Abstract
Aims
Insufficient diuretic response frequently occurs in patients admitted for acute heart failure (HF) and is associated with worse clinical outcomes. Recent studies have shown that measuring natriuresis early after hospital admission could reliably identify patients with a poor diuretic response during hospitalization who might require enhanced diuretic treatment. This study will test the hypothesis that natriuresis‐guided therapy in patients with acute HF improves natriuresis and clinical outcomes.
Methods
The Pragmatic Urinary Sodium‐based treatment algoritHm in Acute Heart Failure (PUSH‐AHF) is a pragmatic, single‐centre, randomized, controlled, open‐label study, aiming to recruit 310 acute HF patients requiring treatment with intravenous loop diuretics. Patients will be randomized to natriuresis‐guided therapy or standard of care. Natriuresis will be determined at set time points after initiation of intravenous loop diuretics, and treatment will be adjusted based on the urinary sodium levels in the natriuresis‐guided group using a pre‐specified stepwise approach of increasing doses of loop diuretics and the initiation of combination diuretic therapy. The co‐primary endpoint is 24‐h urinary sodium excretion after start of loop diuretic therapy and a combined endpoint of all‐cause mortality or first HF rehospitalization at 6 months. Secondary endpoints include 48‐ and 72‐h sodium excretion, length of hospital stay, and percentage change in N‐terminal pro brain natriuretic peptide at 48 and 72 h.
Conclusion
The PUSH‐AHF study will investigate whether natriuresis‐guided therapy, using a pre‐specified stepwise diuretic treatment approach, improves natriuresis and clinical outcomes in patients with acute HF.
Introduction
Acute heart failure (HF) is one of the leading causes of hospitalization in the world, is associated with significant morbidity and mortality, and as such responsible for a large proportion of health care expenses. 1 , 2 Treatment of congestion in acute HF remains the Achilles' heel of contemporary HF management and is mostly limited to the administration of loop diuretics. A large number of acute HF patients display an insufficient diuretic response, which is associated with residual congestion and an increased risk of mortality and HF rehospitalization. 3 , 4 , 5 It is therefore important to identify patients with a poor diuretic response early after hospital admission. Given the mode of action of loop diuretics, natriuresis might be a sensitive, objective, quantifiable, and reliable marker to assess response. Recently, a small number of studies have shown that insufficient natriuretic response in acute HF patients was associated with an increased risk of poor outcome. 6 , 7 , 8 , 9 , 10 Furthermore, even early assessment of natriuresis (1–2 h after initiation of loop diuretics) in acute HF patients has been shown to be an accurate marker of insufficient diuretic response during hospitalization. 11 Based on these observational findings, it has been hypothesized that interventions aimed at improving decongestion using a stepwise intensified diuretic treatment approach based on natriuresis, has the potential to significantly improve effectiveness of decongestion, speed up in‐hospital treatment, and prevent readmissions for HF. The Heart Failure Association (HFA) of the European Society of Cardiology (ESC) has already incorporated early measurement of urinary sodium as a marker of diuretic response and as guidance of diuretic treatment in acute HF patients in a recent position paper, and this has furthermore recently been endorsed and included in the 2021 ESC HF guidelines. 12 , 13 However, to date, no prospective randomized natriuresis‐guided studies have been performed in patients with acute HF. The Pragmatic Urinary Sodium‐based treatment algoritHm in Acute Heart Failure (PUSH‐AHF) study has been designed to evaluate the hypothesis that natriuresis‐guided therapy in patients with acute HF improves natriuresis and clinical outcomes compared with standard of care.
Methods
Study design
PUSH‐AHF is a pragmatic, single‐centre, randomized, controlled, open‐label trial to evaluate the effect of natriuresis‐guided therapy compared with standard of care on diuretic response, decongestion and clinical outcomes in patients with acute HF (Figure 1 ). The trial is approved by the ethics committee of the University Medical Centre Groningen, the Netherlands, and is conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonization Guidelines for Good Clinical Practice. All participants provide written informed consent. The trial is registered at ClinicalTrials.gov (NCT04606927). Enrolment has started in February 2021 and is expected to be completed in September 2023 (www.pushahf.nl).
