Abstract
Background
The REStORing health of acutely unwell adulTs (RESORT) is an observational longitudinal cohort, including geriatric rehabilitation inpatients aged ≥65 years admitted to a geriatrician‐led rehabilitation service at a tertiary hospital. The aim of this study is to describe a home‐based bed‐substitution rehabilitation model for geriatric inpatients, including patient phenotype, and health outcomes at preadmission, admission, discharge, and three‐month follow‐up.
Methods
A standardized Comprehensive Geriatric Assessment was performed on admission and discharge, including demographics (home situation, cognitive impairment, medical diagnoses, etc.), frailty (Clinical Frailty Scale (CFS)), mobility (patient‐reported and Functional Ambulation Classification), physical performance (Short Physical Performance Battery (SPPB), handgrip strength), and functional independence (Activities of Daily Living (ADL), Instrumental ADL (IADL)). Service provision data (health care staff visits, length of stay (LOS), and negative events (e.g., falls)) were extracted from medical records. Three‐month outcomes included mobility, ADL and IADL scores, institutionalization, and mortality.
Results
Ninety‐two patients were included with a mean age of 81.1 ± 7.8 years, 56.5% female. Twenty‐nine (31.5%) patients lived alone, 39 (42.4%) had cognitive impairment and the commonest geriatric rehabilitation admission reason was falls (n = 30, 32.6%). Patients received care from nurses, physicians, and a median of four (interquartile range (IQR) 3–6) allied health disciplines for a median LOS of 13.0 days (IQR 10.0–15.0). On a population level, patient mobility and functional independence worsened from preadmission to admission. CFS, SPPB, ADL, and IADL scores improved from admission to discharge, and seven (7.6%) patients fell. At three‐month follow‐up, patient‐reported mobility was comparable to preadmission baseline, but functional independence (ADL, IADL) scores worsened for 27/69 (39.1%) and 28/63 (44.4%), respectively.
Conclusions
Hospitalization‐associated decline in mobility and functional independence improved at discharge and three‐months, but was not fully reversed in the multidisciplinary home‐based geriatric rehabilitation bed‐substitution service. Future research should compare outcomes to equivalent hospital‐based geriatric rehabilitation and evaluate patient perspectives.
Keywords: geriatrics, home care services, hospital‐based, hospital at home, rehabilitation
Short abstract
See related Editorial by William J. Hall in this issue.
Key points
Despite decreased mobility, cognitive impairment, and functional dependence on admission, most patients in our multidisciplinary geriatric home‐based service completed rehabilitation at home.
The loss of mobility and functional independence that occurred in association with hospitalization improved at three‐months but was not fully reversed.
Frequency of negative events, such as falls or admission to a hospital bed, was not unexpected.
Why does this paper matter?
Home‐based rehabilitation can offer patient‐centered care outside of hospital walls for older adults.
INTRODUCTION
Older adults are frequently deconditioned by acute illness and hospitalization and require rehabilitation, with the goal of reducing the associated posthospitalization functional decline, institutionalization, and mortality. 1 , 2 , 3 Aging populations and finite health care resources drive increased focus on health care delivered outside hospital walls. 4 , 5 Home‐based inpatient rehabilitation using a bed‐substitution model (‘home‐based rehabilitation’) is recognized as effective and cost‐efficient compared to hospital‐based rehabilitation for selected patients recovering from acute orthopedic conditions and stroke. 6 , 7 , 8 After hip fracture and knee or hip arthroplasty, home‐based rehabilitation models were equivalent or superior in terms of mobility, pain scores, quality of life, carer burden, hospital length of stay (LOS), complications, and health care costs. 9 , 10 , 11 , 12 , 13 Similarly, after acute stroke, there is little difference in mortality, readmission rates, or carer outcomes between home‐based and hospital‐based rehabilitation. 7 , 14
Home‐based postacute rehabilitation for geriatric inpatients with multiple medical problems has not been widely studied. 1 , 5 , 6 A randomized controlled trial (RCT) of 104 acutely hospitalized geriatric inpatients (mean age 83.9 years), reported lower delirium rates in the home‐based rehabilitation group compared to hospital‐based rehabilitation, whereas the functional independence measure was equivalent in both groups at six‐months postdischarge. 15 Qualitative investigation of rehabilitation service models, including home‐based rehabilitation (mean age 70 years), found patient and staff perceptions of more realistic therapy at home compared to hospital, and that home was more appropriate for culturally or linguistically diverse patients. 16 A retrospective chart review of home‐based rehabilitation patients (no age criteria, 70.6% aged ≥65 years) found that negative outcomes of hospital ward readmission or death occurred more frequently in older adults compared to younger adults. 17 However, this study did not report mobility, physical performance, or postdischarge outcomes, which are important considerations when evaluating hospital care for geriatric inpatients. 18 One retrospective study of a home‐based postacute rehabilitation service (mean age 84.2 years), found that most patients achieved their mobility goals and improved global function, and the authors provide a detailed description of the multidisciplinary care model. 19 However prehospital function, and longitudinal outcomes beyond 30 days, were not assessed.
In Australia, the challenge of caring for increasing numbers of older patients with complex health needs has fueled development of hospital‐led home‐based health services. The ideal model to provide rehabilitation to older patients, however, remains to be determined. Therefore, this study aimed to 1) describe a home‐based bed‐substitution geriatric rehabilitation model for geriatric inpatients, including patient phenotype, and 2) to describe changes in health outcomes at discharge and three‐month follow‐up, including mobility, physical performance, functional independence, institutionalization, and mortality, compared to preadmission and admission.
METHODS
Geriatric home‐based rehabilitation
The home‐based geriatric rehabilitation model is a geriatrician‐led postacute rehabilitation service based at a tertiary hospital in Melbourne, Australia. With a ‘bed’ capacity of up to 15 patients during the study period, the service is designed to substitute hospital‐based rehabilitation in the home.
Hospital‐based rehabilitation is the prevalent model for hospitalized older adults in Australia. Delivered in subacute hospital wards within geographically defined tertiary hospital networks, these public (i.e., nationally funded ‘Medicare’) services are free of charge to citizens and permanent residents. Geriatric (age ≥ 65 years)‐specific multidisciplinary care includes postacute rehabilitation and discharge service coordination. Private (i.e., user‐pay) rehabilitation hospitals are available, but usually do not offer the same breadth of multidisciplinary care and do not accept patients with complex rehabilitation and discharge needs.
