Abstract
Background and objective
The study aimed to evaluate the direct and indirect costs of systemic sclerosis (SSc) in cases with and without interstitial lung disease (ILD).
Methods
Cases diagnosed with SSc (2002–2015) were identified in the Danish National Patient Registry. Cases were matched 1:4 with non‐SSc controls from the general population. Data on costs were obtained from national databases. Excess cost was estimated as the annual cost per case subtracting the costs of the control.
Results
We identified 1869 cases and 7463 controls. Total excess cost (direct healthcare, elderly care and indirect costs) in the SSc‐ILD cohort was €29,725, and €17,905 in the non‐ILD SSc cohort. In‐ and out‐patient contacts and forgone earnings were the key drivers of costs in both cohorts. Healthcare costs were higher before and after the diagnosis compared with the controls. Men incurred higher excess healthcare costs than women. Hospitalization and outpatient services were the key drivers of the gender‐associated differences. Income from employment decreased more rapidly after diagnosis in the SSc‐ILD cohort than in the non‐ILD SSc cohort. Public transfer income increased after diagnosis, with the most pronounced difference in the SSc‐ILD cohort. Disability pension was the key driver of public transfer income.
Conclusion
SSc is associated with a significant individual and societal burden that is evident several years before and after the diagnosis. Total excess costs are higher in SSc‐ILD than in the non‐ILD SSc underlining the severity of pulmonary involvement. Initiatives to maintain work ability and to reduce hospital admissions may reduce the economic burden of SSc.
Keywords: cost of illness, direct healthcare cost, healthcare cost, indirect costs, systemic sclerosis‐associated interstitial lung disease
Short abstract
In a cohort of 1869 cases and 7463 matched controls, we found a significant individual and societal burden of systemic sclerosis that is evident several years before and after the diagnosis. Total excess costs were highest in cases with concomitant interstitial lung disease, which underlines the severity of pulmonary involvement.
INTRODUCTION
Systemic sclerosis (SSc) is an autoimmune connective tissue disease characterized by fibrosis of the skin and internal organs and vasculopathy. 1 SSc is a rare disease with an annual prevalence of 4–44 per 100,000 and associated with high morbidity, premature death and loss of productivity. 1 , 2 , 3 SSc is mostly diagnosed in women between 30 and 50 years. 4 , 5 Pulmonary involvement with interstitial lung disease (SSc‐ILD) is a prevalent complication and clinically significant ILD is seen in up to 40% of patients. 6 SSc‐ILD onset is usually within the first 5 years after diagnosis 1 , 7 , 8 and ranges from subclinical to severe pulmonary fibrosis with reduced lung function and respiratory failure. Moreover, SSc‐ILD is associated with a higher mortality than non‐ILD SSc. 4 , 6 , 9
Few studies have evaluated direct healthcare costs related to SSc‐ILD and they all find higher annual healthcare costs related to SSc‐ILD (€5855–€33.072) compared with non‐ILD SSc (€4406–€5206). 10 , 11 , 12
SSc is associated with high working disability and the rate of patients unable to work increases with disease severity. 3 , 13 , 14 Given that SSc mostly affects patients in their working age, loss of productivity imposes an economic burden on both the affected individuals and society. There is a lack of studies evaluating the economic impact of foregone earnings due to lost working ability and public transfer income (i.e., indirect costs) associated with SSc as such information is sparsely available. The majority of studies evaluating indirect costs of SSc are cross‐sectional and unable to assess how costs vary with disease severity (Table S1 in the Supporting Information). Only one study 12 has evaluated indirect costs of SSc‐ILD. However, the study was small (52 cases) and included only cases, who were insured and employed, thus limiting the generalizability of the results.
In our nationwide registry‐based cohort study with matched controls, we evaluated the excess direct healthcare costs and indirect costs associated with SSc in patients with and without ILD 5 years before and 4 years after the diagnosis.
The aims of the study were (1) to evaluate the annual average direct healthcare costs, elderly care costs and indirect costs in patients with SSc with or without ILD and in matched controls without SSc and (2) to evaluate the change in societal burden of SSc‐ILD and non‐ILD SSc in comparison with matched controls without SSc before and after the diagnosis.
METHODS
Study setting
In Denmark, all citizens have access to tax‐funded universal health care. A unique 10‐digit Civil Registration System (CPR) number is assigned to all Danish citizens upon birth or immigration, enabling precise, individual‐level record linkage across all Danish registries. 15
Data source
The Danish National Patient Registry (DNPR) is an administrative registry that has achieved complete nationwide coverage on all non‐psychiatric admissions since 1978 and outpatient clinic contacts since 1995. 16 Registration is mandatory and submitted by the treating physician. Diagnoses are classified according to the International Classification of Diseases and Related Health problems 10th Revision (ICD‐10).
