Abstract
Aim
To evaluate the effect of bempedoic acid on glycaemic and lipid variables in patients with hypercholesterolaemia.
Methods
A patient‐level pooled analysis of four phase 3, randomized, double‐blind, placebo‐controlled trials evaluated changes in glycaemia, change from baseline in LDL‐C, and adverse events. Patients (N = 3621) on maximally tolerated statins were randomized 2:1 to oral bempedoic acid 180 mg or placebo once daily for 12 to 52 weeks with the results analysed by baseline glycaemic status (diabetes, prediabetes, or normoglycaemia).
Results
The annual rate of new‐onset diabetes for bempedoic acid versus placebo in patients with normoglycaemia at baseline (n = 618) was 0.3% versus 0.8%, and for patients with prediabetes at baseline (n = 1868) it was 4.7% versus 5.9%. In patients with diabetes or prediabetes, bempedoic acid significantly (P < .0001) reduced HbA1c by −0.12% and −0.06%, respectively, and did not worsen fasting glucose versus placebo. Bempedoic acid significantly and consistently lowered LDL‐C levels versus placebo, regardless of baseline glycaemic status (placebo‐corrected difference range, −17.2% to −29.6%; P < .001 for each stratum). The safety of bempedoic acid was comparable with placebo and similar across glycaemic strata.
Conclusions
Bempedoic acid significantly lowered LDL‐C across glycaemic strata and did not worsen glycaemic variables or increase the incidence of new‐onset diabetes versus placebo over a median follow‐up of 1 year.
Keywords: cardiovascular disease, lipid‐lowering therapy, statins, type 2 diabetes
1. INTRODUCTION
Cardiovascular disease (CVD) risk increases in individuals who progress from normoglycaemia to prediabetes and type 2 diabetes. Individuals with type 2 diabetes have a 2‐fold increased risk for coronary heart disease (CHD), including a 2.3‐fold increased risk for CHD death and a 1.7‐fold increased risk for other vascular deaths. 1 Reducing LDL‐C levels, primarily with the use of statins, is recommended for individuals with hypercholesterolaemia irrespective of glycaemic status. 2 , 3 , 4 , 5 , 6 Many individuals with insufficient LDL‐C lowering or higher residual atherogenic risk may require further reduction in LDL‐C, and/or non‐HDL‐C and other apolipoprotein B (Apo B)‐containing lipoproteins. 4 , 5 , 6
Some lipid‐lowering therapies (LLTs) are known to affect glucose levels: bile acid sequestrants 7 , 8 and possibly fibrates 9 , 10 may improve glycaemia, whereas niacin and statins have the potential to worsen glycaemic control. 9 , 11 , 12 In systematic reviews and meta‐analyses of clinical trials, 3‐hydroxy‐3‐methylglutaryl‐coenzyme A (HMG‐CoA) inhibition was associated with a 9% to 12% relative increase in risk for type 2 diabetes depending on statin intensity. 9 , 13 , 14 , 15 Results from population‐based genetic studies show that genetic variants associated with lower HMG‐CoA reductase activity are also associated with higher blood glucose, higher HbA1c, and a greater risk of new‐onset type 2 diabetes. 16 , 17 , 18 Despite the associated increased risk for diabetes and worsening of glycaemic control, the reduction in the risk of CVD and cardiovascular death supports the recommended first‐line use of statins in patients with or without type 2 diabetes. 2 , 4 , 19
Bempedoic acid is a first‐in‐class inhibitor of ATP–citrate lyase (ACL), an enzyme in the cholesterol biosynthesis pathway that is upstream of HMG‐CoA reductase, the target for statin therapy. 20 , 21 Bempedoic acid is a prodrug metabolized in the liver by very long‐chain acyl‐CoA synthetase 1 (ACSVL1) to form the pharmacologically active bempedoic acid‐CoA metabolite, which inhibits hepatic cholesterol synthesis and leads to upregulation of hepatic LDL receptors, increased uptake and clearance of LDL, and lowering of plasma LDL‐C levels. 21 Although ACL (the target of bempedoic acid) and HMG‐CoA reductase (the target of statins) are both involved in hepatic cholesterol synthesis, results from preclinical, clinical, 22 , 23 , 24 , 25 , 26 and Mendelian randomization studies 16 suggest that ACL inhibition may not adversely affect glycaemic control and may be associated with a reduced risk of developing diabetes. The results of a recent meta‐analysis based on published mean incidence rates of adverse events (AEs) from 10 phase 2 or 3 studies also suggested that bempedoic acid might actually have a protective effect against new‐onset diabetes. 27 Because changes in glycaemic status require a larger number of participants than are available in any individual study, we performed a patient‐level analysis of pooled data from four phase 3 clinical studies in patients grouped by baseline glycaemic status (diabetes, prediabetes, normoglycaemia) with the goal of determining if bempedoic acid has an effect on glycaemic variables (HbA1c, fasting glucose, and incidence of new‐onset diabetes), and assessing the efficacy and safety of bempedoic acid in patients who had diabetes, prediabetes, or normoglycaemia.
2. METHODS
2.1. Studies
This was a post hoc patient‐level pooled analysis of four phase 3, randomized, double‐blind, placebo‐controlled trials. 23 , 24 , 25 , 26 In each study, patients were randomized 2:1 to treatment with oral bempedoic acid 180 mg or placebo once daily for 12 weeks (CLEAR Tranquility [N = 269]; NCT03001076), 23 24 weeks (CLEAR Serenity [N = 345]; NCT02988115), 24 or 52 weeks (CLEAR Wisdom [N = 779; NCT02991118] 25 and CLEAR Harmony [N = 2230; NCT02666664]). 26 For each study, the protocol was approved by institutional review boards or an independent ethics committee at each participating institution prior to initiation of the study.
2.2. Patients
Patients were included in the four studies if they had hypercholesterolaemia and required additional LDL‐C lowering despite receiving stable LLT. Details of the other inclusion/exclusion criteria are available in the published articles reporting on each trial. 23 , 24 , 25 , 26 All patients provided written informed consent.
2.3. Background lipid‐lowering therapy
CLEAR Harmony and CLEAR Wisdom enrolled patients who had prior atherosclerotic CVD (ASCVD) and/or heterozygous familial hypercholesterolaemia (HeFH) who were receiving maximally tolerated statin background therapy with or without other LLT. 25 , 26 CLEAR Serenity and CLEAR Tranquility enrolled patients with hypercholesterolaemia with multiple cardiovascular risk factors, and who had a history of statin intolerance and were receiving background therapy with at most a low‐dose statin or no statin therapy. 23 , 24 In CLEAR Serenity, statin intolerance was defined as a patient's inability to tolerate two or more statins (one at the lowest approved starting dose) because of an AE that started or increased during statin therapy and resolved or improved when the statin was discontinued. In CLEAR Tranquility, statin intolerance was defined as a patient's inability to tolerate more than low or very low‐dose (below the lowest approved starting dose) statin therapy. All patients enrolled in CLEAR Tranquility also received background ezetimibe 10 mg once daily 23 ; background ezetimibe use was allowed but not required in the other three studies. 24 , 25 , 26 Background LLT had to be stable at least 4 weeks before screening and throughout the study, although provisions to adjust LLT beginning at week 24 were available if a patient's LDL‐C value exceeded 4.4 mmol/L (170 mg/dl) and increased 25% or more from baseline.
2.4. Assessments
Patients were stratified by baseline glycaemic status into three groups: diabetes (patients with one or more of the following: a history of type 1 or 2 diabetes, receiving antihyperglycaemic medication before baseline, and/or HbA1c ≥ 6.5% [48 mmol/mol] at baseline, or at least one fasting plasma glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization), prediabetes (patients with all of the following: no medical history of diabetes; not receiving antihyperglycaemic medication before baseline; and HbA1c of 5.7% [39 mmol/mol] to 6.4% [46 mmol/mol; inclusive] at baseline; or at least one fasting glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and baseline), and normoglycaemia (patients not fulfilling the above criteria for diabetes or prediabetes). Criteria for the glycaemic control subgroups are consistent with standard of care guidelines and are similar to definitions used for studies investigating the effect of other LLTs on glycaemic control. 28 , 29
Changes from baseline in HbA1c levels and fasting glucose levels were assessed. In the four individual studies, the prespecified primary efficacy endpoint was the change from baseline in LDL‐C levels at week 12. Secondary efficacy endpoints included the change from baseline to week 12 in total cholesterol (TC), non‐HDL‐C, apo B, and high‐sensitivity C‐reactive protein (hsCRP) levels.
