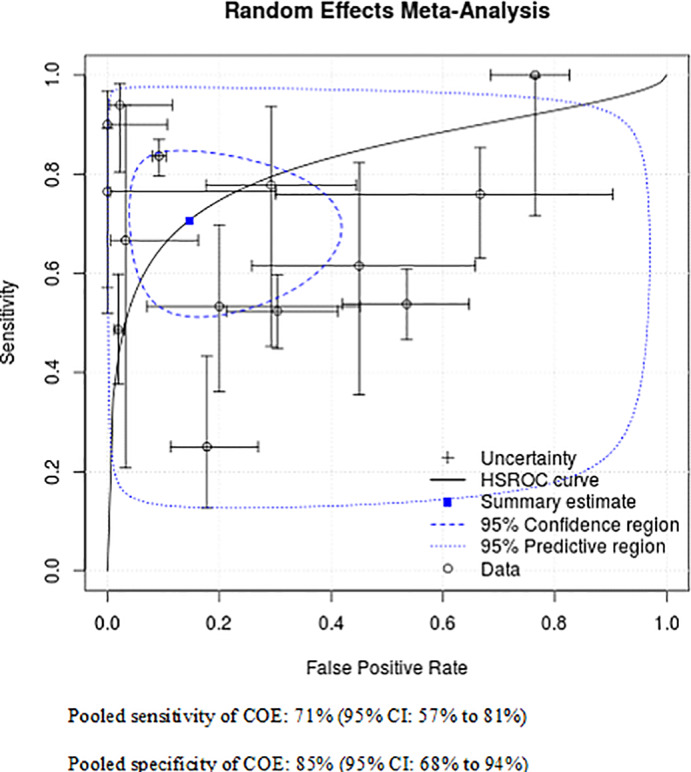
COE versus biopsy: Summary ROC curve for dysplastic and malignant lesions (N = 14 studies) [Color figure can be viewed at wileyonlinelibrary.com]
3.4.2. Diagnostic accuracy in identifying different types of lesions
Studies reporting data on the diagnostic performance of s COE versus incisional or excisional biopsy for the identification of different types of lesions, for example, mild dysplasia, moderate dysplasia, severe dysplasia, micro invasive carcinoma, and carcinoma in situ was scarce. Six studies reported albeit limited data on the true positive and/or true negative findings for different types of lesions (see Table 3). In Jayaprakash et al., 32 the majority of false negative findings were due to misdiagnosis of mild dysplasia or parakeratosis with atypia 68/81 (84%) and 3/81(4%) related to the missed diagnosis of cancer. Similarly in Allegra et al., 20 6/14 (43%) false negative findings were due to a missed diagnosis of mild dysplasia. The remaining false negative findings consisted of moderate dysplasia (n = 3), severe dysplasia (n = 3), carcinoma in situ (n = 1), and cancer (n = 1). While Kammerer et al. 29 and McNamara et al. 29 reported only one false negative finding due to moderate dysplasia and severe dysplasia, respectively. Hanken et al. 28 and Koch et al. 23 did not report on the grading of dysplasia hence the false negative findings could not be explored further. Lastly, Chainani‐Wu et al. 26 categorized mild and moderate dysplasia as negative test. The limited and inconsistency in the data reporting precluded any further analyses.
3.4.3. Sensitivity and specificity for malignant lesions only
Seven studies reported data allowing calculation of sensitivity and specificity of COE versus incisional biopsy for identification of malignant‐only lesions (Table 4). The sensitivity varied from 67% 33 to 100%, 28 , 29 and specificity from 26% 28 to 99%. 33 A meta‐analysis of the seven studies found overall pooled estimates of 88% (95% CI: 73%–95%) and 81% (95% CI: 51%–95%) for sensitivity and specificity, respectively. The false positive rate was 19% (95% CI: 5.5%–49%) and the false negative rate was 12% (95% CI: 5%–27%). The summary ROC curve is presented in Figure 3.
FIGURE 3.
