Abstract
Objective
Gait impairment in persons with Parkinson disease is common and debilitating. Compensation strategies (eg, external cues) are an essential part of rehabilitation, but their underlying mechanisms remain unclear. Using electroencephalography (EEG), we explored the cortical correlates of 3 categories of strategies: external cueing, internal cueing, and action observation.
Methods
Eighteen participants with Parkinson disease and gait impairment were included. We recorded 126‐channel EEG during both stance and gait on a treadmill under 4 conditions: (1) uncued, (2) external cueing (listening to a metronome), (3) internal cueing (silent rhythmic counting), and (4) action observation (observing another person walking). To control for the effects of sensory processing of the cues, we computed relative power changes as the difference in power spectral density between walking and standing for each condition.
Results
Relative to uncued gait, the use of all 3 compensation strategies induced a decrease of beta band activity in sensorimotor areas, indicative of increased cortical activation. Parieto‐occipital alpha band activity decreased with external and internal cueing, and increased with action observation. Only internal cueing induced a change in frontal cortical activation, showing a decrease of beta band activity compared to uncued gait.
Interpretation
The application of compensation strategies resulted in changed cortical activity compared to uncued gait, which could not be solely attributed to sensory processing of the cueing modality. Our findings suggest there are multiple routes to control gait, and different compensation strategies seem to rely on different cortical mechanisms to achieve enhanced central motor activation in persons with Parkinson disease. ANN NEUROL 2022;91:329–341
Gait impairment is a common and disabling manifestation of Parkinson disease. This impairment can be present both continuously (ie, decreased step length, reduced arm swing, and increased gait variability) and episodically (eg, festination or freezing of gait). 1 , 2 Gait impairment limits functional mobility and may lead to falls and subsequent injuries.
The pathophysiology underlying gait impairment in Parkinson disease is complex and presumably involves dysfunction of multiple supraspinal components within the locomotor network, including corticostriatal loops. The pathophysiology of episodic and continuous gait deficits is not identical, but does overlap. 3 Persons with Parkinson disease generally experience more difficulties when walking in an automated manner (ie, without consciously paying attention), compared to when producing goal‐directed behavior (often facilitated by the presence of a clear external or sometimes an internal stimulus). 4 Studies in animals and humans revealed that these differences between automatic and goal‐directed behavior are likely related to greater loss of dopaminergic innervation in the posterior putamen, which has been associated with the control of automatic (habitual) behavior, in contrast to the relatively preserved rostromedial striatum, which is primarily involved in goal‐directed behavior. 5 , 6 Consequently, persons with Parkinson disease may increasingly rely on making a compensatory shift from the automated to the goal‐directed mode of action control to maintain functional mobility. Recently, Gilat et al published an excellent model diagram of gait control in Parkinson disease. 7
The application of compensation strategies forms an essential part of gait rehabilitation. These strategies involve a wide variety of "detours" that are typically spontaneously invented by persons with Parkinson disease to overcome their walking difficulties. Examples of compensation strategies include stepping over lines on the floor, counting while walking, skipping, and mimicking the movements of another person. They can be employed to alleviate freezing of gait episodes, but are also commonly applied in clinical practice to ameliorate gait rhythmicity, gait speed, and step length in persons with Parkinson disease with and without freezing of gait. 8 , 9 A comprehensive framework of 7 distinct categories of compensation strategies was recently proposed, based on a review of hundreds of patient videos collected over a 4‐year period. 10 It is hypothesized that the mechanisms underlying these strategies may be different for each proposed category, potentially explaining why the efficacy of a specific strategy tends to vary between patients. 9 The general idea is that the application of compensation strategies facilitates the shift from automatic to goal‐directed motor control, thereby bypassing the most affected basal ganglia circuitries. This switch to goal‐directed control of gait is postulated to lead to increased recruitment of cortical areas including (pre‐)frontal and parietal areas. 11 , 12
To date, the cortical correlates of compensation strategies for gait impairment in Parkinson disease remain relatively unclear. Recent technological advances now allow for the study of cortical activity during actual walking rather than imaged gait, using brain imaging techniques such as electroencephalography (EEG). The interpretation of earlier EEG studies on this topic is complicated by their lack of control conditions, hampering the ability to distinguish the cortical signature of compensation strategies in motor control from the cortical activity related to the sensory or attentional processing of the cueing modality. In the present study, we overcome this limitation through the use of a novel approach comprising high‐density EEG recordings during both gait and stance to explore the cortical correlates underlying 3 categories of compensation strategies: (1) external cueing, (2) internal cueing, and (3) action observation. We hypothesized that each of the different types of compensation strategy would present with a distinct pattern of cortical activation. 10 Based on previous studies, external cueing was postulated to assist in filtering information and prioritizing a stimulus through improvement of executive attention, regulated by frontostriatal circuitries. 10 Internal cueing was hypothesized to aid in orienting or focusing attention toward gait, and thought to involve prefrontal and parietal areas. 10 , 13 Finally, action observation was hypothesized to compensate for reduced automaticity through activation of the mirror neuron system, involving the supplementary motor area (SMA), dorsal premotor cortex, supramarginal gyrus, and superior parietal lobe. 10 , 14 , 15
Patients and Methods
Participants
Twenty persons with Parkinson disease and self‐reported disabling gait impairment (ie, negatively affecting their ability to perform their usual daily activities) participated in this study. All had previously participated in an experiment aimed at evaluating the efficacy of compensation strategies for gait impairments in Parkinson disease. Persons were eligible for inclusion if they had demonstrated beneficial effects of external cueing, internal cueing, and action observation on gait quality. A beneficial effect was defined as any decrease in stride time variability compared to uncued gait (without any compensation strategy), assessed during 3‐minute trials of continuous overground walking, in combination with a subjective improvement in gait compared to uncued gait according to the participant. Exclusion criteria were inability to walk unaided for 5 minutes consecutively, presence of comorbidities significantly influencing gait capacity (ie, history of stroke, orthopedic ailments), and deep brain stimulation (DBS).
