Abstract
Background
Tezepelumab is a human monoclonal antibody that blocks activity of thymic stromal lymphopoietin (TSLP). In the phase IIb PATHWAY study (NCT02054130), tezepelumab significantly reduced annualized asthma exacerbation rates (AAERs) versus placebo in adults with severe, uncontrolled asthma. We evaluated the effects of tezepelumab in reducing type 2 (T2) inflammatory biomarker levels in the PATHWAY population, and the relationship between baseline T2 biomarker levels and AAER.
Methods
Adults with severe, uncontrolled asthma (n = 550) were randomized to tezepelumab (70 mg or 210 mg every 4 weeks, or 280 mg every 2 weeks) or placebo for 52 weeks. Blood eosinophil count, fractional exhaled nitric oxide (FeNO), and serum total immunoglobulin (Ig)E, interleukin (IL)‐5, IL‐13, periostin, thymus and activation‐regulated chemokine (TARC), and TSLP were measured at baseline and over 52 weeks. AAERs were analyzed by baseline threshold (high/low) biomarker levels.
Results
Positive correlations were observed between T2 inflammatory biomarkers (blood eosinophil count, FeNO, IL‐5, IL‐13 and periostin) at baseline. At Week 52, treatment with tezepelumab 210 mg reduced all biomarker levels measured from baseline versus placebo. Exacerbations were reduced by 55–83% in the pooled tezepelumab cohort versus placebo, irrespective of baseline blood eosinophil count, FeNO, or serum total IgE, IL‐5, IL‐13, periostin, TARC, or TSLP, when these biomarkers were assessed individually.
Conclusion
At baseline, positive correlations between specific T2 inflammatory biomarkers were observed. Tezepelumab reduced multiple T2 inflammatory biomarkers, which indicates decreased airway inflammation, and reduced exacerbations irrespective of baseline T2 biomarker profiles in patients with severe asthma.
Keywords: asthma, biomarkers, inflammation
This phase IIb, randomized, double‐blind, placebo‐controlled clinical trial evaluates the effects of tezepelumab in reducing type 2 inflammatory biomarkers and the relationship between baseline biomarker levels and annualized asthma exacerbation rates. Exacerbations are reduced by 55–83% in the pooled tezepelumab cohort compared to placebo, irrespective of baseline type 2 biomarker levels. At Week 52, treatment with tezepelumab reduces the levels of all measured biomarkers.Abbreviations: FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; TARC, thymus and activation‐regulated chemokine; TSLP, thymic stromal lymphopoietin

Abbreviations
- AAER
annualized asthma exacerbation rate
- CI
confidence interval
- FeNO
fractional exhaled nitric oxide
- Ig
immunoglobulin
- IL
interleukin
- MAD
median absolute deviation
- ppb
parts per billion
- Q2W
every 2 weeks
- Q4W
every 4 weeks
- RR
rate ratio
- T2
type 2
- TARC
thymus and activation‐regulated chemokine
- TSLP
thymic stromal lymphopoietin
1. INTRODUCTION
Asthma affects more than 300 million people worldwide, of whom up to 10% have severe asthma. 1 , 2 Approximately half of people with severe asthma have uncontrolled disease despite adherence to inhaled therapies, including glucocorticoids, long‐acting β2 agonists, and long‐acting muscarinic agonists. 3 The majority of these patients have evidence of type 2 (T2) inflammation, with high blood eosinophil counts and fractional exhaled nitric oxide (FeNO) levels. 4 These patients are candidates for biologic medications directed against specific T2 inflammatory mediators (eg, interleukin [IL]‐5, IL‐4/IL‐13, and immunoglobulin [Ig]E). However, these biologics have indications limited to eosinophilic and allergic asthma, and have been shown to be less effective in patients with low levels of T2 biomarkers. 5 , 6 , 7 Thus, there is a need for a treatment approach that benefits patients across a broad range of inflammatory profiles.
Thymic stromal lymphopoietin (TSLP) is an epithelial cell‐derived cytokine that is implicated in the initiation and persistence of airway inflammation in asthma. 8 , 9 , 10 TSLP is released in response to airborne triggers associated with asthma exacerbations including allergens, viruses, cigarette smoke, and pollutants. 8 , 11 , 12 Levels of TSLP are increased in the airways of patients with asthma and correlate with disease severity. 10 , 13 , 14 TSLP plays a major role in the activation of dendritic cells, leading to the differentiation and maturation of T‐helper 2 cells, 9 , 15 as well as activation of mast cells and type 2 innate lymphoid cells (ILC2s). 8 , 16
Tezepelumab is a human monoclonal antibody (IgG2λ) that binds specifically to TSLP, preventing it from interacting with its heterodimeric receptor, comprised of the TSLP receptor chain and the IL‐7 receptor α‐chain. 17 , 18 , 19 In the phase IIb PATHWAY study, tezepelumab was well tolerated and reduced asthma exacerbations by up to 71% versus placebo in patients with severe, uncontrolled asthma, irrespective of baseline blood eosinophil count, FeNO level, and T2 inflammatory status (T2‐high was defined as IgE >100 IU/ml and a blood eosinophil count ≥140 cells/μl). 18 , 20 This post hoc analysis of the PATHWAY study investigated: (a) correlations between levels of a broad range of T2 inflammatory biomarkers (including cytokines and chemokines) at baseline; (b) the effects of tezepelumab on levels of these biomarkers; and (c) the relationship between baseline T2 biomarker levels and response to tezepelumab as measured by the annualized asthma exacerbation rate (AAER).
