Abstract
Aim
Surgery is an important therapeutic option for Crohn's disease. The need for first bowel surgery seems to have decreased with the introduction of tumour necrosis factor inhibitors (TNFi; adalimumab or infliximab). However, the impact of TNFi on the need for intestinal surgery in Crohn's disease patients irrespective of prior bowel resection is not known. The aim of this work is to compare the incidence of bowel surgery in Crohn's disease patients who remain on TNFi treatment versus those who discontinue it.
Method
We performed a nationwide register‐based observational cohort study in Sweden of all incident and prevalent cases of Crohn's disease who started first‐line TNFi treatment between 2006 and 2017. Patients were categorized according to TNFi treatment retention less than or beyond 1 year. The study cohort was evaluated with regard to incidence of bowel surgery from 12 months after the first ever TNFi dispensation.
Results
We identified 5003 Crohn's disease patients with TNFi exposure: 3748 surgery naïve and 1255 with bowel surgery prior to TNFi initiation. Of these patients, 7% (n = 353) were subjected to abdominal surgery during the first 12 months after the start of TNFi and were subsequently excluded from the main analysis. A majority (62%) continued TNFi for 12 months or more. Treatment with TNFi for less than 12 months was associated with a significantly higher surgery rate compared with patients who continued on TNFi for 12 months or more (hazard ratio 1.26, 95% CI 1.09–1.46; p = 0.002).
Conclusion
Treatment with TNFi for less than 12 months was associated with a higher risk of bowel surgery in Crohn's disease patients compared with those who continued TNFi for 12 months or more.
Keywords: biologics, bowel surgery, Crohn's disease
What does this paper add to the literature?
Previous studies have not unambiguously been able to show reduction of surgery after therapy with tumour necrosis factor inhibitors (TNFi. To our knowledge this is the largest study on real‐life use of TNFi and surgery in Crohn's disease. A majority of patients (62%) continued on TNFi for 12 months or more, and this was associated with a reduced rate of surgery compared to drug survival of TNFi less than 12 months.
INTRODUCTION
The range of therapeutic options for Crohn's disease has grown substantially with the introduction of biologicals. Tumour necrosis factor inhibitors (TNFi) have increased the likelihood of clinical and endoscopic remission in patients with Crohn's disease [1, 2, 3]. However, surgery remains an essential part of the therapeutic arsenal, and is often required to treat disease complications such as stricturing or perforating disease, or when the response to medical treatment is inadequate [4]. In a recent Swedish population‐based study including patients with inflammatory bowel disease (IBD) between 1990 and 2014, the cumulative incidence of first intestinal surgery within 5 years of diagnosis of Crohn's disease decreased from 54.8% between 1990 and 1995 to 17.3% between 2009 and 2014, with ileocaecal resection being the most frequent intervention. No significant decrease was seen in repeat surgery from the year 2000 and later [5]. The question has been raised whether surgery rates have decreased after the introduction of TNFi. Randomized controlled trials (RCTs) have reported fewer hospital admissions as well as lower rates of surgery for Crohn's disease [1, 2, 3, 6]. However, in real‐life observational studies, the impact of biologicals on surgery rates has been ambiguous, with reports on reduction as well as no certain effects of TNFi treatment [7, 8, 9, 10, 11, 12]. Interestingly, several studies suggest that the need for surgery was reduced even before the biological era and has continued to decline after TNFi were introduced, which might reflect a more conservative strategy with regard to bowel resection [5, 7, 13, 14, 15, 16, 17].
We are only aware of one RCT including a generalizable cohort that compared surgery in ileocaecal Crohn's disease with TNFi during a period of 12 months, namely the LIR!C study [18]. The follow‐up study, although retrospective, reported a long‐term (median 63.5 months) frequency of Crohn’s related surgery of 48% in the TNFi arm [19]. We have previously investigated whether taking TNFi drugs for ≥12 months reduced first ever bowel surgery rates compared with <12 months in a cohort of 1856 Crohn's disease patients between 2006 and 2014 [20]. Somewhat unexpectedly, we found similar cumulative surgery rates (28% after 7 years) regardless of treatment retention less than or beyond 1 year. Since this cohort was restricted to surgery‐naïve patients, we have now set out to investigate whether TNFi is associated with reduced rates of surgery in all Crohn's disease patients and whether there are any differences between surgery‐naïve patients and patients with prior bowel surgery. We performed a register‐based cohort study in Sweden between 2006 and 2017 to test the hypothesis that Crohn's disease patients who are still on TNFi after 12 months (indicating that they have responded to and tolerated TNFi) are less likely to undergo bowel surgery than patients who stop TNFi before 12 months of treatment, regardless of their history of abdominal surgery.
METHOD
Study design
We constructed an observational retrospective cohort of Crohn's disease patients based on data recorded prospectively in routine medical practice. The incidence of post‐TNFi bowel surgery and any differences between the frequency of first ever abdominal surgery and repeat operation were compared between patients with continuous treatment for ≥12 months since start of TNFi (presumed sustained response and tolerance to TNFi) and patients who stopped treatment <12 months from initiation, likely because of lack of response or intolerance to TNFi.
Setting
In Sweden, all residents have universal access to publicly funded healthcare irrespective of place of residence, socioeconomic status or severity of disease. Virtually all IBD patients are seen by a gastroenterologist. Through the unique personal identity number issued to all Swedish residents, data from national and virtually complete administrative and clinical registers on demographics, morbidity and mortality can be linked [21, 22, 23, 24].
Study population
All individuals in Sweden registered with a diagnosis of IBD in the Swedish National Patient Register (inpatient care or nonprimary outpatient care) from 1 January 1987 until 31 December 2016 were identified (Figure 1) [22, 25]. The following codes were used to identify IBD patients from 1996 (ICD‐10): Crohn's disease, K50; ulcerative colitis, K51; and indeterminate colitis, K52.3. From 1987 (ICD‐9) the following codes were used: Crohn's disease, 555; ulcerative colitis, 556. The study population was limited to patients who had received two or more IBD diagnoses on two separate occasions, of which at least one IBD diagnosis was a main diagnosis in the departments of internal medicine, gastroenterology or paediatrics [26].
FIGURE 1.