Measurements took place in the morning. Disease severity was assessed at the start of the measurement, in the dopaminergic "ON" phase, using the Movement Disorder Society‐sponsored Unified Parkinson's Disease Rating Scale part III. 16 The presence and severity of freezing of gait was determined using the New Freezing of Gait Questionnaire. 17 Participants were to refrain from taking their scheduled dosages of dopaminergic medication for the duration of the experiment (±4 h). Consequently, due to the long EEG preparation time, EEG recordings were performed while participants were in the dopaminergic end‐of‐dose phase. This was confirmed by debriefing the participants, who all indicated a clear worsening of their symptoms that would normally have necessitated the intake of dopaminergic medication. We specifically designed this element of our study to mimic the daily life situation, as the end‐of‐dose phase would be the time of day at which the application of compensation strategies would be most useful. 18
Informed consent was obtained from each participant, in accordance with the principles of the Declaration of Helsinki. This study was approved by the institutional review board of the Radboud University Medical Center in Nijmegen, the Netherlands, and the local Medical Ethics Committee Arnhem‐Nijmegen (ref: 2019‐5710).
Experimental Protocol
Participants stood on a treadmill in a quiet, nondistracting environment, and were equipped with a safety harness. The experiment consisted of EEG recordings during standing and during gait, under 4 conditions: (1) uncued, (2) external cueing, (3) internal cueing, and (4) action observation (see Fig 1 and the descriptions below). The uncued conditions (uncued stance and gait) were recorded at the beginning of the experiment. The order of the remaining conditions within the stance and gait blocks was counterbalanced across participants. The duration of each recording was 4 minutes, except for uncued stance (quiet stance), which lasted 1 minute. Treadmill speed was set at the participant's preferred comfortable speed and kept constant for all gait recordings. Each condition was individually explained, practiced if necessary, and then recorded. General instructions to the participants included focusing their gaze on a fixation cross projected on the screen in front of them, refraining from talking during the recordings, and refraining from actively suppressing any tremors, dyskinesia, or dystonia that may occur. Participants were encouraged to take unrestricted breaks in between recordings to prevent fatigue.
FIGURE 1.
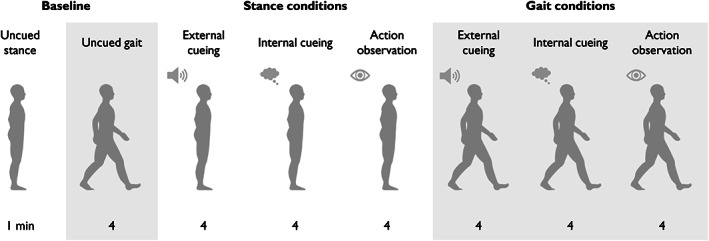
Experimental tasks and conditions. The experimental conditions consisted of external cueing, internal cueing, and action observation during stance and gait. Stance conditions always preceded the gait conditions, but the order of the conditions was counterbalanced within stance/gait blocks and across participants. Each condition lasted 4 minutes, with the exception of uncued stance (commonly referred to as quiet stance). Prior to the main experiment, participants practiced walking on the treadmill at a comfortable speed to determine their preferred cadence. The corresponding belt speed remained constant throughout the experiment. Resting breaks were encouraged between all conditions.
Uncued Condition
During the uncued conditions, participants were explicitly instructed not to apply any compensation strategies. During uncued gait, the participant's natural cadence was estimated by the researcher using a freely available beats‐per‐minute app on a smartphone (BPM, v3.04, CHEEBOW).
External Cueing
During the external cueing conditions, participants listened to the sound of a metronome that was played through speakers. The metronome was developed for an in‐house treadmill operations application (D‐flow; Motek Forcelink, Amsterdam, the Netherlands) and recorded in parallel as a trigger line for data synchronization. The pace of the metronome was set to the uncued gait cadence. During gait, participants were to synchronize their steps to the rhythm of the metronome (ie, make a heel strike at every beat), without counting along, or using any other compensation strategies.
Internal Cueing
During the internal cueing conditions, participants silently counted in a rhythmic manner (eg, 1‐2‐3‐4‐1‐2‐3‐4). During gait, participants were to synchronize their steps to their counting (ie, make a heel strike at every count), without using any other compensation strategies. Due to the nature of the internal cueing condition, synchronization of the cue with motion and EEG data was not possible.
Action Observation
For the action observation conditions, participants watched a prerecorded video of a healthy person walking on the same treadmill. The video was projected onto a large screen in front of them. The person in the video walked on the treadmill, synchronizing their steps with the rhythm of a metronome. A set of videos with cadences between 80 and 120 steps per minute (increments of 5 steps per minute) was available, to ensure the projected video would closely match the natural cadence of every participant, as measured during uncued gait. During gait, participants were to synchronize their steps to the steps of the person in the video, without counting along, or using any other compensation strategies. The audio signal of the metronome in the video was muted, but its digital signal was recorded in parallel as a trigger line for data synchronization.
Data Acquisition
High‐density EEG data were acquired using 126‐channel Ag‐AgCl electrodes embedded in an electrode cap (WaveGuard; ANT Neuro, Hengelo, the Netherlands), with electrode distribution according to the 5% electrode system. 19 The ground electrode was placed on the left mastoid. EEG was sampled at 2,048Hz using a biosignal amplifier (REFA; TMSi, Oldenzaal, the Netherlands) with a built‐in antialiasing low‐pass filter (552Hz) and average reference. Electrode impedance was ≤10kΩ.
Movement data were acquired using a 10‐camera 3‐dimensional motion capture system (Vicon, Oxford, UK), with a sampling rate of 100Hz. Thirty‐five reflective markers were placed on anatomical landmarks as defined by the PlugInGait Full Body Model, 20 excluding the head markers for EEG purposes. In addition to the trigger line for external cueing and action observation, a digital trigger signal was simultaneously recoded by the EEG and motion capture systems for data synchronization.
Data Processing and Analysis
Movement Data
The motion capture data were analyzed to determine the difference in gait variability between uncued gait and each of the 3 gait conditions with compensation strategies. Gait variability is associated with fall risk in a broad variety of populations, including persons with Parkinson disease. 21 , 22 , 23 , 24 Gait variability was expressed as the coefficient of variation (CV) of stride time:
| (1) |
Stride time was computed with a custom MATLAB (MathWorks, Natick, MA) script and was defined as the time between two subsequent right heel strikes (same for the left). Heel strikes were identified as (local) minima of the vertical displacement of the heel markers within a gait cycle. A similar procedure was used to determine toe offs from the toe markers. The sequence of gait events within the gait cycle was checked for order, and aberrant cycles were discarded. The latency between gait events was computed, and outliers were rejected.
Electroencephalogram
EEG data were processed using the EEGLAB toolbox (UC San Diego, Swartz Center for Computational Neuroscience, La Jolla, CA) 25 and custom MATLAB scripts.
Preprocessing and Artifact Reduction
EEG data were combined with gait events (ie, heel strikes and toe offs for each foot). EEG data were bandpass filtered between 2 and 200Hz (5,120th order finite impulse response filter, Hamming window, zero‐phase shift) and downsampled to 512Hz. Afterward, EEG data from all conditions were concatenated.
