Abstract
Background
Iron plays a role in many key processes in the developing brain. During pregnancy, iron supplementation is widely recommended to prevent and treat iron deficiency; however, the prevalence of iron deficiency and the risk of iron overload vary greatly between populations. Evidence on the role of high levels of maternal ferritin, a storage iron marker during pregnancy in relation to offspring neurodevelopment is lacking.
Objective
Our main objective was to examine if maternal ferritin levels during pregnancy are associated with child cognitive and motor abilities.
Methods
We included Dutch mother‐child dyads from the prospective population‐based Generation R Study, born in 2002–2006. We compared children whose mothers had high (standard deviation score >+1) or low (standard deviation score <−1) early‐pregnancy ferritin to children whose mothers had intermediate ferritin (reference group) using linear regression. Children underwent non‐verbal intelligence and language tests at 4–9 years (cognitive abilities), finger‐tapping and balancing tests at 8–12 years (motor abilities), and structural magnetic resonance imaging at 8–12 years (brain morphology). Covariates were child age, sex, maternal intelligence quotient estimate, age, body‐mass‐index, education, parity, smoking and alcohol use.
Results
Of the 2479 mother‐child dyads with data on maternal ferritin and at least one child neurodevelopmental outcome, 387 mothers had low (mean = 20.6 µg/L), 1700 intermediate (mean = 64.6 µg/L) and 392 high (mean = 170.3 µg/L) early‐pregnancy ferritin. High maternal ferritin was associated with 2.54 points (95% confidence interval ‐4.16, ‐0.92) lower child intelligence quotient and 16.02 cm3 (95% confidence interval ‐30.57, ‐1.48) smaller brain volume. Results remained similar after excluding mothers with high C‐reactive protein. Low maternal ferritin was not associated with child cognitive abilities. Maternal ferritin was unrelated to child motor outcomes.
Conclusion
High maternal ferritin during pregnancy was associated with poorer child cognitive abilities and smaller brain volume. Maternal iron status during pregnancy may be associated with offspring neurodevelopment.
Keywords: brain, ferritin, intelligence, iron, motor skills, pregnancy
Synopsis.
Study question
Is maternal ferritin associated with child cognitive and motor abilities and brain morphology?
What's already known
Iron plays a role in many key processes in the developing brain. Ferritin is a commonly used marker of iron stores during pregnancy. Low ferritin indicates iron‐deficiency, whereas high ferritin can indicate high iron intake and reserves but also inflammation.
What this study adds
In this population where maternal iron deficiency was rare, high rather than low maternal ferritin was associated with poorer offspring cognitive functioning and smaller brain volume in school age. These associations remained after addressing potential confounding by factors such as inflammation, maternal cognitive abilities and smoking.
1. BACKGROUND
Epidemiological, clinical and experimental studies suggest that suboptimal conditions during intrauterine life impact foetal development and can influence a range of health outcomes. 1 To diagnose iron‐deficiency, ferritin is often measured during pregnancy, but is this iron storage marker associated with child outcomes?
Iron, while crucial for erythropoiesis, energy metabolism and cell signalling, is highly toxic in its free form and thus stored within ferritin, a protein whose serum concentration decreases as iron stores are depleted. 2 , 3 , 4 Previous studies have linked low maternal ferritin during pregnancy with poorer child cognitive abilities. 5 , 6 On the contrary, evidence from animal models and studies on mostly preterm neonates suggest that high ferritin and iron overload in infancy may also be associated with disturbances in key neurocellular processes and adverse neurodevelopmental sequelae. 7 Like the prevalence of iron deficiency and low ferritin, also the prevalence of elevated ferritin varies between populations: in the United States, for example approximately 6% of 20–49‐year‐old women are estimated to have serum ferritin above 150 µg/L, indicating potential risk of iron overload. 4 , 8 Yet, there is little evidence on what the upper cut‐offs of ferritin should be during pregnancy, and whether high maternal ferritin is associated with adverse sequelae for the offspring.
Some indirect evidence to support maternal iron status may be associated with child neurodevelopmental outcomes comes from studies on maternal haemoglobin, which is widely used to screen for (severe) iron‐deficiency. 4 , 9 Previous studies have linked both low and high maternal haemoglobin during pregnancy with perinatal complications, and some evidence suggests both extremes could be associated with poorer motor outcomes in the offspring. 10 , 11 However, evidence regarding maternal haemoglobin and child developmental outcomes is limited, and should be interpreted with caution, as iron status is only one of many factors that can influence haemoglobin. 4 , 9 , 10
The purpose of the current study was to investigate if maternal ferritin and haemoglobin during early pregnancy are associated with key developmental outcomes in the child: intelligence quotient (IQ) and language and motor abilities in school age. The study was embedded in a population‐based, prospective cohort and included 2549 Dutch mothers and their children. To investigate underlying differences in brain development, we also examined global and regional brain morphology in a subset of children who underwent structural Magnetic Resonance Imaging (MRI). Of the two biomarkers measured in early pregnancy, ferritin is a more reliable indicator of iron status. 4 , 9 We focussed on ferritin as our main exposure of interest, and analysed haemoglobin as a secondary exposure, with the aim of establishing if either low or high maternal ferritin, or low or high maternal haemoglobin during pregnancy are associated with child neurodevelopment.
2. METHODS
2.1. Cohort
This study was conducted within the Generation R Study, a previously described population‐based prospective cohort. 12 The study was approved by the Medical Ethical Committee of Erasmus MC, Rotterdam and conducted according to the Declaration of Helsinki. All participating mothers and parents/legal guardians of participating children gave written informed consent.