2. METHODS
2.1. Study design and patients
PATHWAY was a phase IIb, multicenter, randomized, double‐blind, placebo‐controlled trial (ClinicalTrials.gov identifier: NCT02054130). The full design and inclusion and exclusion criteria have been described previously. 18 Eligible patients were current non‐smokers, 18–75 years old, with severe asthma that was inadequately controlled despite treatment with medium‐dose (250–500 μg/day fluticasone dry powder inhaler or equivalent) or high‐dose (> 500 μg/day fluticasone dry powder inhaler or equivalent) inhaled corticosteroids plus a long‐acting β2 agonist. Patients were required to have experienced at least two asthma exacerbations requiring systemic corticosteroids, or at least one exacerbation requiring hospitalization, in the 12 months before trial entry. Study participants were randomized 1:1:1:1 to receive subcutaneous injections of tezepelumab 70 mg every 4 weeks (Q4W), 210 mg Q4W, 280 mg every 2 weeks (Q2W), or placebo Q2W, for 52 weeks. The primary efficacy end point was the AAER over 52 weeks.
This trial was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation guidelines for good clinical practice, and applicable regulatory requirements. Approvals from independent ethics committees were obtained, and all patients provided written informed consent before participating in the study.
2.2. Biomarker analyses
Correlations between the following biomarkers at baseline were assessed: blood eosinophil count, FeNO level (also measured at Week 8), and serum concentrations of total IgE, IL‐5, IL‐13, periostin, thymus and activation‐regulated chemokine (TARC, also known as chemokine [C‐C motif] ligand 17 [CCL‐17]), and TSLP. Change from baseline to Weeks 4, 12, 20, 28, 40, and 52 in these biomarkers was also assessed.
Blood eosinophil count was determined at a centralized laboratory using a standard clinical hematology analyser with automated or manual differentials using Wright–Giemsa stains. FeNO was measured using a NIOX MINO airway inflammation monitor (Circassia Pharmaceuticals Inc.) to perform a standardized single‐breath test, as per American Thoracic Society recommendations. 21 Immunoassays were used to determine serum total IgE levels (Phadia, Thermo Fisher), IL‐5 (Quanterix), IL‐13 (Quanterix), periostin (Abbott), and TARC (R&D Systems). Post‐baseline IL‐5 and TARC levels were not assessed in the tezepelumab 70 mg Q4W group. An electrochemiluminescence fit‐for‐purpose S‐PLEX assay (Meso Scale Discovery) was used to quantify serum levels of TSLP.
2.3. Analysis of exacerbation rates according to baseline levels of T2 biomarkers
An asthma exacerbation was defined as worsening of asthma that led to use of systemic corticosteroids for at least 3 days, an emergency room visit that required systemic corticosteroids for at least 3 days, or hospitalization. The AAER was defined as the total number of asthma exacerbations in each treatment arm/total person‐year follow‐up. The AAER was analyzed according to baseline biomarker subgroup using standard, clinically used cut‐offs (where available) 22 , 23 , 24 , 25 , 26 or median values.
2.4. Statistical analyses
Correlation values for baseline biomarker levels were generated using the Spearman rank method, and median percentage change from baseline assessments was used for time‐course plots. Nominal p values are presented for correlations between biomarker levels at baseline.
To evaluate the relationship between high and low baseline biomarker status and AAER, we defined patient subgroups with the following baseline biomarker thresholds: a blood eosinophil count of 150 or 300 cells/µl, FeNO levels of 25 or 50 ppb, and levels of IL‐5, IL‐13, IgE, TARC, periostin, and TSLP above or below the median value.
Rate ratios and 95% confidence intervals for AAER within each baseline biomarker category (high or low) were estimated from a negative binomial regression model, with treatment group (new binary indicator: all tezepelumab doses pooled together into one group vs placebo), baseline blood eosinophil count (≥250 or <250 cells/μl), and baseline inhaled corticosteroid dose level (medium or high) as covariates. The results of these analyses were presented graphically using a forest plot.