Data on treatment‐emergent AEs (TEAEs), laboratory tests (including HbA1c and fasting plasma glucose), vital sign measurements, and body weights were collected in each study. Because of the variance in follow‐up among studies (12 to 52 weeks), exposure‐adjusted incidence rates expressed as per 100 person‐years (PY) are reported for TEAEs. Prespecified categories of AEs of special interest included new‐onset type 2 diabetes/hyperglycaemia, hypoglycaemia, elevations in hepatic enzyme levels, metabolic acidosis, muscular disorders, neurocognitive disorders, renal disorders, uric acid elevations/gout, and decreases in haemoglobin levels. New‐onset diabetes was diagnosed by fulfilling the HbA1c or fasting glucose levels used to define diabetes at baseline or an investigator‐reported diabetes‐related AE (including the MedDRA preferred terms of blood glucose abnormal, blood glucose increased, diabetes mellitus, diabetes mellitus inadequate control, diabetic ketoacidosis, glucose tolerance impaired, glucose urine present, glycosuria, glycosylated haemoglobin increased, hyperglycaemia, impaired fasting glucose, ketoacidosis, ketosuria, ketosis, type 2 diabetes mellitus, urine ketone body present), or initiation of an antihyperglycaemic medication at any point after baseline.
2.5. Statistical analysis
In the pooled analysis, the intent‐to‐treat (ITT) population, which included all randomized patients, served as the efficacy analysis population. The safety population included all patients who received at least one dose of bempedoic acid or placebo.
The pooled data were analysed to determine changes in the glycaemic status of all three strata. The percentages of patients transitioning from prediabetes to diabetes or to normoglycaemia, from normoglycaemia to prediabetes or diabetes, or experiencing shifts in HbA1c in patients with diabetes, were assessed. Because of the varied study lengths and patient populations, lipid‐lowering efficacy was evaluated in two study pools, patients with ASCVD and/or HeFH on statins (52‐week studies that included patients on maximally tolerated statins) and a pool of statin‐intolerant patients (12‐ and 24‐week studies in patients on statin doses less than or equal to the lowest indicated dose), both stratified by glycaemic status. Analysis of covariance (ANCOVA) models were used for lipid variables (LDL‐C, TC, non‐HDL‐C, triglycerides, and apo B) change from baseline to week 12 and for HbA1c, fasting glucose, and weight. An ANCOVA subgroup analysis was used to determine any interaction between the change from baseline to week 12 in HbA1c and fasting glucose and baseline LLT use or baseline statin use. Wilcoxon rank sum test was used for hsCRP change from baseline to week 12. A survival analysis was performed for time to postbaseline new‐onset diabetes during the treatment period in patients with prediabetes at baseline and for time to first HbA1c of less than 6.5% (48 mmol/mol) in patients with diabetes who had HbA1c of 6.5% or higher (48 mmol/mol) at baseline during the treatment period.
3. RESULTS
3.1. Patients
Of 3621 patients in the safety analysis population, 1135 patients (31.3%) had diabetes, 1868 patients (51.6%) had prediabetes, and 618 patients (17.1%) had normoglycaemia at baseline. Most patient demographic variables were comparable across the groups (Table 1). Patients with diabetes at baseline had numerically higher fasting glucose, HbA1c, triglycerides, and hsCRP levels, although these differences were not assessed for statistical differences; HDL‐C levels were lower in patients with diabetes. A higher proportion of patients with diabetes had a history of hypertension or ASCVD than did patients with prediabetes or normoglycaemia. A majority of patients in each group (>80%) were treated with statins with or without other LLTs, and more than 60% of patients in each group were using moderate‐ or high‐intensity statins (as defined in the 2018 American Heart Association [AHA]/American College of Cardiology [ACC] guidelines 4 ). The median duration of treatment was 361 to 364 days for both treatment arms in each of the three glycaemic control subgroups. The mean (SD) duration of treatment for patients with normoglycaemia at baseline was 270.3 (131.2) days for patients treated with bempedoic acid and 269.2 (126.3) days for patients receiving placebo; prediabetes at baseline was 276.7 (126.3) days for patients treated with bempedoic acid and 294.0 (114.6) days for patients receiving placebo; and diabetes at baseline was 278.7 (124.6) days for patients treated with bempedoic acid and 292.9 (116.2) days for patients receiving placebo.
TABLE 1.
Baseline demographics and characteristics by baseline glycaemic status
| Variable | Diabetes a | Prediabetes b | Normoglycaemia c | |||
|---|---|---|---|---|---|---|
| BA (n = 755) | Placebo (n = 380) | BA (n = 1259) | Placebo (n = 609) | BA (n = 410) | Placebo (n = 208) | |
| Age, mean (SD), y | 65.7 (8.7) | 67.0 (8.3) | 65.3 (9.1) | 65.8 (8.9) | 64.1 (10.6) | 64.6 (10.2) |
| Male, % (n) | 64.8 (489) | 63.9 (243) | 69.1 (870) | 67.2 (409) | 58.8 (241) | 61.1 (127) |
| White, % (n) | 91.1 (688) | 93.9 (357) | 95.9 (1208) | 94.9 (578) | 95.9 (393) | 93.8 (195) |
| BMI, mean (SD), kg/m2 | 31.7 (5.3) | 31.7 (5.4) | 29.4 (4.8) | 29.4 (4.8) | 27.6 (4.2) | 28.0 (4.1) |
| eGFR category, % (n) | ||||||
| ≥90 ml/min/1.73m2 | 26.8 (202) | 21.1 (80) | 19.3 (243) | 21.2 (129) | 20.7 (85) | 22.1 (46) |
| ≥60 to <90 ml/min/1.73m2 | 54.8 (414) | 59.7 (227) | 66.2 (834) | 65.4 (398) | 69.3 (284) | 63.9 (133) |
| <60 ml/min/1.73m2 | 18.4 (139) | 19.2 (73) | 14.5 (182) | 13.5 (82) | 10.0 (41) | 13.9 (29) |
| Fasting glucose, mean (SD), mg/dl | 130.8 (38.0) | 129.7 (37.4) | 100.3 (10.6) | 100.0 (10.8) | 89.7 (6.2) | 90.1 (6.2) |
| HbA1c, mean (SD) | ||||||
| %, mean (SD) | 6.8 (1.0) | 6.8 (1.0) | 5.8 (0.3) | 5.8 (0.3) | 5.4 (0.2) | 5.4 (0.2) |
| mmol/mol, mean | 51 | 51 | 40 | 40 | 36 | 36 |
| On antihyperglycaemic medication at baseline, d % (n) | 75.5 (570) | 73.9 (281) | — | — | — | — |
