Abstract
Aims
Remote monitoring of pulmonary artery pressure has reduced heart failure (HF) hospitalizations in chronic HF as elevation of pulmonary artery pressure provides information that can guide treatment. The venous system is characterized by high capacitance, thus substantial increases in intravascular volume can occur before filling pressures increase. The inferior vena cava (IVC) is a highly compliant venous conduit and thus a candidate for early detection of change in intravascular volume. We aimed to compare IVC cross‐sectional area using a novel sensor with cardiac filling pressures during experimental manipulation of volume status, vascular tone, and cardiac function.
Methods and results
Experiments were conducted in sheep to manipulate volume status (colloid infusion), vascular tone (nitroglycerin infusion) and cardiac function (rapid cardiac pacing). A wireless implantable IVC sensor was validated ex‐vivo and in‐vivo, and then used to measure the cross‐sectional area of the IVC. Right‐ and left‐sided cardiac filling pressures were obtained via right heart catheterization. The IVC sensor provided highly accurate and precise measurements of cross‐sectional area in ex‐vivo and in‐vivo validation. IVC area changes were more sensitive than the corresponding changes in cardiac filling pressures during colloid infusion (p < 0.001), vasodilatation (p < 0.001) and cardiac dysfunction induced by rapid pacing (p ≤ 0.02).
Conclusions
Inferior vena cava area can be remotely and accurately measured in real time with a wireless implantable sensor. Changes in IVC area are more sensitive than corresponding changes in filling pressures following experimental volume loading and fluid redistribution. Additional research is warranted to understand if remote monitoring of the IVC may have advantages over pressure‐based monitors in HF.
Keywords: Heart failure, Inferior vena cava, Venous pressure, Models, Animal
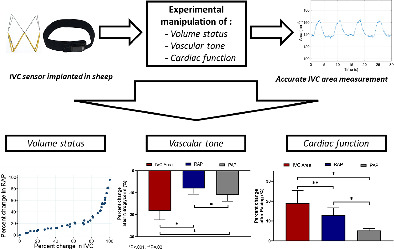
A wireless inferior vena cava (IVC) sensor was implanted in sheep followed by experiments to manipulate volume status, vascular tone, and cardiac function. The IVC sensor provided accurate measurements of cross‐sectional area. IVC area changes were more sensitive than the corresponding changes in cardiac filling pressures during manipulation of volume status, vascular tone, and cardiac function. PAP, mean pulmonary artery pressure; RAP, right atrial pressure.
Introduction
Congestion is a central pathophysiological process and the primary target in the treatment of heart failure (HF). Quantifying and tracking congestion are critical components of acute and chronic HF disease management. 1 , 2 , 3 , 4 Acute decompensated heart failure (ADHF) is one of the most common hospital discharge diagnoses and accounts for more than half of all HF‐related expenditures. 5 , 6 , 7 ADHF is primarily a disease of congestion rather than low cardiac output, making prevention of congestion critical. 1 , 2 , 3 , 4 In the CHAMPION trial (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) monitoring of pulmonary artery pressure (PAP) led to a 37% relative reduction in HF‐related hospitalizations. However, the absolute rate of HF events remained high with death or HF‐related hospitalization occurring in over 30% of the CardioMEMS monitored patients at 1 year. 8 In the GUIDE‐HF trial (Hemodynamic‐Guided Management of Heart Failure), haemodynamic‐guided management of HF did not result in a lower composite endpoint rate. However, there was a significant benefit in the pre‐COVID‐19 period that applied to both New York Heart Association class II and III patients. 9
Symptoms of congestion occur primarily as a consequence of increased systemic and pulmonary venous pressures. Importantly, there is a relative disconnect between pressure and volume in the venous circulation, an evolutionary adaptation that allows maintenance of cardiac preload (filling pressures) over a wide range of volume status. 10 , 11 , 12 The venous system is highly compliant and approximately 70% of intravascular volume resides in this compartment. Given this high compliance, relatively large changes in intravascular volume can be buffered resulting in little change in filling pressures. 12 , 13 Only when either capacitance is exhausted or a significant change in vascular tone reduces capacitance will cardiac filling pressure significantly rise. As a result of this physiology, techniques to monitor the distention of the venous system may offer an earlier and more sensitive signal for monitoring congestion.