The National Income Statistics Registry (ISR) holds information on income composition of the entire Danish population 17 Information on vital status, migration and cohabitation was obtained from the CPR. 18
Study population
Patients ≥18 years of age diagnosed with SSc (ICD‐10: M34) from 1 January 2002 to 31 December 2015 were identified in the DNPR and included as incident cases. Prevalent cases would bias the results and therefore a 2‐year washout period was applied (2000–2001) excluding patients diagnosed before the inclusion period. Cases diagnosed with ILD (ICD‐10: J84) any time before or after SSc diagnosis were included in the SSc‐ILD cohort and cases with no ILD diagnosis were included in the non‐ILD SSc cohort.
Cases were individually matched on the date of the first SSc diagnosis to controls without SSc randomly selected from the background population using the CPR registry. Cases and controls were matched 1:4 by sex, birth year, cohabitating status and municipality.
The Deyo–Charlson Comorbidity Index (DCCI) included comorbidities from the DNPR 3 years before the index date (Table S2 in the Supporting Information). 19 , 20 , 21 The DCCI includes both separate comorbidities and SSc‐related conditions as these cannot be accurately separated using only the ICD‐10 coding system.
The DCCI has a positive predictive value (PPV) of 98% in the DNPR. 22
Socioeconomic status (SES) was defined by source of income in SSc cases and controls as high if employed or enrolled in an educational programme and low if receiving public transfer payment. Cases receiving age pension were not included in either SES group as a universal public pension is paid to all retired Danish citizens above 65 years of age.
Patients and controls were followed up until death, migration or end of follow‐up in 2016. Controls were excluded if the matched case died or migrated and cases were excluded if all four controls died or migrated before the end of follow‐up.
Costing methodology
Cohort studies can measure the costs and work productivity from the time of diagnosis and onwards and thereby display the burden of disease at different disease stages. The present study used an excess cost approach (including costs related to SSc and comorbidities) 23 by estimating the mean annual cost per case compared with the costs of the controls.
Healthcare costs included the average costs of hospital admissions, outpatient care (including emergency department contact), primary care visits and medication. Hospital‐related costs were calculated based on data from the Diagnosis‐Related Groups/Danish Ambulant Grouping System codes. Costs of medication were obtained from the Danish Register of Medicinal Product Statistics, which includes data on all prescription drugs collected in the Danish community pharmacies since 1995. 24 Frequencies and costs of consultations with general practitioners and other specialists were obtained from the National Health Service Register. 25 Psychiatric costs were available from 2007.
Indirect costs were estimated as the differences in earning between cases and controls based on earned income and various social security compensation.
Costs of elderly care and retirement home were available from 2009. Costs were measured annually and adjusted to 2020 prices in euros (€1: DKK 7.45).
Statistical analysis
Costs are presented as annual means for cases and controls. Statistical significance of the cost estimates and DCCI was assessed by non‐parametric bootstrap analysis. A significance level of 0.05 was assumed for all tests. In the before and after analysis, only cases with at least a 5‐year follow‐up period prior to diagnosis were included. A Generalized Estimating Equation (two‐step model) for gamma regression was used to compare healthcare costs, income from employment and public transfer income in female cases and controls with male cases and controls within age groups and to assess gender differences in healthcare costs (adjusted for age). The two‐step model takes individuals with no costs or income (= 0) into account. Statistical analyses were performed using SAS 9.4 TS Level 1M5 (SAS, Inc., Cary, NC, USA).
RESULTS
We identified and extracted data for 1869 SSc cases and 7463 matching controls from the DNPR. Matching was successful in more than 99% of cases. Female cases were overrepresented (75.5%) and the age‐associated incidence peak was 51–70 years. SSc‐ILD was diagnosed in 275 cases (14.7%). The proportion of men was higher in the SSc‐ILD cohort (30.9%) than in the non‐ILD SSc cohort (23.4%). DCCI was higher in cases than in controls (p < 0.01) (Table 1). SES did not differ between the SSc cohort and controls from 5 to 3 years before diagnosis, whereas the SSc cohort had lower (p < 0.01) SES from 2 years before until 4 years after the diagnosis compared with controls (Table S3 in the Supporting Information).
TABLE 1.