| On insulin at baseline, e % (n) | 18.5 (140) | 21.6 (82) | — | — | — | — |
| History of hypertension, % | 86.5 (653) | 89.2 (339) | 77.0 (970) | 76.4 (465) | 62.7 (257) | 67.3 (140) |
| History of ASCVD, % | 83.6 (631) | 84.5 (321) | 80.5 (1013) | 81.8 (498) | 74.9 (307) | 74.5 (155) |
| Lipid variables mean (SD), mg/dl | ||||||
| Total cholesterol | 189.6 (41.2) | 188.4 (40.8) | 194.5 (44.4) | 194.2 (45.5) | 199.1 (45.0) | 193.4 (42.8) |
| Non‐HDL‐C | 142.4 (39.5) | 141.3 (40.2) | 144.0 (42.7) | 143.2 (43.3) | 144.6 (43.6) | 139.2 (39.8) |
| LDL‐C | 110.1 (33.6) | 109.6 (33.7) | 115.2 (37.2) | 115.0 (38.4) | 119.1 (39.1) | 113.9 (35.3) |
| HDL‐C | 47.1 (11.7) | 47.1 (10.4) | 50.5 (12.8) | 51.0 (14.6) | 54.5 (13.8) | 54.2 (12.5) |
| Triglycerides, median (Q1, Q3), mg/dl | 148.0 (114.5, 202.0) | 144.5 (110.8, 192.8) | 131.0 (100.0, 176.5) | 127.0 (97.0, 177.5) | 114.2 (88.0, 154.0) | 114.0 (88.0, 149.2) |
| Apo B, mean (SD), mg/dl | 101.6 (29.6) | 101.3 (30.9) | 102.4 (31.7) | 101.5 (32.0) | 101.9 (31.0) | 97.6 (29.7) |
| hsCRP, median (Q1, Q3), mg/dl | 2.1 (1.0, 4.6) | 2.1 (1.0, 5.2) | 1.6 (0.8, 3.3) | 1.6 (0.8, 3.5) | 1.3 (0.7, 2.8) | 1.3 (0.7, 2.5) |
| Baseline LLT use, % (n) | ||||||
| Statin alone | 71.0 (536) | 70.8 (269) | 70.1 (882) | 71.9 (438) | 69.3 (284) | 67.3 (140) |
| Statin plus other LLT | 14.3 (108) | 13.9 (53) | 13.3 (167) | 12.8 (78) | 12.9 (53) | 13.0 (27) |
| Other LLT alone | 7.9 (60) | 8.2 (31) | 9.3 (117) | 8.9 (54) | 12.7 (52) | 12.5 (26) |
| None | 6.8 (51) | 7.1 (27) | 7.4 (93) | 6.4 (39) | 5.1 (21) | 7.2 (15) |
| Baseline statin intensity, % (n) | ||||||
| None | 14.7 (111) | 15.3 (58) | 16.7 (210) | 15.3 (93) | 17.8 (73) | 19.7 (41) |
| Low f | 8.2 (62) | 6.3 (24) | 7.6 (96) | 7.4 (45) | 10.5 (43) | 12.0 (25) |
| Moderate | 37.4 (282) | 37.1 (141) | 31.1 (392) | 31.9 (194) | 33.2 (136) | 33.2 (69) |
| High | 39.7 (300) | 41.3 (157) | 44.6 (561) | 45.5 (277) | 38.5 (158) | 35.1 (73) |
| Baseline ezetimibe use, % (n) | 11.4 (86) | 12.1 (46) | 16.4 (206) | 14.6 (89) | 17.8 (73) | 20.7 (43) |
Abbreviations: Apo B, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BA, bempedoic acid; BMI, body mass index; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitivity C‐reactive protein; LLT, lipid‐lowering therapy; SD, standard deviation.
Patients with one or more of the following: history of type 1 or type 2 diabetes; receiving diabetes medication prior to baseline; and/or HbA1c ≥ 6.5% at baseline, or at least one fasting plasma glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization.
Patients with all of the following: no medical history of diabetes; not receiving diabetes medication prior to baseline; and HbA1c of 5.7% to 6.4% (inclusive) at baseline, or at least one fasting glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization.
Patients not fulfilling the criteria for diabetes or prediabetes.
Included insulin and non‐insulin medications.
Included insulin and analogues.
Included low‐dose or very‐low‐dose statin regimens in studies that enrolled patients with statin intolerance.
3.2. Glycaemic effects
HbA1c levels were modestly but significantly lower at week 12 compared with baseline among patients treated with bempedoic acid versus placebo in both the diabetes (P < .0001) and prediabetes (P = .0004) groups; no significant changes were observed in patients with normoglycaemia (Figure 1A). There were modest changes from baseline to week 12 in fasting glucose across the three groups regardless of treatment (Figure 1B). Of note, the number of patients and, as expected, baseline fasting glucose levels, varied across the three glycaemic control groups, making comparisons across the three glycaemic control groups difficult. A subgroup analysis showed that the modest HbA1c or fasting glucose changes from baseline to week 12 were not related to stable (at least 4 weeks before screening) baseline LLT or statin use (ANCOVA interaction P ≥ .369 for all comparisons). Patients with diabetes and prediabetes treated with bempedoic acid had mean reductions in body weight, whereas body weight increased modestly in patients with diabetes and prediabetes treated with placebo (P = .090 and P = .008, respectively, between‐group change from baseline) (Figure 1C). No statistically significant differences in glycaemic variables were observed in the normoglycaemia group between bempedoic acid and placebo.
FIGURE 1.
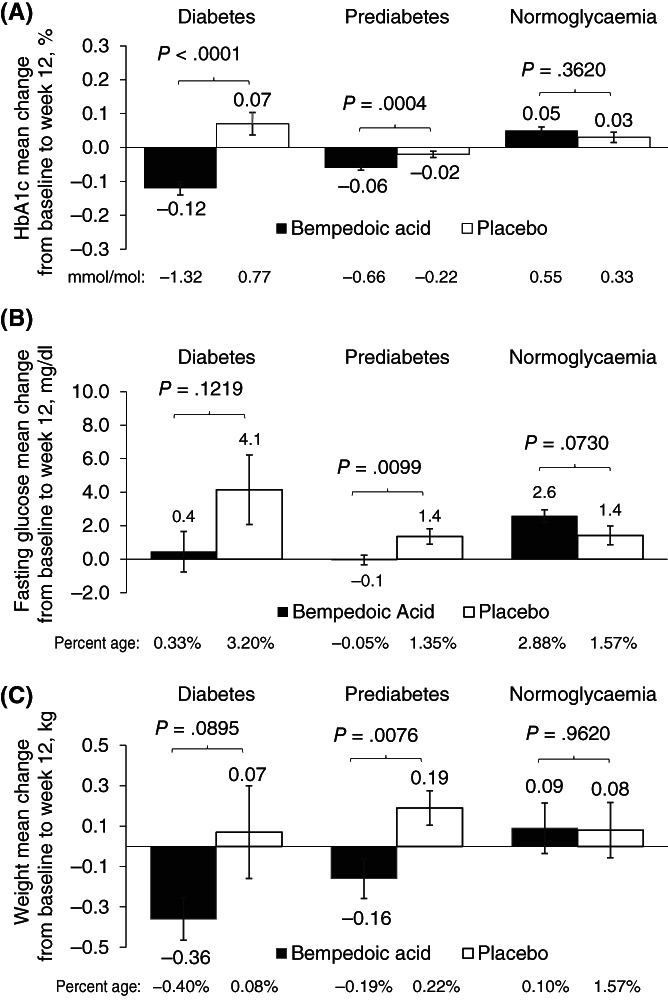
Change in: A, glycated haemoglobin (HbA1c); B, Fasting glucose; and C, Weight. Data are mean ± standard error. P value based on t test for change from baseline (bempedoic acid vs. placebo). Patients were randomized 2:1 to receive bempedoic acid 180 mg or placebo once daily for 12 weeks (CLEAR Tranquility), 24 weeks (CLEAR Serenity) or 52 weeks (in CLEAR Harmony and CLEAR Wisdom). Primary endpoint was the change from baseline in low‐density lipoprotein cholesterol (LDL‐C) levels at week 12
The annual rate of new‐onset diabetes (based on a Kaplan–Meier analysis) was numerically lower for bempedoic acid versus placebo for patients with prediabetes at baseline (4.7% vs. 5.9%) and for patients who were normoglycaemic at baseline (0.3% vs. 0.8%), although this difference was not significant in either case (P > .05). A survival analysis revealed that the risk of developing diabetes was numerically lower in patients with prediabetes treated with bempedoic acid than with placebo, although the difference between treatment groups was not significant (hazard ratio, 0.796; P = .2819; Figure 2A). Similar findings were observed for time to first HbA1c of 6.5% or higher among patients with prediabetes at baseline (Figure 2B). Patients with diabetes who had HbA1c levels of 6.5% or higher (48 mmol/mol) at baseline and who were treated with bempedoic acid were more probable to experience a decrease in HbA1c levels to less than 6.5% (48 mmol/mol) than were patients treated with placebo (hazard ratio, 1.468; P = .0316; Figure 2C).