Referrals to home‐based rehabilitation were accepted from acute inpatient hospitals, hospital‐based rehabilitation wards (providing they had ongoing rehabilitation goals), and the community (e.g., general practitioners). A senior nurse or medical staff with experience in geriatric rehabilitation and postacute care confirmed suitability. Eligibility criteria for admission were: complex, chronic, or multiple health conditions requiring multidisciplinary input, suitable home environment within the catchment area (travel time ≤30 min), suitable for the program as assessed by a geriatrician or rehabilitation physician, that is, medically stable, able to transfer and toilet safely (independently or with assistance from a carer who is always present), and adequate cognitive ability to participate in rehabilitation. Aged care home residents are ineligible due to government funding restrictions.
Patients received minimum daily visits from the multidisciplinary team, including nurses, physicians, physiotherapists, occupational therapists, allied health assistants, dietitians, social workers, and speech pathologists, coordinated by a senior nurse. Initial visits included development of individual rehabilitation goals and safety assessment of the home environment. Personalized home therapy programs, prescribed by physiotherapists and occupational therapists, were implemented by allied health assistants. Duration of therapy sessions was tailored to patient ability. Where safe to do so, patients were given exercises to be done independent of rehabilitation staff. Assistive medical equipment (e.g., over the toilet frames, bed sticks) could be provided, and referrals made to relevant community providers for recommended home modifications and ongoing equipment provision. Personal care assistants could be brokered (e.g., for showering assistance) and interpreters were available. Pathology collection in the home and transfer to imaging services could be arranged. A daily team ‘huddle’ reviewed day‐to‐day service provision and a weekly multidisciplinary case conference reviewed patient progress and care plans. On discharge from the service, tailored referrals are made to less‐intensive community rehabilitation programs and agencies providing in‐home care (e.g., gardening, home cleaning, showering).
Clinical phone support was available 24 h per day to patients and their carers. The home rehabilitation senior nurse and/or physician were available from 8 am to 5 pm. After 5 pm, a senior rehabilitation nurse or doctor was contactable but home visits were not available. Ambulance transfer to the affiliated tertiary hospital emergency department could be arranged if urgent assessment and/or admission to an acute or subacute hospital bed was required. Government funding regulations meant that patients were not eligible for a rebate to see their primary care physician (i.e., general practitioner) while admitted to the hospital‐affiliated home rehabilitation service.
Study design
The REStORing health of acutely unwell adulTs (RESORT) is a multicenter, longitudinal, observational cohort of geriatric rehabilitation patients using a standardized multidisciplinary Comprehensive Geriatric Assessment (CGA) on admission to and discharge from geriatric rehabilitation at a tertiary hospital. Three‐months postdischarge, patients were telephoned by a researcher for follow‐up assessment. If the patient or their proxy were not contactable, medical records were reviewed. These assessments were implemented in the home‐based bed‐substitution rehabilitation service in August 2019. Patients admitted to home‐based rehabilitation were recruited between 26th August 2019 and 18th March 2020. Recruitment closed when the coronavirus disease of 2019 (COVID‐19) pandemic began impacting service delivery and study activities.
Home‐based geriatric rehabilitation patients were included if they were aged ≥65 years, or, if they were aged <65 years and had an age‐related condition. Patients were excluded if they were palliative at admission or if they could not consent and had no proxy to consent on their behalf. Discharge measures were not available if patients were discharged unplanned (e.g., transfer to hospital), they declined to complete assessments when health care staff visited, or, if patients had already met their rehabilitation goals, and therefore, did not receive discharge assessments. Ethics approval was granted by the local Human Research Ethics Committee (HREC/17/MH/103), and written consent was obtained from all patients or their nominated proxy.
Patient characteristics
Baseline demographic information was collected on admission using surveys with patients and/or carers or extracted from medical records, including age, sex, living situation before hospital admission, receipt of formal home services, and language spoke at home.
Disease and frailty
Physicians recorded morbidity using the Charlson Comorbidities Index (CCI), a weighted summary measure combining 19 comorbidities. 20 Physicians and nurses assessed frailty using the Clinical Frailty Scale (CFS) on rehabilitation admission and discharge, which rates patients from ‘very fit’ (score = 0) to ‘terminally ill’ (score = 9). 21 Cognitive impairment was defined as a composite of diagnosed dementia, cognitive impairment, or abnormal testing (score <24 on the standardized Mini‐Mental State Examination (MMSE), 22 <26 points on the Montreal Cognitive Assessment (MoCA), 23 or <23 on the Rowland Universal Dementia Assessment Scale (RUDAS) 24 ). Nurses administered the Malnutrition Screening Tool (MST), on a scale from zero to five with higher scores indicating higher malnutrition risk. 25
Reasons for hospital admission
The patient's primary reason for hospital admission was classified as surgical or nonsurgical, and into the following categories: musculoskeletal, neurological, cardiac, psychiatric, respiratory, and other. The reasons for geriatric rehabilitation admission included all active medical issues requiring management during home‐based rehabilitation (i.e., patients may have multiple diagnoses) and included the following categories: musculoskeletal, neurological, cardiac, psychiatric, respiratory, endocrine/metabolic/breast, hematological, vascular, genitourinary, and other. Subcategories used are given in Table 1, and the commonest condition in each is presented, and any condition affecting five or more patients.
TABLE 1.
