Abstract
Narcolepsy type 1 (NT1) is a chronic sleep disorder correlated with loss of hypocretin(orexin). In NT1 post‐mortem brains, we observed 88% reduction in corticotropin‐releasing hormone (CRH)‐positive neurons in the paraventricular nucleus (PVN) and significantly less CRH‐positive fibers in the median eminence, whereas CRH‐neurons in the locus coeruleus and thalamus, and other PVN neuronal populations were spared: that is, vasopressin, oxytocin, tyrosine hydroxylase, and thyrotropin releasing hormone‐expressing neurons. Other hypothalamic cell groups, that is, the suprachiasmatic, ventrolateral preoptic, infundibular, and supraoptic nuclei and nucleus basalis of Meynert, were unaffected. The surprising selective decrease in CRH‐neurons provide novel targets for diagnostics and therapeutic interventions. ANN NEUROL 2022;91:282–288
Narcolepsy type 1 (with cataplexy, NT1) is a chronic sleep disorder characterized by excessive daytime sleepiness, cataplexy, and disturbed nocturnal sleep. 1 NT1 research has focused on loss of hypocretin‐producing neurons in the hypothalamus. 2 , 3 Notable findings supporting neural loss were decreased hypothalamic expression of neuronal activity‐regulated pentraxin and dynorphin, both co‐expressed by hypocretin neurons. 4 , 5 A systematic search for involvement of hypothalamic neurons other than hypocretin neurons has never been performed in NT1.
In this study, we therefore systematically examined other hypothalamic cell groups, in a well‐defined post‐mortem collection of NT1 (n = 5) and controls (n = 5), including the suprachiasmatic nucleus (SCN), ventrolateral preoptic nucleus (VLPO), paraventricular nucleus (PVN), infundibular nucleus (IFN), supraoptic nucleus (SON), and nucleus basalis of Meynert (NBM).
Methods
Hypothalamic and locus coeruleus samples were obtained from the Netherlands Brain Bank (NBB). Permission for the autopsy, and use of brain material and clinical data for research purposes was obtained from the patient or next of kin. Five NT1 subjects, one of which had been treated chronically with opiates, and 5 matched controls free from neuropsychiatric disorders were included. All brains were systematically neuropathologically investigated. 6 The patients with NT1 had been treated by experienced somnologists (authors G.J.L. and R.F.; Table 1 and Table S1). The subject with NT1 who had been treated with opiates, after which symptoms improved and cataplexy disappeared, has been reported in a prior study, 7 where the hypocretin cell counts of NBB2008‐023 and NBB2010‐064 were determined. Because the median eminence of the 4 control was not available, 4 alternative controls (marked with * in the Table 1) were included. All potential confounding factors of patients and controls were matched for (see the Table 1; p ≥ 0.41). 8
TABLE 1.
Clinical‐Pathological Information
| NBB | Age | Sex | PMD | pH | BW | B‐AD | A | B‐PD | Cause of death | Narcolepsy medication |
|---|---|---|---|---|---|---|---|---|---|---|
| Narcolepsy type 1 | ||||||||||
| 2008–023 | 66 | F | 420 | 6.6 | 1,175 | 1 | A | 0 | Heart failure | Amphetamine; modafinil; sodium oxybate |
| 2018–018 | 82 | F | 330 | 6.5 | 1,165 | 2 | O | 0 | Pneumonia | Modafinil |
| 2018–091 | 71 | F | 395 | 6.3 | 1,215 | 4 | A | 0 | Heart failure, renal insufficiency | Sodium oxybate; modafinil |
| 2021–046 | 83 | M | 442 | 6.9 | 1,355 | NA | NA | NA | Severe heart failure, died within 24 h after fall with hip fracture | Sodium oxybate, paroxetine |
| Narcolepsy type 1 with chronic opiates | ||||||||||
| 2010–064 | 85 | F | 220 | 6.8 | 1,206 | 1 | O | 0 | Chronic pain syndrome with palliative sedation |
Morphine (9 yr); modafinil; morphine Midazolam (last 24 h) |
| Control | ||||||||||
| 2012–052 | 64 | F | 340 | 6.4 | 1,221 | 0 | O | 0 | Legal euthanasia | |
| 2000–022 | 83 | F | 470 | 6.5 | 1,072 | 2 | O | 0 | Heart failure, cachexia | |
| 2012–005 | 84 | F | 336 | 6.7 | 1,027 | 2 | A | 0 | Heart failure | |
| 2009–001 | 88 | M | 283 | 6.2 | 1,418 | 2 | A | 0 | Gastrointestinal bleeding | |
| 1998–104 | 74 | F | 445 | 7.0 | 1,167 | 2 | O | 0 | Cachexia in endstage pancreas carcinoma | |
| 2012–049* | 70 | F | 455 | 6.0 | 1,188 | 2 | A | 0 | Cachexia in endstage pancreas carcinoma | |
| 2000–142* | 82 | F | 330 | 6.6 | 1,280 | 1 | O | 0 | Myocardial infarction | |
| 1999–046* | 89 | F | 310 | 6.6 | 1,158 | 2 | O | 0 | Cardiac arrest | |
| 1994–076* | 78 | M | 505 | 6.6 | 1,407 | 2 | NA | NA | Cardiac arrhythmia | |
Intergroup differences in clock time and month of death were evaluated using the Mardia‐Watson‐Wheeler test.