Baseline characteristics of the SSc cases (all), SSc‐ILD cases, non‐SSc cases and matched controls
| SSc‐all | Controls | ILD‐SSc | Controls | Non‐ILD SSc | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Total (n) | 1869 | 7463 | 275 | 1098 | 1594 | 6365 | ||||||
| Age | ||||||||||||
| 18–30 | 116 | 6.2 | 464 | 6.2 | 9 | 3.3 | 36 | 3.3 | 107 | 6.7 | 428 | 6.7 |
| 31–40 | 176 | 9.4 | 702 | 9.4 | 18 | 6.5 | 71 | 6.5 | 158 | 9.9 | 631 | 9.9 |
| 41–50 | 316 | 16.9 | 1264 | 16.9 | 56 | 20.4 | 224 | 20.4 | 260 | 16.3 | 1040 | 16.3 |
| 51–60 | 462 | 24.7 | 1848 | 24.8 | 70 | 25.5 | 280 | 25.5 | 392 | 24.6 | 1568 | 24.6 |
| 61–70 | 455 | 24.3 | 1815 | 24.3 | 73 | 26.5 | 292 | 26.6 | 382 | 24.0 | 1523 | 23.9 |
| >70 | 344 | 18.4 | 1370 | 18.4 | 49 | 17.8 | 195 | 17.8 | 295 | 18.5 | 1175 | 18.5 |
| Sex | ||||||||||||
| Male | 458 | 24.5 | 1831 | 24.5 | 85 | 30.9 | 340 | 31.0 | 373 | 23.4 | 1491 | 23.4 |
| Female | 1411 | 75.5 | 5632 | 75.5 | 190 | 69.1 | 758 | 69.0 | 1221 | 76.6 | 4874 | 76.6 |
| Cohabiting | 1179 | 63.1 | 4699 | 63.0 | 183 | 66.5 | 731 | 66.6 | 996 | 62.5 | 3968 | 62.3 |
| CCI score a | ||||||||||||
| 0 | 1172 | 62.7 | 6430 | 86.2 | 167 | 60.7 | 951 | 86.6 | 1005 | 63.0 | 5479 | 86.1 |
| 1 | 352 | 18.8 | 556 | 7.5 | 60 | 21.8 | 80 | 7.3 | 292 | 18.3 | 476 | 7.5 |
| 2 | 188 | 10.1 | 299 | 4.0 | 28 | 10.2 | 43 | 3.9 | 160 | 10.0 | 256 | 4.0 |
| 3+ | 157 | 8.4 | 178 | 2.4 | 20 | 7.3 | 24 | 2.2 | 137 | 8.6 | 154 | 2.4 |
| Mean CCI score (SD) | 0.7 (1.2) | 0.3 (0.9) | 0.7 (1.2) | 0.3 (1.0) | 0.7 (1.3) | 0.3 (0.9) | ||||||
Abbreviations: CCI, Charlson comorbidity index; ILD, interstitial lung disease; SSc, systemic sclerosis.
Chi‐square test for difference in CCI between cases and controls: p < 0.001 in all three groups.
Healthcare costs were markedly higher in both genders within all age groups in the total SSc cohort compared with the controls (Figure 1).
FIGURE 1.
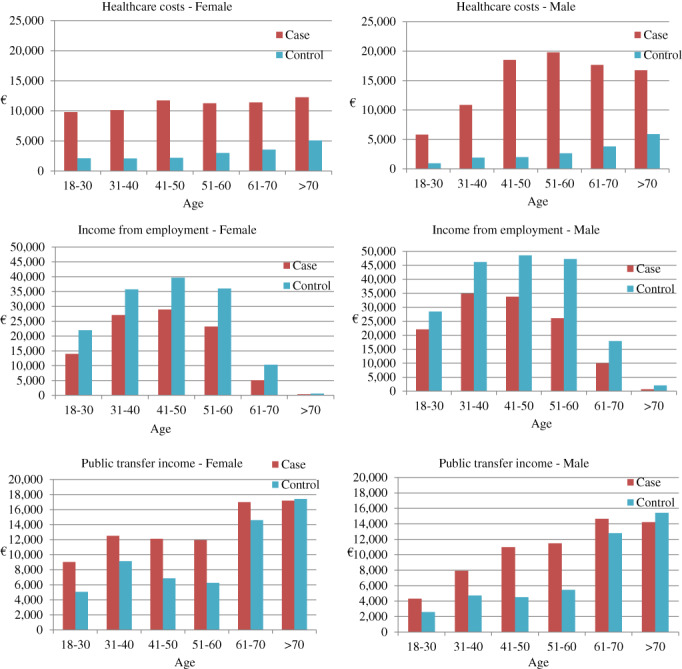
Healthcare costs, income from employment and public transfer income in euros (€) of all systemic sclerosis cases and matched controls distributed by age and sex
Males aged 41–70 years incurred higher healthcare costs compared with controls than women compared with controls (Table S4 in the Supporting Information). Males aged 41–70 years incurred significantly higher healthcare costs than women compared with the controls in the non‐ILD cohort, whereas a difference in costs between genders was only seen from 51 to 60 years in the SSc‐ILD cohort (Table S4 in the Supporting Information). The differences in costs related to hospitalization and outpatient services were significantly higher in men compared with women for the SSc and non‐ILD cohorts, whereas no gender differences were seen in the ILD‐SSc cohort (Table S5 in the Supporting Information).
