Abstract
BACKGROUND
Fosnetupitant (FosNTP), an intravenous neurokinin 1 receptor antagonist, demonstrated a favorable safety profile with a potentially low risk of injection site reactions (ISRs) and promising antiemetic efficacy in patients receiving cisplatin‐based highly emetogenic chemotherapy in a previous phase 2 study. We conducted a randomized, double‐blind safety study to evaluate the safety profile of FosNTP, including ISRs, in patients receiving doxorubicin‐cyclophosphamide or epirubicin‐cyclophosphamide (AC/EC) chemotherapy.
METHODS
Patients scheduled to receive AC/EC were randomized 1:1 to receive 235 mg of FosNTP or 150 mg of fosaprepitant (FosAPR), both in combination with 0.75 mg of intravenous palonosetron and 9.9 mg of dexamethasone on day 1. The stratification factors were age category (<55 vs ≥55 years) and study site. The primary end point was the incidence of treatment‐related adverse events (TRAEs) with FosNTP.
RESULTS
Overall, 102 patients were randomized to FosNTP (n = 52) or FosAPR (n = 50), and all were treated with the study drug and evaluated for safety. The primary end point, the incidence of TRAEs, was similar with FosNTP (21.2%; 95% confidence interval [CI], 11.1%‐34.7%) and FosAPR (22.0%; 95% CI, 11.5%‐36.0%), with any‐cause ISRs observed in 5.8% and 26.0% of patients, respectively, and treatment‐related ISRs observed in 0% and 10.0%, respectively. The overall (0‐120 hour) complete response (defined as no emetic event and no rescue medication) rate, standardized by age category in the full analysis set, was 45.9% (23 of 51 patients) with FosNTP and 51.3% (25 of 49 patients) with FosAPR.
CONCLUSIONS
FosNTP demonstrated a favorable safety profile with a very low risk of ISRs in the AC/EC setting.
Keywords: anthracyclines, cyclophosphamide, fosaprepitant, injection site reaction, nausea, neurokinin 1 receptor antagonists, vomiting
Short abstract
Fosnetupitant shows a favorable safety profile in patients receiving doxorubicin‐cyclophosphamide or epirubicin‐cyclophosphamide chemotherapy. As an intravenous neurokinin 1 receptor antagonist with a low risk of causing injection site reactions, fosnetupitant may be used for protecting patients with cancer from experiencing chemotherapy‐induced nausea and vomiting.
Introduction
Anthracycline‐cyclophosphamide chemotherapy is a key treatment regimen commonly used for many cancers, including breast cancer, but it is known to have a high risk of causing chemotherapy‐induced nausea and vomiting (CINV) and hence is positioned as a highly emetogenic chemotherapy (HEC) by various antiemetic guidelines. 1 , 2 , 3 , 4 CINV should be prevented to maintain patients' quality of life and to continue their planned cancer treatment.
Per antiemetic guidelines, triplet therapy with a neurokinin 1 (NK1) receptor antagonist (RA), a 5‐hydroxytryptamine3 (5‐HT3) RA, and dexamethasone (DEX) is basically recommended for patients receiving HEC, and quartet therapy with olanzapine in addition to the aforementioned triplet therapy is recommended or considered as a treatment option depending on the guidelines. 1 , 2 , 3 , 4 Among the NK1 RAs, injectable fosaprepitant (FosAPR) is widely used but has been reported to be associated with an increased risk/incidence of injection site reactions (ISRs), particularly in patients receiving anthracycline‐based chemotherapy regimens, necessitating FosAPR dosing interruptions, injection site changes, or switching to oral aprepitant; this illustrates clinical concerns with the use of injectable FosAPR, which is an unmet medical need. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
Fosnetupitant (FosNTP) is the intravenously administered phosphorylated prodrug of netupitant. The active drug netupitant has a high binding affinity and selectivity for the NK1 receptor, with an elimination half‐life of approximately 70 hours in plasma. 13 In the United States and the European Union, a fixed‐dose combination of 235 mg of FosNTP and 0.25 mg of the 5‐HT3 RA palonosetron (PALO) for intravenous use (IV NEPA) has been approved for CINV prophylaxis. 14 , 15
In Japan, the development of FosNTP focused on its evaluation as a single‐agent product. To date, a phase 2 study has been conducted to evaluate FosNTP in comparison with placebo in combination with PALO (0.75 mg) and DEX in patients receiving cisplatin‐based HEC; the percentage of patients with a complete response (CR; defined as no emetic event and no rescue medication) during the overall phase (0‐120 hours after the start of HEC administration) was used as the primary end point. The study demonstrated the superiority of FosNTP (235 mg) to placebo, with a favorable safety profile and a potentially low risk of developing ISRs. 13 Based on the phase 2 study results, a head‐to‐head, randomized, phase 3 study was conducted in patients receiving cisplatin‐based HEC to evaluate the efficacy and safety of FosNTP compared with FosAPR in combination with PALO (0.75 mg) and DEX (JapicCTI‐194611).
