Abstract
Environmental exposure plays a major role in the development of allergic diseases. The exposome can be classified into internal (e.g., aging, hormones, and metabolic processes), specific external (e.g., chemical pollutants or lifestyle factors), and general external (e.g., broader socioeconomic and psychological contexts) domains, all of which are interrelated. All the factors we are exposed to, from the moment of conception to death, are part of the external exposome. Several hundreds of thousands of new chemicals have been introduced in modern life without our having a full understanding of their toxic health effects and ways to mitigate these effects. Climate change, air pollution, microplastics, tobacco smoke, changes and loss of biodiversity, alterations in dietary habits, and the microbiome due to modernization, urbanization, and globalization constitute our surrounding environment and external exposome. Some of these factors disrupt the epithelial barriers of the skin and mucosal surfaces, and these disruptions have been linked in the last few decades to the increasing prevalence and severity of allergic and inflammatory diseases such as atopic dermatitis, food allergy, allergic rhinitis, chronic rhinosinusitis, eosinophilic esophagitis, and asthma. The epithelial barrier hypothesis provides a mechanistic explanation of how these factors can explain the rapid increase in allergic and autoimmune diseases. In this review, we discuss factors affecting the planet’s health in the context of the ‘epithelial barrier hypothesis,’ including climate change, pollution, changes and loss of biodiversity, and emphasize the changes in the external exposome in the last few decades and their effects on allergic diseases. In addition, the roles of increased dietary fatty acid consumption and environmental substances (detergents, airborne pollen, ozone, microplastics, nanoparticles, and tobacco) affecting epithelial barriers are discussed. Considering the emerging data from recent studies, we suggest stringent governmental regulations, global policy adjustments, patient education, and the establishment of individualized control measures to mitigate environmental threats and decrease allergic disease.
Keywords: air pollution, climate change, epithelial barrier, exposome, nutrition
1. INTRODUCTION
Research over the years shows growing evidence that environmental factors play an increasingly dominant role in human health. 1 , 2 As the genetics of allergic diseases have not thoroughly explained the considerable increases observed in the past decades, gene‐environment interaction studies, as well as Mendelian randomization techniques, underline the basis of environmental triggers for allergic diseases, making them primarily considered to be environmental diseases. 3 The exposome encompasses all environmental exposures such as chemicals, pollutants, tobacco smoke as well as lifestyle factors, dietary habits, and infectious agents that a person encounters throughout their lifetime, from conception to death. 4 , 5 , 6 The concept was first introduced by Wild in 2005 to highlight the impact of the entire environment on human health by complementing the genome. 7 Wild classified the individual's contact with external environmental factors as the eco‐exposome and the internal effects that occur after interaction with the exposome as the endo‐exposome. 8 Afterward, three overlapping domains have been defined as general external environment (climate, biodiversity, urban environment, and socioeconomic factors); specific external environment (allergens, microbes, diet, tobacco, and pollutants); and host‐dependent internal environment (metabolic factors, inflammation, and oxidative stress). 2 , 4 , 9 Further, as a novel but a potentially broader explanatory approach, Renz et al. put forth the concept of the meta‐exposome, which takes into consideration the bidirectional effect of the environment on human subjects and influence of humans on all other living systems and their genomes. 10 Species diversity in the natural environment in which humans live is of great importance in enriching the human microbiome, ensuring immune balance, and for preventing the development of allergic and inflammatory diseases. 11 The recently introduced ‘epithelial barrier hypothesis’ proposes that exposure to the urban environment and significant changes in the urban exposome by modernization, industrialization, and urbanization damages and initiates inflammation of the epithelium, the cellular layer that covers the surface of the skin, as well as the respiratory, urogenital, and gastrointestinal tract. 12 The epithelial barrier concept is initially introduced by assigning the epithelial tasks of keeping away the noxious environmental insults, such as the epithelial barrier, secretory IgA, and lamina reticularis thickening. The second set of events consist of washing away the inflammation by draining from tissues toward the lumen, ciliary movement, mucus production, and the immune regulatory function exerted by various regulatory cells, their cytokines, and cell surface factors. 13 Activation of epithelial cells and release of epithelial cell cytokines, such as IL‐25, IL‐33, and thymic stromal lymphopoietin (TSLP), followed by type 2 inflammation play major roles in the development and exacerbation of allergic diseases. 14 Local epithelial damage to the skin and mucosal barriers lead to type 2 inflammation in the tissues and development of allergic conditions presenting as atopic dermatitis (AD), asthma, allergic rhinitis (AR), chronic rhinosinusitis (CRS), and eosinophilic esophagitis. These diseases and the epithelial barrier damage always develop in association with changes in microbiome. 12 In the gut, leaky epithelial barriers and microbial imbalance may contribute to the onset or development of many chronic autoimmune and metabolic diseases, such as diabetes, obesity, rheumatoid arthritis, multiple sclerosis, or ankylosing spondylitis. 12 These diseases may be triggered or aggravated by distant inflammatory responses and changes in the gut or lung microbiome. Moreover, defective epithelial barriers have also been linked to neurodegenerative and psychiatric diseases such as Parkinson’s disease, Alzheimer’s disease, autism spectrum disorders, and chronic depression. 12 Environmental exposures can directly disrupt the epithelial barriers of the gut, skin, and respiratory tract and alter the structure of the microbiome. Thus, leaky epithelium and impaired immunoregulation in the affected organs influence the development of a chronic ongoing inflammation. 9 , 15 Furthermore, changes and loss of microbial biodiversity in urban environments secondary to delivery by cesarean section, early antibiotic exposures, reduced exposure to farm‐life, and lack of pets in the houses leading to low endotoxin exposures have been suggested to increase allergic diseases due to the development of microbial dysbiosis in early life. 16 , 17
The industrial revolution in the 19th century affected our planet leading to drastic changes in environmental homeostasis, which is defined as the healthy interrelationship of living organisms with their environment. Humans have been exposed to more than 200,000 new molecules since the industrial revolution, particularly during the last 60 years, without a clear understanding of their toxicity or means to mitigate their effects. The concepts of chronic exposure, molecular and microscopic changes, epigenetic changes, and their synergistic and additive affects were not considered for regulation of these new molecules. 18 , 19 , 20 Various emerging health threats including dramatic increases in air pollutants such as particulate matter (PM), diesel exhaust, nitrogen dioxide (NO2), ozone (O3), and tobacco smoke, the alarming effects of global warming, changes and loss of biodiversity, and the complex interactions between all these factors are affecting all living beings. 15 , 19 Recent studies have shown that climate change and global warming have many consequences on respiratory health by increasing airborne allergen concentrations (i.e., pollen, 21 , 22 fungi, 23 ) and allergenicity, 24 , 25 duration of pollination, and season length of airborne pollens. 26 , 27 Another important environmental insult that negatively affects health is the change in dietary habits due to increases in the consumption of dietary fatty acids and processed foods, usage of emulsifiers, and decrease of antioxidant content in western‐type diet, which is widely consumed (Figure 1).
FIGURE 1.
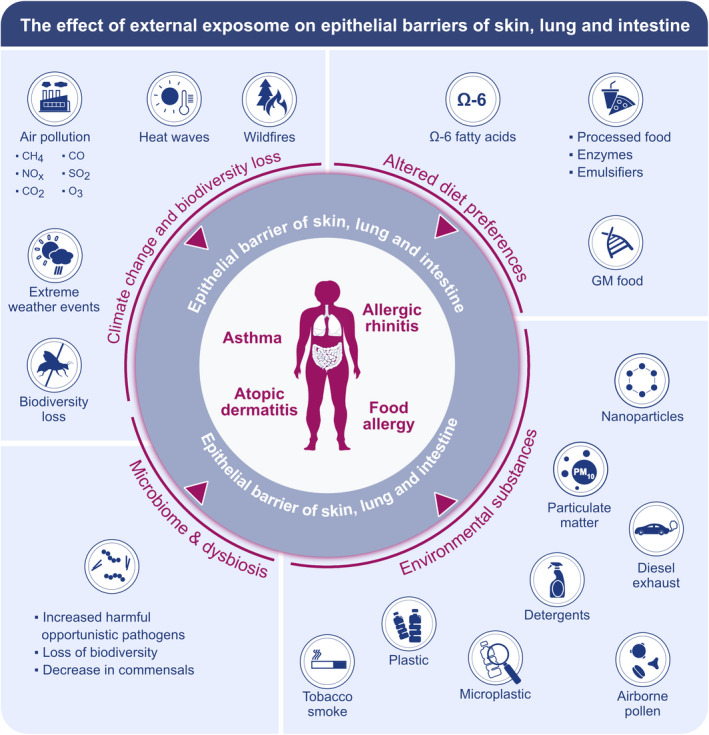
Effect of external exposome on epithelial barriers of skin, lung, and intestine. Extreme weather events, wild fires, global warming due to the climate change, air pollution and changes and loss of biodiversity; increased consumption of processed foods, n‐6 fatty acids and genetically modified food; exposure to environmental substances; and the increase in harmful opportunistic pathogens, loss of microbiome diversity and decrease in commensals; disrupts the barriers of skin, lung, and intestine and causes allergic diseases such as asthma, atopic dermatitis, food allergy, and allergic rhinitis. CH4: methane, NOx: nitric oxides, CO2: carbon dioxide, CO: carbon monoxide, SO2: sulfur dioxide, O3: ozone, GM: genetically modified
Here, we discuss the impacts of climate change and the exposome, the relationship between the microbiome of skin, gut, oropharynx, lung, and exposome; the effect of increased fatty acid consumption due to the changes in dietary habits; environmental agents (detergents, disinfectants, household cleaners, airborne pollen, PM, O3, microplastics, nanoparticles, and tobacco) that affect epithelial barriers and finally the epithelial barrier hypothesis. We review the changes in the external exposome within the last decades and their effects on epithelial barriers in relation to allergic diseases.
2. GLOBAL WARMING, CLIMATE CHANGE, AND EXPOSOME
Climate change refers to any change in climate and weather patterns altered for an extended time period. Some authorities (Framework Convention on Climate Change) define climate changes as that which attributable directly or indirectly to human activities which alter the composition of the global atmosphere, in natural climate variability observed over comparable time periods. 28 , 29 It is noteworthy that although it took nearly a century to convince the scientific authorities that human actions could alter the climate of the entire world, there is now consensus on the levels of carbon dioxide (CO2), methane (CH4), nitrous oxide (NO), and fluorinated gases that are increased as a result of industrialization, urbanization, and population growth and are accumulating in the atmosphere trapping heat leading to the greenhouse effect and anthropogenic climate change. 30 , 31 Anthropogenic global warming and climate change are considered the major growing threats to global biodiversity and ecosystems, leading to the extinction of thousands of species over the next century. 32
The greenhouse effect and air pollution together increase average temperatures around the world. 33 Air pollution is defined as a major driver for global warming. As a consequence of a warmer Earth, water temperatures of the oceans increase, glaciers melt, sea levels rise, and the snow and ice cover in the Northern Hemisphere diminish. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Ozone layer depletion and climate change also impact ultraviolet (UV) radiation. 42 , 43 , 44 Extreme weather events, such as heatwaves, droughts, floods, blizzards, thunderstorms, sandstorms, wildfires, and hurricanes, are happening more frequently and intensely due to climate change. 34 , 45 , 46 , 47 , 48 Urbanization is linked to the rising levels of pollutants in the air, as well as water and soil. These environmental changes are modifying spatial and transient dissemination of aeroallergens such as pollens and dust mites causing recurrence of respiratory allergic diseases over a long period of time in most industrialized countries as well as in developing countries. 19 , 33 , 49 , 50
It is well documented that climate change negatively affects many aspects of human health, both physically and mentally 51 , 52 (Figure 2). In the perspective of allergic disease, this phenomenon alters the timing, dispersion, quantity, and quality of aeroallergens classified as bio‐contaminants, leading to an increase in the frequency and severity of allergies. 9 Higher temperatures have been shown to prolong the pollen season, and higher CO2 levels lead to an increase in the biomass of pollen and pollen production, which makes the plants produce more pollen and allergens. Likewise, the overall pollen season of all pollen types has been indeed extended. Most pollen types have shifted toward earlier times of the year for pollen outputs (i.e., ragweed), possibly aggravating the burden on pollen‐allergic patients. 21 , 53 Air pollutants, especially NO2, which is more prevalent in urban locations, also collaborate with airborne allergens and alter the biological functions of pollens by decreasing viability, altering the physicochemical characteristics of the pollen surface, and increasing their allergenic potential through pro‐inflammatory properties by acting as an adjuvant, and therefore pose a greater risk for the development of atopic sensitization and symptoms in sensitized individuals. 24 , 54 Elevated pollutants have been shown to change the transcriptome of the ragweed pollen. 54 , 55 Proteases derived from pollens irreversibly damage the airway epithelial barriers by disrupting intercellular junctions and anchorage of respiratory epithelial cells 56 (Figure 3).
FIGURE 2.
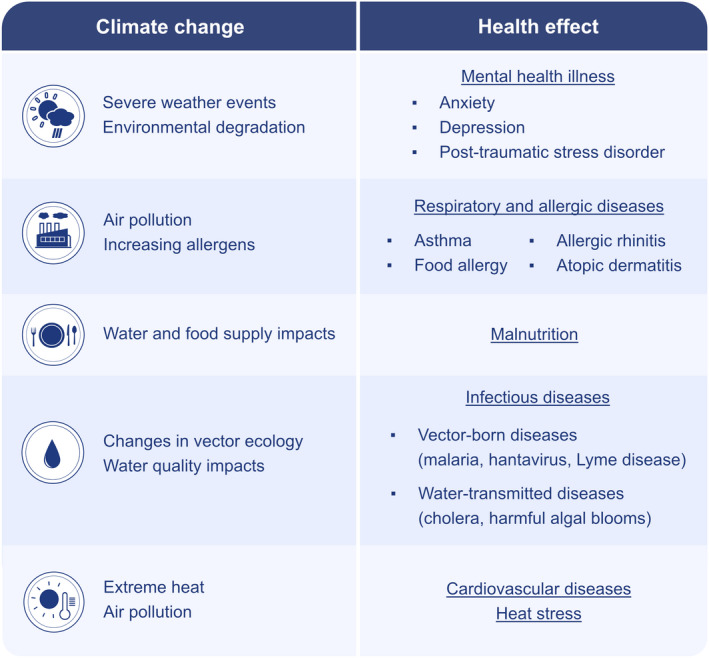
Health effects of climate change. Climate change causes mental health illness such as anxiety, depression, and post‐traumatic stress; causes respiratory and allergic diseases through air pollution and increased allergens; causes malnutrition through affecting water and food supplies; causes infectious diseases such as vector‐borne (malaria, hantavirus, lyme disease) and water transmitted diseases (cholera, harmful algal blooms); causes cardiovascular diseases, and heat stress due to extreme heat and air pollution
FIGURE 3.
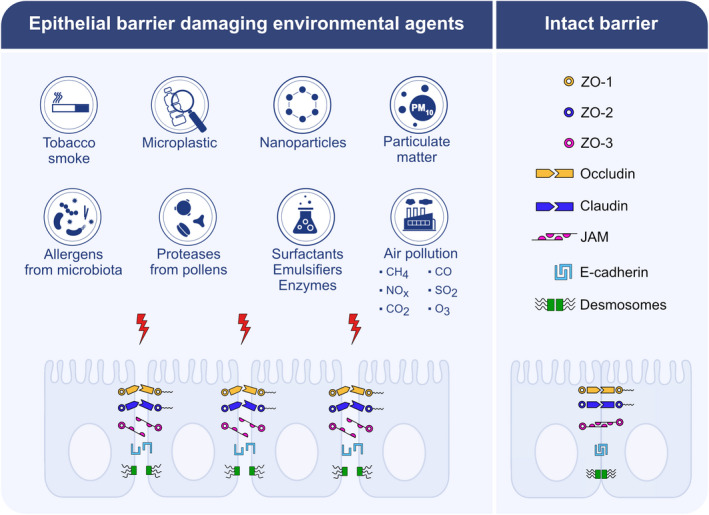
Epithelial barrier damaging agents from the environment. Allergens derived from bacteria, virus, and fungus; protease activity of allergens; surfactant, emulsifiers, and enzymes used as food additives; cigarette smoke, nanoparticles, particulate matter, and pollutant gases including nitric oxides, sulfur dioxide, carbon monoxide, carbondioxide, methane, ozone; microplastics irreversibly damage epithelial barriers by disrupting intercellular connections and anchoring of epithelial cells. Zonula occludens 1–3, occludin, claudins, junctional adhesion molecules, E‐cadherin and desmosomes are depicted as damaged epithelial molecules. CH4: methane, NOx: nitric oxides, CO2: carbondioxide, CO: carbon monoxide, SO2: sulfur dioxide, O3: ozone, ZO: Zonula occludens, JAM: junctional adhesion molecules
It is even claimed that climate change may have played a pivotal role in the emergence of COVID‐19 by forcing species to change their habitats and their geographic range, and serving as a tool to bring wild animals closer to humans and farm animals. 57 It was mentioned that SARS‐CoV‐2 cell entry factor (SCEF) is important for the entrance of COVID‐19 virus to the upper airway epithelium, while in smokers it was also shown that SARS‐CoV‐2 can easily penetrate both the upper and lower airways epithelium. 58 Moreover, a significant positive correlation between COVID‐19 infection and airborne pollen concentration was reported by Damialis et al. The authors have mentioned that the interaction of the coronavirus and pandemic viruses with similar potential in the future may be exacerbated by the increased abundances of airborne pollen, because pollen exposure weakens antiviral immune response. 59 The association between climate change and infectious disease is well established, but burden of the effects and affected pathogens remain under‐studied. In 2017, McIntyre et al. published the first large‐scale systematic assessment of climate effects on pathogens, concluding that zoonotic pathogens may be more climate sensitive as 75% of emerging diseases are zoonotic. 60
There is scientific evidence suggesting that it is crucial to take timely action against air pollution and greenhouse gas production to control urbanization‐induced climate change and biodiversity loss and change, which will contribute to the higher burden of allergic diseases in the near future. 61 Healthcare facilities as well as healthcare professionals should play significant roles as practitioners, and role models against this threat. 52 Healthcare professionals should now take leadership and responsibility to guide policy decision makers for bringing solutions to lessen the harm to our exposome in an evidence‐based manner. Any attempt to reduce PM and CO2 emission would address both air pollution and climate change, which can be achieved by strict policy decisions to obtain long‐lasting health effects. Moreover, appropriate controls for reducing greenhouse gases and air pollution may also diminish the negative health aspects of changing bioaeresols (i.e., pollens, fungi). In addition, artificial plantation and transportation of new species may also increase the negative health risks associated with bioaeresols. This issue can be discussed in the future larger reports. It is also crucial to prioritize the surveillance for pathogens that may respond to climate change and contribute to strengthening climate change resilience for infectious diseases in order to act against new epidemics such as Zika virus in South America or even the COVID‐19 pandemic.
