Abstract
The use of natural microorganisms in biotransformations is frequently constrained by their limited tolerance to the high concentrations of metabolites and solvents required for effective industrial production. In many cases, more robust strains have to be generated by random mutagenesis and selection. This process of directed evolution can be accelerated in mutator strains, which carry defects in one or more of their DNA repair genes. However, in order to use mutator strains, it is essential to restore the normal low mutation rate of the selected organisms immediately after selection to prevent the accumulation of undesirable spontaneous mutations. To enable this process, we constructed temperature-sensitive plasmids that temporarily increase the mutation frequency of their hosts by 20- to 4,000-fold. Under appropriate selection pressure, microorganisms transformed with mutator plasmids can be quickly evolved to exhibit new, complex traits. By using this approach, we were able to increase the tolerance of three bacterial strains to dimethylformamide by 10 to 20 g/liter during only two subsequent transfers. Subsequently, the evolved strains were returned to their normal low mutation rate by curing the cells of the mutator plasmids. Our results demonstrate a new and efficient method for rapid strain improvement based on in vivo mutagenesis.
Recent advances in genomics and protein evolution have dramatically improved our ability to introduce novel catalytic functions or entire metabolic pathways into microorganisms. However, the utilization of such engineered strains in industrial processes is often constrained by their limited tolerance to the high concentrations of metabolites and solvents required for the efficient production of biomaterials. The generation of more robust strains (that can tolerate production conditions) usually requires the accumulation of multiple favorable mutations. Classical strain improvement methods rely on UV radiation or chemical mutagenesis. These methods are rather inefficient because they are usually discontinuous and they lead to significant cell damage.
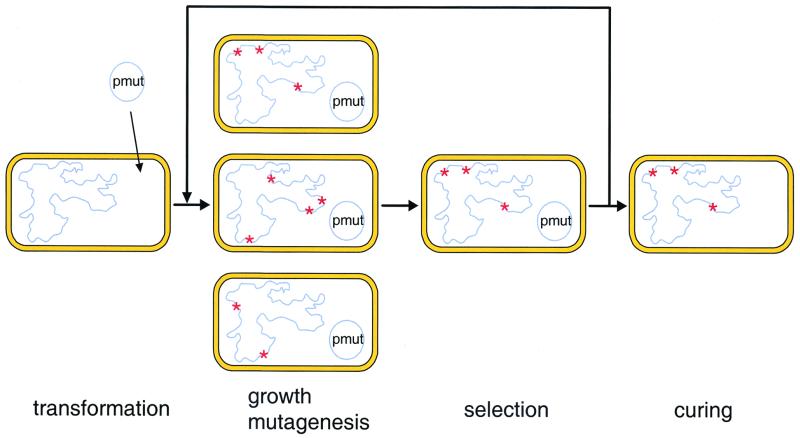
Most natural microorganisms have a very low rate of spontaneous mutagenesis to prevent the accumulation of deleterious mutations (4). However, strains with elevated mutation rates arise spontaneously under conditions of prolonged selection pressure (1, 8, 10, 16, 17, 19). A number of such mutator strains that carry defects in one or more DNA repair genes have been described in the literature (11), but their use is limited by their genetic instability. Nevertheless, mutator strains like XL1-Red (Stratagene) are commonly used for the mutagenesis of individual genes. To mutate a gene, it must be cloned into a plasmid or phagemid and propagated for a limited time in a mutator strain (6, 9). In contrast, we demonstrate here the mutagenesis of the entire genome of an organism by temporarily moving a mutator gene into that organism (Fig. 1). Our strategy is based on the mutD (or dnaQ) gene, which encodes the ε subunit of DNA polymerase III, which is responsible for proofreading. We used the mutD5 allele of Escherichia coli that carries two amino acid substitutions (18). Although the MutD5 protein lacks catalytic activity, it can still bind effectively to DNA polymerase III. If cells harbor mutD5 on a plasmid, then the plasmid-generated nonfunctional MutD5 protein effectively competes with the functional MutD protein that is produced from the chromosomal copy of the mutD gene (2). We reasoned that this dominant mutator phenotype conferred by mutD5 could be utilized to temporarily increase the mutation frequency of E. coli, allowing the rapid evolution of novel traits. Cells carrying a mutD5 gene on a plasmid accumulate a broad spectrum of base substitutions and even frameshift mutations, which makes them a very versatile source of genetic diversity. Once the desired trait(s) has been selected, curing the cells of the mutator plasmid can stabilize the new phenotype. To facilitate plasmid curing, we used the temperature-sensitive origin of replication of pSc101. By using this methodology, we have demonstrated significant acceleration of strain evolution in the presence of a mutator plasmid.