FIGURE 2.
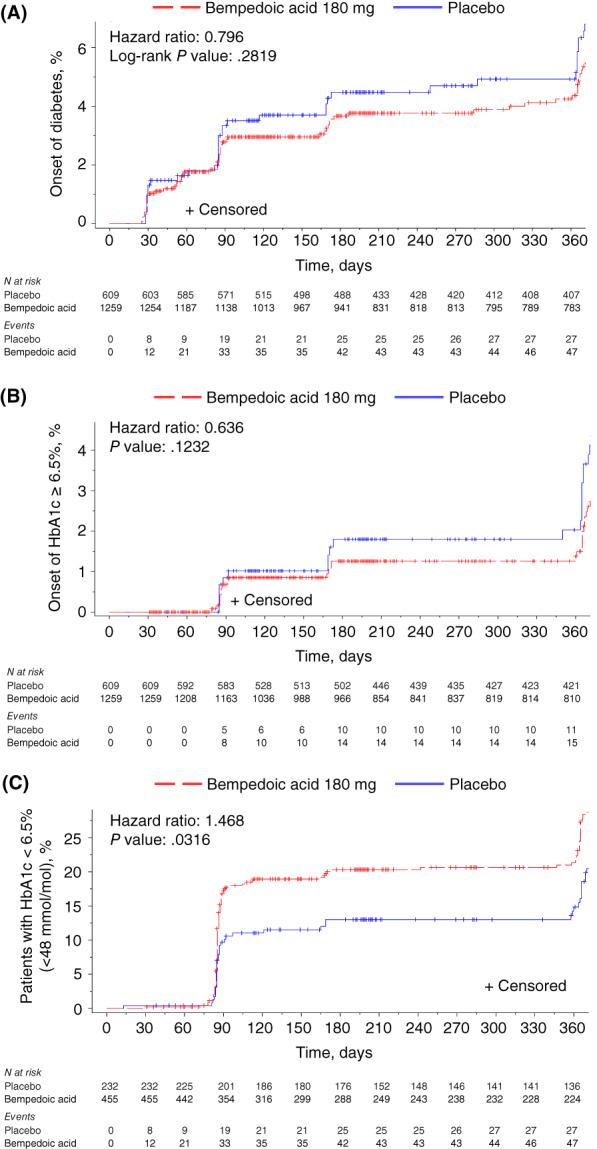
Development of diabetes among patients with prediabetes and improvement to glycated haemoglobin (HbA1c) less than 6.5% (48 mmol/mol) among patients with diabetes. A, Time to postbaseline diabetes (defined as at least one HbA1c value of ≥6.5%; two or more fasting serum glucose values of ≥126 mg/dl; an investigator‐reported diabetes‐related adverse event; and/or initiation of diabetes medication at any point after baseline) among patients with prediabetes at baseline; B, Time to first HbA1c of 6.5% or higher among patients with prediabetes at baseline; and C, Time to first HbA1c less than 6.5% (48 mmol/mol) among patients with diabetes and HbA1c of 6.5% or higher (48 mmol/mol) at baseline. Patients were randomized 2:1 to receive bempedoic acid 180 mg or placebo once daily for 52 weeks in CLEAR Harmony and CLEAR Wisdom and 24 weeks in CLEAR Tranquility and CLEAR Serenity
The crude incidences of transitioning from one baseline glycaemic control category to another based on maximum postbaseline patient values are summarized in Table S1. Transition from prediabetes to diabetes occurred in 4.5% of patients treated with bempedoic acid and 5.9% of patients receiving placebo. Transitions in patients with normoglycaemia at baseline were similar between treatment arms, with prediabetes occurring in 22.9% of patients treated with bempedoic acid and 21.6% of patients treated with placebo, and new‐onset diabetes occurring in 0.5% of patients in both treatment groups. Patients with diabetes and HbA1c of 6.5% or higher at baseline were analysed for transitions to the last HbA1c of 5.7% to 6.4%, or 5.6% or less. Among patients treated with bempedoic acid, 15.2% had a last postbaseline HbA1c of 5.7% to 6.4%, and 1.1% had a last HbA1c of 5.6% or less, whereas 14.2% of patients receiving placebo achieved the last postbaseline HbA1c of 5.7% to 6.4%, and no patients receiving placebo had a last HbA1c of 5.6% or less.
3.3. Efficacy
In both the ASCVD and/or HeFH on statins pool and the statin‐intolerant pool, LDL‐C lowering from baseline was significantly greater in patients taking bempedoic acid versus those taking placebo (placebo‐corrected difference range, −17.2% to −29.6%; P < .001 for each glycaemic stratum in both pools), regardless of patients' glycaemic status at baseline (interaction P value: ASCVD and/or HeFH on statins pool, P = .77; statin‐intolerant pool, P = .03) (Table 2). More patients reached target LDL‐C levels of less than 70 mg/dl at 12 weeks when treated with bempedoic acid compared with placebo, regardless of their glycaemic status at baseline (bempedoic acid vs. placebo: diabetes 37.6% vs. 11.4%, prediabetes 26.9% vs. 6.3%, and normoglycaemia 18.5% vs. 6.4%). Regardless of glycaemic status at baseline, in both pools, patients receiving bempedoic acid versus placebo also experienced significant reductions from baseline in TC, non‐HDL‐C, apo B, and hsCRP (P < .01 for all). The interaction effect for most variables was lower in the ASCVD and/or HeFH on statins pool (interaction P values: total cholesterol, .64; non‐HDL‐C, .93; apo B, .92; hsCRP, .02) than in the statin‐intolerant pool (interaction P values: TC, .03; non‐HDL‐C, .04; apo B, .02; hsCRP, .85).
TABLE 2.