The inferior vena cava (IVC) is a highly compliant venous conduit known to undergo large changes in dimension with minimal change in pressure over a wide operating range. 14 , 15 These characteristics make the IVC an excellent candidate for monitoring volume status in HF with a more sensitive signal expected for volume expansion than pressure‐based metrics. A wireless implantable IVC sensor has been developed that when energized with radiofrequency (RF) energy reports its cross‐sectional area with high temporal and spatial fidelity. The overarching objective of the current study was to better understand the relative performance of IVC dimension versus invasively determined filling pressures to detect intravascular volume expansion, volume redistribution, and worsening cardiac performance. To accomplish this, we first validated a wireless implantable IVC sensor that measures IVC cross‐sectional area with high temporal and spatial resolution. We then characterized the performance of this sensor during experimental manipulation of volume status, vascular tone, and experimental acute HF.
Methods
Animals and procedure preparation
Experiments were conducted at American Preclinical Services (APS, Minneapolis, MN, USA) and the experimental protocol was approved by the APS Institutional Animal Care and Use Committee. Studies were conducted in nine farm sheep (∼70 kg) in accordance with the Animal Welfare Act. Animals were induced with a combination of propofol, midazolam, ketamine, and buprenorphine, and then underwent endotracheal intubation with mechanical ventilation and maintenance anaesthesia using isoflurane. Venous and arterial access were obtained by vascular cut down or percutaneous approach via catheters placed in the iliac vein, iliac artery, carotid artery and jugular vein. Pressures were monitored by fluid filled catheters in the carotid artery and with a Swan–Ganz pulmonary artery catheter (Edwards Lifesciences, CA, USA) and recorded using Powerlab (AD Instruments, CO, USA). In a subset of sheep, a pacing lead was inserted into the right ventricle and connected to an external pulse generator. IVC area was measured using the implantable sensor (see below) which was placed in the IVC using fluoroscopy, and intravascular ultrasound (Boston Scientific, MA, USA). Briefly, via femoral venous access, the introducer sheath was positioned between renal and hepatic veins, and the landing zone length IVC diameter was confirmed using fluoroscopy. The loader containing the sensor was inserted into the introducer sheath. The pusher was inserted through the loader to advance the sensor to the tip of the introducer sheath. The self‐expanding sensor was deployed keeping the pusher position fixed while retracting the introducer sheath. The pusher and sheath were retracted once the sensor was deployed (see online supplementary Videos S1 and S2 ).
Experimental protocol
Nitroglycerin infusion
The purpose of the nitroglycerin experiment was to acutely manipulate vascular (venous) tone in the setting of stable intravascular volume. Nitroglycerin was infused at a rate of 1000 µg/min as a vasodilatory stimulus. IVC area measured by the sensor, as well as right atrial pressure (RAP), and PAPs were monitored throughout the experiment.
Volume experiment
First, blood was extracted from the animal to bring the animals to an initial starting RAP of approximately 2 mmHg. All extracted blood was stored in standard citrate phosphate dextrose adenine blood collection bags to maintain the blood stable for re‐infusion. After an approximate 30‐min period of equilibration allowing plasma refill, the extracted blood and hydroxyethyl starch (1:1 mixture) was injected in progressively larger boluses (50 ml, 100 ml, 250 ml, etc.) with 2 min of equilibration after each bolus. Volume loading was continued until a goal RAP of 15 mmHg or clinical instability (e.g. progressive hypoxia) occurred. When the removed blood was exhausted, hydroxyethyl starch was infused alone. IVC area measured by the implanted sensor, as well as RAP and PAPs were monitored throughout the infusion period.
Rapid cardiac pacing
The purpose of the cardiac pacing experiment was to manipulate cardiac function in the setting of a stable intravascular volume. Rapid cardiac pacing can induce acute systolic and diastolic cardiac dysfunction; in addition, it is a stimulus for vasoconstriction with subsequent redistribution of fluid from unstressed to stressed compartments. In a subset of animals, rapid right ventricular pacing was performed via transvenous pacing at 170 bpm. The threshold of 170 bpm was chosen because preliminary experiments showed that at this rate, mean arterial pressure decreased to <70 mmHg and cardiac index decreased to <3.0 L/min.
Inferior vena cava sensor
The IVC sensor (FIRE1, Dublin, Ireland) is designed as a chronic implantable passive monitor to measure area of the IVC over time. The resulting signal is analysed for IVC area and change in area during the respiratory cycle. The system is comprised of three components: the sensor, which is implanted in the IVC, the delivery system (pusher and loader), and the external detection system (belt, hardware unit and laptop) as depicted in Figure 1 .
