Abstract
Background
Altered cholinergic innervation plays a putative role in cognitive impairment in Parkinson's disease (PD) at least in advanced stages. Identification of the relationship between cognitive impairment and cholinergic innervation early in the disease will provide better insight into disease prognosis and possible early intervention.
Objective
The aim was to assess regional cholinergic innervation status in de novo patients with PD, with and without cognitive impairment.
Methods
Fifty‐seven newly diagnosed, treatment‐naive, PD patients (32 men, mean age 64.6 ± 8.2 years) and 10 healthy controls (5 men, mean age 54.6 ± 6.0 years) were included. All participants underwent cholinergic [18F]fluoroethoxybenzovesamicol positron emission tomography and detailed neuropsychological assessment. PD patients were classified as either cognitively normal (PD‐NC) or mild cognitive impairment (PD‐MCI). Whole brain voxel‐based group comparisons were performed.
Results
Results show bidirectional cholinergic innervation changes in PD. Both PD‐NC and PD‐MCI groups showed significant cortical cholinergic denervation compared to controls (P < 0.05, false discovery rate corrected), primarily in the posterior cortical regions. Higher‐than‐normal binding was most prominent in PD‐NC in both cortical and subcortical regions, including the cerebellum, cingulate cortex, putamen, gyrus rectus, hippocampus, and amygdala.
Conclusion
Altered cholinergic innervation is already present in de novo patients with PD. Posterior cortical cholinergic losses were present in all patients independent of cognitive status. Higher‐than‐normal binding in cerebellar, frontal, and subcortical regions in cognitively intact patients may reflect compensatory cholinergic upregulation in early‐stage PD. Limited or failing cholinergic upregulation may play an important role in early, clinically evident cognitive impairment in PD. © 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: Parkinson's disease, cognition, acetylcholine, positron emission tomography imaging
Abbreviations
- [18F]FEOBV
[18F]fluoroethoxybenzovesamicol
- FDR
false discovery rate
- HC
healthy control
- MDS‐UPDRS‐III
Movement Disorder Society‐Revised Unified Parkinson's Disease Rating Scale, Part III
- PD
Parkinson's disease
- PD‐MCI
Parkinson's disease mild cognitive impairment
- PD‐NC
Parkinson's disease normal cognition
- PET
positron emission tomography
- VAChT
vesicular acetylcholine transporter
- 18F‐FDOPA
3,4‐dihydroxy‐6‐18F‐fluoro‐1‐phenylalaninie
- BF
basal forebrain
- CT
computed tomography
- DVR
Distributed Volume Ratio
- iRBD
idiopathic rapid‐eye‐movement sleep behavior disorder
- MDS
Movement Disorder Society
- MRI
Magnatic Resonance Imaging
- MoCA
Montreal Cognitive Assessment
- MVN
medial vestibular nucleus
- PDD
Parkinson's disease dementia
- PIGD
postural instability and gait difficulty
- PPN/LDTC
peduncupontine‐laterodorsal tegmental complex
- SD
standard deviation
- TD
tremor dominant
- VOI
Volume of Interest
Cognitive impairment is common in Parkinson's disease (PD) and a major source of disability and lower quality of life. 1 , 2 Mild cognitive impairment (PD‐MCI) is already present in 25% to 30% of newly diagnosed patients and is a major risk factor for the development of PD dementia (PDD). 3 , 4 , 5 Cognitive impairment in PD is heterogeneous, with multiple domains affected and great variability in onset and progression. 6
It has been increasingly recognized that comorbid cholinergic dysfunction is a major contributor to the pathophysiology of cognitive impairment in PD. 7 , 8 The four major human brain cholinergic systems are the basal forebrain (BF) corticopetal projection system, cholinergic efferents of the peduncupontine‐laterodorsal tegmental complex (PPN/LDTC), medial vestibular nucleus (MVN) cholinergic neurons projecting to the cerebellum, and striatal cholinergic interneurons. 9 , 10 , 11 , 12
Previous in vivo neuroimaging assessment of cholinergic innervation, using acetylcholinesterase positron emission tomography (PET), has demonstrated predominantly posterior cholinergic denervation in PD, with more severe cholinergic degeneration in PDD. 13 , 14 , 15 , 16 There is additional evidence that the cholinergic system is a major driver of cognitive impairment in PD even in the absence of dementia. 17 We previously showed that deficits in attention, executive functioning, and memory correlated with loss of cholinergic activity on both a global cortical level 16 , 18 and a regional (sub)cortical level. 19 Although cholinergic denervation has been shown in cognitively impaired patients with PD, less is known about cholinergic innervation changes in early‐stage disease and before the onset of cognitive changes. Elucidation of the relationship between cognitive impairment and cholinergic integrity in a very early stage of the disease may provide new clues that may inform novel therapeutic strategies.
