Abstract
Objectives
We aimed to assess prevalence and age at menopause, identify factors associated with early menopause and explore the provision and utilization of healthcare in women living with HIV in Switzerland.
Methods
This was a retrospective Swiss HIV Cohort Study analysis from January 2010 to December 2018. Descriptive statistics to characterise the population and menopause onset. Logistic regression analysis to identify risk factors for early menopause.
Results
Of all women in the SHCS, the proportion of postmenopausal women tripled from 11.5% (n = 274) in 2010 to 36.1% (n = 961) in 2018. The median age at menopause was 50 years. Early menopause (< 45 years) occurred in 115 (10.2%) women and premature ovarian insufficiency (POI) (< 40 years) in 23 (2%) women. Early menopause was associated with black ethnicity (52.2% vs. 21.6%, p < 0.001), but not with HIV acquisition mode, CDC stage, viral suppression, CD4 cell count, hepatitis C, smoking or active drug use. While 92% of the postmenopausal women underwent a gynaecological examination during the 36 months before menopause documentation, only 27% received a bone mineral density measurement within 36 months after the last bleed and 11% were on hormone replacement therapy at the time of menopause documentation.
Conclusions
The median age of women living with HIV at menopause is around 2 years lower than that reported for HIV‐negative women in Switzerland. HIV care providers need to adapt their services to the requirements of the increasing number of women living with HIV transitioning through menopause. They should be able to recognize menopause‐associated symptoms and improve access to bone mineral density measurement as well as hormone replacement therapy.
Keywords: bone mineral density, early menopause, HIV, hormone replacement therapy, menopause
INTRODUCTION
Over recent decades, antiretroviral treatment (ART) has transformed HIV into a long‐term condition with normal life expectancy for those receiving early diagnosis and consistent treatment. Consequently, there has been a demographic shift, with a growing number of older women living with HIV in the Swiss HIV Cohort Study (SHCS).
These demographic changes are not unique to Switzerland, as they are observed in high‐ as well as middle‐ and low‐income settings [1] The literature reports conflicting results on whether menopause occurs at an earlier age in women living with HIV [2, 3, 4, 5, 6].
Approximately 85% of perimenopausal HIV‐negative women report menopause symptoms [7] with a negative impact on quality of life [8, 9].
Peri‐ and postmenopausal women living with HIV might face particular risks due to loss of the protective effects of oestrogen for dyslipidaemia, cardiovascular disease (CVD) [10] and bone density loss, requiring proactive screening and advice about preventive measures. There is little awareness of the benefits of hormone replacement therapy (HRT) in women living with HIV, in the context of preventing osteoporosis and CVD and treating perimenopausal symptoms [11]. In women living with HIV, the prevalence of psychological symptoms in the perimenopause seems very high, especially in relation to depression and anxiety [12, 13, 14]. Furthermore, disentangling perimenopausal symptoms from HIV‐related or antiretroviral treatment (ART)‐related symptoms is challenging. Difficulties accessing appropriate gynaecological menopause care, and the possible negative impact of perimenopausal symptoms on adherence to ART are additional challenges when providing care for perimenopausal women living with HIV [15, 16, 17].
The aim of this study was to determine the prevalence and age at menopause in the SCHS, to assess whether early menopause is prevalent, and if so, to identify the associated risk factors. Furthermore, we aimed to explore healthcare provision and utilization around menopause.
METHODS
The Swiss HIV Cohort Study
The SHCS is a nationwide prospective cohort study With a coverage of at least 75% of all patients receiving ART in Switzerland it is considered to be representative [18]. Around 25% of SHCS participants are female. Patients are followed 6‐monthly with information collected on demographics, behaviour, clinical events and treatment. Local ethics committees of all study sites have approved the study and written informed consent is obtained from all participants. Information on obstetric events has been routinely collected since July 2005, and information on hormonal therapies since June 2007.
Study population and definitions
We included all women with menopause onset between January 2010 and December 2018 registered in the SHCS. We excluded transgender persons and, for the assessment of menopause age, also women after ovarectomy/hysterectomy, as well as women with documented menopause onset after 55 years (as they have a high probability of SHCS inclusion after menopause onset and we hypothesize a cohort inclusion bias). Menopause was defined as the absence of menstrual bleeding for a duration of 12 months and had to be confirmed as the reason for amenorrhoea by the treating physician.