Figure 1.
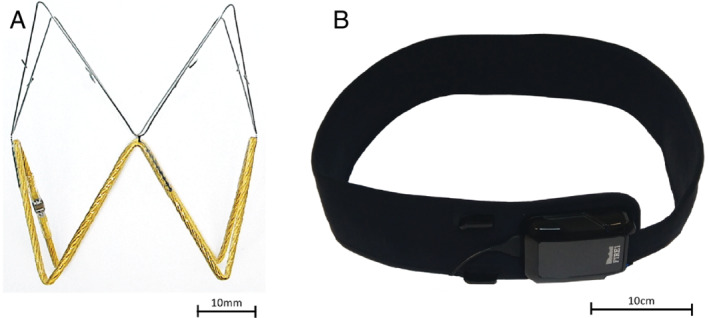
FIRE1 remote monitoring device components. (A) The sensor is an electro‐magnetic resonator comprising of a coil of wire and a capacitor. (B) A hardware unit generates radiofrequency energy, which is transmitted to the belt, worn around the patient's abdomen at elbow height. The energized sensor resonates at a frequency that is detected by the belt.
The sensor is delivered to the implantation site during a percutaneous procedure via femoral access using a standard commercially available 16 Fr sheath. The detection system generates RF energy, which is transmitted to the belt placed along the subjects' abdomen at the approximate level of the sensor. This energy creates an alternating magnetic field that energizes the implanted sensor. The sensor is an electro‐magnetic resonator comprised of a coil of wire and a capacitor. The energized sensor resonates at a frequency that is detected by the belt. The resonant frequency is dependent on its inductance, which in turn is dependent on the area of the sensor. The resonant frequency of the sensor is detected externally by the belt and decoded by the hardware unit. Upon analysis, the results will indicate the cross‐sectional area of the sensor and thus, the cross‐sectional IVC area in the sensor location of the patient.
Validation of the inferior vena cava sensor
The sensor resonance frequency relates directly to the sensor's cross‐sectional area and hence a characterization curve can be used to link the frequency received to the area of the sensor itself. For establishing the model, 18 white acetal calibration tubes of varying inner diameters (13–28 mm in 1 mm steps, plus 30 mm, 34 mm, and 38 mm) manufactured to within ± 0.1 mm were used. The exact inner diameter was measured using a calibrated digital Vernier caliper (150 mm, model 500‐19630, Tokyo, Japan, calibrated accuracy ± 0.01 mm) and the measured diameters were converted to areas. A common characterization curve was derived from 22 sensors from the same manufacturing batch which were used for the current study. The common characterization curve linking sensor resonance frequency and cross‐sectional area was read into Matlab R2019b (The MathWorks, Inc., Natick, MA, USA) for further processing. The accuracy of the sensors used in this study was tested by comparing actual known tube area to area estimated using the common characterization curve data. The frequency measured for any given study sensor in one of the 18 tubes was recorded and converted to area for testing purposes.
After the sensors were implanted in animals, intravascular ultrasound (IVUS) images were obtained from caudal, mid, and cranial positions within the sensor during a pause in ventilation, and the average area was recorded. In addition, a second set of IVUS images was recorded with the IVUS catheter at the centre of the sensor and monitored for ∼10s while the animals were ventilated. IVUS images (∼600) were marked semi‐automatically and then mapped against the sensor area measured at corresponding time points. IVUS areas were averaged, and the mean value was determined. Then, mean IVUS areas were compared with mean areas measured by the sensor matching the time the IVUS images were obtained.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation or median (quartile 1–quartile 3) according to the observed distribution. Categorical variables were described as frequency (percentage). For the validation of the sensor, the IVC area measured by the sensor was compared with the known area of the tubes or the measured area by the IVUS, respectively. Pearson correlation coefficients and the difference between them were reported. For the animal experiments we aimed to compare changes in IVC area, RAP and PAPs. Given that there is significant heterogeneity in the size of the IVC between animals, normalization of the IVC area was necessary. For this comparison, the range (the maximum minus the minimum) of each of the three parameters was calculated for each animal. Then, the percentile change in each parameter was calculated as: (target measurement − baseline measurement)/range. For instance, the percentile change for the IVC area after nitroglycerin infusion was calculated as the IVC area after nitroglycerin infusion – IVC area before infusion (baseline)/IVC area range. Therefore, the percentile change describes how much the parameter (IVC, or pressure) moved from the minimum to the maximum. A percentile change of 50% means the parameter moved from the percentile 1 to the percentile 50. For the volume experiment, the association between IVC area and RAP or PAPs (all in percent change units) was assessed for non‐linearity with linear regression using splines with 3 cubic knots. Because there were repeated measures for each animal, we used linear mixed models to account for the absence of independence of observations. For the pre‐post comparisons of the nitroglycerin and cardiac pacing experiments, the difference in percent change between IVC area and RAP or PAPs was assessed with paired t‐tests. The pre‐ to post‐volume comparisons shown in the online supplementary Table S1 were done with the Wilcoxon matched‐pairs signed‐rank test. A p‐value <0.05 was considered statistically significant. Stata SE 14.0 (StataCorp, College Station, TX, USA) was used for statistical analysis.
