Abstract
The causative agent of Q fever, Coxiella burnetii, is endemic to Queensland and is one of the most important notifiable zoonotic diseases in Australia. The reservoir species for C. burnetii are classically ruminants, including sheep, cattle and goats. There is increasing evidence of C. burnetii exposure in dogs across eastern and central Australia. The present study aimed to determine if pig‐hunting dogs above the Tropic of Capricorn in Queensland had similar rates of C. burnetii exposure to previous serosurveys of companion dogs in rural north‐west New South Wales. A total of 104 pig‐hunting dogs had serum IgG antibody titres to phase I and phase 2 C. burnetii determined using an indirect immunofluorescence assay test. Almost one in five dogs (18.3%; 19/104; 95% confidence interval 9.6%–35.5%) were seropositive to C. burnetii, with neutered dogs more likely to test positive compared to entire dogs (P = 0.0497). Seropositivity of the sampled pig‐hunting dogs was one of the highest recorded in Australia. Thirty‐nine owners of the pig‐hunting dogs completed a survey, revealing 12.8% (5/39) had been vaccinated against Q fever and 90% (35/39) were aware that both feral pigs and dogs could potentially be sources of C. burnetii. Our findings indicate that pig hunters should be aware of the risk of exposure to Q fever during hunts and the sentinel role their dogs may play in C. burnetii exposure.
Keywords: coxiellosis, dogs, pig hunting, Q fever, Queensland, veterinary science
Abbreviations
- CI
confidence interval
- ELISA
enzyme‐linked immunosorbent assay
- F
female
- FITC
fluorescein isothiocyanate
- FN
spayed female
- IFA
immunofluorescence assay
- IgG
immunoglobulin G
- IQR
interquartile range
- M
male
- MN
castrated male
- NSW
New South Wales
- NT
Northern Territory
- PCR
polymerase chain reaction
- PPE
personal protective equipment
- Qld
Queensland
- X
crossbred
Coxiella burnetii, the causative agent of Q fever, is an obligate intracellular bacterium responsible for one of the most important zoonotic diseases in Australia. 1 , 2 , 3 , 4 Originally called ‘Query fever’, the disease was first described in Queensland (Qld), Australia in the 1930s. 5 Ruminants, such as cattle, sheep and goats, remain the primary animal reservoir for the bacterium, 3 , 6 , 7 , 8 experiencing largely subclinical infections with occasional reproductive impairment, 9 , 10 including abortion, dystocia, reduced fertility and neonatal deaths. 7 , 9 Indeed, contact with aborted ruminant reproductive materials and the normal products of parturition are considered high risk exposures for Q fever in humans. 6 , 8 , 11 , 12 , 13 , 14 , 15 , 16
The stable nature of the bacterium in the environment means it can remain viable in soil for more than 4 months and persist as an aerosol for a fortnight. 10 , 17 , 18 , 19 Cases of Q fever have been recorded in humans with no direct exposure to ruminants and are thought to have originated through aerosol or environmental contamination. 4 , 17 , 19 , 20 , 21 , 22
In humans, Q fever causes variable symptoms. About 60% of cases are asymptomatic infections, 23 with up to 40% of people experiencing severe flu‐like symptoms including fever, chills, headaches, muscle and joint pain, fatigue and malaise. 24 Complications include hepatitis, pneumonia, valvular endocarditis, osteomyelitis and post‐Q fever fatigue syndrome. 25 Acute symptoms in humans generally start 2–3 weeks after initial contact with infective propagules of the organism. 1 , 3 , 26 , 27
Q fever is a nationally notifiable disease in humans, with 86% of cases arising from Qld and New South Wales (NSW), 1 , 28 , 29 and Qld responsible for more than half of all Q fever notifications in Australia annually. 3 , 30 There are 6.3 cases per 100,000 people in Qld, which is more than double the rate of the next highest state (NSW). The human Q fever notification rate for regions above the Tropic of Capricorn in Qld between 2016 and 2021 is shown in Figure 1. 31
Figure 1.
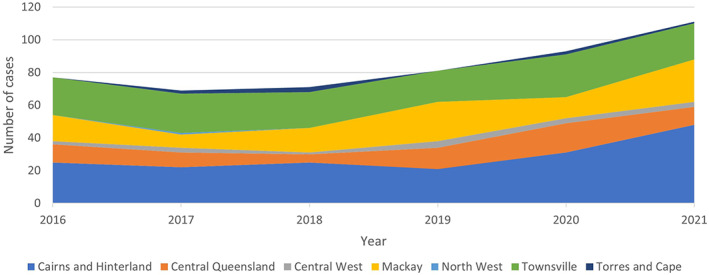
Human Q fever notifications to Queensland Health from Hospital and Health Services regions above the tropic of Capricorn in Queensland, Australia from January 2016 to 19 December 2021.