FosNTP may be associated with a low risk of causing ISRs, and this suggests the potential to overcome the unmet medical need associated with FosAPR. However, no studies have yet reported the safety of FosNTP in patients receiving anthracycline‐based chemotherapy regimens in comparison with FosAPR. Therefore, we conducted a study to evaluate the safety profile, including ISRs, of FosNTP, and we performed an exploratory comparison with FosAPR in patients receiving doxorubicin‐cyclophosphamide or epirubicin‐cyclophosphamide (AC/EC) chemotherapy administered in combination with PALO (0.75 mg) and DEX.
Materials and Methods
Study Design and Patients
This was a double‐blind, randomized, parallel‐group, multicenter, phase 3 study to evaluate the safety of a single intravenous dose of FosNTP in combination with PALO (0.75 mg) and DEX in patients receiving AC/EC chemotherapy. An exploratory FosAPR group was also included to help interpret the safety findings of FosNTP.
Eligible patients who provided informed consent to participate in the study were enrolled by the investigator via an interactive web response system and randomized 1:1 to either the FosNTP group or the FosAPR group by dynamic allocation using stratification factors for randomization (age category [≥55 vs <55 years] and study site).
The study was approved by the institutional review board of each study site and was conducted in compliance with the Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written consent before enrollment.
Key eligibility criteria are shown in Supporting Text 1.
Treatment
In the FosNTP group, starting 60 minutes before the initiation of AC/EC administration, a placebo matching FosAPR was intravenously infused for 30 minutes, and this was followed by an intravenous infusion of a mixture of FosNTP (235 mg), PALO (0.75 mg), and DEX (9.9 mg) for 30 minutes. In the FosAPR group, starting 60 minutes before the initiation of AC/EC administration, FosAPR (150 mg) was intravenously infused for 30 minutes, and this was followed by an intravenous infusion of a mixture of a placebo matching FosNTP, PALO (0.75 mg), and DEX (9.9 mg) for 30 minutes because of the possibility of an incompatibility risk of FosAPR with PALO. 16 In both groups, AC/EC administration was started 30 minutes after the initiation of the administration of FosNTP or a placebo matching FosNTP. DEX was administered at 9.9 mg only on day 1 in both groups, with dose sparing from day 2.
FosNTP and FosAPR were prepared with 100 mL of normal saline and were administered into a peripheral vein.
End Points
The primary end point was the incidence of treatment‐related adverse events (TRAEs) with FosNTP administered in combination with PALO and DEX in patients receiving AC/EC chemotherapy. The main secondary safety end points were the incidence of adverse events (AEs) and ISRs with FosNTP.
The main secondary efficacy end points were the percentages of overall CR, total control (defined as no emetic event, no rescue medication, and no nausea), and complete protection (defined as no emetic event, no rescue medication, and no significant [no more than mild] nausea).
Assessments
AEs were recorded with verbatim terms, coded with the Medical Dictionary for Regulatory Activities (version 23.0), and tabulated by system organ class and preferred term. The severity of AEs was graded by the investigator per the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 5.0). The causal relationship between the study drug and each AE was assessed on a 5‐point scale (ie, definite, probably related, possibly related, unlikely related, and not related). AEs assessed as causally “definite,” “probably related,” or “possibly related” to the study drug were regarded as TRAEs.