FIG. 1.
Acceleration of the evolution of a microorganism by using a mutator plasmid. The starting strain is transformed with mutator plasmid pmut to increase its mutation rate. Subsequently, the cells are subjected to multiple rounds of growth and selection, leading to the establishment of the desired phenotype. The resulting strains can be stabilized by curing them of the mutator plasmid.
MATERIALS AND METHODS
Construction of mutD and mutD5 plasmids and testing in three bacterial strains.
mutD and mutD5 genes were amplified by PCR using primers mutd1 (5′-CGCCTCCAGCGCGACAATAGCGGCCATC-3′) and mutd2 (5′-CCGACTGAACTACCGCTCCGCGTTGTG-3′) from genomic DNA of E. coli FM5 and E. coli CSH116 (11), respectively. The PCR products were cloned into the pCR-Blunt vector (Invitrogen, Carlsbad, Calif.). Plasmids containing inserts with the correct orientation were isolated and digested with the SmaI and HindIII restriction enzymes. The overhangs were filled by using T4 polymerase and cloned into plasmid pMAK705 (12) digested with SmaI and PvuII. The ligation products were transformed into competent JM101 cells. The resulting plasmids had the temperature-sensitive origin of replication, carried a kanamycin resistance marker, and were named pMutD-wt (control plasmid, wild-type genotype) and pMutD5 (mutator plasmid).
The plasmids were successfully tested in E. coli MM294 (F− endA1 hsdR17 [rk− mk+] supE44 thi-1 relA1), E. coli W1485 (F+ λ−), and E. blattae ATCC 33429 for the ability to accelerate the generation of solvent-tolerant mutants. All evolution experiments were performed with LB medium (14). Mutation frequencies were determined by plating 100-μl cell suspension samples on LB plates containing rifampin at 100 μg/ml. The mutation frequency was calculated by dividing the number of resistant cells by the total number of plated cells.
Selection for solvent tolerance.
Evolution experiments were performed with LB agar plates to which dimethylformamide (DMF) at 50, 60, 70, 80, and 90 g/liter and kanamycin at 25 μg/ml had been added. The size of every evolving population was limited to 106 cells. Colonies were counted after 3 days of growth, and 10 colonies were selected for the next plating. Cells from selected colonies were mixed together, and samples containing 106 cells were transferred onto fresh plates containing the same or higher concentrations of DMF. After two consecutive rounds, the cells were cured of the plasmids by growth at elevated temperatures. E. blattae 33429 and E. coli MM294 were cured at 41 and 43°C, respectively. Three to four subculturing steps at the indicated temperatures were sufficient for 87 to 100% curing. Individual cured clones were selected by parallel growth of clones in selective (LB supplemented with kanamycin at 25 μg/ml) and nonselective (LB) media. Clones that lost the ability to grow on plates supplemented with kanamycin were isolated and further analyzed. The curing was confirmed by standard plasmid purification from selected clones and gel analysis.
RESULTS
Evolution of DMF resistance.
To test the application of plasmid pMutD5 to generate new phenotypes, we selected for tolerance to DMF. DMF increases the solubility of many organic compounds, which makes it versatile for biotransformations. However, organic solvents such as DMF are toxic to bacteria even at low concentrations. The toxicity of solvents significantly limits the use of microorganisms in industrial biotechnology. In general, solvent molecules are incorporated into bacterial membranes, disrupting their structure and ultimately leading to cell death (3, 7). In order to increase their solvent tolerance, we subjected two E. coli strains, MM294 and W1485, and one strain of E. blattae, EB33429, carrying a mutator (pMutD5) or control (pMutD-wt) plasmid to selective pressure on DMF-containing plates.
Results of the solvent tolerance evolution experiment are summarized in Table 1. We plated 106 cells carrying the mutator or control plasmid onto agar medium containing various concentrations of DMF. For all three strains, we observed that cells carrying the mutator plasmid showed stronger growth in the presence of DMF than did cells carrying the control plasmid. Subsequently, we isolated 10 of the most DMF-tolerant colonies of each strain and transferred them onto fresh plates. As a result, we observed another significant increase in the DMF tolerance of the mutator strains but little progress in the control cells. These results indicate that the mutator plasmid leads to significant acceleration of the evolution of solvent tolerance in all three strains. Interestingly, the mutD5 allele of E. coli is effective in E. blattae, which shows that the mutD5-encoded protein from E. coli can effectively bind to DNA polymerase III of E. blattae.
TABLE 1.