The clean_rawdata plugin (v2.3) from EEGLAB was used to reject channels with low correlation (<0.6) with neighboring channels and to correct for bursts of high‐amplitude activity (eg, muscle artifacts) using artifact subspace reconstruction (ASR v0.13; threshold: standard deviation [SD] = 15). 26 The artifact‐reduced participant‐specific EEG dataset was segmented into consecutive, nonoverlapping epochs (0.5 seconds). Epochs containing high‐amplitude artifacts were removed from the dataset using the pop_jointprob function from EEGLAB (threshold: SD = 6). Finally, using Infomax independent component analysis (ICA), 27 EEG data were decomposed to estimate source‐resolved brain activity and reduce the influence of physiological noise. 28 ICA performs a blind source decomposition of the dataset based on the assumption that the EEG sources are instantaneously near‐independent. Each independent component is associated with a scalp map, representing the scalp projection of synchronous neural activity in a cortical domain. This map was used to approximate the cortical source of a given component by fitting an equivalent current dipole using the dipfit plugin (v3.7) from EEGLAB, with standard electrode coordinates and a standard 3‐shell boundary element head model. Independent components with an associated equivalent current dipole and a residual variance of <15% were visually inspected considering their mean power spectra to exclude nonbrain activity.
EEG datasets were segmented according to the participant‐specific mean gait cycle duration, to compute the condition‐specific mean power spectral density (PSD; average across the gait cycle) and mean gait cycle spectrograms. PSD was computed between 2 and 48Hz (45 frequencies, linearly distributed) using Morlet wavelets (1.2 cycles at lowest frequency, increasing 0.2 cycles with each step). Gait cycle spectrograms for individual gait cycles were time‐warped via linear interpolation to standardize the gait cycle across participants. Gait events were aligned to 0–10–50–60–100% of the gait cycle, corresponding to the right heel strike, left toe off, left heel strike, right toe off, and right heel strike, respectively. 29
Clustering Independent Components across Participants
For group‐level analysis, independent components were clustered across participants. Feature vectors were created by concatenating information about the location of the corresponding equivalent current dipole, the scalp projection, and the mean power spectral density (3–48Hz, across all conditions). Principal component analysis was used to reduce the feature vectors to 9 principal components before using the k‐means algorithm. The number of clusters was the average number of components per participant (k = 13). Feature vectors located >5 SD from the computed cluster centroids were considered outliers. Only clusters containing independent components from more than half of the participants (n > 9) were considered for further analysis. Condition‐specific PSDs and gait cycle spectrograms were averaged per cluster.
Assessment of Cortical Activation
To obtain a measure of cortical activation, 30 , 31 relative power changes were computed as the difference in PSD between standing and walking per condition. This measure indicates the relative change in cortical activation during the application of a compensation strategy during gait, compared to solely processing the same sensory input (external cueing, action observation), or engaging in a similar cognitive task (internal cueing) during stance. According to the traditional interpretation of event‐related spectral modulations, 30 , 31 , 32 a relative power decrease indicates increased cortical activation, whereas a relative power increase may indicate reduced cortical activation or increased inhibition. Similarly, the difference between condition‐specific spectrograms during gait and condition‐specific PSD during stance resulted in a time‐frequency representation of cortical activation throughout the gait cycle. 33 , 34
Statistical Analysis
Significant differences in cortical activation during the application of a compensation strategy versus uncued gait were evaluated using 2‐tailed t tests for repeated measures and nonparametric permutation testing (5,000 permutations, α = 0.05). 35 This was applied to each frequency line of the PSD (2–48Hz, 45 levels linearly spaced). Significant differences where only considered if present in at least 2 consecutive frequency lines.
Data Availability
Data are available on reasonable request to the corresponding author.
Results
Study Population
Of 20 participants, 18 (10 men and 8 women, aged 66.2 ± 7.6 years) were included in the analysis, as the data from 2 participants had to be excluded (trigger line defect, n = 1; inability to walk on a treadmill, n = 1). Participant characteristics are presented in Table 1.
TABLE 1.
Participant Characteristics
| Characteristic | PD Participants, n = 18 a |
|---|---|
| Age, yr | 66.2 ± 7.6 |
| Sex, M/F | 10/8 |
| Disease duration, yr | 6.4 ± 2.7 |
| LEDD, mg | 894.1 ± 309.5 |
| MDS‐UPDRS III score, median (range) | 29 (11–42) |
| Hoehn & Yahr stage, median (range) | 2 (1–3) |
| Presence of FOG, yes/no b | 6/12 |
Values represent mean ± standard deviation unless indicated otherwise.
Presence of FOG, as defined by a nonzero score on question 1 of the New Freezing of Gait Questionnaire. 13
F = female; FOG = freezing of gait; LEDD = L‐dopa equivalent daily dosage; M = male; MDS‐UPDRS III = Movement Disorder Society‐sponsored Unified Parkinson's Disease Rating Scale part III; PD = Parkinson disease.
Effect of Compensation Strategies on Gait Variability
At group level, the mean stride time variability (in CV, %) was 2.38 (SD = 0.82, range = 1.41–4.48) during uncued gait, 2.30 (SD = 0.73, range = 1.21–4.16) during gait with external cueing, 2.40 (SD = 0.74, range = 1.43–3.76) during gait with internal cueing, and 2.27 (SD = 0.70, range = 1.33–4.13) during gait with action observation. The effect on gait variability was not confounded by changes in gait speed, as gait speed was controlled across all conditions. All participants reported a subjective improvement of gait during the application of all 3 strategies compared to uncued gait.
Clusters of Independent Components
In total, 222 independent components were selected for clustering (mean ± SD = 13 ± 3.5, range = 6–18 per participant). Ten clusters containing independent components from more than half of the participants were identified (Table 2). For descriptive purposes, these clusters were categorized according to their anatomical localization: frontal (n = 1), central/sensorimotor (n = 3), parietal (n = 3), and occipital (n = 3). Cluster‐ and condition‐specific PSDs are presented in the Supplementary Materials.
TABLE 2.