Patient characteristics
| Variable | N | Total |
|---|---|---|
| Demographics | ||
| Age, years, mean (SD) | 92 | 81.1 (7.8) |
| Female, n (%) | 92 | 52 (56.5) |
| Living situation | ||
| Alone, n (%) | 92 | 29 (31.5) |
| With partner, n (%) | 92 | 41 (44.6) |
| With children, n (%) | 92 | 18 (19.6) |
| Receives formal home services, n (%) | 92 | 36 (39.1) |
| English spoken at home, n (%) | 86 | 45 (52.3) |
| Disease and frailty | ||
| CCI score, median (IQR) | 92 | 2 (1–3) |
| Clinical frailty scale score, median (IQR) | 68 | 5 (5–6) |
| Cognitive impairment, n (%) | 92 | 39 (42.4) |
| MST score, median (IQR) | 90 | 0 (0–2) |
| Primary reason for hospital admission | 92 | |
| Surgical, n (%) | 35 (38.0) | |
| Nonsurgical, n (%) | 57 (62.0) | |
| Musculoskeletal, n (%) | 44 (47.8) | |
| Neurological, n (%) | 15 (16.3) | |
| Cardiac, n (%) | 11 (12.0) | |
| Psychiatric, n (%) | 6 (6.5) | |
| Respiratory, n (%) | 5 (5.4) | |
| Other*, n (%) | 11 (12.0) | |
| Reasons for geriatric rehabilitation admission § | 92 | |
| Musculoskeletal, n (%) | 66 (71.7) | |
| Fall(s) | 30 (32.6) | |
| Functional decline | 27 (29.3) | |
| Fracture | 24 (26.1) | |
| Femur | 9 (9.8) | |
| Rib | 4 (4.3) | |
| Humerus | 4 (4.3) | |
| Other || | 16 (17.4) | |
| Joint replacement | 6 (6.5) | |
| Total hip joint replacement | 3 (3.3) | |
| Total knee joint replacement | 3 (3.3) | |
| Psychiatric, n (%) | 28 (30.4) | |
| Delirium | 15 (16.3) | |
| Dementia or cognitive impairment | 13 (14.1) | |
| Neurological, n (%) | 16 (17.4) | |
| Stroke | 11 (12.0) | |
| Cardiac, n (%) | 16 (17.4) | |
| Heart failure | 11 (12.0) | |
| Respiratory, n (%) | 14 (15.2) | |
| Community‐acquired pneumonia | 5 (5.4) | |
| Endocrine/metabolic/breast, n (%) | 11 (12.0) | |
| Malnutrition | 3 (3.3) | |
| Hematological, n (%) | 9 (9.8) | |
| Vascular, n (%) | 8 (8.7) | |
| Genitourinary, n (%) | 6 (6.5) | |
| Other a , n (%) | 11 (12.0) | |
| Mobility and physical performance | ||
| Able to walk | 92 | 91 (98.9) |
| Gait aid used, n (%) | 92 | 77 (83.7) |
| Difficulty climbing a flight of stairs, n (%) | 81 | 70 (86.4) |
| Difficulty walking 100 m, n (%) | 88 | 66 (75.0) |
| FAC score, median (IQR) | 85 | 4 (3–4) |
| Fall (≥1) in the past year, n (%) | 89 | 57 (62.0) |
| SPPB score, median (IQR) | 71 | 4 (3–7) |
| Handgrip strength female (kg), median (IQR) | 36 | 16.5 (12.2–18.8) |
| Handgrip strength male (kg), median (IQR) | 40 | 26.7 (20.0–32.0) |
| Functional independence | ||
| ADL, median (IQR) | 91 | 5.0 (4.0–6.0) |
| IADL, median (IQR) | 89 | 4.0 (2.0–5.0) |
Abbreviations: ADL, activities of daily living; CCI, Charlson comorbidities index; FAC, functional ambulation classification; IADL, instrumental activities of daily living; IQR, interquartile range; MST, malnutrition screening tool; SD, standard deviation; SPPB, short physical performance battery.
Other primary reasons for hospital admission were vascular (n = 3), infection (n = 3), gastrointestinal (n = 3), metabolic (n = 1), and hematological (n = 1).
Reasons for geriatric rehabilitation admission: active medical issues requiring management during the geriatric rehabilitation admission; each patient may have multiple reasons, and this does not record all patient comorbidities.
Other types of fracture were radius (n = 3), spine (n = 3), pelvis (n = 2), fibula (n = 1), tibia (n = 2), metacarpophalangeal (n = 1), skull (n = 1), clavicle (n = 1), knee (n = 1), and foot (n = 1).
Other reasons for geriatric rehabilitation admission were renal (n = 5), gastrointestinal (n = 5), and cancer (n = 1); no patients had a hepatic and pancreatic OR ophthalmological condition.
Mobility and physical performance
Patient‐reported mobility measures of walking ability, gait aid use, difficulty climbing a flight of stairs, and difficulty walking 100 meters were recorded for preadmission, admission, discharge, and three‐month follow‐up. Falls within 12 months before hospital admission were also recorded. Preadmission was defined as two weeks prior to hospitalization for all variables.
On rehabilitation admission and discharge, physiotherapists and/or allied health assistants assessed mobility with the Functional Ambulation Classification (FAC), scored from zero to five with higher scores indicating greater ambulation independence, 26 and physical performance using the Short Physical Performance Battery (SPPB), scored from zero to twelve points with higher scores representing superior performance, 27 and handgrip strength (HGS) measured in kilograms with a handheld dynamometer (JAMAR, Sammons Preston, Inc., Bolingbrook, IL, USA) of which the maximum value of three attempts was used. 28 , 29
Functional independence
Occupational therapists assessed Activities of Daily Living (ADL) using the Katz Index, with scores ranging from zero to six, and Instrumental ADLs (IADLs) using the Lawton and Brody scale, with scores ranging from zero to eight, for preadmission, rehabilitation admission, and discharge. Researchers collected these by telephone at three‐month follow‐up. Higher scores indicate higher levels of independence for both scales, and a change of one point is clinically significant. 30 , 31
ADL and IADL scores were also categorized into independent (ADL score = 6, IADL score = 8) or dependent (ADL score = 0–5, IADL score = 0–7). Lower scores indicate dependence in multiple domains.
Change in health outcomes
Change between preadmission, admission, discharge, and three‐month follow‐up for CFS, FAC, SPPB, ADL, and IADL scores were categorized into ‘improved’, ‘unchanged’, or ‘worsened’. One point or greater change was considered clinically meaningful, with an increase in score for FAC, SPPB, ADL, and IADL representing improvement, whereas a decrease in CFS was ‘improved’. ‘Worsened’ category included patients with a lower score for FAC, SPPB, ADL, and IADL, or a higher CFS score.
Service provision and negative events
LOS during acute hospitalization, hospital‐based rehabilitation, and home‐based rehabilitation were extracted from medical records. Number and discipline of health care staff visits during home‐based rehabilitation were recorded, including the number of different allied health disciplines (physiotherapists, occupational therapists, allied health assistants, dieticians, social workers, and speech pathologists).
Medical records were reviewed for prespecified ‘negative events’ relevant to hospitalized geriatric inpatients during home‐based rehabilitation: falls, infection (new or worsening), delirium (new or worsening), pressure injury, venous thromboembolism, emergency department attendance without transfer to an inpatient hospital bed, transfer to an inpatient hospital bed, and death. Number of patients experiencing negative events, and each type of negative event, were determined.
Other three‐month outcomes
Telephone follow‐up assessment by researchers determined mortality, living situation, receipt of formal home services, institutionalization, and falls.
Statistical analysis
Descriptive statistics were used to analyze patient characteristics and changes in health outcomes using Statistical Package for the Social Sciences (SPSS) (IBM SPSS Advanced Statistics 24.0, Armonk, NY, IBM Corp). Categorical variables were presented as frequencies (n) with percentages (%). Normally distributed continuous variables were reported as means with standard deviations (SDs). Medians with interquartile ranges (IQR) were reported for continuous variables with skewed distribution.