Evolution of solvent tolerance
| Strain | DMF concn (g/liter) | Characterization or no. of coloniesa
|
|
|---|---|---|---|
| Round 1 | Round 2b | ||
| E. coli | |||
| MM294(pMutD5) | 60 | Low-density lawnc | High-density lawnc |
| MM294(pMutD5) | 70 | 11 | 824 |
| MM294(pMutD5) | 80 | 0 | 4 |
| MM294(pMutD-wt) | 60 | 17 | Low-density lawnc |
| MM294(pMutD-wt) | 70 | 0 | 0 |
| E. coli | |||
| W1485(pMutD5) | 50 | High-density lawnc | High-density lawnc |
| W1485(pMutD5) | 60 | 386 | Low-density lawnc |
| W1485(pMutD5) | 70 | 0 | 6 |
| W1485(pMutD-wt) | 50 | High-density lawnc | High-density lawnc |
| W1485(pMutD-wt) | 60 | 0 | 0 |
| E. blattae | |||
| EB33429(pMutD5) | 50 | Low-density lawnc | High-density lawnc |
| EB33429(pMutD5) | 60 | 0 | 968 |
| EB33429(pMutD-wt) | 50 | 793 | High-density lawnc |
| EB33429(pMutD-wt) | 60 | 0 | 0 |
For each experiment, 106 cells were plated on LB agar containing the DMF concentration indicated.
Ten clones from round 1 were selected and mixed, and then 106 cells from this pool were plated on fresh LB agar plates supplemented with various concentrations of DMF.
Cultures that resulted in uniform growth on the plates were characterized as a high-density lawn if growth was similar to that of the no-DMF control and as a low-density lawn if growth was significantly weaker than that of the no-DMF control.
Plasmid curing of evolved strains.
Single colonies of evolved strain EB33429 and MM294 bacteria were easily cured by growth at 41 and 43°C, respectively. Curing had no effect on the level of tolerance on DMF-containing plates. Cured clones of strain EB33429 grew on medium containing DMF at 60 g/liter, and cured clones of strain MM294 grew on medium containing DMF at 80 g/liter, as expected.
Surprisingly, we were not able to cure strain W1485(pMutD5), which had evolved to grow with DMF at 70 g/liter. It should be noted that the mutator plasmid was curable from strain W1485 prior to the evolution of DMF tolerance. Plasmid analysis revealed that pMutD5 formed high-molecular-weight multimers in evolved W1485 cells. A more detailed analysis of this material is required to generate a more robust version of the mutator plasmid.
Influence of plasmids on the mutation rate.
To further study the effect of the mutator plasmid, we measured the formation of rifampin-resistant colonies, which reflects the mutation rate of a strain. Table 2 shows that the mutator plasmid increased the average mutation rate by 2,000- to 4,000-fold in the E. coli strains and by about 200-fold in E. blattae. The mutation rates remained unchanged during the evolution of E. coli but showed a significant reduction in E. blattae. More importantly, the data in Table 2 demonstrate that the mutation rates of evolved MM294 and EB33429 returned to their low wild-type levels when the strains were cured of the mutator plasmid.
TABLE 2.
Mutation frequencies of bacteria harboring mutator and control plasmidsa
| Strain | Avg mutation frequency ± SD
|
||
|---|---|---|---|
| Before evolution | After evolution | After curing | |
| E. coli | |||
| MM294(pMutD5) | (9.3 ± 0.8) × 10−5 | (4.7 ± 4.0) × 10−5 | (1.5 ± 0.5) × 10−8 |
| MM294(pMutD-wt) | (4.2 ± 3.4) × 10−8 | (2.9 ± 2.4) × 10−8 | (6.4 ± 3.2) × 10−8 |
| E. coli | |||
| W1485(pMutD5) | (3.5 ± 2.5) × 10−5 | (4.6 ± 3.7) × 10−5 | No datab |
| W1485(pMutD-wt) | (0.8 ± 0.7) × 10−8 | (4.5 ± 3.6) × 10−8 | No datab |
| E. blattae | |||
| EB33429(pMutD5) | (4.6 ± 2.3) × 10−6 | (1.1 ± 0.9) × 10−7 | <2.5 × 10−8 |
| EB33429(pMutD-wt) | (2.6 ± 2.1) × 10−8 | (4.7 ± 3.8) × 10−8 | <2.5 × 10−8 |
Single colonies were grown in LB medium to an optical density at 620 nm of 0.8 to 1.2 and plated onto LB medium containing rifampin. The frequency of resistant mutants was scored after overnight incubation at 30°C. The experiments were done in triplicate.
Evolved cells could not be cured of the mutator plasmid.
Evaluation of selected strains.