Percentage change from baseline at week 12 in lipid variables
| LDL‐C | TC | Non‐HDL‐C | Apo B | TG | hsCRP | ||
|---|---|---|---|---|---|---|---|
| ASCVD/HeFH on statins pool | |||||||
| Diabetes a | |||||||
| BA | Baseline |
105.3 (30.3) (n = 642) |
183.4 (36.8) (n = 642) |
136.6 (35.4) (n = 642) |
95.5 (26.2) (n = 641) |
163.4 (85.4) (n = 642) |
1.9 (0.9, 4.3) (n = 642) |
| % change |
−18.5 (0.8) (n = 614) |
−11.3 (0.6) (n = 616) |
−12.9 (0.7) (n = 616) |
−9.0 (0.8) (n = 605) |
12.4 (1.7) (n = 616) |
−25.4 (−50.0, 21.5) (n = 606) |
|
| Placebo | Baseline |
105.5 (32.4) (n = 324) |
183.7 (39.7) (n = 324) |
136.7 (39.0) (n = 324) |
96.5 (29.3) (n = 322) |
161.2 (75.9) (n = 324) |
1.9 (0.9, 4.6) (n = 322) |
| % change |
0.6 (1.3) (n = 317) |
0.1 (0.9) (n = 318) |
0.6 (1.2) (n = 318) |
3.4 (1.1) (n = 312) |
4.3 (1.8) (n = 318) |
−3.1 (−36.8, 57.6) (n = 313) |
|
| Difference (95% CI) | −19.1 (−22.1, −16.1) b | −11.4 (−13.5, −9.4) b | −13.5 (−16.2, −10.8) b | −12.3 (−15.0, −9.7) b | 8.2 (3.3, 13.0) b | −18.9 (−27.1, −10.8) b | |
| Prediabetes c | |||||||
| BA | Baseline |
107.5 (31.9) (n = 1037) |
184.9 (38.3) (n = 1037) |
135.1 (37.1) (n = 1037) |
95.1 (26.9) (n = 1034) |
142.8 (67.4) (n = 1037) |
1.5 (0.8, 3.2) (n = 1036) |
| % change |
−16.4 (0.7) (n = 994) |
−10.6 (0.5) (n = 996) |
−11.7 (0.6) (n = 995) |
−9.6 (0.6) (n = 980) |
10.1 (1.5) (n = 996) |
−20.4 (−49.1, 21.3) (n = 971) |
|
| Placebo | Baseline |
108.6 (34.3) (n = 515) |
186.4 (40.7) (n = 515) |
135.9 (38.3) (n = 515) |
95.5 (27.6) (n = 512) |
140.3 (65.7) (n = 515) |
1.6 (0.8, 3.3) (n = 514) |
| % change |
2.1 (1.0) (n = 504) |
1.4 (0.7) (n = 504) |
2.2 (0.9) (n = 504) |
3.3 (0.8) (n = 497) |
6.8 (1.5) (n = 504) |
−1.9 (−32.2, 48.0) (n = 496) |
|
| Difference (95% CI) | −18.6 (−20.9, −16.2) b | −12.0 (−13.6, −10.3) b | −14.0 (−16.1, −11.8) b | −12.9 (−14.9, −10.9) b | 3.2 (−0.9, 7.4) | −17.7 (−24.0, −11.5) b | |
| Normoglycaemia d | |||||||
| BA | Baseline |
112.9 (36.7) (n = 331) |
191.2 (41.8) (n = 331) |
137.9 (41.0) (n = 331) |
95.7 (27.6) (n = 329) |
129.2 (65.3) (n = 331) |
1.2 (0.6, 2.3) (n = 329) |
| % change |
−14.0 (1.0) (n = 314) |
−9.2 (0.7) (n = 314) |
−11.0 (0.9) (n = 314) |
−8.5 (0.9) (n = 309) |
5.0 (2.2) (n = 314) |
−15.9 (−46.4, 34.4) (n = 311) |
|
| Placebo | Baseline |
107.6 (33.4) (n = 160) |
185.5 (40.0) (n = 160) |
132.5 (37.2) (n = 160) |
90.7 (26.6) (n = 159) |
127.3 (56.8) (n = 160) |
1.3 (0.7, 2.5) (n = 160) |
| % change |
3.2 (1.8) (n = 157) |
1.1 (1.2) (n = 157) |
2.2 (1.6) (n = 157) |
4.7 (1.7) (n = 154) |
1.3 (2.1) (n = 157) |
4.2 (−32.5, 57.5) (n = 155) |
|
| Difference (95% CI) | −17.2 (−21.3, −13.0) b | −10.3 (−13.0, −7.6) b | −13.2 (−16.9, −9.5) b | −13.2 (−16.9, −9.5) b | 3.8 (−2.2, 9.7) | −17.1 (−29.3, −5.0) e | |
| Statin‐intolerant pool | |||||||
| Diabetes a | |||||||
| BA | Baseline |
137.0 (38.5) (n = 113) |
224.5 (46.9) (n = 113) |
175.3 (45.0) (n = 113) |
130.9 (31.1) (n = 112) |
198.4 (91.0) (n = 113) |
3.2 (1.6, 6.2) (n = 112) |
| % change |
−21.1 (2.3) (n = 109) |
−13.4 (1.6) (n = 109) |
−15.3 (2.0) (n = 109) |
−11.8 (1.9) (n = 109) |
9.8 (3.8) (n = 109) |
−36.3 (−54.8, 5.6) (n = 109) |
|
| Placebo | Baseline |
133.0 (31.5) (n = 56) |
215.6 (36.4) (n = 56) |
168.0 (36.7) (n = 56) |
129.2 (24.9) (n = 56) |
183.6 (86.3) (n = 56) |
4.0 (1.7, 6.7) (n = 55) |
| % change |
−2.0 (1.8) (n = 52) |
−0.7 (1.3) (n = 52) |
0.0 (1.6) (n = 52) |
−0.2 (1.4) (n = 52) |
9.6 (4.9) (n = 52) |
−4.7 (−26.6, 19.5) (n = 51) |
|
| Difference (95% CI) | −19.1 (−24.9, −13.3) b | −12.7 (−16.7, −8.6) b | −15.4 (−20.4, −10.3) b | −11.6 (−16.3, −6.9) b | 0.2 (−12.1, 12.5) | −27.5 (−41.0, −13.0) b | |
| Prediabetes c | |||||||
| BA | Baseline |
150.8 (39.2) (n = 223) |
238.9 (43.6) (n = 223) |
184.9 (43.1) (n = 223) |
136.5 (30.1) (n = 220) |
173.8 (79.3) (n = 223) |
2.4 (1.1, 4.4) (n = 220) |
| % change |
−24.9 (1.5) (n = 214) |
−16.9 (1.0) (n = 215) |
−20.2 (1.2) (n = 214) |
−16.2 (1.1) (n = 209) |
5.3 (2.9) (n = 215) |
−22.3 (−52.3, 11.8) (n = 210) |
|
| Placebo | Baseline |
149.7 (41.5) (n = 94) |
237.4 (46.6) (n = 94) |
183.2 (47.3) (n = 94) |
135.9 (33.5) (n = 90) |
175.7 (89.8) (n = 94) |
2.4 (1.1, 4.5) (n = 90) |
| % change |
4.7 (2.1) (n = 91) |
2.8 (1.3) (n = 91) |
4.4 (1.8) (n = 91) |
4.3 (1.5) (n = 88) |
8.4 (3.7) (n = 91) |
0.2 (−35.2, 39.8) (n = 88) |
|
| Difference (95% CI) | −29.6 (−34.5, −24.6) b | −19.7 (−22.8, −16.5) b | −24.6 (−28.9, −20.4) b | −20.5 (−24.3, −16.8) b | −3.1 (−12.4, 6.1) | −20.7 (−34.0, −8.0) e | |
| Normoglycaemia d | |||||||
| BA | Baseline |
145.1 (38.2) (n = 79) |
232.3 (43.1) (n = 79) |
172.5 (43.4) (n = 79) |
127.7 (31.3) (n = 79) |
138.1 (66.0) (n = 79) |
2.1 (1.0, 3.7) (n = 79) |
| % change |
−25.2 (2.2) (n = 76) |
−16.4 (1.6) (n = 76) |
−21.1 (2.1) (n = 76) |
−18.3 (1.9) (n = 74) |
3.1 (3.9) (n = 76) |
−32.1 (−53.6, 25.8) (n = 74) |
|
| Placebo | Baseline |
134.2 (33.6) (n = 49) |
219.0 (41.6) (n = 49) |
160.7 (40.3) (n = 49) |
120.8 (28.1) (n = 47) |
133.3 (65.4) (n = 49) |
1.4 (0.8, 3.5) (n = 47) |
| % change |
−1.6 (2.1) (n = 46) |
−1.7 (1.7) (n = 46) |
−0.8 (2.2) (n = 46) |
0.7 (1.9) (n = 45) |
6.2 (5.8) (n = 46) |
10.0 (−19.0, 78.1) (n = 45) |
|
| Difference (95% CI) | −23.6 (−29.7, −17.4) b | −14.7 (−19.4, −10.1) b | −20.4 (−26.5, −14.3) b | −19.0 (−24.4, −13.5) b | −3.1 (−17.1, 10.8) | −43.7 (−65.2, −22.8) b | |
Note: Baseline values are reported as mean (SD) mg/dl, except for hsCRP, which is reported as median (Q1, Q3) mg/L. Percentage change from baseline values for LDL‐C, TC, non‐HDL‐C, Apo B, and TG are reported as LS mean (SE). LS mean, standard error, placebo‐adjusted change from baseline, 95% confidence intervals, and P values are based on ANCOVA with percentage change from baseline as the dependent variable, study and treatment as fixed factors, and baseline as a covariate. Only observed data are included in the analysis. P values for median (IQR) are based on Wilcoxon 2 sample test.