3. EFFECTS OF CLIMATE CHANGE ON ASTHMA AND ALLERGIC RHINITIS
Climate change poses a significant threat to respiratory health by directly generating or exacerbating pre‐existing respiratory disorders. It is vital to highlight that the prevalence of asthma has risen in recent decades and is expected to rise further. 62 Besides, aeroallergens play important roles in the pathophysiology of AR, and their distribution varies by geographical regions depending on the type of climate. Therefore, it can be assumed that the impact brought about by global warming will affect the distribution of aeroallergens and pollen mass, thus, these may effect the prevalence of asthma and AR. 63 , 64 A recent study in Georgia, USA, showed that the concentration of several tree pollen taxa increased over the last 27 years, and multiple species started to release their pollens earlier. The authors have concluded that early pollen discharge of some species could be associated with warmer temperatures. 65 In a retrospective analysis of datasets lasting 20 years or longer from 17 locations in the northern hemisphere showed that increases in daily minimum and maximum temperatures over time were associated with increases in both pollen load and duration of pollen season. 66 In another study, an approximately twenty days earlier start date in pollen season, lengthening of the pollen season by about eight days over the same period and an increase of pollen concentration by twenty‐one percent across North America were found and those findings were associated with increased temperatures. 67 However, it was also reported that a significant increase in the annual amount of airborne pollen for many taxa in urban areas in Europe was not associated with temperature increase. 68
Another effect of climate change can be observed on molds. In contrast to the strong relationship between global warming and increased pollen counts, fungal spores have been shown to decrease with increased temperatures. 23 On the other hand, it is envisioned that climate change may increase the amount of indoor and outdoor molds by increasing humidity in the buildings due to increasing floods and heavy rains. Although, there are not enough studies to validate these associations, in 2005, after the aftermath of hurricanes Katrina and Rita in New Orleans, the levels of mold spores were detected extremely high in the water‐damaged houses. Endotoxins and fungal glucans of predominantly Aspergillus niger, Penicillium spp., Trichoderma, and Paecilomyces. were also high in the environment and were found to be associated with health effects. 69 In another study on fungal exposure of workers participating inpost‐hurricane renovation in the same area, the workers were exposed to increased levels of fungi, but their levels dropped significantly in the first year after hurricanes. Therefore, although the burden of molds increases due to climatic reasons, there are no data showing that this change is permanent. 70 In addition, a study from the San Francisco Bay Area in the USA used time series regression models and noted season length for the most frequent outdoor molds has increased over the last two decades. Finally, the authors suggested that mold spore and pollen activity are connected to variations in observed climate change factors. 71
Increased urbanization and global warming have created a warmer and more humid environment, which is ideal for the growth of house dust mites (HDM). 72 Sensitization to HDM has been most prominent among urbanized Asian regions due to their fast industrialization. 73 A retrospective study from China evaluated a total of 5,486 patients over a 10 years period and demonstrated that HDMs comprised the most common aeroallergen in Guangzhou, which is a rapidly industrializing region. 74
Unfortunately, the concerning outcomes of industrialization, global warming, and climate change are not limited to variations seen in aeroallergens. Air pollution and climate change are inextricably linked. Fossil fuels, the primary source of CO2 emissions, are also major air pollutant that contribute to climate change. A case‐crossover study from Belgium reported that air pollutants cause more severe AR. 75 The results of this study are consistent with the international expert consensus of the World Allergy Organization published in 2020, which in brief, states that pollutants are linked to inflammation and exacerbate allergic airway diseases. 76 The notorious invasive common ragweed (Ambrosia spp), which is a highly allergic species is anticipated to become more widespread and allergenic due to the increase in atmospheric CO2 especially in the Northern Hemisphere. 66 , 77 , 78 Khreis et al. conducted a study across 18 European countries including 63,442,419 children and reported that nearly one‐third of all childhood asthma cases might be linked to exposure to air pollution. Based on their findings, the authors hypothesized that adherence to the recommendations of World Health Organization Air Quality Guidelines could prevent up to 11% of childhood asthma cases each year. 79 , 80 A prospective birth cohort study from the Netherlands analyzed data of 3,687 participants and found a link between the incidence of asthma and exposure to air pollutants from birth. 81 In addition to these recent studies, many older ones establish a connection between exposure to air pollution in early life and developing asthma. 78 , 82 , 83 , 84 , 85 , 86 , 87 , 88
Common limitations mentioned in these studies include the heterogeneity and complexity of asthma, the existence of confounders such as smoking, parental atopy, breastfeeding, and challenges in diagnosing asthma, especially in children. However, over the past decades, some natural disasters like thunderstorms, dust storms, and wildfire smokes have created an opportunity to observe patients with already diagnosed asthma, free from some of these confounding factors during a climate change‐driven event 89 (Figure 4). Thunderstorm‐related asthma epidemics are good demonstrative in vivo models for the impact of heavy rain on pollen's capacity to trigger asthma symptoms whether the patient had symptoms or not in the past. Many studies have shown that thunderstorms increase asthma exacerbations and therefore the number of hospital admissions due to the increased airborne pollen grains and fungal spores. 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 Current hypothesis indicates a mechanical effect with thunderstorms with heavy rain, wind, lightnings, or both, fragmenting pollens into smaller allergenic particles. Sub‐pollen particles ‘attract’ humidity and create droplets, and then descend to the ground drops of water containing potentially allergenic small particles quickly hit the ground and are sprayed into the air, contributing to the creation of bioaerosols. These tiny particles can then easily penetrate deeper into the airways to trigger asthma attacks. 9 , 99 , 100 A study supporting this hypothesis demonstrated that increasing pollen fragment concentrations were associated with thunderstorms, strong downdrafts, and high rates of rainfall and that pollen fragments persisted in the atmosphere for several hours after the storms. 101 The most recent and probably the most catastrophic thunderstorm asthma epidemic struck Melbourne, Australia, in 2016. There was more than a sixfold increase in respiratory‐related presentations to public hospitals in 30 h. A study that evaluated the risk factors for hospital admissions found higher odds ratio among patients with known asthma. 97 Aside from thunderstorms, inhalation of fine particles in smoke during wildfires can induce lung irritation. An analysis in California, USA, showed that the October 2017 wildfires were responsible for over 300 asthma and cardiovascular‐related hospital admissions. 102 In addition, a recent meta‐analysis reported that exposure to fire smoke in both children and adults increased hospital admissions and emergency room visits due to asthma attacks, with a higher incidence in adults. 103 , 104 Air‐liquid interface cultures of bronchial epithelial cells demonstrated that wildfire smoke induces epithelial barrier dysfunction by disrupting tight junction (TJ) proteins, increasing paracellular permeability. 105 Particles found in dust storms are larger than those in wildfires, but they can still induce strong inflammatory responses. 106 A study conducted in Crete, Greece, concluded that extreme desert dust storms increase hospital visits for respiratory symptoms 107 (Figures 3 and 4, Table 1).
FIGURE 4.
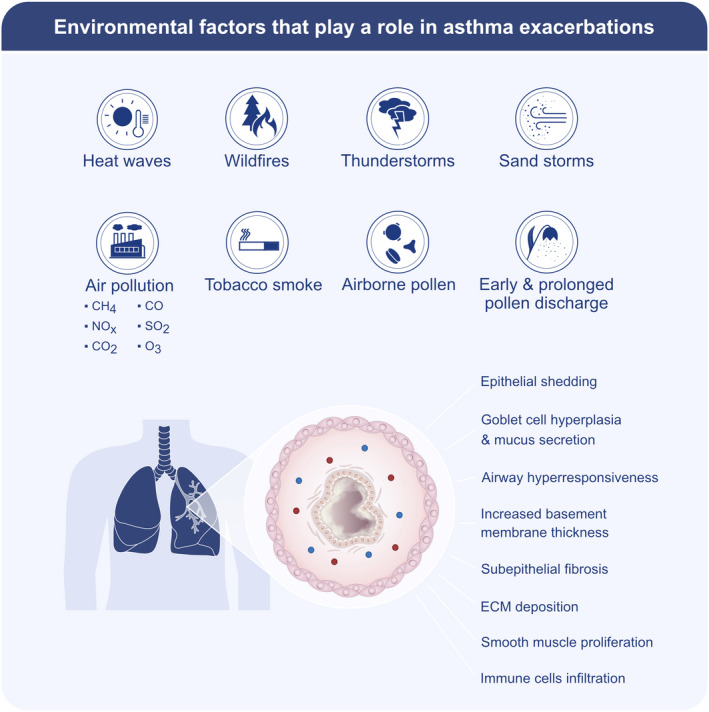
Environmental factors that play a role in asthma exacerbations. Air pollution with gases (NOx, SO2, O3, CO2, CO, CH4) and particulate pollutants (PM2.5 and PM10) emitted from industrial smog and wildfire smog, environmental tobacco smoke, heat waves, sandstorms, and airborne pollen cause asthma exacerbations. Moreover, extreme heat causes early and prolonged pollen discharge, and thunderstorms cause bioaerosols containing potentially allergenic small particles due to the rapid hit of water droplets to the ground. All of these factors may have a direct or indirect effect on epithelial shedding, goblet cell hyperplasia, airway hyperresponsiveness, increased basement thickness, subepithelial fibrosis, extracellular matrix (ECM) deposition, smooth muscle proliferation, and immune cell infiltration in the airways and exacerbate asthma
TABLE 1.
Climate change and environmental exposures associated with allergic diseases
| Allergic disease | Exposure | The effects on disease | Ref. |
|---|---|---|---|
| Asthma and Allergic rhinitis | Global warming |
Increase in the prevalence of asthma and AR Pollen concentrations increase as temperature rise Pollen season length increased Fungal spores decrease as temperature rise With more humid and warmer environments HDM allergy increased AR prevalence increased |
[ 63 ] [ 23 ] |
| Floods | More severe asthma due to mold proliferation | 63, 69 | |
| Rising CO2 levels | Ragweed pollens elicit a stronger allergic lung inflammation and becoming more widespread | [ 55, 77, 78 ] | |
| Air pollutants |
1/3 of childhood asthma cases may be linked to air pollution Increase incidence of asthma in children and young adults Expose to pollution in early life causes asthma development Causing AR to be more severe Cause Ragweed more common and allergenic |
[ 81 ] |
|
|
Thunderstorms |
Trigger asthma exacerbation Increased hospital admissions |
||
|
Wildfires |
Exacerbate asthma Induce epithelial barrier dysfunction |
[ 105 ] |
|
| Dust storms |
Strongly induce inflammatory response Increase respiratory symptoms |
[ 106 ] [ 107 ] |
|
| Food allergy | Rising CO2 levels | Rising peanut and tree nut allergies | [ 110, 111, 112 ] |
| Atopic dermatitis | Floods | Flare‐up of childhood AD | [ 113 ] |
| Air pollutants | Increase in the severity and development of AD | [ 114, 117 ] |
4. CLIMATE CHANGE AND FOOD ALLERGY
The evidence for the relationship between global climate change and an increase in food allergies (FA), such as peanut allergy, is weaker in comparison with other allergic disorders. Peanut and tree nut allergies appear to be on the rise. 108 , 109 , 110 It is still uncertain whether this increase could be attributed to the increases in atmospheric CO2 concentration and temperature. A few studies have investigated the impact of elevated CO2 concentration on peanuts and demonstrated that they are responsive to these factors and suggest that their allergenic characteristics could also be influenced. 110 A study published the first evidence that increased CO2 concentrations can result in a rise in the concentration of the major peanut allergen (Ara h 1). 111 A recent study examined the changes in allergic diseases in asthmatic children over a 25 years period in France and determined that FA with tree pollen sensitization increased. 112 Further research is needed to explore the relationship between climate change and other common causes of food allergies such as egg, shellfish, soy, and cow’s milk (Table 1).
5. CLIMATE CHANGE AND ATOPIC DERMATITIS
Compared to other allergic diseases, it is seen that different consequences of climate change, such as UV lights, cold and dry weather conditions, and floods, have more influence on AD. Global warming is thought to be causing floods by melting polar ice caps, rising sea levels, and heavier rains. Floods have been shown to have an impact on childhood AD flare‐ups, according to a retrospective study conducted in Taiwan. 113 The study hypothesized that increased levels of molds in the indoor environment and prolonged exposure to contaminated water could trigger an AD flare‐up in sensitized children. In an observational study, 60 patients with AD were followed for 18 months and high levels of air pollutants such as PM10, NO2, and O3, as well as an increased pollen counts were found to exacerbate AD symptoms. 114 However, a limitation of the study was a lack of a report of other confounding factors such as UV lights. A recently published retrospective study suggested that UV exposure is beneficial in most patients with AD lesions. 115 This finding is consistent with the literature that supports phototherapy with UV light for AD to reduce inflammatory response in the skin. 116 Although global climate change could allow more harmful UV‐B to reach the Earth’s surface by decreasing stratospheric O3, combined with industrialization and urbanization, it may also decrease UV light penetration by increasing cloud cover, dust, smoke from wildfires, and other airborne particles. A comprehensive study examined the link between air pollution and allergic diseases and found that exposure to oxidants such as O3 and NO2 at birth increased the risk of developing asthma by 17% and eczema by 7% 117 (Table 1).
6. THE EXTERNAL EXPOSOME AND THE MICROBIOME OF SKIN, GUT, OROPHARYNX, AND LUNG
The contribution of the microbiomes of the skin, gastrointestinal and respiratory tracts to health and disease is well established. Studies on allergic diseases and microbiota and external exposome (Cesarean section, feeding with formula, use of prebiotics or probiotics, diets high in fat and low in fiber content, early antibiotic usage in infancy, etc.) have identified that human microbiota has a central role in the regulation of this process. 118 Due to the drastic changes in modern environments, hygiene, and lifestyles, the variance of content of gut and skin microbiota may contribute to the development of various chronic inflammatory diseases including asthma, and other allergic diseases, and may trigger autoimmunity. 9 , 119
Human DNA is assumed to represent only a small percentage of all DNA in the human body. A much higher percentage of the genetic contribution is made by the so‐called human microbiome, which consists mostly of bacteria, fungi, viruses, archaea, and other microorganisms. 120 Host‐microbiota interactions are essential for the evolution of the immune system. While the immune system eliminates the pathogens, it also tolerates the beneficial microbiota that maintains a symbiotic life with the host. 119 The effect of microbiota on immunity‐related diseases is known not only on naturally colonized microorganisms at the barrier sites, such as the gut, skin, lung, and other mucosal surfaces, but also on non‐barrier organs such as liver, kidneys, joints, lungs, eyes, and brain. 119
Microbial dysbiosis has always been reported in areas of epithelial barrier dysfunction, such as gut, esophagus, lung, and sinus mucosa. 12 , 121 , 122 , 123 , 124 , 125 , 126 , 127 One of the main events following epithelial barrier damage is the colonization of opportunistic pathogens, such as Staphylococcus aureus (S.aureus), Moraxella, Hemophilus, and Pneumococcus. S.aureus has become the dominating bacterial species in AD skin lesions and chronic rhinosinusitis. 128 , 129 , 130 , 131 , 132 The percentage of healthy carriers of S. aureus increased to 35% from 4% within the last four decades. 133 These percentages increase to more than 90% in CRS and AD. 134 In the barrier‐damaged areas were opportunistic pathogens start to dominate, relatively non‐inflammatory commensals start to decrease in abundance as well as microbial biodiversity.
Healthy skin consists of different microorganism communities depending on the sampling area. Propionibacterium species are dominant in sebaceous sites, whereas Corynebacterium and Staphylococcus species are found in humid areas. 135 , 136 Changes in these healthy microbiomes may result in allergic sensitizations. 118 The skin harbors many regions with different bacterial communities; therefore, it may also affect local and systemic immune responses. 119 At birth, regulatory T (Treg) cells dominate the skin barrier, and it is necessary to be exposed to commensals in order to develop tolerance against these microorganisms. 137 The importance of interaction with cutaneous microbiota in early life has been proven by observational studies on cutaneous dysbiosis. 119 In the first years of life, early exposure to protective commensals such as Staphylococcus epidermidis prepares tolerogenic Treg cells and contributes to the development of commensal‐specific skin‐resident memory cells and effector T cells that support the innate microbial defense. 135 , 136
Commensals colonizing the oral cavity, a mucosal area of the gastrointestinal system, are well known to contribute to oral health/hygiene and inflammation. 138 Immediately within a few hours of birth, infants undergo rapid colonization of microbiota. Initially, the gut microbiome is generally composed of Escherichia coli and Enterococcus species, followed by anaerobes including Bifidobacteria, Bacterioides, and Clostridium spp. that become predominant in line with decreasing oxygen concentrations in the gut. 139 Clostridium species are more dominant than Bifidobacterium species in cesarean‐born babies, whereas it is vice versa in babies born vaginally. 140 Breast‐fed babies generally have less diversity in the gut in the first few weeks of life and are usually colonized by Bifidobacterium species. 118
In the lungs, the pulmonary blood‐air barrier and colonizing microbiota also play a role in immune‐related diseases. It has been shown that T helper 2 (Th2) cytokine release is decreased as the pulmonary bacterial load increases in newborn mice. 141 Moreover, in the lungs of germ‐free mice, invariant natural killer T (iNKT) cell levels, producing IL‐4 and IL‐13, were also found to increase in response to ovalbumin, suggesting a decisive role in the presence and absence of commensal microbiota. 142 Gut‐lung axis enables the gut microbiome to influence the lungs and protects the host from asthma by shifting the Th2, Treg (Th2‐Treg) balance toward Tregs. 119 Parasitic gut infection is another potential mediator for gut‐lung axis via the altered intestinal microbiota and the induction of pulmonary Tregs. This has been suggested partially to explain the high atopy rates in developed countries with low helminthic exposure. 119 However, for example, in New York, USA, since water sanitation started in 1910, parasite burden significantly decreased, whereas asthma started to increase after the 1960s. 143
Exposure to different microorganisms (bacteria, mold, virus, protozoan, and helminths) may induce epigenetic changes that affect the immune system modulation and result in the development of inflammatory diseases. During the maternal and postnatal periods known as the ‘window of opportunity’, maternal infections, microbiota, diet, drugs, and environmental exposures such as pollutants have a profound importance for the modulation of the immune system. 144 A recently studied model proposed that environmental exposure during pregnancy may remodel the maternal microbiome and immune functions and thus also affect fetal immunity and microbiome development. 145 These effects educate the innate immunity of the newborn and regulate the response‐ability to those microorganisms that pass through maternal vertical transmission and colonize in the body habitats. Depending on the content and functional features, these preliminary microorganisms remodel the composition and accumulation rate of exogenous microorganisms in the first year of life. 146 Antibiotics are among environmental factors that affect the human microbiome and are well known to alter the incidence and severity of autoimmune and allergic diseases. 147 Diets with different fiber, tryptophan, and fatty acid contents may modulate immune‐related diseases through various mechanisms. 119 Furthermore, chemical and physical environmental factors can alter the host‐microbiota interactions. For example, sun exposure has been shown to alter antimicrobial peptides via UV radiation because UV can kill microbes and modulate the skin microbiota. 148
7. MICROBIOME AND ALLERGIC DISEASES
Studies have shown that human health is closely associated with the balance of the common microbial community, the so‐called halobiont homeostasis. Microbial biodiversity and the interactions among various microorganisms have functional outcomes. The change and loss of biodiversity lead to a more unstable and less resistant microbiota, often dominated by one or few microorganisms; this is a phenomenon known as dysbiosis, which can alter the immune balance maintained by the gut, skin, and respiratory microbiomes and cause diseases. 144 , 149 Biodiversity hypothesis states that the increase in allergic diseases may be due to bacterial dysbiosis and decreased biodiversity of commensals. 150 The healthy microbiota on the mucosal surface regulates various aspects of barrier homeostasis such as barrier permeability modulation, TJ expression, angiogenesis, vascular permeability, local micro‐inflammation, and mucosal tolerance (Table 2). On the other hand, dysbiosis together with epithelial barrier leakiness damages immune homeostasis at the affected tissue. 144 In a healthy situation, the microbiome stays above the epithelium to live together, with a homeostatic interaction driven from co‐evolution, however, when the epithelial barrier becomes leaky, dysbiotic commensals and opportunistic pathogens migrate in between the affected epithelial cells and translocate beneath the epithelium. This is easily visible in the CRS epithelium by light microscopy and clearly takes place in the affected gut epithelium in colitis. 151 , 152 , 153 , 154 Therefore, decreased diversity and the changes in the gut and skin microbiota contents are related to several chronic inflammatory diseases, including asthma, AR, AD, and FA. 12 , 17 , 155
TABLE 2.
Microbiome and allergic diseases
| Allergic disease | Current concepts | Ref. |
|---|---|---|
| Asthma | Reduced risk with perinatal and/or early‐life microbial/allergen exposure | [ 161, 162 ] |
| Reduced with endotoxin exposure in childhood | [ 164 ] | |
| Higher abundance of certain gut bacteria was shown in asthmatic subjects | [ 165, 166 ] | |
| Allergic rhinitis | Alteration in normal nasal mucosal bacterial abundance and diversity was shown | [ 169, 170 ] |
| Reduced risk with early‐life exposure to environmental microbiota | [ 155, 171 ] | |
| Atopic dermatitis | Altered abundance and diversity of skin microbiota compared to healthy skin | [ 131, 175, 176, 180 ] |
| Early‐life skin colonization of certain bacteria in AD | [ 182 ] | |
| Increased risk with dysregulated gut‐skin axis | [ 176, 183, 184 ] | |
| Filaggrin mutation can initiate AD | [ 177, 178, 179 ] | |
| Food allergy | Increased risk with dysbiosis in gut environment | [ 189, 191 ] |
| Increased risk with lower gut microbiota diversity at early infancy | [ 190 ] | |
| Reduced risk maternal Mediterranean diet during lactation and gestation | [ 193 ] | |
| Reduced risk with diet consisting of high levels of fruits and vegetables during infancy | [ 194 ] | |
| Increased risk with high‐sugar, high‐fat, low short‐chain fatty acid diets | [ 195 ] |
7.1. Asthma
Asthma is a complex disease and involves several risk factors. Evidence of risk factors in early life that can alter the development of lung immunity associated with dysbiosis, which leads to asthma, was extensively reviewed by Cerata et al. 156 These factors are delivery by cesarean section, usage of antibiotics during the neonatal period, maternal diet, breastfeeding, early‐life allergen exposure, pollution, external microbes, and host microbiome. 17 , 157 , 158 , 159 Some of these risk factors interact with each other to contribute to the pathogenesis of asthma, and some of these interactions are mediated through the microbiome and epithelial barriers. 144 , 160 Perinatal and/or early‐life microbial exposure affects physiological development, and exposure to farming environments, environments with high microbial or allergen loads at this age is associated with a reduced risk of asthma and other allergic diseases in children. 161 , 162 Regular contact with farm animals increases indoor home endotoxin concentrations, which might explain the protective effect of contact with farm animals on atopic outcomes, and it is hypothesized that the farm environment can provide immunomodulatory stimuli. 163 Endotoxin was shown to be a protective factor for asthma in older children. 164 Additionally, peak exposure to specific allergens, bacteria, and certain environmental microbiota, especially in the first year of life, reduces the likelihood of having recurrent wheeze and allergic sensitization. 155 Host microbiota has been primarily linked to asthma pathogenesis via gut microbial metabolites. Having a higher abundance of certain gut bacteria like Faecalibacterium, Lachnospira, Rothia, Bifidobacterium, or Akkermansia, especially during the first month of life, has been associated with protection against allergic sensitization and allergic asthma. 165 , 166 Additionally, there is growing evidence for the role of the gut and lung axis in the development of chronic lung diseases. 167 It is also thought that the composition and function of the upper respiratory tract microbiome may influence the pathogenesis of asthma. 168 It is clear that many external and host‐related factors influence the dynamic nature of the relationship between the host microbiome and asthma (Table 3).