Certain occupations are at greater risk of developing Q fever than others, largely due to interactions with ruminants. Abattoir workers, dairy farmers, veterinarians and saleyard workers are all considered at increased risk compared with the general public. 6 , 7 , 8 , 13 , 16 , 30 Although not recognised as a significant source of human infection, both feral (prevalence rates 1.5%–5.9%) and domestic pigs (prevalence rate 6.8%) overseas have tested positive to C. burnetii with ELISA, immunofluorescence assay (IFA) or PCR testing. 32 , 33 In Australia, one study using an unvalidated ELISA, reported that 22% of feral pigs in northern Queensland were seropositive to C. burnetii. 34
Feral pig hunting is a popular activity in rural and remote Qld, with most hunters using dogs as a hunting aide. 35 , 36 Although pig hunters are not recognised by human health authorities in Qld as being an at‐risk group for Q fever, their exposure to the environment and feral pigs, potential hunting of other wildlife species like macropods and interaction with dogs may be associated with an increased risk. Increasingly, species other than ruminants are being implicated as potential sources of infection including cats and dogs, both of which have been associated with human outbreaks of Q fever, usually linked with attendance at parturition. 9 , 10 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47
There are few epidemiological studies on C. burnetii prevalence in dogs in Australia. Only six serosurveys have been conducted on canids in Australia to date 10 , 19 , 34 , 37 , 38 , 39 (Table 1). The role of dogs in human C. burnetii infections remains unclear, and with limited understanding of the prevalence of C. burnetii in the Australian dog population, humans who interact with parturient dogs such as veterinary staff and owners, may not take appropriate safety precautions to mitigate the potential infectious disease risk.
Table 1.
Summary of canine Coxiella burnetii prevalence data in Australia from published studies until November 2021
| Location | Year | Sample size | Sample source | Method of detection | Prevalence | References |
|---|---|---|---|---|---|---|
| Townsville (Qld) | 1984–1985 | 100 | Pet dogs | ELISA | 16% | 39 |
| Townsville (Qld) | 2006–2007 | 101 | Pet dogs | ELISA | 21.8% | 39 |
| Qld (various) | 2012 | 127 | Dingoes | ELISA | 17.3% | 34 |
| Qld (various) | 2013 | 574 | Pet dogs | PCR | 5% | 19 |
| Sydney (NSW) | 2010–2012 | 309 | Breeding dogs | IFA | 2.3% | 37 |
|
Sydney area and Wagga Wagga (NSW) |
2010–2014 | 328 | Pet dogs | IFA | 3% | 37 |
| Regional NT and NSW | 2000–2014 | 321 | Camp dogs | IFA | 6.5% | 37 |
| Sydney (NSW) | 2011–2012 | 265 | Shelter dogs | IFA | 1.9% | 37 |
| North‐west NSW and NT | 2013–2014 | 96 | Camp dogs | IFA | 4.2% | 38 |
| Remote NSW | 2016–2018 | 330 | Camp dogs | IFA | 26.1% | 10 |
This study aimed to determine if pig‐hunting dogs above the Tropic of Capricorn in Qld had similar rates of C. burnetii exposure to companion dogs in rural NSW. We hypothesised that at least 10% of pig‐hunting dogs would be seropositive to C. burnetii; more than urban companion dogs in NSW (1.9%–2.3%), 37 but less than rural companion dogs in far north‐west NSW (26.1%). 10
Materials and methods
Sample population of pig‐hunting dogs
Veterinary clinics from above the Tropic of Capricorn in central, north and far north Queensland were approached via email and in person, to participate in this study. Clinics were chosen primarily based on location, as it was assumed pig hunters were more likely to utilise clinics in rural and regional areas. Eight clinics opted to participate in the study from the regions around Sarina, Clermont, Proserpine, Charters Towers, Tully, Innisfail, Malanda and the Atherton Tableland (Figure 2).
Figure 2.