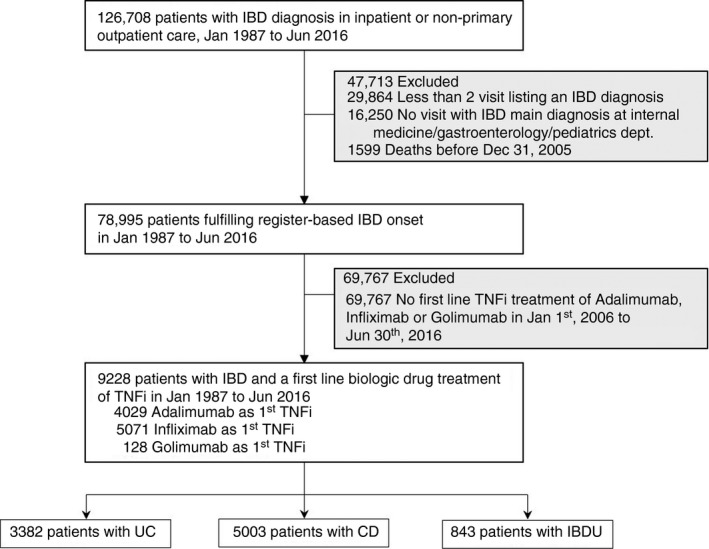
Flow chart of register‐identified patients with inflammatory bowel disease (IBD) and treated with tumour necrosis factor inhibitors (TNFi) (CD, Crohn’s disease; IBDU, inflammatory bowel disease unclassified; UC, ulcerative colitis)
The two most recent IBD diagnoses prior to TNFi therapy were used to categorize patients as either Crohn's disease (two ICD‐10 codes K50, either as primary or secondary diagnosis) or ulcerative colitis (two ICD‐10 codes K51). The rationale was to reduce the influence of incorrect registration in the records or a change in diagnosis. If the two most recent diagnoses were not the same, the patient was classified as inflammatory bowel disease unclassified (IBDU), which also included ICD‐10 code K52.3. Crohn's disease patients were then further selected by identifying those who had been dispensed at least one first‐ever dose of TNFi (infliximab or adalimumab) between 1 January 2006 and 30 June 2016, as registered in the Swedish Prescribed Drug Register which began on 1 July 2005. Since there has been limited off‐label use of golimumab for Crohn's disease in Sweden, this drug was also included in the analysis. Individuals who underwent surgery during the first year of treatment with TNFi were excluded to avoid confounding by surgical indications that were already present before TNFi initiation. Further, main analyses were restricted to patients with 6 months or more of follow‐up time (beginning 1 year after the first TNFi treatment) (Figure 1). The Montreal classification was used to define age of onset, localization and perianal disease [27, 28]. Relevant definitions and diagnostic codes are summarized in Table S1 and Table S2 in the Supporting Information.
Exposure to TNFi
We used nationwide registers to acquire information on use of TNFi in IBD: infliximab (ATC code L04AB02; L04AA12 before 2008), adalimumab (L04AB04; L04AA17 before 2008) and golimumab (L04AB06). The majority of treatment episodes were captured in the national Swedish register for IBD (SWIBREG) [29]. In addition to SWIBREG, TNFi use was captured in the Swedish Prescribed Drug Register (filled prescriptions) and the inpatient and outpatient parts of the National Patient Register (infusions performed in hospital) [24]. According to a recent validation study based on 2323 treatment episodes with biological treatments, more than 80% of episodes were captured when only having access to the Swedish Prescribed Drug Register or National Patient Register, which is why use of SWIBREG was recommended to achieve even better coverage [30].
Treatment retention
Patients with Crohn's disease were categorized into those who stopped TNFi within 12 months and those who continued TNFi for 12 months or more after initiation of TNFi treatment. If a patient was captured in SWIBREG, the start and stop dates in SWIBREG were used. If a stop date in SWIBREG was not followed by a new start date within 90 days (in any of the registers), that treatment episode was considered to have stopped. For patients not in SWIBREG, treatment with TNFi in hospital, as captured in the National Patient Register, was considered ongoing until 90 days after the last infusion. For patients not in SWIBREG or the National Patient Register, filled prescriptions in the Swedish Prescribed Drug Register were regarded as ongoing TNFi treatment until 180 days since the last filled prescription (i.e. patients would need at least three filled prescriptions to be regarded as having been treated with TNFi for 12 months). For individuals captured in multiple registers, the earliest available start date and latest available stop date were used. For patients in both SWIBREG and the Swedish Prescribed Drug Register or National Patient Register and with no stop date in SWIBREG, the treatment was considered to have stopped 90 days after the last treatment episode in the National Patient Register or after the last filled prescription in the Swedish Prescribed Drug Register.
Follow‐up and occurrence of bowel surgery
The outcome measure was abdominal surgery, which was defined according to validated surgical procedure codes in the National Patient Register (for the NOMESCO Classification of Surgical Procedures see Table S2) [22, 31]. Patients with early surgery (within 12 months of the first TNFi dose) were excluded from the long‐term analysis of surgery rates in patients with TNFi ≥ 12 months compared with <12 months. Thus, the long‐term surgery analysis starts 12 months after TNFi initiation. Patients were hence considered at risk of bowel surgery from 12 months after the first TNFi administration and until the first event of emigration, death or end of follow‐up (31 December 2017).
Statistics
Crude incidence rates (events per 1000 person‐years) of bowel surgery were calculated by dividing the number of first bowel resections during follow‐up by the corresponding person‐time at risk. Relative risks of bowel surgery were computed using Cox proportional‐hazard models between patients who were treated with TNFi for more or less than 12 months. The Cox models were stratified by birth year and sex and adjusted for age at index date. We calculated hazard ratios (HRs) overall as well as stratum‐specific HRs for the calendar period of first Crohn's disease diagnosis, sex, age at first Crohn's disease diagnosis and phenotype according to the Montreal and Paris classifications. All analyses were performed using the SAS software package v.9.4 (SAS Institute, Cary, NC, USA).
Sensitivity analyses
The available registers lack detailed information on the reasons for discontinuing TNFi, and therefore patients who were treated for <12 months represent a mix of primary nonresponders, patients with loss of response, side effects, poor compliance or drug discontinuation after achieving remission [32]. Sensitivity analyses of the incidence of bowel surgery were therefore also performed in patients who stopped treatment at ≤6 months (Table S10, Figure S2).
The suboptimal coverage of infliximab in the Swedish Prescribed Drug Register or National Patient Register means that, for example, some adalimumab treatments may seem to be first‐line treatments while they were in fact second‐line treatments. We therefore performed sensitivity analyses in which the study population was restricted to IBD centres with >70% coverage in SWIBREG (the overall coverage in included counties/centres turned out to be >85%) which can be assumed to result in accurate registration of TNFi administered as infusions (Table S3, Figure S1).
RESULTS
Between January 1987 and June 2016, we identified 126,708 patients with IBD of whom 5003 were Crohn's disease patients treated with first‐line TNFi between 2006 and 2017 (Figure 1). Roughly half of the included patients were women, 70% of the patients had received their first Crohn's disease diagnosis after the year 2000 and mean the age at TNFi initiation was 37 years (Table 1). At start of TNFi treatment, 17% had isolated colitis without small bowel involvement, 25% had perianal disease and 25% had a history of bowel resection. Of the included patients, 2584 (52%) received adalimumab and 2391 (48%) infliximab (Table 1).
TABLE 1.