Income from employment was lower in all age groups and income from public transfers higher compared with the controls (Figure 1).
Both cohorts had higher healthcare and indirect costs compared with the controls. The total excess cost in the SSc‐ILD cohort was €29,725, and €17,905 in the non‐ILD SSc cohort. Healthcare costs and forgone earnings were key drivers of excess costs in both cohorts (Table 2).
TABLE 2.
Mean annual healthcare costs, income from employment, social transfer payments and home care costs after SSc diagnosis in (A) SSc‐ILD cases and matched controls and (B) non‐ILD SSc cases and matched controls
| (A) | SSc‐ILD | Controls | Difference | p‐value | |
|---|---|---|---|---|---|
| Number of persons (N) | 275 | 1098 | |||
| Healthcare costs | |||||
| Outpatient services | € | 5398 | 935 | 4463 | <0.001 |
| Inpatient admissions | € | 11,215 | 1522 | 9693 | <0.001 |
| Prescription drugs | € | 1384 | 484 | 900 | <0.001 |
| Primary health sector | € | 917 | 413 | 504 | <0.001 |
| Psychiatric outpatient services | € | 37 | 41 | −4 | 1.000 |
| Psychiatric inpatient admissions | € | 98 | 99 | −1 | 1.000 |
| Healthcare costs total | € | 19,050 | 3495 | 15,555 | <0.001 |
| Home care costs | |||||
| Home care—care | € | 1007 | 607 | 400 | 0.126 |
| Home care—practical help | € | 218 | 85 | 133 | <0.001 |
| Home care total | € | 1225 | 692 | 533 | 0.015 |
| Earned income | € | 14,922 | 24,685 | −9763 | <0.001 |
| Public transfer income | |||||
| Unemployment insurance | € | 237 | 442 | −206 | 0.020 |
| Social security benefit | € | 1318 | 498 | 819 | <0.001 |
| Age pension | € | 4782 | 5385 | −604 | 0.046 |
| Early retirement | € | 1307 | 1087 | 220 | 0.748 |
| Disability pension | € | 4931 | 1731 | 3200 | <0.001 |
| Sick pay (public funded) | € | 693 | 242 | 451 | <0.001 |
| Housing benefits | € | 551 | 483 | 68 | 0.692 |
| Childs benefits | € | 280 | 355 | −75 | 0.161 |
| Public transfer income total | € | 14,097 | 10,223 | 3873 | <0.001 |
| SSc‐ILD excess costs | |||||
| Excess direct healthcare costs | € | 15,555 | |||
| Excess home care costs | € | 533 | |||
| Excess foregone earnings from employment | € | 9763 | |||
| Excess social transfer payments | € | 3873 | |||
| Excess costs, total | € | 29,725 |
| (B) | Non‐ILD SSc | Control | Difference | p‐value | |
|---|---|---|---|---|---|
| Number of persons (N) | 1594 | 6365 | |||
| Healthcare costs | |||||
| Outpatient services | € | 4433 | 1021 | 3411 | <0.001 |
| Inpatient admissions | € | 5435 | 1600 | 3835 | <0.001 |
| Prescription drugs | € | 975 | 427 | 547 | <0.001 |
| Primary health sector | € | 746 | 419 | 326 | <0.001 |
| Psychiatric outpatient services | € | 35 | 36 | −1 | 1.000 |
| Psychiatric inpatient admissions | € | 96 | 96 | 0 | 1.000 |
| Healthcare costs total | € | 11,719 | 3599 | 8119 | <0.001 |
| Home care | |||||
| Home care—care | € | 914 | 535 | 379 | <0.001 |
| Home care—practical help | € | 206 | 100 | 105 | <0.001 |
| Home care total | € | 1120 | 635 | 485 | <0.001 |
| Earned income | € | 15,111 | 21,715 | −6604 | <0.001 |
| Public transfer income | |||||
| Unemployment insurance | € | 266 | 448 | −182 | <0.001 |
| Social security benefit | € | 1043 | 655 | 389 | <0.001 |
| Age pension | € | 5370 | 5855 | −485 | <0.001 |
| Early retirement | € | 974 | 1148 | −174 | 0.019 |
| Disability pension | € | 4518 | 1661 | 256 | <0.001 |
| Sick pay (public funded) | € | 541 | 326 | 215 | <0.001 |
| Housing benefits | € | 733 | 606 | 127 | <0.001 |
| Childs benefits | € | 420 | 470 | −49 | 0.014 |
| Public transfer income total | € | 13,864 | 11,167 | 2697 | <0.001 |
| Non‐ILD SSc excess costs | |||||
| Excess direct healthcare costs | € | 8119 | |||
| Excess home care costs | € | 485 | |||
| Excess foregone earnings from employment | € | 6604 | |||
| Excess social transfer payments | € | 2697 | |||
| Excess costs, total | € | 17,905 |
Note: p‐value from t‐test and bootstrapping.