The effect of treatment on the unadjusted exacerbation rate over 52 weeks according to baseline biomarker level ranges was visualized using locally weighted regression and smoothing scatterplots (LOESS) 27 for placebo and pooled tezepelumab dose groups. LOESS plots were generated to show the relative contribution of each variable to the AAER (DIRECT method with smoothing parameter equal to 1). The x‐axis was truncated to capture clinically relevant values for each baseline biomarker.
Data presented focus primarily on the tezepelumab 210 mg Q4W (selected for phase III clinical studies) and pooled tezepelumab dose groups.
3. RESULTS
3.1. Baseline characteristics in patients from PATHWAY
In total, 550 patients were randomized to receive subcutaneous tezepelumab 70 mg Q4W (n = 138), 210 mg Q4W (n = 137), 280 mg Q2W (n = 137), or placebo Q2W (n = 138). Baseline demographics and clinical characteristics of the overall population have been previously reported and were similar across all treatment groups. 18 Baseline levels of T2 biomarkers are reported in Table 1.
TABLE 1.
Baseline clinical characteristics
|
Placebo (N = 138) |
Tezepelumab 210 mg Q4W (N = 137) |
Pooled tezepelumab (N = 412) |
|
|---|---|---|---|
| Blood eosinophil count, cells/μl | |||
| Mean (±SD) | 380 (328) | 365 (351) | 367 (361) |
| Median (min, max) | 275 (0, 1,870) | 280 (0, 3,180) | 280 (0, 3,990) |
| FeNO, ppb | |||
| Mean (±SD) | 37.8 (39.7) | 31.5 (29.8) | 33.5 (38.1) |
| Median (min, max) | 22.0 (3.5, 276.3) | 22.0 (4.0, 152.5) | 22.0 (2.0, 349.0) |
| IgE, IU/ml | |||
| Mean (±SD) | 474.5 (1,271.6) | 483.9 (1,402.5) | 388.2 (1,018.4) |
| Median (min, max) | 148.2 (6.0, 11,859.6) | 135.4 (2.0, 11,429.6) | 129.6 (2.0, 11,429.6) |
| IL−5, pg/ml | |||
| n | 132 | 126 | 255 |
| Mean (±SD) | 1.13 (1.77) | 1.88 (7.33) | 1.47 (5.23) |
| Median (min, max) | 0.66 (0.03, 15.00) | 0.68 (0.04, 80.16) | 0.60 (0.03, 80.16) |
| IL−13, pg/ml | |||
| n | 101 | 89 | 294 |
| Mean (±SD) | 0.06 (0.08) | 0.06 (0.09) | 0.07 (0.11) |
| Median (min, max) | 0.04 (0.008, 0.63) | 0.03 (0.008, 0.63) | 0.04 (0.008, 1.33) |
| Periostin, ng/ml | |||
| Mean (±SD) | 23.1 (10.1) | 23.2 (10.4) | 22.9 (11.0) |
| Median (min, max) | 20.9 (8.6, 76.1) | 22.0 (6.2, 63.9) | 21.1 (6.2, 107.6) |
| TARC, pg/ml | |||
| n | 137 | 133 | 266 |
| Mean (±SD) | 415.3 (297.0) | 405.7 (254.9) | 411.8 (264.8) |
| Median (min, max) | 315.8 (65.4, 1,795.8) | 342.5 (94.9, 1,300.9) | 351.3 (36.5, 2,000.0) |
| TSLP, fg/ml | |||
| n | 138 | 136 | 408 |
| Mean (±SD) | 355.0 (178.7) | 422.8 (289.8) | 376.3 (353.8) |
| Median (min, max) | 313.6 (66.3, 969.8) | 355.0 (65.5, 2,260.0) | 303.5 (19.5, 4,735.8) |
Abbreviations: FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; ppb, parts per billion; Q4W, every 4 weeks; SD, standard deviation; TARC, thymus and activation‐regulated chemokine; TSLP, thymic stromal lymphopoietin.
3.2. Baseline correlations between biomarkers
At baseline, positive correlations were observed for IL‐5 with blood eosinophil counts (r = .7) and levels of FeNO (r = .5), periostin (r = .4), and IL‐13 (r = .7) (Figure 1A–D; all nominal p < .0001). Positive correlations were also observed at baseline for IL‐13 with blood eosinophil counts (r = .7) and levels of FeNO (r = .4) and periostin (r = .5) (Figure 1E–G; all nominal p < .0001). Blood eosinophil counts and FeNO levels were positively correlated at baseline (Figure 1H; r = .4; nominal p < .0001). No correlations were found between IgE, TARC, or TSLP and any of the other biomarkers assessed.
FIGURE 1.