Emetic events and nausea occurring 0 to 120 hours after the start of AC/EC administration were not regarded as AEs because they were considered as the index for efficacy evaluation. However, emetic events and nausea that continued or newly occurred beyond 120 hours after the start of AC/EC administration were regarded as AEs.
Efficacy assessments were based on records of the following parameters in a patient's diary every 24 hours during 0‐120 hours after the start of AC/EC administration: all emetic events, rescue medication, and severity of nausea (Likert scale: none, mild nausea [able to eat and drink], moderate nausea [able to only drink], or severe nausea [unable to eat or drink]).
Statistical Analysis
The safety analysis was conducted on the as‐treated population, which was defined as all patients who had received the study drug. The efficacy analysis used the full analysis set, which was defined as all patients in the as‐treated population who were administered PALO, DEX, and AC/EC on day 1.
In the primary end point analysis, the numbers and percentages of patients with TRAEs were determined, with 95% confidence interval (CI) calculated by an exact method based on the F distribution.
The target sample size was determined on the basis of the preceding phase 2 study, 13 which showed a 29.2% incidence of TRAEs in the FosNTP (235 mg) group. Under the assumption of a similar incidence of TRAEs in the FosNTP and FosAPR groups in this study, 96 patients were required for study drug administration. To allow for dropouts, the target sample size for the 2 groups was set to 100.
All statistical analyses were performed with SAS version 9.4 and SAS/STAT version 14.2 (SAS Institute, Inc., Cary, North Carolina).
Results
The study enrolled 102 patients across 5 study sites between April 2019 and March 2020. Fifty‐two of these patients were randomized to the FosNTP group, and 50 were randomized to the FosAPR group; all patients received the study drug (Fig. 1). Two patients did not receive protocol‐specified AC/EC on day 1 and were excluded from the full analysis set. Therefore, the efficacy analysis was performed on data from 51 patients in the FosNTP group and 49 patients in the FosAPR group.
Figure 1.
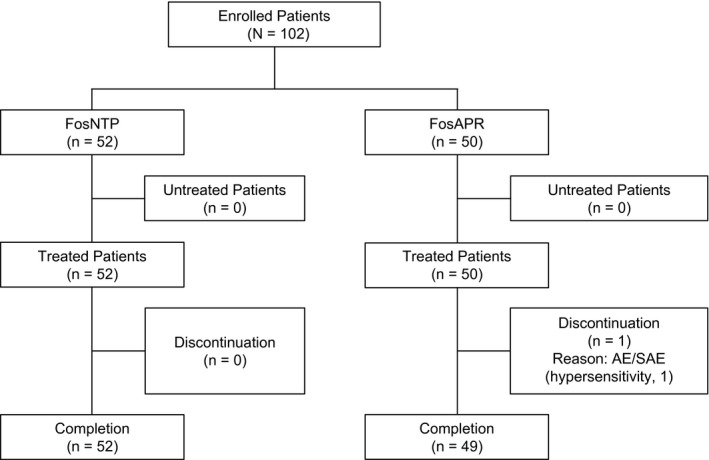
Consolidated Standards of Reporting Trials diagram showing the patient disposition. AE indicates adverse event; FosAPR, fosaprepitant; FosNTP, fosnetupitant; SAE, serious adverse event.
The baseline patient background and disease characteristics are shown in Table 1. All patients were female and had breast cancer. The patient background characteristics were similar between the 2 groups with the exception of higher percentages of patients with motion sickness and patients with no history of smoking in the FosNTP group.
TABLE 1.