Characteristics of Crohn's disease (CD) patients diagnosed in Sweden between 1964 and 2016 at disease onset and start of tumour necrosis factor inhibitors (TNFi). Values in n (%) unless otherwise stated
| Variable | All CD a | Drug survival ≥12 months | Drug survival <12 months | Early surgery b |
|---|---|---|---|---|
| N | 5003 (100%) | 3107 (62%) | 1526 (31%) | 353 (7%) |
| Sex | ||||
| Female | 2435 (49%) | 1390 (45%) | 870 (57%) | 164 (46%) |
| Male | 2568 (51%) | 1717 (55%) | 656 (43%) | 189 (54%) |
| First IBD diagnosis | ||||
| 2011–2016 | 1264 (25%) | 821 (26%) | 363 (24%) | 76 (22%) |
| 2006–2010 | 1269 (25%) | 797 (26%) | 389 (25%) | 82 (23%) |
| 2001–2005 | 979 (20%) | 603 (19%) | 292 (19%) | 81 (23%) |
| 1996–2000 | 476 (10%) | 293 (9%) | 150 (10%) | 32 (9%) |
| 1991–1995 | 318 (6%) | 198 (6%) | 93 (6%) | 25 (7%) |
| 1986–1990 | 270 (5%) | 153 (5%) | 89 (6%) | 27 (8%) |
| 1981–1985 | 196 (4%) | 113 (4%) | 68 (4%) | 14 (4%) |
| 1976–1980 | 125 (2%) | 72 (2%) | 41 (3%) | 8 (2%) |
| 1971–1975 | 90 (2%) | 49 (2%) | 34 (2%) | 7 (2%) |
| 1964–1970 | 16 (0%) | 8 (0%) | 7 (0%) | 1 (0%) |
| Age at disease onset (years) | ||||
| Mean age (SD) | 28.7 (14.2) | 28.1 (13.9) | 29.4 (14.3) | 30.1 (15.6) |
| Median age (IQR) | 25.2 (18.1–36.3) | 24.7 (17.7–35.4) | 25.8 (18.5–37.7) | 25.8 (18.6–38.9) |
| <17 | 1054 (21%) | 686 (22%) | 304 (20%) | 64 (18%) |
| 17–40 | 2993 (60%) | 1865 (60%) | 908 (60%) | 214 (61%) |
| 41–60 | 783 (16%) | 461 (15%) | 260 (17%) | 56 (16%) |
| >60 | 173 (3%) | 95 (3%) | 54 (4%) | 19 (5%) |
| Age at TNFi start (years) | ||||
| Mean age (SD) | 37.3 (16.0) | 36.4 (15.8) | 38.5 (16.2) | 39.0 (16.0) |
| Median age (IQR) | 35.5 (23.6–49.4) | 34.3 (22.9–48.0) | 36.8 (24.6–51.4) | 37.4 (25.6–51.6) |
| <17 | 390 (8%) | 256 (8%) | 116 (8%) | 18 (5%) |
| 17–40 | 2648 (53%) | 1701 (55%) | 757 (50%) | 188 (53%) |
| 41–60 | 1471 (29%) | 873 (28%) | 487 (32%) | 107 (30%) |
| >60 | 494 (10%) | 277 (9%) | 166 (11%) | 40 (11%) |
| Montreal and Paris classification c | ||||
| L1/L3/LX | 4110 (82%) | 2535 (82%) | 1250 (82%) | 309 (88%) |
| L2 | 893 (18%) | 572 (18%) | 276 (18%) | 44 (12%) |
| P | 1230 (25%) | 802 (26%) | 341 (22%) | 84 (24%) |
| Comorbidities | ||||
| PSC | 67 (1%) | 41 (1%) | 22 (1%) | 3 (1%) |
| History of bowel surgery | 1255 (25%) | 747 (24%) | 391 (26%) | 109 (31%) |
| Treatment | ||||
| Adalimumab as first biological | 2584 (52%) | 1616 (52%) | 775 (51%) | 183 (52%) |
| Infliximab as first biological | 2391 (48%) | 1469 (47%) | 746 (49%) | 169 (48%) |
| Golimumab as first biological | 28 (1%) | 22 (1%) | 5 (0%) | 1 (0%) |
| Switch at least once | 448 (9%) | 368 (12%) | 58 (4%) | 22 (6%) |
| Mean time in years from first diagnosis to start of TNFi (SD) | 8.6 (9.6) | 8.3 (9.4) | 9.1 (10.0) | 8.9 (9.6) |
| Mean time in years from last bowel surgery to TNFi start (SD) | 4.8 (4.3) | 4.8 (4.4) | 4.8 (4.3) | 4.7 (4.2) |
| Immunomodulatory drugs during biological therapy | 2671 (53%) | 1888 (61%) | 602 (39%) | 177 (50%) |
Abbreviations: IBD, inflammatory bowel disease; IQR, interquartile range; PSC, primary sclerosing cholangitis; SD, standard deviation.
Seventeen patients died <12 months after the start of TNFi therapy and were only included in the all‐CD patient group.
Underwent bowel surgery <12 months after start of anti‐TNF therapy and were therefore not included in main analysis comparing drug survival ≥ or <12 months.
L, location of CD, according to the last used ICD codes before the start of biological treatment: L1, small bowel disease; L2, colon; L3, small bowel and colon. Patients with codes for an unspecified location are reported as LX. P, perianal disease, according to the corresponding ICD codes, if ever used, before the start of biological treatment. PSC, primary sclerosing cholangitis in CD, according to the corresponding ICD code, if ever used, before the start of biological treatment.
Twelve months after initiation of therapy, 7% of patients had undergone early bowel surgery (<12 months after TNFi initiation), 62% were still being treated with TNFi and 31% had stopped the treatment (Table 1). The early surgery group had a slightly higher proportion of patients with prior bowel resection compared with the whole cohort (31% vs. 25%). Age at TNFi start, phenotypes according to the Montreal and Paris classifications, time between Crohn's disease diagnosis and start of TNFi, and a history of bowel resection were similar in patients with drug survival of more or less than 12 months (Table 1). Patients with TNFi survival ≥12 months more often used immunomodulators at the start of TNFi than patients with shorter drug survival (61% vs. 39%; p < 0.001) (Table 1). The cumulative rates of abdominal surgery (including early surgery) in all Crohn's disease patients treated with TNFi regardless of TNFi retention during the observation period of 2006–2017 were 7% (1 year after initiation of treatment), 16% (3 years after), 22% (5 years after), 27% (7 years after), 32% (9 years after) and 35% (11 years after).
Bowel surgery in all patients
After excluding patients with early surgery, patients who discontinued the drug before 12 months had a significantly higher incidence of abdominal surgery than patients who continued treatment with TNFi for 12 months or more (HR 1.26, 95% CI 1.09–1.46; p = 0.002) (Figure 2). The greatest relative risk of surgery comparing TNFi < 12 months with ≥12 months was in the first year of follow‐up, in patients over 60 years of age at Crohn's disease diagnosis, with TNFi monotherapy and during the latest calendar period (Table 2). The relative risk of surgery was similar in patients irrespective of disease duration before the start of TNFi and across the Montreal and Paris classifications (Table 2). The types of bowel surgery performed between 2006 and 2017 in this Crohn's disease population are reported in Table S4.
FIGURE 2.