Baseline correlations between serum levels of IL‐5 and IL‐13 and other biomarkers of inflammation. Spearman correlation coefficients (r) are shown. Data are presented on the log scale. Abbreviations: FeNO, fractional exhaled nitric oxide; IL, interleukin; ppb, parts per billion
3.3. Effect of tezepelumab treatment on biomarker levels over 52 weeks
Blood eosinophil counts, FeNO levels, and serum levels of total IgE, IL‐5, IL‐13, periostin, and TARC were reduced from baseline in patients who received tezepelumab 210 mg Q4W (Figure 2). Blood eosinophil counts and levels of FeNO, IL‐5, IL‐13, and periostin were reduced at the first post‐baseline measurement time point (Week 4), while serum total IgE and TARC levels decreased gradually until end of treatment at Week 52. Reductions in all biomarkers measured were maintained throughout the 52‐week treatment period. Data on change from baseline to Week 52 in serum TSLP levels are not shown as the assay used to measure TSLP cannot distinguish between unbound TSLP and TSLP bound to tezepelumab.
FIGURE 2.
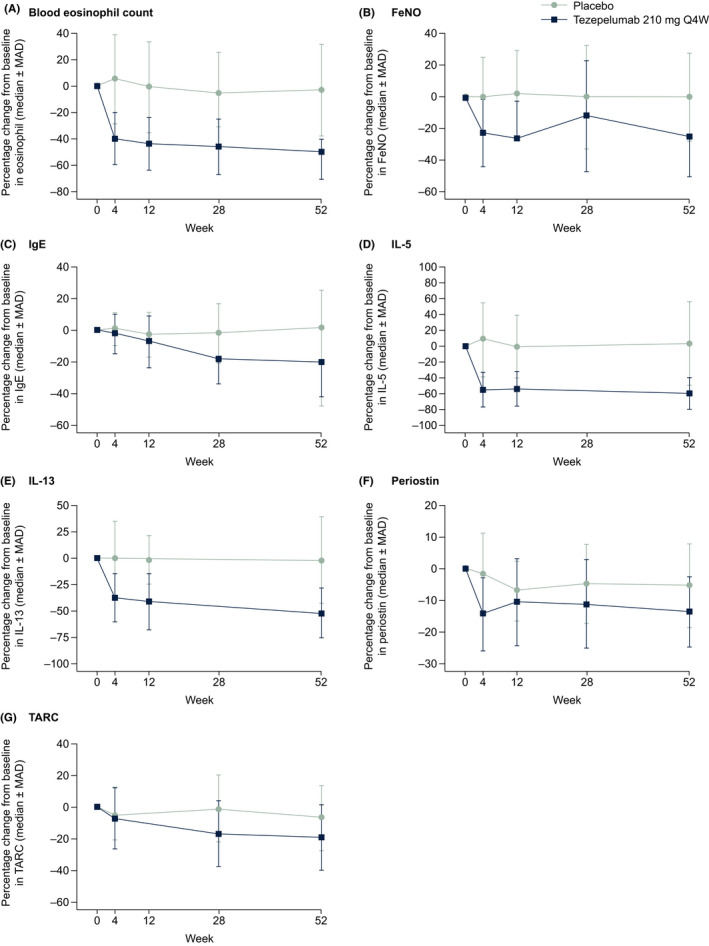
Median percentage change from baseline in biomarker levels over 52 weeks. Abbreviations: FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; MAD, median absolute deviation; Q4W, every 4 weeks; TARC, thymus and activation‐regulated chemokine
At Week 52, median percentage changes from baseline in levels of all biomarkers measured were greater in the tezepelumab 210 mg Q4W group than in the placebo group (Figure 3). At Week 52, the most notable differences between the tezepelumab 210 mg Q4W group and the placebo group were observed in levels of IL‐5 (−60.0% vs 2.8%) and IL‐13 (−51.6% vs −1.5%), blood eosinophil counts (−50.0% vs −3.0%), levels of FeNO (−25.2% vs 0.0%), serum total IgE (–20.1% vs –1.4%), and TARC (−19.0% vs −6.7%). Minimal changes in serum levels of periostin were observed during treatment (−13.6% vs −5.4%). Median percentage changes from baseline to Week 52 in biomarker levels in patients grouped by baseline blood eosinophil counts (<150 cells/µl and ≥150 cells/µl), and FeNO levels (<25 ppb and ≥25 ppb) are presented in Table S1. The greatest reductions from baseline in biomarker levels in the pooled tezepelumab group were observed in patients with baseline blood eosinophil counts of at least 150 cells/µl and FeNO levels of at least 25 ppb, followed by patients with blood eosinophil counts of at least 150 cells/µl and FeNO levels below 25 ppb.
FIGURE 3.