Figure 1.
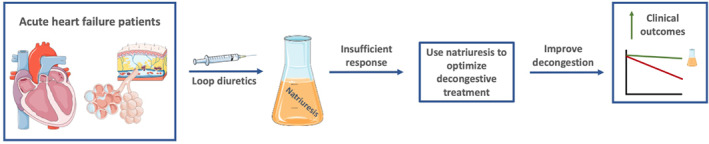
In patients with acute heart failure, loop diuretics are the first and only recommended choice of treatment aimed at relieving congestion by increasing diuresis and natriuresis. Actively assessing natriuresis and using this to optimize diuretic treatment could improve decongestion and clinical outcomes. This figure was created with images adapted from Servier Medical Art licensed under a Creative Commons Attribution 3.0.
Study participants
The study population consists of male and female patients (≥18 years old) presenting with acute HF requiring intravenous loop diuretics (Table 1 ). The diagnosis of acute HF will be assessed by the treating physician based on the current ESC HF guidelines and can be both de novo or an exacerbation of known HF. 14 Patients will be enrolled as soon as the diagnosis of acute HF is made and the first in‐hospital dose of intravenous loop diuretic is administered. Key exclusion criteria include dyspnoea primarily due to non‐cardiac causes or severe renal impairment requiring dialysis or ultrafiltration (Table 1 ). The inclusion and exclusion criteria were intentionally left broad to include a generalizable, real‐life, contemporary acute HF cohort.
Table 1.
Eligibility criteria for the PUSH‐AHF trial
| Inclusion criteria |
|
| Exclusion criteria |
|
ESC, European Society of Cardiology.
Consent procedure
Patients will be enrolled as soon as possible after the initial diagnosis of acute HF. Because of the acute situation and the (low‐risk) nature of the study and intervention, the ethics committee of the University Medical Centre Groningen, the Netherlands, approved the study for deferred consent in order to allow immediate randomization after diagnosis. Study participation and procedures will therefore start immediately after diagnosis of acute HF. Patients will receive information about the study within the first 4 days of hospitalization and will have a maximum of 24 h to consider his/her participation. During this time, written informed consent will be obtained. If patients do not provide written informed consent, they will be considered screen failures and all study data will be destroyed.
Study intervention
Patients presenting with acute HF will be identified at the emergency department and randomized to natriuresis‐guided therapy or standard of care. Patients in the standard of care group will be treated as presented in Figure 2 , according to common clinical practice. Patients randomized to the natriuresis‐guided treatment arm will be treated as presented in Figure 3 . In the natriuresis‐guided arm, decongestive treatment will be adjusted using a stepwise intensified diuretic treatment approach (further specified below and in Figure 3 ) based on the spot urinary sodium values assessed at set time points up until 36 h. The urine collection protocol, both of spot and 24‐h collection urine samples are described in more detail in online supplementary Methods S1 .
Figure 2.