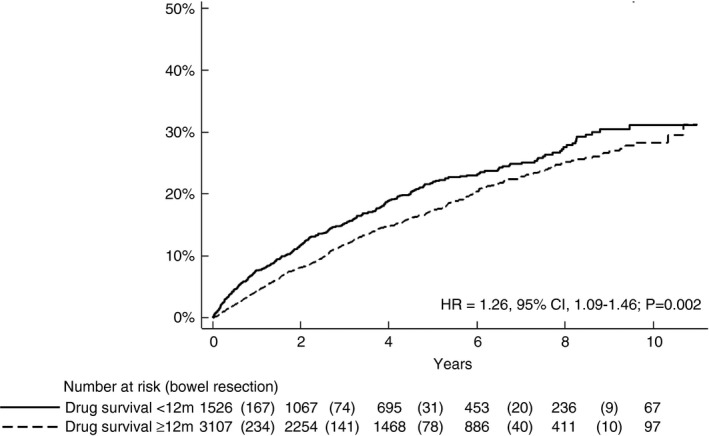
Time to bowel surgery by response assessment at 12 months (i.e. follow‐up started 12 months after start of tumour necrosis factor inhibitor therapy in patients with drug survival ≥12 months and <12 months) (HR, hazard ratio)
TABLE 2.
Number of bowel surgeries, total person‐years at risk and incident rates of intestinal surgery in patients with drug survival <12 months and ≥12 months as well as hazard ratios (HRs) between the groups
| Group | N (%) | Time at risk (years) | No. of surgeries | Incidence rate (95% CI) per 1000 person‐years | HR a (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Drug survival <12 months | Drug survival ≥12 months | Drug survival <12 months | Drug survival ≥12 months | Drug survival <12 months | Drug survival ≥12 months | Drug survival <12 months | Drug survival ≥12 months | ||
| Overall | 1526 (33%) | 3107 (67%) | 6492 | 13360 | 301 | 505 | 46 (41–52) | 38 (35–41) | 1.26 (1.09–1.46) |
| Follow‐up (years) | |||||||||
| 0–1 | 1526 (33%) | 3107 (67%) | 1426 | 2989 | 115 | 131 | 81 (66–95) | 44 (36–51) | 1.79 (1.38–2.32) |
| >1–2 | 1299 (32%) | 2760 (68%) | 1191 | 2504 | 52 | 103 | 44 (32–56) | 41 (33–49) | 1.10 (0.78–1.56) |
| >2–3 | 1067 (32%) | 2252 (68%) | 955 | 2027 | 40 | 82 | 42 (29–55) | 40 (32–49) | 1.04 (0.70–1.54) |
| >3–4 | 858 (32%) | 1827 (68%) | 781 | 1643 | 34 | 59 | 44 (29–58) | 36 (27–45) | 1.28 (0.82–1.99) |
| >4–5 | 695 (32%) | 1467 (68%) | 695 | 1467 | 24 | 40 | 35 (21–48) | 27 (19–36) | 1.34 (0.79–2.27) |
| >5 | 561 (32%) | 1186 (68%) | 1514 | 2869 | 36 | 90 | 24 (16–32) | 31 (25–38) | 0.77 (0.51–1.18) |
| Sex | |||||||||
| Male | 656 (28%) | 1717 (72%) | 2817 | 7289 | 133 | 266 | 47 (39–55) | 36 (32–41) | 1.31 (1.06–1.63) |
| Female | 870 (38%) | 1390 (62%) | 3675 | 6072 | 168 | 239 | 46 (39–53) | 39 (34–44) | 1.22 (0.99–1.49) |
| Age at IBD onset (years) | |||||||||
| <17 | 304 (31%) | 686 (69%) | 1296 | 2984 | 64 | 127 | 49 (37–61) | 43 (35–50) | 1.08 (0.77–1.51) |
| 17–40 | 908 (33%) | 1865 (67%) | 3918 | 8062 | 173 | 283 | 44 (38–51) | 35 (31–39) | 1.30 (1.06–1.59) |
| 41–60 | 260 (36%) | 461 (64%) | 1109 | 1968 | 49 | 81 | 44 (32–57) | 41 (32–50) | 1.05 (0.71–1.55) |
| >60 | 54 (36%) | 95 (64%) | 169 | 346 | 15 | 14 | 89 (44–134) | 40 (19–62) | 3.97 (1.38–11.43) |
| Age at TNFi start (years) | |||||||||
| <17 | 116 (31%) | 256 (69%) | 501 | 1053 | 17 | 33 | 34 (18–50) | 31 (21–42) | 1.17 (0.62–2.22) |
| 17–40 | 757 (31%) | 1701 (69%) | 3208 | 7476 | 153 | 278 | 48 (40–55) | 37 (33–42) | 1.31 (1.07–1.61) |
| 41–60 | 487 (36%) | 873 (64%) | 2147 | 3743 | 96 | 147 | 45 (36–54) | 39 (33–46) | 1.16 (0.89–1.52) |
| >60 | 166 (37%) | 277 (63%) | 635 | 1088 | 35 | 47 | 55 (37–73) | 43 (31–56) | 1.39 (0.86–2.24) |
| Year of TNFi start | |||||||||
| 2014–2016 | 499 (33%) | 1006 (67%) | 797 | 1654 | 54 | 55 | 68 (50–86) | 33 (24–42) | 2.32 (1.53–3.52) |
| 2011–2013 | 396 (29%) | 950 (71%) | 1579 | 3865 | 65 | 149 | 41 (31–51) | 39 (32–45) | 1.00 (0.73–1.37) |
| 2009–2010 | 280 (33%) | 580 (67%) | 1574 | 3468 | 73 | 141 | 46 (36–57) | 41 (34–47) | 1.08 (0.78–1.48) |
| 2006–2008 | 351 (38%) | 571 (62%) | 2542 | 4373 | 109 | 160 | 43 (35–51) | 37 (31–42) | 1.20 (0.91–1.57) |
| TNFi | |||||||||
| Adalimumab | 775 (32%) | 1616 (68%) | 3087 | 6562 | 165 | 264 | 53 (45–62) | 40 (35–45) | 1.39 (1.12–1.71) |
| Infliximab | 746 (34%) | 1469 (66%) | 3392 | 6728 | 136 | 241 | 40 (33–47) | 36 (31–40) | 1.14 (0.90–1.42) |
| Golimumab | 5 (19%) | 22 (81%) | 13 | 70 | 0 | 0 | 0 | 0 | – |
| Time from diagnosis to TNFi start (years) | |||||||||
| ≤1 | 322 (31%) | 722 (69%) | 1208 | 2795 | 60 | 85 | 50 (37–62) | 30 (24–37) | 1.44 (0.96–2.18) |
| 2–5 | 426 (32%) | 891 (68%) | 1838 | 3926 | 73 | 141 | 40 (31–49) | 36 (30–42) | 1.15 (0.84–1.57) |
| 6–10 | 258 (32%) | 545 (68%) | 1135 | 2482 | 56 | 104 | 49 (36–62) | 42 (34–50) | 1.12 (0.76–1.66) |
| >10 | 520 (35%) | 949 (65%) | 2311 | 4156 | 112 | 175 | 48 (39–57) | 42 (36–48) | 1.25 (0.96–1.62) |
| Immunomodulatory drugs during biological therapy | |||||||||
| Yes | 602 (24%) | 1888 (76%) | 2756 | 8487 | 116 | 347 | 42 (34–50) | 41 (37–45) | 1.08 (0.87–1.36) |
| No | 924 (43%) | 1219 (57%) | 3736 | 4874 | 185 | 158 | 50 (42–57) | 32 (27–37) | 1.58 (1.25–2.00) |
| Montreal and Paris classification b | |||||||||
| L1/L3/LX | 1250 (33%) | 2535 (67%) | 5254 | 10758 | 258 | 432 | 49 (43–55) | 40 (36–44) | 1.27 (1.08–1.50) |
| L2 | 276 (33%) | 572 (67%) | 1237 | 2602 | 43 | 73 | 35 (24–45) | 28 (22–34) | 1.20 (0.77–1.88) |
| P | 341 (30%) | 802 (70%) | 1485 | 3440 | 84 | 149 | 57 (44–69) | 43 (36–50) | 1.49 (1.10–2.03) |
| History of bowel surgery | |||||||||
| Yes | 391 (34%) | 747 (66%) | 1666 | 3326 | 108 | 159 | 65 (53–77) | 48 (40–55) | 1.25 (0.94–1.66) |
| No | 1135 (32%) | 2360 (68%) | 4826 | 10034 | 193 | 346 | 40 (34–46) | 34 (31–38) | 1.22 (1.01–1.47) |
Abbreviations: IBD, inflammatory bowel disease; IQR, interquartile range; PSC, primary sclerosing cholangitis; SD, standard deviation.