Graphs were produced using Plotly (Plotly Technologies Inc., Montreal, Québec, Canada) (box plots) and GraphPad Prism version 9.0.2 for Windows (GraphPad Software, San Diego, California USA) (all other graphs).
RESULTS
Out of 127 eligible patients admitted to home‐based rehabilitation, 92 (72.4%) consented to particiapte in the evaluation and were included in the study (Figure 1). Their mean age was 81.1 ± 7.8 years, 52 (56.5%) were female, 29 (31.5%) lived alone, and 36 (39.1%) received formal home services (Table 1). Forty‐five patients (52.3%) spoke English at home, 57 (62.0%) reported fall(s) in the year prior, and 39 (42.4%) had cognitive impairment.
FIGURE 1.
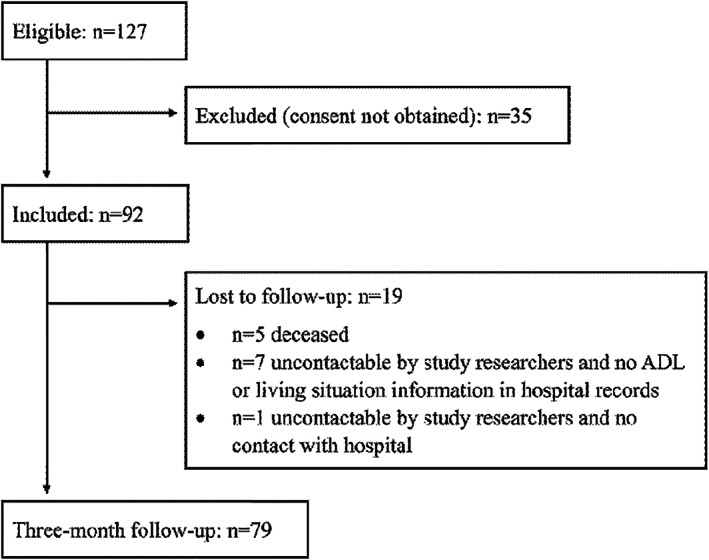
Patient recruitment and follow‐up
The commonest reason for hospital admission was classified as nonsurgical (n = 57, 62.0%), and the commonest category was musculoskeletal (n = 44, 47.8%) (Table 1). The commonest reason for home‐based rehabilitation admission was musculoskeletal (n = 66, 71.7%), of which falls (n = 30, 32.6%), was the most prevalent sub‐category. Psychiatric (n = 28, 30.4%) was the next most common reason, containing delirium (n = 15, 16.3%) and dementia or cognitive impairment (n = 13, 14.1%).
Change in mobility, physical performance, functional independence, and frailty
During home‐based rehabilitation, median CFS and SPPB scores improved (CFS admission: 5 (IQR 5–6), discharge: 4 (IQR 3–6), SPPB admission: 4 (IQR 3–7), discharge: 5.5 (IQR 4–8)) (Figure S1), and over half of patients individually improved (CFS 32/56, 57.1%; SPPB 37/55, 67.5%) (Figure 2A). Median HGS was stable between admission (female: 16.5 kg (12.2–278 18.8 kg), male: 26.7 kg (20.0–32.0 kg)) and discharge (female: 17.8 kg (15.2–20.8 kg), male: 25.2 kg (19.2–31.9 kg)).
On a population level, patient‐reported mobility was worse on admission to geriatric rehabilitation compared to two weeks prehospital admission. This improved at discharge and was close to preadmission results at three‐months (Figure 3A). Median FAC score was stable from admission (4 (IQR3‐4)) to discharge (4 (IQR 4–5)). On an individual level, over half (45/79, 57%) of patients did not change in FAC score, 25/79 (31.6%) improved, and 9/79 (11.4%) worsened.
FIGURE 3.
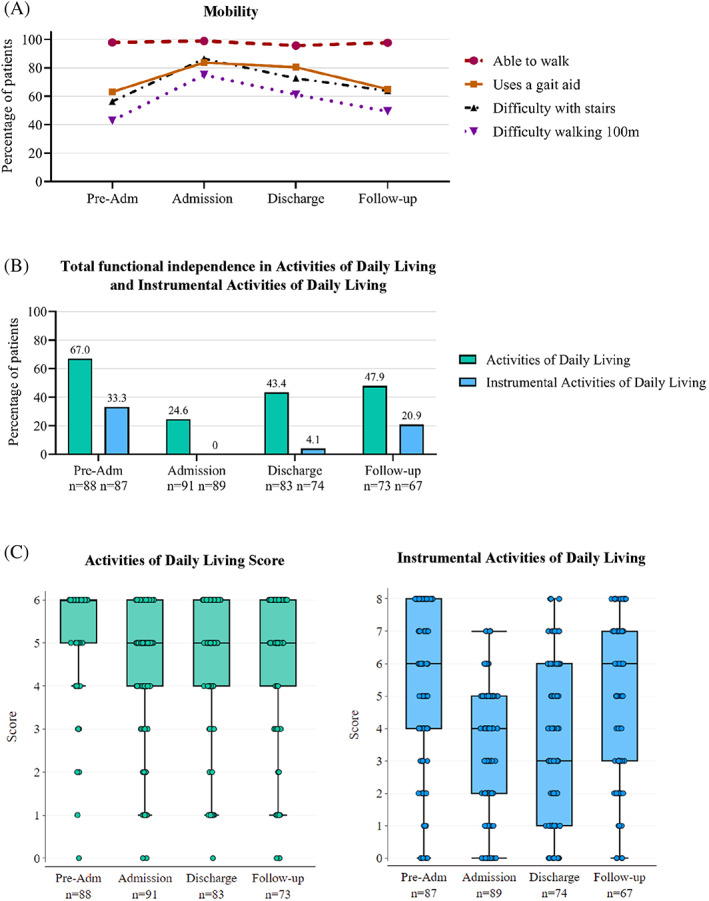
Home‐based rehabilitation patient mobility (A), total functional independence (B) and functional independence scores (C) from preadmission to 3‐month follow‐up. Pre‐Adm: two weeks preadmission to hospital; Admission: admission to home‐based rehabilitation; Discharge: discharge from home‐based rehabilitation; Follow‐up: 3‐month follow‐up postdischarge from home‐based rehabilitation. (A) Patient‐reported mobility. (B) Percentage of patients with total functional independence measured by Activities of Daily Living (ADL and Instrumental Activities of Daily Living (IADL) at each study time point. Patients were considered independent in ADLs and IADLs if no points were lost, that is, ADL score = 6, IADL score = 8. 2.C. Patient functional independence level measured by ADL (0–6) and IADL (0–8) score at each study time point. Median scores are represented within boxes by horizontal lines, and limits of the interquartile range are represented by the top and bottom box borders. Points represent individuals
The median ADL and IADL scores were lower at admission (ADL: 5 (IQR 4–6), IADL: 4 (IQR 2–5)) compared to preadmission (ADL: 6 (IQR 5–6), IADL: 6 (IQR 4–8)), did not increase during home‐based rehabilitation, and only the median IADL score had returned to preadmission level at three‐months (ADL: 5 (IQR 4–6), IADL: 6 (IQR 3–7)) (Figure 3C). The percentage of patients with complete functional independence were highest preadmission (ADL: 59/88 (67.0%), IADL: 29/87 (33.3%)), lowest on admission (ADL: 24/91 (26.4%), IADL: 0/89 (0.0%)), and higher at discharge (ADL: 36/83 (43.4%), IADL: 3/74 (4.1%)), and 293 three‐month follow‐up (ADL: 35/73 (47.9%), IADL: 14/67 (20.9%)) (Figure 3B).