Results
Validation of the inferior vena cava sensor
Nine sensors were tested in 18 different tubes ranging in areas from 133.55 to 1139.79 mm2. The tube areas were known within 0.01 mm2. The correlation between the IVC area measured by the sensors and the tube areas was 1.0 (p < 0.001) (Figure 2 ). The mean difference between the IVC area measured by the sensors and the known area of the tubes was −6.0 mm2 [95% confidence interval (CI) −7.6 to −4.3 mm2] representing a mean difference of −1.4% (95% CI −1.6% to −1.2%). For the in‐vivo validation, a total of 14 IVUS measurements from seven animals were obtained. The correlation between IVC area measured by the sensor and IVUS was r = 0.99 (p < 0.001) (Figure 2 ). The mean difference between the IVC area measured by the sensor and IVUS was −5.5 mm2 (95% CI −13.3 to 2.3 mm2) representing a mean difference of −1.6% (95% CI −4.3% to 1.0%).
Figure 2.
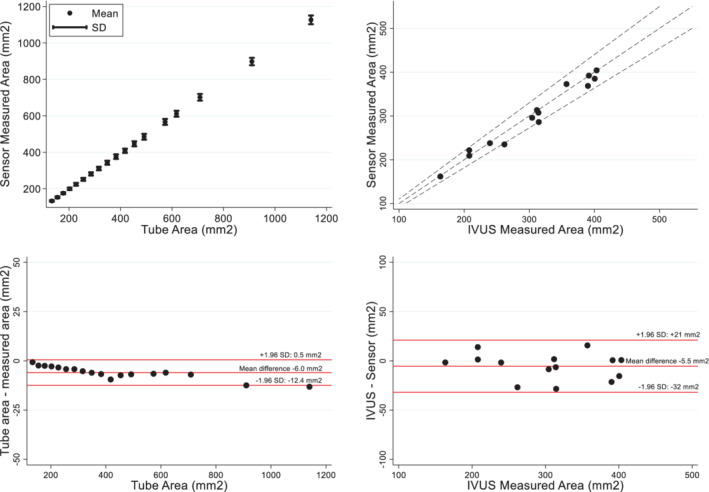
Ex‐vivo and in‐vivo validation of the inferior vena cava sensor. (Top left) The correlation between the known areas of the tubes and the sensor was 1.0 (p < 0.001). (Bottom left) Bland–Altman analysis between known areas of the tubes and sensor; the mean difference was −6.0 mm2. (Top right) The correlation between intravascular ultrasound (IVUS) measured areas and sensor measured was 0.985 (95% confidence interval 0.953–0.995) (p < 0.001). Dashed lines represent the ±10% IVUS area. (Bottom right) Bland–Altman analysis between IVUS areas and sensor; the mean difference was −5.5 mm2.
Animal studies
Nine animals were studied (mean weight 67.1 ± 6.2 kg). Implant of the IVC sensor was successful in all animals without complication. Landing zone was easily visualized, and deployment of sensor was easily completed in all animals. No migrations, fractures or thrombus were observed. External system provided successful continuous wireless monitoring throughout the experiments.
Nitroglycerin experiment
The nitroglycerin experiment was completed in nine animals at baseline prior to manipulation of the fluid status of the animal. The change in IVC area, RAP and PAP was calculated comparing pre‐ to post‐nitroglycerin measurements. Consistent with redistribution of volume into the splanchnic capacitance vessels, IVC area decreased −18.3 ± 14.6% during the nitroglycerin infusion. The corresponding percent decrease in RAP and PAP were −8.1 ± 7.3% and −11.0 ± 8.2%, respectively. Relative changes in RAP and PAP were significantly lower compared with the IVC change (p < 0.001 for both comparisons) (Figure 3 ).