Abbreviations: Apo B, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BA, bempedoic acid; HeFH, heterozygous familial hypercholesterolaemia; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LS, least squares; SE, standard error; TC, total cholesterol; TG, triglycerides.
Patients with one or more of the following: history of type 1 or type 2 diabetes; receiving antihyperglycaemic medication before baseline; and/or HbA1c ≥ 6.5% at baseline, or at least one fasting plasma glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization.
P < .001.
Patients with all of the following: no medical history of diabetes; not receiving antihyperglycaemic medication before baseline; and HbA1c of 5.7% to 6.4% (inclusive) at baseline, or at least one fasting glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization.
Patients not fulfilling the criteria for diabetes or prediabetes.
P < .01.
3.4. Safety
The rates of exposure‐adjusted TEAEs were similar between treatment groups and across baseline glycaemic strata (Table S2). The most common AEs (those that occurred in ≥5/100 PY in any group) irrespective of relationship to study drug included nasopharyngitis, urinary tract infection, myalgia, upper respiratory tract infection, hypoglycaemia, arthralgia, dizziness, diarrhoea, muscle spasms, osteoarthritis, and angina pectoris. TEAEs leading to discontinuations were comparable in patients with diabetes (bempedoic acid, 12.4/100 PY vs. placebo, 10.4/100 PY) and those with normoglycaemia (bempedoic acid, 12.5/100 PY vs. placebo, 12.3/100 PY), but TEAEs leading to discontinuation were numerically higher in patients with prediabetes treated with bempedoic acid (14.4/100 PY) versus placebo (6.8/100 PY).
Prespecified TEAEs of special interest are presented in Table 3. In patients with prediabetes, the incidence of new‐onset diabetes/hyperglycaemia was 1.2/100 PY lower in patients receiving bempedoic acid compared with patients who received placebo. Among patients with prediabetes and diabetes, the incidence of hepatic enzyme elevations was greater in patients treated with bempedoic acid (3.4/100 and 4.1/100 PY, respectively) versus placebo (1.3/100 and 1.8/100 PY, respectively) and the incidence of renal disorders was greater in patients treated with bempedoic acid (3.3/100 and 3.9/100 PY, respectively) versus placebo (0.7/100 and 2.1/100 PY, respectively). The incidence of elevations in blood creatinine levels was greater in patients treated with bempedoic acid (range 0.8/100‐1.5/100 PY) versus placebo (range 0.4/100‐0.6/100 PY), regardless of glycaemic status at baseline. The incidence of elevations in uric acid and/or reports of gout was greater in patients treated with bempedoic acid (range 4.5/100‐6.4/100 PY) versus placebo (range 0.6/100‐2.4/100 PY), regardless of glycaemic status at baseline. Similar incidence of hypoglycaemia, metabolic acidosis, muscular disorders, neurocognitive disorders, and decreases in haemoglobin levels were observed between patients treated with bempedoic acid and placebo.
TABLE 3.
Treatment‐emergent adverse events of special interest
| TEAE a | Exposures‐adjusted incidence, per 100 person‐years (n) | |||||
|---|---|---|---|---|---|---|
| Diabetes b | Prediabetes c | Normoglycaemia d | ||||
| BA (n = 755) | Placebo (n = 380) | BA (n = 1259) | Placebo (n = 609) | BA (n = 410) | Placebo (n = 208) | |
| New‐onset diabetes/hyperglycaemia | 9.2 (59) | 12.5 (42) | 3.2 (34) | 4.4 (24) | 0.9 (3) | 0.6 (1) |
| Diabetes mellitus | 2.8 (18) | 4.5 (15) | 0.2 (2) | 0.2 (1) | 0 | 0 |
| Type 2 diabetes mellitus | 2.8 (18) | 2.7 (9) | 0.8 (8) | 1.1 (6) | 0 | 0 |
| Hyperglycaemia | 1.7 (11) | 1.8 (6) | 0.4 (4) | 0.6 (3) | 0 | 0.6 (1) |
| Blood glucose increased | 1.3 (8) | 0.6 (2) | 0.8 (8) | 1.9 (10) | 0.6 (2) | 0 |
| Diabetes mellitus inadequate control | 0.6 (4) | 1.5 (5) | 0 | 0 | 0 | 0 |
| Glycosuria | 0.2 (1) | 0.6 (2) | 0 | 0 | 0 | 0 |
| Impaired fasting glucose | 0.2 (1) | 0 | 0.5 (5) | 0.4 (2) | 0 | 0 |
| HbA1C increased | 0 | 1.2 (4) | <0.1 (1) | 0.4 (2) | 0 | 0 |
| Glucose tolerance impaired | 0 | 0.3 (1) | 0.6 (6) | 0.2 (1) | 0.3 (1) | 0 |
| Hepatic enzyme elevation | 4.1 (26) | 1.8 (6) | 3.4 (36) | 1.3 (7) | 1.5 (5) | 1.2 (2) |
| Hypoglycaemia | 4.5 (29) | 5.7 (19) | 0.7 (7) | 0.9 (5) | 1.5 (5) | 0.6 (1) |
| Metabolic acidosis | 0.2 (1) | 0 | 0 | 0 | 0 | 0 |
| Muscular disorder | 12.7 (81) | 11.3 (38) | 17.5 (185) | 12.4 (67) | 13.6 (46) | 11.7 (20) |
| Neurocognitive disorder | 1.1 (7) | 1.2 (4) | 0.5 (5) | 0.7 (4) | 1.2 (4) | 0.6 (1) |
| Renal disorder | 3.9 (25) | 2.1 (7) | 3.3 (35) | 0.7 (4) | 2.7 (9) | 2.3 (4) |
| Blood creatinine increased | 0.9 (6) | 0.3 (1) | 0.8 (8) | 0.4 (2) | 1.5 (5) | 0.6 (1) |
| Uric acid elevations/gout | 6.4 (41) | 2.4 (8) | 6.1 (65) | 1.7 (9) | 4.5 (15) | 0.6 (1) |
| Haemoglobin decreased | 4.7 (30) | 3.0 (10) | 2.8 (30) | 1.7 (9) | 2.7 (9) | 1.8 (3) |
Note: TEAE incidence is defined as the number of patients having an event started in a certain period divided by the total person‐time (in 100 PY) at risk during this period.
Abbreviations: BA, bempedoic acid; HbA1C, Glycosylated haemoglobin; PY, person‐years; TEAE, treatment‐emergent adverse event.
TEAEs occurring in more than two patients per 100 PY in any group.
Patients with one or more of the following: history of type 1 or type 2 diabetes; receiving diabetes medication prior to baseline; and/or HbA1c ≥ 6.5% (48 mmol/mol) at baseline, or at least one fasting plasma glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization.
Patients with all of the following: no medical history of diabetes; not receiving diabetes medication prior to baseline; and HbA1c of 5.7% (39 mmol/mol) to 6.4% (46 mmol/mol) (inclusive) at baseline, or at least one value of fasting glucose value of ≥100 mg/dl, but not more than one value of ≥126 mg/dl between screening and randomization.
Patients not fulfilling the criteria for diabetes or prediabetes.
4. DISCUSSION
This patient‐level pooled analysis of patients enrolled in four phase 3 studies shows that bempedoic acid did not worsen fasting glucose compared with placebo and significantly modestly lowered HbA1c levels at week 12 compared with baseline in patients with either diabetes or prediabetes. Bempedoic acid lowered levels of LDL‐C and other lipid variables including TC, non‐HDL‐C, and apo B similarly across glycaemic strata (from baseline to week 12) and did not increase new‐onset diabetes over a median follow‐up of 1 year. The lipid‐lowering efficacy of bempedoic acid in each glycaemic stratum was comparable with the lowering of LDL‐C and other lipid variables reported in the overall pooled analysis 30 and in the individual studies. 23 , 24 , 25 , 26 Further, bempedoic acid lowered LDL‐C and other lipid variables regardless of glycaemic strata, both in patients who were receiving maximally tolerated statins and in those who were statin intolerant. The safety of bempedoic acid was comparable with placebo and similar across glycaemic strata.