Adjusted for age at start of TNFi and stratified by birth year and sex.
L, location of Crohn's disease, according to the last used ICD codes before the start of biological treatment. B is behaviour and P is perianal disease according to the corresponding ICD codes, if ever used, before the start of biological treatment.
Bowel surgery in patients with prior resection
We identified 1255 Crohn's disease patients with a history of bowel surgery prior to TNFi initiation (mean 4.8 years prior to TNFi) and 3748 surgery‐naïve patients. While patients with a record of bowel surgery were older at TNFi initiation, TNFi retention rates were similar in the two groups (Table S5 and Table S6). The cumulative risk of surgery during follow‐up was significantly higher in patients with a history of surgery than in surgery‐naïve patients, regardless of drug survival (p < 0.001) (Figure 3). TNFi drug survival <12 months was associated with an increased HR for bowel surgery in both surgery‐naïve and experienced patients [HR = 1.22 (1.01–1.47) and HR = 1.25 (0.94–1.66), respectively] albeit not statistically significant in the latter group (Figure 4). The highest HR for surgery when stopping TNFi before 12 months was seen during the latest observation period (2014–2016) (Table S7 and Table S8).
FIGURE 3.
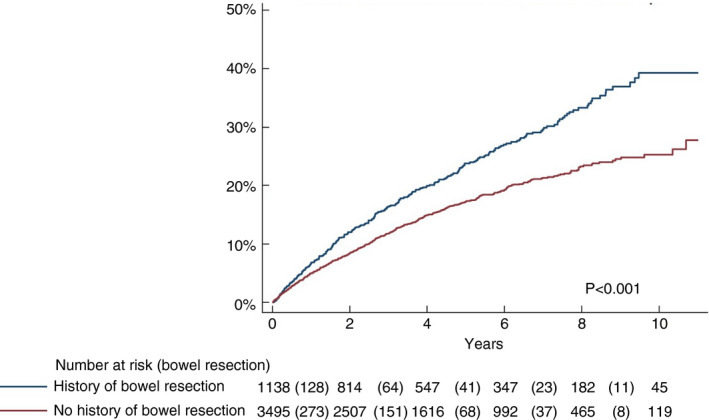
Time to bowel surgery from 12 months after the start of tumour necrosis factor inhibitor therapy in patients stratified by history of abdominal surgery regardless of drug survival
FIGURE 4.
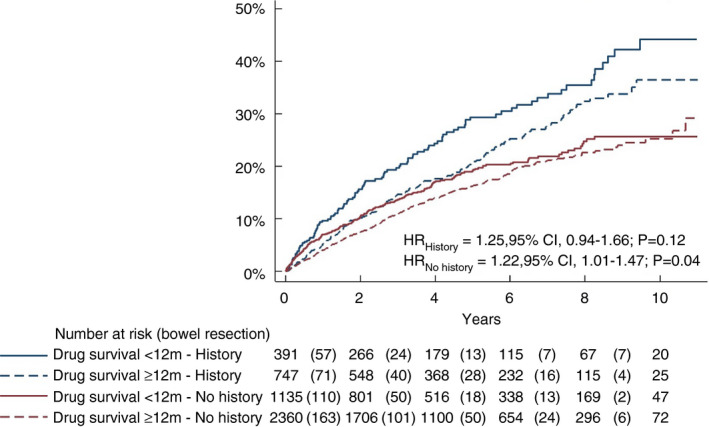
Time to intestinal surgery by response assessment at 12 months (i.e. follow‐up started 12 months after start of tumour necrosis factor inhibitor therapy) in patients stratified by history of bowel surgery and drug survival ≥12 months versus <12 months (HR, hazard ratio)
Sensitivity analyses
We performed a sensitivity analysis restricted to 3206 patients registered in SWIBREG in IBD centres with close to complete coverage (a list of centres is given in Table S3 and patient characteristics in Table S9). In this study population infliximab use was slightly more frequent relative to adalimumab, whereas patient demography and early surgery rates were similar to the nationwide cohort (Tables S9 and 1). The HR for bowel surgery after the start of follow‐up at 12 months was similar to the main results (HR 1.37, 95% CI 1.14–1.64; p = 0.001; Figure S1).
In another sensitivity analysis, in which early surgery was defined as <6 months of TNFi treatment (vs. ≥6 months), 940 (19%) patients had drug survival <6 months and the HR was similar to that in the main analysis (HR 1.34, 95% CI 1.15–1.55; p < 0.001; Table S10, Figure S2).
DISCUSSION
Main findings
In this register‐based study, we used real‐world data to investigate the association of TNFi drug survival more or less than 12 months with the risk of bowel surgery in Crohn's disease patients. Surgery rates were significantly higher among patients who discontinued TNFi before 12 months compared with patients with drug survival ≥12 months.