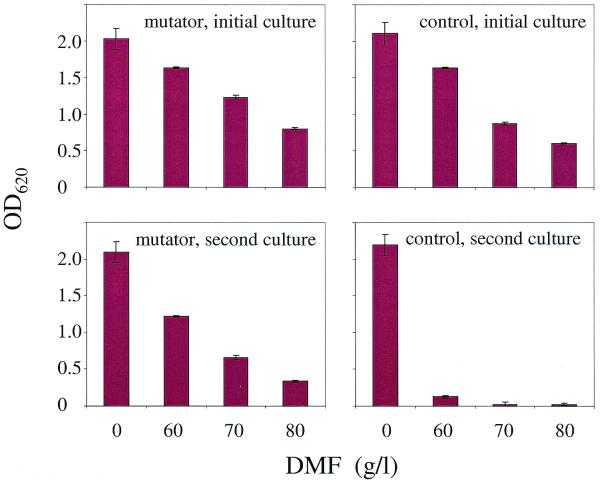
To verify the stability and robustness of the newly evolved traits of MM294 strains, we evaluated their growth in liquid medium supplemented with DMF. It is important to mention that during the curing procedure, the strains were grown in the absence of DMF for more than 30 doublings. Subsequently, the cultures were transferred to LB to which DMF had been added to 0, 60, 70, or 80 g/liter. Initially, growth was observed in all cultures after overnight incubation, although MM294 evolved in the presence of the mutator plasmid reached higher densities in LB containing DMF at 70 and 80 g/liter (Fig. 2). Samples (≈107 cells) taken from the stationary-phase (23 h) cultures grown with DMF at 80 g/liter were transferred to fresh LB medium containing DMF. On this second transfer, the control strain showed very little growth in the presence of DMF. In contrast, evolved strain MM294 was able to grow in all media including LB containing DMF at 80 g/liter. These results demonstrate that the selected phenotype was stable even after the cells had passed through 30 doublings in the absence of any selection pressure.
FIG. 2.
Growth of evolved MM294 strains in the presence of DMF. Evolved MM294 bacteria cured of the mutator or control plasmid were grown for 16 h in LB medium containing various concentrations of DMF (initial culture). Subsequently, cultures obtained with DMF at 80 g/liter were used as the seed for a second set of cultures in the same media. OD620, optical density at 620 nm.
Tuning of the mutation frequency.
Plasmid pMutD5 led to a 4,000-fold increase in the mutation rate of E. coli (Table 2). Such a high mutation frequency may prove to be too high for some applications because large numbers of mutations will accumulate in the strains during prolonged experiments. However, we found that more moderate mutation rates of 20- to 40-fold over the wild-type rate can be achieved by changing the start codon of the mutD5 gene from ATG to TTG or GTG (data not shown), both of which are known to be less efficient in initiating protein synthesis (20).
DISCUSSION
There are many reports in the literature that describe the generation of altered and improved strains by selection or screening. Although spontaneous mutations can lead to substantial diversity in large populations, additional mutations were introduced in many cases by UV radiation or chemical mutagenesis. Unfortunately, most of these mutagenic agents damage multiple cellular components, which leads to substantial cell killing during mutagenesis. It is well known that the evolution of novel traits can be accelerated in mutator strains. Mutator strains eliminate the need for chemical or physical mutagenesis, but their application has been limited by their genetic instability and by the need to stabilize the strains after successful evolution. mutD5 is the strongest known mutator allele in E. coli. It gives raise to a wide spectrum of base pair changes and even frameshift mutations (13, 15, 21). However, strains carrying a defect in mutD are difficult to handle due to their significant genetic instability (5). To make them more useful for strain development, the presence of mutD-derived mutators should be limited to the time during selection. The finding that mutD5 produces a dominant phenotype when present on a plasmid (1) offered a route for the rapid introduction and removal of mutD5. The use of a temperature-sensitive plasmid described here further simplifies the restoration of a normal low mutation rate in an evolved cell. The data reported here demonstrate the significant acceleration of strain evolution in the presence of a mutator plasmid.
Although it is possible to replace the chromosomal copy of mutD by transposon mutagenesis, the use of a mutator plasmid provides a much simpler approach. An additional advantage offered by mutator plasmids is the ability to control the mutation frequency of a strain. Cells carrying mutD5 on a plasmid produce two versions of the mutD protein, which compete for binding to DNA polymerase III. By adjusting the expression level of mutD5 directed by the plasmid, one can control the ratio of the functional to the nonfunctional mutD-encoded protein concentrations in a cell.