HsCRP levels, a biomarker of inflammation, help to classify CVD risk and independently predict future vascular events, including in patients with type 2 diabetes. 31 , 32 Although statins lower hsCRP levels, proprotein convertase subtilisin‐kexin type 9 (PCSK9) inhibitors do not. 33 Based on results from the individual CLEAR studies, hsCRP levels are effectively lowered when bempedoic acid is given alone, with a statin, and/or with ezetimibe. 23 , 24 , 25 , 26 Findings from our study show that bempedoic acid lowered hsCRP across all glycaemic strata. As patients with higher HbA1c levels also tended to have higher median hsCRP levels at baseline, patients with diabetes derived greater absolute reduction in hsCRP than did patients with prediabetes or normoglycaemia.
Bempedoic acid added to background LLT (most of the patients were receiving background moderate‐ or high‐intensity statin therapy) was generally well tolerated, regardless of patients' baseline glycaemic status. Bempedoic acid is associated with small mean increases in uric acid and creatinine levels in some patients that become stable with time and are reversible after treatment is discontinued. 27 , 34 Increases in uric acid and creatinine levels were observed in few patients in our analysis and occurred regardless of glycaemic status at baseline.
Treatment with bempedoic acid did not adversely affect glycaemic control in patients with diabetes, nor did it increase the risk of new‐onset diabetes among patients without diabetes at baseline. Rather, patients with diabetes and prediabetes treated with bempedoic acid experienced a statistically significant but modest reduction from baseline in HbA1c levels and weight compared with patients with diabetes and prediabetes treated with placebo. Patients with prediabetes at baseline treated with bempedoic acid experienced non‐significant lower rates of new‐onset diabetes compared with patients with prediabetes who received placebo. These pooled data are consistent with results from a previous 29‐day phase 2 study, in which 60 patients with diabetes treated with bempedoic acid versus placebo had non‐significant numerical reductions in five glycaemic markers: fasting glucose, fasting insulin, fasting fructosamine, 15‐hour weighted mean plasma glucose, and 24‐hour glucose area under the curve following a glucose tolerance test. 22 These results are also aligned with results from a recent meta‐analysis of 10 randomized controlled trials that suggested bempedoic acid is strongly associated with a decreased risk of new‐onset or worsening diabetes (n = 2498; odds ratio 0.59; 95% CI 0.39‐0.90; P = .01; I 2 = 0%). 27 Our analysis suggests bempedoic acid does not adversely affect glycaemic control.
Statin therapy may increase blood glucose and HbA1c levels in patients with diabetes and is associated with an increased risk of new‐onset diabetes. 35 , 36 Meta‐analyses of clinical trials have revealed approximately an extra 0.1/100 PY new cases of type 2 diabetes in patients taking statins. 13 However, our pooled analysis revealed that new onset of diabetes/hyperglycaemia occurred in 1.2/100 PY fewer cases in patients with prediabetes receiving bempedoic acid compared with placebo while on a background of maximally tolerated statins. Although both statins 13 , 15 and niacin 9 are associated with an increased risk of new‐onset diabetes, other non‐statin LLTs (e.g. bile acid sequestrants, ezetimibe, fibrates, and PCSK9 inhibitors) are not. 9 Several mechanisms for how statins may worsen glycaemic control have been hypothesized. 37 , 38 Simvastatin has been shown to inhibit glucose‐mediated signalling in rat pancreatic islet β‐cells 38 ; and comparison of genetic studies and statin trials found concordance of increases in weight, insulin, glucose, HbA1c, and risk for type 2 diabetes with genetic instruments that simulated the therapeutic HMG‐CoA–lowering activity of pharmacological approaches. 16 , 17 , 18 Although statins and bempedoic acid both have an effect on the cholesterol synthesis pathway, bempedoic acid treatment for up to 1 year appears to result in no worsening of glycaemic control in patients with diabetes, and no increased risk of new‐onset diabetes in patients with prediabetes compared with placebo. In contrast to statins, body weight and measures of glycaemia were either unaffected or modestly improved with bempedoic acid. This is consistent with Mendelian randomization data estimating the effects of lifelong exposure to agents that lower ACL. 16 ACL catalyses the cleavage of citrate into oxaloacetate and acetyl‐CoA, an intersection in lipid and carbohydrate metabolism, and therefore multiple mechanisms may be responsible for improved glycaemic variables associated with ACL inhibition. Studies such as the ongoing CLEAR Outcomes trial (NCT02993406), with a larger number of patients and a longer follow‐up, are needed to confirm our observations. Additional mechanistic studies would also be useful to elucidate the mechanism(s) for the potential improvements in glycaemic measures and reduction in body weight seen with bempedoic acid.
This study is limited by the post hoc nature of the analysis; however, the pooled population provided a comparatively larger amount of individual data from patients in each treatment group and glycaemic strata than if the effect of bempedoic acid on glycaemic control had been assessed for each of the four individual studies. Nonetheless, a low number of patients for some comparisons is a limitation of this post hoc analysis. New‐onset diabetes was evaluated based on AE reporting, HbA1c, fasting glucose, or initiation of antihyperglycaemic medication after baseline; however, other laboratory indices of diabetes, including postprandial glucose/glucose tolerance tests, were not included in the protocols, and background use of antihyperglycaemic medications for disorders other than diabetes cannot be ruled out. Additions and/or changes in doses of antihyperglycaemic medications were not captured during these trials. By definition, a medical history of diabetes could have included patients with type 1 or type 2 diabetes; however, only 12 patients with type 1 diabetes were included in the diabetes stratum, so these patients probably did not overly bias the results. The median follow‐up duration was 1 year and therefore the observed lowered incidence of new‐onset diabetes would be strengthened by studying patients treated with bempedoic acid for extended durations. By comparison, the median follow‐up was 1.9 years for the JUPITER study, which reported an increase in median HbA1c levels and diabetes among patients receiving rosuvastatin. 31 In addition, we did not evaluate the effects of bempedoic acid therapy on CVD outcomes. Data on major adverse cardiovascular events in safety analyses of these trials up to 52 weeks were previously reported, 23 , 24 , 25 , 26 and cardiovascular outcomes with bempedoic acid therapy in high‐risk, statin‐intolerant patients are currently being investigated in the ongoing CLEAR Outcomes trial (NCT02993406; 14 014 patients enrolled, approximately 40% of whom had type 2 diabetes at baseline; the expected duration is approximately 3.5 years). 39
In conclusion, bempedoic acid treatment consistently lowers levels of LDL‐C, other atherogenic lipid levels, and hsCRP in patients with hypercholesterolaemia, regardless of glycaemic status. Bempedoic acid for up to 1 year also did not worsen glycaemic variables or increase the incidence of new‐onset diabetes compared with placebo.