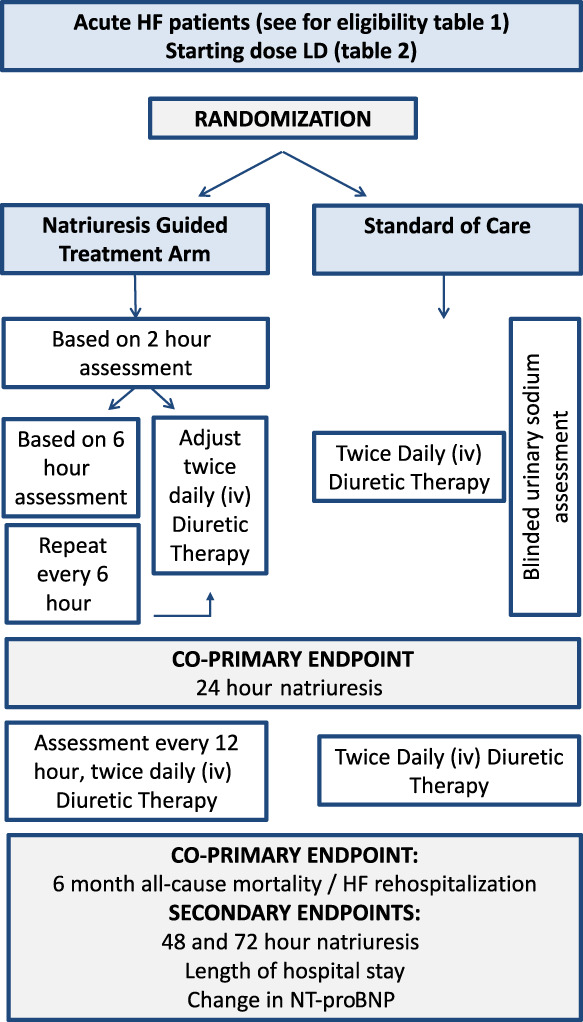
Overview of the PUSH‐AHF study protocol. HF, heart failure; iv, intravenous; LD, loop diuretic; NT‐proBNP, N‐terminal pro brain natriuretic peptide.
Figure 3.
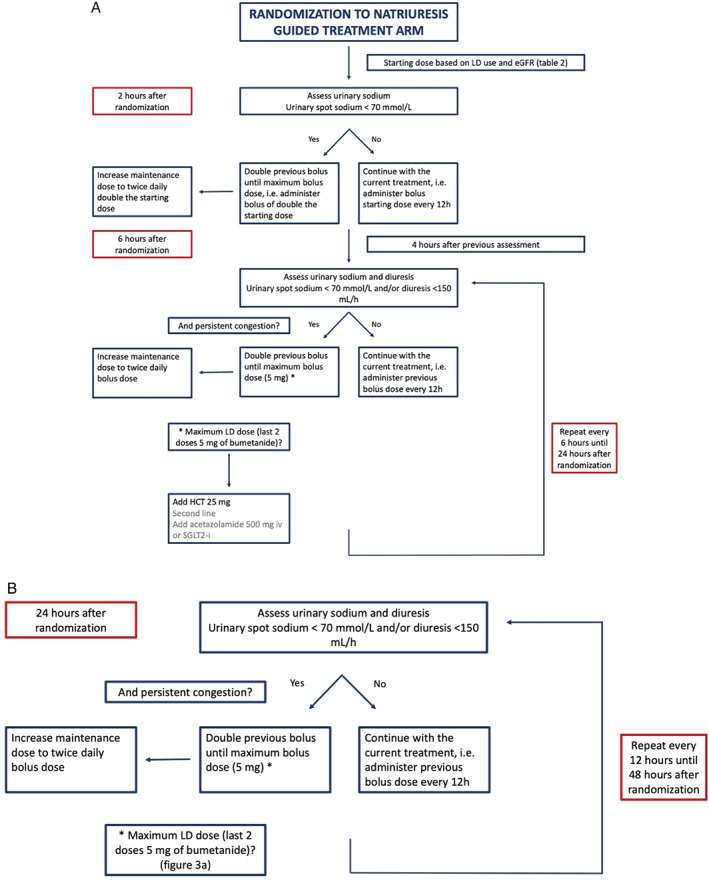
(A) PUSH‐AHF treatment protocol in the natriuresis‐guided arm during the first 24 h (0–24 h after randomization). (B) PUSH‐AHF treatment protocol in the natriuresis‐guided arm during the second 24 h (24–48 h after randomization). eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide; LD, loop diuretic; SGLT2‐i, sodium–glucose co‐transporter inhibitor.