Briefly, all pregnant women living within Rotterdam, the Netherlands, with expected delivery date between 4/2002 and 1/2006 were invited to participate: 8879 pregnant women were recruited. The Generation R Study is a multi‐ethnic cohort: approximately 50% of mothers are of Dutch national origin, that is, both of her parents were born in the Netherlands, while several ethnic minorities make up approximately 50%. 12 To limit confounding and due to the small sample size per ethnic minority group, we only included mothers of Dutch national origin (n = 4096). We excluded women recruited after early pregnancy, when blood samples used to analyse ferritin concentrations were drawn (>18 weeks; n = 602), non‐singleton pregnancies (n = 48) and non‐live births/neonatal deaths (n = 44). 13 After attrition of 248 mother‐child dyads without early‐pregnancy ferritin/haemoglobin data, and 605 without cognitive/motor outcome data at 4–12 years, the final analytical sample included 2549 dyads. Of them, 1550 additionally underwent MRI at 8–12 years: after excluding six participants with incidental findings (e.g., large cysts, confirmed by a neuroradiologist) and low‐quality data (n = 269), neuroimaging data were available for 1275 children (Figure S1). 14 , 15
2.2. Exposure: Maternal ferritin and haemoglobin
Maternal non‐fasting blood samples were drawn at 6–18 weeks of gestation using antecubital venepuncture (mean = 13.2 weeks, SD = 1.8). 13 Maternal ferritin was measured from serum samples stored at −80°C using electrochemiluminescence immunoassay; haemoglobin was measured from fresh plasma.
During early pregnancy, physiological haemodilution occurs, and ferritin and haemoglobin decline (Figure S2). 16 To account for differences in the timing of venepuncture and standardise exposures, we regressed ferritin and haemoglobin on gestational age at venepuncture using linear regression to create standard deviation scores (SDS; mean = 0, SD = 1). Ferritin was square‐transformed to reduce skewness before standardisation. Ferritin and haemoglobin were categorised into three groups each: low (SDS <−1), intermediate (SDS −1 to +1), high (SDS >+1).
2.3. Outcomes
Cognitive abilities were assessed at the 6‐year follow‐up (mean age = 6.1 years, SD = 0.4, range 4.9–9.0). Firstly, non‐verbal IQ was estimated using two subtests (mosaics, categories) of the Dutch non‐verbal intelligence test, Snijders‐Oomen Niet‐Verbale Intelligentietest, 17 as described previously. 18 Raw scores were converted into non‐verbal IQ estimates using normative data tailored to age. 18 Estimates ≤50 and ≥150 were assigned to 50 and 150, respectively, as this test is not designed to differentiate between individuals beyond these limits. Secondly, language ability was measured using the comprehension subtest of the Dutch language development test, Taaltest voor Kinderen. 19 Children were shown two pictures and asked to choose the picture that matched a given word. Number of correct answers was divided by number of items (37) to yield a total score. To attain normality and for ease of interpretation, we square‐transformed and standardised language scores within the cohort.
Motor abilities were assessed at the 9‐year follow‐up (mean = 9.8 years, SD = 0.3, range 8.5–12.0). Firstly, motor control and fine‐motor speed were assessed using a computerised finger‐tapping task. Finger‐tapping is one of the most frequently used neuropsychological instruments, 20 and has been associated with brain lesions and a range of motor dysfunctions. 21 The test included five trials: Participants were asked to tap their index finger as fast as possible for 10 seconds, starting with (1) right, (2) left, (3) both (alternating), (4) right and (5) left hand: average number of taps across tasks was used as the outcome. 14 Secondly, gross motor skills and balance were assessed using a balancing task, the Walking Backwards task from the Body Coordination Test for Children (Körperkoordinationstest für Kinder), validated among school‐aged Dutch children. 22 , 23 Children walked backwards on three 3‐metre‐long, 5‐centimetre‐high beams of different widths (6, 4.5, 3 cm). After a practice trial, each child walked twice backwards along each beam, starting with the widest, finishing with the narrowest. 14 The outcome was the total number of correct steps (until the child stepped off/reached a maximum of 8 steps per trial) across all 6 trials.
On all cognitive and motor outcomes, higher scores reflect better performance.
Children underwent MRI at the mean age of 10.1 years (SD = 0.6, range 8.9–12.0), as described previously. 14 , 15 Images were acquired using the same sequence and scanner (3 Tesla GE 750w Discovery). Following three‐plane localiser scans, a high‐resolution T1‐weighted inversion recovery fast spoiled gradient recalled sequence was acquired (TR = 8.77 ms, TE = 3.4 ms, TI = 600 ms, flip angle = 10°, field of view = 220 × 220 mm, Acquisition Matrix = 220 × 220, slice thickness = 1 mm, number of slices = 230). FreeSurfer v.6.0.0 (http://surfer.nmr.mgh.harvard.edu/) was used to obtain total brain volume, global cortical and subcortical grey matter, global cerebral white matter, total cerebellar volume and total intracranial volume. 14 , 15
2.4. Statistical analysis
In the primary models, we used multivariable linear regression to examine associations between maternal ferritin (high vs. intermediate [reference category] vs. low) and child IQ, language ability, finger‐tapping and balancing (continuous). We corrected for multiple testing using the False‐Discovery‐Rate method (FDR) 24 at α = 0.05 across adjusted primary models, calculating the number of tests (8) based on two exposures (low‐versus‐reference ferritin, high‐versus‐reference ferritin) and four outcomes (IQ, language, finger‐tapping, balancing).
If analyses suggested associations between ferritin and cognitive or motor abilities (adjusted model, surviving multiple‐testing correction), we followed up with analyses of neuroimaging measures to investigate underlying biological differences. We first examined differences in total brain volume. We then examined cortical grey, subcortical grey, cerebellar and total cerebral white matter volumes: in a further model, these were regressed on total intracranial volumes to examine structural specificity.
We used R‐3.3.2 for all analyses.