CONFLICT OF INTEREST
LAL has received research grant(s)/support from AstraZeneca, Amgen, Esperion, Kowa, The Medicines Company, Novartis, and Sanofi/Regeneron. He has also served as an advisor for Amarin, AstraZeneca, Amgen, Esperion, HLS, Merck, Novartis, and Sanofi/Regeneron. MB has received research grant(s)/support from Amgen, Mylan, Sanofi, and Valeant, and has served as a consultant for Amgen, Daiichi Sankyo, Esperion, Freia Pharmaceuticals, Herbapol, KRKA, Mylan, Novartis, Novo Nordisk, Polpharma, Polfarmex, Sanofi‐Aventis, Servier, and Valeant. ALC has received research grant(s)/support from Akcea, Amarin, Amgen, Menarini, Mylan, Sanofi, and Sanofi/Regeneron, and has served as a consultant for Amgen, Amarin, Daiichi‐Sankyo, Eli Lilly, Esperion, Kowa, Ionis Pharmaceuticals, Menarini, MSD, Mylan, Novartis, Recordati, Regeneron, and Sanofi. PBD has received institutional research grant(s)/support from Retrophin/Travere, Regeneron, and Regenxbio, and served as a consultant for Akcea, Amryt, Esperion, Kaneka, and Regeneron. AMG is an Esperion board member and Akcea DSMB chair, and has served as a consultant for Kowa. UL has received an institutional research grant from Daiichi Sankyo and served as a consultant for Amgen, Esperion, Novartis, and Sanofi. GBJM received research grant(s)/support from Amgen, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, and Sanofi, and has served as a consultant for these companies as well as for Esperion, HLS Therapeutics, Novartis, and Servier. KKR has received research grant(s)/support from Amgen, MSD, Pfizer, Regeneron, and Sanofi (all paid to his institution), and has served as a consultant for or received honoraria from AbbVie, Akcea, Algorithm, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Cipla, Dr Reddy's Laboratories, Eli Lilly, Esperion, Kowa, Medco, MSD, Novo Nordisk, Pfizer, Regeneron, Resverlogix, Sanofi, Takeda, and Zuellig Pharma. JCH is an employee of Esperion Therapeutics Inc. and may own Esperion stock or stock options. ZY is a former employee of Esperion Therapeutics Inc. and may own Esperion stock or stock options. HEB has received research grant(s)/support from Acasti, Akcea, Allergan, Amarin, Amgen, AstraZeneca, Esperion, Matinas, Merck, Metavant, Novartis, Pfizer, Regeneron, and Sanofi, and has served as a consultant/advisor for Amarin, Esperion, and Matinas, and as a speaker for Esperion.
AUTHOR CONTRIBUTIONS
LAL, ALC, UL, KKR, and JCH: design, analysis, and writing of the manuscript. MB, PBD, AMG, Jr, GBJM, and HEB: analysis and writing of the manuscript. ZY: statistical analysis and writing of the manuscript. All the authors approved the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14645.
Supporting information
Table S1. Shift Table for Glycaemic Control by Baseline Category (Crude Incidence)
Table S2. Safety overview
ACKNOWLEDGEMENTS
The authors would like to thank Esperion Therapeutics Inc., Ann Arbor, MI, for support of this manuscript. Medical writing support, funded by Esperion Therapeutics Inc., was provided by James Bergstrom, PhD, and Kelly M. Cameron, PhD, CMPP, of JB Ashtin, who developed the first draft based on an author‐approved discussion guide and assisted in implementing author revisions. KKR acknowledges support from the NIHR Imperial Biomedical Research Centre.
Leiter LA, Banach M, Catapano AL, et al. Bempedoic acid in patients with type 2 diabetes mellitus, prediabetes, and normoglycaemia: A post hoc analysis of efficacy and glycaemic control using pooled data from phase 3 clinical trials. Diabetes Obes Metab. 2022;24(5):868‐880. doi: 10.1111/dom.14645
Funding information Esperion Therapeutics Inc.
DATA AVAILABILITY STATEMENT
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedures.
REFERENCES
- 1. Emerging Risk Factors Collaboration , Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375(9733):2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 10. Cardiovascular disease and risk management: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(Suppl 1):S125‐S150. [DOI] [PubMed] [Google Scholar]
- 3. Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285‐e350. [DOI] [PubMed] [Google Scholar]
- 5. Handelsman Y, Jellinger PS, Guerin CK, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm ‐ 2020 executive summary. Endocr Pract. 2020;26(10):1196‐1224. [DOI] [PubMed] [Google Scholar]
- 6. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111‐188. [DOI] [PubMed] [Google Scholar]
- 7. Smushkin G, Sathananthan M, Piccinini F, et al. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes. 2013;62(4):1094‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bays HE, Goldberg RB. The 'forgotten' bile acid sequestrants: is now a good time to remember? Am J Ther. 2007;14(6):567‐580. [DOI] [PubMed] [Google Scholar]
- 9. Collins PD, Sattar N. Glycaemic effects of non‐statin lipid‐lowering therapies. Curr Cardiol Rep. 2016;18(12):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bays HE, Roth EM, McKenney JM, et al. The effects of fenofibric acid alone and with statins on the prevalence of metabolic syndrome and its diagnostic components in patients with mixed dyslipidemia. Diabetes Care. 2010;33(9):2113‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldberg RB, Jacobson TA. Effects of niacin on glucose control in patients with dyslipidemia. Mayo Clin Proc. 2008;83(4):470‐478. [DOI] [PubMed] [Google Scholar]
- 12. Bays HE, Shah A, Lin J, McCrary Sisk C, Paolini JF, Maccubbin D. Efficacy and tolerability of extended‐release niacin/laropiprant in dyslipidemic patients with metabolic syndrome. J Clin Lipidol. 2010;4(6):515‐521. [DOI] [PubMed] [Google Scholar]
- 13. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375(9716):735‐742. [DOI] [PubMed] [Google Scholar]
- 14. Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38‐e81. [DOI] [PubMed] [Google Scholar]
- 15. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305(24):2556‐2564. [DOI] [PubMed] [Google Scholar]
- 16. Ference BA, Ray KK, Catapano AL, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019;380(11):1033‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144‐2153. [DOI] [PubMed] [Google Scholar]
- 18. Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG‐coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364(9435):685‐696. [DOI] [PubMed] [Google Scholar]
- 20. Pinkosky SL, Filippov S, Srivastava RA, et al. AMP‐activated protein kinase and ATP‐citrate lyase are two distinct molecular targets for ETC‐1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinkosky SL, Newton RS, Day EA, et al. Liver‐specific ATP‐citrate lyase inhibition by bempedoic acid decreases LDL‐C and attenuates atherosclerosis. Nat Commun. 2016;7:13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC‐1002, a novel investigational low‐density lipoprotein‐cholesterol‐lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34(3):676‐683. [DOI] [PubMed] [Google Scholar]
- 23. Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin‐intolerant patients with hypercholesterolemia: a randomized, placebo‐controlled study. Atherosclerosis. 2018;277:195‐203. [DOI] [PubMed] [Google Scholar]
- 24. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low‐density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322(18):1780‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022‐1032. [DOI] [PubMed] [Google Scholar]
- 27. Cicero AFG, Fogacci F, Hernandez AV, Banach M, Lipid and Blood Pressure Meta‐Analysis Collaboration (LBPMC) Group and the International Lipid Expert Panel (ILEP) . Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta‐analysis. PLoS Med. 2020;17(7):e1003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY phase 3 studies. Eur Heart J. 2016;37(39):2981‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941‐950. [DOI] [PubMed] [Google Scholar]
- 30. Banach M, Duell PB, Gotto AM Jr, et al. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol. 2020;5(10):1124‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359(21):2195‐2207. [DOI] [PubMed] [Google Scholar]
- 32. van Holten TC, Waanders LF, de Groot PG, et al. Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta‐analyses. PLoS One. 2013;8(4):e62080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahebkar A, Di Giosia P, Stamerra CA, et al. Effect of monoclonal antibodies to PCSK9 on high‐sensitivity C‐reactive protein levels: a meta‐analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol. 2016;81(6):1175‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bays HE, Banach M, Catapano AL, et al. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol. 2020;14(5):649‐659. [DOI] [PubMed] [Google Scholar]
- 35. Erqou S, Lee CC, Adler AI. Statins and glycaemic control in individuals with diabetes: a systematic review and meta‐analysis. Diabetologia. 2014;57(12):2444‐2452. [DOI] [PubMed] [Google Scholar]
- 36. Livingstone SJ, Looker HC, Akbar T, et al. Effect of atorvastatin on glycaemia progression in patients with diabetes: an analysis from the Collaborative Atorvastatin in Diabetes Trial (CARDS). Diabetologia. 2016;59(2):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313(10):1029‐1036. [DOI] [PubMed] [Google Scholar]
- 38. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328‐350. [DOI] [PubMed] [Google Scholar]
- 39. Nicholls S, Lincoff AM, Bays HE, et al. Rationale and design of the CLEAR‐outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104‐112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Shift Table for Glycaemic Control by Baseline Category (Crude Incidence)
Table S2. Safety overview
Data Availability Statement
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedures.