Menopause onset before 45 years was considered as early, and onset before 40 years as premature ovarian insufficiency (POI). Viral suppression was defined as HIV‐RNA < 50 copies/mL, and consuming > 10 g alcohol/day as elevated alcohol consumption. A body mass index (BMI) > 25 kg/m2 was considered as overweight and one < 18 kg/m2 as underweight. Non‐intravenous drug use (NIVDU) was defined as consuming illicit substances orally, by inhalation or sniffing. Depression diagnosis was made by the treating physicians or psychiatrists, who used various diagnostic tools. Bone mineral density was measured with dual‐energy X‐ray absorptiometry (DXA).
For the assessment of clinical and behavioural characteristics at the time of menopause, the 6 months preceding the first interview after menopause onset were taken into account.
Statistical analyses
Descriptive statistics were used to determine the prevalence and age at menopause and to characterize postmenopausal women.
Uni‐ and multivariate logistic regression analyses were performed to identify risk factors for the early onset of menopause with independent variables for ethnicity, overweight, underweight, psychiatric care, depression, smoking, alcohol consumption, ongoing intravenous drug use (IVDU), ongoing NIVDU, hepatitis C or B coinfection (active, resolved or immunologically controlled), suppressed viral load, CD4 count < 200 cells/μL.
All statistical analyses were performed with R v.3.6.1. and Stata v.16.0 [19, 20].
RESULTS
The ageing population of women living with HIV in Switzerland
The proportion of postmenopausal women in the SHCS tripled from 11.5% (n = 274) in 2010 to 36% (n = 961) in 2018 (Figure 1a). The age distribution of women living with HIV under follow‐up indicates that 31% (n = 838) are between 39 and 49 years old and expected to be transitioning through menopause over the next 10 years(Figure 1b).
FIGURE 1.
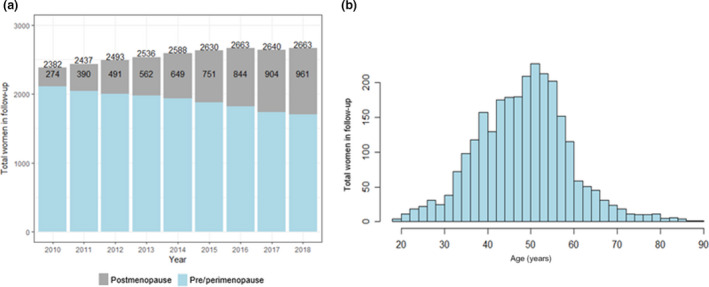
Demographic shift of women participating in the Swiss HIV Cohort Study (SHCS) between 2010 and 2018. (a) Proportion of postmenopausal women over time. (b) Age histogram of women participating in the SHCS in 2018
Age and other characteristics of women at time of menopause
In all, 1130 postmenopausal women met the inclusion criteria. The median age at menopause onset was 50 [interquartile range (IQR): 32.0–55.0] years. Early menopause (< 45 years) occurred in 115 (10.2%) women and POI (< 40 years) in 23 (2%) women.
One‐third of postmenopausal women were of non‐Caucasian ethnicity, mostly black (25%). There was a high hepatitis coinfection rate, with 31% having hepatitis C antibodies (active or cleared hepatitis C infection) and 45% with hepatitis B coinfection (active or immunologically controlled). Also, mental health problems were frequent, with 27% of the participants diagnosed with depression and/or being in psychiatric care. The percentage who were overweight (BMI > 25 kg/m2) was 41%, whereas only 8% were underweight (BMI < 18 kg/m2); 45% were active smokers, 18% reported an elevated alcohol consumption and 15% reported NIVDU at the time, but only 3% injected drugs (Table 1).
TABLE 1.