Patient Baseline and Disease Characteristics (As‐Treated Population)
| Characteristic | FosNTP (n = 52) | FosAPR (n = 50) |
|---|---|---|
| Age, median (range), y | 56.0 (34‐79) | 56.0 (30‐77) |
| Age, No. (%) | ||
| <55 y | 24 (46.2) | 21 (42.0) |
| ≥55 y | 28 (53.8) | 29 (58.0) |
| Sex, No. (%) | ||
| Male | 0 (0.0) | 0 (0.0) |
| Female | 52 (100.0) | 50 (100.0) |
| Drinking history, No. (%) | ||
| No | 32 (61.5) | 31 (62.0) |
| Rarely (once per month) | 10 (19.2) | 12 (24.0) |
| Occasionally (once per week) | 3 (5.8) | 3 (6.0) |
| Regularly (once per day) | 7 (13.5) | 4 (8.0) |
| Smoking history, No. (%) | ||
| Nonsmoker | 41 (78.8) | 32 (64.0) |
| Stopped smoking prior to 180 d before registration | 4 (7.7) | 9 (18.0) |
| Stopped smoking within 180 d before registration | 1 (1.9) | 5 (10.0) |
| Smoker | 6 (11.5) | 4 (8.0) |
| Motion sickness, No. (%) | ||
| No | 24 (46.2) | 28 (56.0) |
| Yes | 28 (53.8) | 22 (44.0) |
| Malignant tumor, No. (%) | ||
| Breast | 52 (100.0) | 50 (100.0) |
| Other | 0 (0.0) | 0 (0.0) |
| Chemotherapy regimen, No. (%) | ||
| AC | 23 (44.2) | 22 (44.0) |
| EC | 29 (55.8) | 28 (56.0) |
| Actual dose, median (range), mg/m2 | ||
| Doxorubicin | 59.90 (56.8‐60.2) | 60.00 (50.3‐60.7) |
| Epirubicin | 88.00 (86.2‐101.3) | 87.90 (84.2‐100.6) |
| Cyclophosphamide | 590.30 (493.4‐656.7) | 591.50 (497.9‐608.1) |
Abbreviations: AC, doxorubicin‐cyclophosphamide; EC, epirubicin‐cyclophosphamide; FosAPR, fosaprepitant; FosNTP, fosnetupitant.
Safety
The primary end point, the incidence of TRAEs, was 21.2% (11 of 52 patients; 95% CI, 11.1%‐34.7%) in the FosNTP group and 22.0% (11 of 50 patients; 95% CI, 11.5%‐36.0%) in the FosAPR group (Table 2).
TABLE 2.
Safety Summary (As‐Treated Population)
| Event | FosNTP (n = 52), No. (%) | FosAPR (n = 50), No. (%) |
|---|---|---|
| AEs | 52 (100.0) | 49 (98.0) |
| Grade 3 or higher AEs | 43 (82.7) | 36 (72.0) |
| TRAEs | 11 (21.2) | 11 (22.0) |
| Grade 3 or higher TRAEs | 5 (9.6) | 0 (0.0) |
| Serious AEs | 2 (3.8) | 0 (0.0) |
| Serious TRAEs | 1 (1.9) | 0 (0.0) |
| AEs leading to discontinuation | 0 (0.0) | 1 (2.0) |
| AEs leading to death | 0 (0.0) | 0 (0.0) |
| TRAEs leading to discontinuation | 0 (0.0) | 1 (2.0) |
| TRAEs leading to death | 0 (0.0) | 0 (0.0) |
Abbreviations: AE, adverse event; FosAPR, fosaprepitant; FosNTP, fosnetupitant; TRAE, treatment‐related adverse event.
In the FosNTP group, TRAEs reported in ≥5% of patients were headache, diarrhea, urticaria, malaise, and decreased appetite (5.8% each; 3 of 52 patients each). Grade 3 or higher TRAEs occurred in 9.6% of the patients (5 of 52 patients); those reported in 2 or more patients were neutrophil count decreased and white blood cell count decreased (3.8% each; 2 of 52 patients each). One serious TRAE (urticaria) was reported in the FosNTP group but resolved 6 days after its onset with appropriate treatment. No AEs leading to death or discontinuation from the study were reported.
In the FosAPR group, the TRAEs reported in ≥5% of patients were constipation (6.0%; 3 of 50 patients) and injection site pain (8.0%; 4 of 50 patients), and no grade 3 or higher TRAEs were reported.