Baseline loop diuretic dose
Baseline loop diuretic dose (the first in‐hospital dose of loop diuretics administered at the emergency department, irrespective of loop diuretic administration in the pre‐hospital setting) in both groups will be determined based on the estimated glomerular filtration rate (eGFR) of the patient at presentation and his/her outpatient loop diuretic dose (Table 2 ). A maximum bolus dose of 5 mg of bumetanide will be used. The bolus dose will be continued in twice daily dosing (every 12 h). Use of continuous administration of loop diuretics is actively discouraged. In the standard of care group this is not adjusted according to protocol, yet can be altered by the treating physician if clinically indicated, for instance based on congestion status or fluid balance according to best practice consensus. The urinary sodium values (of all spot and timed urine collections) in the standard of care group will be blinded till the end of the study.
Table 2.
Determination of loop diuretic starting dose in all patients
| Loop diuretic naive | Chronic loop diuretic use a | |
|---|---|---|
| eGFR ≥60 ml/min/1.73 m2 | Bolus of 1 mg of bumetanide | Bolus equal to total daily loop diuretic dose at home b |
| eGFR <60 ml/min/1.73 m2 | Bolus of 2 mg of bumetanide | Bolus double the total daily loop diuretic dose at home b |
| Maintenance dose is twice daily bolus dose | ||
eGFR, estimated glomerular filtration rate.
40 mg of furosemide is considered equal to 1 mg of bumetanide.
Maximum bolus dose is 5 mg of bumetanide.
Natriuresis‐guided treatment protocol
Based on the spot urinary sodium values obtained from 2 h onwards in the natriuresis‐guided group, decongestive therapy will be adjusted using the PUSH‐AHF treatment algorithm (Figure 3 ). In brief, if spot urinary sodium is <70 mmol/L and/or diuresis <150 ml/h, patients will be eligible (if still congested) for an additional bolus of loop diuretic, which is double the previous dose with a maximum bolus of 5 mg bumetanide. If a patient has received two doses of 5 mg of bumetanide at the two previous time points and has insufficient natriuresis or diuresis at two consecutive time points, the initiation of combination diuretic therapy is indicated (Figure 3A ). More details are provided in online supplementary Methods S1 . The above protocol will be repeated at 12, 18, 24, and 36 h (Figure 3B ). At every time point physicians will first assess the congestion status before administering additional doses of diuretics. After 48 h, adjustment of the decongestive therapy is left at the discretion of the treating physician.
Study endpoints
The primary endpoint of the PUSH‐AHF trial is a combined endpoint of two distinct co‐primary endpoints, namely (i) total 24‐h natriuresis and (ii) the first occurrence of the combined endpoint of all‐cause mortality or HF rehospitalization at 6 months. Secondary endpoints include total 48‐ and 72‐h natriuresis, length of hospital stay, and percentage change in N‐terminal pro brain natriuretic peptide (NT‐proBNP) at 48 and 72 h. Safety endpoints include doubling of serum creatinine at 24 or 48 h, and occurrence of worsening HF, defined as requiring inotropes or vasopressors, mechanical ventilation, or palliative care. The endpoint adjudication committee will adjudicate all rehospitalizations to judge whether a hospitalization is due to HF. The adjudication committee will be blinded to treatment allocation.
Sample size and power calculation
The PUSH‐AHF is powered for its co‐primary endpoint of total 24‐h natriuresis and the first occurrence of all‐cause mortality or HF rehospitalization at 6 months. Based on our previous study in acute HF patients, the mean 24‐h natriuresis was 398 ± 246 mmol. 10 In this population, 36% of patients had an insufficient natriuretic response (defined as urinary sodium <90 mmol or urine output <900 ml) 6 h after initiation of loop diuretic. Assuming a 40% improvement in these 36% of patients and a conservative 15% improvement in 24‐h natriuresis in the remaining patients because of closer monitoring, this assumes an overall 24% improvement in 24‐h urinary sodium excretion. Therefore, to obtain a power of at least 80%, at a two‐sided significance level of 0.025 (Bonferroni correction), a sample size of 125 patients in each group would be sufficient for the primary endpoint of 24‐h natriuresis. To prevent being underpowered due to dropout or missing data, which is expected to be higher than average in this patient population and given the delicate nature of urine collections, enrolment will be increased by 10%, therefore requiring 140 patients per group. Based on this sample size, we will have 81% power with a two‐sided significance level of 0.025 to detect a hazard ratio of 0.49 for the other co‐primary endpoint of all‐cause mortality and HF rehospitalization at 6 months. However, also accounting for 10% missing follow‐up data, we will increase the number of patients to 310 (155 patients per group).