Figure 3.
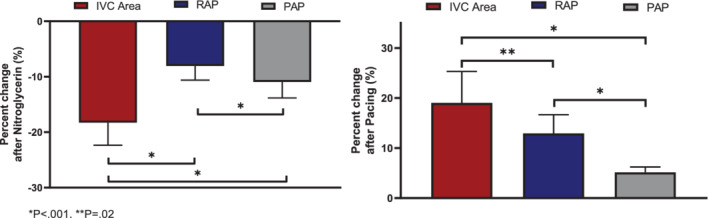
Change in inferior vena cava (IVC) area versus right atrial pressure (RAP) or mean pulmonary artery pressure (PAP) after nitroglycerin and cardiac pacing experiments.
Volume experiment
The volume experiment was completed in all nine animals. In total, an average of 2781 ± 544 ml of blood‐hydroxyethyl starch mixture was infused into each animal, corresponding to 65% of typical total blood volume in sheep. Figure 4 shows that the relationship between IVC area and invasively measured RAP was non‐linear (p‐value for non‐linearity <0.001). Online supplementary Figure S1 shows the corresponding non‐linear association between IVC area and diastolic PAP (dPAP). In the low pressure range, there were large changes in IVC area with relatively small changes in RAP and PAPs. Once RAP and dPAP reached ∼6 and 15 mmHg, respectively, the relationship became significantly steeper, with small increases of volume infusion resulting in larger increases of pressure. For instance, with the initial infusion of 500 ml of fluid, IVC area increased ∼60% compared to only a ∼20% increases of RAP and dPAP. At higher volumes and pressure having infused ∼1500 ml, RAP and dPAP increased by ∼50% compared to a 10% increase of IVC area. The relationship between IVC area and pulmonary capillary wedge pressure was similarly non‐linear but with IVC increasing with less volume infused than required to see increases of pulmonary capillary wedge pressure (online supplementary Figure S2 ). Online supplementary Figure S3 shows the association between IVC respiratory collapse with volume. Online supplementary Figure S4 shows the association between RAP in mmHg and IVC in mm2.
Figure 4.
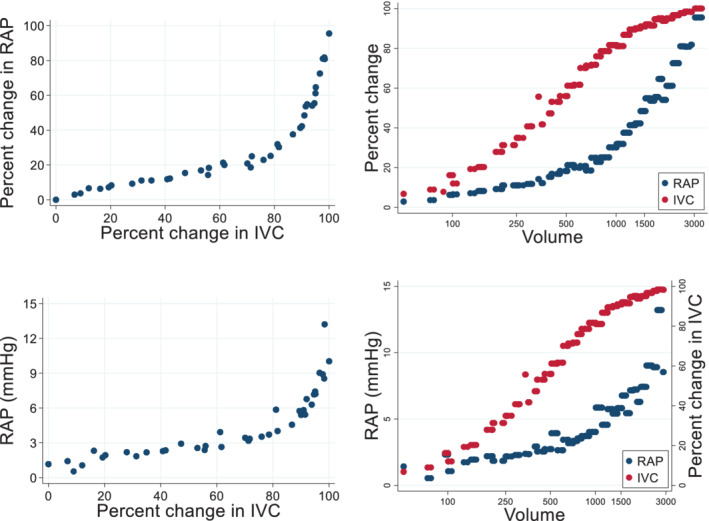
Change in inferior vena cava (IVC) area versus right atrial pressure (RAP) in the nine experimental animals. (Left panels) Association between IVC area and RAP. Top panels show RAP in percent change and bottom panels show RAP in mmHg. Non‐linear associations (p < 0.001 for non‐linearity) was observed between IVC area and RAP. (Right panels) Association of IVC and RAP with volume injected into the animal (ml). IVC was more sensitive than RAP. For instance, at 500 ml of injected volume the percent change in IVC area was ∼60% compared to only ∼20% for RAP. The scatters represent the average of the RAP and IVC of the nine animals at specific volumes that were given into the animal. Volume was injected into the animal in progressively larger boluses (50 ml, 100 ml, 250 ml, etc.) with 2 min of equilibration after each bolus.