Clusters of Independent Sources Obtained with ICA
| Cluster | Location of Cluster Centroid | MNI Coordinates, x, y, z | Brodmann Area | Subjects and ICs Included, n |
|---|---|---|---|---|
| Frontal | ||||
| 1 | Central frontal cortex | 11, 32, 36 | BA 32 | 10 |
| Sensorimotor | ||||
| 2 | Left sensorimotor cortex | −29, −7, 57 | BA 6 | 13 |
| 3 | Central sensorimotor cortex | 1, −22, 61 | BA 4 | 12 |
| 4 | Right sensorimotor cortex | 38, −8, −47 | BA 6 | 14 |
| Parietal | ||||
| 5 | Left parietal cortex | −30, −46, 43 | BA 40 | 15 |
| 6 | Central parietal cortex | 1, −57, 37 | BA 7 | 13 |
| 7 | Right parietal cortex | 35, −44, 34 | BA 40 | 14 |
| Occipital | ||||
| 8 | Left occipital cortex | −34, 60, 11 | BA 37 | 11 |
| 9 | Central occipital cortex | 1, −78, 19 | BA 18 | 11 |
| 10 | Right occipital cortex | 33, −64, 9 | BA 19 | 12 |
IC = independent component; ICA = IC analysis; MNI = Montreal Neurological Institute.
Cortical Activation during the Application of Compensation Strategies
Cortical activation spectra and time‐frequency maps during the application of compensatory strategies relative to uncued gait are presented per cluster in Figures 2, 3, 4, 5. We highlight the most important findings (all p < 0.05) in this section.
FIGURE 2.
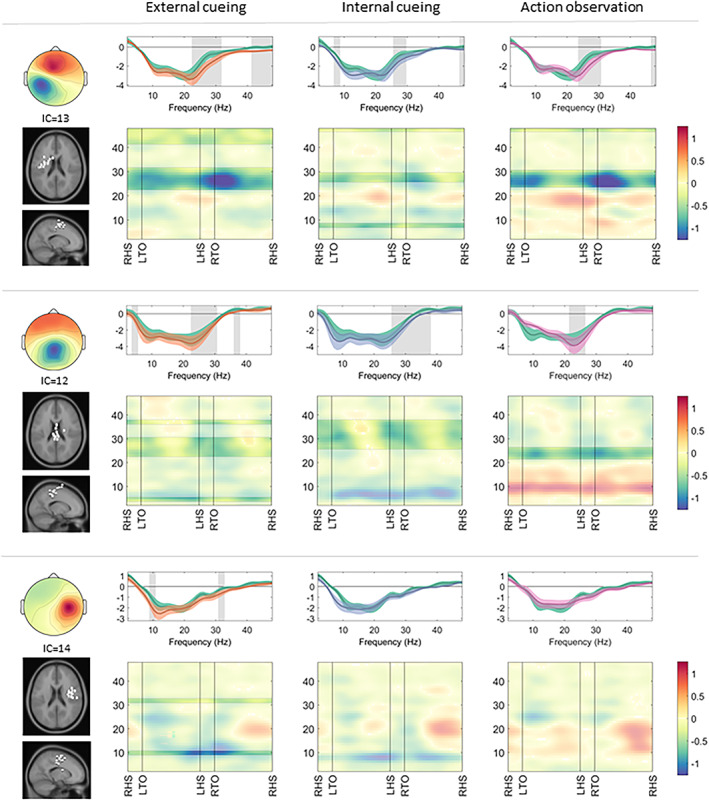
Somatosensory clusters: cortical activation during gait with a compensation strategy. Cortical activation spectra (top rows) show power changes during gait conditions relative to stance conditions (mean ± standard error), and cortical activation time‐frequency maps (bottom rows) illustrate relative differences (compensation strategy gait minus uncued gait) across the gait cycle. The negative values around sensorimotor alpha (8–12Hz) and beta (13–35Hz) frequency bands indicate increased cortical activation during gait (top row, all conditions). Similarly, negative values around the beta frequency band (bottom row, all conditions) indicate stronger cortical activation during application of the compensation strategies. Significant effects (p < 0.05) of a given compensation strategy (external cueing: orange; internal cueing: blue; action observation: pink) in contrast to uncued gait (green) are highlighted (spectra: gray background; maps: unmasked colors). IC = independent component; LHS = left heel strike; LTO = left toe off; RHS = right heel strike; RTO = right toe off.
FIGURE 3.
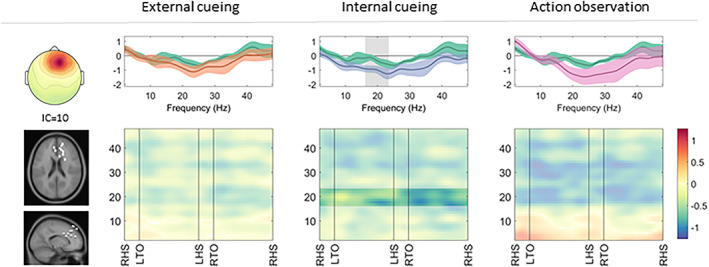
Frontal cluster: cortical activation during gait with a compensation strategy. Cortical activation spectra (top row) show power changes during gait conditions relative to stance conditions (mean ± standard error), and cortical activation time‐frequency maps (bottom row) illustrate relative differences (compensation strategy gait minus uncued gait) across the gait cycle. Significant effects (p < 0.05) of a given compensation strategy (external cueing: orange; internal cueing: blue; action observation: pink) in contrast to uncued gait (green) are highlighted (spectra: gray background; maps: unmasked colors). IC = independent component; LHS = left heel strike; LTO = left toe off; RHS = right heel strike; RTO = right toe off.
FIGURE 4.