Abbreviations: ILD, interstitial lung disease; SSc, systemic sclerosis.
The total healthcare costs were higher in both cohorts before and after the diagnosis compared with the controls with a peak around the time of diagnosis (Figure 2). Three years after diagnosis, healthcare costs seemed to increase again in the SSc‐ILD, whereas healthcare cost continued to decrease in the non‐ILD SSc cohort. Income from employment was lower in both cohorts compared with the controls 5 years before diagnosis, although this did not reach statistical significance until 2 years after diagnosis in the SSc‐ILD cohort (Figure 2 and Table S6 in the Supporting Information). Income from employment decreased more rapidly after diagnosis in the SSc‐ILD cohort than in the non‐ILD SSc cohort (Figure 2). Income from public transfer was higher in both cohorts before diagnosis compared with the controls, although not statistically significant until the year of diagnosis (Table S6 in the Supporting Information). After diagnosis, public transfer income increased markedly with the most pronounced difference in the SSc‐ILD cohort (Figure 2)
FIGURE 2.
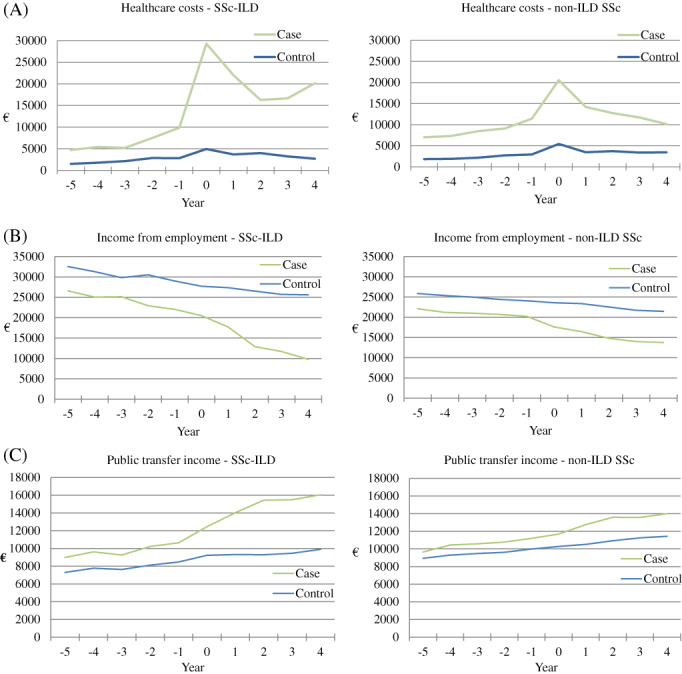
(A) Total healthcare costs, (B) income from employment and (C) public transfer income in euros (€) before and after the diagnosis of systemic sclerosis (SSc) in the SSc‐interstitial lung disease (SSc‐ILD) and non‐ILD SSc cohorts compared with the matched controls. Year 0 = year of SSc diagnosis
DISCUSSION
Our study is the first European study to evaluate indirect costs associated with SSc‐ILD in a follow‐up study. We saw that SSc‐ILD was associated with a 40% higher total excess cost than non‐ILD SSc compared with the controls (€29,725 vs. €17,905). The study provides important information on the individual and societal impact of SSc with and without ILD, not only at the time of diagnosis, but also in the years before and after the initial diagnosis of SSc.