TABLE 3.
Does microbial dysbiosis or epithelial barrier disruption proceeds the development of allergic diseases?
| Allergic disease | Evidence for microbial dysbiosis starts first | Evidence for barrier disruption starts first | Ref. |
|---|---|---|---|
| Asthma | Reduced risk of asthma with perinatal and/or early‐life microbial/allergen exposure |
Increased risk of asthma with epithelial barrier disruption due to exposure to cleaning products |
|
|
Reduced risk of asthma with increased prevalence of early‐life Faecalibacterium, Lachnospira, Rothia, Bifidobacterium, or Akkermansia |
AD patients with epithelial barrier disruption secondary to filaggrin mutation conferred an overall asthma risk | ||
| Reduced risk of asthma with endotoxin exposure in childhood | |||
| Higher abundance of certain gut bacteria was shown in asthmatic subjects | |||
| Allergic rhinitis/ CRS | Alteration in normal nasal mucosal bacterial abundance and diversity was shown | Dysregulation of TJs observed both in biopsy specimens and epithelial cultures in the absence of any inflammatory stimulus | |
| Reduced risk of AR with early‐life exposure to environmental microbiota | |||
| Increased prevalence of S.aureus, Propionibacterium, Corynebacterium, Peptoniphilus and decreased prevalence of Prevotella and Streptococcus in AR | |||
| Atopic dermatitis | Altered abundance and diversity of skin microbiota compared to healthy skin | Genetic mutations in the epidermal barrier‐related genes | |
| Early‐life skin colonization of certain bacteria in AD | Reduced expression of Claudin−1 in AD might enhance the penetration of altered microbial flora | ||
| Increased early‐life prevalence of S. aureus and decreased commensal microbes, eg. S. epidermidis in infants with AD | While the correlations do not imply a causative relation, S aureus negatively correlated with TJ genes only in the lesional skin. Further studies needed | ||
| Gut bacterial dysbiosis has effect on the skin immune system | |||
| Food allergy | Increased risk of FA with dysbiosis in gut environment | Barrier defect secondary to filaggrin mutation is thought to facilitate peanut allergy | |
| Increased early‐life prevalence of Clostridium species and decreased Bifidobacterium and Lactobacillus species in FA | |||
| Increased prevalence of Clostridium species over Bifidobacterium species in infants born by cesarean section | |||
| Presence of Prevotella in maternal stool is associated with a decreased risk of their infant developing FA | |||
| Reduced risk of FA with diet consisting of high levels of fruits and vegetables during infancy | [ 194 ] a , b | ||
| Increased risk of FA with high‐sugar, high‐fat, low short‐chain fatty acid diets | [ 195 ] a , b | ||
References for the first column.
References for the second column.
7.2. Allergic rhinitis
Although there is still a limited link in the relationship between nasal microbiome dysbiosis and the development of AR, the nasal microbiome potentially holds an important role in the modulation of localized immune responses. In the normal nasal mucosa S. aureus, Propionibacterium, Prevotella, Corynebacterium, Bacteroides, and Streptococcus are common. However, in AR, the abundance of S. aureus, Propionibacterium, Corynebacterium, and Peptoniphilus is considerably increased, whereas the numbers of Prevotella and Streptococcus are decreased. 169 It was shown that in patients with seasonal AR, during the season, the variety of organisms in the middle meatus had significantly increased, and there was a correlation between bacterial diversity and nasal lavage eosinophil counts. 170 In a study, 180 children, aged between 7 and 11 years, from Finnish and Russian Karelia (both have similar climates, the former is a more modernized area and latter is a rural environment) were followed up for 10 years, and atopic sensitization and allergic diseases were found to be up to 10‐fold higher in Finnish Karelia. Bacterial and fungal populations in the nasal mucosa were more abundant and diverse in Russian participants than Finnish peers, and it was stated that early‐life exposure to environmental microbiota might be biologically related to allergic manifestations at a younger age (Table 3). 171
7.3. Atopic dermatitis
According to current knowledge, the pathogenesis of AD is defined by the interplay between genetic background and epithelial barrier defects, epigenetic changes, immunologic factors, dysbiosis in the skin and gut microbiota, and external risk factors. 172 , 173 It has been demonstrated several times that the composition and diversity of microorganisms on the skin differ between people with eczema and those healthy ones. 131 , 174 , 175 , 176 It is a major question whether barrier disruption in the affected organs starts first or proceeds microbial dysbiosis (Table 2). In other words, it is still unclear whether dysbiosis of the skin microbiome is one of the pathogenetic factors of AD or the cause of the onset of AD. On the other hand, skin barrier disruption due to genetic defects in barrier molecules such as filaggrin mutations can initiate AD. 177 , 178 , 179 The same question is also valid for asthma and CRS. 177 In atopic skin, there has been a reduction in commensal bacteria and in patients with AD, a higher colonization index and increased pathogen density show a positive correlation with the skin lesions’ severity and the severity of the disease. 180 , 181 Early‐life skin colonization may also occur before the disease’s clinical manifestations. 182 Gut‐skin axis is another potential pathway in the pathogenesis of AD. Gut and skin microbes can interact with each other through immunologic and metabolic pathways. 183 Certain microbial metabolites from the gut have effects on skin microbiota, and gut bacterial dysbiosis has an effect on the skin immune system via a systemic imbalance in the Th2‐Treg lymphocyte ratio 173 , 184 (Table 3).
7.4. Food allergy
The gut microbiome is a dynamic environment constantly being influenced and modified by external factors, such as diet. Mechanisms of immune tolerance to food antigens appear with these modifications in this process. Disruptions in the immune responses and dysbiosis of the gut microbiome are associated with the development of FA. 185 , 186 , 187 , 188 Gut microbiome and bacterial diversity vary with factors such as maternal health, maternal diet, mode of delivery, dietary change with increasing age from the intrauterine stage to the first 3–4 years of life and intestinal colonization during infancy, which may affect the development of FA. 189 , 190 At 3 months, lower microbiota richness was associated with increased food sensitization by age 1, but microbiota richness was no longer associated with food sensitization after 12 months of age. 190 Although breastfeeding is a major source of immune factors and beneficial bacterial species, the direct relationship between breastfeeding and food sensitization is still unclear. 191 , 192 A maternal mediterranean diet during lactation and gestation and an infant diet consisting of high levels of fruits and vegetables were found to be protective against the subsequent development of FA. 193 , 194 In contrast, high‐sugar and high‐fat diets and diets with low levels of fecal short‐chain fatty acids have been associated with the development of FA. 195 In the end, poor food, altered bacterial diversity, and lack of protective factors from certain bacterial species like prevotella may result in allergic diseases, including FA. 191 , 196 Children with egg allergy were found to have increased diversity and different taxa in the early‐life gut microbiome compared to children without allergies. 197 This suggests that the specific microbiota associated with individual food allergies may differ depending on the food (Table 3).
8. CHANGE IN DIETARY HABITS AND ALLERGIC DISEASES
In recent years, many dietary hypotheses have been put forward in relation to allergic diseases, and changing dietary content is considered one of the most important environmental factors that cause allergies. Increased consumption of processed and fatty foods as a result of the conversion of the traditional diet to the western diet is often associated with the increase in the prevalence of allergic diseases. 198 , 199 The effects of fatty acids are contradictory. While n‐3 (omega‐3) fatty acids have potential protective effects, n‐6 (omega‐6) fatty acids have potentially harmful effects. 200 , 201 In a combination study in humans and mouse models to show the counterregulatory actions of docosahexaenoic acid (DHA; C22:6, w‐3)‐derived protectin D1 (PD1) in allergic airway inflammation, it was shown that PD1 administration decreased airway eosinophil count, T lymphocyte recruitment, airway mucus, levels of IL‐13, cysteinyl leukotrienes, PGD2, and airway hyperresponsiveness to inhaled methacholine. These studies present the importance of PD1 in reducing airway inflammation and the importance of an omega‐3‐rich diet in terms of maintaining airway homeostasis. 201 In an in vitro study, the D‐series resolvins PD1 and resolvin D1 derived from the omega‐3 (v‐3) fatty acid DHA have been shown to have potent pro‐resolution activities in allergic airway inflammation by intranasal administration. PD1 and resolvin D1 have been shown to reduce the total number of inflammatory cells and eosinophils in bronchoalveolar lavage and lung tissue and to cause goblet cell metaplasia in the airways of mice. 202 In particular, the increased n‐6/n‐3 ratio is considered one of the main factors that increase the allergic response. 199 , 203 The n‐6/n‐3 ratio of the human diet in the Paleolithic period was 0.79 and remained approximately the same for a long time. 204 However, within the last century, this ratio has increased up to 10–20:1 due to nutritional changes. This rapid change in the n‐6/n‐3 ratio, which is recommended to be approximately 5:1, also increased the negative impacts of n‐6 fatty acids and their metabolites. It is known that arachidonic acid (AA), one of the n‐6 fatty acid metabolites, increases the inflammatory response via eicosanoids. The increase in thromboxane A2, prostaglandin E2, and leukotriene B4 eicosanoid levels in the body also increase allergic sensitivity. 205 Unlike n‐6, n‐3 fatty acids compete with AA and prevent the formation of inflammatory agents. Moreover, eicosapentaenoic acid (EPA)‐derived resolvins (especially resolvin E1) induce an anti‐inflammatory effect by attenuating NF‐κB activation. 206
Another nutritional factor frequently associated with allergic diseases is antioxidants. In the past, foods were delivered to the consumer shortly after production, but today they are replaced mainly by processed foods. 207 Decreased levels of antioxidant A, C, and E vitamins in processed foods are thought to increase susceptibility to allergic diseases. 208 In addition, genetically modified (GM) organisms, which is the general name given to plants, animals, or microorganisms whose specific characteristics have been modified by transferring genes from species other than their own by biotechnological methods, is another important problem of our era. In recent years, GM plants have been increasingly used for food production and industrial applications. Some studies have suggested that transgenic crops may have allergic effects. 209 , 210 , 211 Although there are no clear results, GM food does not appear to be more allergenic than natural food, and so far there are no data to suggest that GM proteins cause allergies. 212 , 213 However, it should be noted that the reported studies are limited to short‐term follow‐ups, and long‐term results are unknown. In addition, a new protein is transferred by gene transfer technology, and most of the allergens are in protein structure. Therefore, longer‐term studies about the effects of GM foods on humans are needed. Another current issue is that rural life, contact with farm animals, and consumption of non‐pasteurized milk at an early age prevent the development of allergic diseases as a result of increased exposure to nonmicrobial‐derived N‐glycolylneuraminic acid (Neu5GC). 214 , 215 Below, the complex interactions between changing dietary habits linked to asthma, AR, AD, and FA will be discussed.
8.1. Asthma
The increase in the prevalence of asthma in parallel with the increased processed/fast food consumption in recent years has suggested that these two conditions may be related. 216 Especially, in western countries, as a result of the widespread consumption of fast food, the increased use of predominantly n‐6 containing vegetable oils is proposed to be one of the main factors in the increase of asthma. 217 High n‐6/n‐3 ratio and n‐6‐derived AA metabolites induce asthma by causing airway inflammation and bronchoconstriction. 218 , 219 In contrast, n‐3 fatty acids have beneficial effects by reducing airway inflammation and severity of bronchoconstriction and are also sources of pro‐resolving mediators that have been shown to reduce airway inflammation. 220 , 221 Furthermore, maternal intake of n‐3 fatty acids during pregnancy has been shown to have protective effects against asthma in children. 222 , 223 However, in a systematic review of 8 studies on the consumption of n‐3 fatty acids involving 3,366 women and their 3,175 children, the evidence was limited to recommend supplementation of n‐3 fatty acids during pregnancy and/or lactation to reduce allergic disease in children. 224 In subsequent studies, maternal fish oil consumption has been reported to increase histone acetylation of anti‐inflammatory gene regions (such as FOXP3, IL10RA, and IL7R) and may be protective against asthma; however, further studies are needed. 225 , 226 , 227 In the chromatographic analysis of the fecal samples of 301 one‐year‐old children, the highest fecal butyrate and propionate levels (≥95th percentile) were associated with less atopic sensitization and development of asthma between 3 and 6 years of age. 228 In addition to the protective effects of n‐3 fatty acids, the salicylic acid Neu5GC exposure has been shown to reduce airway inflammation and protect against the development of asthma. 214
Another factor that plays an important role in the pathophysiology of asthma is oxidative stress. 229 Insufficient intake of antioxidant vitamins (A, C, and E) and the resulting imbalance between antioxidant capacity and reactive oxygen species in the body make individuals more susceptible to asthma. 230 Two systematic reviews and meta‐analyses reported that the beneficial effects of vitamins A, D, C, E, and, zinc on asthma outcomes were weak, but low dietary intakes of vitamins A and C were associated with a statistically significant likelihood of asthma and wheezing. Unlike vitamins A and C, vitamin E intake was not associated with asthma. 231 , 232 Vitamin A is metabolized to retinoic acid (RA) by CD103+ dendritic cells (DCs). This DC‐derived RA has significant effects on DC activity, and depending on its concentration, promotes Th17 cells or Tregs. So far, specific microbial strains such as Bifidobacterium longum subsp infantes have been shown to promote RA metabolism, fork head box P3 (FoxP3)+ Treg cells, induce mucosal immune tolerance, and protect against inflammatory diseases. 233 , 234 , 235 Moreover, a number of in vivo studies demonstrated that vitamin D and RA, inhibit the formation of inflammatory Th17 and favor the generation of FoxP3 Tregs, and confer tolerogenic responses (Table 4). 236
TABLE 4.
Change in dietary habits and allergic diseases
| Allergic disease | Dietary habits | The effects on disease | Ref. |
|---|---|---|---|
|
Asthma |
High n−6/n−3 ratio | Airway inflammation and bronchoconstriction ↑ | [ 219 ] |
|
n−3 fatty acids |
Airway inflammation and severity of bronchoconstriction ↓ | [ 201, 221 ] | |
| Maternal intake of n−3 fatty acids | Protective effects against asthma in children | [ 222, 223, 224 ] | |
|
Maternal fish oil consumption |
Histone acetylation of anti‐inflammatory gene regions ↑ | [ 225, 226, 227 ] | |
| High Butyrate and Propionate | Less atopic sensitization and asthma development between 3–6 years of age | [ 228 ] | |
| Neu5GC exposure |
Reduce airway inflammation Protect against development of asthma |
[ 214 ] | |
|
Vit. A, D, C, E, zinc Low dietary intakes of Vit A and C |
Weak beneficial effect on asthma Statistically significant likelihood of asthma and wheezing |
[ 231, 232 ] | |
| Increased RA, Vit D consumption |
Induce mucosal immune tolerance Inhibit Th17, favors the generation of FoxP3+ Tregs Protect against inflammatory diseases |
[ 233, 234, 235, 236 ] | |
| Allergic rhinitis | Consumption of junk food/fast food | The risk of AR ↑ | [ 237 ] |
|
Fish (n−3) consumption during pregnancy |
The prevalence of AR ↓ | [ 242, 243, 244, 245 ] | |
| Supplementation of Vit. A, C, and E |
AR symptoms ↓ No positive effects on AR |
[ 249, 250, 251, 252 ] | |
|
Atopic dermatitis |
Processed foods and some food additives | The occurrence and severity of AD ↑ | [ 253, 255 ] |
| Breastfeeding for the first four months | The risk of eczema in the first four years ↓ | [ 257 ] | |
| Feeding infants with intensive eHF in the first 4–6 months, avoiding milk and dairy products |
Prevent the development of AD |
[ 257 ] | |
| Feeding eHF after the sixth month | Not suppress the development of AD | [ 258 ] | |
|
Monounsaturated fatty acid |
Allergic sensitization in females, mostly no significant associations for males | [ 258 ] | |
| High n−6/n−3 ratio | Moderate to severe AD ↑ | [ 260 ] | |
| The intake of n−6 fatty acids | Lower in the severe AD group | [ 261 ] | |
| n−3 PUFA docosahexaenoic acid | Beneficial impact on AD | [ 262 ] | |
| Supplementation with polyunsaturated fatty acids of the omega−3 | The mean SCORAD improved in 14 of 17 patients by more than 50% after 8 weeks and 16 weeks of treatment | [ 263 ] | |
| Dietary supplementation with very long‐chain n−3 fatty acids | No significant difference, the possibility of a placebo effect | [ 264 ] | |
| Vitamin C and E | Protective effects against AD | [ 266, 267, 268 ] | |
|
Food allergy |
Consumption of highly processed foods during pregnancy |
Food allergies in infants ↑ |
[ 270, 271 ] |
| Food additives and preservatives | Food allergies ↑ | [ 272, 273 ] | |
| Starting fish oil supplementation early in pregnancy and continuing during lactation | Allergic sensitization to food proteins in offspring ↓ | [ 274 ] | |
| Maternal intake of vitamins A, C, and E together with food |
Protective effect against FA in childhood |
[ 275 ] | |
| Taking Vit. A, C, and E supplements | Not protective effect against FA | [ 276 ] |
8.2. Allergic rhinitis
Studies on adults and children reported that consumption of junk food/fast food increases the risk of AR. 237 Although the results are unclear, food additives and artificial sweeteners often found in processed foods can trigger AR symptoms. 238 , 239 , 240 Limited number of studies have investigated the effects of dietary fatty acids on AR development. Accordingly, increased n‐6/n‐3 ratio and n‐6‐derived AA metabolites have been suggested to play an important role in the pathophysiology of AR. 241 While there is no evidence to support a protective association of fish (n‐3 rich) consumption during pregnancy with AR symptoms from infancy to 8 years of age, early intake of fish (before 9 months) has been shown to reduce the prevalence of AR. 242 , 243 , 244 , 245 In adults, results are conflicting. Dietary n‐3 fatty acid intake showed to be both protective and non‐effective. 243 , 246 However, dietary n‐3 fatty acids have been shown to dampen AR in a mouse model. 247 Oxidative stress and low total antioxidant levels also increase the occurrence and symptoms of AR. 248 In some studies, it was observed that serum levels of vitamins A, C, and E were low in individuals with AR, and supplementation of these vitamins reduced AR symptoms. 249 , 250 However, there are also studies in which the positive effects of supplementation were not observed (Table 4). 251 , 252
8.3. Atopic dermatitis
The prevalence of AD, a common, chronic, and recurrent inflammatory disease characterized by skin barrier impairment, has increased globally across all age groups, and this increase has also been associated with the westernization of dietary patterns and increased consumption of processed food. 253 Processed foods and some food additives such as monosodium glutamate (popular flavor enhancer) could act as pseudo‐allergens and increase the occurrence and severity of AD. 254 , 255 Maternal diet and antenatal nutrition could affect fetal development by altering fetal programming, which may in turn alter immune response and atopy. 256 Nutrition during infancy and childhood is very important for the development of AD, and breastfeeding for the first 6 months is thought to be effective in preventing the development of atopic diseases. A study conducted with 4,089 patients showed that breastfeeding for the first 4 months reduces the risk of eczema in the first 4 years. 257 In addition, it has been pointed out that feeding infants with extensively hydrolyzed formula (eHF) in the first 4–6 months, avoiding cow's milk and dairy products, and starting solid foods after the 4th month prevent the development of AD. 257 However, it was reported that feeding with eHF after the 6th month did not suppress the development of AD. 258
Studies investigating the effects of dietary fatty acids on AD in adults are quite limited and conflicting. Although some studies have indicated that low n‐3 intake is inversely correlated with AD in women and one randomized control trial noted that AD severity decreases after n‐3 supplementation; another studies have found no association between n‐3 intake and AD, and other clinical studies reported that n‐3 supplementation in adults did not show any benefit over placebo in AD. 259 , 260 , 261 , 262 , 263 , 264 Recently, oxidative stress has been shown to induce AD by increasing the pro‐inflammatory response. 265 Studies conducted on children and adults have found an inverse relationship between serum vitamin C and E levels with AD, and supplementation of vitamin E reduced AD symptoms (Table 4). 266 , 267 , 268
8.4. Food allergy
As a result of changes in diet, the increase in the prevalence of FA is inevitable. 269 Maternal nutrition and consumption of highly processed foods during pregnancy have been shown to increase FA in infants. 270 , 271 In addition, food additives and preservatives, which are often found in processed foods, increase the susceptibility to food allergies. 272 , 273 Clinical studies investigating the effect of n‐3 fatty acids on FA during pregnancy and/or lactation are contradictory but, starting fish oil supplementation early in pregnancy and continuing during lactation has been shown to reduce allergic sensitization to food proteins in offspring. 274 In a study that measured fatty acids in the feces with high‐performance liquid chromatography indicated that children with the highest fecal butyrate levels were less likely to develop food allergies. 228 Antioxidant intake can also affect food allergies through its effects on the immune response. Maternal intake of vitamins A, C, and E together with food has a protective effect against FA in childhood. 275 However, taking these vitamins as supplements did not show similar effects 276 (Table 4).