Characteristics of women living with HIV at the time of menopause onset
| Women at time of menopause onset (n = 1130) | |
|---|---|
| Age at menopause (years) [median (range)] | 50 (32.0–55.0) |
| Early menopause (age < 45 years) | 115 (10%) |
| Premature ovarian insufficiency (age < 40 years) | 23 (2%) |
| Demographic and clinical characteristics | |
| Ethnicity | |
| Caucasian | 753 (67%) |
| Black | 279 (25%) |
| Other | 98 (9%) |
| Suppressed viral load (<50 copies/mL) | 975 (87%) |
| CD4 count < 200 cells/μL | 58 (5%) |
| Underweight (BMI < 18 kg/m2) | 86 (8%) |
| Overweight (BMI > 25 kg/m2) | 468 (41%) |
| Depression and/or being in psychiatric care | 300 (27%) |
| Behavioural characteristics | |
| Elevated alcohol consumption | 200 (18%) |
| Smoking | 513 (45%) |
| Active IVDU | 33 (3%) |
| Active non‐IVDU | 173 (15%) |
| Having sex with a stable sex partner | 746 (66%) |
| Having sex with an occasional sex partner | 394 (34.9%) |
Abbreviations: BMI, body mass index; IVDU, intravenous drug use.
Black women differed considerably from the other women by being more often overweight (BMI > 25 kg/m2 in 70% vs. 32%), smoking less (10% vs. 57%), drinking less alcohol (13% vs. 20%) and reporting less NIVDU (1% vs. 20%) (data not shown).
Demographic and clinical characteristics of women with early menopause
Women of black ethnicity were four times more likely to experience an early menopause compared with women of other ethnicities [adjusted odds ratio (aOR) = 4.2, 95% CI: 2.5–7.2, p < 0.001]. Smoking status, drug use and body weight showed no significant associations with early menopause. In addition, HIV‐associated factors, such as low CD4 status, viral suppression or hepatitis coinfection, did not influence age of menopause. Transmission through IVDU, smoking and hepatitis C were associated with a later menopause onset in univariate logistic regression, but did not remain significant in the adjusted model. Being underweight was also associated with a later menopause, but was excluded from the adjusted model because of confounding with black ethnicity (Tables 2, S1; Figure 2). Women of black ethnicity had a slightly lower median menopause age compared with women of other ethnicities (49 vs. 50 years). Moreover, 20 of the 23 women experiencing menopause under the age of 40 were of black ethnicity (data not shown).
TABLE 2.
Comparison of demographic characteristics between women with early menopause and women with normal age of menopause onset
|
Women with menopause onset at age ≥ 45 years (n = 1015) |
Women with early menopause onset (age < 45 years) (n = 115) |
p (normal vs. early) |
|
|---|---|---|---|
| Ethnicity | |||
| Other than black | 796 (78.4%) | 55 (47.8%) | < 0.001 |
| Black | 219 (21.6%) | 60 (52.2%) | |
| Transmission group | |||
| Heterosexual | 680 (67.0%) | 83 (72.2%) | 0.003 |
| IVDU | 293 (28.9%) | 21 (18.3%) | |
| Other | 42 (4.1%) | 11 (9.6%) | |
| BMI | |||
| Normal weight | 516 (50.8%) | 60 (52.2%) | 0.20 |
| Underweight (BMI < 18 kg/m2) | 82 (8.1%) | 4 (3.5%) | |
| Overweight (BMI > 25 kg/m2) | 417 (41.1%) | 51 (44.3%) | |
| HIV and comorbidities | |||
| CD4 count < 200 cells/µL | 51 (5.0%) | 7 (6.1%) | 0.62 |
| Viral load < 50 copies/mL | 871 (85.8%) | 104 (90.4%) | 0.073 |
| Hepatitis C (active or cleared) | 339 (33.4%) | 24 (20.9%) | 0.006 |
| Hepatitis B (active or immunologically controlled) | 461 (45.4%) | 50 (43.5%) | 0.76 |
| Depression or in psychiatric care | 273 (26.9%) | 27 (23.5%) | 0.43 |
| Behaviour | |||
| Elevated alcohol consumption | 182 (17.9%) | 18 (15.7%) | 0.54 |
| Smoking | 472 (46.5) | 41 (35.7%) | 0.031 |
| Active IVDU | 30 (3%) | 3 (2.6%) | 0.83 |
| Active non‐IVDU | 159 (15.7%) | 14 (12.2%) | 0.32 |
Abbreviations: BMI, body mass index; IVDU, intravenous drug use; NIVDU, non‐intravenous drug use.
FIGURE 2.