With regard to direct healthcare costs, we found considerably higher healthcare costs among SSc cases compared with the controls in all age groups and both genders. Healthcare costs for both the SSc‐ILD and the non‐ILD SSc cohorts were largely driven by hospitalizations and outpatient visits. Noteworthy, the excess costs due to inpatient admissions were vastly higher in the SSc‐ILD cohort (€9693) than in the non‐ILD SSc cohort (€3835) indicating the severity of this complication. Two previous cohort studies also found higher excess healthcare costs in SSc‐ILD compared with non‐ILD SSc, with costs driven by outpatient pharmacy claims and services, 11 hospitalization and increasing ILD severity. 10 An American study found healthcare costs to be significantly higher in an SSc‐ILD cohort ($37,505/€33.072) compared with non‐SSc controls ($4997/€4406) during 12 months of follow‐up. 12
Interestingly, we find that although healthcare costs decline after diagnosis in the non‐ILD cohort, healthcare costs seem to increase in the 3 years following diagnosis in the SSc‐ILD cohort. This is in concordance with Fischer et al. 11 who found healthcare cost to increase in cases with SSc‐ILD 5 years after diagnosis. This likely reflects the high morbidity and progressive course of SSc‐ILD seen in a high proportion of patients.
Because of the low prevalence and heterogeneous presentation of SSc, the time to diagnosis is often prolonged 26 which might be reflected in our data by the higher healthcare costs several years before diagnosis in both cohorts compared with the controls. Moreover, comorbidity 3 years prior to SSc diagnosis was higher in SSc cases than in the controls, which likely contributes to the observed excess healthcare costs. Two years prior to diagnosis, we also observed lower SES in SSc cases compared with the controls. Low SES is associated with poor health, and poor health is vice versa a risk factor for low SES. 27 It is unclear from our data to what extend comorbidities contribute to this socioeconomic disadvantage and low SES in the SSc cases. Future research is needed to detangle this interaction between SES and comorbidities and the impact on SSc.
Excess healthcare costs in the total SSc cohort were considerably higher in men compared with women. Surprisingly, the difference in costs between genders could not be explained by the high share of men with SSc‐ILD (Figure 2 and Table S4 in the Supporting Information). The gender‐associated difference in costs was related to hospitalization and outpatient services, whereas we saw no difference in costs related to medication and primary health care (Table S5 in the Supporting Information). These findings might reflect that men suffer more severe disease than women, which is supported by several studies that find worse prognosis among men. 28
To our knowledge, this is the first study to evaluate indirect costs associated with SSc‐ILD in a population‐based nationwide cohort. We find that indirect costs contributed markedly to the total costs associated with both ILD‐SSc and non‐ILD SSc, which is in line with previous cross‐sectional studies on productivity loss associated with SSc that found annual costs from €5500 to €10,500. 29 , 30 , 31 , 32 A cohort study from the United States evaluated insured and working SSc cases and matched non‐SSc controls and found excess costs of $3103 (€2736) due to productivity loss in the first year following diagnosis. 33 In a subset cohort from the study, a difference of $5846 (€5155) was found between SSc‐ILD cases (n = 52) and matched non‐SSc controls during 6 months of follow‐up. 12 This is in line with our study where we saw reduced earned income in both cohorts compared with the matched controls, with the largest difference in the SSc‐ILD cohort (€9763) compared with the non‐ILD SSC cohort (€6604). Importantly, our results reflect foregone earnings of not only working cases, but also the entire Danish SSc population.
Public transfer income after diagnosis was higher and increased in both cohorts compared with the controls, with the most significant difference seen in the SSc‐ILD cohort. Disability pension was the key driver of excess public transfer income in both cohorts after diagnosis, which is in line with Sandqvist et al. who found increasing rates of working disability after SSc onset. 13
As expected, the difference in earned income and public transfer income levelled out in the age groups >70 years because of the universal public pension (Figure 2).
The strengths of the present study are the large and nationwide study population and the 17‐year study period. The population‐based study design, within a tax‐funded, uniformly organized healthcare system and virtually complete follow‐up on all cases, reduces the risk of selection bias. 18 The population‐based matched control cohort enabled us to control for some important demographic confounders. We expect the risk of SSc misclassification to be minimal as SSc have a PPV of 94% in the DNPR. 34 Importantly, most cases with SSc and ILD are diagnosed in the hospital settings; hence, we expect our data to be highly representative of the entire Danish SSc population and our findings to be generalizable to populations with similar standard of medical care as in Denmark.
The study has several limitations. The DNPR does not provide information on disease severity, BMI or smoking; thus, we were not able to evaluate the impact of these important factors.
Low SES may contribute to the excess costs found in this study. We partly accounted for socioeconomic factors by matching on cohabitation and residency. Furthermore, stratification analysis showed no difference in SES between SSc cases and controls 5 to 3 years before diagnosis, whereas the observed low SES 2 years before diagnosis in the SSc cohorts is likely influenced by increased morbidity prior to SSc diagnosis hindering cases to maintain their job.