On admission to rehabilitation, ADL and IADL scores had worsened for 52/87 (58.6%) and 66/87 (75.9%) patients, respectively, compared to preadmission (Figure 2B). At discharge, ADL ability was unchanged from preadmission levels for 41/79 (51.9%) patients, and 31/79 (39.2%) worsened. IADL independence was worse for 50/71 (70.4%) patients at discharge and 28/73 (44.4%) patients at three‐month follow‐up, compared to preadmission. When compared to admission scores, most frequent patients had improved IADLs both at discharge (28/73, 38.4%) and three‐month follow‐up (33/54, 61.1%).
FIGURE 2.
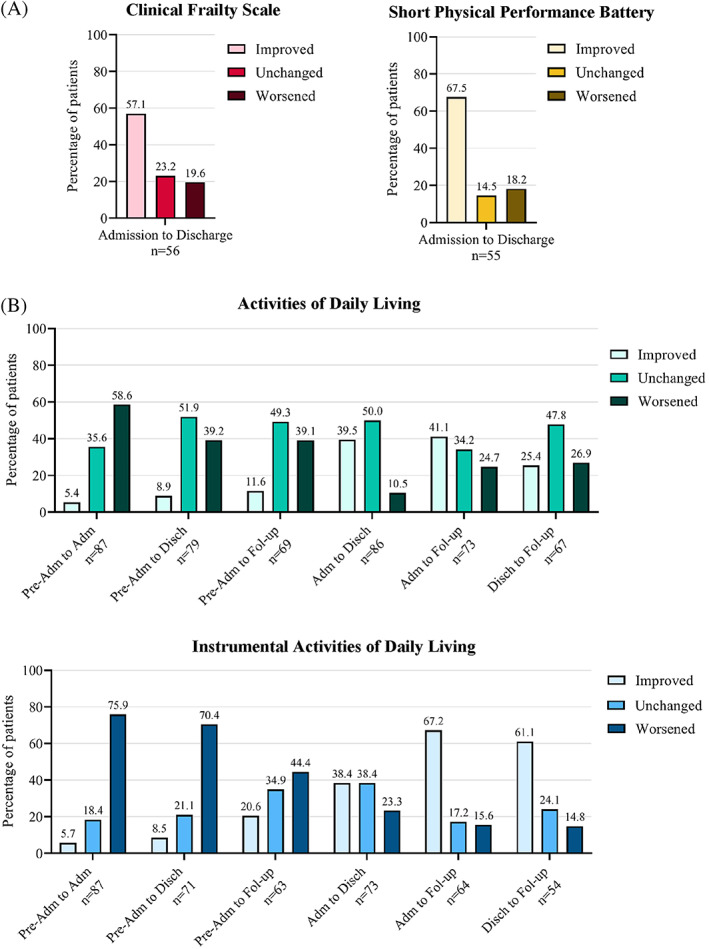
Change in home‐based rehabilitation patient frailty and physical performance from admission to discharge (A), and change in functional independence from preadmission to 3‐month follow‐up (B). Pre‐Adm: two weeks preadmission to hospital; Adm: admission to home‐based rehabilitation; Disch: discharge from home‐based rehabilitation; Fol‐up: 3‐month follow‐up postdischarge from home‐based rehabilitation. Patients were considered improved or worsened in the measured ability if their score improved or worsened by ≥1 point between the specified study time points. Patients were considered unchanged in the measured ability if there was no change in score. (A) Frailty measured by the Clinical Frailty Scale and physical performance measured by the Short Physical Performance Battery. (B) Functional independence measured by Activities of Daily Living and Instrumental Activities of Daily Living scores
Service provision and negative events
Median LOS in home‐based rehabilitation was 13.0 (IQR 10.0–15.0) days (Table 2). Prior to home‐based rehabilitation, 90 (97.8%) patients were admitted to an acute hospital bed for a median of 6.0 (IQR 4.0–12.3) days, and 50 (54.3%) patients spent a median of 15.0 (IQR 8.0–24.0) days in hospital‐based rehabilitation before being admitted to home‐based rehabilitation.
TABLE 2.