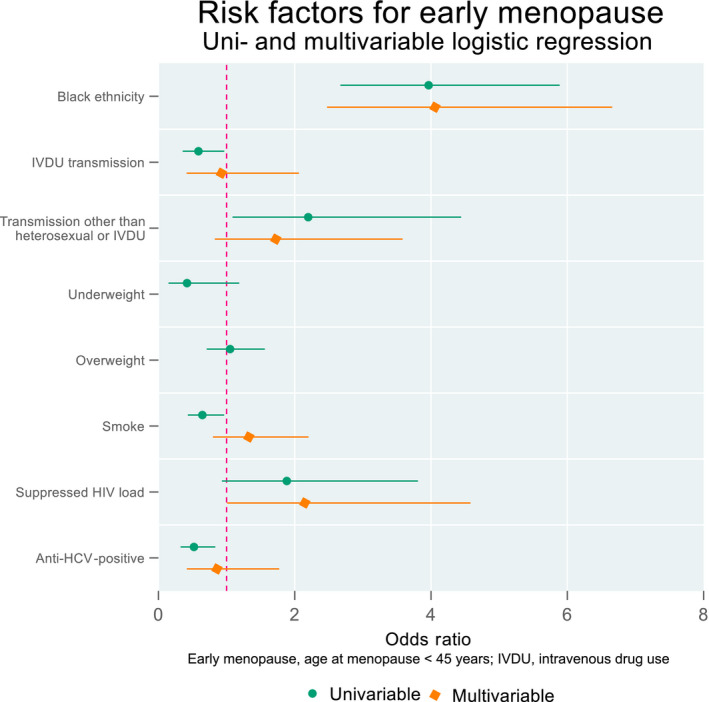
Uni‐ and multivariate logistic regression of risk factors for an early menopause
Health care utilization and provision around menopause
In all, 7% of the women reported missing an ART dose more than once a month. Forty‐one per cent underwent gynaecological examinations during the 6 months preceding menopause documentation and 92% during the 36 months before documentation. HRT was given to 11% during the 6 months preceding the first visit after menopause onset. A bone mineral density measurement (BDM) within 36 months after the last bleeding was only performed in 27% of the patients, and even less frequently in women of black ethnicity (21% vs. 29%, p = 0.02) (Table 3).
TABLE 3.
Healthcare provision and utilization during perimenopause
| Health care provision and utilization |
Ethnicity other than black (n = 851) |
Black ethnicity (n = 279) |
p‐value |
Total (n = 1130) |
|---|---|---|---|---|
| Gynaecological examination during the 6 months preceding menopause | 353 (42%) | 114 (41%) | 0.87 | 467 (41%) |
| Gynaecological examination during the 36 months preceding menopause | 784 (92%) | 250 (90%) | 0.19 | 1034 (92%) |
| Received a BDM within 36 months after the last bleeding | 243 (29%) | 59 (21%) | 0.02 | 302 (27%) |
| ART missed more than once a month | 57 (7%) | 20 (7%) | 0.78 | 77 (7%) |
| On HRT during the 6 months preceding menopause documentation a | 98 (12%) | 27 (10%) | 0.38 | 125 (11%) |
Abbreviations: BDM, bone mineral density measurement measured with dual‐energy X‐ray absorptiometry; HRT, hormone replacement therapy.
Refers to the 6 months preceding the first interview after menopause onset.
DISCUSSION
The number of HIV‐positive women transitioning through menopause in the SHCS is steadily increasing, with menopause onset occurring approximately 2 years earlier than reported for HIV‐negative women in Switzerland [21] Women of black ethnicity were four times more likely to experience early menopause than those of other ethnicities, but HIV‐related factors or smoking seem not to have an influence on menopause age. One in 10 women were using HRT, and only one in four had a BDM within 3 years of her last period.
An ageing population with significant comorbidities
Over the study period, the proportion of postmenopausal women in the SHCS tripled to 36% and another third is expected to transition through menopause during the next 10 years. The ageing of people living with HIV is not a Swiss or high‐income‐country phenomenon, but has been reported from all world regions [22, 23] and has major implications for HIV care providers, who during the past 30 years have been used to providing care for young and middle‐aged patients. Equally, research concerning women living with HIV has mainly focused on those of reproductive age, namely on pregnancy.
The clinical and behavioural characteristics of women at menopause onset illustrate the complexity of this population. Although the vast majority have a good immune function and viral suppression, psychiatric comorbidities were present in one in four women (27%), more than 40% were overweight, 45% were active smokers and almost 20% reported an elevated alcohol intake. Not surprisingly, all mentioned behaviours were considerably more frequent in the study population than in HIV‐negative women of a similar age living in Switzerland [24].