* = included for median eminence measurements; A = amyloid; BW = brain weight gram; B‐AD = Braak AD (PMID,8307060); B‐PD = Braak PD (PMID,12498954); f = female; m = male; NA = not available; NBB = Netherlands Brain Bank identification number; PMD = post mortem delay in minutes.
Immunohistochemistry, Cell Counting
All formalin‐fixed paraffin‐embedded hypothalamus and locus coeruleus tissue blocks were coronally serially sectioned at 6 μm. Details of antigen retrieval procedures and specificity of primary antibodies are summarized in Table S2. Immunoreactivity was visualized by the avidin‐biotin complex method using DAB–nickel. 9 Six sections were treated with silver‐gold to enhance immunohistochemical staining. 10 The coefficient of variation (SD/mean × 100%) of 20% random sampling was 7.8% (calculated by counting one control subject 3 times).
As in our group's previous study, 11 neuronal cell numbers were determined blind for the patient information by counting the nucleolus as a unique marker for each neuron. The total number of neurons on one side of the hypothalamus was determined using the Cavalieri principle. 11 Completeness of the corticotropin‐releasing hormone (CRH), SCN vasopressin (AVP), and hypocretin cell counts were confirmed by graphically presenting the number of neurons counted in every section from rostral to caudal. In contrast, due to scarcity of hypothalamic sections, AVP, oxytocin, tyrosine hydroxylase (TH), and thyrotropin releasing hormone (TRH) staining were performed only in the peak section of the PVN. The CRH peak level had an excellent correlation with total CRH cell numbers (r = 0.926, p = 0.000***). The density of neuronal populations in the VLPO, NBM, SON, and IFN was similarly determined.
Prohormone convertase 1/3 (PC1/3) and secretagogin (SCGN) have both been reported to be partially co‐expressed in CRH neurons. 12 , 13 Double labeling of CRH with PC1/3 or SCGN was confirmed by immunofluorescent confocal imaging using the Leica microscope TSA SP5 at 20 times magnification.
Integrated Optical Density Image Analysis
Two microglia markers, ionized calcium‐binding adaptor molecule 1 (IBA1) 14 and human leukocyte antigen (HLA), and an enzyme for cortisol synthesis (11β‐hydroxysteroid‐dehydrogenase [11bHSD1]) were investigated. This enzyme was tested because high local cortisol levels may inhibit CRH‐expressing neurons in the PVN. Because glia cells have no nucleolus, the neuronal counting procedure could not be applied and the integrated optical density (IOD) was measured for IBA1, HLA, 11bHSD1, and CRH fibers in the median eminence. The collected images were transformed into OD images using a standard transformation curve, corrected for background. The image analysis system and procedures have been described in detail previously. 15 The IOD was calculated by multiplying the percentage of the positive‐stained masked area by the OD of the immunoreactivity signal in each section.
Statistical Analyses
NT1 and controls were compared using the exact Wilcoxon‐Mann‐Whitney U test (P) followed by Benjamini‐Hochberg corrections (q). The subject with NT1 with opiates was descriptively listed. Correlations were tested by Spearman's correlation coefficient. All p values are 2‐sided with 0.05 (*) as the significance threshold using SPSS Statistics 25. Percentage changes were calculated using the median values.
Results
We confirmed the substantial loss of 97% of the total number of hypocretin‐1 immunoreactive neurons in NT1 compared to the matched controls (p = 0.016*, q = 0.021*; Fig). In addition, most of the surviving hypocretin neurons appeared to be localized in the dorsomedial or peri‐fornical area, as has been described previously. 16 The subject with NT1 with chronic opiate use exhibited relatively more hypocretin neurons than the 3 typical subjects with NT1, as described before. 7
FIGURE 1.