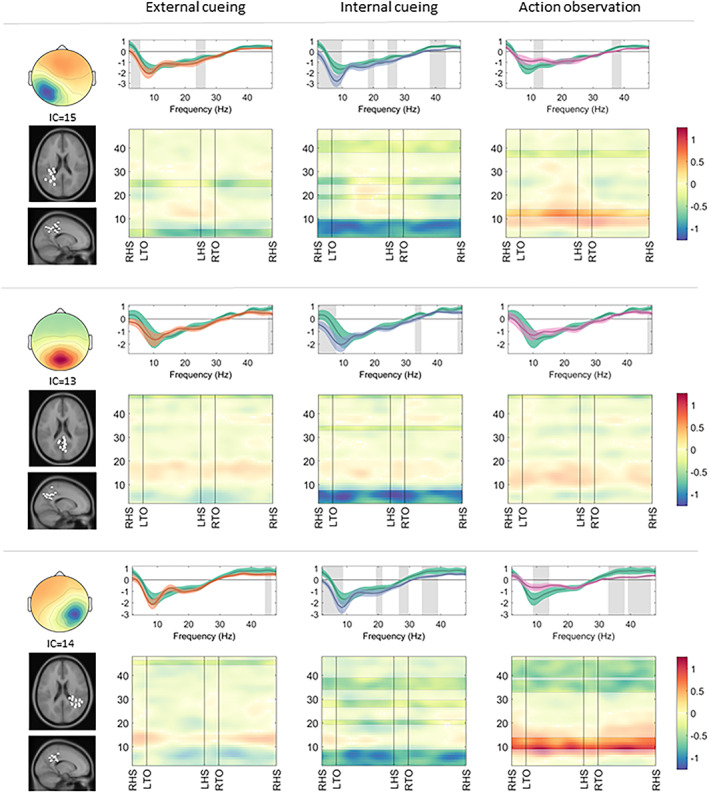
Parietal clusters: cortical activation during gait with a compensation strategy. Cortical activation spectra (top rows) show power changes during gait conditions relative to stance conditions (mean ± standard error), and cortical activation time‐frequency maps (bottom rows) illustrate relative differences (compensation strategy gait minus uncued gait) across the gait cycle. The negative values, primarily around theta (3–7Hz) and alpha (8–12Hz) frequency bands, indicate increased cortical activation during gait (top row, all conditions), which is sustained across the gait cycle (bottom rows). Significant effects (p < 0.05) of a given compensation strategy (external cueing: orange; internal cueing: blue; action observation: pink) in contrast to uncued gait (green) are highlighted (spectra: gray background; maps: unmasked colors). IC = independent component; LHS = left heel strike; LTO = left toe off; RHS = right heel strike; RTO = right toe off.
FIGURE 5.
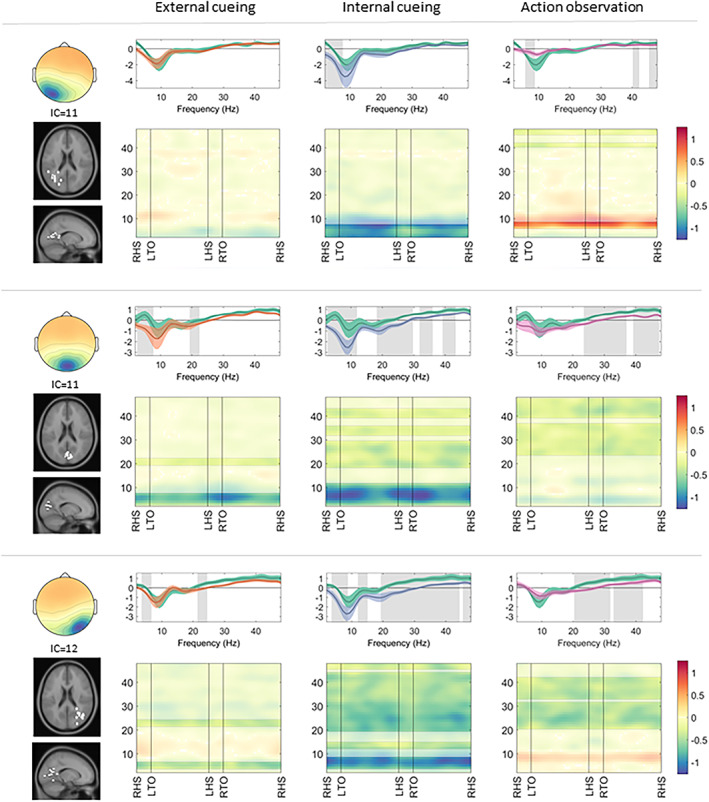
Occipital clusters: cortical activation during gait with a compensation strategy. Cortical activation spectra (top rows) show power changes during gait conditions relative to stance conditions (mean ± standard error), and cortical activation time‐frequency maps (bottom rows) illustrate relative differences (compensation strategy gait minus uncued gait) across the gait cycle. Significant effects (p < 0.05) of a given compensation strategy (external cueing: orange; internal cueing: blue; action observation: pink) in contrast to uncued gait (green) are highlighted (spectra: gray background; maps: unmasked colors). IC = independent component; LHS = left heel strike; LTO = left toe off; RHS = right heel strike; RTO = right toe off.
Relative to uncued gait, all 3 compensation strategy modalities induced a stronger decrease of beta band activity in the sensorimotor left (external cueing: 23–31Hz, t[12] = 3.19; internal cueing: 26–29Hz, t[12] = 2.93; action observation: 24–30Hz, t[12] = 3.33) and central (external cueing: 23–30Hz, t[11] = 2.65; internal cueing: 26–37Hz, t[11] = 2.45; action observation: 22–26Hz, t[11] = 2.87) clusters during gait, indicative of increased cortical activation. Beta band activity displayed a distinct modulation across the gait cycle, with the largest appearing increase in cortical activation during the double support phase (see Fig 2).
In the frontal cluster, applying internal cueing during gait induced a stronger decrease of beta band activity (17–23Hz, t[9] = 2.39) compared to uncued gait, indicative of increased cortical activation. External cueing and action observation did not induce a significant change in cortical activation of the frontal cluster compared to uncued gait (see Fig 3).
All parietal clusters displayed a stronger decrease in theta/alpha band activity during gait with internal cueing (left: 2–9Hz, t[14] = 3.27; central: 2–7Hz, t[12] = 2.85; right: 2–8Hz, t[13] = 2.76), indicative of increased cortical activation compared to uncued gait. The parietal left (19–20Hz, t[14] = 2.94 and 25–27Hz, t[14] = 2.65) and parietal right (33–38Hz, t[13] = 2.19) clusters also displayed a stronger decrease in beta band activity during gait with internal cueing. Contrastingly, the parietal left (11–14Hz, t[14] = −2.29) and parietal right (9–14Hz, t[13] = −2.84) clusters displayed a weaker decrease in alpha band activity during gait with action observation compared to uncued gait. Alpha and beta band activity of the parietal clusters was not significantly changed by the application of external cueing during gait (see Fig 4).
In the occipital clusters, a decrease in alpha band activity was apparent for gait with internal cueing (left: 2–7Hz, t[10] = 2.71; central: 3–11Hz, t[10] = 4.85; right: 4–8Hz, t[11] = 2.62) and external cueing (central: 3–7Hz, t[10] = 3.47; right: 4–6Hz, t[11] = 2.50), but not for gait with action observation. Gait with action observation induced a weaker decrease in alpha band activity in the left occipital cluster (6–8Hz, t[10] = −2.50), indicative of a relative decrease in cortical activity in this area compared to uncued gait. Beta band activity showed a stronger decrease in the occipital central (external cueing: 20–22Hz, t[10] = 3.39; internal cueing: 19–29Hz, t[10] = 3.28 and 32–35Hz, t[10] = 2.76; action observation: 24–37Hz, t[10] = 2.82) and occipital right (external cueing: 22–24Hz, t[11] = 2.28; internal cueing: 12–15Hz, t[11] = 2.49 and 20–44Hz, t[11] = 3.21; action observation: 21–31Hz, t[11] = 2.59 and 33–42Hz, t[11] = 2.01) clusters during the application of all 3 compensation strategies during gait compared to uncued gait (see Fig 5).