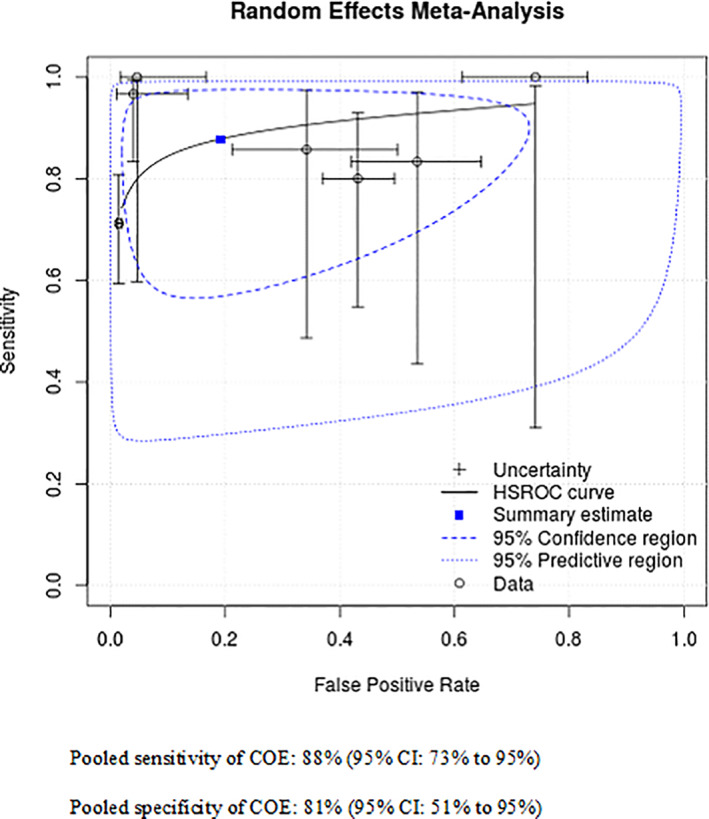
COE versus biopsy: Summary ROC curve for malignant lesions only (N = 7 studies) [Color figure can be viewed at wileyonlinelibrary.com]
3.4.4. Diagnostic accuracy of different types of dental professional in identifying dysplastic or malignant lesions
Three studies compared the diagnostic accuracy of different dental professionals performing COE to identify dysplastic or malignant lesions (Table 3). Brocklehurst et al. 18 compared primary care dentists, hygienists/therapists, hospital‐based dentists, and dental nurses. Sensitivity and specificity were reported as median and range for each dental professional, with a higher sensitivity (median 90%) and specificity (median 76%) being achieved when COE was performed by hospital‐based dentists, followed by primary care dentists (sensitivity 81%, specificity 73%); hygienists/therapists (sensitivity 77%, specificity 69%) and dental nurses (sensitivity 68%, specificity 59%). Patel et al. 33 also compared diagnostic accuracy between general dental practitioners and specialist dentists (a dentist with registered postgraduate training and qualifications). In contrast to Brocklehurst et al., 18 Patel et al. 33 reported higher sensitivity (88%) and specificity (98%) for general dental practitioners compared with specialist dentists (sensitivity 83%, specificity 89%). Forman et al. 30 did not report sensitivity or specificity data for each dental professional but reported that the overall accuracy of identifying benign lesions from non‐benign lesions was greater for experienced surgeons with 25–30 years of experience compared with surgeons with less than 5 years' experience (96% vs. 94%, respectively).
3.4.5. Diagnostic accuracy of different types of dental professional in identifying malignant lesions only
Only one study, Patel et al. 33 assessed this outcome by dental professional (Table 4). The study showed no difference in specificity (99%) between general dental practitioners and specialist dentists with registered postgraduate qualifications. However, a slightly higher sensitivity (72% vs. 67%) was observed with specialist dentists.
4. DISCUSSION
4.1. Summary of results
In this systematic review, 18 studies were identified that assessed the diagnostic performance of COE versus incisional or excisional biopsy for identification of dysplastic or malignant lesions. Of these, 14 studies were included in a random effects meta‐analysis giving a summary estimate for COE of 71% sensitivity and a slightly better specificity of 85% with a false positive and false negative rate of 15% and 29%, respectively. The diagnostic accuracy of identifying malignant‐only lesions was reported in seven studies, giving a pooled estimate of 88% sensitivity and 81% specificity. The false positive rate was 19% and the false negative rate was 12%. However, it should be noted that due to the multifocal nature of oral premalignant lesions, pathologic analysis of random biopsies often does not provide accurate information of the status of the entire clinically evident lesion hence caution should be applied when looking at the sensitivity and specificity estimates of COE versus incisional or excisional biopsy. In addition, we identified three studies that compared the diagnostic accuracy of different dental professionals performing COE to identify dysplastic or malignant lesions. Varying sensitivity and specify was observed depending on who performed the assessment with higher accuracy observed when performed by a dentist with registered postgraduate training and qualifications. Unfortunately, due to the limited number of studies and evidence, this did not allow for a definitive conclusion to be made regarding the most appropriate dental or medical professional to conduct COE.