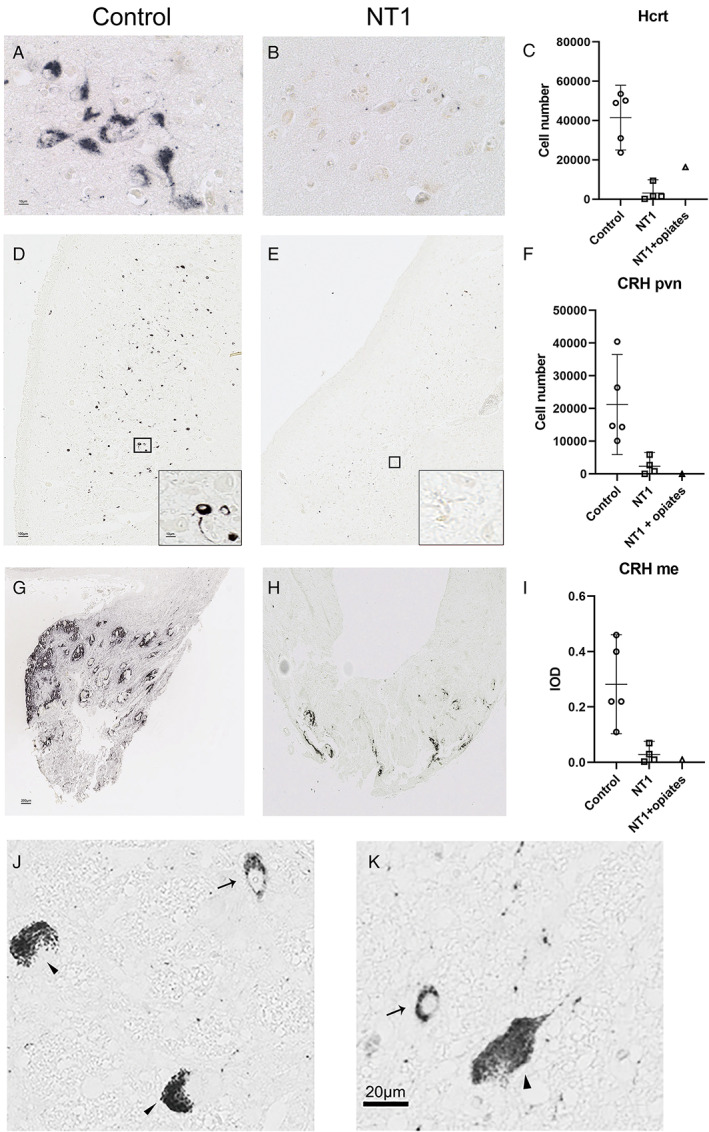
Patients with narcolepsy type 1 (NT1) have not only a loss of 97% of hypocretin/orexin (Hcrt) neurons but also an 88% reduction of corticotropin‐releasing hormone (CRH) expressing neurons in the paraventricular nucleus (pvn) and an 91% reduction of CRH‐positive fiber staining in the median eminence (me). (A) Control and (B) NT1 Hcrt immunoreactive cells. (C) The total number of Hcrt neurons is more than 97% reduced in patients with NT1 compared to controls. (D) Control and (E) NT1 CRH immunoreactive cells in the pvn. (F) The total number of CRH neurons in the PVN is 88% lower in patients with NT1 than in controls. The subject with NT1 with chronic opiates (NT1 + opiates) also showed few CRH neurons. (G) Control and (H) NT1 fiber immunoreactivity in the median eminence. (I) The total optical density of CRH in the peak level of median eminence is 91% lower in NT1 than in controls. (J, K) Photomicrographs showing pigmented locus coeruleus neurons (indicated with an arrow head) and (J) an example of the positive CRH staining (indicated with an arrow) in the locus coeruleus of control, (K) a positive CRH staining (indicated with an arrow) in the locus coeruleus of the subject with NT1. The 6 μm sections of the locus coeruleus area contained up to 20 CRH neurons, in both, the controls and the patients with NT1. Scale bar represents 10 μm for A; 100 μm for D and 10 μm for insert; 200 μm for G and H, and 20 μm for K. Bar plots show the mean and the lower Bound‐Upper Bound of the 95% confidence intervals in C, F, and I. [Color figure can be viewed at www.annalsofneurology.org]
The total number of CRH‐positive neurons in the PVN of the patients with NT1 was significantly reduced by 88% compared to the controls (p = 0.016*, q = 0.021*; see Fig). In addition, the subject with NT1 with chronic opiate use clearly showed a lower number of CRH‐positive neurons than the controls (see Fig). In agreement with the CRH‐cell staining loss, CRH fibers in the median eminence were significantly reduced by 91% in patients with NT1 compared to controls (p = 0.016*, q = 0.021*; see Fig). In contrast, the CRH neurons in the thalamus (ie, the paraventricular and parathenial thalamic nuclei and around the medial dorsal thalamic nucleus) and in the locus coeruleus and were unaffected (see Fig).