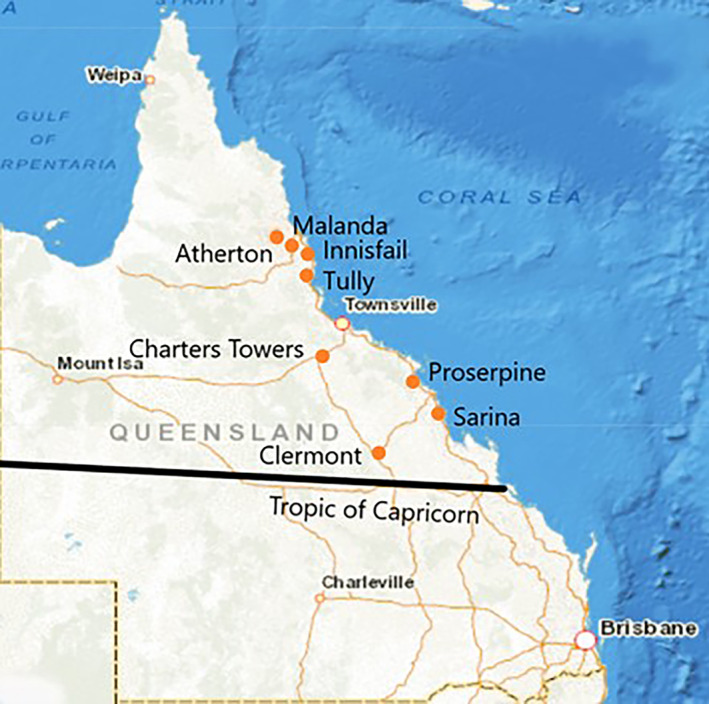
Location of participating veterinary clinics in a 2018 serosurvey of pig‐hunting dogs from above the tropic of Capricorn in Queensland, Australia.
All dogs enrolled in the study were older than 6 months‐of‐age at the time of sampling. The key inclusion criteria for the study specified that the dog must be currently used for pig hunting. Veterinary clinics provided medical histories for each patient, and the following data were captured at the time of sampling: the dog's age, breed, sex, reproductive state (sexually intact versus neutered) and vaccination history. Animal ethics approval was obtained from The University of Sydney Animal Ethics Committee (approval number 2018/1341).
A registered veterinarian performed a physical examination and determined each dog as clinically normal prior to sampling. Whole blood (3–5 mL) was collected via cephalic venepuncture, stored on ice and allowed to clot. Serum was then collected from the sample, aliquoted and stored at −20°C until IFA testing was performed at the Sydney School of Veterinary Science, The University of Sydney, New South Wales, Australia.
Owner survey
Dog owners self‐identified their dogs as pig‐hunting dogs and completed a survey while their dogs were examined by a veterinarian. The paper‐based survey (Appendix S1) comprised of 34 questions: 30 closed or semi‐closed questions and four open ended questions. There were five themes in the survey: demographic information about the owner; information about the dog; hunting style and geographic region of hunts with their dog; the health of their dogs; and their knowledge and awareness of zoonotic disease risks during hunts. The survey obtained human ethics approval from The University of Sydney Human Research Ethics Committee (approval number 2018/317).
IFA testing
Indirect IFA testing for phase I and phase II IgG antibodies was conducted using a commercially available, human indirect IFA kit (Vircell, Spain), with phase I and phase II antigens separated into different wells and adapted and verified for use in dogs, as described previously. 10 , 37 The adaptations included replacing the supplied human fluorescein isothiocyanate (FITC) conjugate with anticanine IgG FITC conjugate (CJ‐F‐CANG‐10ML, Veterinary Medical and Research Development [VMRD]), and utilising positive control sera from a dog at the centre of a Q fever outbreak, 37 as well as the supplied human positive and negative controls.
Dogs were initially screened with sera diluted at 1/64, with this cut‐off determined by previous studies as it provides a clear distinction between bacterial fluorescence and background fluorescence at this titre. 37 Positive sera then underwent twofold serial dilutions to determine the final titre for both phase I and phase II antibodies based on fluorescence. A BX60 epifluorescence microscope (Olympus, Melville, NY, USA) was used to read slides at a 400× magnification (wavelength: excitation 490 nm, emission 530 nm). Two of the authors (BO and JN) independently read each slide. Samples were considered seropositive if phase I and/or phase II antibody titres were 1/64 or greater.