Discussion
We conducted a high‐density EEG gait study of 18 persons with Parkinson disease and gait impairment, aiming to explore the cortical correlates of 3 categories of compensation strategies: external cueing, internal cueing, and action observation. The main findings of the study are: (1) compared to uncued gait, the application of compensation strategies during gait resulted in altered cortical activity, which could not be solely attributed to sensory processing of the cueing modality; (2) beta band activity in the sensorimotor areas was decreased during gait while applying compensation strategies, indicating increased recruitment of this cortical area compared to uncued gait; and (3) cortical activation patterns differed depending on the type of compensation strategy that was applied, suggesting that each of the strategies engages a distinct cortical network.
Compensation Strategies Change Cortical Activation
Compared to uncued gait, the application of external cueing, internal cueing, and action observation during gait resulted in spectral power changes over sensorimotor, frontal, parietal, and occipital cortical areas, which is in agreement with previous findings of walking under goal‐directed conditions (eg, following internal or external cues). 33 , 36 In contrast to earlier work, we were able to confirm that the altered cortical activation we found was not merely attributable to increased cortical recruitment due to processing sensory input related to the cueing modality (ie, listening to a metronome, 37 watching another person walking 38 ) or engaging in a cognitive task (such as rhythmic counting 39 ). By including control conditions during stance (during which the same compensation strategies were applied) into our experimental protocol, we were able to correct for the stimulus‐related cortical activity, and consequently distil the cortical activation patterns that were most likely contributing to gait control. To our knowledge, we are the first to apply this approach to study the cortical correlates of compensation strategies for gait impairment in Parkinson disease.
Compensation Strategies Facilitate the Recruitment of Sensorimotor Areas
Gait is controlled through a complex supraspinal network. 40 It was previously concluded, from an exploratory activation of likelihood estimation meta‐analysis of functional magnetic resonance imaging studies, that persons with Parkinson disease have more difficulties recruiting cortical motor areas during gait compared to healthy controls, as illustrated by decreased cortical activation of the SMA. 41 Hypoactivity of the SMA has been associated with gait disturbances including increased cadence, decreased step length, and reduced arm swing in persons with Parkinson disease. 42 , 43 , 44 Importantly, after normalization of SMA activity (eg, through dopaminergic medication, DBS, or transcranial stimulation of the motor cortex), movement amplitude improves. 45 , 46 , 47 A recent study revealed that walking with instructed arm swing (which is a type of compensation strategy 10 ) increased step length and gait speed in persons with Parkinson disease and restored deficient cortical activation over the putative SMA. 48 Movement execution (eg, finger tapping, foot dorsiflexion, and walking) is associated with a relative power decrease in sensorimotor beta rhythm. 31 , 49 In the present study, we found a consistently larger decrease of beta band activity in sensorimotor clusters during the application of all 3 compensation strategies compared to uncued gait. This implies that the application of a compensatory strategy facilitates the recruitment of sensorimotor areas in persons with Parkinson disease and gait impairment. Our findings provide evidence for the hypothesis that central motor activation could be achieved through cues by making use of alternative motor pathways. 7 , 10 , 50 These alternative pathways likely involve corticostriatal loops that rely on different modes of gait control (ie, goal‐directed or emotional) compared to the primary automatic mode of gait control via the posterior putamen, which is most affected by dopaminergic denervation in Parkinson disease. 5 , 51
Cortical Correlates Differ between Compensation Strategies
Another important result of the present study is the finding that the 3 types of compensatory strategies produced different patterns of cortical activation. This implies that specific compensation strategies have unique underlying cortical mechanisms. This is in line with earlier hypotheses regarding the distinctive underpinnings of different categories of gait compensation strategies, 10 as specified in the introductory section.
Differences in cortical activation between strategies became most apparent through our finding that solely internal cueing elicited increased engagement of the frontal cluster compared to uncued gait. The use of auditory cues during walking did not significantly alter frontal brain activation compared to uncued gait, which contradicts the presumed major role of executive brain areas in external cueing. 10 However, our findings are in agreement with a recent EEG study investigating the cortical correlates of external (visual) cueing in Parkinson disease, which did not demonstrate involvement of the frontal cortices during gait with visual cues. 36 Furthermore, a functional near‐infrared spectroscopy pilot study on tactile cueing in persons with Parkinson disease also revealed that the use of (external) somatosensory cues does not increase activation of the prefrontal cortex compared to uncued gait. 52 Previously, the mechanisms underlying external cueing were postulated to improve gait by targeting frontostriatal circuitries. 5 , 10 Our findings do not provide support for this hypothesis, suggesting external cueing does not seem to rely on increased involvement of frontal executive areas.
Cortical activation in the parietal and occipital areas also differed between the 3 compensation strategies, especially in their elicited alpha band responses. Relative to uncued gait, alpha activity decreased during gait with internal and external cueing, but increased during gait with action observation. Alpha band oscillations in parieto‐occipital areas are essential for attentional processes, by facilitating the selection of relevant information. 53 External and internal cueing strategies have been hypothesized to work through aiding in filtering information and allocating attention to gait. 10 The relative alpha activity increase during gait with action observation may reflect active top‐down inhibition or disengagement of visual areas to suppress the processing of visual information irrelevant to the task (ie, anything other than the observed person's feet). 54
Given the wide variety of unique compensation strategies within each of the 7 proposed categories, 10 even different strategies within the same category may have distinct neural mechanisms (eg, visual vs tactile vs auditory cues in external cueing). Notably, patients often employ highly personalized strategies in daily life, comprising a combination of different categories of compensation strategies (eg, counting in combination with lifting the knees up high) rather than a "pure" form of external cueing, internal cueing, or action observation as assessed in the present study. 10 The EEG correlates of these personalized strategies may differ from the strategies examined in this study, but this remains to be uncovered by future studies.