Randomization and blinding
Subjects will be randomized to natriuresis‐guided therapy or standard of care by use of the electronic health record (EHR) (EPIC, Verona, WI, USA). Treatment allocation will be maintained as a fixed variable in the EHR, and a study specific orderset consistent with the treatment allocation will be ordered upon start of intravenous loop diuretic therapy. To prevent contamination and cross‐over between treatment arms, physicians will be blinded entirely to all urinary sodium measurements (timed collections as well as spot urinary sodium) in the standard of care arm. More details on the use of the EHR and blinding can be found in online supplementary Methods S1 .
Discussion
The primary treatment goal of patients admitted with acute HF is achieving euvolaemia with the use of loop diuretics. Unfortunately, a large number of patients with acute HF show insufficient response to diuretics, resulting in residual congestion and poor outcomes, such as high rates of HF rehospitalizations. 5 Natriuresis is a sensitive marker of response to loop diuretic therapy and allows for early identification of patients with insufficient diuretic response. 10 , 11 Urinary sodium therefore has all the characteristics required for a marker that can be used to actively assess diuretic response and to consequently guide decongestive treatment, using a stepwise approach. The PUSH‐AHF is the first trial to assess the effect of natriuresis‐guided enhanced diuretic therapy compared with standard of care on total natriuresis and clinical outcomes. If the PUSH‐AHF is able to show superiority of natriuresis‐guided therapy over standard of care, this will pave the way for more individualized diuretic therapy in patients with acute HF.
Current treatment of patients with acute heart failure and the rationale for the enhanced diuretic treatment protocol
Acute HF is characterized by signs and symptoms due to redistribution and excessive fluid retention, for which loop diuretics are the first and only guideline‐recommended treatment. 14 Loop diuretics inhibit the sodium–chloride–potassium co‐transporter in the ascending limb of the loop of Henle, resulting in sodium and chloride excretion with concomitant water excretion. 5 , 15 Several mechanisms, such as impaired resorption, neurohormonal activation and compensatory proximal and distal tubular sodium reabsorption, contribute to loop diuretic resistance in patients with acute HF. 12 , 16 , 17 , 18 , 19 Despite it being well known that higher diuretic doses are required in patients with HF, due to the above described mechanisms, increasing diuretic doses will over time become less effective. 20 Insufficient response to diuretics is therefore common and a large number of patients are discharged with residual congestion after an admission for acute HF. Yet, evidence‐based data on dosing and adjustment of loop diuretics in acute HF are currently lacking. The Diuretic Optimization Strategies Evaluation (DOSE) trial failed to show a benefit of high doses of loop diuretics compared with low‐dose loop diuretics using a randomized, double‐blind approach. 21 A possible explanation of the neutral results of the DOSE study might be due to the enrolment and randomization of patients with both a good and an insufficient diuretic response whereas no additional effect of higher doses is expected in patients with a good diuretic response. In contrast, in patients with an insufficient diuretic response, intensification of the diuretic treatment could lead to improved decongestion and consequent better outcomes.