Data analysis
The age and serostatus of pig‐hunting dogs, the relationship between housing type and seropositivity as well as the likelihood of positive cases being from the same household, were compared using Mann–Whitney U tests. Two‐tailed Fisher's exact tests were used to compare the likelihood of male and female dogs recording seropositive titres as well as the role of neuter status in seropositivity. A significance level of P < 0.05 was used for all statistical tests.
Results
Pig‐hunting dogs
A total of 104 pig‐hunting dogs were sampled from above the Tropic of Capricorn in Qld, Australia. Nineteen dogs (18.3%, 95% CI 9.6%–35.5%) were seropositive to C. burnetii on IFA. Twelve dogs had IgG titres to both phase I and phase II, seven dogs had IgG titres to phase II only, while no dogs were seropositive to phase I IgG titres alone (Table 2).
Table 2.
Signalment and IgG titre for Coxiella burnetii seropositive pig‐hunting dogs tested in 2018 from above the tropic of Capricorn, Queensland using an indirect immunofluorescence assay
| Breed or type | Age (years) | Sex | Location | Phase I IgG titre | Phase II IgG titre |
|---|---|---|---|---|---|
| Bull Arab X | 6 | MN | Malanda | Negative | 1/256 |
| Border Collie X | 11 | FN | Malanda | Negative | 1/256 |
| Bull Arab X | 6 | MN | Malanda | Negative | 1/256 |
| Pit Bull Terrier X | 7 | MN | Proserpine | 1/256 | 1/128 |
| Bull Arab X | 10 | FN | Clermont | 1/128 | 1/128 |
| Wolfhound X | 2 | MN | Charters towers | 1/256 | 1/256 |
| Staffordshire Terrier X | 2 | FN | Charters towers | 1/256 | 1/256 |
| Bull Arab X | 0.5 | M | Malanda | Negative | 1/256 |
| Wolfhound X | 2 | F | Malanda | Negative | 1/64 |
| Bull Arab X | 3 | F | Malanda | Negative | 1/64 |
| NQ Bullhound | 2 | F | Malanda | 1/64 | 1/64 |
| Cattle Dog X | 0.5 | M | Malanda | 1/256 | 1/256 |
| Bull Terrier X Boxer | 0.9 | M | Malanda | 1/128 | 1/64 |
| Jack Russell Terrier | 2 | M | Malanda | 1/256 | 1/256 |
| Bull Arab X | 4 | F | Tully | Negative | 1/64 |
| Cattle Dog X | 2 | M | Tully | 1/128 | 1/128 |
| Cattle Dog X | 2 | F | Clermont | 1/64 | 1/256 |
| Ridgeback X | 7 | M | Clermont | 1/256 | 1/128 |
| Bull Arab X Wolfhound | 5 | M | Charters towers | 1/64 | 1/128 |
F, female intact; FN, spayed female; M, male intact; MN male castrated; X, crossbred.
There was no significant difference in age between seropositive (median age 2 years; IQR 2–6 years) and seronegative dogs (median age 3 years; IQR 2–5 years) (P = 0.99; Mann–Whitney U test). Likewise, there was no relationship between sex and seropositivity (11/60 males vs 8/44 females; P = 1; Fisher's exact test, two‐tailed). However, neutered dogs (7/20; median age 6; IQR 4–8.5) were more likely to be seropositive than entire dogs (12/84; median age 2; IQR 1.7–3.25) (P = 0.0497; Fisher's exact test, two‐tailed).
Seropositive dogs were more likely to come from households where multiple dogs tested seropositive (P < 0.00001; Mann–Whitney U test). Some regions had higher rates of seropositivity than others (Table 3). Three regions – Charters Towers, Malanda and Tully – had more than 30% of dogs test positive for Coxiella burnetii antibodies.
Table 3.
Coxiella burnetii seropositive results in pig‐hunting dogs tested in 2018 from veterinary clinics above the tropic of Capricorn in Queensland, Australia
| Region | Number of seropositive dogs | Total dogs | Percentage of seropositive dogs (%) |
|---|---|---|---|
| Atherton | 0 | 5 | 0 |
| Clermont | 3 | 36 | 8.3 |
| Charters towers | 3 | 6 | 50 |
| Innisfail | 0 | 5 | 0 |
| Malanda | 10 | 30 | 33 |
| Proserpine | 1 | 19 | 5.5 |
| Tully | 2 | 3 | 66.6 |
Owner survey
A total of 39 owners returned the survey (Appendix S1). Just over one third of owners (35.8%; 14/39) indicated they kept their pig‐hunting dogs in their home or house yard, with the remainder (64.2%; 25/39) noting their dogs lived primarily in separate kennels. No relationship between housing type and seropositivity was found (P = 0.86; Mann–Whitney U test).