4.2. Interpretation of results and comparison to the existing literature
The findings of our review showed wide variation in sensitivity and specificity between studies, though the pooled diagnostic accuracy of COE is comparable with detection of other cancers (e.g., colon–rectum, cervix, or breast cancer) by clinical inspection or other noninvasive approaches. 35 , 36 , 37 While there are many screening programs for OCC or OPMDs, a systematic review by Warnakulasuriya et al. 38 found that visual screening by dentists in primary care or in extended health care facilities can accurately identify OCC and/or OPMDs with reliability when using established guidelines. Walsh et al. 39 reported that COE was better at correctly classifying the absence of OPMDs or oral cavity cancer in disease‐free individuals than classifying the presence in diseased individuals. The number of false negative and false positive findings are a potential concern. In the case of the false negative outcomes, where the disease is not detected and left untreated, the cancer may reach an advanced stage which could lead to late diagnosis and poorer prognosis. This would be especially detrimental in the case where a high‐grade dysplastic lesion or a malignant lesion was left undetected in contrast to a less invasive lesion such as a mild dysplastic lesion with a very low risk of cancer development. Thus further exploration of the number of false negative outcomes across malignant and premalignant lesions was undertaken. Unfortunately only a limited number of studies reported the false negative outcomes across the different types of lesions (see Tables 3 and 4), precluding further analysis. The major surgical treatment needed for the advanced stage disease could result in disfigurement, social isolation, increased levels of morbidity, and infrequently, death. 40 , 41 , 42 There is a consensus view that early detection, diagnosis and treatment of OCC can significantly enhance survival rates and reduce morbidity. 43 Silverman et al. 44 researched the relationship between delay in diagnosis, stage, and mortality and state that survival rates would increase by 80% if malignancies were identified and treated earlier. In addition, patients with false positive findings will be undergoing unnecessary biopsy, causing unnecessary anxiety, worry and cost and impacting the patients' quality of life. Commonly observed diagnoses among false positive findings within and across the included studies were not reported. However, Chainani Wu et al. 26 reported that the clinical sign of speckled appearance had a high sensitivity for the detection of carcinoma in situ or carcinoma though the specificity for speckled appearance was low. Meanwhile, Forman et al. 30 hypothesized that there were certain common oral lesions that were associated with a high degree of clinical diagnostic accuracy. Fibromas (99.2%), mucoceles (97.2%), pyogenic granulomas (96.8%), and squamous papillomas (96.3%) showed a high level of concordance. Traumatic ulcerations were associated with a clinical impression accuracy rate of 83.6%. In addition, the authors reported that older patients and patients who received radiation therapy were most likely to be misdiagnosed clinically and men were 1.5 times more likely to be misdiagnosed.
4.3. Strengths and limitations
The strength of our review lies in the robust systematic reviewing methodology used, including the comprehensive and reproducible search strategy. There are a number of limitations to our review. Primarily, there was significant heterogeneity in all aspects of study design across the studies, including patient characteristics, previous history of cancer, prevalence of dysplastic and malignant lesions among the study sample, the range of clinical lesions, the types and expertise of the dental and medical professional performing the COE and the methodology used for undertaking biopsy across the included studies. In addition, there was lack of consistency and reporting across the included studies in the clinical criteria used to define levels of “suspicion” for making a clinical assessment of benign versus malignant. Another potential limitation of our review could be the inclusion of retrospective studies, especially the inclusion of Patel et al., 33 which was a large study with a cohort of 3067 participants. However a sensitivity analysis with the exclusion of this study made minimal impact on the sensitivity (69% vs. 71%) and specificity (85% vs. 85%) results, see Appendix S3. Furthermore, the sensitivity and specificity of COE in this review might be overestimated as the majority of the included studies have been conducted by specialists with an interest in OCC with extensive experience, unlike a general dental or medical clinician, hence limiting the applicability of the review findings to general practice. It is important to note that these limitations are principally sourced in the evidence base, rather than the methods used to interrogate, and evaluate it.