9. ENVIRONMENTAL SUBSTANCES AFFECTING THE EPITHELIAL BARRIERS
Following the industrial revolution in the 19th century, environmental health threats such as air pollution and chemical hazards have increased worldwide. With the increase in PM, diesel exhaust, O3, nanoparticles, and cigarette smoke, the air that we breathe has been dangerously polluted. The toxic burden faced by humans has increased with the introduction of cleaning products, detergents, and surfactants, as well as with increases in the use of processed foods and emulsifiers. 277 , 278 , 279 , 280
The steep increase in type 2 inflammatory diseases, that is, asthma and AD, coincide with the usage of surfactants and enzymes in laundry detergents and household cleaners in the 1960s, and food allergy and eosinophilic esophagitis coincide with the use of food emulsifiers and dishwasher detergents after 1990s. Besides, the increasing load of nanoparticles and microplastics in the seas, soils, and nowadays in the indoor and inner‐city air pose a significant threat to living beings. Recent research has revealed that environmental exposures, climate change, and global warming adversely affect airborne pollens and increase their allergenicity. 5 , 281
9.1. Particulate matter, nanoparticles, nitrogen dioxide, and ozone
Particulate matter is a mixture of solid particles and liquid droplets generated by human activity. Besides, it is formed in the atmosphere through chemical reactions of gases such as sulfur dioxide, nitrogen oxides, and certain organic compounds often emitted from industrial processes, motor vehicle exhaust, diesel and coal combustion, house heating, and wildfires. All types of PM (PM0.1, <0.1 μm in diameter; PM 2.5, <2.5 μm in diameter; PM10, <10 μm in diameter) particles behave like gases due to their small sizes and cause diseases, especially in the respiratory tract. 282 However, PM2.5 is considered to be the greatest problem, because it can diffuse deeper into the terminal bronchioles and alveoli. 283 It has been demonstrated in vitro that PM 2.5 can disrupt the epithelial barrier by degrading TJ proteins in the lower and upper airways, downregulating occludin and claudin‐1 expressions, suppressing E‐cadherin levels, decreasing transepithelial electric resistance, and increasing paracellular permeability 282 , 284 , 285 , 286 (Figure 3). Wildfires are a major source of ambient air PM2.5 in different studies and wildfire exposure has been associated with worsening asthma symptoms and increases in emergency room visits. 287 Moreover, short‐term and long‐term exposures to high levels PM2.5 can cause increased FoxP3 methylation, a key transcription factor in immune tolerance. 288 , 289 Furthermore, PM2.5 causes increased lysosomal membrane permeability, oxidative stress, and lipid peroxidation at low doses, while at high doses it causes necrosis in airway epithelial cells. 290 In vivo and in vitro studies have revealed that PM2.5 is also associated with skin diseases such as AD, skin allergies, and eczema by causing DNA damage, irreversible lipid peroxidation, protein carbonation, and loss of structural epidermal proteins such as cytokeratin, filaggrin, and E‐cadherin in the skin epithelial barrier. 291 , 292 , 293 , 294 , 295 On the other hand, PM10 is also a significant contributor causing damage to airway epithelial cells. PM10 was reported to induce alveolar epithelial dysfunction by reducing occludin at the plasma membrane and dissociation of ZO‐1 in human and primary rat alveolar epithelial cells. 296 In another recently published study, cellular DNA damage and aberrant gene expression patterns associated with PM10 were shown in the airways cells. 297 Furthermore, PM10 strongly stimulated messenger RNA expression and secretion of the pro‐inflammatory cytokines IL‐6 and CXCL1 in mouse airway epithelial cells and it induced the expression of IL‐6, IL‐8, and IL1B in human airway epithelial cells. 298 It must be noted that a link has been reported with PM 10 exposure not only to asthma but also to other inflammatory diseases. Exposure to airborne PM is associated with increases in multiple sclerosis in Stockholm, Sweden. Increased numbers of circulating myeloid DCs that express cytokines such as IL‐1β, IL‐6, and IL‐23, which stimulate the development of Th17 cells, were reported in these patients. 286 , 296 , 299 Their findings were associated with an increase in CCR6+ CD4+ T cells with the migratory capacity to pass through the blood–brain barrier. These findings suggest that PM causes chronic respiratory diseases, especially asthma, and exacerbate existing ones. Although the harmful effects of ultrafine particles such as PM0.1 are less well known, in an animal model, it was demonstrated that PM0.1 caused increase of lysosomal membrane permeability, oxidative stress, and lipid peroxidation at low doses and it caused necrosis in airway epithelial cells at high doses. 290 It was also reported that PM0.1 induced autophagic cell death of human neuronal cells. 300
In recent years, nanoparticles (NPs), organic or inorganic, smaller than 100 nm, are increasingly used in various industries and contribute significantly to air pollution. NPs can produce quantum effects by confining their electrons and entering the human body through inhalation, ingestion, skin, or injection. Inhaled NPs pass through the pores in the alveolar‐capillary membrane, enter the interstitium and even into the systemic circulation via the blood and lymphatics. 301 , 302 Carbon nanotubes directly stimulate epithelial cells, macrophages, and fibroblasts to produce pro‐inflammatory and profibrotic mediators, causing increased collagen production and deposition in the extracellular matrix, leading to fibrosis. 303 Titanium dioxide (TiO2) and silicon (SiO2), the most ubiquitous NPs, induce unbalanced overexpression of immature neurotrophins and lead to apoptotic death of lung epithelial cells. 304 Moreover, with their high lipid affinity, NPs coat and disrupt phospholipid membranes, interfere with lipid‐rich structures in the pulmonary circulation such as surfactants and endothelial cell junctions, and even destabilize lysosomal membranes triggering cell death. 305 Although initial studies reported that TiO2 did not penetrate the stratum corneum, a recent study indicated that the cubic and about 25nm size sample was cytotoxic to human epidermal keratinocytes. 306 Similarly, acicular TiO2‐NP is shown to interact with human epidermal keratinocytes, induce secretion of pro‐inflammatory cytokines and disrupt the skin barrier by altering cell junctions. 307 The intestinal toxicity of NPs is less known, cationic liposome NPs containing ZnO, silver, aluminum, and nickel, as well as TiO2 and SiO2 NPs, accumulate across the intestinal epithelial barrier and then translocate by endocytosis by the M‐cell, or by disrupting the integrity of the cell membrane, or by phagocytosis by macrophages. 305 , 308 It has been shown in vitro that cellular uptake of Nickel Oxide‐NPs causes cytotoxicity by disrupting the mitochondrial and lysosomal functions and TiO2‐NPs lead to increased paracellular permeability in human intestinal epithelium 309 , 310 (Figure 3).
Nitrogen dioxide (NO2) is a major component of air pollution, especially an important component of the traffic‐related air pollution. However, due to the use of gas stoves, it is accepted as an important indoor pollutant as well as an outdoor pollutant. 80 Exposure to NO2 is associated with an increased risk of developing respiratory diseases due to its deep penetration into the lungs. This effect is thought to be via epithelial barrier damage. In an in vitro study investigating the effect of NO2 on airway epithelial defense functions; ciliary activity, mucociliary transport velocity, and epithelial permeability were significantly impaired in the NO2 exposed group of fifty‐two healthy rabbits. 311 In another animal model, it was suggested that after exposure to ≤1 ppm NO2 level, active ion transport across the airway epithelium was significantly increased without change in paracellular pathways for diffusion, and NO2 altered the cell membrane function. 312 A randomized controlled trial examining the effects of indoor NO2 in asthmatic children demonstrated that increased NO2 exposure was associated with a dose‐related increase in risk of higher asthma severity score, wheeze, night symptoms, and rescue medication use. 313 NO2 can cause epithelial barrier dysfunction in the upper as well as the lower airways. In an in vivo study evaluating the effects of 2 ppm NO2 exposure on human nasal epithelium by electron microscopy, the luminal margin membranes of ciliary cells were ultrastructurally altered in six of seven nasal epithelial samples after NO2 exposure. 314 , 315
Ozone gas has a variable lifetime and occurs both in the upper and lower atmospheres, almost at ground level. Ground‐level O3 is the main component of photochemical smog and is formed in the presence of sunlight by chemical reactions between oxides of nitrogen and volatile organic compounds emitted by motor vehicles, power plants, industrial boilers, refineries, and chemical plants. Even during colder months, O3 can reach high levels and be carried long distances by wind and spread to rural areas. 316 , 317 Due to its poor water solubility, inhaled O3 can penetrate deep into the lungs. Acute exposure can damage alveolar cells, bronchiolar epithelium, and capillary endothelium initially with cell stress, desquamation, followed by protein leakage, neutrophil, and macrophage influx, and production of IL‐1α and IL‐33 from epithelial and myeloid cells. 318 , 319 , 320 Ehile acute exposure caused airway inflammation and airway hyperresponsiveness, chronic inflammatory process presenting with collagen deposition in epithelial and subepithelial areas led to peribronchial fibrosis and emphysema. 318 , 321 Recent studies suggest that chronic O3 exposure is responsible for bronchial hyperreactivity, asthma, asthma exacerbation, chronic obstructive pulmonary disease, and even pulmonary fibrosis and respiratory death 322 (Table 5).
TABLE 5.
Environmental substances affecting the epithelial barriers
| Environmental factors | Mechanism | Ref. |
|---|---|---|
| PM | Increase Fox P3 methylation (especially PM2.5) |
[ 282, 284, 285, 286, 288, 289, 290, 291, 292, 293, 294, 295, 296, 297, 298, 300 ] |
|
Degrade TJ proteins, downregulate occludin and claudin−1 expression, suppress E‐cadherin levels (especially PM 2.5 &PM10) | ||
| Increase paracellular permeability (especially PM 2.5) | ||
| Increase lysosomal membrane permeability, oxidative stress, and lipid peroxidation (especially PM0.1 & PM 2.5) | ||
| Cause DNA damage, protein carbonation (especially PM 2.5 & PM10) | ||
|
Cause loss of structural epidermal proteins such as cytokeratin, filaggrin (especially PM2.5) | ||
| Reduce occluding, dissociate ZO−1, stimulate mRNA expression, and secretion of pro‐inflammatory cytokines (especially PM10) | ||
| Cause necrosis in airway epithelial cells, at high doses cause autophagic cell death of human neuronal cells (especially PM0.1) | ||
| Nanoparticles |
Stimulate collagen production and deposition in the extracellular matrix, lead to Fibrosis |
|
| Overexpress of immature neurotrophins and lead to apoptotic death | ||
| Disrupt phospholipid membranes | ||
| Destabilize lysosomal membranes and trigger cell death | ||
| Alter cell junctions and disrupt cell membrane integrity | ||
|
Disrupt the mitochondrial and lysosomal functions and increase paracellular Permeability | ||
| Nitrogen dioxide | Damages upper and lower airway epithelial barrier | [ 311, 312, 313, 314 ] |
| Altered cell membrane functions | ||
| Dose‐related increase in asthma risk due to deep penetration into lungs | ||
| Ozone | Have high penetration in airways | |
| Lead to cell stress, desquamation, and cell death with oxidative stress | ||
| Induce IL−1α and IL−33 production from epithelial and myeloid cells | ||
| Increase protein leakage, neutrophil, and macrophage influx | ||
|
Induce collagen deposition in epithelial and subepithelial areas and cause peri bronchial fibrosis in chronic process | ||
| Detergents and emulsifiers | Have direct detrimental effects on epithelial barrier integrity |
[ 229, 230, 264, 266, 267, 268, 269, 270, 271, 272, 273, 274, 275, 276 ] |
| Increase trans epidermal water loss and decrease stratum corneum hydration | ||
| Damage TJs and related molecules of the airways | ||
| Induce secreting IL−25, IL−33 and TSLP | ||
| Alter the microbiota and disrupt mucus–bacterial interactions in intestinal epithelial barrier | ||
| Microplastic | Penetrate tissues and interact with cellular structural molecules | |
| Cause the proteins to fold, alter structure, and denaturate | ||
| Interact lipid bilayers to alter cell membranes | ||
|
Induce inflammatory gene transcription, pro‐inflammatory cytokines, and pro‐apoptotic protein expression | ||
| Cause endoplasmic reticulum, mitochondrial dysfunction and induce cell death by oxidative stress | ||
| Tobacco and e‐cigarettes | Disrupt epithelial cell barrier integrity | |
| Cause rapid lipid peroxidation | ||
| Increase alveolar epithelial permeability | ||
| Impair alveolar clearance | ||
| Alter apoptotic cell recognition receptors and cytokine secretion pathways | ||
| Cause epithelial cell death and dysfunction of macrophages | ||
| Protease allergens | Increase the permeability of epithelium | |
| Damage the tight junction molecules ZO−1, occludin, and E‐cadherin | ||
| Stimulate Th2 differentiation and IL−4 and IL−13 secretion | ||
|
Increase the formation of collagen under the epithelial tissue and induce airway Remodeling |
9.2. Detergents and emulsifiers
At the beginning of this century, the scarcity of oils used in soap production and the desire to find stronger cleaning agents led to the start of work to produce the first synthetic detergent. Within the last several decades, the use of detergents for laundry, dishwashing, household, or industrial area cleaning has tremendously increased, with a change in their formulation, including cosmetics and personal care products. The increase in the incidence of allergic diseases in the same period suggested that detergents may trigger the formation of allergic diseases by affecting the associated epithelial barriers, especially the skin and respiratory tract 9 , 323 (Figure 3). Surfactants, particularly sulfated anionic surfactants and commercial detergents, have proven to have direct detrimental effects on skin and bronchial epithelial barrier integrity, even in trace concentrations. 278 , 279 In a clinical trial, the insult of sodium lauryl sulfate, an anionic surfactant, to the stratum corneum was demonstrated by both increased transepidermal water loss (TEWL) and decreased stratum corneum hydration. 324 , 325 Recently, a study showed that detergents cause high TEWL in cleaning personnel due to reduced barrier function, related to the increased risk of work‐related hand dermatitis. 326 Furthermore, it has been observed that with the increased need for hand cleaning during the COVID‐19 pandemic, disinfectants worsen the disease in individuals with a previous history of AD or contact dermatitis and even also cause eczema in healthy individuals. 327 , 328 In addition, isothiazolinone derivatives used in water‐based detergents also cause contact dermatitis by direct contact or airborne. 329 , 330
The airway epithelial barrier is as sensitive as the skin barrier to detergents. Detergent residues on newly washed clothes and on the surface of the floor can be easily inhaled, and even at very low concentrations, they directly damage TJs and related molecules of the airways. 278 In the same study, RNA sequencing demonstrated an increase in cytokines such as IL‐25, IL‐33, and TSLP that initiate type 2 immunity, even in 50,000 times diluted commercial laundry detergent damaged epithelial tissue. As a result, as reported in different occupational studies, the development of asthma or exacerbation of respiratory symptoms in adults seems inevitable by inhaling sprays or vapors, namely aerosolized forms of detergents that are widely used in every field. 331 , 332
Emulsifiers such as lecithin, carboxymethyl cellulose, and sorbitol monostearate, which are food additives, are frequently used to reduce the surface tension with a detergent‐like effect, to maintain a homogeneous dispersion. 333 , 334 They are thickening the mucosal surface fluid, trapping commensal bacteria, avoiding a healthy interaction between the epithelium and commensals and alter the microbiota and disrupt mucus–bacterial interactions in mouse models to induce intestinal inflammation. 333 , 335 Dishwasher detergent residue left on rinsed clean dishes when used for food consumption are being currently studied to determine if they are damaging the esophageal or gastrointestinal epithelial barriers 15 (Table 5).
9.3. Microplastics
Water‐insoluble polymeric particles derived from petroleum are called microplastics (<5mm in size) and nanoplastics (1 nm‐1µm in size). Due to their cost‐effectiveness, small size, light, and compatible nature, these substances are used in many sectors. Secondary microplastics are formed when larger‐sized ‘macroplastics’ found in nature are degraded into smaller‐sized fragments by ultraviolet rays from the sun, waves, rain wind, and other decaying organisms. Humans are easily exposed to micro‐ and nanoplastics in daily life through contact, inhalation, and ingestion from water, soil, and air. These products can easily penetrate tissues and interact with cells and cellular structural molecules. 336 , 337 The nanoplastic protein interaction causes the proteins to fold, alters their secondary structure and denatures them, and can also interact with lipid bilayers to alter cell membranes. 338 , 339 In a study with 25 nm and 70 nm nanoplastics, they were shown to induce inflammatory gene transcription, upregulate pro‐inflammatory cytokines, and alter the expression of proteins connected with cell cycle and pro‐apoptosis. 340 Of the nanoplastic varieties, nano polystyrene induces metabolic changes related to autophagy and endoplasmic reticulum stress, while nano ZnO causes oxidative stress‐induced cell death due to mitochondrial dysfunction. 341 Occupational studies have shown that workers in different fields exposed to plastics and their products develop increased respiratory symptoms, decreased lung capacity, interstitial fibrosis, allergic alveolitis, and granulomatous lesions. 336
The amount of plastic produced per year exceeds 100 million tons and increasing plastic waste load in the seas and oceans poses a threat to the environment and the health of humans and affected animals. Today, increased levels of plastic are detected in fish and sea creatures. Microplastics have been detected even in drinking water, foods such as mussels, shrimp, fish, salt, sugar, honey, and beer. They have a high absorption capacity in the gastrointestinal tract, and the effects of microplastics have been shown both in vitro and in vivo. 337 In a mouse model, polystyrene microplastics have been shown to reduce intestinal mucus secretion and cause damage the intestinal barrier function 342 (Figure 3). Different sizes of spherical fluorescent polystyrene particles (1, 4, and 10 µm) were used in the culture media, and it was observed that the one μm plastic particle is the most cytotoxic due to its high surface volume ratio. The study concluded that the smallest particles (<1.5 µm) might penetrate the gastrointestinal epithelial barrier 343 (Table 5).
9.4. Tobacco and e‐cigarettes
Tobacco, one of the most common toxic substances in the environment with its content of approximately 5,000 chemicals, causes toxicity especially to the respiratory system. In vitro studies have shown that tobacco causes rapid lipid peroxidation in the rat tracheal epithelium and is associated with alveolar epithelial damage in guinea pigs. 344 , 345 In lung transplantation studies, impaired alveolar clearance in the lungs of a smoker or ex‐smoker donor, expressed ex vivo, indicated alveolar epithelial damage and was associated with primary graft failure. 346 , 347 Even though e‐cigarettes are marketed as alternatives to aid smoking cessation, there is not enough evidence to show that they help. Moreover, their safety is questionable as they contain a number of toxic chemicals such as benzene, ethanol, iron, aluminum, cadmium, tobacco‐specific nitrosamines, polycyclic aromatic hydrocarbons, and formaldehyde, in addition to their four main components: nicotine, propylene glycol, glycerol, and food flavorings. 348 An in vitro study showed that sub‐chronic exposure to e‐cigarette aerosols disrupt human bronchial epithelial cell barrier integrity similar to cigarette smoke. 349 Furthermore, flavorings in tobacco products have been shown to cause airway epithelial cell death, apoptosis, and dysfunction of macrophages through altering apoptotic cell recognition receptors and bronchial epithelial cell cytokine secretion pathways 350 (Table 5).
9.5. Airborne pollens
The protease activity of allergens such as molds, pollens, cockroaches, HDMs, and food contributes to epithelial integrity disruption and initiates the innate immune response 351 , 352 (Figure 3). Group 1 HDM allergens called Der p 1, Der f 1, Blo t 1, Eur m 1, and Der m 1 have cysteine protease properties that increase the permeability of the epithelium. 353 Stimulation with the cockroach Per a 10 allergen with serine protease activity was found to be detrimental on the TJ molecules zonula occludens‐1 (ZO‐1) and occludin and stimulated Th2 differentiation in the airway epithelium. 354 It has been found that Der p 3, Der p 6, and Der p 9 destroy ZO‐1, occludin, and E‐cadherin in lung epithelial cells with trypsin, chymotrypsin, and collagenolytic‐like protease effects and are effective in airway remodeling. 355 Asp f 5, an allergen with metalloprotease properties from Aspergillus fumigatus, has been shown to increase IL‐4 and IL‐13 levels in mouse lung tissue and play a role in airway remodeling by changing the amount of collagen under the lung epithelial tissue. 356 Besides, it has been reported that food cysteine proteases impair intestinal barrier function and increase intestinal permeability with mechanisms similar to inhalant allergens in both mice and in vitro studies. 357 To conclude, protease active allergens facilitate the presentation of allergens, induce an allergic inflammatory response, thus facilitating sensitivity to secondary allergens 358 , 359 (Table 5).