Covariates were chosen based on prior literature with the help of directed acyclic graphs (see Figure S3). 4 , 25 , 26 , 27 , 28 , 29 , 30 In the age‐and‐sex‐adjusted model, we included child age at follow‐up and sex (male/female) as covariates. In the adjusted model, we added potential maternal confounders. These included age at enrolment, pre‐pregnancy body‐mass‐index (kg/m2), prospectively self‐reported smoking (none/quit when pregnancy was known/continued) and alcohol consumption (none/quit when pregnancy was known/continued occasionally/continued weekly), education at enrolment (tertiary/lower), parity (primipara/multipara) and maternal IQ assessed 5 years after delivery (using Raven's Standard Progressive Matrices 31 ).
2.4.1. Missing data
We imputed forty datasets using multiple imputation by chained equations (MICE) to handle missing covariate data within the analytical sample (n = 2549), using predictive mean matching, logistic regression and polytomous logistic regression methods to impute continuous, binary and non‐binary nominal covariates, respectively. 32 All available exposure, outcome and covariate data were used for imputing covariate data (Table S1). In all models, estimates were pooled across multiple imputation datasets using Rubin's rules.
2.4.2. Sensitivity analyses
To address unmeasured confounding, we calculated E‐values. 33 , 34 E‐values reflect the minimum strength of association to exposure and outcome that an unmeasured confounder would need to fully explain exposure‐outcome associations. 34
Inflammatory status may increase ferritin independently of iron status: in sensitivity analyses, we only included participants with early‐pregnancy C‐reactive protein (CRP) <5 mg/L. 4 , 35
Folic acid and vitamin supplements can contain iron: in sensitivity analyses, we added self‐reported prenatal maternal folic acid (no/yes) or multivitamin (no/yes) supplement use as covariates, to investigate if these factors explained any observed associations.
To further facilitate comparisons between our results and findings from other populations, we re‐ran the primary models using maternal ferritin re‐categorised into low (<20 µg/L), intermediate (20–150 µg/L) or high (>150 µg/L), to predict child cognitive and motor abilities, based on previously used cut‐offs. 3 , 4
To test for linear associations, we used ferritin SDS as a continuous predictor. To further assess if associations between iron status and neurodevelopment are U‐shaped, 11 we used quadratic terms (ferritin SDS 2 ).
Furthermore, we re‐ran the primary models after replacing ferritin with haemoglobin, a more widely available yet less specific screener for iron status, categorised into low (<−1 SD), intermediate or high (>1 SD). Iron deficiency ultimately leads to decreased haemoglobin, however, in our sample anaemia (haemoglobin <11g/dl, n = 54) and iron‐deficiency anaemia (haemoglobin <11 g/dl and ferritin <20 µg/L, n = 8) were rare, and we refrained from analysing these as predictors. 9
We describe the characteristics of the Generation R Study cohort members who were otherwise eligible for inclusion in the current study but could not be included in the analytical sample because of missing exposure or outcome data (n = 853) (see Figure S1). In sensitivity analyses, we addressed the potential for selection bias owing to non‐response by using baseline data (predictors: maternal characteristics during pregnancy, child sex; see Table 1) to calculate inverse probability of attrition weights (outcome: inclusion or exclusion from the analytical sample), which were then used to re‐estimate the primary linear regression models. 36
TABLE 1.
Characteristics of the analytical sample, stratified by low, intermediate and high maternal ferritin during pregnancy
| Low ferritin (SDS <−1, n = 387) |
Intermediate ferritin ( n = 1700) |
High ferritin (SDS >1, n = 392) | |
|---|---|---|---|
| Maternal characteristics | |||
| Ferritin, µg/L, mean (SD) | 20.6 (6.6) | 64.6 (24.3) | 170.3 (53.6) |
| Haemoglobin, g/dl, mean (SD) | 12.5 (0.8) | 12.6 (0.8) | 12.6 (0.8) |
| Age at enrolment, years, mean (SD) | 31.7 (4.6) | 31.4 (4.1) | 31.4 (3.6) |
| Pre‐pregnancy BMI, kg/m2, mean (SD) | 23.0 (3.6) | 23.2 (4.0) | 23.6 (4.0) |
| Estimated IQ, mean (SD) | 101.7 (12.5) | 100.9 (12.7) | 100.2 (12.5) |
| Education, tertiary, n (%) | 237 (61.6) | 1032 (61.6) | 248 (64.1) |
| Parity, primiparous, n (%) | 138 (35.8) | 1076 (63.4) | 325 (82.9) |
| Smoking during pregnancy | |||
| None, n (%) | 290 (80.8) | 1144 (73.6) | 261 (73.5) |
| Quit in early pregnancy, n (%) | 18 (5.0) | 160 (10.3) | 49 (13.8) |
| Continued, n (%) | 51 (14.2) | 250 (16.1) | 45 (12.7) |
| Alcohol use during pregnancy | |||
| None, n (%) | 125 (33.3) | 473 (29.2) | 84 (22.6) |
| Quit in early pregnancy | 60 (16.0) | 272 (16.8) | 71 (19.1) |
| Occasional, n (%) | 150 (40.0) | 678 (41.9) | 156 (41.9) |
| Weekly, n (%) | 40 (10.7) | 196 (12.1) | 61 (16.4) |
| Prenatal folic acid supplementation, n (%) | 288 (88.9) | 1270 (91.4) | 298 (94.3) |
| Prenatal multivitamin supplementation, n (%) | 101 (29.8) | 577 (38.8) | 122 (35.9) |
| Early‐pregnancy CRP <5 mg/L, n (%) | 232 (61.9) | 973 (58.6) | 214 (55.6) |
| Child characteristics | |||
| Sex, female, n (%) | 183 (47.3) | 863 (50.8) | 196 (50.0) |
| Characteristics at the 6‐year follow‐up | |||
| Age at cognitive assessment, years, mean (SD) | 6.1 (0.4) | 6.1 (0.4) | 6.1 (0.4) |
| Non‐verbal IQ, points, mean (SD) | 106.4 (13.2) | 105.1 (14.4) | 103.0 (15.0) |
| Language ability score, points, mean (SD) | 0.24 (0.93) | 0.26 (0.86) | 0.21 (0.94) |
| Characteristics at the 9‐year follow‐up | |||
| Age at motor assessment, years, mean (SD) | 9.8 (0.3) | 9.8 (0.3) | 9.8 (0.3) |
| Finger‐tapping score, taps, mean (SD) | 38.2 (6.5) | 38.3 (6.4) | 37.9 (6.5) |
| Balance performance score, steps, mean (SD) | 27.1 (8.1) | 26.8 (8.6) | 26.0 (8.9) |
| Characteristics at the MRI visit | |||
| Age at MRI, years, mean (SD) | 10.1 (0.6) | 10.1 (0.6) | 10.2 (0.6) |
| Total brain volume, cm3, mean (SD) | 1232.2 (102.6) | 1235.2 (105.8) | 1223.1 (117.4) |
| Cortical grey matter volume, cm3, mean (SD) | 592.4 (49.3) | 592.7 (51.5) | 586.6 (55.6) |
| Subcortical grey matter volume, cm3, mean (SD) | 60.8 (4.3) | 61.2 (4.4) | 60.9 (4.7) |
| Cerebral white matter volume, cm3, mean (SD) | 430.5 (47.3) | 432.2 (48.2) | 427.6 (52.6) |
| Cerebellar volume, cm3, mean (SD) | 146.3 (13.3) | 147.0 (12.6) | 145.7 (14.0) |
Abbreviations: %, proportion of cases in relation to all participants with data available; BMI, Body‐mass‐index; CRP, C‐reactive protein; IQ, Intelligence Quotient; MRI, Magnetic resonance imaging; n, number of cases; SD, Standard deviation; SDS, standard deviation score (ferritin and haemoglobin were standardised for gestational age at the time of venepuncture).