Findings compared with earlier studies
We have previously published the only report on first ever bowel surgery rates in Crohn's disease patients on maintenance TNFi ≥12 months compared with patients who stopped treatment before 12 months [20]. The drug survival rates in our previous report and the current one were almost identical at 12 months (62% in the present cohort and 65% in the earlier report) [20]. In contrast to the earlier published cohort of resection‐naïve patients, in which TNFi <12 months was not significantly associated with an increased surgery rate (HR 1.17, 95% CI 0.88–1.56), the current larger and more recent study, including both surgery‐naïve patients and patients with prior bowel surgery, demonstrated statistically significant higher rates of surgery for TNFi drug survival <12 months compared with ≥12 months (HR 1.26, 95% CI 1.08–1.46), an association which was even more pronounced in the sensitivity analyses. The somewhat higher HR and tighter confidence intervals in the latter cohort is logical, since a larger number of patients were included in the current study with data retrieved from the Swedish National Patient Register and the Swedish Prescribed Drug Register, but importantly also from the Swedish national quality register SWIBREG. Moreover, the increased use of TNFi in more recent years further improved our statistical power. Considering the very similar point estimates and the overlapping CIs between the two studies, increased statistical power is likely be the most important reason for this. Since the publication of our previous study, we have also assessed how well the national registers capture TNFi use, which has resulted in an improved algorithm for capturing infliximab [30]. The treat‐to‐target strategy with dose optimization of TNFi by therapeutic drug monitoring, which is used in clinical routines in some Swedish centres, might also result in improved clinical efficacy in patients with drug survival >12 months compared with <12 months. However, treatment strategies or dose escalation cannot be caught in this study due to the lack of such granularity in the data.
Moreover, the current study found higher rates of drug survival ≥12 months among users of immunomodulators than among nonusers, an observation that may reflect the results from earlier studies demonstrating the higher clinical efficacy of combining infliximab with thiopurines [33, 34]. In our previous smaller study, HRs for bowel surgery were similar across strata of TNFi treatment with or without immunomodulators. In the current study, drug survival <12 months was not a risk factor for bowel surgery within the group of TNFi‐treated patients exposed to immunomodulators, whereas among patients only exposed to TNFi monotherapy it was (Table 2).
As expected, the cumulative rates of first bowel surgery during follow‐up were significantly higher in the current cohort of patients with at least one previous abdominal resection compared with the surgery‐naïve patient population (42% vs. 21% 9 years after introduction of TNFi; p < 0.001). Previous reports have shown that inflammatory recurrence can be observed within 2 years of ileocaecal resection in up to 80% of Crohn's disease patients, and after first resection, repeat surgery rates of 11%–32% have been reported within 5 years and 20%–44% within 10 years, which is in keeping with our results [35, 36, 37, 38]. However, the impact of prior surgery per se on further resections is difficult to assess in the current study since this group was older and exhibited longer disease duration than the surgery‐naïve patients. Moreover, the indication for TNFi after surgery is prophylactic to prevent recurrent resection, in distinction to treating surgery‐naïve patients with TNFi, which may introduce a bias in any comparison between the patient groups.
While we found no significantly increased risk of bowel resections in surgery‐experienced patients with drug survival <12 months, the efficacy of postoperative TNFi prophylaxis with regards to endoscopic recurrence in patients undergoing ileocaecal resection has been demonstrated in several studies [39, 40, 41]. The PREVENT study showed a protective role of infliximab in endoscopic but not clinical recurrence, whereas a significant effect on repeat surgery could not be seen within the 76‐week follow‐up [39]. The POCER study investigated the role of immunosuppressants and adalimumab as postoperative prophylaxis. The authors demonstrated superior efficacy of adalimumab compared with thiopurines in preventing endoscopic recurrence within 6 months, but no patients in the study underwent repeat surgery during the observation period [40, 41]. In addition to these two pivotal RCTs, a meta‐analysis including eight additional small studies of medical prevention of postoperative recurrence suggested that TNFi was superior to both immunosuppressants and mesalamine (312 patients) [42]. However, to our knowledge no study has demonstrated a significant effect of TNFi treatment on repeat bowel resections. Moreover, in contrast to previous trials of highly selected study populations followed for a relatively short time, our study reflects real‐life use of TNFi in Crohn's disease patients with prior bowel surgery and includes 1255 patients, compared with the next largest study, the PREVENT trial, which included 297 patients [39]. Finally, when comparing the cumulative rates of abdominal surgery in patients without prior surgery and who continued TNFi for more than 12 months with the resection rates of all TNFi‐treated patients regardless of TNFi retention, the following question arises: is surgery prevented in surgery‐naïve patients or only delayed in a substantial proportion of these patients? Such postponing of surgery may of course be of value in many cases, as long as simultaneous slow deterioration is avoided as this may affect the results from surgery when needed [43].
Limitations
We were unable to compare degree of disease activity or symptom severity across exposure groups since such variables are not at all available in the Swedish National Patient Register and are currently poorly covered in SWIBREG. The limited nationwide coverage of infliximab in the Swedish National Patient Register and Prescribed Drug Register mainly concerns the identification of second‐line adalimumab patients wrongly categorized as first‐line treatment with adalimumab since prior infliximab infusions might be missed. Similarly, adalimumab‐treated patients who have switched to infliximab might have been misclassified in the nationwide sample as stopping biologicals. There is also a risk that the few patients treated with biologicals before the start of SWIBREG and the Prescribed Drug Register in 2005 have been mistakenly identified as first‐time users. However, while infliximab use had suboptimal coverage nationwide in the National Patient Register and Prescribed Drug Register, this limitation was compensated by the supplemental SWIBREG data from IBD centres with close to complete coverage (Table S3). The fact that patients from the high‐coverage hospitals had very similar patient characteristics and results to the national cohort is reassuring. Additional limitations are that the data set does not allow for more refined analysis, such as the use of therapeutic drug monitoring and optimization of TNFi therapy. Finally, although the coverage of prescribed TNFi (adalimumab) is complete, we only have information on the syringes patients have obtained at the pharmacy and not how many syringes any given patient actually administered.
Strengths
Through the National Patient Register, we were able to identify all Crohn's disease patients without being limited to restrictive inclusion criteria of randomized trials or the selected patient population in highly specialized university or tertiary referral clinics. Thus, generalizability was optimized [22]. Moreover, the size of the study has allowed for more accurate risk estimates, and the long observation period of 11 years made it possible to estimate the long‐term risk of surgery. In Sweden, clinical data have been collected prospectively through the national registers based on routine clinical care with nearly complete coverage for most parameters, and the validity of the main variables has been shown to be excellent [22, 24, 31].
CONCLUSION
Treatment with TNFi for less than 12 months was associated with more frequent bowel surgery in Crohn's disease patients compared with patients who were treated with TNFi for 12 months or more.
CONFLICT OF INTEREST
ME has received honoraria for lectures and consultancy from AbbVie, Merck (MSD), Takeda, Ferring, Orion Pharma, Otsuka, Tillotts, ITH, Novartis, Pfizer and Janssen, and received research funding from AbbVie and MSD. JKS has served as an external consultant to Parexel and Janssen. OO has been Principal Investigator on projects at Karolinska Institutet partly financed by investigator‐initiated grants from Janssen and Ferring, and also reports a grant from Pfizer in the context of a national safety monitoring program. None of those studies have any relation to the present study. Karolinska Institutet also has received fees for OO's lectures and participation on advisory boards from Janssen, Ferring, Takeda and Pfizer regarding topics not related to the present study. PM has received honoraria for lectures from Ferring, AbbVie and Takeda and has served as an external consultant to Janssen and AbbVie and has been Principal Investigator for a research project partly funded by Takeda. CH has received honoraria for lectures from Ferring, AbbVie, Janssen and Takeda and has served as an external consultant to Pfizer. ÅHE has worked on projects at Karolinska Institutet and SWIBREG partly financed by grants from Ferring and Jansen.