10. EPITHELIAL BARRIER HYPOTHESIS AND ALLERGIC DISEASES
The increase in understanding the underlying multiple and complex immune mechanisms, additional to the development of novel detailed diagnostic techniques and ease of access to comparable data have led to the proposal of several hypotheses explaining the complexity of chronic conditions, including allergic disorders. Such hypotheses can provide a framework toward understanding the relations between the immune system, allergens, environmental triggers and epigenetics, signs and symptoms of diseases, and demographics in the field of allergy. Among these ‘Hygiene hypotheses’ has initially focused on explaining the increase in allergic disorders with decreased incidence of infections in westernized countries, which is mainly based upon epidemiological data. With the evolution of our understanding about innate and adaptive arms of the immune system and its regulation, the impacts of microbiome, exposome, genome, and epigenome, ‘atopic march’ have found their illustrative backbones. 360 Precise definition of endotypes, phenotypes, and even theratypes in addition to several biomarkers has advanced existing paradigms into tailored novel approaches including therapies. 361
Skin, in addition to respiratory and gastrointestinal mucosa, is the contact surface of the host with the external environment lined by epithelial barriers (epidermal barrier and epithelial mucosa). Functional derangement of the epithelial barrier, which acts as a first‐line physical defense and active site of the immune response, has been regarded as a factor responsible for the development of many diseases, including allergies and autoimmune diseases. Recently described ‘Epithelial barrier hypothesis’ emphasizes that environmental exposure to substances that are mainly toxic and hazardous have deleterious impacts onto these outermost access points, the barriers of the skin, airways, and gastrointestinal mucosa. 12
11. REGULATION OF THE EPITHELIAL BARRIERS
Epithelial cells play critical roles in the maintenance of homeostasis through a wide range of physiologic functions. There is a complex interplay between environmental factors, epithelium, and the immune system. Epithelial cells can secrete IL‐25, IL‐33, and TSLP in response to several stimuli, leading to a skewing of the immune response into Th2 type. 362 These cytokines activate DCs and group 2 innate lymphoid cells (ILC2). ILC2s share many functional similarities with Th2 cells, such as the production of IL‐4, IL‐5, IL‐9, and IL‐13 as well as other effector molecules potentiating the Th2 immune response. 363 It has been reported that ILC2s disrupt epithelial barrier integrity by IL‐13 364 (Figure 5).
FIGURE 5.
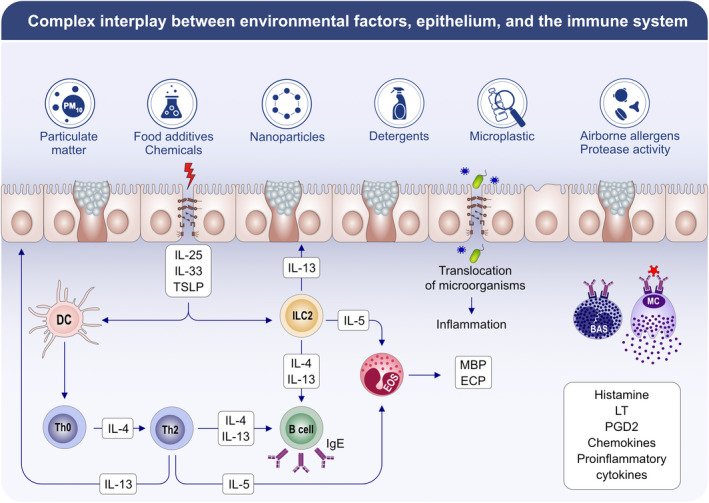
Complex interplay between environmental factors, epithelium, and the immune system. Epithelial cells can secrete IL‐25, IL‐33, and TSLP in response to various stimuli from the environment resulting in a Th2 type shift of the immune response. These cytokines activate dendritic cells and group 2 innate lymphoid cells). ILC2s share many functional similarities with Th2 cells such as the production of IL‐5, and IL‐13 as well as other effector molecules that enhance the Th2 immune response. Eosinophils, basophils, and mast cells are attracted to the area and degranulate. Dysregulation of the epithelial barrier has been hypothesized to cause a leaky epithelium, which causes dysbiosis of the microbial content, decrease of commensals and increase of opportunistic pathogens. The translocation of microorganisms to interepithelial and subepithelial compartments induces inflammation. IL: interleukin, TSLP: thymic stromal lymphopoietin, DC: dendritic cell, ILC2: innate lymphoid cell‐2, EOS: eosinophil, BAS: basophil, MC: mast cell, MBP: major basic protein, ECP: eosinophilic cationic protein, LT: leukotriens, PGD2: Prostaglandin D2, Th0: naive T cell, Th2: T helper 2, Ig E: immunoglobulin E
It has been postulated that derangement of the epithelial barrier causes a leaky epithelium that results in dysbiosis of microbial content, including commensals and opportunistic pathogens and translocation of this content into interepithelial and subepithelial compartments, which induces micro‐inflammation. 12 It is well‐accepted that the microbiome plays a crucial role in the shaping of the immune system and tissue homeostasis.
12. EPITHELIAL BARRIER DAMAGE AND THE LINK BETWEEN ALLERGIC DISEASES
There are several similarities and differences in the structure and function of epithelial barriers between the skin, the gastrointestinal system, and the respiratory tract. 15 A disruption in the epithelial barrier could be a common pathway in the development of multiple allergic conditions. 365 , 366 The interaction between the epithelium and immune cells in addition to crosstalk with microbiota and environmental factors enroll in the pathogenesis of atopic disorders, including AD, AR, asthma, CRS as well as eosinophilic esophagitis and FA 367 , 368 (Table 2). Dysbiosis in the gut microbiota has effects on the immunity of distal organs, such as the lungs, in addition to modulating the immune responses of the gastrointestinal tract itself. 369 Increasing evidence has shown the link between gut‐lung axis, which suggests that the host‐microbe interactions exist beyond the local environment to distal tissues. 370
In the skin, the epidermal barrier is composed of the stratum corneum and TJs. Change in the composition and dysfunction of lipids (ceramides, free fatty acids, and cholesterol) and structural proteins (filaggrin), elevated skin pH, and loss of microbiome diversity all may lead to impairment of epidermal barrier as seen in patients with AD. 285 , 371 While the transmembrane proteins including claudins, occludins and adhesion molecules compromising the TJs, their barrier functions are weakened by altered lipid and profilaggrin processing. Filaggrin is a structural protein that is essential for the regulation of epidermal homeostasis. Loss‐of‐function mutations in filaggrin have been proposed to cause epithelial barrier defects, which reduces epidermal defense against allergens, microorganisms, and other environmental insults and plays a role in the development of AD, as well as bearing risk for FA, AR, and asthma. This defect results in a skewing of immune response into Th2 type immune response with resultant chronic inflammation of the skin. 372 There is reduced expression of the TJ proteins claudin‐1 and claudin‐23 in patients with AD. 127 The importance of the epithelial barrier can be marked even in the early days of life. It has been revealed that the development of allergy to several foods in the absence of oral feeding may be linked to inflamed and disrupted epithelial barrier in infants with severe AD. 373
Also, airway epithelial cells not only comprise a physical barrier but also play key roles in immune, inflammatory, repair, and remodeling responses upon encounters with triggers, including inhaled allergens, pathogens, PMs, and chemicals. Additional to mucociliary clearance, epithelial cells can produce antimicrobial peptides, several chemokines, and cytokines that recruit and activate other effector cells and sustain clearance of pathogenic insults. 374 TJs in the airway epithelium have been extensively studied in healthy individuals. Studies have revealed that the airway epithelium in asthma and upper airway diseases is dysfunctional due to disturbed TJ formation. 375 In asthmatic children, expression of claudin‐1, occludin, and ZO‐1 proteins was significantly reduced compared to non‐asthmatic children. 376 Claudin‐18 is required for the airway epithelial permeability barrier. Loss of claudin‐18 was sufficient to impair epithelial barrier function in human bronchial epithelial cells. IL‐13 decreases claudin‐18 expression in epithelial cells. Claudin‐18 mRNA levels were found to be lower in asthmatics than in healthy controls. 377 It has also been reported that TJ protein expressions were decreased in patients with CRS with nasal polyps, which highlights the role of the defective epithelial barrier in the disease pathogenesis. 124
Airway epithelium acts as an initial defense barrier to inhaled particulates, including allergens. The protease activity of several allergens may contribute to sensitization by disrupting the integrity of the airway epithelial barrier. 378 It has been understood that the common allergens such as HDMs and several pollens have cysteine and serine proteases that can disrupt the epithelial barrier. Similarly, molds including aspergillus and penicillium exert serine protease activity. Additional insults to these activities have dampened the immune response. A disrupted barrier is a site for dysbiosis in the airway epithelium as well. Change in the content of the microbiome also shapes the altered immune response. Infectious triggers have several impacts on airway epithelium. It is well known that human rhinovirus (HRV) infection is a trigger of asthma exacerbations. It has been reported that HRV increases inflammatory cytokine production, reduces IFN‐β production, and plays a role in wound repair by causing defective repair response in the epithelial cells of asthmatic children. 379 Besides, it has been reported that low‐dose chronic endotoxin or farm dust exposure has reduced epithelial cytokines that can activate DCs, protecting asthma development in mice models. 380 On the other hand, it has been suggested that chlorination products promote allergic sensitization by compromising the permeability or the immunoregulatory function of epithelial barriers. 381 In animal models of asthma, hypochlorite‐induced asthma was linked to impairment of airway barrier to this irritant. 382 It has also been reported that laundry detergents at a very high dilution or detergent residue after rinsing have disruptive effects on the TJ barrier integrity in human bronchial epithelial cells. 278 Detailed discussions related to these are mentioned in this manuscript elsewhere.
The intestinal epithelium is the largest mucosal surface in contact with the external environment. The gastrointestinal epithelial barrier has to defend against the passage of foreign antigens, several microbes, and their toxins, while it has to act as a selective barrier to absorb and exchange essential dietary nutrients, water, and electrolytes. 383 Protein‐protein networks that form desmosomes, adherence junctions, and TJs compromise the intestinal epithelial barrier. 383 Early studies have delineated the role of the gut epithelial barrier on the development of inflammatory bowel disease and celiac disease. 384 , 385 The number of diseases related to the leaky gut has increased within the last few years and now include diseases such as type 1 diabetes, irritable bowel syndrome, and colorectal cancer. 386 It has been proposed that the breakdown of oral tolerance to food could also be associated with a downregulated intestinal barrier function. In several experimental animal models and human clinical trials, the association between food allergy and defects in the intestinal barrier function has been emphasized. 370 , 386 , 387 , 388 There is also evidence that an abnormal epithelial barrier is also present in patients with eosinophilic esophagitis. 368
13. CONCLUSION
The current exposome concept is a promising model to improve the uncertain data on the impact of environmental factors on the development of allergic diseases. External exposome has vital effects on human health related to climate change, air pollution, change and loss of biodiversity, change in dietary habits, and dysbiosis. These external factors that cause damage to the epithelium, and the disruption in the epithelial barrier constitutes a common pathway in the development of multiple allergic conditions. Moreover, the interaction between the epithelium and immune cells in addition to crosstalk with microbiota and environmental factors take part in the pathogenesis of atopic disorders including AD, AR, asthma, and FA. As a result, an increase in the incidence of allergic diseases secondary to external changes is expected in the medium and long term.
In this scenario, the task of scientific society is to give priority to external exposome research and establish the extensive usage of the exposome‐based approach for allergic diseases and asthma. The relationship between environmental exposure and allergic diseases should be assessed in longitudinal cohort studies with standardized exposure models. It is essential to understand the risk factors and preventive measures to be taken against them in multifactorial, chronic diseases such as allergic conditions. There is no doubt that these developments will ultimately lead to better prevention strategies.
From a public health perspective, the education of the population and the emergence of governmental decisions to prevent climate change, changes and loss of biodiversity, unhealthy dietary habits, and exposure to environmental substances (detergents, airborne pollen, O3, microplastics, nanoparticles, tobacco) are urgent measures to be considered throughout the world. In this global fight, mitigation measures can be undertaken to limit the harmful effects of all these threats. Furthermore, adaptation measures should be taken for the impacts of global warming.
These growing environmental threats need actions throughout the world with united forces of all capabilities. The unresponsiveness of governmental institutions, the resistance of the general population, lack of infrastructure, and poverty are all barriers to these efforts. Physicians have the most crucial role in promoting and activating the people on the health effects of environmental changes. A multidimensional approach involving all stakeholders will be necessary to overcome these environmental problems and develop a better future for our planet.
CONFLICTS OF INTEREST
Dr. Akdis reports grants from Allergopharma, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission’s Horizon's 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, Glaxo Smith‐Kline, Scibase, advisory role in Sanofi/Regeneron, Scibase, Novartis, Glaxo Smith Kline. Other authors declare no conflicts of interest in relation to this work.
AUTHOR CONTRIBUTION
ZCS, DM, and CA conceptualized the scope of the article, decided the headings and subheadings, and distributed the tasks among all authors. BOO wrote the part of the environmental substances. PC and SA wrote the part of the effects of climate change. MT and IY wrote the part of microbiome, BG and UO wrote the part of the nutritional factors. CO wrote the part of the epithelial barrier hypothesis. ZCS reviewed and combined the contributions from all authors. ZCS, MA, IO, YM, KN, CO, DM, and CA reviewed the article and figures, contributed different sections, and harmonized the final version.
ACKNOWLEDGEMENTS
We thank Anna Globinska for developing the figures in the style of Allergy. Open Access Funding provided by Universitat Zurich.
Celebi Sozener Z, Ozdel Ozturk B, Cerci P, et al. Epithelial barrier hypothesis: Effect of external exposome on microbiome and epithelial barriers in allergic disease. Allergy. 2022;77:1418–1449. doi: 10.1111/all.15240
Funding information
There is no funding specific for this manuscript
Contributor Information
Zeynep Celebi Sozener, Email: zeynepsozener@gmail.com.
Cezmi A. Akdis, Email: akdisac@siaf.uzh.ch.
REFERENCES
- 1. Vermeulen R, Schymanski EL, Barabasi AL, Miller GW. The exposome and health: where chemistry meets biology. Science. 2020;367(6476):392‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim KN, Hong YC. The exposome and the future of epidemiology: a vision and prospect. Environ Health Toxicol. 2017;32:e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S81‐S94. [DOI] [PubMed] [Google Scholar]
- 4. Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014;69(9):876‐878. [DOI] [PubMed] [Google Scholar]
- 5. Agache I, Miller R, Gern JE, et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy. 2019;74(3):449‐463. [DOI] [PubMed] [Google Scholar]
- 6. Vineis P, Robinson O, Chadeau‐Hyam M, Dehghan A, Mudway I, Dagnino S. What is new in the exposome? Environ Int. 2020;143:105887. [DOI] [PubMed] [Google Scholar]
- 7. Wild CP. Complementing the genome with an ‘exposome’: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847‐1850. [DOI] [PubMed] [Google Scholar]
- 8. Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41(1):24‐32. [DOI] [PubMed] [Google Scholar]
- 9. Cecchi L, D'Amato G, Annesi‐Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol. 2018;141(3):846‐857. [DOI] [PubMed] [Google Scholar]
- 10. Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: early‐life events and immune development in a changing world. J Allergy Clin Immunol. 2017;140(1):24‐40. [DOI] [PubMed] [Google Scholar]
- 11. Haahtela T. A biodiversity hypothesis. Allergy. 2019;74(8):1445‐1456. [DOI] [PubMed] [Google Scholar]
- 12. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021. Nov;21(11):739‐751. doi: 10.1038/s41577-021-00538-7. [DOI] [PubMed] [Google Scholar]
- 13. Akdis CA. Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol. 2006;18(6):718‐726. [DOI] [PubMed] [Google Scholar]
- 14. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL‐1 to IL‐38), interferons, transforming growth factor beta, and TNF‐alpha: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984‐1010. [DOI] [PubMed] [Google Scholar]
- 15. Sözener ZC, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol. 2020;145(6):1517‐1528. [DOI] [PubMed] [Google Scholar]
- 16. Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. 2017;140(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking bigger: how early‐life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy. 2019;74(11):2103‐2115. [DOI] [PubMed] [Google Scholar]
- 18. Cecchi L, D’Amato G, Annesi‐Maesano I. Climate change and outdoor aeroallergens related to allergy and asthma: taking the exposome into account. Allergy. 2020;75(9):2361‐2363. [DOI] [PubMed] [Google Scholar]
- 19. Biagioni B, Annesi‐Maesano I, D’Amato G, Cecchi L. The rising of allergic respiratory diseases in a changing world: from climate change to migration. Expert Rev Respir Med. 2020;14(10):973‐986. [DOI] [PubMed] [Google Scholar]
- 20. Delgado‐Dolset MI, Obeso D, Rodríguez‐Coira J, et al. Understanding uncontrolled severe allergic asthma by integration of omic and clinical data. Allergy. 2021. doi: 10.1111/all.15192. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 21. Ziska LH, Makra L, Harry SK, et al. Temperature‐related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: a retrospective data analysis. Lancet Planet Health. 2019;3(3):e124‐e131. [DOI] [PubMed] [Google Scholar]
- 22. Ziska L, Knowlton K, Rogers C, et al. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc Natl Acad Sci. 2011;108(10):4248‐4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damialis A, Mohammad AB, Halley JM, Gange AC. Fungi in a changing world: growth rates will be elevated, but spore production may decrease in future climates. Int J Biometeorol. 2015;59(9):1157‐1167. [DOI] [PubMed] [Google Scholar]
- 24. Zhao F, Elkelish A, Durner J, et al. Common ragweed (Ambrosia artemisiifolia L.): allergenicity and molecular characterization of pollen after plant exposure to elevated NO2. Plant, Cell Environ. 2016;39(1):147‐164. [DOI] [PubMed] [Google Scholar]
- 25. Lang‐Yona N, Shuster‐Meiseles T, Mazar Y, Yarden O, Rudich Y. Impact of urban air pollution on the allergenicity of Aspergillus fumigatus conidia: outdoor exposure study supported by laboratory experiments. Sci Total Environ. 2016;541:365‐371. [DOI] [PubMed] [Google Scholar]
- 26. Kolářová E, Nekovář J, Adamík P. Long‐term temporal changes in central European tree phenology (1946–2010) confirm the recent extension of growing seasons. Int J Biometeorol. 2014;58(8):1739‐1748. [DOI] [PubMed] [Google Scholar]
- 27. Buters J, Prank M, Sofiev M, et al. Variation of the group 5 grass pollen allergen content of airborne pollen in relation to geographic location and time in season. J Allergy Clin Immunol. 2015;136(1):87‐95.e6. [DOI] [PubMed] [Google Scholar]
- 28. Shukla P, Skea J & Calvo Buendia E et al. IPCC, 2019: Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. 2019.
- 29. Shankar HM, Rice MB. Update on climate change: its impact on respiratory health at work, home, and at play. Clin Chest Med. 2020;41(4):753‐761. [DOI] [PubMed] [Google Scholar]
- 30. Blanco G, Gerlagh R, Suh S, et al. Trends and Mitigation. 2014.
- 31. Gulzar A, Mehmood M, Ganie S, Showqi I. A brief review on global warming and climate change: consequences and mitigation strategies. Int J Adv Res Sci Eng. 2018;7:2146‐2156. [Google Scholar]
- 32. Weiskopf SR, Rubenstein MA, Crozier LG, et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci Total Environ. 2020;733:137782. [DOI] [PubMed] [Google Scholar]
- 33. D'Amato G, Holgate ST, Pawankar R, et al. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ J. 2015;8(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D'Amato G, Akdis CA. Global warming, climate change, air pollution and allergies. Allergy. 2020;75(9):2158‐2160. [DOI] [PubMed] [Google Scholar]
- 35. Levitus S, Antonov JI, Boyer TP, Locarnini RA, Garcia HE, Mishonov AV. Global ocean heat content 1955–2008 in light of recently revealed instrumentation problems. Geophys Res Lett. 2009;36(7):L07608. doi: 10.1029/2008GL037155 [DOI] [Google Scholar]
- 36. Church JA, White NJ. A 20th century acceleration in global sea‐level rise. Geophys Res Lett. 2006;33(1):L10602. doi: 10.1029/2005GL024826 [DOI] [Google Scholar]
- 37. Hausfather Z, Cowtan K, Clarke DC, Jacobs P, Richardson M, Rohde R. Assessing recent warming using instrumentally homogeneous sea surface temperature records. Sci Adv. 2017;3(1):e1601207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwok R, Rothrock D. Decline in Arctic sea ice thickness from submarine and ICESat records: 1958–2008. Geophys Res Lett. 2009;36(15):L15501. doi: 10.1029/2009GL039035 [DOI] [Google Scholar]
- 39. Jacob T, Wahr J, Pfeffer WT, Swenson S. Recent contributions of glaciers and ice caps to sea level rise. Nature. 2012;482(7386):514‐518. [DOI] [PubMed] [Google Scholar]
- 40. Zemp M, Frey H, Gärtner‐Roer I, et al. Historically unprecedented global glacier decline in the early 21st century. J Glaciol. 2015;61(228):745‐762. [Google Scholar]
- 41. Kunkel KE, Robinson DA, Champion S, Yin X, Estilow T, Frankson RM. Trends and extremes in Northern Hemisphere snow characteristics. Curr Clim Change Rep. 2016;2(2):65‐73. [Google Scholar]
- 42. Bernhard GH, Neale RE, Barnes PW, et al. Environmental effects of stratospheric ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2019. Photochem Photobiol Sci off J Eur Photochem Ass Eur Soc Photobiol. 2020;19(5):542‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bais AF, McKenzie RL, Bernhard G, et al. Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci. 2015;14(1):19‐52. [DOI] [PubMed] [Google Scholar]
- 44. Bornman JF, Barnes PW, Robson TM, et al. Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochem Photobiol Sci. 2019;18(3):681‐716. [DOI] [PubMed] [Google Scholar]
- 45. Peterson TC, Karl TR, Kossin JP, et al. Changes in weather and climate extremes: State of knowledge relevant to air and water quality in the United States. J Air Waste Manag Assoc. 2014;64(2):184‐197. [DOI] [PubMed] [Google Scholar]
- 46. Allan RP, Soden BJ. Atmospheric warming and the amplification of precipitation extremes. Science. 2008;321(5895):1481‐1484. [DOI] [PubMed] [Google Scholar]
- 47. Bell TL, Rosenfeld D, Kim KM, Yoo JM, Lee MI, Hahnenberger M. Midweek increase in US summer rain and storm heights suggests air pollution invigorates rainstorms. J Geophys Res Atm. 2008;113:D02209. doi: 10.1029/2007JD008623 [DOI] [Google Scholar]
- 48. Changnon SA Jr. Urban effects on severe local storms at St. Louis. J Appl Meteorol Climatol. 1978;17(5):578‐586. [Google Scholar]
- 49. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heinrich J, Guo F, Fuertes E. Traffic‐Related air pollution exposure and asthma, hayfever, and allergic sensitisation in birth cohorts: a systematic review and MetaAnalysis. 2016.