2.5. Ethics approval
The study was approved by the Medical Ethical Committee of Erasmus MC, Rotterdam.
3. RESULTS
Table 1 shows participant characteristics stratified by low, intermediate and high ferritin. Compared to mothers with intermediate ferritin (n = 1700, ferritin mean = 64.6 µg/L), mothers with low ferritin (n = 387, mean = 20.6 µg/L) were more often multiparous and older, and less often smoked or used alcohol, multivitamins and folic acid, whereas mothers with high ferritin (n = 392, mean = 170.3 µg/L) were more often primiparous, had higher BMI and higher education, used alcohol and folic acid supplements more often, and more often had elevated CRP. Table S1 shows characteristics in the entire analytical sample. Table S2 shows characteristics stratified by low, intermediate and high haemoglobin. Spearman's correlation between maternal haemoglobin and ferritin was 0.03.
We report results from adjusted models (see: ‘Statistical analysis’), unless otherwise stated.
3.1. Maternal ferritin and child cognitive abilities
Figure 1 depicts the associations of maternal early‐pregnancy ferritin with child IQ and language abilities. Children exposed to high maternal ferritin had 2.54 points lower IQ (95% CI −4.16, −0.92), compared to children whose mothers had intermediate ferritin (Figure 1). This association survived correction for multiple testing. High maternal ferritin was not associated with child language abilities (−0.08 SD units, 95% CI −0.19, 0.02) (Figure 1).
FIGURE 1.
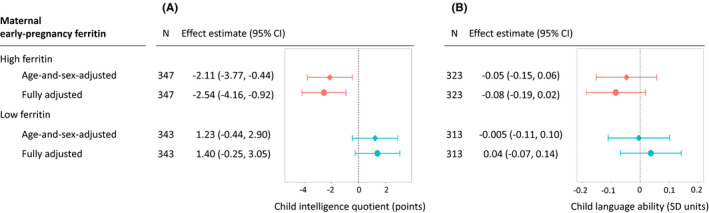
Maternal early‐pregnancy ferritin level and child intelligence quotient (panel A) and language ability (panel B) at the 6‐year‐follow‐up. Panel A. Child intelligence quotient (points). Panel B. Child language ability (SD units). In the age‐and‐sex‐adjusted model (squares), associations were adjusted for child sex and age at the time of assessment. In the adjusted model (circles), we added as covariates the following maternal factors: age at enrolment (years), pre‐pregnancy body‐mass‐index (kg/m2), prospectively self‐reported smoking (none/quit when pregnancy was known/continued) and alcohol consumption (none/quit when pregnancy was known/continued occasionally/continued weekly), education at enrolment (tertiary/lower), parity (primipara/multipara) and maternal intelligence quotient estimate (continuous). Maternal ferritin was standardised for gestational age at the time of venepuncture. CI, Confidence Interval; Effect estimate: difference in intelligence quotient points (panel A) or language ability SD units (panel B), among children exposed to high ferritin (ferritin standard deviation score >1), or low ferritin (ferritin standard deviation score <−1), compared to reference group with intermediate ferritin (ferritin standard deviation score between −1 and +1; n = 1517 and n = 1415 for intelligence quotient and language ability analyses, respectively); N, group size; SD, standard deviation
Children whose mothers had low ferritin had 1.40 points higher IQ (95% CI −0.25, 3.05) and 0.04 SD units higher language scores (95% CI −0.07, 0.14) (Figure 1). This suggests that low maternal ferritin was not associated with adverse child cognitive outcomes.
E‐value point estimates were 1.63 and 1.41 for the associations of child IQ with high and low maternal ferritin, respectively, and 1.41 and 1.24 for the associations of language ability with high and low maternal ferritin, respectively. These describe the effect magnitude of an unmeasured confounder needed to explain away the associations. Of note, the changes in effect estimates in the age‐and‐sex‐adjusted versus fully adjusted models were small (Figure 1).
In sensitivity analyses, we excluded mothers with CRP ≥5 mg/L (Table S3), added maternal antenatal folic acid or multivitamin supplements as covariates (Table S4), and used alternative cut‐offs that are not tailored to gestational age, to categorise maternal ferritin into low (<20 µg/L, n = 176), intermediate (20–150 µg/L, n = 2085) or high (>150 µg/L, n = 218) (Table S5). The results remained similar: children whose mothers had high ferritin had lower IQ.