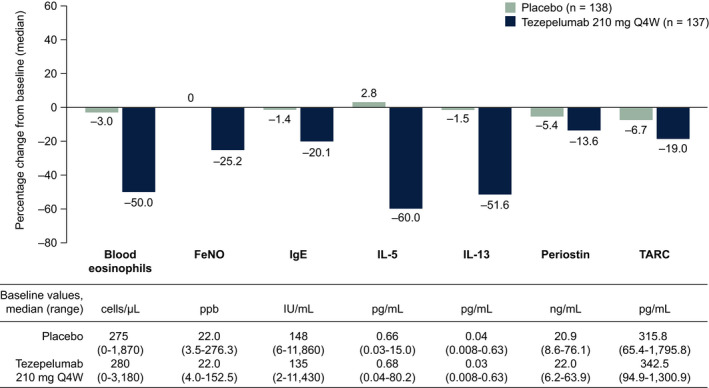
Median percentage change from baseline to Week 52 in biomarker levels. FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; ppb, parts per billion; Q4W, every 4 weeks; TARC, thymus and activation‐regulated chemokine
3.4. Effect of tezepelumab treatment on AAER: relationship to baseline levels of T2 biomarkers
Owing to the small number of patients in the biomarker subgroups, results are reported for the pooled tezepelumab treatment group only. At Week 52, the AAER was reduced from baseline by 66% in the pooled tezepelumab group versus placebo. Reductions from baseline in AAER at Week 52 were observed irrespective of baseline high and low T2 inflammatory status, as determined by cut‐offs applied for blood eosinophil count, FeNO, serum total IgE, IL‐5, IL‐13, periostin, TARC, and TSLP. Reductions versus placebo ranged from 55% to 83% in the pooled tezepelumab cohorts (Figure 4).
FIGURE 4.

AAER by baseline biomarker category at Week 52. AAER = the total number of asthma exacerbations/total person‐year follow‐up in each group. *p values are nominal. Gray shaded area shows the response of the overall population. a n refers to the number of patients within each subgroup based on baseline status. bAAER reduction from baseline at Week 52 vs placebo was 62% with tezepelumab 70 mg Q4W, 71% with tezepelumab 210 mg Q4W, and 66% with tezepelumab 280 mg Q2W. cData based on tezepelumab 210 mg Q4W and 280 mg Q2W data only; IL‐5 and TARC were not measured in the tezepelumab 70 mg Q4W cohort. AAER, annualized asthma exacerbation rate; CI, confidence interval; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; ppb, parts per billion; Q2W, every 2 weeks; Q4W, every 4 weeks; RR, rate ratio; TARC, thymus and activation‐regulated chemokine; TSLP, thymic stromal lymphopoietin
The effect of tezepelumab treatment on AAER in patients with high and low biomarker levels was corroborated by LOESS plots showing AAER at Week 52 versus baseline biomarker levels for pooled tezepelumab dose groups (Figure S1). In the placebo group, there was a trend toward a higher AAER with increasing baseline levels of FeNO and IL‐5, and to a lesser extent with increasing baseline blood eosinophil counts, while in the pooled tezepelumab group reductions in AAER were maintained across the range of baseline blood eosinophil counts and levels of FeNO and IL‐5. AAERs in the placebo group were similar across the range of baseline levels of serum total IgE, IL‐13, periostin, TARC, and TSLP. In the pooled tezepelumab treatment group, AAER was consistently lower than in the placebo group across the range of baseline levels of serum total IgE, IL‐13, periostin, TARC, and TSLP.
4. DISCUSSION
This post hoc analysis of the PATHWAY study population extended previous investigations of the effect of tezepelumab on levels of T2 biomarkers (blood eosinophil count, FeNO, and serum total IgE) through evaluation of inflammatory mediators including serum IL‐5, IL‐13, periostin, TARC, and TSLP. The effect of tezepelumab on these biomarkers and cytokines throughout the treatment period, and in relation to the AAER, was evaluated in a cohort of patients with severe asthma.
Baseline levels of serum IL‐5, IL‐13, and periostin correlated significantly with baseline blood eosinophil counts and FeNO levels, which suggests that these circulating mediators are associated with inflammation in this study population of patients with severe, uncontrolled asthma. After 52 weeks of treatment with tezepelumab, levels of these T2 inflammatory mediators were substantially reduced from baseline. When assessed in patients grouped by baseline blood eosinophil counts and FeNO levels, the greatest reductions in biomarker levels with tezepelumab were observed in patients with baseline blood eosinophil counts of at least 150 cells/µl and FeNO levels of at least 25 ppb, followed by patients with baseline blood eosinophil counts of at least 150 cells/µl and FeNO levels below 25 ppb. These data further demonstrate that blocking TSLP impacts multiple key downstream inflammatory mediators and is effective in reducing airway inflammation in patients with severe asthma.