- 51. Clayton S. Climate change and mental health. Curr Environ Health Rep. 2021;8(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 52. Nikendei C, Bugaj TJ, Nikendei F, Kuhl SJ, Kuhl M. Climate change: Causes, consequences, solutions and public health care implications Z Evid Fortbild Qual Gesundhwes. 2020;156–157:59‐67. [DOI] [PubMed] [Google Scholar]
- 53. Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol. 2003;111(2):290‐295. [DOI] [PubMed] [Google Scholar]
- 54. Zhao F, Durner J, Winkler JB, et al. Pollen of common ragweed (Ambrosia artemisiifolia L.): Illumina‐based de novo sequencing and differential transcript expression upon elevated NO2/O3. Environ Pollut. 2017;224:503‐514. [DOI] [PubMed] [Google Scholar]
- 55. El Kelish A, Zhao F, Heller W, et al. Ragweed (Ambrosia artemisiifolia) pollen allergenicity: SuperSAGE transcriptomic analysis upon elevated CO 2 and drought stress. BMC Plant Biol. 2014;14(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Cleemput J, Poelaert KCK, Laval K, et al. Pollens destroy respiratory epithelial cell anchors and drive alphaherpesvirus infection. Sci Rep. 2019;9(1):4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zang SM, Benjenk I, Breakey S, Pusey‐Reid E, Nicholas PK. The intersection of climate change with the era of COVID‐19. Public Health Nurs. 2021;38(2):321‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aliee H, Massip F, Qi C, et al. Determinants of expression of SARS‐CoV‐2 entry‐related genes in upper and lower airways. Allergy. 2021;77(2):690‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Damialis A, Gilles S, Sofiev M, et al. Higher airborne pollen concentrations correlated with increased SARS‐CoV‐2 infection rates, as evidenced from 31 countries across the globe. Proc Nat Acad Sci. 2021;118(12):e2019034118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McIntyre KM, Setzkorn C, Hepworth PJ, Morand S, Morse AP, Baylis M. Systematic assessment of the climate sensitivity of important human and domestic animals pathogens in Europe. Sci Rep. 2017;7(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barry M, Annesi‐Maesano I. Ten principles for climate, environment and respiratory health. Eur Respir J. 2017;50:1701912. [DOI] [PubMed] [Google Scholar]
- 62. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. D’Amato G, Chong‐Neto HJ, Monge Ortega OP, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy. 2020;75(9):2219‐2228. [DOI] [PubMed] [Google Scholar]
- 64. Smith AM, Ramirez RM, Harper N, et al. Large‐scale provocation studies identify maladaptive responses to ubiquitous aeroallergens as a correlate of severe allergic rhinoconjunctivitis and asthma. Allergy. 2022. doi: 10.1111/all.15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manangan A, Brown C, Saha S, et al. Long‐term pollen trends and associations between pollen phenology and seasonal climate in Atlanta, Georgia (1992‐2018). Ann Allergy Asthma Immunol. 2021;127(4):471‐480.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ziska LH, Makra L, Harry SK, et al. Temperature‐related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: a retrospective data analysis. Lancet Plan Health. 2019;3(3):e124‐e131. [DOI] [PubMed] [Google Scholar]
- 67. Anderegg WR, Abatzoglou JT, Anderegg LD, Bielory L, Kinney PL, Ziska L. Anthropogenic climate change is worsening North American pollen seasons. Proc Natl Acad Sci. 2021;118(7):e2013284118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ziello C, Sparks TH, Estrella N, et al. Changes to airborne pollen counts across Europe. PLoS One. 2012;7(4):e34076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rao CY, Riggs MA, Chew GL, et al. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl Environ Microbiol. 2007;73(5):1630‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rando RJ, Kwon C‐W, Lefante JJ. Exposures to thoracic particulate matter, endotoxin, and glucan during post‐hurricane Katrina restoration work, New Orleans 2005–2012. J Occup Environm Hygiene. 2014;11(1):9‐18. [DOI] [PubMed] [Google Scholar]
- 71. Paudel B, Chu T, Chen M, Sampath V, Prunicki M, Nadeau KC. Increased duration of pollen and mold exposure are linked to climate change. Sci Rep. 2021;11(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Acevedo N, Zakzuk J, Caraballo L. House dust mite allergy under changing environments. Allergy Asthma Immunol Res. 2019;11(4):450‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang Y, Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2019;11(2):156‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang W, Huang X, Chen Z, et al. Prevalence and trends of sensitisation to aeroallergens in patients with allergic rhinitis in Guangzhou, China: a 10‐year retrospective study. BMJ Open. 2016;6(5):e011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stas M, Aerts R, Hendrickx M, et al. Exposure to green space and pollen allergy symptom severity: a case‐crossover study in Belgium. Sci Total Environ. 2021;781:146682. [DOI] [PubMed] [Google Scholar]
- 76. Naclerio R, Ansotegui IJ, Bousquet J, et al. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: impact of air pollution on patients with AR: current knowledge and future strategies. World Allergy Org J. 2020;13(3):100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vitalpur G, Ahmad HA, Slaven JE. Weed pollen season trends in relation to atmospheric co2 changes in Indiana and Ohio. Ann Allergy Asthma Immunol. 2019;123(3):306‐307. [DOI] [PubMed] [Google Scholar]
- 78. Rauer D, Gilles S, Wimmer M, et al. Ragweed plants grown under elevated CO2 levels produce pollen which elicit stronger allergic lung inflammation. Allergy. 2021;76(6):1718‐1730. [DOI] [PubMed] [Google Scholar]
- 79. Khreis H, Cirach M, Mueller N, et al. Outdoor air pollution and the burden of childhood asthma across Europe. Eur Respir J. 2019;54(4). [DOI] [PubMed] [Google Scholar]
- 80. Organization WH . WHO global air quality guidelines: particulate matter (PM2. 5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide: executive summary. 2021. [PubMed]
- 81. Gehring U, Wijga AH, Koppelman GH, Vonk JM, Smit HA, Brunekreef B. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J. 2020;56(1):2000147. [DOI] [PubMed] [Google Scholar]
- 82. Esposito S, Tenconi R, Lelii M, et al. Possible molecular mechanisms linking air pollution and asthma in children. BMC Pulmon Med. 2014;14(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of US women. Am J Respir Crit Care Med. 2014;190(8):914‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bowatte G, Lodge C, Lowe AJ, et al. The influence of childhood traffic‐related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta‐analysis of birth cohort studies. Allergy. 2015;70(3):245‐256. [DOI] [PubMed] [Google Scholar]
- 85. Jacquemin B, Siroux V, Sanchez M, et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE). Environ Health Perspect. 2015;123(6):613‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic‐related air pollution and risk of development of childhood asthma: a systematic review and meta‐analysis. Environ Int. 2017;100:1‐31. [DOI] [PubMed] [Google Scholar]
- 87. Rancière F, Bougas N, Viola M, Momas I. Early exposure to traffic‐related air pollution, respiratory symptoms at 4 years of age, and potential effect modification by parental allergy, stressful family events, and sex: a prospective follow‐up study of the PARIS birth cohort. Environ Health Perspect. 2017;125(4):737‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rice MB, Rifas‐Shiman SL, Litonjua AA, et al. Lifetime air pollution exposure and asthma in a pediatric birth cohort. J Allergy Clin Immunol. 2018;141(5):1932–1934. e1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Deng S‐Z, Jalaludin BB, Antó JM, Hess JJ, Huang C‐R. Climate change, air pollution, and allergic respiratory diseases: a call to action for health professionals. Chin Med J. 2020;133(13):1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dehdari Rad H, Assarehzadegan M‐A, Goudarzi G, et al. Do Conocarpus erectus airborne pollen grains exacerbate autumnal thunderstorm asthma attacks in Ahvaz, Iran? Atmos Environ. 2019;213:311‐325. [Google Scholar]
- 91. Thien F, Beggs PJ, Csutoros D, et al. The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health. 2018;2(6):e255‐e263. [DOI] [PubMed] [Google Scholar]
- 92. Thien F. Thunderstorm asthma: potential danger but a unique opportunity. Asia Pacific Allergy. 2017;7(2):55‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Damialis A, Bayr D, Leier‐Wirtz V, et al. Thunderstorm Asthma: in search for relationships with airborne pollen and fungal spores from 23 sites in Bavaria, Germany. A rare incident or a common threat? J Allergy Clin Immunol. 2020;145(2):AB336. [Google Scholar]
- 94. Packe G, Ayres J. Asthma outbreak during a thunderstorm. Lancet. 1985;326(8448):199‐204. [DOI] [PubMed] [Google Scholar]
- 95. Marks GB, Colquhoun JR, Girgis ST, et al. Thunderstorm outflows preceding epidemics of asthma during spring and summer. Thorax. 2001;56(6):468‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. D'Amato G, Liccardi G, Frenguelli G. Thunderstorm‐asthma and pollen allergy. Allergy. 2007;62(1):11‐16. [DOI] [PubMed] [Google Scholar]
- 97. Hew M, Lee J, Susanto NH, et al. The 2016 Melbourne thunderstorm asthma epidemic: Risk factors for severe attacks requiring hospital admission. Allergy. 2019;74(1):122‐130. [DOI] [PubMed] [Google Scholar]
- 98. Hew M, Lee J, Varese N, et al. Epidemic thunderstorm asthma susceptibility from sensitization to ryegrass (Lolium perenne) pollen and major allergen Lol p 5. Allergy. 2020;75(9):2369‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sénéchal H, Visez N, Charpin D, et al. A Review of the effects of major atmospheric pollutants on pollen grains, pollen content, and allergenicity. Sci World J. 2015;2015:940243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. D'Amato G, Annesi‐Maesano I, Cecchi L, D'Amato M. Latest news on relationship between thunderstorms and respiratory allergy, severe asthma, and deaths for asthma. Allergy. 2019;74(1):9‐11. [DOI] [PubMed] [Google Scholar]
- 101. Hughes DD, Mampage CB, Jones LM, Liu Z, Stone EA. Characterization of atmospheric pollen fragments during springtime thunderstorms. Environm Sci Technol Lett. 2020;7(6):409‐414. [Google Scholar]
- 102. Cleland SE, Serre ML, Rappold AG, West JJ. Estimating the acute health impacts of fire‐originated PM2. 5 exposure during the 2017 California Wildfires: Sensitivity to choices of inputs. GeoHealth. 2021;5(7):e2021GH000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Arriagada NB, Horsley JA, Palmer AJ, Morgan GG, Tham R, Johnston FH. Association between fire smoke fine particulate matter and asthma‐related outcomes: systematic review and meta‐analysis. Environ Res. 2019;179:108777. [DOI] [PubMed] [Google Scholar]
- 104. Kondo M, De Roos A, White L, et al. Meta‐analysis of heterogeneity in the effects of wildfire smoke exposure on respiratory health in North America. Int J Environ Res Public Health. 2019;16(6):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Roscioli E, Hamon R, Lester SE, Jersmann HP, Reynolds PN, Hodge S. Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD. Respir Res. 2018;19(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Atafar Z, Pourpak Z, Yunesian M, et al. Proinflammatory effects of dust storm and thermal inversion particulate matter (PM 10) on human peripheral blood mononuclear cells (PBMCs) in vitro: a comparative approach and analysis. J Environm Health Sci Eng. 2019;17(1):433‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lorentzou C, Kouvarakis G, Kozyrakis GV, et al. Extreme desert dust storms and COPD morbidity on the island of Crete. Int J Chron Obstruct Pulmon Dis. 2019;14:1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lieberman JA, Gupta RS, Knibb RC, et al. The global burden of illness of peanut allergy: a comprehensive literature review. Allergy. 2021;76(5):1367‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Warren C, Lei D, Sicherer S, Schleimer R, Gupta R. Prevalence and characteristics of peanut allergy in US adults. J Allergy Clin Immunol. 2021;147(6):pp. 2263–2270. e2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Beggs PJ, Walczyk NE. Impacts of climate change on plant food allergens: a previously unrecognized threat to human health. Air Qual Atmos Health. 2008;1(2):119‐123. [Google Scholar]
- 111. Ziska LH, Yang J, Tomecek MB, Beggs PJ. Cultivar‐specific changes in peanut yield, biomass, and allergenicity in response to elevated atmospheric carbon dioxide concentration. Crop Sci. 2016;56(5):2766‐2774. [Google Scholar]
- 112. Loraud C, de Ménonville CT, Bourgoin‐Heck M, Cottel N, Wanin S, Just J. Emergence of pollen food allergy syndrome in asthmatic children in Paris. Pediatr Allergy Immunol. 2021;32(4):702‐708. [DOI] [PubMed] [Google Scholar]
- 113. Chen N‐T, Chen M‐J, Wu C‐D, Guo YL. Emergency room visits for childhood atopic dermatitis are associated with floods? Sci Total Environ. 2021;773:145435. [DOI] [PubMed] [Google Scholar]
- 114. Patella V, Florio G, Palmieri M, et al. Atopic dermatitis severity during exposure to air pollutants and weather changes with an Artificial Neural Network (ANN) analysis. Pediatr Allergy Immunol. 2020;31(8):938‐945. [DOI] [PubMed] [Google Scholar]
- 115. Napolitano M, Monfrecola G, Fabbrocini G, Fattore D, Patrí A, Patruno C. Impact of sun exposure on adult patients affected by atopic dermatitis. Giornale Italiano di Dermatologia e Venereologia: Organo Ufficiale, Societa Italiana di Dermatologia e Sifilografia. 2020.
- 116. Patrizi A, Raone B, Ravaioli GM. Management of atopic dermatitis: safety and efficacy of phototherapy. Clin Cosmet Investig Dermatol. 2015;8:511‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. To T, Zhu J, Stieb D, et al. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur Respir J. 2020;55(2):1900913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Aguilera AC, Dagher IA, Kloepfer KM. Role of the microbiome in allergic disease development. Curr Allergy Asthma Rep. 2020;20(9):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ruff WE, Greiling TM, Kriegel MA. Host‐microbiota interactions in immune‐mediated diseases. Nat Rev Microbiol. 2020;18(9):521‐538. [DOI] [PubMed] [Google Scholar]
- 120. Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(Suppl 1):S38‐S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219‐232. [DOI] [PubMed] [Google Scholar]
- 122. Caruso R, Lo BC, Nunez G. Host‐microbiota interactions in inflammatory bowel disease. Nat Rev Immunol. 2020;20(7):411‐426. [DOI] [PubMed] [Google Scholar]
- 123. Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two‐way street. Mucosal Immunol. 2017;10(2):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN‐gamma and IL‐4. J Allergy Clin Immunol. 2012;130(5):1087‐1096 e1010. [DOI] [PubMed] [Google Scholar]
- 125. Masterson JC, Biette KA, Hammer JA, et al. Epithelial HIF‐1alpha/claudin‐1 axis regulates barrier dysfunction in eosinophilic esophagitis. J Clin Invest. 2019;129(8):3224‐3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93‐103. [DOI] [PubMed] [Google Scholar]
- 127. De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773‐786.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dainichi T, Kitoh A, Otsuka A, et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286‐1298. [DOI] [PubMed] [Google Scholar]
- 129. Kim Y‐C, Won H‐K, Lee JW, et al. Staphylococcus aureus nasal colonization and asthma in adults: systematic review and meta‐analysis. J Allergy Clin Immunol Pract. 2019;7(2):606‐615 e609. [DOI] [PubMed] [Google Scholar]
- 130. Ong PY. New insights in the pathogenesis of atopic dermatitis. Pediatr Res. 2014;75(1–2):171‐175. [DOI] [PubMed] [Google Scholar]
- 131. Altunbulakli C, Reiger M, Neumann AU, et al. Relations between epidermal barrier dysregulation and Staphylococcus species–dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol. 2018;142(5):pp. 1643–1647. e1612. [DOI] [PubMed] [Google Scholar]
- 132. Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Chronic rhinosinusitis: pathogenesis, therapy options, and more. Expert Opin Pharmacother. 2018;19(16):1805‐1815. [DOI] [PubMed] [Google Scholar]
- 133. Sintobin I, Siroux V, Holtappels G, et al. Sensitisation to staphylococcal enterotoxins and asthma severity: a longitudinal study in the EGEA cohort. Eur Respir J. 2019;54(3):1900198. [DOI] [PubMed] [Google Scholar]
- 134. Teufelberger AR, Broker BM, Krysko DV, Bachert C, Krysko O. Staphylococcus aureus Orchestrates Type 2 Airway diseases. Trends Mol Med. 2019;25(8):696‐707. [DOI] [PubMed] [Google Scholar]
- 135. Naik S, Bouladoux N, Linehan JL, et al. Commensal‐dendritic‐cell interaction specifies a unique protective skin immune signature. Nature. 2015;520(7545):104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Linehan JL, Harrison OJ, Han S‐J, et al. Non‐classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell. 2018;172(4):784‐796 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Scharschmidt T, Vasquez K, Truong H‐A, et al. A wave of regulatory t cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015;43(5):1011‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(6):852. [DOI] [PubMed] [Google Scholar]
- 140. Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511‐521. [DOI] [PubMed] [Google Scholar]
- 141. Gollwitzer ES, Saglani S, Trompette A, et al. Lung microbiota promotes tolerance to allergens in neonates via PD‐L1. Nat Med. 2014;20(6):642‐647. [DOI] [PubMed] [Google Scholar]
- 142. Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Platts‐Mills T, Erwin E, Heymann P, Woodfolk J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy. 2005;60:25‐31. [DOI] [PubMed] [Google Scholar]
- 144. Fiuza BSD, Fonseca HF, Meirelles PM, Marques CR, da Silva TM, Figueiredo CA. Understanding asthma and allergies by the lens of biodiversity and epigenetic changes. Front Immunol. 2021;12:623737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lynch SV, Microbiota VD. Epigenetics, and trained immunity. convergent drivers and mediators of the asthma trajectory from pregnancy to childhood. Am J Respir Crit Care Med. 2021;203(7):802‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gilbert JA, Lynch SV. Community ecology as a framework for human microbiome research. Nat Med. 2019;25(6):884‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18(2):105‐120. [DOI] [PubMed] [Google Scholar]
- 148. Patra V, Laoubi L, Nicolas JF, Vocanson M, Wolf P. A perspective on the interplay of ultraviolet‐radiation, skin microbiome and skin resident memory TCRalphabeta+ Cells. Front Med. 2018;5:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O'Mahony L. Recent developments and highlights in mechanisms of allergic diseases: Microbiome. Allergy. 2018;73(12):2314‐2327. [DOI] [PubMed] [Google Scholar]
- 150. Haahtela T, Holgate S, Pawankar R, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Altunbulakli C, Costa R, Lan F, et al. Staphylococcus aureus enhances the tight junction barrier integrity in healthy nasal tissue, but not in nasal polyps. J Allergy Clin Immunol. 2018;142(2):665‐668.e8. [DOI] [PubMed] [Google Scholar]
- 152. Mouries J, Brescia P, Silvestri A, et al. Microbiota‐driven gut vascular barrier disruption is a prerequisite for non‐alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kim J‐W, Kwok S‐K, Choe J‐Y, Park S‐H. Recent advances in our understanding of the link between the intestinal microbiota and systemic lupus erythematosus. Int J Mol Sci. 2019;20(19):4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Earley ZM, Akhtar S, Green SJ, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10(7):e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lynch SV, Wood RA, Boushey H, et al. Effects of early‐life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134(3):593‐601 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cereta AD, Oliveira VR, Costa IP, et al. Early life microbial exposure and immunity training effects on asthma development and progression. Front Med. 2021;8: 662262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gollwitzer ES, Marsland BJ. Impact of early‐life exposures on immune maturation and susceptibility to disease. Trends Immunol. 2015;36(11):684‐696. [DOI] [PubMed] [Google Scholar]
- 158. Tang HHF, Teo SM, Sly PD, Holt PG, Inouye M. The intersect of genetics, environment, and microbiota in asthma‐perspectives and challenges. J Allergy Clin Immunol. 2021;147(3):781‐793. [DOI] [PubMed] [Google Scholar]
- 159. Siracusa A, De Blay F, Folletti I, et al. Asthma and exposure to cleaning products–a European A cademy of A llergy and C linical I mmunology task force consensus statement. Allergy. 2013;68(12):1532‐1545. [DOI] [PubMed] [Google Scholar]
- 160. Natividad JM, Rytz A, Keddani S, Bergonzelli G, Garcia‐Rodenas CL. Blends of human milk oligosaccharides confer intestinal epithelial barrier protection in vitro. Nutrients. 2020;12(10):3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136(4):860‐865. [DOI] [PubMed] [Google Scholar]
- 162. Radon K, Windstetter D, Eckart J, et al. Farming exposure in childhood, exposure to markers of infections and the development of atopy in rural subjects. Clin Exp Allergy. 2004;34(8):1178‐1183. [DOI] [PubMed] [Google Scholar]