To test the possibility that this was a staining sensitivity issue, we performed 3 additional control experiments, none of which changed the results: (1) increasing the concentration of the same CRH antibody 10 times either with or without antigen retrieval with microwave; (2) using another CRH antibody, a rabbit polyclonal antibody Cat#5Bo; (3) using silver‐gold enhancement, 10 a method to enhance the visibility of immunohistochemical staining.
In contrast, densities of the other major neuronal cell types in the PVN were stable, including AVP, oxytocin, TH, and TRH. Microglia markers, IBA1 14 and HLA, and 11bHSD1, were similar in patients with NT1 and controls in the PVN. Additionally, all other quantified hypothalamic cell groups were stable (Table 2). Moreover, PC1/3 and SCGN staining was unaltered in the PVN of the subjects with NT1.
TABLE 2.
Immunocytochemistry and Quantification Procedures
| Structure | Primary antibody | Q | NT1 | Control | NT1 (opiates) | R (95% CI) |
|---|---|---|---|---|---|---|
| SCN | AVP | TC | 11,749 ± 7,679 | 11,786 ± 3,926 | 18,700 | 1.00 (0.08 1.93) |
| VLPO | Galanin | CD | 44 ± 17 | 35 ± 16 | 15 | 0.80 (−0.20 1.81) |
| MLH | Hcrt | TC | 4,175 ± 2,639 | 41,493 ± 5,931 | 16,384 | 13.10 (7.90 18.29) |
| NBM | ChAT | CD | 5,155 ± 189 | 4,954 ± 797 | 3,713 | 0.96 (0.47 1.45) |
| PVN | CRH | TC | 2,862 ± 1,704 | 21,197 ± 5,517 | 0 | 9.02 (2.50 15.54) |
| PVN/SON | AVP | CD |
PVN (70 ± 30) SON (96 ± 20) |
PVN (49 ± 11) SON (127 ± 27) |
PVN (86) SON (137) |
1.34 (0.30 2.37) 1.16 (0.46 1.85) |
| PVN/SON | Oxy | CD |
PVN (34 ± 4) SON (0.056 ± 0.05) |
PVN (48 ± 10) SON (0.03 ± 0.01) |
PVN (16) SON (0.02) |
2.75 (0.51 5.00) 0.46 (−0.12 1.04) |
| PVN | Th | CD | 14 ± 6 | 27 ± 9 | 4 | 1.89 (0.22 3.56) |
| TRH | CD | 0.005 ± 0.008 | 0.014 ± 0.012 | 0.0000 | 3.33 (0.38 6.29) | |
| PVN | SCG | CD | 2 ± 2 | 15 ± 12 | 0.010 | 6.12 (−8.11 20.35) |
| PVN | PC1/3 | CD | 7 ± 3 | 14 ± 9 | 0.002 | 2.10 (−1.53 5.73) |
| IFN | NPY | CD | 10 ± 3 | 16 ± 6 | 35 | 1.59 (0.03 3.15) |
| IFN | POMC | CD | 16 ± 6 | 28 ± 11 | 40 | 2.01 (−0.25 4.27) |
| PVN | 11bHSD1 | IOD | 0.03 ± 0.001 | 0.003 ± 0.001 | 0.003 | 1.11 (−0.28 2.50) |
| PVN | HLA | IOD | 0.026 ± 0.006 | 0.030 ± 0.020 | 0.015 | 1.01 (−0.86 2.88) |
| PVN | IBA1 | IOD | 0.010 ± 0.003 | 0.010 ± 0.004 | 0.018 | 1.00 (−0.11 2.11) |
Data of the groups are expressed as mean ± standard error of the mean (SEM).