Levels of IL‐5, IL‐13, and FeNO, and blood eosinophil counts, were reduced during the first 4 weeks of treatment with tezepelumab 210 mg Q4W. These early responses to tezepelumab suggest that TSLP‐induced release of T2 inflammatory mediators may be inhibited and the reduction is sustained over a 52‐week treatment period. By contrast, serum total IgE levels gradually decreased over the 52‐week treatment period. Although IL‐4 was not directly measured in this study, the gradual reduction in IgE levels may be a consequence of reduced IL‐4 and IL‐13 levels, resulting in a progressive reduction in immunoglobulin class switching from IgM to IgE isotype production. 28
In this study, we show that reductions in exacerbation rates were observed after treatment with tezepelumab in the pooled dose group, irrespective of baseline levels of T2 inflammatory biomarkers, such as blood eosinophil count (including in patients with blood eosinophil counts of at least 150 cells/µl and below 150 cells/µl), FeNO, and serum levels of IgE, IL‐5, IL‐13, periostin, TARC, and TSLP, when these biomarkers were assessed individually. Interestingly, a recent study found no association between periostin levels and exacerbations. 29 Using LOESS plots, we have also shown that exacerbation rates in the placebo cohort were strongly dependent upon baseline levels of FeNO and IL‐5. Furthermore, exacerbation rates in the placebo group were slightly higher among patients with high baseline blood eosinophil counts than those with low baseline blood eosinophil counts. This observation is consistent with findings of other studies of biologics. 22 , 23
There are few published studies in which circulating levels of IL‐5 and IL‐13 have been assessed in patients with severe asthma. While blood eosinophil counts and FeNO levels have been shown to be correlated with IL‐5 mRNA expression in the sputum of patients with asthma, 30 this is the first study to measure both circulating levels of IL‐5 and IL‐13 in a large cohort. The significant relationship between these baseline biomarkers observed in the current study further supports the use of IL‐5 and IL‐13, in addition to blood eosinophil counts and levels of FeNO and IgE, in the initial evaluation of patients with severe, uncontrolled asthma.
At baseline, serum TSLP levels were in the femtogram range, which, to our knowledge, has not been reported before in patients with asthma. This may be owing to the high sensitivity of the TSLP assay used in PATHWAY compared with assays used in previous studies. 31 TSLP levels were similar between the tezepelumab and placebo groups at baseline. Treatment with tezepelumab reduced exacerbation rates irrespective of baseline TSLP levels, demonstrating that baseline levels of circulating TSLP were not predictive of response. No correlations were observed between TSLP levels and blood eosinophil count and levels of FeNO, serum total IgE, IL‐5, or IL‐13. Circulating levels of TSLP may not reflect expression levels in the lung owing to the episodic and proximal release of TSLP. 32
The changes in biomarker levels observed during treatment with tezepelumab in our study provide important insights into the effects of TSLP in asthma. As an epithelial cell‐derived cytokine that plays a key role in polarizing naive T cells toward a T‐helper 2 inflammatory phenotype, 9 as well as activating T‐helper 2 cells and ILC2s, 16 , 33 TSLP has been shown to induce high local and systemic concentrations of IL‐4, IL‐5, and IL‐13 in animal models. 15 , 34 Blocking TSLP with tezepelumab in the current study resulted in substantial reductions in blood eosinophil counts and their mediator, IL‐5, 35 as well as in IL‐13 and its downstream biomarkers, FeNO, and TARC. 36 , 37 Although IL‐33 and IL‐25 levels were not measured in this study, both of these biomarkers, as well as TSLP, have been described as alarmins of T2 inflammation. 38 , 39 , 40 , 41 Thus, the partial suppression of IL‐5 and IL‐13 levels suggests that other epithelial cell‐derived mediators of T2 inflammation may remain active and continue to release mediators and cytokines, including IL‐33 or IL‐25, which continue to be functional during therapeutic inhibition of TSLP and may contribute to T2 cytokine release. Furthermore, T‐helper 2 memory cells, ILC2s, eosinophils, and mast cells 42 may also continue to release these cytokines for periods of time in the absence of elevated circulating TSLP.
A limitation of this study is that patients in PATHWAY were receiving medium‐ or high‐dose inhaled corticosteroids, which may have affected baseline blood eosinophil counts and FeNO levels. 43 , 44 Furthermore, this study did not assess transcriptomic data; therefore, protein levels could not be compared with gene expression levels. In addition, samples were not collected from lung tissue, or eosinophil counts from bronchoalveolar lavage fluid, which may have provided insight into inflammation localized to the lung. However, the effect of tezepelumab on airway inflammation was investigated in the recently completed phase 2 CASCADE study. 45 Tezepelumab was found to reduce airway eosinophil counts versus placebo in bronchial biopsy samples from patients with moderate‐to‐severe uncontrolled asthma, irrespective of levels of inflammatory biomarkers (blood eosinophils, FeNO, and serum total IgE, IL‐5, IL‐13) at baseline. This was associated with a reduction in T2 biomarkers, including blood eosinophil count and FeNO. The improvements in exacerbation rates and other clinical outcomes observed with tezepelumab in clinical studies including the PATHWAY study are therefore likely to be at least partly driven by reductions in eosinophilic airway inflammation. Interestingly, tezepelumab treatment was also associated with a reduction in airway hyperresponsiveness, indicating possible additional benefits of tezepelumab beyond reducing T2 airway inflammation. 45 In a phase II study of patients with uncontrolled asthma despite treatment with inhaled corticosteroids, tezepelumab reduced the proportion of patients with airway hyperresponsiveness and decreased eosinophilic inflammation in bronchoalveolar lavage fluid and airway tissue. 46
In conclusion, positive correlations between specific T2 inflammatory biomarkers that were observed at baseline demonstrate the robust relationships between several biomarkers of T2 inflammation. We have further demonstrated that tezepelumab reduces levels of inflammatory mediators, which may be reflective of decreased airway inflammation, and reduces exacerbations irrespective of baseline levels of a broad range of T2 inflammatory biomarkers when biomarkers were assessed individually. Treatment with tezepelumab reduced exacerbation rates irrespective of baseline TSLP levels, demonstrating that baseline levels of circulating TSLP were not predictive of response. Future studies of tezepelumab are needed to confirm these findings.