- 163. Waser M, Schierl R, Von mutius E, et al. Determinants of endotoxin levels in living environments of farmers’ children and their peers from rural areas. Clin Exp Allergy. 2004;34(3):389‐397. [DOI] [PubMed] [Google Scholar]
- 164. Mendy A, Gasana J, Vieira ER, et al. Endotoxin exposure and childhood wheeze and asthma: a meta‐analysis of observational studies. J Asthma. 2011;48(7):685‐693. [DOI] [PubMed] [Google Scholar]
- 165. Arrieta M‐C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. [DOI] [PubMed] [Google Scholar]
- 166. Martinez FD. Childhood asthma inception and progression: role of microbial exposures, susceptibility to viruses and early allergic sensitization. Immunol Allergy Clin North Am. 2019;39(2):141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lee J‐J, Kim S‐H, Lee M‐J, et al. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy. 2019;74(4):709‐719. [DOI] [PubMed] [Google Scholar]
- 169. Lal D, Keim P, Delisle J, et al. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int Forum Allergy Rhinol. 2017;7(6):561‐569. [DOI] [PubMed] [Google Scholar]
- 170. Choi CH, Poroyko V, Watanabe SO, et al. Seasonal allergic rhinitis affects sinonasal microbiota. Am J Rhinol Allergy. 2014;28(4):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ruokolainen L, Paalanen L, Karkman A, et al. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin Exp Allergy. 2017;47(5):665‐674. [DOI] [PubMed] [Google Scholar]
- 172. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62(2):151‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Stefanovic N, Irvine AD, Flohr C. The role of the environment and exposome in atopic dermatitis. Curr Treat Options Allergy. 2021;1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nakamura Y, Oscherwitz J, Cease KB, et al. Staphylococcus delta‐toxin induces allergic skin disease by activating mast cells. Nature. 2013;503(7476):397‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kim J, Kim BE, Ahn K, Leung DY. Interactions between atopic dermatitis and Staphylococcus aureus infection: clinical implications. Allergy, Asthma Immunol Res. 2019;11(5):593‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Pessemier BD, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut‐Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms. 2021;9(2):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315‐1327. [DOI] [PubMed] [Google Scholar]
- 178. Elias MS, Long HA, Newman CF, et al. Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J Allergy Clin Immunol. 2017;140(5):1299‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Muhandes L, Pippel M & Chapsa M, et al. Filaggrin deficient mice have a lower threshold for cutaneous allergen sensitization but do not develop spontaneous skin inflammation or atopy. bioRxiv. 2020. doi: 10.1101/2020.09.11.293688 [DOI] [Google Scholar]
- 180. Paller AS, Kong HH, Seed P, et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta‐analysis. Br J Dermatol. 2016;175(4):687‐695. [DOI] [PubMed] [Google Scholar]
- 182. Meylan P, Lang C, Mermoud S, et al. Skin colonization by staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol. 2017;137(12):2497‐2504. [DOI] [PubMed] [Google Scholar]
- 183. Park DH, Kim JW, Park HJ, Hahm DH. Comparative analysis of the microbiome across the gut‐skin axis in atopic dermatitis. Int J Mol Sci. 2021;22(8):4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy. 2015;45(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 185. Savage JH, Lee‐Sarwar KA, Sordillo J, et al. A prospective microbiome‐wide association study of food sensitization and food allergy in early childhood. Allergy. 2018;73(1):145‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark‐Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39(4):518‐526. [DOI] [PubMed] [Google Scholar]
- 187. Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646‐652.e5. [DOI] [PubMed] [Google Scholar]
- 188. Bunyavanich S. Food allergy: could the gut microbiota hold the key? Nat Rev Gastroenterol Hepatol. 2019;16(4):201‐202. [DOI] [PubMed] [Google Scholar]
- 189. Kumbhare SV, Patangia DVV, Patil RH, Shouche YS, Patil NP. Factors influencing the gut microbiome in children: from infancy to childhood. J Biosci. 2019;44(2):49. [PubMed] [Google Scholar]
- 190. Azad MB, Konya T, Guttman DS, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632‐643. [DOI] [PubMed] [Google Scholar]
- 191. Jungles KN, Jungles KM, Greenfield L, Mahdavinia M. the infant microbiome and its impact on development of food allergy. Immunol Allergy Clin North Am. 2021;41(2):285‐299. [DOI] [PubMed] [Google Scholar]
- 192. Jarvinen KM, Martin H, Oyoshi MK. Immunomodulatory effects of breast milk on food allergy. Ann Allergy Asthma Immunol. 2019;123(2):133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Castro‐Rodriguez JA, Garcia‐Marcos L. What are the effects of a mediterranean diet on allergies and asthma in children? Front Pediatr. 2017;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Grimshaw KEC, Maskell J, Oliver EM, et al. Diet and food allergy development during infancy: birth cohort study findings using prospective food diary data. J Allergy Clin Immunol. 2014;133(2):511‐519. [DOI] [PubMed] [Google Scholar]
- 195. Sandin A, Braback L, Norin E, Bjorksten B. Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatr. 2009;98(5):823‐827. [DOI] [PubMed] [Google Scholar]
- 196. Vuillermin PJ, O’Hely M, Collier F, et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat Commun. 2020;11(1):1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fazlollahi M, Chun Y, Grishin A, et al. Early‐life gut microbiome and egg allergy. Allergy. 2018;73(7):1515‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15(5):308‐322. [DOI] [PubMed] [Google Scholar]
- 199. Venter C, Meyer RW, Nwaru BI, et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. 2019;74(8):1429‐1444. [DOI] [PubMed] [Google Scholar]
- 200. Miles EA, Calder PC. Omega‐6 and omega‐3 polyunsaturated fatty acids and allergic diseases in infancy and childhood. Curr Pharm Des. 2014;20(6):946‐953. [DOI] [PubMed] [Google Scholar]
- 201. Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178(1):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Koltsida O, Karamnov S, Pyrillou K, et al. Toll‐like receptor 7 stimulates production of specialized pro‐resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 2013;5(5):762‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Nwaru BI, Erkkola M, Lumia M, et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. Br J Nutr. 2012;108(4):720‐732. [DOI] [PubMed] [Google Scholar]
- 204. Thang CL, Boye JI, Shi HN, Zhao X. Effects of supplementing different ratios of omega‐3 and omega‐6 fatty acids in western‐style diets on cow's milk protein allergy in a mouse model. Mol Nutr Food Res. 2013;57(11):2029‐2038. [DOI] [PubMed] [Google Scholar]
- 205. Innes JK, Calder PC. Omega‐6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41‐48. [DOI] [PubMed] [Google Scholar]
- 206. Kytikova O, Novgorodtseva T, Denisenko Y, Antonyuk M, Gvozdenko T. Pro‐resolving lipid mediators in the pathophysiology of asthma. Medicina. 2019;55(6):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Gref A, Rautiainen S, Gruzieva O, et al. Dietary total antioxidant capacity in early school age and subsequent allergic disease. Clin Exp Allergy. 2017;47(6):751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. van Neerven R, Savelkoul H. Nutrition and allergic diseases. Nutrients. 2017;9(7):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Helm RM. Food biotechnology: is this good or bad? Implications to allergic diseases. Ann Allergy Asthma Immunol. 2003;90(6):90‐98. [DOI] [PubMed] [Google Scholar]
- 210. Lupi R, Denery‐Papini S, Rogniaux H, et al. How much does transgenesis affect wheat allergenicity?: Assessment in two GM lines over‐expressing endogenous genes. J Proteomics. 2013;80:281‐291. [DOI] [PubMed] [Google Scholar]
- 211. Panda R, Ariyarathna H, Amnuaycheewa P, et al. Challenges in testing genetically modified crops for potential increases in endogenous allergen expression for safety. Allergy. 2013;68(2):142‐151. [DOI] [PubMed] [Google Scholar]
- 212. Ladics GS. Assessment of the potential allergenicity of genetically‐engineered food crops. J Immunotoxicol. 2019;16(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 213. Dunn SE, Vicini JL, Glenn KC, Fleischer DM, Greenhawt MJ. The allergenicity of genetically modified foods from genetically engineered crops: A narrative and systematic review. Ann Allergy Asthma Immunol. 2017;119(3):214‐222 e213. [DOI] [PubMed] [Google Scholar]
- 214. Frei R, Ferstl R, Roduit C, et al. Exposure to nonmicrobial N‐glycolylneuraminic acid protects farmers’ children against airway inflammation and colitis. J Allergy Clin Immunol. 2018;141(1):382‐390 e387. [DOI] [PubMed] [Google Scholar]
- 215. Frei R, Roduit C, Ferstl R, O'Mahony L, Lauener RP. Exposure of Children to Rural Lifestyle Factors Associated With Protection Against Allergies Induces an Anti‐Neu5Gc Antibody Response. Front Immunol. 2019;10:1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Adams S, Lopata AL, Smuts CM, Baatjies R, Jeebhay MF. Relationship between serum omega‐3 fatty acid and asthma endpoints. Int J Environ Res. 2019;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Nelson KA. The Effect of Fiber and Omega‐6: Omega‐3 Fatty Acid Ratio on Asthma and Asthma‐related Symptoms in an Adult US Population. Capstone Experience. 2021;135. https://digitalcommons.unmc.edu/coph_slce/135 [Google Scholar]
- 218. Hogenkamp A, Ehlers A, Garssen J, Willemsen LEM. Allergy modulation by N‐3 long chain polyunsaturated fatty acids and fat soluble nutrients of the mediterranean diet. Front Pharmacol. 2020;11:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J Allergy Clin Immunol. 2014;133(5):1255‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kytikova O, Novgorodtseva T, Denisenko Y, Antonyuk M, Gvozdenko T. Pro‐resolving lipid mediators in the pathophysiology of asthma. Medicina (Kaunas). 2019;55(6):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Maslova E, Rifas‐Shiman SL, Oken E, Platts‐Mills TAE, Gold DR. Fatty acids in pregnancy and risk of allergic sensitization and respiratory outcomes in childhood. Ann Allergy Asthma Immunol. 2019;122(1):120‐122 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Lumia M, Luukkainen P, Tapanainen H, et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr Allergy Immunol. 2011;22(8):827‐835. [DOI] [PubMed] [Google Scholar]
- 224. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n‐3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;2015(7):CD010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Gomez JL. Epigenetics in Asthma. Curr Allergy Asthma Rep. 2019;19(12):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Alhamwe BA, Alhamdan F, Ruhl A, Potaczek DP, Renz H. The role of epigenetics in allergy and asthma development. Curr Opin Allergy Clin Immunol. 2020;20(1):48‐55. [DOI] [PubMed] [Google Scholar]
- 227. Acevedo N, Frumento P, Harb H, et al. Histone acetylation of immune regulatory genes in human placenta in association with maternal intake of olive oil and fish consumption. Int J Mol Sci. 2019;20(5):1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74(4):799‐809. [DOI] [PubMed] [Google Scholar]
- 229. Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J. 2011;4(10):151‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Qu J, Li Y, Zhong W, Gao P, Hu C. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis. 2017;9(1):E32‐E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Allen S, Britton J, Leonardi‐Bee J. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta‐analysis. Thorax. 2009;64(7):610‐619. [DOI] [PubMed] [Google Scholar]
- 232. Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta‐analysis. J Allergy Clin Immunol. 2011;127(3):724‐733.e30. [DOI] [PubMed] [Google Scholar]
- 233. Konieczna P, Akdis CA, Quigley EM, Shanahan F, O'Mahony L. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes. 2012;3(3):261‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Konieczna P, Ferstl R, Ziegler M, et al. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid‐dependent and independent mechanisms. PLoS One. 2013;8(5):e62617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Konieczna P, Groeger D, Ziegler M, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61(3):354‐366. [DOI] [PubMed] [Google Scholar]
- 236. Hufnagl K, Jensen‐Jarolim E. Vitamin A and D in allergy: from experimental animal models and cellular studies to human disease. Allergo J Int. 2018;27(3):72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wee JH, Min C, Park MW, Park I‐S, Park B, Choi HG. Energy‐drink consumption is associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Eur J Clin Nutr. 2020;75(7):1077‐1087. [DOI] [PubMed] [Google Scholar]
- 238. Pestana S, Moreira M, Olej B. Safety of ingestion of yellow tartrazine by double‐blind placebo controlled challenge in 26 atopic adults. Allergol Immunopathol (Madr). 2010;38(3):142‐146. [DOI] [PubMed] [Google Scholar]
- 239. Wang X, Liu W, Hu Y, Zou Z, Shen L, Huang C. Home environment, lifestyles behaviors, and rhinitis in childhood. Int J Hyg Environ Health. 2016;219(2):220‐231. [DOI] [PubMed] [Google Scholar]
- 240. Maslova E, Strom M, Olsen SF, Halldorsson TI. Consumption of artificially‐sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS One. 2013;8(2):e57261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Magnusson J, Kull I, Westman M, et al. Fish and polyunsaturated fat intake and development of allergic and nonallergic rhinitis. J Allergy Clin Immunol. 2015;136(5):pp. 1247–1253 e1241–1242. [DOI] [PubMed] [Google Scholar]
- 242. Alm B, Goksör E, Thengilsdottir H, et al. Early protective and risk factors for allergic rhinitis at age 4½ yr. Pediatr Allergy Immunol. 2011;22(4):398‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Miyake Y, Sasaki S, Tanaka K, et al. Fish and fat intake and prevalence of allergic rhinitis in Japanese females: the Osaka Maternal and Child Health Study. J Am Coll Nutr. 2007;26(3):279‐287. [DOI] [PubMed] [Google Scholar]
- 244. Stratakis N, Roumeliotaki T, Oken E, et al. Fish and seafood consumption during pregnancy and the risk of asthma and allergic rhinitis in childhood: a pooled analysis of 18 European and US birth cohorts. Int J Epidemiol. 2017;46(5):1465‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Maslova E, Strøm M, Oken E, et al. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis–longitudinal evidence from the Danish National Birth Cohort. Brit J Nutr. 2013;110(7):1313‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Thien FCK, Mencia‐Huerta J‐M, Lee TH. Dietary fish oil effects on seasonal hay fever and asthma in pollen‐sensitive subjects. Am Rev Respir Dis. 1993;147:1138‐1143. [DOI] [PubMed] [Google Scholar]
- 247. Sawane K, Nagatake T, Hosomi K, et al. Dietary omega‐3 fatty acid dampens allergic rhinitis via eosinophilic production of the anti‐allergic lipid mediator 15‐hydroxyeicosapentaenoic acid in mice. Nutrients. 2019;11(12):2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Ozkaya E, Akduman H, Erenberk U, Demir A, Dundaroz MR. Plasma paraoxonase activity and oxidative stress and their relationship to disease severity in children with allergic rhinitis. Am J Rhinol Allergy. 2013;27(1):13‐17. [DOI] [PubMed] [Google Scholar]
- 249. Vollbracht C, Raithel M, Krick B, Kraft K, Hagel AF. Intravenous vitamin C in the treatment of allergies: an interim subgroup analysis of a long‐term observational study. J Int Med Res. 2018;46(9):3640‐3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Wang SY, Wang YF, Pan CC, Sun JW. Serum level and clinical significance of vitamin E in children with allergic rhinitis. BMC Pediatr. 2020;20(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Tongtako W, Klaewsongkram J, Mickleborough TD, Suksom D. Effects of aerobic exercise and vitamin C supplementation on rhinitis symptoms in allergic rhinitis patients. Asian Pac J Allergy Immunol. 2018;36(4):222‐231. [DOI] [PubMed] [Google Scholar]
- 252. Dunstan J, Breckler L, Hale J, et al. Supplementation with vitamins C, E, β‐carotene and selenium has no effect on anti‐oxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy. 2007;37(2):180‐187. [DOI] [PubMed] [Google Scholar]
- 253. Park S, Bae J‐H. Fermented food intake is associated with a reduced likelihood of atopic dermatitis in an adult population (Korean National Health and Nutrition Examination Survey 2012–2013). Nutr Res. 2016;36(2):125‐133. [DOI] [PubMed] [Google Scholar]
- 254. Park S, Choi HS, Bae JH. Instant noodles, processed food intake, and dietary pattern are associated with atopic dermatitis in an adult population (KNHANES 2009–2011). Asia Pac J Clin Nutr. 2016;25(3):602‐613. [DOI] [PubMed] [Google Scholar]
- 255. Anil H, Harmanci K. Evaluation of contact sensitivity to food additives in children with atopic dermatitis. Postepy Dermatol Alergol. 2020;37(3):390‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Finch J, Munhutu MN, Whitaker‐Worth DL. Atopic dermatitis and nutrition. Clin Dermatol. 2010;28(6):605‐614. [DOI] [PubMed] [Google Scholar]
- 257. Kull I, Bohme M, Wahlgren CF, Nordvall L, Pershagen G, Wickman M. Breast‐feeding reduces the risk for childhood eczema. J Allergy Clin Immunol. 2005;116(3):657‐661. [DOI] [PubMed] [Google Scholar]
- 258. Høst A, Halken S, Muraro A, et al. Dietary prevention of allergic diseases in infants and small children: Amendment to previous published articles in Pediatric Allergy and Immunology 2004, by an expert group set up by the Section on Pediatrics, European Academy of Allergology and Clinical Immunology. Pediatr Allergy Immunol. 2008;19(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 259. Trak‐Fellermeier MA, Brasche S, Winkler G, Koletzko B, Heinrich J. Food and fatty acid intake and atopic disease in adults. Eur Respir J. 2004;23(4):575‐582. [DOI] [PubMed] [Google Scholar]
- 260. Solvoll K, Søyland E, Sandstad B, Drevon C. Dietary habits among patients with atopic dermatitis. Eur J Clin Nutr. 2000;54(2):93‐97. [DOI] [PubMed] [Google Scholar]
- 261. Ito M, Morita T, Okazaki S, et al. Dietary habits in adult Japanese patients with atopic dermatitis. J Dermatol. 2019;46(6):515‐521. [DOI] [PubMed] [Google Scholar]
- 262. Koch C, Dölle S, Metzger M, et al. Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double‐blind, controlled trial. Br J Dermatol. 2008;158(4):786‐792. [DOI] [PubMed] [Google Scholar]
- 263. Eriksen BB, Kare DL. Open trial of supplements of omega 3 and 6 fatty acids, vitamins and minerals in atopic dermatitis. J Dermatolog Treat. 2006;17(2):82‐85. [DOI] [PubMed] [Google Scholar]
- 264. Søyland E, Funk J, Rajka G, et al. Dietary supplementation with very long‐chain n‐3 fatty acids in patients with atopic dermatitis. A double‐blind, multicentre study. Br J Dermatol. 1994;130(6):757‐764. [DOI] [PubMed] [Google Scholar]
- 265. Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016;2016:2721469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Shin J, Kim YJ, Kwon O, Kim N‐I, Cho Y. Associations among plasma vitamin C, epidermal ceramide and clinical severity of atopic dermatitis. Nutr Res Pract. 2016;10(4):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Jaffary F, Faghihi G, Mokhtarian A, Hosseini SM. Effects of oral vitamin E on treatment of atopic dermatitis: a randomized controlled trial. J Res Med Sci. 2015;20(11):1053‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Oh SY, Chung J, Kim MK, Kwon SO, Cho BH. Antioxidant nutrient intakes and corresponding biomarkers associated with the risk of atopic dermatitis in young children. Eur J Clin Nutr. 2010;64(3):245‐252. [DOI] [PubMed] [Google Scholar]
- 269. Hussain M, Bonilla‐Rosso G, Kwong Chung CKC, et al. High dietary fat intake induces a microbiota signature that promotes food allergy. J Allergy Clin Immunol. 2019;144(1):157‐170.e8. [DOI] [PubMed] [Google Scholar]
- 270. Kim YH, Kim KW, Lee S‐Y, et al. Maternal perinatal dietary patterns affect food allergy development in susceptible infants. J Allergy Clin Immunol Pract. 2019;7(7):2337‐2347 e2337. [DOI] [PubMed] [Google Scholar]
- 271. Berdi M, de Lauzon‐Guillain B, Forhan A, et al. Immune components of early breastmilk: association with maternal factors and with reported food allergy in childhood. Pediatr Allergy Immunol. 2019;30(1):107‐116. [DOI] [PubMed] [Google Scholar]
- 272. Jerschow E, McGinn AP, de Vos G, et al. Dichlorophenol‐containing pesticides and allergies: results from the US National Health and Nutrition Examination Survey 2005–2006. Ann Allergy Asthma Immunol. 2012;109(6):420‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Yamashita H, Matsuhara H, Miotani S, et al. Artificial sweeteners and mixture of food additives cause to break oral tolerance and induce food allergy in murine oral tolerance model for food allergy. Clin Exp Allergy. 2017;47(9):1204‐1213. [DOI] [PubMed] [Google Scholar]