AUTHOR CONTRIBUTIONS
Guarantors of the article: JKS and ME. Author contributions: JKS had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: ME, AE, OO, PM. Acquisition of data: OO. Analysis and interpretation of data: all authors. First draft of manuscript: ME, PM. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: OO.
ETHICAL APPROVAL
The study was approved by the regional ethics committee in Stockholm (Dnr 2007/785‐31/5, 2011/1509‐32, 2014/1287‐31/4, 2015/0004‐31, 2016/192‐31/2). Since this was a strictly register‐based study, individual informed consent was not required.
Supporting information
Supplementary Material
APPENDIX A.
STROBE statement
| Item no. | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found OK | ||
| INTRODUCTION | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported OK |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses OK |
| METHODS | ||
| Study design | 4 | Present key elements of study design early in the paper OK |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow‐up, and data collection OK |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow‐up OK |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable OK |
| Data sources/measurement | 8 a | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group OK |
| Bias | 9 | Describe any efforts to address potential sources of bias OK |
| Study size | 10 | Explain how the study size was arrived at NA |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why OK |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding |
| (b) Describe any methods used to examine subgroups and interactions | ||
| (c) Explain how missing data were addressed | ||
| (d) If applicable, explain how loss to follow‐up was addressed | ||
| (e) Describe any sensitivity analyses OK | ||
| RESULTS | ||
| Participants | 13 a | (a) Report numbers of individuals at each stage of study, e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow‐up, and analysed OK |
| (b) Give reasons for nonparticipation at each stage OK | ||
| (c) Consider use of a flow diagram OK | ||
| Descriptive data | 14 a | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders OK |
| (b) Indicate number of participants with missing data for each variable of interest NA | ||
| (c) Summarize follow‐up time (e.g. average and total amount) OK | ||
| Outcome data | 15 a | Report numbers of outcome events or summary measures over time OK |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder‐adjusted estimates and their precision (e.g. 95% confidence interval). Make clear which confounders were adjusted for and why they were included OK |
| (b) Report category boundaries when continuous variables were categorized | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||
| Other analyses | 17 | Report other analyses done, e.g. analyses of subgroups and interactions, and sensitivity analyses OK |
| DISCUSSION | ||
| Key results | 18 | Summarize key results with reference to study objectives OK |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias OK |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence OK |
| Generalizability | 21 | Discuss the generalisability (external validity) of the study results OK |
| OTHER INFORMATION | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based OK |
Give information separately for exposed and unexposed groups.
Eberhardson M, Myrelid P, Söderling JK, Ekbom A, Hallqvist Everhov Å, Hedin C, et al; the SWIBREG Study Group . Tumour necrosis factor inhibitors in Crohn's disease and the effect on surgery rates. Colorectal Dis. 2022;24:470–483. 10.1111/codi.16021
SWIBREG Study Group collaborators: Hans Strid, Henrik Hjortswang, Malin Olsson, Jan Björk, Jonas L. Bengtsson, Jonas Halfvarson, Marie A. Andersson, Pontus Karling, Martin Rejler, Susanna Jäghult, Ulrika L. Fagerberg, Olof Grip, and Caroline Nordenvall
Funding information
ME was supported by the Bengt Ihre Research Fellowship and Stockholm County Council ALF (project number 20180565). OO was supported by the SFO Young Scholar Award at Karolinska Institutet, the Swedish Research Council (Dnr 2020‐02002) and the Swedish Society of Medicine. OO was funded by Stockholm County Council ALF (project numbers 20170720 and 20190638 respectively). CH was funded by a Bengt Ihre Fellowship and a Stockholm County Council postdoctoral fellowship. ÅHE was supported by grants from the Bengt Ihre Foundation and the Bengt Ihre Research Fellowship. None of the funding organizations has had any role in the design and conduct of the study; in the collection, management and analysis of the data; or in the preparation, review and approval of the manuscript.
Contributor Information
Michael Eberhardson, Email: michael.eberhardson@liu.se.
the SWIBREG Study Group:
Hans Strid, Henrik Hjortswang, Malin Olsson, Jan Björk, Jonas L. Bengtsson, Jonas Halfvarson, Marie A. Andersson, Pontus Karling, Martin Rejler, Susanna Jäghult, Ulrika L. Fagerberg, Olof Grip, and Caroline Nordenvall
DATA AVAILABILITY STATEMENT
The data underlying this article were provided by the Swedish National Patient Register, Swedish Prescribed Drug Register and SWIBREG by permission. Data can be obtained with permission of these registers.
REFERENCES
- 1. Colombel JF, Rutgeerts PJ, Sandborn WJ, Yang M, Camez A, Pollack PF, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12(3):414–422.e5. [DOI] [PubMed] [Google Scholar]
- 2. Panaccione R, Colombel JF, Sandborn WJ, Rutgeerts P, D'Haens GR, Robinson AM, et al. Adalimumab sustains clinical remission and overall clinical benefit after 2 years of therapy for Crohn's disease. Aliment Pharmacol Ther. 2010;31(12):1296–309. [DOI] [PubMed] [Google Scholar]
- 3. Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128(4):862–9. [DOI] [PubMed] [Google Scholar]
- 4. Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A. ECCO guidelines on therapeutics in crohn’s disease: surgical treatment. Journal of Crohn's and Colitis. 2020;14(2):155–68. 10.1093/ecco-jcc/jjz187 [DOI] [PubMed] [Google Scholar]
- 5. Kalman TD, Everhov ÅH, Nordenvall C, Sachs MC, Halfvarson J, Ekbom A, et al. Decrease in primary but not in secondary abdominal surgery for Crohn's disease: nationwide cohort study, 1990–2014. Br J Surg. 2020;107(11):1529–38. [DOI] [PubMed] [Google Scholar]
- 6. Feagan BG, Panaccione R, Sandborn WJ, D'Haens GR, Schreiber S, Rutgeerts PJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn's disease: results from the CHARM study. Gastroenterology. 2008;135(5):1493–9. [DOI] [PubMed] [Google Scholar]
- 7. Bernstein CN, Loftus EV Jr, Ng SC, Lakatos PL, Moum B, Epidemiology , et al. Hospitalisations and surgery in Crohn's disease. Gut. 2012;61(4):622–9. [DOI] [PubMed] [Google Scholar]
- 8. Limketkai BN, Parian AM, Chen P‐H, Colombel J‐F. Treatment with biologic agents has not reduced surgeries among patients with Crohn’s disease with short bowel syndrome. Clin Gastroenterol Hepatol. 2017;15(12):1908–14.e2. [DOI] [PubMed] [Google Scholar]
- 9. Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjær M, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003–2005: a population‐based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101(6):1274–82. [DOI] [PubMed] [Google Scholar]