Secondary analyses of maternal ferritin SDS as a continuous variable supported the primary findings. Per 1 SD‐unit increase in maternal ferritin, child IQ decreased by 0.61 points (95% CI −1.21, −0.002) and language score by 0.04 SD units (95% CI −0.07, 0.002). Adding quadratic terms did not suggest U‐shaped associations between ferritin and IQ or language abilities.
3.2. Maternal ferritin and child motor abilities
We found no associations between maternal ferritin and child finger‐tapping or balancing scores (Figure 2). E‐value point estimates were 1.17 and 1.27 for associations of finger‐tapping with high and low maternal ferritin, respectively, and 1.30 and 1.15 for associations of balancing with high and low maternal ferritin, respectively.
FIGURE 2.
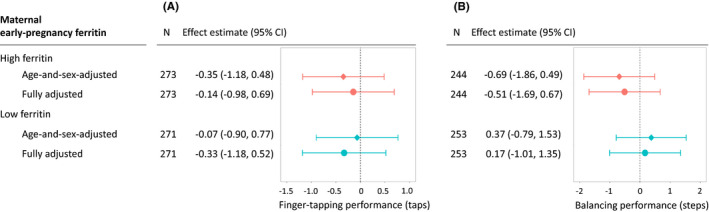
Maternal early‐pregnancy ferritin level and child motor abilities: finger‐tapping (panel A) and balancing task (panel B) performance at the 9‐year‐follow‐up. Panel A. Child finger‐tapping performance (taps). Panel B. Child balancing performance (steps). In the age‐and‐sex‐adjusted model (squares), associations were adjusted for child sex and age at the time of assessment. In the adjusted model (circles), we added as covariates the following maternal factors: age at enrolment (years), pre‐pregnancy body‐mass‐index (kg/m2), prospectively self‐reported smoking (none/quit when pregnancy was known/continued) and alcohol consumption (none/quit when pregnancy was known/continued occasionally/continued weekly), education at enrolment (tertiary/lower), parity (primipara/multipara) and maternal intelligence quotient estimate (continuous). Maternal ferritin was standardised for gestational age at the time of venepuncture. CI, Confidence Interval; Effect estimate: difference in taps (panel A) or steps (panel B), among children exposed to high ferritin (ferritin standard deviation score >1), or low ferritin (ferritin standard deviation score <−1), compared to reference group with intermediate ferritin (ferritin standard deviation score between −1 and +1; n = 1200 and n = 1109 for finger‐tapping and balancing analyses, respectively); N, group size; SD, standard deviation
The results remained similar when excluding mothers with CRP ≥5 mg/L (Table S3), adding maternal antenatal folic acid or multivitamin supplements as covariates (Table S4), or using alternative cut‐offs to categorise maternal ferritin into low (<20 µg/L), intermediate or high (>150 µg/L) (Table S5). There was no indication of linear associations between ferritin SDS and finger‐tapping (per 1 SD increase in ferritin, score increased by 0.09 taps, 95% CI −0.23, 0.40) or balancing (0.13 steps, 95% CI −0.31, 0.57). Adding quadratic terms did not suggest U‐shaped associations between ferritin and finger‐tapping or balancing.
3.3. Maternal ferritin and child brain morphology
After observing an association between maternal high ferritin and child IQ, we tested for associations between high maternal ferritin and child brain morphology (Table 2). Children exposed to high maternal ferritin had 16.02 cm3 smaller total brain volume (95% CI −30.57, −1.48 cm3) compared to children in the intermediate ferritin (reference) group. E‐value point estimate for this model was 1.56. When examining brain structures separately, the association (of high ferritin) with cortical grey matter volume seemed most pronounced; however, after residualising the outcomes on total intracranial volume, no evidence of structural specificity remained (Table 2).
TABLE 2.
Brain morphology at the mean age of 10.1 years among children exposed to high maternal ferritin (n = 200), compared to intermediate maternal ferritin (n = 848) during early pregnancy
| Brain outcome | Outcome treated as natural units (cm3) | Outcome regressed on intracranial volume (SD units) |
|---|---|---|
| Effect estimate (95% CI) | Effect estimate (95% CI) | |
| Total brain volume | −16.02 (−30.57, −1.48) | n/a |
| Cortical grey matter | −7.43 (−14.64, −0.22) | −0.06 (−0.22, 0.10) |
| Subcortical grey matter | −0.41 (−1.03, 0.22) | 0.02 (−0.13, 0.18) |
| Cerebral white matter | −6.51 (−1327, 0.25) | −0.05 (−0.21, 0.11) |
| Cerebellar volume | −1.75 (−3.59, 0.10) | −0.07 (−0.23, 0.09) |
All associations were adjusted for child sex and age at the time of assessment, and the following maternal factors: age at enrolment (years), pre‐pregnancy body‐mass‐index (kg/m2), prospectively self‐reported smoking (none/quit when pregnancy was known/continued) and alcohol consumption (none/quit when pregnancy was known/continued occasionally/continued weekly), education at enrolment (tertiary/lower), parity (primipara/multipara) and intelligence quotient (continuous, assessed among mothers approximately 5 years after delivery).
Abbreviation: CI, Confidence Interval;
Effect estimate: difference in brain volumetric outcomes from magnetic resonance imaging data in natural units (cm3), or in SD units (after the outcome in question has been regressed on total intracranial volume to reveal tissue‐ and region‐specific differences), among children exposed to high maternal ferritin (ferritin SD score >1), compared intermediate ferritin (ferritin SD score between −1 and +1). Maternal ferritin was standardised for gestational age at the time of venepuncture.