- 274. Hoppenbrouwers T, Cvejic Hogervorst JH, Garssen J, Wichers HJ, Willemsen LEM. Long chain polyunsaturated fatty acids (LCPUFAs) in the prevention of food allergy. Front Immunol. 2019;10:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Kuśmierek M, Sardecka I, Łoś‐Rycharska E, Krogulska A. The impact of immunomodulatory factors from maternal diet during pregnancy on cow's milk allergy in offspring–A pilot study in the paediatric population of the Kuyavian‐Pomeranian Voivodship. Allergol Immunopathol. 2019;47(6):570‐578. [DOI] [PubMed] [Google Scholar]
- 276. Tuokkola J, Lamminsalo A, Metsälä J, et al. Maternal antioxidant intake during pregnancy and the development of cows’ milk allergy in the offspring. Br J Nutr. 2021;125(12):1386‐1393. [DOI] [PubMed] [Google Scholar]
- 277. Folletti I, Siracusa A, Paolocci G. Update on asthma and cleaning agents. Curr Opin Allergy Clin Immunol. 2017;17(2):90‐95. [DOI] [PubMed] [Google Scholar]
- 278. Wang M, Tan GE, Eljaszewicz A, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892‐1903. [DOI] [PubMed] [Google Scholar]
- 279. Xian MU, Wawrzyniak P, Rückert B, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol. 2016;138(3):890‐893.e9. [DOI] [PubMed] [Google Scholar]
- 280. Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. J Crohn's Colitis. 2013;7(4):338‐341. [DOI] [PubMed] [Google Scholar]
- 281. Eguiluz‐Gracia I, Mathioudakis AG, Bartel S, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75(9):2170‐2184. [DOI] [PubMed] [Google Scholar]
- 282. Chuang H‐C, Ho K‐F, Cao J‐J, et al. Effects of non‐protein‐type amino acids of fine particulate matter on E‐cadherin and inflammatory responses in mice. Toxicol Lett. 2015;237(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 283. Yang L, Li C, Tang X. The impact of PM2.5 on the host defense of respiratory system. Front Develop Biol. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Liu J, Chen X, Dou M, et al. Particulate matter disrupts airway epithelial barrier via oxidative stress to promote Pseudomonas aeruginosa infection. J Thorac Dis. 2019;11(6):2617‐2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Zhao R, Guo Z, Zhang R, et al. Nasal epithelial barrier disruption by particulate matter </=2.5 mum via tight junction protein degradation. J Appl Toxicol. 2018;38(5):678‐687. [DOI] [PubMed] [Google Scholar]
- 286. Xian MU, Ma S, Wang K, et al. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol Res. 2020;12(1):56‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Pacheco SE, Guidos‐Fogelbach G, Annesi‐Maesano I, et al. Climate change and global issues in allergy and immunology. J Allergy Clin Immunol. 2021;148(6):1366‐1377. [DOI] [PubMed] [Google Scholar]
- 288. Prunicki M, Stell L, Dinakarpandian D, et al. Exposure to NO2, CO, and PM2.5 is linked to regional DNA methylation differences in asthma. Clin. Epigenetics. 2018;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Prunicki M, Kelsey R, Lee J, et al. The impact of prescribed fire versus wildfire on the immune and cardiovascular systems of children. Allergy. 2019;74(10):1989‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Thevenot PT, Saravia J, Jin N, et al. Radical‐containing ultrafine particulate matter initiates epithelial‐to‐mesenchymal transitions in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;48(2):188‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Piao MJ, Ahn MJ, Kang KA, et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch Toxicol. 2018;92(6):2077‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Wang TY, Libardo MDJ, Angeles‐Boza AM, Pellois JP. Membrane oxidation in cell delivery and cell killing applications. ACS Chem Biol. 2017;12(5):1170‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Pan TL, Wang PW, Aljuffali IA, Huang CT, Lee CW, Fang JY. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J Dermatol Sci. 2015;78(1):51‐60. [DOI] [PubMed] [Google Scholar]
- 294. Ngoc LTN, Park D, Lee Y, Lee YC. Systematic review and meta‐analysis of human skin diseases due to particulate matter. Int J Environ Res Public Health. 2017;14(12):1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Tang KT, Ku KC, Chen DY, Lin CH, Tsuang BJ, Chen YH. Adult atopic dermatitis and exposure to air pollutants‐a nationwide population‐based study. Ann Allergy Asthma Immunol. 2017;118(3):351‐355. [DOI] [PubMed] [Google Scholar]
- 296. Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology. 2011;16(2):340‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Abayalath N, Malshani I, Ariyaratne R, et al. Characterization of airborne PAHs and metals associated with PM10 fractions collected from an urban area of Sri Lanka and the impact on airway epithelial cells. Chemosphere. 2022;286:131741. [DOI] [PubMed] [Google Scholar]
- 298. Kumar RK, Shadie AM, Bucknall MP, et al. Differential injurious effects of ambient and traffic‐derived particulate matter on airway epithelial cells. Respirology. 2015;20(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 299. Cortese A, Lova L, Comoli P, et al. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J Neuroinflammation. 2020;17(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Jeon YM, Lee MY. Airborne nanoparticles (PM0. 1) induce autophagic cell death of human neuronal cells. J Appl Toxicol. 2016;36(10):1332‐1342. [DOI] [PubMed] [Google Scholar]
- 301. Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26‐49. [DOI] [PubMed] [Google Scholar]
- 302. Poh TY, Ali NABM, Mac Aogáin M, et al. Inhaled nanomaterials and the respiratory microbiome: clinical, immunological and toxicological perspectives. Part Fibre Toxicol. 2018;15(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Vietti G, Lison D, van den Brule S. Mechanisms of lung fibrosis induced by carbon nanotubes: towards an Adverse Outcome Pathway (AOP). Part Fibre Toxicol. 2016;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Chakraborty S, Castranova V, Perez MK, Piedimonte G. Nanoparticles‐induced apoptosis of human airway epithelium is mediated by proNGF/p75(NTR) signaling. J Toxicol Environ Health A. 2017;80(1):53‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Urbančič I, Garvas M, Kokot B, et al. Nanoparticles can wrap epithelial cell membranes and relocate them across the epithelial cell layer. Nano Lett. 2018;18(8):5294‐5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Zhang LW, Monteiro‐Riviere NA. Toxicity assessment of six titanium dioxide nanoparticles in human epidermal keratinocytes. Cutan Ocul Toxicol. 2019;38(1):66‐80. [DOI] [PubMed] [Google Scholar]
- 307. Hiroike M, Sakabe J‐I, Kobayashi M, et al. Acicular, but not globular, titanium dioxide nanoparticles stimulate keratinocytes to produce pro‐inflammatory cytokines. J Dermatol. 2013;40(5):357‐362. [DOI] [PubMed] [Google Scholar]
- 308. Meng H, Leong W, Leong KW, Chen C, Zhao Y. Walking the line: the fate of nanomaterials at biological barriers. Biomaterials. 2018;174:41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Vita AA, Royse EA, Pullen NA. Nanoparticles and danger signals: Oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J Leukoc Biol. 2019;106(1):95‐103. [DOI] [PubMed] [Google Scholar]
- 310. Abudayyak M, Güzel E, Özhan G. Genotoxic, and apoptotic effects of nickel oxide nanoparticles in intestinal epithelial cells. Turk J Pharm Sci. 2020;17(4):446‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Kakinoki Y, Ayaki T, Yushi W, et al. Nitrogen dioxide compromises defence functions of the airway epithelium. Acta Otolaryngol. 1998;118(538):221‐226. [PubMed] [Google Scholar]
- 312. Robison TW, Kim KJ. Dual effect of nitrogen dioxide on barrier properties of guinea pig tracheobronchial epithelial monolayers cultured in an air interface. J Toxicol Environ Health Part A. 1995;44(1):57‐71. [DOI] [PubMed] [Google Scholar]
- 313. Belanger K, Holford TR, Gent JF, Hill ME, Kezik JM, Leaderer BP. Household levels of nitrogen dioxide and pediatric asthma severity. Epidemiology (Cambridge, Mass). 2013;24(2):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Carson JL, Collier AM, Hu S, Devlin RB. Effect of nitrogen dioxide on human nasal epithelium. Am J Respir Cell Mol Biol. 1993;9:264. [DOI] [PubMed] [Google Scholar]
- 315. Lopez DJ, Lodge CJ, Bui DS, et al. Association between ambient air pollution and development and persistence of atopic and non‐atopic eczema in a cohort of adults. Allergy. 2021;76(8):2524‐2534. [DOI] [PubMed] [Google Scholar]
- 316. Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Publ Health. 2020;8(14):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Schultz MG, Schröder S, Lyapina O, et al. Tropospheric ozone assessment report: database and metrics data of global surface ozone observations. Elem Sci Ant. 2017;5(58). [Google Scholar]
- 318. Michaudel C, Fauconnier L, Jule Y, Ryffel B. Functional and morphological differences of the lung upon acute and chronic ozone exposure in mice. Sci Rep. 2018;8(1):10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Michaudel C, Mackowiak C, Maillet I, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL‐33. J Allergy Clin Immunol. 2018;142(3):942‐958. [DOI] [PubMed] [Google Scholar]
- 320. Sokolowska M, Quesniaux VFJ, Akdis CA, Chung KF, Ryffel B, Togbe D. Acute respiratory barrier disruption by ozone exposure in mice. Front Immunol. 2019;10:2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Wiegman CH, Li F, Ryffel B, Togbe D, Chung KF. Oxidative stress in ozone‐induced chronic lung inflammation and emphysema: a facet of chronic obstructive pulmonary disease. Front Immunol. 2020;11:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Kim SY, Kim E, Kim WJ. health effects of ozone on respiratory diseases. Tuberc Respir Dis (Seoul). 2020;83(1):6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Kogawa AC, Cernic BG, do Couto LGD, Salgado HRN. Synthetic detergents: 100 years of history. Saudi Pharm J. 2017;25(6):934‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Leoty‐Okombi S, Gillaizeau F, Leuillet S, et al. Effect of sodium lauryl sulfate (SLS) applied as a patch on human skin physiology and its microbiota. Cosmetics. 2021;8(1):6. [Google Scholar]
- 325. Wilhelm KP, Freitag G, Wolff HH. Surfactant‐induced skin irritation and skin repair. Evaluation of the acute human irritation model by noninvasive techniques. J Am Acad Dermatol. 1994;30(6):944‐949. [DOI] [PubMed] [Google Scholar]
- 326. Douwes J, Slater T, Shanthakumar M, et al. Determinants of hand dermatitis, urticaria and loss of skin barrier function in professional cleaners in New Zealand. Int J Occup Environ Health. 2017;23(2):110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Guertler A, Moellhoff N, Schenck TL, et al. Onset of occupational hand eczema among healthcare workers during the SARS‐CoV‐2 pandemic: Comparing a single surgical site with a COVID‐19 intensive care unit. Contact Dermatitis. 2020;83(2):108‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Kendziora B, Guertler A, Ständer L, et al. Evaluation of hand hygiene and onset of hand eczema after the outbreak of SARS‐CoV‐2 in Munich. Eur J Dermatol. 2020;30(6):668‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Aerts O, Goossens A, Lambert J, Lepoittevin JP. Contact allergy caused by isothiazolinone derivatives: an overview of non‐cosmetic and unusual cosmetic sources. Eur J Dermatol. 2017;27(2):115‐122. [DOI] [PubMed] [Google Scholar]
- 330. Van Steenkiste E, Goossens A, Meert H, Apers S, Aerts O. Airborne‐induced lymphomatoid contact dermatitis caused by methylisothiazolinone. Contact Derm. 2015;72(4):237‐240. [DOI] [PubMed] [Google Scholar]
- 331. Vizcaya D, Mirabelli MC, Gimeno D, et al. Cleaning products and short‐term respiratory effects among female cleaners with asthma. Occup Environ Med. 2015;72(11):757‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Carder M, Seed MJ, Money A, Agius RM, van Tongeren M. Occupational and work‐related respiratory disease attributed to cleaning products. Occup Environ Med. 2019;76(8):530‐536. [DOI] [PubMed] [Google Scholar]
- 333. Viennois E, Chassaing B. First victim, later aggressor: How the intestinal microbiota drives the pro‐inflammatory effects of dietary emulsifiers? Gut Microbes. 2018;9(3):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Pressman P, Clemens R, Hayes W, Reddy C. Food additive safety: a review of toxicologic and regulatory issues. Toxicol Res Appl. 2017;1:1‐22. [Google Scholar]
- 335. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Wright SL, Kelly FJ. Plastic and human health: a micro issue? Environ Sci Technol. 2017;51(12):6634‐6647. [DOI] [PubMed] [Google Scholar]
- 337. Yee M‐L, Hii L‐W, Looi CK, et al. Impact of microplastics and nanoplastics on human health. Nanomaterials. 2021;11(2):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Holloczki O, Gehrke S. Can nanoplastics alter cell membranes? ChemPhysChem. 2020;21(1):9‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Holloczki O, Gehrke S. Nanoplastics can change the secondary structure of proteins. Sci Rep. 2019;9(1):16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Xu M, Halimu G, Zhang Q, et al. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019;694:133794. [DOI] [PubMed] [Google Scholar]
- 341. Lim SL, Ng CT, Zou LI, et al. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology. 2019;13(8):1117‐1132. [DOI] [PubMed] [Google Scholar]
- 342. Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308‐317. [DOI] [PubMed] [Google Scholar]
- 343. Stock V, Böhmert L, Lisicki E, et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol. 2019;93(7):1817‐1833. [DOI] [PubMed] [Google Scholar]
- 344. Burns AR, Hosford SP, Dunn LA, Walker DC, Hogg JC. Respiratory epithelial permeability after cigarette smoke exposure in guinea pigs. J Appl Physiol. 1989;66(5):2109‐2116. [DOI] [PubMed] [Google Scholar]
- 345. Churg A, Cherukupalli K. Cigarette smoke causes rapid lipid peroxidation of rat tracheal epithelium. Int J Exp Pathol. 1993;74(2):127‐132. [PMC free article] [PubMed] [Google Scholar]
- 346. MacGowan GA, Dark JH, Corris PA, Nair AR. Effects of drug abuse, smoking and alcohol on donor hearts and lungs. Transpl Int. 2019;32(10):1019‐1027. [DOI] [PubMed] [Google Scholar]
- 347. Ware LB, Lee JW, Wickersham N, et al. Donor smoking is associated with pulmonary edema, inflammation and epithelial dysfunction in ex vivo human donor lungs. Am J Transplant. 2014;14(10):2295‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Chadi N, Hadland SE, Harris SK. Understanding the implications of the "vaping epidemic" among adolescents and young adults: a call for action. Subst Abus. 2019;40(1):7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Ghosh B, Reyes‐Caballero H, Akgün‐Ölmez SG, et al. Effect of sub‐chronic exposure to cigarette smoke, electronic cigarette and waterpipe on human lung epithelial barrier function. BMC Pulm Med. 2020;20(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Ween MP, Hamon R, Macowan MG, Thredgold L, Reynolds PN, Hodge SJ. Effects of E‐cigarette E‐liquid components on bronchial epithelial cells: Demonstration of dysfunctional efferocytosis. Respirology. 2020;25(6):620‐628. [DOI] [PubMed] [Google Scholar]
- 351. Jacquet A. Interactions of airway epithelium with protease allergens in the allergic response. Clin Exp Allergy. 2011;41(3):305‐311. [DOI] [PubMed] [Google Scholar]
- 352. Souza PMD, Bittencourt MLDA, Caprara CC, et al. A biotechnology perspective of fungal proteases. Braz J Microbiol. 2015;46(2):337‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Wan H, Winton HL, Soeller C, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104(1):123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Kale SL, Agrawal K, Gaur SN, Arora N. Cockroach protease allergen induces allergic airway inflammation via epithelial cell activation. Sci Rep. 2017;7:42341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Post S, Nawijn MC, Hackett TL, et al. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax. 2012;67(6):488‐495. [DOI] [PubMed] [Google Scholar]
- 356. Namvar S, Warn P, Farnell E, et al. Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model. Clin Exp Allergy. 2015;45(5):982‐993. [DOI] [PubMed] [Google Scholar]
- 357. Grozdanovic MM, Čavić M, Nešić A, et al. Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions. Biochimica Et Biophysica Acta (BBA)‐General Sub. 2016;1860(3):516‐526. [DOI] [PubMed] [Google Scholar]
- 358. Hosoki K, Boldogh I, Sur S. Innate responses to pollen allergens. Curr Opin Allergy Clin Immunol. 2015;15(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Vinhas R, Cortes L, Cardoso I, et al. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. 2011;66(8):1088‐1098. [DOI] [PubMed] [Google Scholar]
- 360. Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. The concepts of asthma endotypes and phenotypes to guide current and novel treatment strategies. Expert Rev Respir Med. 2018;12(9):733‐743. [DOI] [PubMed] [Google Scholar]
- 361. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Akdis CA, Arkwright PD, Brüggen M‐C, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582‐1605. [DOI] [PubMed] [Google Scholar]
- 363. Pasha MA, Patel G, Hopp R & Yang Q. Role of innate lymphoid cells in allergic diseases. Allergy asthma proc. 2019;40(3):138‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Sugita K, Steer CA, Martinez‐Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL‐13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300‐310.e11. [DOI] [PubMed] [Google Scholar]
- 365. Ho JS, Li CH, Wang A, Asai Y. It's no skin off my nose: the relationship between the skin and allergic rhinitis. Ann Allergy Asthma Immunol. 2021;127(2):176–182. [DOI] [PubMed] [Google Scholar]
- 366. Singh N, Diebold Y, Sahu SK, Leonardi A. Epithelial barrier dysfunction in ocular allergy. Allergy. 2022;1360–1372. doi: 10.1111/all.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Sugita K, Soyka MB, Wawrzyniak P, et al. Outside‐in hypothesis revisited: the role of microbial, epithelial, and immune interactions. Ann Allergy Asthma Immunol. 2020;125(5):517‐527. [DOI] [PubMed] [Google Scholar]
- 368. Simon D, Page B, Vogel M, et al. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid‐treated eosinophilic esophagitis. Allergy. 2018;73(1):239‐247. [DOI] [PubMed] [Google Scholar]
- 369. Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S. The gut microbiota and respiratory diseases: new evidence. J Immunol Res. 2020;2020:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Ventura MT, Polimeno L, Amoruso AC, et al. Intestinal permeability in patients with adverse reactions to food. Digestive Liver Dis. 2006;38(10):732‐736. [DOI] [PubMed] [Google Scholar]
- 371. Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol. 2013;14(5):389‐399. [DOI] [PubMed] [Google Scholar]
- 372. O'Regan GM, Sandilands A, McLean WI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clinical Immunol. 2008;122(4):689‐693. [DOI] [PubMed] [Google Scholar]
- 373. Nowak‐Węgrzyn A, Chatchatee P. Mechanisms of tolerance induction. Ann Nutr Metab. 2017;70(Suppl. 2):7‐24. [DOI] [PubMed] [Google Scholar]
- 374. Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134(3):509‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145(6):1499‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Looi K, Buckley AG, Rigby PJ, et al. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin Exp Allergy. 2018;48(5):513‐524. [DOI] [PubMed] [Google Scholar]
- 377. Sweerus K, Lachowicz‐Scroggins M, Gordon E, et al. Claudin‐18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139(1) :72‐81.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Hassim Z, Maronese SE, Kumar RK. Injury to murine airway epithelial cells by pollen enzymes. Thorax. 1998;53(5):368‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Kicic A, Stevens PT, Sutanto EN, et al. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin Exp Allergy. 2016;46(11):1441‐1455. [DOI] [PubMed] [Google Scholar]
- 380. Schuijs MJ, Willart MA, Vergote K, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106‐1110. [DOI] [PubMed] [Google Scholar]
- 381. Bernard A. Chlorination products: emerging links with allergic diseases. Curr Med Chem. 2007;14(16):1771‐1782. [DOI] [PubMed] [Google Scholar]
- 382. Van Den Broucke S, Pollaris L, Vande Velde G, et al. Irritant‐induced asthma to hypochlorite in mice due to impairment of the airway barrier. Arch Toxicol. 2018;92(4):1551‐1561. [DOI] [PubMed] [Google Scholar]
- 383. Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Schulzke J‐D, Bentzel CJ, Schulzke I, Riecken E‐O, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res. 1998;43(4):435‐441. [DOI] [PubMed] [Google Scholar]
- 385. Wyatt J, Vogelsang H, Hübl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohri's disease. The Lancet. 1993;341(8858):1437‐1439. [DOI] [PubMed] [Google Scholar]
- 386. Samadi N, Klems M, Untersmayr E. The role of gastrointestinal permeability in food allergy. Ann Allergy Asthma Immunol. 2018;121(2):168‐173. [DOI] [PubMed] [Google Scholar]
- 387. Järvinen KM, Konstantinou GN, Pilapil M, et al. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr Allergy Immunol. 2013;24(6):589‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Dupont C, Barau E, Molkhou P, Raynaud F, Barbet J, Dehennin L. Food‐induced alterations of intestinal permeability in children with cow's milk‐sensitive enteropathy and atopic dermatitis. J Pediatr Gastroenterol Nutr. 1989;8(4):459‐465. [DOI] [PubMed] [Google Scholar]