The rationale for using urinary sodium as response variable
In recognition of this problem, several studies have aimed to better understand and early identify insufficient diuretic response over the last years. Initially, these studies used either net fluid balance, urine output or weight loss indexed to administered loop diuretic dose as surrogates of diuretic response. 3 , 4 However, all of these are, to a certain extent, unreliable in daily clinical practice. Given the mode of action of loop diuretics, natriuresis might not only be a sensitive, objective, accurate and quantifiable marker of response, but also the most reliable marker to assess response. 22 To date, multiple studies have demonstrated that impaired natriuretic response to loop diuretic treatment is associated with markers of persistent congestion, and with higher rates of HF rehospitalization and mortality. 6 , 8 , 9 , 10 Additionally, even early assessment of natriuresis in a spot urine sample 1 to 2 h after initiation of intravenous loop diuretic treatment has been shown to be an accurate marker of longer term (6 h) natriuretic response. 11 This led to the development of a natriuretic response prediction equation which was additionally validated and tested in a prospective study showing that loop diuretic‐guided treatment based on this natriuretic response predication equation resulted in significant increases in urine output, net fluid loss and weight loss. 23 These findings suggest that natriuresis could be a reliable tool to assess diuretic response, select non‐responders, and consequently titrate diuretic therapy accordingly. To date, the value of natriuresis‐guided therapy and its effect on decongestion or outcomes, has however not been studied in a randomized, controlled setting.
The possible value of natriuresis‐guided enhanced diuretic therapy and the PUSH‐AHF study treatment protocol
As illustrated above, natriuresis has all the characteristics required for a marker that can be used to actively guide decongestive treatment and move toward a personalized treatment approach in acute HF. This is important as current treatment is limited, in many cases insufficient and recent studies investigating novel therapies in acute HF were neutral. This might in part be due to patient selection and the timing of initiation of the novel therapies. In these trials, acute HF patients were not enrolled immediately at presentation and irrespective of their response to standard therapy, that is, loop diuretics. In approximately half of acute HF patients, response to this therapy will however be adequate, limiting the potential, additional effect of a novel therapy. Furthermore, we know that early treatment with intravenous loop diuretics, as well as good diuretic response in the first 24 h are associated with better outcomes. 24 , 25 By assessing natriuresis early after initiation of loop diuretic therapy, patients with an insufficient response (non‐responders) will be identified and will receive (early) intensified therapy. This could furthermore prove to be a set‐up for future acute HF trials where initial non‐responders will be eligible for novel treatment options early after admission.
Additionally, PUSH‐AHF will implement a treatment protocol that includes combination diuretic therapy in patients with an insufficient natriuretic response. This treatment protocol is an adaptation of the proposed algorithm in the position statement from the HFA of the ESC on the use of diuretics in HF with congestion, which has also been incorporated in the recently published 2021 ESC HF guidelines. 12 , 13 Post hoc analyses from the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS‐HF) suggest that such a stepped pharmacological care approach in acute HF patients with persistent congestion may result in greater decongestion without negative effects on renal function. 26 The PUSH‐AHF will provide additional information on the effects of combination diuretic therapy in increasing natriuretic response and possible side‐effects such as electrolyte disturbances or decreases in renal function, which will be closely monitored throughout the trial. If positive, this could become a validated framework for the intensified treatment of acute HF patients with an initial insufficient response.