Geriatric home‐based rehabilitation service provision: length of stay, multidisciplinary care, negative events, and three‐month outcomes
| Variable | n | Total |
|---|---|---|
| Length of stay, days, median (IQR) | ||
| Acute hospital care* | 90 | 6.0 (4.0–12.3) |
| Home‐based geriatric rehabilitation | 92 | 13.0 (10.0–15.0) |
| Hospital‐based rehabilitation | 50 | 15.0 (8.0–24.0) |
| Total inpatient hospital (acute and rehabilitation wards)* | 90 | 16.0 (6.8–28.0) |
| Total admission (acute hospital and hospital‐based rehabilitation and home‐based rehabilitation) | 92 | 29.0 (20.0–40.5) |
| Multidisciplinary health care, number of visits, median (IQR) | ||
| Number of visits per day (any health care staff) | 92 | 1.6 (1.5–1.8) |
| Number of visits per week (any health care staff) | 92 | 11.4 (10.5–12.8) |
| Number of health care staff visits per week, by discipline | ||
| Nurse | 92 | 6.0 (5.4–6.7) |
| Physician † | 88 | 0.6 (0.5–0.8) |
| Physiotherapist | 91 | 1.6 (1.2–2) |
| Occupational therapist | 87 | 1.2 (0.8–1.6) |
| Allied health assistant | 79 | 1.7 (1.2–2.4) |
| Dietitian | 36 | 0.7 (0.5–1.0) |
| Social worker | 32 | 0.8 (0.5–1.2) |
| Speech pathologist | 5 | 0.6 (0.5–1.6) |
| Number of different allied health disciplines ‡ | 92 | 4 (3–6) |
| Negative events, n (%) | ||
| Patients who experienced any negative event(s) | 92 | 22 (23.9) |
| Single negative event | 15 (16.3) | |
| Multiple negative events (≥2 events) | 7 (7.6) | |
| Fall | 92 | 7 (7.6) |
| Single fall | 5 (5.4) | |
| Multiple falls (≥2 falls) | 2 (1.2) | |
| Fall‐related injury | 1 (1.1) | |
| Infection (new or worsening) | 92 | 4 (4.4) |
| Delirium (new or worsening) | 92 | 2 (2.2) |
| Developed prior to admission | 1 (1.1) | |
| Developed during admission | 1 (1.1) | |
| Pressure injury | 92 | 1 (1.1) |
| Venous thromboembolism | 92 | 0 (0) |
| Emergency department attendance | 92 | 3 (3.3) |
| Transfer to an inpatient hospital bed | 92 | 13 (14.1) |
| Acute hospital | 11 (12.0) | |
| Hospital‐based geriatric rehabilitation | 2 (2.2) | |
| Other | 92 | 1 (1.1) |
| Death | 92 | 0 (0) |
| Three‐month outcomes, n (%) | ||
| Deceased | 91 | 5 (5.4) |
| Living at home | 79 | 75 (81.5) |
| Living alone | 27 (29.3) | |
| Institutionalized (living in residential aged care home) | 79 | 4 (4.3) |
| Fall | 69 | 11 (12.0) |
| Single fall | 7 (7.6) | |
| Multiple falls (≥2 falls) | 4 (4.3) | |
Abbreviation: IQR, interquartile range.
Two patients were admitted to home‐based rehabilitation direct from the community; therefore, total inpatient hospital length of stay is relevant to 90 patients.
Four patients were not seen by a home‐based rehabilitation physician, as they were recently reviewed by another physician and the home‐based rehabilitation team judged that their physician review was not required.
Number of different allied health disciplines includes physiotherapists, occupational therapists, allied health assistants, dietitians, social workers, and speech pathologists.
Patients received visits from a median of 1.6 (IQR1.5–1.8) health care staff daily. All were seen by a nurse, 88 (95.7%) by a physician, and were visited by a median of 4 (IQR 3–6) different allied health disciplines. Patients received nurse visits a median of 6 (IQR 5.4–6.7) times per week during their admission, followed by allied health assistants (1.7 (IQR 1.2–2.4) visits), physiotherapists (1.6 (IQR 1.2–2) visits), and occupational therapists (1.2 (IQR 0.8–1.6) visits).
Transfer to an inpatient hospital bed (n = 13, 14.1%) was the commonest negative event during rehabilitation. Seven (7.6%) patients fell, with one (1.1%) sustaining a fall‐related injury, which was managed in their home.
Other three‐month outcomes
Three‐months after discharge from rehabilitation, five (5.4%) patients were deceased (Table 2). Seventy‐nine (85.9%) lived at home, 27 (29.3%) lived alone, and four (4.3%) had entered residential aged care. Eleven (12.0%) reported at least one fall since discharge.
DISCUSSION
On admission to home‐based rehabilitation, patients were predominantly in their 8th and 9th decade of life, frail, had multiple comorbidities, including cognitive impairment, low muscle strength, and dependency in ADLs and IADLs. Despite this phenotype, most patients completed their rehabilitation at home and improved their mobility, physical performance, functional independence, and frailty scores. This challenges some assumptions of hospital ward‐based clinicians about patient suitability for home‐based inpatient bed‐substitution care. In a study of hospitalized inpatients, ward‐based clinicians most frequently reported functional and cognitive disability (63.5%), and inappropriate home environment or social supports (45.2%), as barriers to their patients receiving home‐based bed‐substitution care. 32 However, clinicians experienced in home‐based care identified many more suitable patients than their ward‐based colleagues.
Improvement in functional independence and frailty achieved in home‐based rehabilitation may also reduce health care system costs, as poor performance in these variables has been associated with higher health care costs after hospital discharge for older adults. 18 Advanced age and the potential for geriatric syndromes, including cognitive dysfunction, has been proposed as a contributor to negative outcomes during home‐based rehabilitation. 17 The frequency of patient transfer to a hospital bed from home‐based rehabilitation was higher in our cohort than in an Australian retrospective chart review (6.3%), however, their service did not include nurses and patients were younger (29.4% were aged ≤65 years), and therefore, patients were likely less frail or dependent. 17 A demographically similar (mean age 84.2 years, >100 different diagnostic related groups, mean LOS 14.2 days) American home‐based postacute rehabilitation service, however, reported a similar rate of patients requiring Emergency Department review without hospital admission (2.5%) and readmission to a hospital bed (11.8%). 19
A fall was the commonest reason for admission to home‐based rehabilitation—an important finding given the significant association of falls with morbidity, mortality, and new institutionalization for older adults. 33 Falls prevalence in the previous 12 months was also higher in our cohort than the falls rate in community‐dwelling older adults (~34% in people ≥85 years old), 34 and in another home‐based rehabilitation cohort where only 4.9% of patients reported fall(s). 17 A RCT comparing home‐based rehabilitation to hospital‐based rehabilitation in older patients after a fall with hip fracture found no difference in falls rates at four‐month follow‐up (18%). 10 Falls rate between discharge and three‐month follow‐up in our study was lower, possibly affected by one‐month shorter follow‐up period, and was comparable to another study of three‐month outcomes for acutely hospitalized older adults. 35 Surprisingly, falls during rehabilitation were not reported in any of the home‐based rehabilitation studies discussed. 10 , 15 , 16 , 17 , 19 However there is objective evidence that older adults are more active when they receive rehabilitation at home instead of hospital, 36 which may predispose to falls. Future home‐based rehabilitation studies, including older adults must include falls reporting, and service provision should include falls prevention and management.