Regarding Q fever vaccination, five respondents (12.8%; 5/39) indicated they had received a Q fever vaccination, while 27 said they had not been vaccinated against Q fever (60.2%; 27/39) and seven were unsure. To the best of their knowledge, no owner had contracted Q fever previously. Most respondents (35/39; 90%) were aware that both feral pigs and pig‐hunting dogs could be sources of C. burnetii.
Discussion
Almost one in five pig‐hunting dogs in our research cohort from above the Tropic of Capricorn in Qld, Australia was seropositive for C. burnetii (Table 2). This rate of seropositivity is high compared to previous canine serosurveys which used IFA to investigate seroprevalence in breeding dogs, urban companion dogs and dogs from remote First Nations communities across NSW and the Northern Territory (NT). 37 , 38 The seropositivity rate of pig‐hunting dogs in Qld, however, was lower than companion dogs in far north‐west NSW, which to date has recorded the highest seropositivity in canines in Australia. 10 Issues with the prevalence rates found in earlier serosurveys using ELISA above the Tropic of Capricorn in Qld have been discussed previously and include using an ELISA optimised for mice and using pooled samples as positive and negative controls. 10 , 37 This was the first study examining C. burnetii exposure in pig‐hunting dogs and adds to our growing understanding of the epidemiology of C. burnetii in dogs in Australia. Pig‐hunting dogs in Qld have been shown to be at high risk for canine heartworm disease 48 and Leptospira spp. exposure, 49 and their exposure rate to C. burnetii suggests another increased infectious disease risk.
Although the average seropositivity rate across the pig‐hunting dog cohort was 18.3%, the regions of Malanda, Charters Towers and Tully had more than one in three dogs test positive to C. burnetii antibodies (Table 3). It was established that dogs from the same household had an increased risk of testing seropositive to C. burnetii. This is not unexpected, as dogs living and hunting together would face similar environmental sources of exposure. There was no relationship between age or sex and seropositivity, however neutered dogs were found to have an increased risk of C. burnetii seropositivity compared to entire dogs. This may be due to the increased age of neutered dogs, reflecting a potential cumulative lifetime exposure risk. Establishing the validity of this increased risk requires further investigation with a larger, more diverse sample of dogs from across Queensland to determine if this relationship holds true.
Feral pigs have been found to shed C. burnetii in Australia and overseas. 19 , 32 The increased rates of seropositivity in pig‐hunting dogs may be due to direct contact with feral pigs, although it is more likely their increased environmental exposure during hunts, and rural lifestyle, increases their exposure risk compared to urban dogs. 10 Like humans, dogs living in rural and agricultural regions appear to have an increased risk of seropositivity to C. burnetii, possibly due to environmental contamination of dust and soil from livestock. 10 , 17 , 27 In addition, exposure to marsupials and ruminants may be indirectly increased by hunting in livestock paddocks and environments where animals converge, 50 and there are anecdotal reports of pig hunters shooting kangaroos to feed their dogs, with feeding fresh kangaroo meat a potential risk factor for Coxiella exposure in cats and dogs. 51
The single time point nature of this serosurvey is associated with several limitations. We can determine past exposure to C. burnetii from this study, but we are unable to establish the timing of exposure or whether the exposure resulted in clinical or subclinical disease for the animal such as abortion in entire female pig‐hunting dogs. Previous research conducted on companion dogs in far north‐west NSW found no detectable C. burnetii in blood or reproductive organs. 10 Future investigations of C. burnetii exposure in pig‐hunting dogs should include a ‘control’ population of nonhunting farm or companion dogs to isolate the risk posed by hunting compared to other environmental interactions such as herding livestock. As pig‐hunting dogs travel widely with their owners, 35 , 52 it is challenging to identify the exact location of C. burnetii exposure.
A key finding from our research was the rates of Q fever vaccination amongst the pig hunters who enrolled their dogs in our serosurvey. None of the owners surveyed indicated they had previously been diagnosed with Q fever, although the majority were aware that feral pigs and dogs may be a source of infection. Five (12.8%) of the respondents indicated they had been previously vaccinated against Q fever. It is likely this rate of Q fever vaccination is much higher than the general public, however as Q fever vaccination data are not collected on a state or national basis, we were unable to make this comparison. Although a Q Fever Register exists, it is privately run and does not capture data on vaccination rates. 53 Given our results demonstrate a relatively high rate of C. burnetii seropositivity in pig‐hunting dogs, it seems prudent to encourage this vaccination trend and recommend all pig hunters in Qld to strongly consider Q fever vaccination.
