Abstract
Identification of physiological and environmental factors that limit efficient growth of hyperthermophiles is important for practical application of these organisms to the production of useful enzymes or metabolites. During fed-batch cultivation of Sulfolobus solfataricus in medium containing l-glutamate, we observed formation of l-pyroglutamic acid (PGA). PGA formed spontaneously from l-glutamate under culture conditions (78°C and pH 3.0), and the PGA formation rate was much higher at an acidic or alkaline pH than at neutral pH. It was also found that PGA is a potent inhibitor of S. solfataricus growth. The cell growth rate was reduced by one-half by the presence of 5.1 mM PGA, and no growth was observed in the presence of 15.5 mM PGA. On the other hand, the inhibitory effect of PGA on cell growth was alleviated by addition of l-glutamate or l-aspartate to the medium. PGA was also produced from the l-glutamate in yeast extract; the PGA content increased to 8.5% (wt/wt) after 80 h of incubation of a yeast extract solution at 78°C and pH 3.0. In medium supplemented with yeast extract, cell growth was optimal in the presence of 3.0 g of yeast extract per liter, and higher yeast extract concentrations resulted in reduced cell yields. The extents of cell growth inhibition at yeast extract concentrations above the optimal concentration were correlated with the PGA concentration in the culture broth. Although other structural analogues of l-glutamate, such as l-methionine sulfoxide, glutaric acid, succinic acid, and l-glutamic acid γ-methyl ester, also inhibited the growth of S. solfataricus, the greatest cell growth inhibition was observed with PGA. We also observed that unlike other glutamate analogues, N-acetyl-l-glutamate enhanced the growth of S. solfataricus. This compound was stable under cell culture conditions, and replacement of l-glutamate with N-acetyl-l-glutamate in the medium resulted in increased cell density.
Recently, hyperthermophiles have attracted the attention of many workers in the biotechnology research community because of the potential industrial applications of these organisms and because they may provide a better understanding of how cell components are stabilized when they are heated (1, 13, 18, 28, 29). Despite the biotechnological potential of hyperthermophiles, thus far the uses of these organisms have been limited because of the low cell yields when they are cultivated, which are attributed mainly to a lack of knowledge concerning the physiological characteristics and high-temperature cultivation techniques (5, 17). Therefore, identification of physiological and environmental factors that limit efficient growth of hyperthermophiles and development of strategies to obtain high cell densities under high-temperature conditions are particularly important for increasing the biomass yields of these microorganisms in environments different from their natural habitats.
Sulfolobus solfataricus P2 is a hyperthermophilic archaeon which normally grows at 75 to 85°C and pH 2.0 to 4.0 (4, 7, 32). Since physiological and genomic studies of this species have been carried out most intensively among various members of the order Sulfolobales (11, 15, 20, 21, 27), we selected S. solfataricus as a model archaeon and have been investigating the factors that affect the growth of this microorganism in a laboratory-scale fermentor (22–25). Unlike most other hyperthermophiles, S. solfataricus grows under aerobic conditions, and thus it is anticipated that this archeon can be cultivated as efficiently as other aerobes, such as Escherichia coli and yeast cells, if physiological and environmental factors unfavorable for cell growth are identified and eliminated.
Recently, we studied the effects of low-molecular-weight solutes, such as compatible solutes and l amino acids, on the growth of S. solfataricus (25). Of the low-molecular-weight solutes tested, l-glutamate was found to be the best growth enhancer for S. solfataricus. When cells were grown in medium containing both glucose and l-glutamate, S. solfataricus preferentially utilized l-glutamate instead of glucose. In this regard, it is noteworthy that complex nutrients, such as hydrolyzed peptone and yeast extract, which are essential for efficient growth of most heterotrophic hyperthermophiles (14), contain l-glutamate as a major component. Growth stimulation and reduction of the lag time by yeast extract in the medium are attributable to the role of l-glutamate as a compatible solute that facilitates adaptation of cells to stressful environmental factors, such as high temperatures and extremely acidic conditions.