CONFLICT OF INTEREST
Dr. Corren received grants from AstraZeneca during the conduct of the study. He has also received grants from Genentech, Novartis, Optinose, Regeneron, Sanofi, and Teva, and has received personal fees from AstraZeneca, Genentech, Regeneron, and Sanofi. Ms Pham, Dr. Ren, Dr. Colice, and Dr. Griffiths are employees of AstraZeneca and own stock and stock options in AstraZeneca. Dr. Garcia Gil was an employee of AstraZeneca at the time of this study; she is a present employee of Almirall. Ms Sałapa is an employee of AstraZeneca. Dr. Parnes is an employee of Amgen Inc., and owns stock and stock options in Amgen Inc.
AUTHOR CONTRIBUTION
All authors contributed to the conception and design of the study, the analysis and interpretation of data, and the writing of the manuscript. KS provided statistical advice and oversaw the statistical analysis. All authors revised the manuscript critically for intellectual content and provided approval of the version to be published.
Supporting information
App S1
ACKNOWLEDGEMENTS
The authors thank the participants of the PATHWAY study, Karin Bowen, MSc, for her contributions to the analysis and critical review, and Claudia Chen, MS, MPh, for her contribution to the statistical analysis. Medical writing support was provided by Madeleine Wynn, M. Res., of PharmaGenesis London, London, UK, and funded by AstraZeneca and Amgen Inc. This study was co‐sponsored by AstraZeneca and Amgen Inc.
Corren J, Pham T‐H, Garcia Gil E, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. 2022;77:1786–1796. doi: 10.1111/all.15197
(ClinicalTrials.gov identifier: NCT02054130)
Funding information
This study was funded by AstraZeneca and Amgen Inc
REFERENCES
- 1. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343‐373. [DOI] [PubMed] [Google Scholar]
- 2. Global Initiative for Asthma . Global initiative for asthma management and prevention 2020 guidelines. https://ginasthma.org/wp‐content/uploads/2020/06/GINA‐2020‐report_20_06_04‐1‐wms.pdf. Assessed February 26, 2021.
- 3. Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896‐902. [DOI] [PubMed] [Google Scholar]
- 4. Jackson DJ, Busby J, Pfeffer PE, et al. Characterisation of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2021;76(3):220‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Busse WW, Maspero JF, Rabe KF, et al. Liberty asthma QUEST: phase 3 randomized, double‐blind, placebo‐controlled, parallel‐group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate‐to‐severe asthma. Adv Ther. 2018;35(5):737‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Busse WW, Bleecker ER, FitzGerald JM, et al. Long‐term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1‐year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7(1):46‐59. [DOI] [PubMed] [Google Scholar]
- 7. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673‐680. [DOI] [PubMed] [Google Scholar]
- 10. Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):104‐111. [DOI] [PubMed] [Google Scholar]
- 11. Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle‐exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol. 2008;28(2):147‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calvén J, Yudina Y, Hallgren O, et al. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG‐I‐like helicases. Innate Immun. 2012;4(1):86‐99. [DOI] [PubMed] [Google Scholar]
- 13. Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2‐attracting chemokines and disease severity. J Immunol. 2005;174(12):8183‐8190. [DOI] [PubMed] [Google Scholar]
- 14. Ying S, O'Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790‐2798. [DOI] [PubMed] [Google Scholar]
- 15. Kitajima M, Lee H‐C, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Camelo A, Rosignoli G, Ohne Y, et al. IL‐33, IL‐25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017;1(10):577‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gauvreau GM, O'Byrne PM, Boulet L‐P, et al. Effects of an anti‐TSLP antibody on allergen‐induced asthmatic responses. N Engl J Med. 2014;370(22):2102‐2110. [DOI] [PubMed] [Google Scholar]
- 18. Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936‐946. [DOI] [PubMed] [Google Scholar]
- 19. Verstraete K, Peelman F, Braun H, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun. 2017;8:14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corren J, Garcia Gil E, Parnes J, Pham T, Griffiths J. Tezepelumab treatment effect on annualized rate of exacerbations by baseline biomarkers in uncontrolled severe asthma patients: phase 2b PATHWAY study. J Allergy Clin Immunol. 2019;199:A2621. [Google Scholar]