- 10. Vester‐Andersen MK, Prosberg MV, Jess T, Andersson M, Bengtsson BG, Blixt T, et al. Disease course and surgery rates in inflammatory bowel disease: a population‐based, 7‐year follow‐up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014;109:705. [DOI] [PubMed] [Google Scholar]
- 11. Ma C, Moran GW, Benchimol EI, Targownik LE, Heitman SJ, Hubbard JN, et al. Surgical rates for Crohn's disease are decreasing: a population‐based time trend analysis and validation study. Am J Gastroenterol. 2017;112(12):1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toh JWT, Wang N, Young CJ, Rickard MJFX, Keshava A, Stewart P, et al. Major abdominal and perianal surgery in Crohn's disease: long‐term follow‐up of Australian patients with Crohn's disease. Dis Colon Rectum. 2018;61(1):67–76. [DOI] [PubMed] [Google Scholar]
- 13. Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn's disease in a population‐based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59(9):1200–6. [DOI] [PubMed] [Google Scholar]
- 14. Lakatos PL, Golovics PA, David G, Pandur T, Erdelyi Z, Horvath A, et al. Has there been a change in the natural history of Crohn's disease? Surgical rates and medical management in a population‐based inception cohort from western Hungary between 1977–2009. Am J Gastroenterol. 2012;107(4):579–88. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen GC, Nugent Z, Shaw S, Bernstein CN. Outcomes of patients with Crohn's disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology. 2011;141(1):90–7. [DOI] [PubMed] [Google Scholar]
- 16. Annese V, Duricova D, Gower‐Rousseau C, Jess T, Langholz E. Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the epidemiology committee of ECCO. J Crohns Colitis. 2016;10(2):216–25. [DOI] [PubMed] [Google Scholar]
- 17. Olivera P, Spinelli A, Gower‐Rousseau C, Danese S, Peyrin‐Biroulet L. Surgical rates in the era of biological therapy: up, down or unchanged? Curr Opin Gastroenterol. 2017;33(4):246–53. [DOI] [PubMed] [Google Scholar]
- 18. Ponsioen CY, de Groof EJ, Eshuis EJ, Gardenbroek TJ, Bossuyt PMM, Hart A, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: a randomised controlled, open‐label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2(11):785–92. [DOI] [PubMed] [Google Scholar]
- 19. Stevens TW, Haasnoot ML, D'Haens GR, Buskens CJ, de Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: retrospective long‐term follow‐up of the LIR!C trial. Lancet Gastroenterol Hepatol. 2020;5(10):900–7. [DOI] [PubMed] [Google Scholar]
- 20. Eberhardson M, Söderling JK, Neovius M, Cars T, Myrelid P, Ludvigsson JF, et al. Anti‐TNF treatment in Crohn's disease and risk of bowel resection – a population based cohort study. Aliment Pharmacol Ther. 2017;46(6):589–98. [DOI] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J‐L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Almqvist C, Bonamy A‐K, Ljung R, Michaëlsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–36. [DOI] [PubMed] [Google Scholar]
- 24. Wettermark B, Hammar N, MichaelFored C, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–35. [DOI] [PubMed] [Google Scholar]
- 25. Jakobsson GL, Sternegård E, Olén O, Myrelid P, Ljung R, Strid H, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–21. [DOI] [PubMed] [Google Scholar]
- 26. Busch K, Ludvigsson JF, Ekstrom‐Smedby K, Ekbom A, Askling J, Neovius M. Nationwide prevalence of inflammatory bowel disease in Sweden: a population‐based register study. Aliment Pharmacol Ther. 2014;39(1):57–68. [DOI] [PubMed] [Google Scholar]
- 27. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shrestha S, Olén O, Eriksson C, Everhov ÅH, Myrelid P, Visuri I, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish National Patient Register. Scand J Gastroenterol. 2020;55(4):430–5. [DOI] [PubMed] [Google Scholar]
- 29. Ludvigsson JF, Andersson M, Bengtsson J, Eberhardson M, Fagerberg UL, Grip O, et al. Swedish Inflammatory Bowel Disease Register (SWIBREG) – a nationwide quality register. Scand J Gastroenterol. 2019;54(9):1089–101. [DOI] [PubMed] [Google Scholar]
- 30. Bröms G, Söderling J, Sachs MC, Halfvarson J, Myrelid P, Ludvigsson JF, et al. Capturing biologic treatment for IBD in the Swedish Prescribed Drug Register and the Swedish National Patient Register – a validation study. Scand J Gastroenterol. 2021;56(4):410–21. [DOI] [PubMed] [Google Scholar]
- 31. Forss A, Myrelid P, Olén O, Everhov ÅH, Nordenvall C, Halfvarson J, et al. Validating surgical procedure codes for inflammatory bowel disease in the Swedish National Patient Register. BMC Med Inform Decis Mak. 2019;19(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gecse KB, Végh Z, Lakatos PL. Optimizing biological therapy in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2016;10(1):37–45. 10.1586/17474124.2016.1096198 [DOI] [PubMed] [Google Scholar]
- 33. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362(15):1383–95. [DOI] [PubMed] [Google Scholar]
- 34. Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400 e3. [DOI] [PubMed] [Google Scholar]
- 35. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99(4):956–63. [DOI] [PubMed] [Google Scholar]
- 36. Olaison G, Smedh K, Sjödahl R. Natural course of Crohn's disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms.. Gut. 1992;33(3):331–335. 10.1136/gut.33.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Landsend E, Johnson E, Johannessen HO, Carlsen E. Long‐term outcome after intestinal resection for Crohn's disease. Scand J Gastroenterol. 2006;41(10):1204–8. [DOI] [PubMed] [Google Scholar]
- 38. Tsai L, Ma C, Dulai PS, Prokop LJ, Eisenstein S, Ramamoorthy SL, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn's disease: a meta‐analysis of population‐based cohorts. Clin Gastroenterol Hepatol. 2021;19(10):2031–2045.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn's disease after ileocolonic resection. Gastroenterology. 2016;150(7):1568–78. [DOI] [PubMed] [Google Scholar]
- 40. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet. 2015;385(9976):1406–17. [DOI] [PubMed] [Google Scholar]
- 41. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Efficacy of thiopurines and adalimumab in preventing Crohn's disease recurrence in high‐risk patients – a POCER study analysis. Aliment Pharmacol Ther. 2015;42(7):867–79. [DOI] [PubMed] [Google Scholar]
- 42. Carla‐Moreau A, Paul S, Roblin X, Genin C, Peyrin‐Biroulet L. Prevention and treatment of postoperative Crohn's disease recurrence with anti‐TNF therapy: a meta‐analysis of controlled trials. Dig Liver Dis. 2015;47(3):191–6. [DOI] [PubMed] [Google Scholar]
- 43. Iesalnieks I, Kilger A, Glass H, Obermeier F, Agha A, Schlitt HJ. Perforating Crohn's ileitis: delay of surgery is associated with inferior postoperative outcome. Inflamm Bowel Dis. 2010;16(12):2125–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data underlying this article were provided by the Swedish National Patient Register, Swedish Prescribed Drug Register and SWIBREG by permission. Data can be obtained with permission of these registers.