The finding that exogenously supplied l-glutamate can promote the growth of S. solfataricus in batch cultures prompted us to investigate whether cell densities can be further increased by continuous addition of l-glutamate to the medium in a fed-batch operation. In the present study, we found that prolonged incubation of l-glutamate under culture conditions (high temperature and low pH) resulted in conversion of l-glutamate to l-pyroglutamic acid (2-pyrrolidone-5-carboxylic acid) (PGA) and that PGA is a potent inhibitor of growth of S. solfataricus. Although it has been known for decades that thermal conversion of l-glutamate to PGA occurs (31) and although hyperthermophiles require temperatures that produce PGA, the effect of PGA production on the growth of hyperthermophiles has not been investigated previously. Our data indicate that thermal decomposition of a medium component at an elevated temperature can be one of the major factors that limit efficient growth of hyperthermophiles.
MATERIALS AND METHODS
Microorganism.
S. solfataricus P2 (= DSM 1617), which was isolated from a volcanic hot spring in Italy, was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany).
Culture methods.
Batch cultivation was carried out in screw-cap flasks (working volume, 50 ml) at 78°C in a shaking water bath with agitation at 100 oscillations per min. The glucose minimal medium (GM medium) used for seed culture and control experiments was composed of glucose (3.0 g/liter) and modified Allen's basal salt solution. Modified Allen's basal salt solution contained (per liter of distilled water) 1.3 g of (NH4)2SO4, 0.28 g of KH2PO4, 0.25 g of Mg SO4 · 7H2O, 70 mg of CaCl2 · 2H2O, 20 mg of FeCl3 · 6H2O, 4.5 mg of Na2B4O7 · 10H2O, 1.8 mg of MnCl2 · 4H2O, 0.05 mg of ZnSO4 · 7H2O, 0.05 mg of CuCl2 · 2H2O, 0.04 mg of VOSO45H2O, 0.03 mg of Na2MoO4 · 5H2O, and 0.01 mg of CoSO4 · 7H2O (15). GYM medium was prepared by adding 3.0 g of yeast extract per liter to GM medium. The pH of the culture medium was adjusted to 3.0 with sulfuric acid. Yeast extract was obtained from Difco Laboratories (Detroit, Mich.), and all other chemicals used in this work were obtained from Sigma (St. Louis, Mo.).
Fed-batch cultivation was conducted in a bench top fermentor with a working volume of 2.3 liters (KLF2000; Bioengineering AG, Wald, Switzerland) as described previously (22, 23). The pH of the culture broth was automatically adjusted to 3.0, and the dissolved oxygen level was maintained at more than 30% of air saturation. GM medium supplemented with l-glutamate at a specified concentration was used as a base medium. The feed rate was controlled to maintain constant residual concentrations of l-glutamate and glucose by using the constant-volume fed-batch protocol (22).
Analytical methods.
The cell densities of S. solfataricus cultures were determined by measuring turbidity at 540 nm and were correlated to cell dry weights. To determine cell dry weight, cells were washed twice with distilled water and dried for 48 h at 110°C. The concentrations of PGA, l-glutamate, and N-acetyl-l-glutamate were determined by using a high-performance liquid chromatograph (HPLC) (Knauer, Berlin, Germany) equipped with a UV detector (210 nm) and a reverse-phase C18 column (Waters, Milford, Mass.). Deionized water adjusted to pH 2.1 with phosphoric acid was used as the eluent (flow rate, 1.0 ml/min). The specific growth rate was calculated from the slope of a straight line on a semilog plot in which the logarithm of cell concentration was plotted versus the culture time. The rate constant for decomposition of l-glutamate was determined by assuming that first-order reaction kinetics were operating. The I50 of PGA was the concentration of PGA at which the growth rate of S. solfataricus was one-half the growth rate in medium without PGA.