Pragmatic trial design
As incorporated in the acronym, the PUSH‐AHF is considered a pragmatic study for several reasons. First, the trial is incorporated in the clinical care of acute HF patients at our hospital. The study protocol starts at the moment of the start of loop diuretic therapy at admission at the emergency department, where patients are automatically randomized to one of the two treatment groups by the EHR. This is performed using an automated random number generator, as has been used before in a multicentre, randomized clinical trial and is currently being used in a large pragmatic HF trial. 27 , 28 When the diagnosis of acute HF is made at the emergency department and intravenous diuretics are prescribed, the assessments (and orders) associated with the allocated treatment group are automatically activated. This incorporation in the EHR results in effective and accurate uptake of the study assessments and the treatment protocol in clinical care. Second, the PUSH‐AHF trial has intentionally been designed to enrol a generalizable acute HF population. In traditional randomized clinical trials, highly selected patient populations are enrolled to ensure ‘perfect’ conditions. However, this not only leads to non‐generalizable trial results, it also limits patient enrolment. With the PUSH‐AHF we strive to enrol a generalizable acute HF patient population hopefully resulting in high enrolment rates and results that can be extended to almost all acute HF patients. Third, the use of deferred consent allows us to enrol and randomize patients immediately at the start of diagnosis and treatment of acute HF, thereby enabling uncompromised research in this very early phase that is usually excluded from randomized clinical acute HF trials. Fourth, as all in‐hospital assessments are part of clinical care, this results in a reduced burden of trial participation for both the study staff, as well as the patients. Additionally, the final follow‐up visit will be executed by telephone call further reducing the burden of trial participation for these patients. Using the Pragmatic Explanatory Continuum Indicator Summary 2 (PRECIS‐2) tool, which is designed to help trialists make informed decisions on pragmatic trial design consistent with the intended purpose of the trial, the PUSH‐AHF study scores a 35 out of a maximum of 40 points (where a higher score indicates a more pragmatic trial) (Figure 4 ). 29 Although pragmatic trials have a great number of advantages, they also have inherent limitations. For the PUSH‐AHF the potential risks are non‐adherence to the treatment protocol despite incorporation in the EHR and extensive training of the clinical care staff (including physicians, and nurses at the emergency department, cardiac care unit and the cardiology wards), as well as missing data. Given the number of ongoing pragmatic trials, this decade may provide the answer whether pragmatic trials might indeed be the best of both worlds. 30
Figure 4.
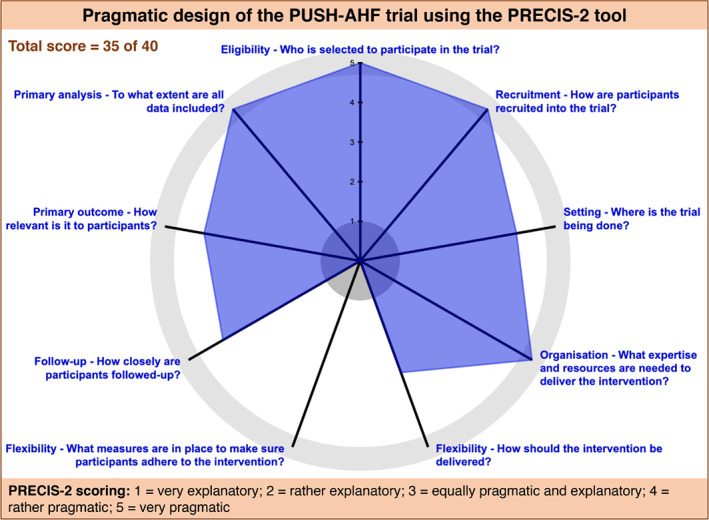
PRECIS‐2 wheel diagram for the PUSH‐AHF study.PRECIS‐2, Pragmatic Explanatory Continuum Indicator Summary 2; PUSH‐AHF, Pragmatic Urinary Sodium‐based treatment algoritHm in Acute Heart Failure.
Conclusions
Insufficient diuretic response is one of the main reasons for high rehospitalization rates and poor clinical outcomes in patients with acute HF and therefore optimized and individualized treatment of these patients is warranted. Natriuresis might be a sensitive, objective, quantifiable and reliable marker to assess diuretic response and to subsequently guide diuretic treatment in acute HF patients. The PUSH‐AHF is a pragmatic, randomized, controlled, open‐label study designed to study the value of natriuresis‐guided therapy in improving natriuresis and clinical outcomes in acute HF patients.
Funding
Dr. J.M. ter Maaten is supported by a Dekker grant of the Dutch Heart Foundation for this study.
Conflict of interest: P.v.d.M. received consultancy fees and/or research grants from Astra Zeneca, Ionis, Novartis, Pfizor, Pharmacosmos, Pharma Nord and Vifor Pharma. A.A.V. and/or his institution received grants and/or consultancy reimbursements from Amgen, Astra Zeneca, Bayer AG, BMS, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novartis, Novo Nordisk, and Roche Diagnostics. K.D. received speaker fees from Astra Zeneca and Boehringer Ingelheim. All other authors have nothing to disclose.