Study Limitations
The following limitations should be considered when interpreting the results of this study. First, treadmill gait differs from overground walking. Because the EEG amplifier was too heavy to achieve true mobile recordings, we had to resort to treadmill walking to enable the acquisition of high‐density EEG data during actual gait. Moreover, treadmill walking allowed us to control for gait speed across conditions. However, walking on a treadmill most probably caused a substantial deflation of the positive effect of the compensation strategies on stride time variability, as stride time variability during gait on a treadmill is conceivably considerably lower compared to the variability during self‐paced overground walking. 55 This deflation in the positive effect on gait variability may, however, be beneficial for the purpose of this study, as the mental effects of sudden gait improvement with a strategy (eg, a decrease in anxiety compared to uncued gait) may also affect EEG results. Lastly, it can be argued that walking on a treadmill may act as a tactile cue for persons with Parkinson disease and gait impairment, therefore causing an overestimation of sensorimotor recruitment during uncued treadmill gait compared to uncued overground gait. Combined, our results are likely to underestimate the actual increase in cortical activation evoked by applying a compensation strategy during self‐paced overground gait.
Second, whereas EEG has excellent temporal resolution, spatial resolution is limited. 56 Consequently, the interpretation of the source localizations of brain activity only provides a rough estimation. It is difficult to reliably distinguish between the relative contributions of specific cortical areas of interest (ie, the SMA, premotor cortex, and primary motor cortex), and virtually impossible to explore the role of deeper, subcortical structures (ie, the cerebellum and basal ganglia) in gait control and compensation. Regardless, the most important advantage of using EEG rather than neuroimaging techniques with greater spatial resolution is the ability to measure cortical activity during actual gait, instead of imagined gait in a scanner.
Future Directions
The insights into the cortical correlates of compensation strategies may eventually be translated into more targeted therapeutic interventions for gait impairment in Parkinson disease, either as standalone treatments, such as closed‐loop DBS, or in conjunction with physical therapy (eg, by studying the potential benefits of the training of compensation strategies combined with transcranial direct current stimulation of relevant cortical areas 57 ). At present, the results of this study can already be implemented in clinical practice in support of much‐needed patient education on this topic. 9
A topic of future investigations could be the exploration of the EEG correlates of alleviating a freezing episode with the use of a variety of compensation strategies. The cortical mechanisms at play may be different when strategies are applied episodically as a way to alleviate a freezing episode, compared to when they are being applied during continuous walking. Another interesting topic of further research could be the evaluation of gait compensation strategies in persons with and without (severe) cognitive impairment using EEG. With disease progression, cognitive dysfunction may hamper the efficient switching from automated to goal‐directed gait control, 58 potentially hindering a person's ability to benefit from the application of compensation strategies.
Conclusions
The present study highlights that compensation strategies in Parkinson disease are likely to share an overarching working mechanism: using alternative pathways to achieve enhanced central motor activation. Our study also suggests that there is more than one route to control gait, and that different compensation strategies may rely on different cortical mechanisms. It is likely that humans in general use multiple routes to control gait (eg, in the context of urgent situations, or when playing tennis), 59 but that the presence of such alternative routes to motor control only becomes apparent in persons with Parkinson disease when the primary automatic motor pathway fails. 50
Author Contributions
A.T., V.W., B.R.B., T.S.‐E., and J.N. contributed to the conception and design of the study; A.T., T.B., and T.S.‐E. contributed to the acquisition and analysis of data; A.T., T.B., T.S.‐E., and J.N. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interests
Nothing to report.
Supporting information
FIGURE S1: Scalp projections and condition‐specific power spectral densities per cluster.
Acknowledgments
This study was supported by a ZonMW Veni grant to J.N. (16.196.022) and a ZonMW Vidi grant to V.W. (91.717.369). The Center of Expertise for Parkinson & Movement Disorders was supported by a center of excellence grant awarded by the Parkinson Foundation.
We thank Dr P. Praamstra for his contributions to this work.
References
- 1. Mirelman A, Bonato P, Camicioli R, et al. Gait impairments in Parkinson's disease. Lancet Neurol 2019;18:697–708. [DOI] [PubMed] [Google Scholar]
- 2. Nutt JG, Bloem BR, Giladi N, et al. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss D, Schoellmann A, Fox MD, et al. Freezing of gait: understanding the complexity of an enigmatic phenomenon. Brain 2020;143:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hallett M. The intrinsic and extrinsic aspects of freezing of gait. Mov Disord 2008;23:S439–S443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Redgrave P, Rodriguez M, Smith Y, et al. Goal‐directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 2010;11:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 1988;318:876–880. [DOI] [PubMed] [Google Scholar]
- 7. Gilat M, Ginis P, Zoetewei D, et al. A systematic review on exercise and training‐based interventions for freezing of gait in Parkinson's disease. NPJ Parkinsons Dis 2021;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait‐related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry 2007;78:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tosserams A, Wit L, Sturkenboom I, et al. Perception and use of compensation strategies for gait impairment by persons with Parkinson disease. Neurology 2021;97:e1404–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nonnekes J, Ruzicka E, Nieuwboer A, et al. Compensation strategies for gait impairments in Parkinson disease: a review. JAMA Neurol 2019;76:718–725. [DOI] [PubMed] [Google Scholar]
- 11. Herz DM, Eickhoff SB, Lokkegaard A, Siebner HR. Functional neuroimaging of motor control in Parkinson's disease: a meta‐analysis. Hum Brain Mapp 2014;35:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenderoth N, Toni I, Bedeleem S, et al. Information processing in human parieto‐frontal circuits during goal‐directed bimanual movements. Neuroimage 2006;31:264–278. [DOI] [PubMed] [Google Scholar]
- 13. Fan J, McCandliss BD, Fossella J, et al. The activation of attentional networks. Neuroimage 2005;26:471–479. [DOI] [PubMed] [Google Scholar]
- 14. Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 2001;14:S103–S109. [DOI] [PubMed] [Google Scholar]