The most important advantage of using a mutator plasmid for strain evolution may be the reduced risk of generating spontaneous mutators. It is well documented that under strong selection pressure, bacterial strains can turn spontaneously into mutators by accumulating defects in their DNA repair pathways. Such spontaneous mutators are favored under strong selection pressure, as they have an increased chance to accumulate beneficial mutations (10, 16). However, restoring DNA repair in such spontaneous mutators is difficult, as the origin of any repair defect needs to be identified before it can be removed. Strains carrying mutator plasmids are not mutation limited, even under strong selection pressure, and consequently, mutator phenotypes will not be enriched during strain evolution. In conclusion, in this paper, we describe a new and efficient method for rapid strain improvement based on in vivo mutagenesis.
ACKNOWLEDGMENT
We thank Roopa Ghirnikar for help with the preparation of the manuscript.
REFERENCES
- 1.Chao L, Cox E C. Competition between high and low mutating strains of Escherichia coli. Evolution. 1983;37:125–134. doi: 10.1111/j.1558-5646.1983.tb05521.x. [DOI] [PubMed] [Google Scholar]
- 2.Cox E C, Horner D L. Structure and coding properties of a dominant Escherichia coli mutator gene, mutD. Proc Natl Acad Sci USA. 1983;80:2295–2299. doi: 10.1073/pnas.80.8.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bont J A M. Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol. 1998;16:493–499. [Google Scholar]
- 4.Drake J W. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci USA. 1991;88:7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funchain P, Yeung A, Stewart J L, Lin R, Slupska M M, Miller J H. The consequences of growth of a mutator strain of Escherichia coli as measured by loss of function among multiple gene targets and loss of fitness. Genetics. 2000;154:959–970. doi: 10.1093/genetics/154.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greener A, Callahan M. XL1-Red: a highly efficient random mutagenesis strain. Strategies. 1994;7:32–34. [Google Scholar]
- 7.Isken S, de Bont J A M. Bacteria tolerant to organic solvents. Extremophiles. 1998;2:229–238. doi: 10.1007/s007920050065. [DOI] [PubMed] [Google Scholar]
- 8.Liao H, McKenzie T, Hageman R. Isolation of a thermostable enzyme variant by cloning and selection in a thermophile. Proc Natl Acad Sci USA. 1986;83:576–580. doi: 10.1073/pnas.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long-McGie J, Liu A D, Schellenberger V. Rapid in vivo evolution of a β-lactamase using phagemids. Biotechnol Bioeng. 2000;68:121–125. doi: 10.1002/(sici)1097-0290(20000405)68:1<121::aid-bit15>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Mao E F, Lane L, Lee J, Miller J H. Proliferation of mutators in a cell population. J Bacteriol. 1997;179:417–422. doi: 10.1128/jb.179.2.417-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 12.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piechocki R, Kupper D, Quinones A, Langhammer R. Mutational specificity of a proof-reading defective Escherichia coli dnaQ49 mutator. Mol Gen Genet. 1986;202:162–168. doi: 10.1007/BF00330533. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Schaaper R. Mechanisms of mutagenesis in the Escherichia coli mutator mutD5: role of DNA mismatch repair. Proc Natl Acad Sci USA. 1988;85:8126–8130. doi: 10.1073/pnas.85.21.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sniegowski P D, Gerrish P J, Lenski R E. Evolution of high mutation rates in experimental populations of E. coli. Nature. 1997;387:703–705. doi: 10.1038/42701. [DOI] [PubMed] [Google Scholar]
- 17.Taddei F, Radman M, Maynard-Smith J, Toupance B, Gouyon P H, Godelle B. Role of mutator alleles in adaptive evolution. Nature. 1997;387:700–702. doi: 10.1038/42696. [DOI] [PubMed] [Google Scholar]
- 18.Takano K, Nakabeppu Y, Maki H, Horiuchi T, Sekiguchi M. Structure and function of dnaQ and mutD mutators of Escherichia coli. Mol Gen Genet. 1986;205:9–13. doi: 10.1007/BF02428026. [DOI] [PubMed] [Google Scholar]
- 19.Troebner W, Piechocki R. Competition between isogenic mutS and mut+ populations of Escherichia coli K12 in continuously growing populations. Mol Gen Genet. 1984;198:175–176. doi: 10.1007/BF00328719. [DOI] [PubMed] [Google Scholar]
- 20.Vellanoweth R L, Rabinowitz J C. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol Microbiol. 1992;6:1105–1114. doi: 10.1111/j.1365-2958.1992.tb01548.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu T H, Clarke C H, Marinus M G. Specificity of Escherichia coli mutD and mutL mutator strains. Gene. 1990;87:1–5. doi: 10.1016/0378-1119(90)90488-d. [DOI] [PubMed] [Google Scholar]