RESULTS
PGA formation from l-glutamate under culture conditions.
Based on our previous finding that l-glutamate enhances the growth of S. solfataricus in batch cultures (25), we attempted fed-batch cultivation of S. solfataricus by continuously adding l-glutamate to the culture medium. Since the cell density and growth rate were maximal at an l-glutamate concentration of 1.0 g/liter (25), the initial fed-batch experiments were performed by controlling the residual concentration of l-glutamate so that it was at this level. In contrast to our expectation, however, continuous addition of l-glutamate resulted in a very marginal increase in the cell density. Moreover, growth of S. solfataricus in fed-batch cultures was suppressed as the residual glutamate concentration was increased to 3.0 g/liter, and no cell growth was observed in the presence of 5.0 g of l-glutamate per liter. During our study of fed-batch cultivation of S. solfataricus in glutamate-supplemented media, we also observed that the concentrations of l-glutamate in culture media gradually decreased even in the absence of cell growth.
It has been determined previously that l-glutamate can be converted to PGA at a high temperature (31). To explore the possibility that l-glutamate is spontaneously converted to PGA, GM medium containing l-glutamate was maintained under the conditions employed for the fed-batch experiments. Samples were withdrawn at appropriate times and analyzed by using a reverse-phase HPLC. From HPLC chromatograms, we confirmed that l-glutamate in GM medium was dehydrated to PGA under the cultivation conditions used; the peak corresponding to l-glutamate (2.21 min) decreased gradually with incubation time, whereas a new peak (7.74 min), whose retention time was identical to that of authentic PGA, increased simultaneously. The stoichiometry of the conversion was as follows: 1 mol of l-glutamate → 1 mol of PGA + 1 mol of H2O. To quantify dehydration of l-glutamate in GM medium, the rates of PGA formation were determined at different temperatures and pHs. It was found that the first-order dehydration rate of l-glutamate was markedly influenced by pH and temperature. Glutamate dehydration was rather modest at pH 7.0, but the dehydration rates were significantly higher at pH 3.0 and 10.0. At 78°C, for example, the glutamate dehydration rate at pH 3.0 (1.08 × 10−2 h−1) was 36 times higher than that at pH 7.0 (0.03 × 10−2h−1). On the other hand, the dehydration rate of l-glutamate was almost unaffected by the presence of the modified Allen's salts and glucose in GM medium (data not shown).
Effect of PGA on growth of S. solfataricus.
When the PGA concentrations in samples from fed-batch experiments were analyzed by the HPLC method, the PGA level in the culture medium increased with the l-glutamate concentration. In addition, there was an inverse relationship between the maximal cell densities and the PGA concentrations in the media (data not shown). These results suggest that cell growth is adversely affected by accumulation of PGA in fed-batch cultures with l-glutamate.
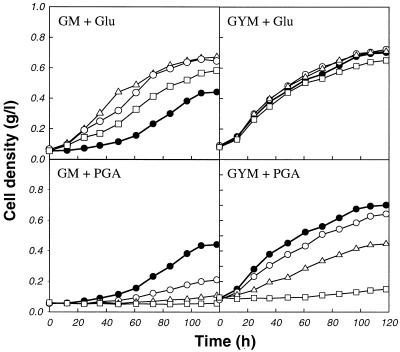
In order to investigate the effect of PGA on growth of S. solfataricus, various amounts of PGA were added to two media: a glucose minimal medium (GM medium) and a complex medium (GYM medium). For comparison, growth of S. solfataricus was also monitored after l-glutamate was added to the same medium. As shown in Fig. 1, PGA significantly inhibited the growth of S. solfataricus, whereas l-glutamate enhanced cell growth. In GYM medium, reduced inhibitory effects of PGA were observed compared to the results obtained with GM medium. Irrespective of the medium composition, however, S. solfataricus did not grow in the presence of 15.5 mM PGA.
FIG. 1.