- 21. American Thoracic S, European RS. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912‐930. [DOI] [PubMed] [Google Scholar]
- 22. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486‐2496. [DOI] [PubMed] [Google Scholar]
- 23. Goldman M, Hirsch I, Zangrilli JG, Newbold P, Xu X. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: subanalyses of the phase III SIROCCO and CALIMA studies. Curr Med Res Opin. 2017;33(9):1605‐1613. [DOI] [PubMed] [Google Scholar]
- 24. Jeppegaard M, Veidal S, Sverrild A, Backer V, Porsbjerg C. Validation of ATS clinical practice guideline cut‐points for FeNO in asthma. Respir Med. 2018;144:22‐29. [DOI] [PubMed] [Google Scholar]
- 25. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651‐659. [DOI] [PubMed] [Google Scholar]
- 26. Soma T, Iemura H, Naito E, et al. Implication of fraction of exhaled nitric oxide and blood eosinophil count in severe asthma. Allergol Int. 2018;67S:S3‐S11. [DOI] [PubMed] [Google Scholar]
- 27. Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829‐836. [Google Scholar]
- 28. Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: the immune system in health and disease, 5th Edition. Garland Science; 2001. The production of IgE. https://www.ncbi.nlm.nih.gov/books/NBK27117/. Accessed July 19, 2020. [Google Scholar]
- 29. Buhl R, Korn S, Menzies‐Gow A, et al. Prospective, single‐arm, longitudinal study of biomarkers in real‐world patients with severe asthma. J Allergy Clin Immunol Pract. 2020;8(8):2630‐2639 e2636. [DOI] [PubMed] [Google Scholar]
- 30. Truyen E, Coteur L, Dilissen E, et al. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61(3):202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pham TH, Kearley J, Parnes JR, Leung L, Goleva E, Griffiths JM. Development of a highly sensitive assay to quantitate circulating thymic stromal lymphopoietin (TSLP) levels in blood. J Allergy Clin Immunol. 2020;145:AB30. [Google Scholar]
- 32. Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci USA. 2007;104(3):914‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rochman Y, Dienger‐Stambaugh K, Richgels PK, et al. TSLP signaling in CD4(+) T cells programs a pathogenic T helper 2 cell state. Sci Signal. 2018;11(521):eaam8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Omori M, Ziegler S. Induction of IL‐4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178(3):1396‐1404. [DOI] [PubMed] [Google Scholar]
- 35. Schleich F, Demarche S, Louis R. Biomarkers in the management of difficult asthma. Curr Top Med Chem. 2016;16(14):1561‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Syed F, Huang CC, Li K, et al. Identification of interleukin‐13 related biomarkers using peripheral blood mononuclear cells. Biomarkers. 2007;12(4):414‐423. [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Wang W, Lv Z, et al. Elevated expression of IL‐33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253‐2262. [DOI] [PubMed] [Google Scholar]
- 39. Khaitov MR, Gaisina AR, Shilovskiy IP, et al. The role of interleukin‐33 in pathogenesis of bronchial asthma. New experimental data. Biochemistry (Mosc). 2018;83(1):13‐25. [DOI] [PubMed] [Google Scholar]
- 40. Bianchetti L, Marini MA, Isgro M, Bellini A, Schmidt M, Mattoli S. IL‐33 promotes the migration and proliferation of circulating fibrocytes from patients with allergen‐exacerbated asthma. Biochem Biophys Res Commun. 2012;426(1):116‐121. [DOI] [PubMed] [Google Scholar]
- 41. Beale J, Jayaraman A, Jackson DJ, et al. Rhinovirus‐induced IL‐25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6(256):256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doran E, Cai F, Holweg CTJ, Wong K, Brumm J, Arron JR. Interleukin‐13 in asthma and other eosinophilic disorders. Front Med. 2017;4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neelamegan R, Saka V, Tamilarasu K, Rajaram M, Selvarajan S, Chandrasekaran A. Clinical utility of fractional exhaled nitric oxide (FeNO) as a biomarker to predict severity of disease and response to inhaled corticosteroid (ICS) in asthma patients. J Clin Diagn Res. 2016;10(12):FC01‐FC06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lommatzsch M, Klein M, Stoll P, Virchow JC. Impact of an increase in the inhaled corticosteroid dose on blood eosinophils in asthma. Thorax. 2019;74(4):417‐418. [DOI] [PubMed] [Google Scholar]
- 45. Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate‐to‐severe uncontrolled asthma (CASCADE): a double‐blind, randomised, placebo‐controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299‐1312. [DOI] [PubMed] [Google Scholar]
- 46. Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J. 2021:2101296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