3.4. Maternal haemoglobin and child outcomes
Maternal haemoglobin was mostly unrelated to child cognitive and motor abilities (Table S6). Children of mothers with low haemoglobin (SDS <−1, n = 257) had lower language abilities scores (−0.13 SD, 95% CI −0.24, −0.01) than children whose mothers had intermediate haemoglobin (n = 1123).
3.5. Non‐response analyses
Figure S1 is a flowchart describing the exclusions and loss to follow‐up. Table S7 shows that compared to eligible mother‐child dyads who were lost to follow‐up (n = 853), mothers in the analytical sample (n = 2549) more often had tertiary education, were primiparous, and used folic acid supplements, were older, and less often continued smoking; children were more often girls. When we used inverse probability of attrition weights, calculated based on maternal baseline characteristics and child sex, to re‐estimate the associations between maternal ferritin and child neurodevelopment, the results remained unchanged (Table S8).
4. COMMENT
4.1. Principal findings
The current study showed that high maternal ferritin in early pregnancy is associated with lower child IQ in school age. This association remained when adjusting for maternal IQ, age, BMI, smoking, alcohol use, education and parity, and survived correction for multiple testing. The finding was supported by neuroimaging measures, which suggested that children whose mothers had high ferritin during pregnancy had smaller brain volume in preadolescence. High maternal ferritin was not associated with child language or motor abilities. In contrast to high maternal ferritin, low maternal ferritin was not associated with child neurodevelopmental outcomes.
4.2. Strengths of the study
The large, prospective cohort study design and population‐based neuroimaging approach are strengths of the current study. Further, we had detailed, high‐quality follow‐up data including cognitive, motor and neuroimaging outcomes in school age and were able to control for many maternal factors that have been proposed to affect both ferritin levels and offspring development.
4.3. Limitations of the data
Ethnic homogeneity, while a strength in controlling for confounding, limits generalisability to other ethnicities. Follow‐up rates were higher among more highly educated mothers, but the potential for selective attrition that could be addressed based on available data had little impact on the findings. While using pooled estimates from multiple imputation models partly addresses selective attrition and estimation uncertainty, it assumes data are missing at random given all observed values. This assumption cannot be tested, and its violation could lead to biased estimates. Maternal inflammation, metabolic diseases and substance use could elevate ferritin and influence child neurodevelopment, and we may not fully capture these phenomena by adjusting for self‐reported smoking and alcohol use and BMI or excluding women with elevated CRP. While unmeasured confounders would need to have a considerable effect on both the exposure and the outcome to explain away the association between high ferritin and child IQ or brain volume—which changed only little when observed potential confounders were added as covariates—the risk of residual confounding inevitably remains, and non‐observational data would be needed to overcome this limitation. Further, we had no data on iron supplements, and iron overload or its aetiology could not be confirmed with liver biopsies, genetic analyses or complimentary parameters such as fasting transferrin saturation or soluble transferrin receptor. Finally, extreme iron overload, for example due to hereditary haemochromatosis, where average serum ferritin can be >1000 µg/L, were beyond the scope of this study: studies with access to clinical rather than population‐based samples may be better suited to investigate such rare disorders. 3 , 4 , 37
4.4. Interpretation
High ferritin can indicate high storage iron levels, for example due to an iron‐rich diet, and ferritin is generally considered a reliable marker of iron status during pregnancy. 3 , 4 , 37 However, as an active phase protein, ferritin can also increase through inflammation, thus measurements of inflammatory markers including CRP can be used in combination with ferritin when assessing iron status. 4 In the current study, findings remained similar after excluding mothers with elevated CRP, which makes it less likely that inflammation‐related increase in ferritin explained the findings. Further, factors such as overweight and alcohol are associated with higher ferritin. 4 , 30 In our study, associations between maternal ferritin and child neurodevelopment remained similar after adding maternal BMI, IQ, age, smoking, alcohol use, education and parity as covariates. Taken together, these results offer some support that maternal iron status is associated with child neurodevelopment; however, we advise caution in drawing causal conclusions based on observational data.
Excess iron exposure could lead to oxidative stress, interact with other nutrients and decrease key neurotransmitter levels in the developing brain. 7 However, prior evidence mostly comes from animal models and postnatal iron overload among preterm infants. 7 Some studies suggest that low maternal iron intake/anaemia during pregnancy is associated with neonatal alterations in hippocampal morphogenesis, 38 serum brain‐derived neurotrophic factor levels, 38 and white matter maturation. 39 In our sample, high maternal ferritin during pregnancy was associated with smaller child brain volume, which is in line with our finding that high maternal ferritin during pregnancy is associated with lower IQ. This is in accordance with the consistent finding in the neuroscience of intelligence that larger brain volume is associated with better cognitive abilities; however, the basis of this correlation is not fully understood. 27 We found no evidence of structural specificity (differences specific to cortical or subcortical grey matter, white matter or cerebellar volume), after taking into account an overall reduction in brain volume, which is plausible considering the early timing of the exposure. Taken together, our neuroimaging and IQ findings are consistent and support the hypothesis that maternal iron status is associated with offspring brain development.
High maternal ferritin during pregnancy was associated with poorer child cognitive abilities in the current study, while some previous studies have suggested that low maternal ferritin during pregnancy predicts poorer child cognitive abilities. 5 , 6 This may seem like a discrepancy; however, some differences in study populations may help elucidate how our findings add to the existing literature, rather than contradict it. The current study was carried out in a population that was well‐suited to detect associations with high ferritin: a relatively high proportion of mothers (~9%) had ferritin concentrations >150 µg/L. 4 Conversely, severe iron deficiency was rare, thus we may be unable to detect adverse effects driven by severe iron‐deficiency, which may be a true concern in more iron‐deficient populations. 40 Further, our population had access to routine haemoglobin controls during pregnancy and, if anaemic, to iron supplementation through the Dutch antenatal care system. 41 This may have mitigated potentially adverse effects of iron deficiency on offspring neurodevelopment.