We cannot dismiss that other markers of SES (e.g., highest achieved educational level and occupational type) are associated with SSc and thus affect income level.
ILD is not validated in the DNPR which could lead to misclassification and an over‐ or under‐estimation of the share of cases with SSc‐ILD. However, validation studies of the DNPR generally show a high PPV, particularly for diseases that are primarily followed in specialized departments such as ILD; thus, we expect this information bias to be limited. 22
In conclusion, we found that SSc is associated with a significant individual as well as societal burden that is evident several years before and after the diagnosis. SSc‐ILD is associated with 40% higher total excess costs compared with non‐ILD SSc, thus underlining the severity of pulmonary involvement. Importantly, we find that men impose higher excess healthcare‐related costs than women, and hospitalization and outpatient services are the key drivers of gender differences in healthcare costs.
Given that the key driver of healthcare costs in SSc‐ILD was inpatient admissions, initiatives to reduce admissions could potentially reduce costs associated with SSc‐ILD. Furthermore, since the majority of patients are diagnosed with SSc in their working age, the decrease in working ability and increasing costs due to public transfer income underline the importance of timely diagnosis and treatment in order to maintain employment for patients.
CONFLICT OF INTEREST
Ole Hilberg has received an unrestricted grant from Boehringer Ingelheim A/S for the current work. Outside the current work, Malene Knarborg has received a fee from AstraZenica A/S. Melina Gade Sikjær, Anders Løkke and Rikke Ibsen declare no competing interests.
AUTHOR CONTRIBUTION
Malene Knarborg: Conceptualization (equal); methodology (lead); project administration (lead); visualization (equal); writing – original draft (supporting); writing – review and editing (equal). Anders Løkke: Conceptualization (equal); methodology (equal); project administration (equal); supervision (equal); writing – review and editing (lead). Ole Hilberg: Conceptualization (equal); methodology (equal); project administration (supporting); supervision (lead); writing – review and editing (equal). Rikke Ibsen: Conceptualization (equal); data curation (lead); formal analysis (lead); methodology (equal); software (lead); validation (lead); visualization (equal); writing – review and editing (supporting). Melina Gade Sikjær: Conceptualization (equal); methodology (equal); project administration (equal); visualization (equal); writing – original draft (lead).
HUMAN ETHICS APPROVAL DECLARATION
The study was approved by the Danish Data Protection Agency as the research only involved register‐based data and therefore human research ethical approval was not required.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
Research funding: The study received an unrestricted grant from Boehringer Ingelheim A/S for the current work. Boehringer Ingelheim A/S had no role in study design, data collection, data analyses, data interpretation, manuscript writing or decision‐making where to submit for publication.
Knarborg M, Løkke A, Hilberg O, Ibsen R, Sikjær MG. Direct and indirect costs of systemic sclerosis and associated interstitial lung disease: A nationwide population‐based cohort study. Respirology. 2022;27:341–349. 10.1111/resp.14234
Associate Editor: Michael P. Keane; Senior Editor: Chris Grainge
Funding information Boehringer Ingelheim
DATA AVAILABILITY STATEMENT
Data were anonymized from the national databases and are not publicly available.
REFERENCES
- 1. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–99. [DOI] [PubMed] [Google Scholar]
- 2. Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis‐associated interstitial lung disease. Clin Epidemiol. 2019;11:257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hudson M, Steele R, Lu Y, Thombs BD, Baron M. Work disability in systemic sclerosis. J Rheumatol. 2009;36(11):2481–6. [DOI] [PubMed] [Google Scholar]
- 4. Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–15. [DOI] [PubMed] [Google Scholar]
- 5. Mayes MD, Lacey JV Jr, Beebe‐Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48(8):2246–55. [DOI] [PubMed] [Google Scholar]
- 6. Perelas A, Silver RM, Arrossi AV, Highland KB. Systemic sclerosis‐associated interstitial lung disease. Lancet Respir Med. 2020;8(3):304–20. [DOI] [PubMed] [Google Scholar]
- 7. Benan M, Hande I, Gul O. The natural course of progressive systemic sclerosis patients with interstitial lung involvement. Clin Rheumatol. 2007;26(3):349–54. [DOI] [PubMed] [Google Scholar]