Our service provided true multidisciplinary health care in the home, with patients receiving care from a nurse, physician, and four different allied health disciplines. This enabled integration of standardized assessments into routine care. Nurses formed the backbone of our program as the most frequent visitors to patients; this allowed us to care for a cohort with high rates of functional dependency. Other studies have not described their patients in similar detail for direct comparison, however. 10 , 15 , 16 , 17
Three‐months after discharge, patients had improved functional independence and mobility compared to their home‐based rehabilitation admission status but did not return to preadmission levels. This contrasts a study of similarly aged acutely hospitalized patients, which found comparable rates of ADL and IADL independence at hospital admission and three‐months postdischarge. 37 However, lower prevalence of cognitive impairment (17.6%) and absence of need for hospital‐based rehabilitation indicates different patient characteristics to our cohort. Persistence of increased dependency three‐months after discharge highlights the need to offer long‐term support services to hospitalized older adults, and to identify, which strategies are most effective in recovering preadmission function long‐term.
Our broad study inclusion criteria and noninterventional study design were highly acceptable to patients, which met our primary aim of describing the home‐based rehabilitation population. Loss of choice about where care is received has been identified as a barrier to recruiting hospitalized patients, especially geriatric populations, to RCTs of home‐based rehabilitation. 9 , 38 There are limitations, however, to the conclusions that can be drawn from our study. As an observational study, effectiveness and safety of home‐based rehabilitation compared to hospital‐based rehabilitation for geriatric inpatients cannot be determined. Cost and patient and carer perspectives, were also not captured. The single‐center location and relatively small sample size limited our ability to perform statistical tests, which might influence the conclusions; however, we contribute to the groundwork needed for future research into this seldom‐studied model of bed‐substitution home‐based rehabilitation.
In summary, despite frailty, decreased mobility, cognitive impairment, and increased functional dependence on admission, most patients in our multidisciplinary home‐based rehabilitation service completed rehabilitation at home, and frequency of negative events, such as falls, was not unexpected. The loss of mobility and functional independence that occurred in association with hospitalization improved at three‐months but was not fully reversed. Comparison with a matched group of patients in hospital‐based geriatric rehabilitation is required to determine if home‐based rehabilitation is a true bed‐substitution model. Understanding the patient and carer perspective, cost analysis, and longer‐term outcomes should be evaluated in future studies.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All authors have read and approved the submission of this manuscript. All authors contributed to study concept and design. Paula M. Loveland, Esmee M. Reijnierse, and Louis Island contributed to acquisition of data. Analysis and interpretation of data were performed by all authors. Paula M. Loveland and Esmee M. Reijnierse drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content.
SPONSOR'S ROLE
This research was supported by an unrestricted grant from the University of Melbourne (unrestricted grant received by Prof. Andrea B. Maier), and the Medical Research Future Fund) provided by the Melbourne Academic Centre for Health. None of these organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Figure S1. Home‐based rehabilitation patient frailty and physical performance from admission to discharge. Home‐based rehabilitation patient frailty and mobility scores at admission to, and discharge from, home‐based rehabilitation. Median scores are represented in within boxes by horizontal lines, and limits of the interquartile range are represented by the top and bottom box borders. Points represent individuals.
ACKNOWLEDGMENTS
The authors thank the multidisciplinary team members of the Royal Melbourne Hospital, particularly the RMH@Home subacute team involved in the RESORT cohort and the @AgeMelbourne team for their role in the data collection.
Loveland PM, Reijnierse EM, Island L, Lim WK, Maier AB. Geriatric home‐based rehabilitation in Australia: Preliminary data from an inpatient bed‐substitution model. J Am Geriatr Soc. 2022;70(6):1816‐1827. doi: 10.1111/jgs.17685
Funding information This work was supported by an unrestricted grant from the University of Melbourne (unrestricted grant received by Prof. Andrea B. Maier), and the Medical Research Future Fund) provided by the Melbourne Academic Centre for Health).
NB: parts of this manuscript data were presented in poster format at the Australia and New Zealand Society of Geriatric Medicine Annual Scientific Meeting held 19th–21st May in Melbourne, Australia.
See related Editorial by William J. Hall in this issue.
REFERENCES
- 1. Kortebein P. Rehabilitation for hospital‐associated deconditioning. Am J Phys Med Rehabil. 2009;88(1):66‐77. doi: 10.1097/PHM.0b013e3181838f70 [DOI] [PubMed] [Google Scholar]
- 2. Kosse NM, Dutmer AL, Dasenbrock L, Bauer JM, Lamoth CJC. Effectiveness and feasibility of early physical rehabilitation programs for geriatric hospitalized patients: a systematic review. BMC Geriatr. 2013;13:107. doi: 10.1186/1471-2318-13-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartley P, Romero‐Ortuno R, Wellwood I, Deaton C. Changes in muscle strength and physical function in older patients during and after hospitalisation: a prospective repeated‐measures cohort study. Age Ageing. 2021;50(1):153‐160. doi: 10.1093/ageing/afaa103 [DOI] [PubMed] [Google Scholar]
- 4. DeCherrie LV, Wajnberg A, Soones T, et al. Hospital at Home‐Plus: a platform of facility‐based care. J Am Geriatr Soc. 2019;67(3):596‐602. doi: 10.1111/jgs.15653 [DOI] [PubMed] [Google Scholar]
- 5. Ward D, Drahota A, Gal D, Severs M, Dean TP. Care home versus hospital and own home environments for rehabilitation of older people. Cochrane Database Syst Rev. 2008;(4):CD003164. doi: 10.1002/14651858.CD003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stolee P, Lim SN, Wilson L, Glenny C. Inpatient versus home‐based rehabilitation for older adults with musculoskeletal disorders: a systematic review. Clin Rehabil. 2011;26(5):387‐402. doi: 10.1177/0269215511423279 [DOI] [PubMed] [Google Scholar]