Rapid cardiac pacing experiment
The cardiac pacing experiment was successfully completed in seven animals. The change in IVC area, RAP and PAP was calculated comparing pre‐cardiac pacing versus cardiac pacing. The percent change in IVC area was +19.0 ± 20.0%; that is, IVC area increased after cardiac pacing compared with baseline. The percent change in RAP and PAP were +12.9 ± 12.2% and +5.1 ± 4.4%, respectively (both RAP and PAP increased after cardiac pacing). Changes in RAP and PAP were significantly lower compared to the IVC change (p ≤ 0.02 for both comparisons) (Figure 3 ). Online supplementary Table S1 summarizes haemodynamic and IVC measurements across studies.
Discussion
The primary observations of this study are: (i) a passive resonance‐based IVC sensor provides highly accurate and precise measurements of cross‐sectional area of the IVC; (ii) this sensor can be placed in the IVC of an intact animal and provides real‐time information on IVC size during provocative manoeuvres; (iii) increases in IVC area during volume loading were more sensitive than increases in cardiac filling pressures during colloid infusion, indicating that intravascular volume expansion can be detected at an earlier stage using this approach; and (iv) changes in IVC dimensions were more sensitive than change in cardiac filling pressures induced by vasodilatation and rapid cardiac pacing, indicating value in detecting fluid redistribution (Graphical Abstract). These observations support further research on remote IVC area monitoring as a new candidate to improve clinical management of HF by allowing for earlier detection of incipient congestion.
The finding that IVC area is more sensitive to detect volume changes than pressure‐based metrics is expected based on established physiologic principles. The venous system is comprised of the most compliant vessels in the body (approximately 30 times that of the arteries) and >70% of total blood volume resides in the venous compartment. 12 , 13 This great compliance facilitates a relative pressure–volume disconnect allowing large changes in blood volume to be associated with small changes in pressure. In line with this physiology, filling pressures such as central venous pressure and pulmonary capillary wedge pressure have repeatedly demonstrated limited correlation with measures of volume status such as circulating blood volume and haemodynamic response to fluid challenge. 10 , 13 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Given that the IVC is a highly compliant venous structure, it is not surprising that its behaviour paralleled that known of the venous system. At the lower end of intravascular volumes, IVC area changed dramatically with little to no change in pressure. With severe hypervolaemia, as the capacitance of the venous system was exhausted, little change in IVC area was observed with large changes in pressure. Thus, the value for IVC area monitoring is to identify early increases in central vascular volume that may be more sensitive than increases in PAPs.
A less obvious but also predictable finding was that IVC area would also be more sensitive for redistributive shifts in volume than pressure‐based metrics, even when total blood volume remained constant. Blood volume can be conceptually divided into stressed (Vs) and unstressed (Vu) compartments. Vu is the volume of blood that would fill the vascular space at a pressure of zero, and this is normally thought to represent ∼70% of venous blood volume. If the vascular space were to continue to be filled, that volume that further stretches the vessels generating a pressure defines Vs. In addition to the inherently compliant nature of the venous system, the capacitance can be greatly altered by changes in vascular tone. With stimuli such as sympathetic activation, increased tone leads to a significantly altered pressure–volume relationship moving blood from Vu to Vs. 11 , 12 , 19 , 24 , 25 In the current study, we demonstrated that vasodilatation with nitroglycerin and vasoconstriction with rapid cardiac pacing, stimuli known to alter vascular tone and redistribute blood, significantly altered the IVC area with relatively small changes in pressures. These observations, combined with the anatomic location of the IVC in the central circulation, suggest that the IVC may act as a surrogate for changes in stressed blood volume.
The clinical implications of the current study are that chronic monitoring of an IVC sensor may provide incremental/additive information to pressure‐based sensors. The total blood volume of the sheep used in this experiment is estimated to be approximately 4 L. Thus, infusing 500 ml of blood/hydroxyethyl starch represents a meaningful relative expansion of blood volume. However, this only translated into ∼20% change in dPAP when starting from a normal intravascular volume. Thus, when a patient with HF is adequately decongested, relatively large increases in intravascular volume may occur prior to increases in dPAP. These would be detected earlier using IVC area. These observations warrant testing chronic IVC remote monitoring in HF patients. The first in human clinical investigation of the FIRE1 device/system is ongoing (FUTURE‐HF, NCT04203576) and will evaluate the feasibility and safety of implanting the FIRE1 system in stable HF patients.