With regard to the safety point of particular interest, AEs relevant to ISRs were observed in 5.8% of the patients (3 of 52 patients, 3 events) in the FosNTP group and in 26.0% of the patients (13 of 50 patients, 18 events) in the FosAPR group (Table 3). Furthermore, TRAEs relevant to ISRs were observed in 0% of the patients in the FosNTP group and in 10.0% of the patients (5 of 50 patients, 5 events) in the FosAPR group. Four of the 5 ISR events occurred during the FosAPR infusion (3 events of injection site pain and 1 event of injection site vasculitis).
TABLE 3.
Summary of ISRs (As‐Treated Population)
| Preferred Term per MedDRA (Version 23.0) | NCI CTCAE Grade | AEs Relevant to ISRs, No. (%) | TRAEs Relevant to ISRs, No. (%) | ||
|---|---|---|---|---|---|
| FosNTP (n = 52) | FosAPR (n = 50) | FosNTP (n = 52) | FosAPR (n = 50) | ||
| Total injection site reactions | 3 (5.8) | 13 (26.0) | 0 | 5 (10.0) | |
| Injection site erythema | Grade 1 | 2 (3.8) | 2 (4.0) | 0 | 0 |
| Grade 2 | 0 | 0 | 0 | 0 | |
| Any grade | 2 (3.8) | 2 (4.0) | 0 | 0 | |
| Injection site pain | Grade 1 | 1 (1.9) | 7 (14.0) | 0 | 3 (6.0) |
| Grade 2 | 0 | 1 (2.0) | 0 | 1 (2.0) | |
| Any grade | 1 (1.9) | 8 (16.0) | 0 | 4 (8.0) | |
| Injection site induration | Grade 1 | 0 | 2 (4.0) | 0 | 0 |
| Grade 2 | 0 | 0 | 0 | 0 | |
| Any grade | 0 | 2 (4.0) | 0 | 0 | |
| Injection site swelling | Grade 1 | 0 | 1 (2.0) | 0 | 0 |
| Grade 2 | 0 | 0 | 0 | 0 | |
| Any grade | 0 | 1 (2.0) | 0 | 0 | |
| Injection site phlebitis | Grade 1 | 0 | 0 | 0 | 0 |
| Grade 2 | 0 | 2 (4.0) | 0 | 0 | |
| Any grade | 0 | 2 (4.0) | 0 | 0 | |
| Injection site vasculitis | Grade 1 | 0 | 2 (4.0) | 0 | 1 (2.0) |
| Grade 2 | 0 | 1 (2.0) | 0 | 0 | |
| Any grade | 0 | 3 (6.0) | 0 | 1 (2.0) | |
Abbreviations: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; FosAPR, fosaprepitant; FosNTP, fosnetupitant; ISR, injection site reaction; MedDRA, Medical Dictionary for Regulatory Activities; NCI, National Cancer Institute; TRAE, treatment‐related adverse event.
Efficacy
The results of the main secondary efficacy end points are shown in Supporting Table 1.
The overall CR rate standardized by age category was 45.9% (23 of 51 patients; 95% CI, 33.2%‐58.6%) in the FosNTP group and 51.3% (25 of 49 patients; 95% CI, 37.3%‐65.2%) in the FosAPR group.
Discussion
This study evaluated the safety, including ISRs, of a single dose of FosNTP administered in combination with PALO and DEX in patients receiving AC/EC chemotherapy.
The incidence of TRAEs with FosNTP—the primary end point—was similar to that with FosAPR. The incidence of grade 3 or higher TRAEs was numerically higher in the FosNTP group compared with the FosAPR group; however, the difference did not appear to be clinically meaningful because no TRAEs led to discontinuation from the study in the FosNTP group, and many of the reported events were well known to occur with and were attributable to anticancer drugs.