This study does not offer proof of underlying causal mechanisms, nor should its results be directly translated into clinical recommendations. Importantly, our results are not evidence that iron supplementation during pregnancy is unnecessary or harmful. It remains important to identify and treat iron‐deficiency anaemia. 42 For non‐targeted antenatal iron supplementation, the long‐term effects remain unclear and most likely vary by population: in low‐income settings with high rates of iron‐deficiency, routine antenatal iron supplementation may be associated with some benefits. 40 , 43 We encourage future studies to analyse data on maternal iron intake, particularly from randomised controlled trials, to see if there is an optimal level of intake—not too low and not too high—that we should target, to optimise maternal and child health.
We are not aware of prior studies showing an association between high ferritin during pregnancy and child cognitive abilities or brain morphology and encourage replication of our findings. Prenatal MRI studies could elucidate the underlying mechanisms further: we refrained from testing mediation of ferritin on IQ through morphology, as children underwent MRI after cognitive tests. Repeated maternal measures and measuring maternal and child ferritin could elucidate timing‐specificity and mediation through child iron stores.
In our study, higher ferritin was associated with lower IQ and smaller brain volume, while associations with language abilities were less clear, and motor skills seemed unrelated to maternal ferritin. This could suggest outcome‐specificity; however, IQ and language abilities were assessed earlier in life, and the sensitivity of tests aimed at measuring different phenotypes may vary. Nonetheless, we encourage research into possible outcome‐specificity, and note that offspring mental health outcomes could also be of interest. 44 , 45
Finally, in the current study, we focussed on maternal ferritin; however, we also tested if haemoglobin was associated with child neurodevelopmental outcomes. While iron deficiency ultimately leads to low haemoglobin, haemoglobin is a less specific marker of iron status, compared to ferritin. In the current study, we observed an association between low maternal haemoglobin and child language abilities. This association, while in line with some studies linking maternal anaemia with offspring neurodevelopment, 46 , 47 seemed not to be driven by iron status. Thus, to understand the associations between maternal haemoglobin or anaemia and child neurodevelopment, we encourage research into the many genetic and environmental factors that can affect haemoglobin, beyond just iron‐deficiency. 42
5. CONCLUSIONS
In the current study, we showed that high maternal ferritin during pregnancy was associated with lower IQ and smaller brain volume among school‐aged children. The results support the hypothesis that maternal iron status is associated with offspring brain development, but these associations may be more complex than previous research on iron deficiency has shown.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to express their deep gratitude to all families who participated in this study, and to all the research staff, medical personnel and other contributors who made the Generation R Study possible.
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest to report.
Sammallahti S, Tiemeier H, Reiss IKM, Muckenthaler MU, EL Marroun H, Vermeulen M. Maternal early‐pregnancy ferritin and offspring neurodevelopment: A prospective cohort study from gestation to school age. Paediatr Perinat Epidemiol. 2022;36:425–434. doi: 10.1111/ppe.12854
Funding information
This study was supported by the Netherlands Organization for Scientific Research (NWO) and the Dutch Ministry of Health, Welfare and Sport (general design of the Generation R Study); LEaDing Fellows EU Marie Skłodowska‐Curie COFUND Programme (Dr Sammallahti); NWO Vici Grant 016.VICI.170.200 (Dr Tiemeier); and Stichting Volksbond Rotterdam, the Brain & Behavior Research Foundation NARSAD Young Investigator Grant 27853, and the European Union's Horizon 2020 Research and Innovation Program LifeCycle grant No. 733206 (Dr El Marroun). Funding sources had no role in study design, data collection, analysis or interpretation, reporting or submission for publication
DATA AVAILABILITY STATEMENT
The individual‐level data are not publicly available due to privacy and ethical restrictions. Requests for data access can be send to datamanagementgenr@erasmusmc.nl and will be discussed in the Generation R Study Management Team.
REFERENCES
- 1. Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75:951‐970. [DOI] [PubMed] [Google Scholar]
- 2. Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721‐1741. [DOI] [PubMed] [Google Scholar]
- 3. Daru J, Allotey J, Peña‐Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med. 2017;27:167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. World Health Organization; 2020. [PubMed] [Google Scholar]
- 5. Arija V, Hernández‐Martínez C, Tous M, et al. Association of iron status and intake during pregnancy with neuropsychological outcomes in children aged 7 years: the prospective birth cohort Infancia y Medio Ambiente (INMA) Study. Nutrients. 2019;11:2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tran TD, Biggs BA, Tran T, et al. Impact on infants’ cognitive development of antenatal exposure to iron deficiency disorder and common mental disorders. PLoS One. 2013;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Wu Y, Li T, Wang X, Zhu C. Iron metabolism and brain development in premature infants. Front Physiol. 2019;10:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Centers for Disease Control and Prevention . Trace elements. In: Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 2012. National Center for Environmental Health; 2012. [Google Scholar]
- 9. World Health Organization . Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 10. Young MF, Oaks BM, Tandon S, Martorell R, Dewey KG, Wendt AS. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta‐analysis. Ann N Y Acad Sci. 2019;1450:47‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mireku MO, Davidson LL, Koura GK, et al. Prenatal hemoglobin levels and early cognitive and motor functions of one‐year‐old children. Pediatrics. 2015;136:e76‐e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaddoe VWV, Mackenbach JP, Moll HA, et al. The generation R study: design and cohort profile. Eur J Epidemiol. 2006;21:475‐484. [DOI] [PubMed] [Google Scholar]