Supporting information
Methods S1. Supplementary methods.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 3. Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O'Connor CM, et al. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–93. [DOI] [PubMed] [Google Scholar]
- 4. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure‐pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12:184–92. [DOI] [PubMed] [Google Scholar]
- 6. Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, et al. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long‐term outcomes in acute decompensated heart failure. J Card Fail. 2014;20:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70:265–73. [DOI] [PubMed] [Google Scholar]
- 8. Biegus J, Zymliński R, Sokolski M, Todd J, Cotter G, Metra M, et al. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail. 2019;21:624–33. [DOI] [PubMed] [Google Scholar]
- 9. Hodson DZ, Griffin M, Mahoney D, Raghavendra P, Ahmad T, Turner J, et al. Natriuretic response is highly variable and associated with 6‐month survival: insights from the ROSE‐AHF trial. JACC Heart Fail. 2019;7:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damman K, Ter Maaten JM, Coster JE, Krikken JA, van Deursen VM, Krijnen HK, et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur J Heart Fail. 2020;22:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail. 2016;9:e002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–55. [DOI] [PubMed] [Google Scholar]
- 13. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al.; Authors/Task Force Members, Document Reviewers . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 15. Boorsma EM, Ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol. 2020;17:641–55. [DOI] [PubMed] [Google Scholar]
- 16. Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–9. [DOI] [PubMed] [Google Scholar]
- 17. Kim EJ, Lee MG. Pharmacokinetics and pharmacodynamics of intravenous bumetanide in mutant Nagase analbuminemic rats: importance of globulin binding for the pharmacodynamic effects. Biopharm Drug Dispos. 2001;22:147–56. [DOI] [PubMed] [Google Scholar]
- 18. Ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M, et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail. 2017;19:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco‐Bacik MA, Wilson FP, et al. Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol. 2017;28:3414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96:132–43. [DOI] [PubMed] [Google Scholar]
- 21. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al.; NHLBI Heart Failure Clinical Research Network . Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ter Maaten JM, Damman K. Down the road from challenges in acute heart failure trials. Eur J Heart Fail. 2019;21:1423–5. [DOI] [PubMed] [Google Scholar]
- 23. Rao VS, Ivey‐Miranda JB, Cox ZL, Riello R, Griffin M, Fleming J, et al. Natriuretic equation to predict loop diuretic response in patients with heart failure. J Am Coll Cardiol. 2021;77:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, et al. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:3042–51. [DOI] [PubMed] [Google Scholar]
- 25. ter Maaten JM, Dunning AM, Valente MA, Damman K, Ezekowitz JA, Califf RM, et al. Diuretic response in acute heart failure‐an analysis from ASCEND‐HF. Am Heart J. 2015;170:313–21. [DOI] [PubMed] [Google Scholar]
- 26. Grodin JL, Stevens SR, de Las Fuentes L, Kiernan M, Birati EY, Gupta D, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail. 2016;22:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson FP, Martin M, Yamamoto Y, Partridge C, Moreira E, Arora T, et al. Electronic health record alerts for acute kidney injury: multicenter, randomized clinical trial. BMJ. 2021;372:m4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmad T, Yamamoto Y, Biswas A, Ghazi L, Martin M, Simonov M, et al. REVeAL‐HF: design and rationale of a pragmatic randomized controlled trial embedded within routine clinical practice. JACC Heart Fail. 2021;9:409–19. [DOI] [PubMed] [Google Scholar]
- 29. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS‐2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 30. Greene SJ, Velazquez EJ, Anstrom KJ, Eisenstein EL, Sapp S, Morgan S, et al.; TRANSFORM‐HF Investigators . Pragmatic design of randomized clinical trials for heart failure: rationale and design of the TRANSFORM‐HF trial. JACC Heart Fail. 2021;9:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1. Supplementary methods.