Several limitations should be noted. The present study was designed to provide proof of concept that the IVC area shows earlier changes compared to invasively obtained filling pressures. The study was conducted using healthy sheep with experimental manipulation of volume, venous tone, and cardiac function. As such, findings of this study cannot be directly extrapolated to humans and particularly humans with HF. The three experiments were done on the animals the same day; thus, we cannot exclude some potential effects of the first experiments on the subsequent ones. Animals in these experiments were under general anaesthesia, blunting cardiovascular reflexes and thus changes in vascular tone with the interventions. Changes in IVC area during volume infusions could be affected by changes in vascular tone. Animals were endotracheally intubated receiving positive pressure mechanical ventilation. As such, insights into respiratory variations of the IVC are limited. Changes in IVC area are not a pure reflection of change in total blood volume and likely are variably influenced also by venous capacitance, IVC compliance and tone, and transmural pressure. The ‘volume experiment’ was designed to evaluate the pressure–IVC relationships starting at a low normal central venous pressure to an elevated central venous pressure, and the reported observations are correct within this construct. However, given the well‐known pressure–volume disconnect and the effect of mechanical ventilation and anaesthesia on filling pressures, the absolute volume status of the animal was unknown and likely varied somewhat between animals. Future research in HF patients will be required to anchor these observations to clinically relevant volume categories such as hypovolaemia and hypervolaemia. IVC areas were found to be variable between individual sheep and thus, dimensions were normalized to the range of IVC observed during the experiment to reduce this variability and focus on the underlying physiological pressure–volume relationship. Future research may be necessary to determine if normalization provides similar superior sensitivity of the IVC to pressure in human HF patients. Therefore, these findings should be regarded as hypothesis‐generating and additional research in chronic HF patients will be required to truly understand the potential of chronic IVC monitoring for HF.
In conclusion, the present study shows for the first time that a wireless passive RF‐based sensor can be safely deployed in the IVC, allowing for remote assessment of IVC area in real time. We further show that changes in IVC area are more sensitive than the corresponding changes in pressure both to experimental volume loading and fluid redistribution, with greater dynamic changes in the operating range of normal to moderately high venous pressures. These data call for further study of chronic remote home monitoring using IVC implanted devices to improve clinical management of patients with chronic HF.
Funding
This study was funded by FIRE1. The sponsor participated in data generation, analysis of the raw signal data, and in the review and approval of the manuscript.
Conflict of interest: J.B. declares that he serves as a consultant for Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, FIRE1, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, and Vifor. M.F. was supported by NHLBI K23HL151744 from the National Heart, Lung, and Blood Institute (NHLBI), the American Heart Association grant No 20IPA35310955, Mario Family Award, Duke Chair's Award, Translating Duke Health Award, Bayer and BTG Specialty Pharmaceuticals. He receives consulting fees from AxonTherapies, Bodyport, CVRx, Daxor, Edwards LifeSciences, NXT Biomedical, Zoll, Viscardia. J.M.T. reports grants and personal fees from 3ive labs, Boehringer Ingelheim, Reprieve medical, FIRE1, Sanofi, Sequana Medical, Merck, personal fees from Bayer, Bristol Myers Squibb, AstraZeneca, Novartis, Cardionomic, MagentaMed, W.L. Gore, Windtree Therapeutics, Lexicon pharmaceuticals, Regeneron, Edwards, BD, Precardia, grants from Otsuka, Abbott, outside the submitted work; In addition, J.M.T. has a patent Treatment of diuretic resistance US20200079846A1 issued to Yale and Corvidia Therapeutics Inc. and a patent Methods for measuring renalase WO2019133665A2 issued to Yale. All other authors have nothing to disclose.
Supporting information
Table S1. Haemodynamic and inferior vena cava parameters.
Figure S1. Change in inferior vena cava area versus diastolic pulmonary artery pressure in the nine experimental animals.
Figure S2. Change in inferior vena cava area versus pulmonary capillary wedge pressure in the nine experimental animals.
Figure S3. Inferior vena cava respiratory collapse changed with changes in intravascular volume – at higher volume inferior vena cava collapse decreased significantly.
Figure S4. Association between right atrial pressure and inferior vena cava with injected volume into the animal.
Video S1. Video shows the self‐expanding sensor is deployed in the inferior vena cava.
Video S2. Intravascular ultrasound of the inferior vena cava with the sensor deployed.