- 13. Kruithof CJ, Kooijman MN, van Duijn CM, et al. The generation R study: Biobank update 2015. Eur J Epidemiol. 2014;29:911‐927. [DOI] [PubMed] [Google Scholar]
- 14. White T, Muetzel RL, El Marroun H, et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol. 2018;33:99‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muetzel RL, Mulder RH, Lamballais S, et al. Frequent bullying involvement and brain morphology in children. Front Psychiatry. 2019;10:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fenton V, Cavjll I, Fisher J. Iron stores in pregnancy. Br J Haemotol. 1977;37:145‐149. [PubMed] [Google Scholar]
- 17. Tellegen P, Winkel M, Wijnberg‐Williams B, Laros J. Snijders‐Oomen Niet‐Verbale Intelligentietest SON‐R 2 1/2‐7. [Snijders‐Oomen Non‐Verbal Intelligence Test SON‐R 2 1/2‐7]. Boom Testuitgevers; 2005. [Google Scholar]
- 18. Verlinden M, Veenstra R, Ghassabian A, et al. Executive functioning and non‐verbal intelligence as predictors of bullying in early elementary school. J Abnorm Child Psychol. 2014;42:953‐966. [DOI] [PubMed] [Google Scholar]
- 19. van Bon WHJ, Hoekstra J. Taaltests voor kinderen. Swets & Zetlinger; 1982. [Google Scholar]
- 20. Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Arch Clin Neuropsychol. 2005;20:33‐65. [DOI] [PubMed] [Google Scholar]
- 21. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests ‐ Adminstration, Norms, and Commentary.. 3rd ed. Oxford University Press; 2006. [Google Scholar]
- 22. Kiphard E, Schilling F. Körperkoordinationstest für Kinder 2, überarbeitete und ergänzte Aufgabe. Beltz Test GmbH; 2007. [Google Scholar]
- 23. Iivonen S, Kaarina Sääkslahti A, Laukkanen A. A review of studies using the Körperkoordinationstest für Kinder (KTK). Eur J Adapt Phys Act. 2016;8:18‐36. [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol). 1995;57:289‐300. [Google Scholar]
- 25. Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta‐analysis. Alcohol Clin Exp Res. 2014;38:214‐226. [DOI] [PubMed] [Google Scholar]
- 26. Sanchez CE, Barry C, Sabhlok A, et al. Maternal pre‐pregnancy obesity and child neurodevelopmental outcomes: a meta‐analysis. Obes Rev. 2018;19:464‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201‐211. [DOI] [PubMed] [Google Scholar]
- 28. Barclay KJ. A within‐family analysis of birth order and intelligence using population conscription data on Swedish men. Intelligence. 2015;49:134‐143. [Google Scholar]
- 29. Clifford A, Lang L, Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol Teratol. 2012;34:560‐570. [DOI] [PubMed] [Google Scholar]
- 30. McKinnon EJ, Rossi E, Beilby JP, Trinder D, Olynyk JK. Factors that affect serum levels of ferritin in Australian adults and implications for follow‐up. Clin Gastroenterol Hepatol. 2014;12:101‐108.e4. [DOI] [PubMed] [Google Scholar]
- 31. Prieler J. Raven’s advanced progressive matrices, vol. 24.00. Schufried; 2003. [Google Scholar]
- 32. van Buuren S, Groothuis‐Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1‐67. [Google Scholar]
- 33. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E‐values. Epidemiology. 2018;29:e45‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268‐274. [DOI] [PubMed] [Google Scholar]
- 35. Watts DH, Krohn MA, Wener MH, Eschenbach DA. C‐reactive protein in normal pregnancy. Obstet Gynecol. 1991;77:176‐180. [DOI] [PubMed] [Google Scholar]
- 36. Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018;97:407‐416. [DOI] [PubMed] [Google Scholar]
- 37. Garcia‐Casal MN, Pasricha SR, Martinez RX, Lopez‐Perez L, Peña‐Rosas JP. Are current serum and plasma ferritin cut‐offs for iron deficiency and overload accurate and reflecting iron status? A systematic review. Arch Med Res. 2018;49:405‐417. [DOI] [PubMed] [Google Scholar]
- 38. Basu S, Kumar D, Anupurba S, Verma A, Kumar A. Effect of maternal iron deficiency anemia on fetal neural development. Obstet Gynecol Surv. 2018;73:445‐447. [DOI] [PubMed] [Google Scholar]
- 39. Monk C, Georgieff MK, Xu D, et al. Maternal prenatal iron status and tissue organization in the neonatal brain. Pediatr Res. 2016;79:482‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jayasinghe C, Polson R, van Woerden HC, Wilson P. The effect of universal maternal antenatal iron supplementation on neurodevelopment in offspring: a systematic review and meta‐analysis. BMC Pediatr. 2018;18:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wildschut HIJ, Ten H‐B, Borkent‐Polet M, et al. Practice variation of test procedures reportedly used in routine antenatal care in The Netherlands. Acta Obstet Gynecol Scand. 1999;78:27‐32. [PubMed] [Google Scholar]
- 42. ACOG Committee on Practice Bulletins—Obstetrics . Practice bulletin no. 95: anemia in pregnancy. Obstet Gynecol. 2008;112:201‐207. [DOI] [PubMed] [Google Scholar]
- 43. Peña‐Rosas J, De‐Regil L, Garcia‐Casal M, Dowswell T. Daily oral iron supplementation during pregnancy. Cochr Database Syst Rev. 2015(7):CD004736. 10.1002/14651858.CD004736.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parsons AG, Zhou SJ, Spurrier NJ, Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: Long‐term follow‐up of a randomised controlled trial. Br J Nutr. 2008;99:1133‐1139. [DOI] [PubMed] [Google Scholar]
- 45. Zhou S, Gibson R, Crowther C, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long‐term follow‐up of a randomized controlled trial. Am J Clin Nutr. 2006;83:1112‐1117. [DOI] [PubMed] [Google Scholar]
- 46. Wiegersma AM, Dalman C, Lee BK, Karlsson H, Gardner RM. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry. 2019;76:1294‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leonard H, de Klerk N, Bourke J, Bower C. Maternal health in pregnancy and intellectual disability in the offspring: a population‐based study. Ann Epidemiol. 2006;16:448‐454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The individual‐level data are not publicly available due to privacy and ethical restrictions. Requests for data access can be send to datamanagementgenr@erasmusmc.nl and will be discussed in the Generation R Study Management Team.