It is unknown why Q fever vaccination rates were high for our pig hunters. It may be that pig hunters are more likely to be engaged in agricultural sectors where vaccination is either mandatory or strongly recommended, such as abattoirs and livestock saleyards. Alternatively, it might reflect the rural nature of the activity, being a popular recreation in nonmetropolitan regions of Qld. 54 , 55 Further research into Q fever vaccination rates by occupation may provide additional insights.
There is mounting evidence of C. burnetii exposure in dogs in Australia. 10 , 37 , 38 , 39 The zoonotic disease risk posed by these dogs remains poorly understood, and it would be prudent to continue investigations and surveillance on C. burnetii in dogs to further explore this possibility. In the interim, pig hunters and veterinary staff handling parturient pig‐hunting bitches and neonates should be cautious and take appropriate risk mitigation measures such as wearing PPE and vaccination against Q fever.
Conflicts of interest and sources of funding
The authors declare no conflicts of interest or sources of funding for the work presented here.
Supporting information
Appendix S1. Survey completed by pig‐hunting dog owners.
Acknowledgments
The authors thank all participating veterinary clinics and dog owners for engaging with this research study. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Orr, B. , Malik, R. , Westman, ME. and Norris, JM. , Seroprevalence of Coxiella burnetii in pig‐hunting dogs from north Queensland, Australia. Aust Vet J. 2022;100:230–235. 10.1111/avj.13151
References
- 1. Eldin C, Mélenotte C, Mediannikov O et al. From Q fever to Coxiella burnetii infection: A paradigm change. Clin Microbiol Rev. 2017;30:115–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oskam C, Owens J, Codello A et al. Rethinking Coxiella infections in Australia. Microbiol Aust. 2018;39:223. [Google Scholar]
- 3. Department of Health . Q fever [Internet]. CDNA National Guidelines for Public Health Units. 2018. Available at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-q-fever.htm. Cited 10 November 2021.
- 4. Gidding HF, Peng CQ, Graves S et al. Q fever seroprevalence in Australia suggests one in twenty people have been exposed. Epidemiol Infect. 2020;148:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Derrick EH, Director MD. ‘Q’ fever, a new fever entity: clinical features. Diagn Lab Investig. 1937;12:281–299. [Google Scholar]
- 6. Gunther MJ, Heller J, Hayes L et al. Dairy goat producers' understanding, knowledge and attitudes towards biosecurity and Q‐fever in Australia. Prev Vet Med. 2019;170:104742. [DOI] [PubMed] [Google Scholar]
- 7. Cooper A, Hedlefs R, McGowan M et al. Serological evidence of Coxiella burnetii infection in beef cattle in Queensland. Aust Vet J. 2011;89:260–264. [DOI] [PubMed] [Google Scholar]
- 8. Woldeyohannes SM, Gilks CF, Baker P et al. Seroprevlance of Coxiella burnetii among abattoir and slaughterhouse workers: a meta‐analysis. One Health. 2018;6:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agerholm JS. Coxiella burnetii associated reproductive disorders in domestic animals‐a critical review. Acta Vet Scand. 2013;55:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma GC, Norris JM, Mathews KO et al. New insights on the epidemiology of Coxiella burnetii in pet dogs and cats from New South Wales, Australia. Acta Trop. 2020;205:105416. [DOI] [PubMed] [Google Scholar]
- 11. Mangena M, Gcebe N, Pierneef R et al. Q fever: seroprevalence, risk factors in slaughter livestock and genotypes of Coxiella burnetii in South Africa. Pathogens. 2021;10:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark NJ, Tozer S, Wood C et al. Unravelling animal exposure profiles of human Q fever cases in Queensland, Australia, using natural language processing. Transbound Emerg Dis. 2020;67:2133–2145. [DOI] [PubMed] [Google Scholar]
- 13. Hansman D, Murphy AM, Wannan JS et al. Q fever, brucellosis and leptospirosis among abattoir workers in New South Wales. Med J Aust. 1966;2:20–23. [DOI] [PubMed] [Google Scholar]