References
- 1. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 Suppl 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 2. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–33. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–73. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 5. Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. [DOI] [PubMed] [Google Scholar]
- 6. Fonarow GC, Corday E. Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry. Heart Fail Rev. 2004;9:179–85. [DOI] [PubMed] [Google Scholar]
- 7. Bradley SM, Levy WC, Veenstra DL. Cost‐consequences of ultrafiltration for acute heart failure: a decision model analysis. Circ Cardiovasc Qual Outcomes. 2009;2:566–73. [DOI] [PubMed] [Google Scholar]
- 8. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al.; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. [DOI] [PubMed] [Google Scholar]
- 9. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, et al. Haemodynamic‐guided management of heart failure (GUIDE‐HF): a randomised controlled trial. Lancet. 2021;398:991–1001. [DOI] [PubMed] [Google Scholar]
- 10. Baek SM, Makabali GG, Bryan‐Brown CW, Kusek JM, Shoemaker WC. Plasma expansion in surgical patients with high central venous pressure (CVP); the relationship of blood volume to hematocrit, CVP, pulmonary wedge pressure, and cardiorespiratory changes. Surgery. 1975;78:304–15. [PubMed] [Google Scholar]
- 11. Cohn JN. Central venous pressure as a guide to volume expansion. Ann Intern Med. 1967;66:1283–7. [DOI] [PubMed] [Google Scholar]
- 12. Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–48. [DOI] [PubMed] [Google Scholar]
- 13. Yamauchi H, Biuk‐Aghai EN, Yu M, Ho HC, Chapital AD, Koss W, et al. Circulating blood volume measurements correlate poorly with pulmonary artery catheter measurements. Hawaii Med J. 2008;67:8–11. [PubMed] [Google Scholar]
- 14. Katzarski KS, Nisell J, Randmaa I, Danielsson A, Freyschuss U, Bergstrom J. A critical evaluation of ultrasound measurement of inferior vena cava diameter in assessing dry weight in normotensive and hypertensive hemodialysis patients. Am J Kidney Dis. 1997;30:459–65. [DOI] [PubMed] [Google Scholar]
- 15. Moreno AH, Katz AI, Gold LD, Reddy RV. Mechanics of distension of dog veins and other very thin‐walled tubular structures. Circ Res. 1970;27:1069–80. [DOI] [PubMed] [Google Scholar]
- 16. Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29:352–60. [DOI] [PubMed] [Google Scholar]
- 17. Janssens U, Graf J. Volume status and central venous pressure. Anaesthesist. 2009;58:513–9. [DOI] [PubMed] [Google Scholar]
- 18. Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–8. [DOI] [PubMed] [Google Scholar]
- 19. Noble BJ, Drinkhill MJ, Myers DS, Hainsworth R. Reflex control of splanchnic blood volume in anaesthetized dogs. J Physiol. 1998;513:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinsky MR, Teboul JL. Assessment of indices of preload and volume responsiveness. Curr Opin Crit Care. 2005;11:235–9. [DOI] [PubMed] [Google Scholar]
- 21. Rex S, Brose S, Metzelder S, Huneke R, Schalte G, Autschbach R, et al. Prediction of fluid responsiveness in patients during cardiac surgery. Br J Anaesth. 2004;93:782–8. [DOI] [PubMed] [Google Scholar]
- 22. Shoukas AA, Sagawa K. Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ Res. 1973;33:22–33. [DOI] [PubMed] [Google Scholar]
- 23. Wiesenack C, Fiegl C, Keyser A, Laule S, Prasser C, Keyl C. Continuously assessed right ventricular end‐diastolic volume as a marker of cardiac preload and fluid responsiveness in mechanically ventilated cardiac surgical patients. Crit Care. 2005;9:R226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drees JA, Rothe CF. Reflex venoconstriction and capacity vessel pressure‐volume relationships in dogs. Circ Res. 1974;34:360–73. [DOI] [PubMed] [Google Scholar]
- 25. Rothe CF, Drees JA. Vascular capacitance and fluid shifts in dogs during prolonged hemorrhagic hypotension. Circ Res. 1976;38:347–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Haemodynamic and inferior vena cava parameters.
Figure S1. Change in inferior vena cava area versus diastolic pulmonary artery pressure in the nine experimental animals.
Figure S2. Change in inferior vena cava area versus pulmonary capillary wedge pressure in the nine experimental animals.
Figure S3. Inferior vena cava respiratory collapse changed with changes in intravascular volume – at higher volume inferior vena cava collapse decreased significantly.
Figure S4. Association between right atrial pressure and inferior vena cava with injected volume into the animal.
Video S1. Video shows the self‐expanding sensor is deployed in the inferior vena cava.
Video S2. Intravascular ultrasound of the inferior vena cava with the sensor deployed.