11bHSD1 = 11β‐hydroxysteroid‐dehydrogenase; AVP = arginine vasopressin; ChAT = choline acetyltransferase; CD = cell density (cell/mm2); CRH = corticotropin‐releasing hormone; Hcrt = hypocretin‐1 /orexin A; HLA = human leukocyte antigen‐DP, DQ, DR; IBA1 = Ionized calcium binding adaptor molecule 1; IFN = infundibular nucleus; IOD = integrated optical density; NBM = nucleus basalis of Meynert; NPY = neuropeptide Y; NT1 = narcolepsy type 1 (narcolepsy with cataplexy); NT1 with opiates = NBB2010‐064; Oxy = oxytocin; PC1/3 = prohormone convertase 1/3 double stain with CRH; POMC = proopiomelanocortin; POMC = proopiomelanocortin; PVN = paraventricular nucleus; Q = quantification; SCN = suprachiasmatic nucleus; SCG = secretagogin double stain with CRH; SON = supraoptic nucleus; TC = total cell number; Th = tyrosine hydroxylase; TRH = thyrotropin releasing hormone; MLH = medial lateral hypothalamus; VLPO = ventrolateral preoptic nucleus; R = mean of ratio (controls/NT1 mean); 95% CI = 95% confidence interval (lower bound upper bound).
Discussion
We found that patients with NT1 show not only 97% loss of hypocretin neurons but also an 88% reduction of CRH‐expressing neurons in the PVN compared with controls, whereas CRH‐positive neurons in the thalamus and in the locus coeruleus were unaffected. It should be noted that, in the future, it is of interest to also study other CRH‐containing brain regions, such as the amygdala. A 91% reduction of CRH‐staining fibers in the median eminence was observed. The CRH‐positive neurons in the PVN are the primary group of neurons of the HPA axis and project to the median eminence containing the portal capillaries of the pituitary. 8 In contrast, the other major neuronal types intermingled with the CRH‐positive neurons in the PVN were spared, including AVP‐positive, oxytocin‐positive, TH‐positive, and TRH‐positive neurons. AVP and oxytocin neurons in both the PVN and SON all were stable. No changes were observed in the neuronal densities in VLPO, 17 SCN, NBM, or IFN.
Theoretically, the observed low number of CRH neurons might be due to extremely low CRH concentrations below the threshold for immunohistochemical detection. However, we tried 3 different ways to enhance the staining signal, none of which changed the results. Earlier findings showed that chronic treatment with opiates can increase the number of immunohistochemically stained hypocretin cells, whereas cataplexy was ameliorated. 7 Of interest, opiate treatment had no effect on the CRH‐neuron number in the PVN of this subject.
A limitation of the present study is the low number of subjects. Because NT1 is a rare disorder, 1 there are very few postmortem brains available for research. However, it is unlikely that this has influenced our conclusions, because the reduction in CRH neurons was so striking. In addition, even with this small size, we confirmed the greatly reduced number of hypocretin cells. 2 , 3 In addition, it is unclear whether current narcolepsy animal models have diminished CRH neuron numbers in the PVN. Another question is whether the loss of hypocretin‐expressing and CRH‐expressing neurons are functionally related.
In conclusion, 2 decades ago, the discovery that hypocretin production is almost completely lost in human NT1 2 , 3 led to the successful establishment of the diagnostic standard (i.e. low/undetectable hypocretin‐1 levels in CSF). 18 Our study indicates that besides hypocretin deficiency, there is also a marked loss of CRH‐positive neurons selectively in the PVN. This may explain earlier findings, such as a 60% reduction in the basal secretion of ACTH in patients with NT1, 19 and lower plasma levels of cortisol after dexamethasone suppression. 20 The unaffected number of AVP neurons in the biological clock may explain the normal circadian rhythm of adrenocorticotropic hormone. 19 The marked loss of CRH‐positive neurons in the PVN may provide a novel target for diagnostics and therapeutic interventions, and for identifying the target of the presumed auto‐immune cause of narcolepsy.
Author Contributions
L.S., R.F., G.J.L., and D.F.S. contributed to the conception and design of the study. L.S., D.F.S., R.B., R.F., and G.J.L. contributed to the acquisition and analysis of data. L.S., R.F., G.J.L., and D.F.S. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
L.S., R.F., and G.J.L. have received research support from Jazz Pharma (Solriamfetol is a Jazz Pharma medication used in the treatment of excessive sleepiness) and Bioprojet (Pitolisant, manufactured by Bioprojet, is used in the treatment of narcolepsy). R.F. and G.J.L. have received consultancy fees from Bioprojet.