- 14. Kennedy JM, Lulham CR, Gordon D. Occupational fevers, Queensland, 1950‐1951. Med J Aust. 1952;1(11):360–364. [PubMed] [Google Scholar]
- 15. Rahaman MR, Marshall H, Milazzo A et al. Q fever prevention and vaccination: Australian livestock farmers' knowledge and attitudes to inform a one Health approach. One Health. 2021;12:100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKelvie P. Q fever in a Queensland meatworks. Med J Aust. 1980;1:590–593. [DOI] [PubMed] [Google Scholar]
- 17. Archibald J. Disease in the dust: experiences of Q fever during drought in Australia. Perspect Public Health. 2019;139:77–78. [DOI] [PubMed] [Google Scholar]
- 18. Wiley KE, Walker J, Lower T et al. Australian beef industry worker's knowledge, attitudes and practices regarding Q fever: a pilot study. Vaccine. 2019;37:6336–6341. [DOI] [PubMed] [Google Scholar]
- 19. Tozer SJ, Lambert SB, Strong CL et al. Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses Public Health. 2014;61:105–112. [DOI] [PubMed] [Google Scholar]
- 20. Gidding HF, Faddy HM, Durrheim DN et al. Seroprevalence of Q fever among metropolitan and non‐metropolitan blood donors in New South Wales and Queensland, 2014–2015. Med J Aust. 2019;210:309–315. [DOI] [PubMed] [Google Scholar]
- 21. Buckley B. Q fever epidemic in Victorian general practice. Med J Aust. 1980;1:593–595. [DOI] [PubMed] [Google Scholar]
- 22. Gale M, Ketheesan N, Govan B et al. Q fever cases at a North Queensland Centre during 1994‐2006. Intern Med J. 2007;37:644–646. [DOI] [PubMed] [Google Scholar]
- 23. Maurin M, Raoult D. Q Fever. Clin Microbiol Rev. 1990;12:518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marrie TJ. Q fever ‐ a review. Can Vet J. 1990;31:555–563. [PMC free article] [PubMed] [Google Scholar]
- 25. Million M, Raoult D. Recent advances in the study of Q fever epidemiology, diagnosis and management. J Infect. 2015;71:S2–S9. [DOI] [PubMed] [Google Scholar]
- 26. Gsell O. Clinical aspect of Queensland fever. Asp Clin Fievre Qld. 1950;8:243–245. [PubMed] [Google Scholar]
- 27. Lindsay PJ, Rohailla S, Miyakis S. Q fever in rural Australia: education versus vaccination. Vector‐Borne Zoonotic Dis. 2018;18:632–634. [DOI] [PubMed] [Google Scholar]
- 28. Hirschmann JV. The discovery of Q fever and its cause. Am J Med Sci. 2019;358:3–10. [DOI] [PubMed] [Google Scholar]
- 29. NNDSS Annual Report Working Group . Australia's notifiable disease status, 2016: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell. 2021;45:153–155. Available at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/8FA6078276359430CA257BF0001A4C42/$File/australia_s_notifiable_disease_status_2016_annual_report_of_the_national_notifiable_diseases_surveillance_system.pdf. Cited 14 November 2021. [DOI] [PubMed] [Google Scholar]
- 30. Eastwood K, Graves SR, Massey PD et al. Q fever: A rural disease with potential urban consequences. Aust J Gen Pract. 2018;47:112–116. [DOI] [PubMed] [Google Scholar]
- 31. Queensland Health . Notifiable conditions annual reporting [internet]. Brisbane, Queensland, Queensland Health, 2021. Available at: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/diseases-infection/surveillance/reports/notifiable/annual. Cited 7 January 2022. [Google Scholar]
- 32. González‐Barrio D, Martín‐Hernando MP, Ruiz‐Fons F. Shedding patterns of endemic Eurasian wild boar (Sus scrofa) pathogens. Res Vet Sci. 2015;102:206–211. [DOI] [PubMed] [Google Scholar]
- 33. Seo M‐G, Ouh I‐O, Lee S‐H et al. Detection and genotyping of Coxiella burnetii in pigs, South Korea, 2014–2015. Emerg Infect Dis. 2016;22:2192–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooper A, Goullet M, Mitchell J et al. Serological evidence of Coxiella burnetii exposure in native marsupials and introduced animals in Queensland, Australia. Epidemiol Infect. 2012;140:1304–1308. [DOI] [PubMed] [Google Scholar]