The high prevalence of psychiatric comorbidities and recreational drug use reveals the importance of integrating mental health and harm‐reducing interventions into HIV care. In addition, the high prevalence of increased BMI does imply an increased metabolic and cardiovascular risk for those women and needs attention too.
Is the earlier menopause onset related to HIV or to other factors?
Studies reporting age at menopause onset of women living with HIV often noted an earlier menopause onset compared with our study, but also when compared with HIV‐negative women in the respective countries [2, 5, 6, 25]. Due to the ethnic influence on menopause onset, it is difficult to compare studies. HIV cohorts from the US have higher proportions of African American women compared with those who are HIV‐negative, and also our cohort has a considerably higher proportion of women of black ethnicity compared with the general Swiss population. Ethnicity as a factor influencing menopause age has been well described [26]. Our data, with black ethnicity as an independent factor for an early menopause, confirm these findings. Nonetheless, when excluding women of black ethnicity, the median menopause age remains below that reported for HIV‐negative women in Switzerland, suggesting other causal factors.
Previous studies have hypothesized that the HIV infection per se may lead to an earlier menopause due to a continuous inflammatory state, early ovarian insufficiency and concurrent opportunistic infections. In our study, we could not identify any HIV‐related risk factors for an early menopause. This might be due to the high ART coverage, with only 5% of women with CD4 counts < 200 cells/μL and very few with viral replication. Studies examining the influence of CD4 count on menopause age have conflicting results, and are not easy to compare due to inconsistent definitions regarding low CD4 count and time point of measurement [2, 4, 6, 25, 27, 28].
The association of cigarette smoking with earlier menopause has been described by others, irrespective of the HIV status [26, 27, 29]. We did not find an association when investigating all menopausal women, but could see a slight trend of earlier menopause being more frequent in women who smoke (65% vs. 57%, p = 0.3), when excluding the women of black ethnicity (who have an earlier menopause, but smoke less). Similarly, we had to exclude weight from the multivariate regression analysis due to it being confounded with black ethnicity. These examples show how ethnic and social characteristics are interrelated, and how difficult it is to disentangle their individual influences on menopause onset. Irrespective of the exact mechanisms leading to an early menopause, and without being able to provide a universal model to predict the exact age at which menopause will occur, HIV care providers need to keep in mind that menopause onset in their patients is likely to occur earlier and with increased vulnerability for comorbidities.
Health care utilization and provision
Screening for menopause symptoms once yearly in women living with HIV aged ≥ 40 years is recommended by the European AIDS Clinical Society [30]. It is unclear how well this recommendation is followed in SHCS, as it is not recorded. Observational HIV studies from other countries show that menopause screening in women living with HIV is often poor [31, 32], although menopause symptoms are highly prevalent and probably more frequent and more severe compared with HIV‐negative women [5, 13, 15, 32]. Women with severe menopausal symptoms do not only experience reduced quality of life, but also seem less likely to engage in medical care and to be adherent to their ART [16, 17].
A majority of perimenopausal women followed in the SHCS are under gynaecological follow‐up, but only 11% were using HRT at the time of menopause documentation. Similar low rates have been reported from women living with HIV in the US and the UK [3, 4, 28, 32].
Menopause as well as HIV are established risk factors for osteoporosis. HIV treatment guidelines recommend either a BDM in all menopausal women [33] or at least to consider a BDM [30]. This is in contrast to the Swiss Association Against Osteoporosis, which does not explicitly mention HIV or menopause as an indication for a BDM [34]. While being regularly followed in their HIV clinics, only one in four of the women received a BDM within 3 years of the last bleeding. Although having a higher osteoporosis risk [35], in black women this was even more prominent, with only one in five women receiving a BDM. This might be explained by the lower age at menopause onset and by the lower prevalence of other risk factors such as smoking and being underweight in women of black ethnicity, which could prompt the treating physicians to omit BDMs. Hirst et al. similarly reported a considerable non‐compliance with national HIV osteoporosis screening guidelines in the UK, while Gilleece et al. found high rates of osteopenia (41%) and osteoporosis (21%) in their women's HIV clinic [36, 37]. With regard to the morbidity associated with osteoporosis and the availability of effective treatment, it is especially important to improve diagnosis of osteoporosis.