The incidence of ISRs associated with administration of the study drug was of particular interest in this study. The incidence of AEs and TRAEs relevant to ISRs in the FosNTP group was 5.8% and 0%, respectively. During the conduct of this study, a phase 3b study of IV NEPA in patients with breast cancer receiving AC/EC chemotherapy in the United States and Europe showed a low incidence of infusion site AEs (1.5%; 3 of 200 patients) in the IV NEPA group, with no treatment‐related infusion site AEs reported. 17 Incidentally, the results of a phase 3 study of triplet antiemetic therapy including FosAPR in patients with breast cancer receiving AC/EC chemotherapy were published; the incidence of ISRs was similar to that in the FosAPR group reported in this study. 18 Furthermore, a pivotal phase 3 study comparing FosNTP and FosAPR in patients receiving cisplatin‐based HEC conducted in parallel with this study showed that the incidences of AEs/TRAEs relevant to ISRs were statistically significantly lower in the FosNTP group than the FosAPR group (AEs, 11.0% with FosNTP vs 20.6% with FosAPR [P < .001]; TRAEs, 0.3% with FosNTP vs 3.6% with FosAPR [P < .001]). 19 These data suggest that FosNTP has a very low risk of causing ISRs not only in patients receiving cisplatin but also in patients receiving AC/EC chemotherapy, who are known to have a higher risk of ISRs 5 , 6 , 7 , 8 , 9 , 10 , 18 ; this suggests that FosNTP could help in minimizing this discomfort, an unmet medical need associated with FosAPR.
A possible reason for the lower incidence of ISRs with FosNTP may be the very small amount of insoluble netupitant instantaneously produced from FosNTP in the blood at the local infusion site for less damage to the vascular endothelial cell. Indeed, an in vitro study demonstrated that the rate of conversion to active netupitant was as low as 0.9% for 15 minutes and 1.3% for 30 minutes when FosNTP was incubated in blood (data on file). This finding suggests that although systemic blood contains a certain level of netupitant produced from FosNTP by dephosphorylase in the liver and other organs in the body, the blood at the local infusion site contains almost no netupitant converted from FosNTP without systemic metabolism, and this may have contributed to the reduced risk of ISRs with FosNTP infusion.
Starting 60 minutes before anticancer therapy administration, FosAPR should be administered as an intravenous infusion for approximately 30 minutes. 20 In contrast, FosNTP is expected to be effective when it is administered as an intravenous infusion for 30 minutes just before anticancer therapy administration. Furthermore, as was done in this study, FosNTP was administered with PALO/DEX mixed in 1 infusion bag because of no incompatibility risk; this enabled a shortening of the total infusion time in comparison with the sequential dosing of FosAPR and PALO/DEX.
The efficacy was evaluated as a secondary end point, and FosNTP was shown to be effective in patients with CINV receiving AC/EC as well. An exploratory comparison with FosAPR showed a slightly lower overall CR rate in the FosNTP group. This could be because of the insufficient sample size for efficacy comparison and partly because a history of motion sickness, a suggested risk factor for CINV, 1 , 2 was noted in more patients in the FosNTP group (54.9% [28 of 51 patients]) than the FosAPR group (44.9% [22 of 49 patients]). Besides, in the pivotal phase 3 study of patients receiving cisplatin‐based HEC, the primary end point of the overall CR rate was 75.2% in the FosNTP group and 71.0% in the FosAPR group; this demonstrated the noninferiority of FosNTP to FosAPR. 19
Because this study was conducted in patients receiving AC/EC to evaluate the safety of a single dose of FosNTP, the safety of repeated dosing was not evaluated. Another phase 3 study conducted in patients receiving cisplatin‐based HEC evaluated the safety of repeated doses of FosNTP for up to 4 cycles of chemotherapy. 19
In conclusion, FosNTP administered as a single intravenous dose in combination with PALO and DEX showed a favorable safety profile in patients receiving AC/EC chemotherapy. Hence, as an intravenous NK1 RA with a low risk of causing ISRs, FosNTP may be used for protecting patients with cancer from experiencing CINV.
Funding Support
Funding for this trial was provided by Taiho Pharmaceutical Co, Ltd.