- 7. Langhorne P, Baylan S. Early supported discharge services for people with acute stroke. Cochrane Database Syst Rev. 2017;7(7):CD000443. doi: 10.1002/14651858.CD000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karlsson A, Berggren M, Gustafson Y, Olofsson B, Lindelöf N, Stenvall M. Effects of geriatric interdisciplinary home rehabilitation on walking ability and length of hospital stay after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2016;17(5):464‐464.e15. doi: 10.1016/j.jamda.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 9. Naylor JM, Hart A, Mittal R, Harris I, Xuan W. The value of inpatient rehabilitation after uncomplicated knee arthroplasty: a propensity score analysis. Med J Aust. 2017;207(6):250‐255. doi: 10.5694/mja16.01362 [DOI] [PubMed] [Google Scholar]
- 10. Crotty M, Whitehead CH, Gray S, Finucane PM. Early discharge and home rehabilitation after hip fracture achieves functional improvements: a randomized controlled trial. Clin Rehabil. 2002;16(4):406‐413. doi: 10.1191/0269215502cr518oa [DOI] [PubMed] [Google Scholar]
- 11. Buhagiar MA, Naylor JM, Harris IA, et al. Effect of inpatient rehabilitation vs a monitored home‐based program on mobility in patients with Total knee arthroplasty: the HIHO randomized clinical trial. JAMA. 2017;317(10):1037‐1046. doi: 10.1001/jama.2017.1224 [DOI] [PubMed] [Google Scholar]
- 12. Berggren M, Karlsson Å, Lindelöf N, et al. Effects of geriatric interdisciplinary home rehabilitation on complications and readmissions after hip fracture: a randomized controlled trial. Clin Rehabil. 2019;33(1):64‐73. doi: 10.1177/0269215518791003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahomed NN, Davis AM, Hawker G, et al. Inpatient compared with home‐based rehabilitation following primary unilateral total hip or knee replacement: a randomized controlled trial. J Bone Joint Surg Am. 2008;90(8):1673‐1680. doi: 10.2106/JBJS.G.01108 [DOI] [PubMed] [Google Scholar]
- 14. Gonçalves‐Bradley DC, Iliffe S, Doll HA, et al. Early discharge hospital at home. Cochrane Database Syst Rev. 2017;6, 2021 (6):CD000356‐CD. doi: 10.1002/14651858.CD000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caplan GA, Coconis J, Board N, Sayers A, Woods J. Does home treatment affect delirium? A randomised controlled trial of rehabilitation of elderly and care at home or usual treatment (the REACH‐OUT trial). Age Ageing. 2006;35(1):53‐60. doi: 10.1093/ageing/afi206 [DOI] [PubMed] [Google Scholar]
- 16. Dow B, Black K, Bremner F, Fearn M. A comparison of a hospital‐based and two home‐ based rehabilitation programmes. Disabil Rehabil. 2007;29(8):635‐641. doi: 10.1080/09638280600902760 [DOI] [PubMed] [Google Scholar]
- 17. Bharadwaj S, Bruce D. Effectiveness of 'rehabilitation in the home' service. Aust Health Rev. 2014;38(5):506‐509. doi: 10.1071/AH14049 [DOI] [PubMed] [Google Scholar]
- 18. Ribbink ME, van Seben R, Reichardt LA, et al. Determinants of post‐acute care costs in acutely hospitalized older adults: the hospital‐ADL study. J Am Med Dir Assoc. 2019;20(10):1300‐1306.e1. doi: 10.1016/j.jamda.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 19. Augustine MR, Davenport C, Ornstein KA, et al. Implementation of post‐acute rehabilitation at home: a skilled nursing facility‐substitutive model. J Am Geriatr Soc. 2020. Jul;68(7):1584‐1593. doi: 10.1111/jgs.16474 [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 21. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489‐495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 24. Storey JE, Rowland JT, Basic D, Conforti DA, Dickson HG. The Rowland universal dementia assessment scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr. 2004;16(1):13‐31. doi: 10.1017/s1041610204000043 [DOI] [PubMed] [Google Scholar]
- 25. Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15(6):458‐464. doi: 10.1016/s0899-9007(99)00084-2 [DOI] [PubMed] [Google Scholar]
- 26. Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl‐Baker L. Clinical gait assessment in the neurologically impaired: reliability and meaningfulness. Phys Ther. 1984;64(1):35‐40. doi: 10.1093/ptj/64.1.35 [DOI] [PubMed] [Google Scholar]
- 27. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85‐M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 28. Massy‐Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population‐based study. BMC Res Notes. 2011;4(1):127. doi: 10.1186/1756-0500-4-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reijnierse EM, de Jong N, Trappenburg MC, et al. Assessment of maximal handgrip strength: how many attempts are needed? J Cachexia Sarcopenia Muscle. 2017;8(3):466‐474. doi: 10.1002/jcsm.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20‐30. doi: 10.1093/geront/10.1_part_1.20 [DOI] [PubMed] [Google Scholar]
- 31. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179‐186. [PubMed] [Google Scholar]
- 32. Lim SM, Island L, Horsburgh A, Maier AB. Home first! Identification of hospitalized patients for home‐based models of care. J Am Med Dir Assoc. 2021;22(2):413‐417.e1. doi: 10.1016/j.jamda.2020.05.061 [DOI] [PubMed] [Google Scholar]
- 33. Close JCT, Lord SR, Antonova E, et al. Older people presenting to the emergency department after a fall: a population with substantial recurrent healthcare use. Emerg Med J. 2012;29(9):742‐747. doi: 10.1136/emermed-2011-200380 [DOI] [PubMed] [Google Scholar]
- 34. Moreland B, Kakara R, Henry A. Trends in nonfatal falls and fall‐related injuries among adults aged ≥65 years — United States, 2012–2018. MMWR Morb Mortal Wkly Rep. 2020;69(27):875‐881. doi: 10.15585/mmwr.mm6927a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reijnierse EM, Verlaan S, Pham VK, Lim WK, Meskers CGM, Maier AB. Lower skeletal muscle mass at admission independently predicts falls and mortality 3 months post‐discharge in hospitalized older patients. J Gerontol A Biol Sci Med Sci. 2019;74(10):1650‐1656. doi: 10.1093/gerona/gly281 [DOI] [PubMed] [Google Scholar]
- 36. Ramsey KA, Loveland P, Rojer AGM, et al. Geriatric rehabilitation inpatients roam at home! A matched cohort study of objectively measured physical activity and sedentary behavior in home‐based and hospital‐based settings. J Am Med Dir Assoc. 2021;S1525‐8610(21):00396–0. doi: 10.1016/j.jamda.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 37. Meskers CGM, Reijnierse EM, Numans ST, et al. Association of Handgrip Strength and Muscle Mass with dependency in (instrumental) activities of daily living in hospitalized older adults ‐the EMPOWER study. J Nutr Health Aging. 2019;23(3):232‐238. doi: 10.1007/s12603-019-1170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crotty M, Kittel A, Hayball N. Home rehabilitation for older adults with fractured hips: how many will take part? J Qual Clin Pract. 2000;20(2–3):65‐68. doi: 10.1046/j.1440-1762.2000.00367.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Home‐based rehabilitation patient frailty and physical performance from admission to discharge. Home‐based rehabilitation patient frailty and mobility scores at admission to, and discharge from, home‐based rehabilitation. Median scores are represented in within boxes by horizontal lines, and limits of the interquartile range are represented by the top and bottom box borders. Points represent individuals.