The organization of HIV care in Switzerland, with gynaecological care not being integrated into HIV care, is an important reason for the low rates of HRT and BDMs. While HIV specialists might not feel confident to discuss menopause with their patients, many gynaecologists are not confident with HIV‐related aspects and may withhold HRT fearing drug–drug interactions [38] This has been confirmed by Tariq et al. whose study participants reported being sent to and fro between HIV specialists and general practitioners for their menopause complaints as both sides were unfamiliar with menopause and HIV infection issues at the same time [32].
Strengths and limitations
This is the first analysis of menopause in women living with HIV in Switzerland. The study provides detailed descriptions of clinical and behavioural characteristics and healthcare utilization, which has direct implications for healthcare providers.
Nonetheless this study has limitations. Determining the exact time of menopause onset is difficult because it is based on the self‐reported cessation of menses, which can be confounded by irregular bleeding, anovulatory cycles or hormonal contraception, among other things. Moreover, menopause is recorded as a reason for amenorrhoea during a biannual visit, which does not correspond to the exact date on which a woman has not bled for 12 months.
Parity and menarche were not included in the logistic regression and might represent unmeasured bias.
Our data do not discriminate among hormonal contraception, hormonally induced pregnancy termination and HRT – we assumed that hormones administered during perimenopause corresponded to HRT – and might have misclassified some hormonal contraceptives.
CONCLUSIONS
The number of women living with HIV transitioning through menopause in the SHCS is constantly growing and occurs earlier than reported for HIV‐negative women in Switzerland. Few women receive menopause‐specific healthcare provision in the form of HRT and osteoporosis screening. To provide adequate care to peri‐ and postmenopausal women, routine assessment of menopause status, symptoms and HRT provision, along with interdisciplinary coordination between HIV specialists, gynaecologists and mental health specialists, is necessary.
CONFLICTS OF INTEREST
None of the authors has declared a possible conflict of interest related to this study. AH’s institution has received travel grants, congress and advisory fees from MSD, Viiv and Gilead, unrelated to this work. BBF has undertaken Advisory Boards or ad hoc consultancy for Merck/MSD, Melinta/Menarini, Shionogi and Pfizer, unrelated to this work. KD's institution has received sponsorship for specialist meetings from MSD, outside the scope of this work, and additional research funding unrelated to this publication from Gilead. PET's institution has received grants and advisory fees from Gilead and ViiV, outside the submitted work. KA‐P’s institution has received travel grants and advisory fees from MSD, Gilead and ViiV Healthcare.
AUTHOR CONTRIBUTIONS
AH, KA‐P and AA developed and designed the study. AA planned and performed the statistical analyses. AH wrote the manuscript with supervision and inputs from KA‐P and AA. PS, AC, PET, KD, BBF, CP, LS‐B and IA contributed with their professional expertise, and reviewed and discussed the analyses and the manuscript.
Supporting information
Tab S1
ACKNOWLEDGEMENTS
We thank all patients, doctors and nurses associated with the SHCS. Open Access Funding provided by Universitat Bern.
Members of the Swiss HIV Cohort Study
Abela A I, Aebi‐Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Hachfeld A, Haerry D (deputy of ‘Positive Council’), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Kusejko K (Head of Data Centre), Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nemeth J, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Schmid P, Speck R, Stöckle M (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, Wandeler G, Yerly S.
Hachfeld A, Atkinson A, Stute P, et al. Women with HIV transitioning through menopause: Insights from the Swiss HIV Cohort Study (SHCS). HIV Med. 2022;23:417–425. doi: 10.1111/hiv.13255
Anna Hachfeld and Andrew Atkinson equally contributed to the project.
The members of ‘the Swiss HIV Cohort Study (SHCS)’ is in Appendix section.
Portions of these data were presented as ‘Menopause impacts drug use and mental health in women living with HIV in Switzerland’ in an E‐poster presentation at EACS 2019 Basel, Switzerland.
Funding information
This study has been financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant no. 177499), by SHCS project no. 854 and by the SHCS research foundation. The data are gathered by the five Swiss university hospitals, two cantonal hospitals, 15 affiliated hospitals and 36 private physicians (listed in http://www.shcs.ch/180‐health‐care‐providers).