Effects of l-glutamate and PGA on growth of S. solfataricus. l-Glutamate (Glu) or PGA was added to GM medium and GYM medium. The following amounts of glutamate were added: none (●), 3.4 mM (○), 6.8 mM (▵), and 20.4 mM (□). The following amounts of PGA were added: none (●), 3.9 mM (○), 7.7 mM (▵), and 15.5 mM (□).
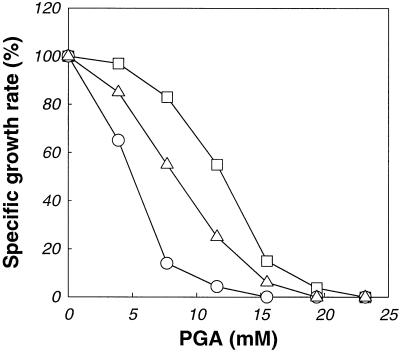
Since both l-glutamate and l-aspartate enhance the growth of S. solfataricus (25), we investigated the effects of these two amino acids on the inhibitory effect of PGA by measuring the growth rate in GM medium supplemented with 10 mM l-glutamate or 10 mM l-aspartate in the presence of various concentrations of PGA. As shown in Fig. 2, inhibition of S. solfataricus growth by PGA was relieved by the addition of l-glutamate or l-aspartate. While the I50 of PGA for cell growth was 5.1 mM in GM medium (with no amino acid added), the I50s of PGA were 8.4 and 12.1 mM in the presence of l-aspartate and l-glutamate, respectively. No amino acid other than l-glutamate and l-aspartate resulted in an appreciable reduction in growth inhibition by PGA (data not shown).
FIG. 2.
Effects of PGA on growth of S. solfataricus in GM medium (○) and in medium supplemented with 10 mM l-aspartate (▵) or 10 mM l-glutamate (□). The specific growth rates for each PGA concentration were normalized to those obtained in the absence of PGA.
PGA formation in a yeast extract solution and its effect on growth of S. solfataricus.
l-Glutamate is one of the most abundant amino acids in yeast extract (14). For this reason, we investigated whether PGA can be produced from yeast extract and whether there is any relationship between PGA formation and inhibition of S. solfataricus growth in the presence of high yeast extract concentrations.
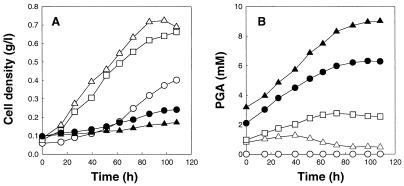
To examine spontaneous formation of PGA under S. solfataricus culture conditions, we incubated a yeast extract solution at 78°C and pH 3.0 without cells and analyzed the culture broth by using a reverse-phase HPLC. From this HPLC analysis, we found that the yeast extract used in this work originally contained 2.2% (wt/wt) PGA. After 80 h of incubation of the yeast extract solution under cell culture conditions, the PGA content was 8.5% (wt/wt). In order to examine the relationship between growth inhibition by yeast extract and PGA formation, cells were cultivated in GM medium supplemented with different amounts of yeast extract. The cell densities and PGA concentrations in culture broth are shown in Fig. 3. As reported previously (4, 15), growth of S. solfataricus was stimulated by addition of a small amount (3.0 g/liter) of yeast extract to GM medium. When the yeast extract concentration exceeded 6.0 g/liter (Fig. 3A), however, significant growth inhibition was observed. From time course profiles of PGA formation, it was found that accumulation of PGA in the medium was significant at high yeast extract concentrations (Fig. 3B). The results described above indicate that the growth inhibition observed in the presence of high yeast extract concentrations is due at least in part to accumulation of PGA in the culture broth.
FIG. 3.
(A) Batch growth of S. solfataricus in GM medium supplemented with yeast extract. (B) Time course profiles for residual PGA concentrations in the culture broth. The following amounts of yeast extract were added: none (○), 3.0 g/liter (▵), 6.0 g/liter (□), 9.0 g/liter (●), and 12.0 g/liter (▴).