Conflict of Interest Disclosures
Junji Tsurutani has received grants or contracts from Eisai, Daiichi Sankyo, Kyowa Kirin, Chugai, Nihon Kayaku, Pfizer, Eli Lilly, Boehringer Ingelheim, and MSD; consulting fees from Daiichi Sankyo, AstraZeneca, and Eli Lilly; payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Taiho, Eisai, Daiichi Sankyo, Chugai, Kyowa Kirin, Nihon Kayaku, Eli Lilly, Pfizer, and Novartis; and support for attending meetings and/or travel from Daiichi Sankyo, Chugai, and Kyowa Kirin. Kenichi Inoue has received grants or contracts from Novartis, Pfizer, Chugai, Daiichi Sankyo, Parexel, MSD, Bayer, Eli Lilly, AstraZeneca, Sanofi, Eisai, and Taiho and payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Eisai, Chugai, Pfizer, and Eli Lilly. Yuko Tanabe has received payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Daiichi Sankyo and Pfizer. Tetsuhiko Taira has received payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Kyowa Kirin, Chugai, Eli Lilly Japan, Taiho, Daiichi Sankyo, and Novartis. Kaoru Kubota has received grants or contracts from Ono and Boehringer Ingelheim; consulting fees from Taiho; and payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Ono, Boehringer Ingelheim, Chugai, MSD, AstraZeneca, Eli Lilly, Daiichi Sankyo, Bristol‐Myers Squibb, Taiho, Kyowa Hakko Kirin, Takeda, Nihon Kayaku, Sawai, and Pfizer. Tomohide Tamura has received payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Chugai, Boehringer Ingelheim, Ono, Taiho, MSD, CMIC, Lilly, and Nihon Kayaku. Toshiaki Saeki has received grants or contracts from Eisai, Kyowa Kirin, Taiho, Chugai, Nippon Kayaku, and MSD KK and payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Taiho. The other author made no disclosures.
Author Contributions
Kazuo Matsuura: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Junji Tsurutani: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Kenichi Inoue: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Yuko Tanabe: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Tetsuhiko Taira: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Kaoru Kubota: Conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Tomohide Tamura: Conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work. Toshiaki Saeki: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript, and accountability for all aspects of the work.
Supporting information
Table S1
Matsuura K, Tsurutani J, Inoue K, Tanabe Y, Taira T, Kubota K, Tamura T, Saeki T. A phase 3 safety study of fosnetupitant as an antiemetic in patients receiving anthracycline and cyclophosphamide: CONSOLE‐BC. Cancer.2022. 10.1002/cncr.34088
We thank the patients, their families, and the investigators who participated in this study. We also thank Ali Nasermoaddeli, MD, PhD (Taiho Pharmaceutical Co, Ltd), for his important medical advice. Editorial support was provided by Cactus Life Sciences (part of Cactus Communications) and Kotaro Suto of Taiho Pharmaceutical Co, Ltd, and was funded by Taiho Pharmaceutical Co, Ltd.
Clinical trial information: A randomized, double‐blind, multicenter, phase III study of Pro‐NETU for the prevention of chemotherapy induced nausea and vomiting (CINV) in patients receiving doxorubicin‐cyclophosphamide/epirubicin‐cyclophosphamide (AC/EC) based highly emetogenic chemotherapy: CONSOLE‐BC (JapicCTI‐194691).
Data Availability
The sponsor's policy on data sharing can be found at https://www.taiho.co.jp/en/science/policy/clinical_trial_information_disclosure_policy/index.html.