- 15. Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta‐analysis. Hum Brain Mapp 2001;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 17. Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture 2009;30:459–463. [DOI] [PubMed] [Google Scholar]
- 18. Nonnekes J, Snijders AH, Nutt JG, et al. Freezing of gait: a practical approach to management. Lancet Neurol 2015;14:768–778. [DOI] [PubMed] [Google Scholar]
- 19. Oostenveld R, Praamstra P. The five percent electrode system for high‐resolution EEG and ERP measurements. Clin Neurophysiol 2001;112:713–719. [DOI] [PubMed] [Google Scholar]
- 20. Davis RB, Õunpuu S, Tyburski D, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci 1991;10:575–587. [Google Scholar]
- 21. Schaafsma JD, Giladi N, Balash Y, et al. Gait dynamics in Parkinson's disease: relationship to parkinsonian features, falls and response to levodopa. J Neurol Sci 2003;212:47–53. [DOI] [PubMed] [Google Scholar]
- 22. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community‐living older adults: a 1‐year prospective study. Arch Phys Med Rehabil 2001;82:1050–1056. [DOI] [PubMed] [Google Scholar]
- 23. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–1707. [DOI] [PubMed] [Google Scholar]
- 24. DeMott TK, Richardson JK, Thies SB, Ashton‐Miller JA. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med Rehabil 2007;86:125–132. [DOI] [PubMed] [Google Scholar]
- 25. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- 26. Mullen TR, Kothe CA, Chi YM, et al. Real‐time neuroimaging and cognitive monitoring using wearable dry EEG. IEEE Trans Biomed Eng 2015;62:2553–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bell AJ, Sejnowski TJ. An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 1995;7:1129–1159. [DOI] [PubMed] [Google Scholar]
- 28. Onton J, Makeig S. Information‐based modeling of event‐related brain dynamics. Prog Brain Res 2006;159:99–120. [DOI] [PubMed] [Google Scholar]
- 29. Los Amigos R. Observational gait analysis. Los Amigos Research and Education Institute, Rancho Los Amigos National Rehabilitation Center: Downey, CA, 2001. [Google Scholar]
- 30. Pfurtscheller G. The cortical activation model (CAM). Prog Brain Res 2006;159:19–27. [DOI] [PubMed] [Google Scholar]
- 31. Pfurtscheller G, Lopes da Silva FH. Event‐related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842–1857. [DOI] [PubMed] [Google Scholar]
- 32. Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 2006;159:211–222. [DOI] [PubMed] [Google Scholar]
- 33. Wagner J, Solis‐Escalante T, Grieshofer P, et al. Level of participation in robotic‐assisted treadmill walking modulates midline sensorimotor EEG rhythms in able‐bodied subjects. Neuroimage 2012;63:1203–1211. [DOI] [PubMed] [Google Scholar]
- 34. Wagner J, Solis‐Escalante T, Scherer R, et al. It's how you get there: walking down a virtual alley activates premotor and parietal areas. Front Hum Neurosci 2014;8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maris E, Oostenveld R. Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 2007;164:177–190. [DOI] [PubMed] [Google Scholar]
- 36. Stuart S, Wagner J, Makeig S, Mancini M. Brain activity response to visual cues for gait impairment in Parkinson's disease: an EEG study. Neurorehabil Neural Repair 2021;35(11):996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujioka T, Ross B. Auditory processing indexed by stimulus‐induced alpha desynchronization in children. Int J Psychophysiol 2008;68:130–140. [DOI] [PubMed] [Google Scholar]
- 38. Kaneko N, Yokoyama H, Masugi Y, et al. Phase dependent modulation of cortical activity during action observation and motor imagery of walking: an EEG study. Neuroimage 2021;225:117486. [DOI] [PubMed] [Google Scholar]
- 39. Lu L, Wang Q, Sheng J, et al. Neural tracking of speech mental imagery during rhythmic inner counting. Elife 2019;8:e48971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord 2013;28:1483–1491. [DOI] [PubMed] [Google Scholar]
- 41. Snijders AH, Takakusaki K, Debu B, et al. Physiology of freezing of gait. Ann Neurol 2016;80:644–659. [DOI] [PubMed] [Google Scholar]
- 42. Hanakawa T, Katsumi Y, Fukuyama H, et al. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain 1999;122:1271–1282. [DOI] [PubMed] [Google Scholar]
- 43. Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain 1994;117:1169–1181. [DOI] [PubMed] [Google Scholar]
- 44. Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain 2000;123:394–403. [DOI] [PubMed] [Google Scholar]
- 45. Tani N, Saitoh Y, Kishima H, et al. Motor cortex stimulation for levodopa‐resistant akinesia: case report. Mov Disord 2007;22:1645–1649. [DOI] [PubMed] [Google Scholar]
- 46. Fasano A, Piano C, De Simone C, et al. High frequency extradural motor cortex stimulation transiently improves axial symptoms in a patient with Parkinson's disease. Mov Disord 2008;23:1916–1919. [DOI] [PubMed] [Google Scholar]
- 47. Ballanger B, Lozano AM, Moro E, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: a [(15)O] H2O PET study. Hum Brain Mapp 2009;30:3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weersink JB, Maurits NM, van Laar T, de Jong BM. Enhanced arm swing improves Parkinsonian gait with EEG power modulations resembling healthy gait. Parkinsonism Relat Disord 2021;91:96–101. [DOI] [PubMed] [Google Scholar]
- 49. Pfurtscheller G, Aranibar A. Event‐related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 1977;42:817–826. [DOI] [PubMed] [Google Scholar]
- 50. Duysens J, Nonnekes J. Parkinson's kinesia paradoxa is not a paradox. Mov Disord 2021;36:1115–1118. [DOI] [PubMed] [Google Scholar]
- 51. Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol Dis 2015;82:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stuart S, Mancini M. Prefrontal cortical activation with open and closed‐loop tactile cueing when walking and turning in Parkinson disease: a pilot study. J Neurol Phys Ther 2020;44:121–131. [DOI] [PubMed] [Google Scholar]
- 53. Foxe JJ, Snyder AC. The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2011;2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9‐12 Hz) increase with memory load during retention in a short‐term memory task. Cereb Cortex 2002;12:877–882. [DOI] [PubMed] [Google Scholar]
- 55. Hollman JH, Watkins MK, Imhoff AC, et al. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture 2016;43:204–209. [DOI] [PubMed] [Google Scholar]
- 56. Pfister H, Kaynig V, Botha C, et al. Visualization in connectomics. Math Vis 2012;37.
- 57. Gilat M, Silva L, de Lima A, et al. Freezing of gait: promising avenues for future treatment. Parkinsonism Relat Disord 2018;52:7–16. [DOI] [PubMed] [Google Scholar]
- 58. Shine JM, Matar E, Ward PB, et al. Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 2013;136:3671–3681. [DOI] [PubMed] [Google Scholar]
- 59. Ballanger B, Thobois S, Baraduc P, et al. “Paradoxical kinesis” is not a hallmark of Parkinson's disease but a general property of the motor system. Mov Disord 2006;21:1490–1495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1: Scalp projections and condition‐specific power spectral densities per cluster.
Data Availability Statement
Data are available on reasonable request to the corresponding author.