Effects of structural analogues of l-glutamate on growth of S. solfataricus.
We examined the effects of various structural analogues of l-glutamate on growth of S. solfataricus. In this experiment, cells were cultivated in GM medium supplemented with each analogue at a concentration of 10 mM, and the specific growth rate was determined from the growth curve. As shown in Table 1, the growth inhibition was greatest with PGA among the glutamate analogues tested in this work. Significant growth inhibition was also observed in the presence of l-methionine sulfoxide, glutaric acid, succinic acid, and l-glutamic acid γ-methyl ester. When N-acetyl-l-glutamic acid was added, on the other hand, the growth rate of S. solfataricus increased about 1.7-fold compared to the growth rate in GM medium.
TABLE 1.
Effects of l-glutamate analogues on the growth rate of S. solfataricus
| l-Glutamate analogue | Specific growth rate (102 h−1) |
|---|---|
| N-Acetyl-l-glutamic acid | 4.1 |
| Control (GM medium) | 2.6 |
| N-Carbamy-l-glutamic acid | 2.6 |
| γ-Aminobutyric acid | 2.5 |
| l-Glutamic acid γ-benzyl ester | 2.1 |
| N-p-Aminobenzoyl-l-glutamic acid | 2.0 |
| l-Glutamic acid γ-ethyl ester | 2.0 |
| l-Glutamic acid γ-methyl ester | 1.6 |
| Succinic acid | 1.1 |
| Glutaric acid | 1.1 |
| l-Methionine sulfoxide | 0.8 |
| PGA | 0.5 |
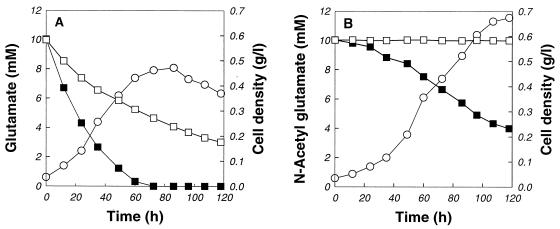
We also investigated cell growth in GM medium supplemented with l-glutamate or N-acetyl-l-glutamate. As shown in Fig. 4, there was an increase in the maximal cell density when N-acetyl-l-glutamate was used in place of l-glutamate. In addition, the biomass yield was much higher with N-acetyl-l-glutamate (Yx/s = 0.59) than with l-glutamate (Yx/s = 0.31). We also examined the stability of N-acetyl-l-glutamate under the culture conditions used (78°C and pH 3.0). In contrast to l-glutamate, N-acetyl-l-glutamate was stably maintained and was not spontaneously converted to other compounds under the test conditions.
FIG. 4.
Growth of S. solfataricus in GM medium supplemented with l-glutamate (A) or N-acetyl-l-glutamate (B). Symbols: □, l-glutamate or N-acetyl-l-glutamate concentration in medium without cells; ○, cell density; ■, l-glutamate or N-acetyl-l-glutamate concentration in inoculated culture.
DISCUSSION
The thermal lability of nutrients has been known for several decades, especially as it relates to food processing (12, 16). Many nutrient instability problems are related to thermochemical modifications of carbohydrates and their effects on the growth of microorganisms (9, 10). In this study, we showed that l-glutamate, one of the most abundant amino acids in yeast extract, can be spontaneously converted to PGA during cultivation of hyperthermophiles and that PGA significantly suppresses growth of S. solfataricus. Although thermal conversion of l-glutamate to PGA was originally described several decades ago (31) and the role of PGA as an agonist for the glutamate receptor has been investigated with nerve cells (6, 19), there have been no previous reports concerning the effects of PGA on the growth of microorganisms.