- 8. Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long‐term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66(6):1625–35. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann‐Vold AM, Fretheim H, Halse AK, Seip M, Bitter H, Wallenius M, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med. 2019;200(10):1258–66. [DOI] [PubMed] [Google Scholar]
- 10. Morrisroe K, Stevens W, Sahhar J, Ngian GS, Ferdowsi N, Hansen D, et al. The clinical and economic burden of systemic sclerosis related interstitial lung disease. Rheumatology (Oxford). 2020;59(8):1878–88. [DOI] [PubMed] [Google Scholar]
- 11. Fischer A, Kong AM, Swigris JJ, Cole AL, Raimundo K. All‐cause healthcare costs and mortality in patients with systemic sclerosis with lung involvement. J Rheumatol. 2018;45(2):235–41. [DOI] [PubMed] [Google Scholar]
- 12. Zhou Z, Fan Y, Thomason D, Tang W, Liu X, Zhou ZY, et al. Economic burden of illness among commercially insured patients with systemic sclerosis with interstitial lung disease in the USA: a claims data analysis. Adv Ther. 2019;36(5):1100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandqvist G, Hesselstrand R, Petersson IF, Kristensen LE. Work disability in early systemic sclerosis: a longitudinal population‐based cohort study. J Rheumatol. 2015;42(10):1794–800. [DOI] [PubMed] [Google Scholar]
- 14. Sharif R, Mayes MD, Nicassio PM, Gonzalez EB, Draeger H, McNearney TA, et al. Determinants of work disability in patients with systemic sclerosis: a longitudinal study of the GENISOS cohort. Semin Arthritis Rheum. 2011;41(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–5. [DOI] [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–3. [DOI] [PubMed] [Google Scholar]
- 17. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7 Suppl):103–5. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–9. [DOI] [PubMed] [Google Scholar]
- 19. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 21. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol. 2004;57(12):1288–94. [DOI] [PubMed] [Google Scholar]
- 22. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larg A, Moss JR. Cost‐of‐illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29(8):653–71. [DOI] [PubMed] [Google Scholar]
- 24. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. [DOI] [PubMed] [Google Scholar]
- 25. Andersen JS, Olivarius Nde F, Krasnik A. The Danish National Health Service Register. Scand J Public Health. 2011;39(7 Suppl):34–7. [DOI] [PubMed] [Google Scholar]
- 26. Delisle VC, Hudson M, Baron M, Thombs BD, Canadian Scleroderma Research Group A . Sex and time to diagnosis in systemic sclerosis: an updated analysis of 1,129 patients from the Canadian scleroderma research group registry. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S‐10‐4. [PubMed] [Google Scholar]
- 27. Hoffmann R, Kröger H, Geyer S. Social causation versus health selection in the life course: does their relative importance differ by dimension of SES? Soc Indic Res. 2019;141(3):1341–67. [Google Scholar]
- 28. Pokeerbux MR, Giovannelli J, Dauchet L, Mouthon L, Agard C, Lega JC, et al. Survival and prognosis factors in systemic sclerosis: data of a French multicenter cohort, systematic review, and meta‐analysis of the literature. Arthritis Res Ther. 2019;21(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chevreul K, Brigham KB, Gandré C, Mouthon L. The economic burden and health‐related quality of life associated with systemic sclerosis in France. Scand J Rheumatol. 2015;44(3):238–46. [DOI] [PubMed] [Google Scholar]
- 30. Bernatsky S, Hudson M, Panopalis P, Clarke AE, Pope J, Leclercq S, et al. The cost of systemic sclerosis. Arthritis Rheum. 2009;61(1):119–23. [DOI] [PubMed] [Google Scholar]
- 31. López‐Bastida J, Linertová R, Oliva‐Moreno J, Posada‐de‐la‐Paz M, Serrano‐Aguilar P. Social economic costs and health‐related quality of life in patients with systemic sclerosis in Spain. Arthritis Care Res (Hoboken). 2014;66(3):473–80. [DOI] [PubMed] [Google Scholar]
- 32. Kawalec PP, Malinowski KP. The indirect costs of systemic autoimmune diseases, systemic lupus erythematosus, systemic sclerosis and sarcoidosis: a summary of 2012 real‐life data from the Social Insurance Institution in Poland. Expert Rev Pharmacoecon Outcomes Res. 2015;15(4):667–73. [DOI] [PubMed] [Google Scholar]
- 33. Zhou Z, Fan Y, Tang W, Liu X, Thomason D, Zhou ZY, et al. Economic burden among commercially insured patients with systemic sclerosis in the United States. J Rheumatol. 2019;46(8):920–7. [DOI] [PubMed] [Google Scholar]
- 34. Butt SA, Jeppesen JL, Fuchs C, Mogensen M, Engelhart M, Torp‐Pedersen C, et al. Trends in incidence, mortality, and causes of death associated with systemic sclerosis in Denmark between 1995 and 2015: a nationwide cohort study. BMC Rheumatol. 2018;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data were anonymized from the national databases and are not publicly available.