Supporting information
Table S1 Clinical‐pathological information
Table S2 Immunocytochemistry and quantification procedures
Acknowledgments
L.S. has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant (Agreement No. 707404) and has been awarded the Young Scientist Award 2020 by the European Narcolepsy Network (supported by the Klaus‐Grawe Foundation). D.F.S. and L.S. were supported by Friends of the Netherlands Institute for Neuroscience Foundation. This work was partially supported by research grants from the Bioprojet‐Biotech, Saint Gregoire, France. The funding agencies had no role in the design of the study, nor in the collection, analysis, or interpretation of data.
The authors are greatly indebted to the Netherlands Brain Bank and the following persons for their invaluable support: R.W.H. Verwer, J. Coppens, J. van Heerikhuize, M. Kooreman, M. Groot, J.R. Zhou, J. Shen, S. Linssen, Z. Harteman, and P. Reinbold. The authors are grateful to Professor I. Huitinga from the Netherlands Institute for Neuroscience for providing the brain samples and material from multiple sclerosis brain tissue that served as positive controls. Dr. D. Roelen from the LUMC did the HLA genotyping. We thank Professors G. Martens and J. Homberg from the Radboud University for the suggestions and valuable discussion.
References
- 1. Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy — clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol 2019;15:519–539. [DOI] [PubMed] [Google Scholar]
- 2. Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000;6:991–997. [DOI] [PubMed] [Google Scholar]
- 3. Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blouin AM, Thannickal TC, Worley PF, et al. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology 2005;65:1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crocker A, Espana RA, Papadopoulou M, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 2005;65:1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van de Nes JA, Kamphorst W, Ravid R, Swaab DF. Comparison of beta‐protein/A4 deposits and Alz‐50‐stained cytoskeletal changes in the hypothalamus and adjoining areas of Alzheimer's disease patients: amorphic plaques and cytoskeletal changes occur independently. Acta Neuropathol 1998;96:129–138. [DOI] [PubMed] [Google Scholar]
- 7. Thannickal TC, John J, Shan L, et al. Opiates increase the number of hypocretin‐producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 2018;10:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swaab DF. The human hypothalamus: basic and clinical aspects. Part 1: nuclei of the human hypothalamus. Amsterdam: Elsevier, 2003. [Google Scholar]
- 9. Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain 2007;130:1577–1585. [DOI] [PubMed] [Google Scholar]
- 10. Vujovic N, Gooley JJ, Jhou TC, Saper CB. Projections from the subparaventricular zone define four channels of output from the circadian timing system. J Comp Neurol 2015;523:2714–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shan L, Bossers K, Unmehopa U, et al. Alterations in the histaminergic system in Alzheimer's disease: a postmortem study. Neurobiol Aging 2012;33:2585–2598. [DOI] [PubMed] [Google Scholar]
- 12. Dong W, Seidel B, Marcinkiewicz M, et al. Cellular localization of the prohormone convertases in the hypothalamic paraventricular and supraoptic nuclei: selective regulation of PC1 in corticotrophin‐releasing hormone parvocellular neurons mediated by glucocorticoids. J Neurosci 1997;17:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romanov RA, Alpár A, Zhang M, et al. A secretagogin locus of the mammalian hypothalamus controls stress hormone release. EMBO J 2015;34:36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honda M, Arai T, Fukazawa M, et al. Absence of ubiquitinated inclusions in hypocretin neurons of patients with narcolepsy. Neurology 2009;73:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao S‐F, Qi X‐R, Zhao J, et al. Decreased NOS1 expression in the anterior cingulate cortex in depression. Cereb Cortex 2013;23:2956–2964. [DOI] [PubMed] [Google Scholar]
- 16. Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol 2003;13:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gavrilov YV, Ellison BA, Yamamoto M, et al. Disrupted sleep in narcolepsy: exploring the integrity of galanin neurons in the ventrolateral preoptic area. Sleep 2016;39:1059–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000;355:39–40. [DOI] [PubMed] [Google Scholar]
- 19. Kok SW, Roelfsema F, Overeem S, et al. Dynamics of the pituitary‐adrenal ensemble in hypocretin‐deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time‐keeping by the master pacemaker. J Clin Endocrinol Metab 2002;87:5085–5091. [DOI] [PubMed] [Google Scholar]
- 20. Maurovich‐Horvat E, Keckeis M, Lattová Z, et al. Hypothalamo‐pituitary‐adrenal axis, glucose metabolism and TNF‐α in narcolepsy. J Sleep Res 2014;23:425–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical‐pathological information
Table S2 Immunocytochemistry and quantification procedures