Formation of PGA from l-glutamate during cultivation can adversely affect the growth of S. solfataricus in two ways: the level of the growth enhancer (l-glutamate) can decrease and the growth inhibitor (PGA) can accumulate. As reported previously (25), l-glutamate is an important growth enhancer for S. solfataricus, and hence a loss of l-glutamate during cultivation should accompany a decrease in cell growth. Since the rate of dehydration of l-glutamate was higher at a low pH, acidophilic hyperthermophiles, such as Sulfolobus sp., Metallosphaera sp., Acidianus sp., and Stygiolobus sp., might be much more vulnerable to growth suppression by decomposition of l-glutamate than neutrophilic hyperthermophiles are.
It was observed in this study that PGA was lethal to S. solfataricus. There was no cell growth in the presence of PGA concentrations greater than 15.5 mM in GM medium. According to a recent study of a high-density culture of S. solfataricus in which a microfiltration bioreactor was used (26), toxic compounds with molecular masses less than 1 kDa were produced during fermentation and reduced the maximum cell density. In view of the results presented in this paper, it is very probable that PGA (molecular weight, 129.1) is one of the toxic compounds that accumulate in culture broth. It is noteworthy that a complex nutrient, such as yeast extract, which contains a large amount of l-glutamate, was added continuously in the study of Schiraldi et al. (26).
The inhibitory effect of PGA produced from l-glutamate explains in part the growth inhibition in the presence of high yeast extract concentrations observed with batch cultures of S. solfataricus and Thermoplasma acidophilum (2, 4, 15). Previously, we reported that addition of excess yeast extract to fed-batch cultures of S. solfataricus results in low maximum cell densities, and the inhibitory effect was attributed to accumulation of mineral ions in yeast extract (24). However, at that time it was difficult to explain the inhibitory effect of yeast extract in batch cultures in which the concentrations of mineral ions were not as high as those in fed-batch cultures. From the results obtained in this study, it is clear that formation of PGA is one of major reasons for growth inhibition in the presence of high yeast extract concentrations in batch cultures of thermoacidophiles.
In our study, the inhibition caused by PGA was reduced when acidic amino acids, such as l-aspartate and l-glutamate, were added. In many cases, the carrier protein for l-glutamate transport is similar to that for l-aspartate transport, and l-aspartate works as a competitive inhibitor of l-glutamate transport among amino acids (8, 30). Considering this, it is likely that PGA is a competitive inhibitor for transport of l-glutamate or l-aspartate in S. solfataricus. Although our results imply that PGA functions as a structural analogue of l-glutamate, it remains to be seen how PGA causes death of S. solfataricus upon uptake.
Various structural analogues of l-glutamate were examined, and inhibitory effects were observed in the presence of l-glutamic acid γ-benzyl ester, l-glutamic acid γ-methyl ester, glutaric acid, l-methionine sulfoxide, PGA, and succinic acid. There was not as much correlation between affinity of the analogues for uptake of l-glutamate by nerve cells (3) and the extent of inhibition of growth of S. solfataricus. For example, glutaric acid, l-methionine sulfoxide, PGA, and succinic acid were reported to have negligible affinity for l-glutamate transport in nerve cells. It is interesting that N-acetyl glutamate did not decay to another form under S. solfataricus culture conditions. The amine group of N-acetyl-l-glutamate was presumed to be protected from internal cyclization between amine and carboxylic groups by attachment of an acetyl group moiety. S. solfataricus utilized N-acetyl-l-glutamate as well as l-glutamate, and growth was greatly enhanced by addition of N-acetyl-l-glutamate to GM medium. Furthermore, the cell mass yield was much higher when N-acetyl-l-glutamate was used instead of l-glutamate. Considering these results, N-acetyl-l-glutamate might be substituted for l-glutamate during cultivation of S. solfataricus.
ACKNOWLEDGMENTS
This work was supported by the Korean Ministry of Science and Technology and by in-house grants from the Pohang University of Science and Technology.