- 35. Orr B, Malik R, Norris J et al. The welfare of pig‐hunting dogs in Australia. Animals. 2019;9:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meurk C. Loving nature, killing nature, and the crises of caring: an anthropological investigation of conflicts affecting feral pig management in Queensland, Australia [Internet]. School of Social Science: The University of Queensland, Brisbane, Australia, 2011. Available at: https://espace.library.uq.edu.au/view/UQ:247462. Cited 10 November 2021. [Google Scholar]
- 37. Shapiro AJ, Norris JM, Heller J et al. Seroprevalence of Coxiella burnetii in Australian dogs. Zoonoses Public Health. 2016;63:458–466. [DOI] [PubMed] [Google Scholar]
- 38. Shapiro AJ, Brown G, Norris JM et al. Vector‐borne and zoonotic diseases of dogs in north‐West New South Wales and the Northern Territory, Australia. BMC Vet Res. 2017;13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper A, Hedlefs R, Ketheesan N et al. Serological evidence of Coxiella burnetii infection in dogs in a regional centre. Aust Vet J. 2011;89:385–387. [DOI] [PubMed] [Google Scholar]
- 40. Shapiro AJ, Norris JM, Bosward KL et al. Q fever (Coxiella burnetii) knowledge and attitudes of Australian cat breeders and their husbandry practices. Zoonoses Public Health. 2017;64:252–261. [DOI] [PubMed] [Google Scholar]
- 41. Rezaei M, Khalili M, Saberi M et al. Are dogs and cats possible reservoirs for human Q fever in Iran? Acta Vet Eurasia. 2021;47:37–43. [Google Scholar]
- 42. Stefanetti V, Compagnone A, Sordini C et al. Retrospective biomolecular investigation of Coxiella burnetii and Leptospira spp. DNA in cases of abortion, stillbirth and neonatal mortality in dogs and cats. Top Companion Anim Med. 2018;33:122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shapiro AJ, Bosward KL, Heller J et al. Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Vet Microbiol. 2015;177:154–161. [DOI] [PubMed] [Google Scholar]
- 44. Malo JA, Colbran C, Young M et al. An outbreak of Q fever associated with parturient cat exposure at an animal refuge and veterinary clinic in Southeast Queensland. Aust N Z J Public Health. 2018;42:451–455. [DOI] [PubMed] [Google Scholar]
- 45. Kopecny L, Bosward KL, Shapiro A et al. Investigating Coxiella burnetii infection in a breeding cattery at the centre of a Q fever outbreak. J Feline Med Surg. 2013;15:1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laughlin T, Waag D, Williams J et al. Q fever: From deer to dog to man. Lancet. 1991;337:676–677. [DOI] [PubMed] [Google Scholar]
- 47. Gibbons GC, White PJ. Q fever in a veterinary hospital ‐ an unusual epidemiology. In: Proceedings of the Australasian Society for Infectious Diseases. 2012;35. [Google Scholar]
- 48. Orr B, Ma G, Koh WL et al. Pig‐hunting dogs are an at‐risk population for canine heartworm (Dirofilaria immitis) infection in eastern Australia. Parasit Vectors. 2020;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orr B, Westman ME, Malik R et al. Leptospirosis is an emerging infectious disease of pig‐hunting dogs and humans in North Queensland. PLoS Negl Trop Dis. 2022;16:e0010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stevenson S, Gowardman J, Tozer S et al. Life‐threatening Q fever infection following exposure to kangaroos and wallabies. BMJ Case Rep 2015:bcr2015210808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shapiro A, Bosward K, Mathews K et al. Molecular detection of Coxiella burnetii in raw meat intended for pet consumption. Zoonoses Public Health. 2020;67:443–452. [DOI] [PubMed] [Google Scholar]
- 52. Gabriele‐Rivet V, Brookes V, Arsenault J et al. Hunting practices in northern Australia and their implication for disease transmission between community dogs and wild dogs. Aust Vet J. 2019;97:268–276. [DOI] [PubMed] [Google Scholar]
- 53. AMPC . Q Fever Register [Internet]. 2021. Available at: https://www.qfever.org/home/abouttheregister. Cited 2 November 2021.
- 54. Meurk C. Contesting death: conservation, heritage and pig killing in far North Queensland, Australia. Environ Values. 2015;24:79–104. [Google Scholar]
- 55. Bengsen AJ, Sparkes J. Can recreational hunting contribute to pest mammal control on public land in Australia? Mammal Rev. 2016;46:297–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Survey completed by pig‐hunting dog owners.