4.4. Implications for policy, practice, and future research
Our review highlights the need to identify ways to improve the accuracy of detecting OCC and OPMDs at an early stage of the disease. Improvements in making a diagnosis may be sought through continuous training in accurate interpretation of diagnostic approaches, care during examination, building experience, expertise, and confidence of dental or medical professionals in detecting OCC or dysplasia, increasing patients' awareness and acceptance of the disease and developing reliable adjunctive tools to improve findings and accuracy of COE. Currently, research is ongoing into the effectiveness of the use of adjunctive aids such as toluidine blue, chemiluminescence, loss of tissue autofluorescence and other aids. 22 , 24 , 43 , 45 , 46 However, as yet there is insufficient evidence to justify their use as adjuncts to COE. 43 , 47 , 48 While there is a growing body of research investigating detection technologies and new therapies, psychosocial research into how these new developments may be accepted and utilized is also required. 43 A review by Ford et al. 43 looked at a theoretical model 49 to explore behavioral influences on the early detection of OCC. The model comprised four time intervals (appraisal; help seeking; diagnostic; and pretreatment) that made up the total time between the appearance of signs or symptoms of a cancer and the commencement of treatment. The authors 43 proposed that unless future theory‐based studies target these aspects of OCC, and consider the structural and psychosocial parameters that surround it, then efforts to improve its timely detection will have limited effectiveness. In addition they also suggested addressing health inequity at a government policy level and focusing on improved access and affordability since low socioeconomic status is a risk factor for OCC. 43 The most important limitation identified in our review was the inconsistency in the clinical assessment of negative and positive test and differentiation between the level of suspicion, that is, low or high risk lesion. Therefore, further research in developing standardized assessment criteria is needed as well as potentially exploring the use of artificial intelligence approaches.
5. CONCLUSIONS
The pooled diagnostic accuracy of COE versus incisional or excisional biopsy for identifying dysplastic and/or malignant lesions is 71% sensitivity and 85% specificity, with a slightly better sensitivity of 88% and specificity of 81% for identifying malignant lesions only. The evidence on diagnostic accuracy for different types of dental or medical professional in identifying dysplastic or malignant lesions was inconclusive due to the limited number of studies identified. This review highlights the need to improve the diagnostic accuracy of detecting malignant and/or potentially malignant oral lesions at an early stage of the disease in order to improve prognosis and outcomes. In addition, further well‐designed studies are needed to compare the accuracy of different members of the dental and medical team in detecting malignant and nonmalignant oral lesions.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Munira Essat, Katy Cooper, and Mark Clowes. The first draft of the manuscript was written by Munira Essat and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supporting information
Appendix S1: Example search strategy
Appendix S2: Eligibility criteria for study selection
Appendix S3: COE versus biopsy: Summary ROC curve for dysplastic and malignant lesions without Patel et al. 33 (N = 13 studies)
Essat M, Cooper K, Bessey A, Clowes M, Chilcott JB, Hunter KD. Diagnostic accuracy of conventional oral examination for detecting oral cavity cancer and potentially malignant disorders in patients with clinically evident oral lesions: Systematic review and meta‐analysis. Head & Neck. 2022;44(4):998-1013. doi: 10.1002/hed.26992
Funding information Small Business Research Initiative (SBRI) to Zilico Ltd
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Dissanayaka WL, Pitiyage G, Kumarasiri PVR, Liyanage RLPR, Dias KD, Tilakaratne WM. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(4):518‐525. [DOI] [PubMed] [Google Scholar]
- 3. Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life‐style changes that influence survival. Oral Oncol. 2010;46(6):407‐410. [DOI] [PubMed] [Google Scholar]
- 4. Sciubba JJ. Oral cancer. The importance of early diagnosis and treatment. Am J Clin Dermatol. 2001;2(4):239‐251. [DOI] [PubMed] [Google Scholar]