Contributor Information
Anna Hachfeld, Email: anna.hachfeld@insel.ch.
the Swiss HIV Cohort Study (SHCS):
A I Abela, K Aebi‐Popp, A Anagnostopoulos, M Battegay, E Bernasconi, DL Braun, HC Bucher, A Calmy, M Cavassini, A Ciuffi, G Dollenmaier, M Egger, L Elzi, J Fehr, J Fellay, H Furrer, CA Fux, HF Günthard, A Hachfeld, D Haerry, B Hasse, HH Hirsch, M Hoffmann, I Hösli, M Huber, CR Kahlert, L Kaiser, O Keiser, T Klimkait, RD Kouyos, H Kovari, K Kusejko, G Martinetti, B Martinez de Tejada, C Marzolini, KJ Metzner, N Müller, J Nemeth, D Nicca, P Paioni, G Pantaleo, M Perreau, A Rauch, P Schmid, R Speck, M Stöckle, P Tarr, A Trkola, G Wandeler, and S Yerly
REFERENCES
- 1. AIDSinfo | UNAIDS [Internet]. https://aidsinfo.unaids.org/. Accessed July 7, 2021.
- 2. Boonyanurak P, Bunupuradah T, Wilawan K, et al. Age at menopause and menopause‐related symptoms in human immunodeficiency virus–infected Thai women. Menopause. 2012;19(7):820‐824. [DOI] [PubMed] [Google Scholar]
- 3. Clark RA, Cohn SE, Jarek C, et al. Perimenopausal symptomatology among HIV‐infected women at least 40 years of age. JAIDS J Acquir Immune Defic Syndr. 2000;23(1):99‐100. [DOI] [PubMed] [Google Scholar]
- 4. Fantry LE, Zhan M, Taylor GH, Sill AM, Flaws JA. Age of menopause and menopausal symptoms in HIV‐infected women. AIDS Patient Care STDs. 2005;19(11):703‐711. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira CE, Pinto‐Neto AM, Conde DM, Costa‐Paiva L, Morais SS, Magalhães J. Menopause symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol. 2007;23(4):198‐205. [DOI] [PubMed] [Google Scholar]
- 6. de Pommerol M, Hessamfar M, Lawson‐Ayayi S, et al. Menopause and HIV infection: age at onset and associated factors, ANRS CO3 aquitaine cohort. Int J STD AIDS. 2011;22(2):67‐72. [DOI] [PubMed] [Google Scholar]
- 7. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103‐115. [DOI] [PubMed] [Google Scholar]
- 8. Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause‐specific questionnaire assessment in US population‐based study shows negative impact on health‐related quality of life. Maturitas. 2009;62(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 9. Woods NF, Mitchell ES. Symptom interference with work and relationships during the menopausal transition and early postmenopause: observations from the Seattle Midlife women’s health study. Menopause. 2011;18(6):654‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Looby SED. Menopause‐associated metabolic manifestations and symptomatology in HIV infection: a brief review with research implications. J Assoc Nurses AIDS Care. 2012;23(3):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howells P, Modarres M, Samuel M, Taylor C, Hamoda H. Experience of hormone replacement therapy in postmenopausal women living with HIV. Post Reprod Health. 2019;25(2):80‐85. [DOI] [PubMed] [Google Scholar]
- 12. Lui‐Filho JF, Valadares ALR, Gomes DdeC, Amaral E, Pinto‐Neto AM, Costa‐Paiva L. Menopausal symptoms and associated factors in HIV‐positive women. Maturitas. 2013;76(2):172‐178. [DOI] [PubMed] [Google Scholar]
- 13. Miller SA, Santoro N, Lo Y, et al. Menopause symptoms in HIV‐infected and drug‐using women. Menopause. 2005;12(3):348‐356. [DOI] [PubMed] [Google Scholar]
- 14. Maki PM, Rubin LH, Cohen M, et al. Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse HIV+ and HIV− Women. Menopause N Y N. 2012;19(11):1215‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Looby SE. Symptoms of menopause or symptoms of HIV? Untangling the knot. Menopause. 2018;25(7):728‐730. [DOI] [PubMed] [Google Scholar]
- 16. Solomon D, Sabin CA, Burns F, et al. The association between severe menopausal symptoms and engagement with HIV care and treatment in women living with HIV. AIDS Care. 2021;33(1):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duff PK, Money DM, Ogilvie GS, et al. Severe menopausal symptoms associated with reduced adherence to antiretroviral therapy among perimenopausal and menopausal women living with HIV in metro vancouver. Menopause N Y N. 2018;25(5):531‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swiss HIV Cohort Study , Schoeni‐Affolter F, Ledergerber B, et al. Cohort profile: the Swiss HIV cohort study. Int J Epidemiol. 2010;39(5):1179‐1189. [DOI] [PubMed] [Google Scholar]
- 19. R Core Team, R Foundation for Statistical Computing . R: a language and environment for statistical computing [Internet]. 2019. http://www.R‐project.org/. Accessed September 19, 2019.