REFERENCES
- 1.Adams M W W, Kelly R M. Finding and using hyperthermophilic enzymes. Trends Biotechnol. 1998;16:329–332. doi: 10.1016/s0167-7799(98)01193-7. [DOI] [PubMed] [Google Scholar]
- 2.Belly R T, Bohlool B B, Brock T D. The genus Thermoplasma. Ann NY Acad Sci. 1973;225:94–107. [Google Scholar]
- 3.Bennett J P, Logan W J, Snyder S H. Amino acids as central nervous transmitters: the influence of ions, amino acid analogues, and ontogeny on transport systems for glutamic and aspartic acids and glycine into central nervous synaptosomes of the rat. J Neurochem. 1973;21:1533–1550. doi: 10.1111/j.1471-4159.1973.tb06037.x. [DOI] [PubMed] [Google Scholar]
- 4.Brock T D, Brock K M, Belly R T, Weiss R L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 5.Cowan D A. Biotechnology of the archaea. Trends Biotechnol. 1992;10:315–323. doi: 10.1016/0167-7799(92)90257-v. [DOI] [PubMed] [Google Scholar]
- 6.De Mello C F, De La Vega D D, Pizutti L T, Lopes F P, Rubin M A, Homerich J G, Melo C R, Somer J E, Souza D O, Wajner M. Neurochemical effects of pyroglutamic acid. Neurochem Res. 1995;20:1437–1441. doi: 10.1007/BF00970591. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa M, Gambacorta A, Bu'Lock J D. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus acidocaldarius. J Gen Microbiol. 1975;86:156–164. doi: 10.1099/00221287-86-1-156. [DOI] [PubMed] [Google Scholar]
- 8.De Vrij W, Bulthuis R A, Van Iwaarden P R, Konings W N. Mechanism of glutamate transport in membrane vesicles from Bacillus stearothermophilus. J Bacteriol. 1989;171:1118–1125. doi: 10.1128/jb.171.2.1118-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driskill L E, Kusy K, Bauer M W, Kelly R M. Relationship between glycosyl hydrolase inventory and growth physiology of the hyperthermophile Pyrococcus furiosus on carbohydrate-based media. Appl Environ Microbiol. 1999;65:893–897. doi: 10.1128/aem.65.3.893-897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einarsson H, Snygg B G, Eriksson C. Inhibition of bacterial growth by Maillard reaction products. J Agric Food Chem. 1983;31:1043–1046. [Google Scholar]
- 11.Grogan D W. Phenotype characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J Bacteriol. 1989;171:6710–6719. doi: 10.1128/jb.171.12.6710-6719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodge J E. Dehydrated foods: chemistry of browning reactions in model systems. J Agric Food Chem. 1953;1:928–943. [Google Scholar]
- 13.Huber H, Stetter K O. Hyperthermophiles and their possible potential in biotechnology. J Biotechnol. 1998;64:39–52. [Google Scholar]
- 14.Jannasch H W, Wirsen C O, Hoaki T. Isolation and cultivation of heterotrophic hyperthermophiles from deep-sea hydrothermal vents. Thermophiles. In: Robb F T, editor. Archaea: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. p. 9-13. [Google Scholar]
- 15.Kan E S, Park C B, Lee S B. Optimization of culture conditions for hyperthermophilic archaeon Sulfolobus solfataricus. Kor J Biotechnol Bioeng. 1997;12:121–126. [Google Scholar]
- 16.Kelly F H C, Brown D W. Thermal decomposition and colour formation in aqueous sucrose solutions. Sugar Technol Rev. 1979;6:1–48. [Google Scholar]