- 5. Epstein J, Silverman S Jr, Epstein J, Lonky S, Bride M. Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 2008;44(6):538‐544. [DOI] [PubMed] [Google Scholar]
- 6. Epstein JB, Gorsky M, Cabay RJ, Day T, Gonsalves W. Screening for and diagnosis of oral premalignant lesions and oropharyngeal squamous cell carcinoma: role of primary care physicians. Can Fam Physician. 2008;54(6):870‐875. [PMC free article] [PubMed] [Google Scholar]
- 7. Huber MA, Bsoul SA, Terezhalmy GT. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Quintessence Int. 2004;35(5):378‐384. [PubMed] [Google Scholar]
- 8. Epstein JB, Villines D, Drahos G, Kaufman E, Gorsky M. Oral lesions in patients participating in an oral examination screening week at an urban dental school. J Am Dent Assoc. 2008;139(10):1338‐1344. [DOI] [PubMed] [Google Scholar]
- 9. Giovannacci I, Vescovi P, Manfredi M, Meleti M. Non‐invasive visual tools for diagnosis of oral cancer and dysplasia: a systematic review. Med Oral Patol Oral Cir Bucal. 2016;21(3):e305‐e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macey R, Walsh T, Brocklehurst P, et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. 2015;5:CD010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rashid A, Warnakulasuriya S. The use of light‐based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med. 2015;44(5):307‐328. [DOI] [PubMed] [Google Scholar]
- 12. Centre for Reviews and Dissemination . Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. Centre for Reviews and Dissemination; 2009. [Google Scholar]
- 13. Deeks JJ, Bossuyt PM, Gatsonis C. Cochrane handbook for systematic reviews of diagnostic test accuracy, version 1.0.0. The Cochrane Collaboration; 2013. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. [DOI] [PubMed] [Google Scholar]
- 16. Freeman SC, Kerby CR, Patel A, Cooper NJ, Quinn T, Sutton AJ. Development of an interactive web‐based tool to conduct and interrogate meta‐analysis of diagnostic test accuracy studies: MetaDTA. BMC Med Res Methodol. 2019;19(81):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piazza C, Del Bon F, Paderno A, et al. The diagnostic value of narrow band imaging in different oral and oropharyngeal subsites. Eur Arch Otorhinolaryngol. 2016;273(10):3347‐3353. [DOI] [PubMed] [Google Scholar]
- 18. Brocklehurst P, Pemberton MN, Macey R, Cotton C, Walsh T, Lewis M. Comparative accuracy of different members of the dental team in detecting malignant and non‐malignant oral lesions. Br Dent J. 2015;218(9):525‐529. [DOI] [PubMed] [Google Scholar]
- 19. Gillenwater A, Jacob R, Ganeshappa R, et al. Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Arch Otolaryngol Head Neck Surg. 1998;124(11):1251‐1258. [DOI] [PubMed] [Google Scholar]
- 20. Allegra E, Lombardo N, Puzzo L, Garozzo A. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngol Ital. 2009;29(4):187‐190. [PMC free article] [PubMed] [Google Scholar]
- 21. Bhoopathi V, Mascarenhas AK. Effectiveness of oral surgeons compared with OralCDx brush biopsy in diagnosing oral dysplastic lesions. J Oral Maxillofac Surg. 2011;69(2):428‐431. [DOI] [PubMed] [Google Scholar]
- 22. Farah CS, McIntosh L, Georgiou A, McCullough MJ. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck. 2012;34(6):856‐862. [DOI] [PubMed] [Google Scholar]
- 23. Koch FP, Kaemmerer PW, Biesterfeld S, Kunkel M, Wagner W. Effectiveness of autofluorescence to identify suspicious oral lesions—a prospective, blinded clinical trial. Clin Oral Investig. 2011;15(6):975‐982. [DOI] [PubMed] [Google Scholar]
- 24. McIntosh L, McCullough MJ, Farah CS. The assessment of diffused light illumination and acetic acid rinse (Microlux/DL) in the visualisation of oral mucosal lesions. Oral Oncol. 2009;45(12):e227‐e231. [DOI] [PubMed] [Google Scholar]
- 25. McNamara KK, Martin BD, Evans EW, Kalmar JR. The role of direct visual fluorescent examination (VELscope) in routine screening for potentially malignant oral mucosal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):636‐643. [DOI] [PubMed] [Google Scholar]
- 26. Chainani‐Wu N, Madden E, Cox D, Sroussi H, Epstein J, Silverman S Jr. Toluidine blue aids in detection of dysplasia and carcinoma in suspicious oral lesions. Oral Dis. 2015;21(7):879‐885. [DOI] [PubMed] [Google Scholar]
- 27. Epstein JB, Feldman R, Dolor RJ, Porter SR. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck. 2003;25(11):911‐921. [DOI] [PubMed] [Google Scholar]