- 20. StataCorp . Stata Statistical Software, College Station, TX, USA. 2012.
- 21. Dratva J, Zemp E, Staedele P, et al. Variability of reproductive history across the Swiss SAPALDIA cohort – Patterns and main determinants. Ann Hum Biol. 2007;34(4):437‐453. [DOI] [PubMed] [Google Scholar]
- 22. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smit M, Cassidy R, Cozzi‐Lepri A, et al. Projections of non‐communicable disease and health care costs among HIV‐positive persons in Italy and the U.S.A.: a modelling study. PLoS One. 2017;12(10):e0186638. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5653300/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Statistik B für . Gesundheitsdeterminanten [Internet]. https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/determinanten.html. Accessed July 7, 2021.
- 25. Schoenbaum EE, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41(10):1517‐1524. [DOI] [PubMed] [Google Scholar]
- 26. Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 27. Calvet GA, Grinsztejn BGJ, de Quintana M, et al. Predictors of early menopause in HIV‐infected women: a prospective cohort study. Am J Obstet Gynecol. 2015;212(6):765.e1‐765.e13. [DOI] [PubMed] [Google Scholar]
- 28. Cejtin HE. Care of the human immunodeficiency virus‐infected menopausal woman. Am J Obstet Gynecol. 2012;207(2):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dratva J, Gómez Real F, Schindler C, et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population‐based studies. Menopause. 2009;16(2):385‐394. [DOI] [PubMed] [Google Scholar]
- 30. EACS B . EACS guidelines [Internet]. EACSociety. https://www.eacsociety.org/guidelines/eacs‐guidelines/eacs‐guidelines.html. Accessed July 7, 2021.
- 31. Munatsi S, Pammi M, Chadwick S, Gamoudi D, Taylor R. The Menopause Experience: A Quality Improvement Project, BHIVA conference Poster Presentation P151. 2019. BHIVA via website, conference report. https://www.bhiva.org/file/5ca732511644f/P151.pdf. Accessed February 18, 2022.
- 32. Tariq S, Burns F, Gilson R, Rolland A, Sabin C. Positive tRansItions through Menopause (PRIME) [Internet]. Institute for Global Health. 2018. https://www.ucl.ac.uk/global‐health/research/a‐z/PRIME. Accessed July 7, 2021.
- 33. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis off Publ Infect Dis Soc Am. 2015;60(8):1242‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schweizerische Vereinigung gegen die Osteoporose . Osteoporose Empfehlungen 2015: Prävention, Diagnostik, Behandlung [Internet]. 2015. https://www.svgo.ch/?Broschueren. Accessed August 30, 2021.
- 35. Sharma A, Flom PL, Rosen CJ, Schoenbaum EE. Racial differences in bone loss and relation to menopause among HIV‐infected and uninfected women. Bone. 2015;77:24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirst F, Walker‐Bone K, Samraj S, Sundaram S. Assessment of bone health of women living with HIV aged > 50 years in clinical practice: are we doing enough?, BHIVA conference Poster Presentation P052. 2019. BHIVA‐conference. https://www.bhiva.org/file/5ca73250c2b47/P052.pdf. Accessed February 18, 2022.
- 37. Gilleece Y, Mundowa G, Roberts J. No Bones about it: high rates of osteopenia and osteoporosis in women with HIV in the UK, BHIVA conference poster presentation P068. BHIVA‐conference. 2019. https://www.bhiva.org/file/5ca73250cf798/P068.pdf. Accessed February 18, 2022.
- 38. Chirwa M, Ma R, Guallar C, Tariq S. Managing menopause in women living with HIV: a survey of primary care practitioners. Post Reprod Health. 2017;23(3):111‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1