- 17.Kelly R M, Brown B H, Blumetals I I, Adams M W W. Characterization of enzymes from high-temperature bacteria. In: Adams M W W, Kelly R M, editors. Biocatalysis at extreme temperatures. Washington, D.C.: American Chemical Society; 1992. pp. 23–41. [Google Scholar]
- 18.Konig H, Stetter K O. Biomolecules are unstable under “black smoker” conditions. Naturwissenschaften. 1984;71:583–586. [Google Scholar]
- 19.Mornet C, Briley M. The ‘forgotten’ amino acid pyroglutamate. Trends Pharmacol Sci. 1988;9:278–279. doi: 10.1016/0165-6147(88)90006-5. [DOI] [PubMed] [Google Scholar]
- 20.Nicolaus B, Tricone A, Lama L, Romano I, Marsiglia F, Gambacorta A. Adaptation of Sulfolobus solfataricus on minimal media. Biotechnol Lett. 1991;13:667–670. [Google Scholar]
- 21.Ozbas T, Durusoy T, Tanyolac A, Yurum Y. The factors affecting the growth kinetics of Sulfolobus solfataricus, a sulfur removing bacterium. Fuel Process Technol. 1993;33:61–75. [Google Scholar]
- 22.Park C B, Lee S B. Constant-volume fed-batch operation for high density cultivation of hyperthermophilic aerobes. Biotechnol Tech. 1997;11:277–281. [Google Scholar]
- 23.Park C B, Lee S B. Inhibitory effect of mineral ion accumulation on high density growth of the hyperthermophilic archaeon Sulfolobus solfataricus. J Biosci Bioeng. 1999;87:315–319. doi: 10.1016/s1389-1723(99)80038-3. [DOI] [PubMed] [Google Scholar]
- 24.Park C B, Lee S B. Cultivation of the hyperthermophilic archaeon Sulfolobus solfataricus in low-salt media. Biotechnol Bioprocess Eng. 1999;4:21–25. [Google Scholar]
- 25.Park C B, Lee S B. Effects of exogenous compatible solutes on growth of the hyperthermophilic archaeon Sulfolobus solfataricus. J Biosci Bioeng. 2000;89:318–322. doi: 10.1016/s1389-1723(00)88952-5. [DOI] [PubMed] [Google Scholar]
- 26.Schiraldi C, Marulli F, Di Lernia I, Martino A, De Rosa M. A microfiltration bioreactor to achieve high cell density in Sulfolobus solfataricus fermentation. Extremophiles. 1999;3:199–204. doi: 10.1007/s007920050117. [DOI] [PubMed] [Google Scholar]
- 27.Sensen C W, Charlebois R L, Chow C, Glausen I G, Curtis B, Doolittle W F, Duguet M, Erauso G, Gaasterland T, Garrett R A, Gordon P, De Jong I H, Jeffries A C, Kozera C, Medina N, Moors A D, van der Oost J, Phan H, Ragan M A, Schenk M E, She Q, Singh R K, Tolstrup N. Completing the sequence of the Sulfolobus solfataricus P2 genome. Extremophiles. 1998;2:305–312. doi: 10.1007/s007920050073. [DOI] [PubMed] [Google Scholar]
- 28.Stetter K O. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999;452:22–25. doi: 10.1016/s0014-5793(99)00663-8. [DOI] [PubMed] [Google Scholar]
- 29.Takai K, Sako Y. A molecular view of archaeal diversity in marine and terrestrial hot water environments. FEMS Microbiol Ecol. 1999;28:177–188. [Google Scholar]
- 30.Tolner B, Ubbink-Kok T, Poolman B, Konings W N. Characterization of proton/glutamate symport protein of Bacillus subtilis and its functional expression in Escherichia coli. J Bacteriol. 1995;177:2863–2869. doi: 10.1128/jb.177.10.2863-2869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson H, Cannan R K. The glutamic acid-pyrrolidonecarboxylic acid system. J Biol Chem. 1937;119:309–331. [Google Scholar]
- 32.Zillig W, Stetter K O, Wunderl S, Schulz W, Priess H, Scholz J. The Sulfolobus-“Caldariella” group: taxanomy on the basis of the structure of DNA-dependent RNA polymerase. Arch Microbiol. 1980;125:259–269. [Google Scholar]