- 28. Hanken H, Kraatz J, Smeets R, et al. The detection of oral pre‐malignant lesions with an autofluorescence based imaging system (VELscopeTM)—a single blinded clinical evaluation. Head Face Med. 2013;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kammerer PW, Rahimi‐Nedjat RK, Ziebart T, et al. A chemiluminescent light system in combination with toluidine blue to assess suspicious oral lesions‐clinical evaluation and review of the literature. Clin Oral Investig. 2015;19(2):459‐466. [DOI] [PubMed] [Google Scholar]
- 30. Forman MS, Chuang SK, August M. The accuracy of clinical diagnosis of oral lesions and patient‐specific risk factors that affect diagnosis. J Oral Maxillofac Surg. 2015;73(10):1932‐1937. [DOI] [PubMed] [Google Scholar]
- 31. Jayaprakash V, Reid M, Frustino J, et al. Autofluorescence visualization for detecting potentially malignant white oral mucosal lesions. Oral Oncol. 2013;49(SUPPL. 1):S50. [Google Scholar]
- 32. Jayaprakash V, Sullivan M, Merzianu M, et al. Autofluorescence‐guided surveillance for oral cancer. Cancer Prev Res (Phila). 2009;2(11):966‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel KJ, De Silva HL, Tong DC, Love RM. Concordance between clinical and histopathologic diagnoses of oral mucosal lesions. J Oral Maxillofac Surg. 2011;69(1):125‐133. [DOI] [PubMed] [Google Scholar]
- 34. Marzouki HZ, Vu TTV, Ywakim R, Chauvin P, Hanley J, Kost KM. Use of fluorescent light in detecting malignant and premalignant lesions in the oral cavity: a prospective, single‐blind study. J Otolaryngol Head Neck Surg. 2012;41(3):164‐168. [PubMed] [Google Scholar]
- 35. Brem RF, Baum J, Lechner M, et al. Improvement in sensitivity of screening mammography with computer‐aided detection: a multiinstitutional trial. AJR Am J Roentgenol. 2003;181(3):687‐693. [DOI] [PubMed] [Google Scholar]
- 36. Tepus M, Yau TO. Non‐invasive colorectal cancer screening: an overview. Gastrointest Tumors. 2020;7(3):62‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vahedpoor Z, Behrashi M, Khamehchian T, Abedzadeh‐Kalahroudi M, Moravveji A, Mohmadi‐Kartalayi M. Comparison of the diagnostic value of the visual inspection with acetic acid (VIA) and Pap smear in cervical cancer screening. Taiwan J Obstet Gynecol. 2019;58(3):345‐348. [DOI] [PubMed] [Google Scholar]
- 38. Warnakulasuriya S, Fennell N, Diz P, Seoane J, Rapidis A, Programme LLL. An appraisal of oral cancer and pre‐cancer screening programmes in Europe: a systematic review. J Oral Pathol Med. 2015;44(8):559‐570. [DOI] [PubMed] [Google Scholar]
- 39. Walsh T, Liu JL, Brocklehurst P, et al. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst Rev. 2013;11:1‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grafton‐Clarke C, Chen KW, Wilcock J. Diagnosis and referral delays in primary care for oral squamous cell cancer: a systematic review. Br J Gen Pract. 2019;69(679):e112‐e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kerawala C. Complications of head and neck cancer surgery—prevention and management. Oral Oncol. 2010;46(6):433‐435. [DOI] [PubMed] [Google Scholar]
- 42. Shah JP, Gil Z. Current concepts in management of oral cancer—surgery. Oral Oncol. 2009;45(4–5):394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ford P, Farah C. Early detection and diagnosis of oral cancer: strategies for improvement. J Cancer Policy. 2013;1(1–2):e2‐e7. [Google Scholar]
- 44. Silverman S, Kerr AR, Epstein JB. Oral and pharyngeal cancer control and early detection. J Cancer Educ. 2010;25(3):279‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farah CS, McCullough MJ. A pilot case control study on the efficacy of acetic acid wash and chemiluminescent illumination (ViziLite) in the visualisation of oral mucosal white lesions. Oral Oncol. 2007;43(8):820‐824. [DOI] [PubMed] [Google Scholar]
- 46. McCullough M, Prasad G, Farah C. Oral mucosal malignancy and potentially malignant lesions: an update on the epidemiology, risk factors, diagnosis and management. Aust Dent J. 2010;55:61‐65. [DOI] [PubMed] [Google Scholar]
- 47. Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44(1):10‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc. 2008;139(7):896‐894. [DOI] [PubMed] [Google Scholar]
- 49. Walter F, Webster A, Scott S, Emery J. The Andersen model of total patient delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17(2):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Example search strategy
Appendix S2: Eligibility criteria for study selection
Appendix S3: COE versus biopsy: Summary ROC curve for dysplastic and malignant lesions without Patel et al. 33 (N = 13 studies)
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