References
- 1. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119‐v133. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology: Antiemesis. Version 1. National Comprehensive Cancer Network; 2021. [Google Scholar]
- 3. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:3240‐3261. [DOI] [PubMed] [Google Scholar]
- 4. Aogi K, Takeuchi H, Saeki T, et al. Optimizing antiemetic treatment for chemotherapy‐induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol. 2021;26:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leal AD, Kadakia KC, Looker S, et al. Fosaprepitant‐induced phlebitis: a focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer. 2014;22:1313‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Candelario N, Lu MLR. Fosaprepitant dimeglumine for the management of chemotherapy‐induced nausea and vomiting: patient selection and perspectives. Cancer Manag Res. 2016;8:77‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sato Y, Kondo M, Inagaki A, et al. Highly frequent and enhanced injection site reaction induced by peripheral venous injection of fosaprepitant in anthracycline‐treated patients. J Cancer. 2015;5:390‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujii T, Nishimura N, Urayama KY, et al. Differential impact of fosaprepitant on infusion site adverse events between cisplatin‐ and anthracycline‐based chemotherapy regimens. Anticancer Res. 2015;35:379‐383. [PubMed] [Google Scholar]
- 9. Hegerova LT, Leal AD, Grendahl DC, et al. An analysis of fosaprepitant‐induced venous toxicity in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2015;23:55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuda T, Kiyomori C, Mizukami T, et al. Infusion site adverse events in breast cancer patients receiving highly emetic chemotherapy with prophylactic anti‐emetic treatment with aprepitant and fosaprepitant: a retrospective comparison. Mol Clin Oncol. 2016;4:603‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saito H, Yoshizawa H, Yoshimori K, et al. Efficacy and safety of single‐dose fosaprepitant in the prevention of chemotherapy‐induced nausea and vomiting in patients receiving high‐dose cisplatin: a multicentre, randomised, double‐blind, placebo‐controlled phase 3 trial. Ann Oncol. 2013;24:1067‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundberg JD, Crawford BS, Phillips G, Berger MJ, Wesolowski R. Incidence of infusion‐site reactions associated with peripheral intravenous administration of fosaprepitant. Support Care Cancer. 2014;22:1461‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugawara S, Inui N, Kanehara M, et al. Multicenter, placebo‐controlled, double‐blind, randomized study of fosnetupitant in combination with palonosetron for the prevention of chemotherapy‐induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Cancer. 2019;125:4076‐4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Highlights of prescribing information . AKYNZEO® (netupitant and palonosetron) capsules, for oral use. AKYNZEO® (fosnetupitant and palonosetron) for injection, for intravenous use. US Food and Drug Administration. Accessed June 3, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210493s000lbl.pdf
- 15. Summary of product characteristics . Akynzeo 300 mg/0.5 mg hard capsules. Akynzeo 235 mg/0.25 mg powder for concentrate for solution for infusion. European Medicines Agency. Accessed June 3, 2021. https://www.ema.europa.eu/en/documents/product‐information/akynzeo‐epar‐product‐information_en.pdf
- 16. Sun S, Schaller J, Placek J, Duersch B. Compatibility of intravenous fosaprepitant with intravenous 5‐HT3 antagonists and corticosteroids. Cancer Chemother Pharmacol. 2013;72:509‐513. [DOI] [PubMed] [Google Scholar]
- 17. Schwartzberg L, Navari R, Clark‐Snow R, et al. Phase IIIb safety and efficacy of intravenous NEPA for prevention of chemotherapy‐induced nausea and vomiting (CINV) in patients with breast cancer receiving initial and repeat cycles of anthracycline and cyclophosphamide (AC) chemotherapy. Oncologist. 2020;25:e589‐e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsumoto K, Takahashi M, Sato K, et al. A double‐blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy‐induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Med. 2020;9:3319‐3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shiraishi Y, Hata A & Inui N et al. A phase III, randomized, double‐blind, multicenter, active control study of fosnetupitant for the prevention of chemotherapy‐induced nausea and vomiting (CINV) in patients receiving cisplatin based highly emetogenic chemotherapy (HEC): CONSOLE study [abstract 12099]. Abstract presented at: Annual Meeting of the American Society of Clinical Oncology; June 4‐8, 2021; Chicago, IL.
- 20. Highlights of prescribing information . EMEND (fosaprepitant) for injection, for intravenous use. US Food and Drug Administration. Accessed June 3, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022023s019lbl.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The sponsor's policy on data sharing can be found at https://www.taiho.co.jp/en/science/policy/clinical_trial_information_disclosure_policy/index.html.