The aim of this study was to characterize cholinergic innervation status in newly diagnosed, treatment‐naive PD patients with and without cognitive deficits. We compared vesicular acetylcholine transporter (VAChT) [18F]fluoroethoxybenzovesamicol ([18F]FEOBV) PET imaging between de novo patients (with and without MCI) and healthy controls (HCs). Unlike previously used acetylcholinesterase PET ligands, [18F]FEOBV PET allows for detailed assessment of not only low‐level cortical but also high‐binding subcortical structures, such as the basal ganglia and the cerebellum. 20 , 21 , 22
Patients and Methods
Participants
Fifty‐seven newly diagnosed patients with PD and 10 HCs were included in this cross‐sectional study. Patients were enrolled in the Dutch Parkinson Cohort study between 2017 and 2019 (for details, see Boertien et al). 23 Inclusion criteria for patients consisted of PD diagnosis by a movement disorders specialist according to Movement Disorder Society (MDS) Clinical Diagnosis Criteria for PD 24 and with a confirmed dopaminergic striatal deficit on 3,4‐dihydroxy‐6‐18F‐fluoro‐1‐phenylalaninie (18F‐FDOPA) PET. HCs had a normal neurological examination and did not have a history of neurological or psychiatric disorders. Exclusion criteria for both PD and HC subjects included the inability to provide written informed consent, the use of dopaminergic and (anti‐)cholinergic medication, and an estimated low premorbid intelligence level (estimated IQ <70, on the Dutch Adult Reading test 25 ). All subjects provided written informed consent, and the study was approved by the local ethics committee.
Clinical Examination
All patients underwent a comprehensive neuropsychological assessment covering all cognitive domains. 23 A selection of outcome measures of tests and subtests of the cognitive test battery was made a priori, meeting level II criteria for PD‐MCI, 26 , 27 presented in Table 1. Subject scores for each of the cognitive tests were compared to established test‐specific normative data generated by age, gender, and education. A performance of >1.5 standard deviation (SD) below normative values was considered abnormal. Patients were categorized as either PD with normal cognition (PD‐NC) or PD‐MCI. PD‐MCI was based on level II criteria for PD‐MCI and required below‐threshold performance on at least two neuropsychological tests. 26 Any patient or clinical characteristics possibly influencing performance on the neuropsychological assessments and MCI grouping, including visual difficulties, color blindness, speech problems, and significant mood disorders, were considered during assessment and before data analysis and, if necessary, excluded. HCs underwent cognitive testing using the Montreal Cognitive Assessment (MoCA) test.
TABLE 1.
Selection of cognitive tasks used for the assessment of PD‐MCI
| Domain | Function | (Sub)test |
|---|---|---|
| Learning and memory | Verbal learning | RAVLT immediate recalla |
| Verbal recall | RAVLT delayed recalla | |
| Visual learning | LLT immediate recallb | |
| Visual recall | LLT delayed recallb | |
| Attention and processing speed | Basic processing speed | Vienna Test System Reaction Time Test(53): S1 decision timec |
| Complex information processing speed | Vienna Test System Reaction Time Test: S3 decision timec | |
| Selective attention | Stroop III: color word | |
| Attention and memory span | Digit Span | |
| Executive function | Set shifting | WCST perseverative errorsd |
| Problem solving | WCST trials to complete first categoryd | |
| Flexibility | Trail Making Test part B | |
| Visual perception | Visuospatial perception | Judgment of line orientation |
| Visual search and attention | TEA Map search | |
| Language | Verbal fluency | Category fluency: animal naming |
| Naming ability | Boston Naming Test |
Abbreviations: PD‐MCI, Parkinson's disease mild cognitive impairment; RAVLT, Rey Auditory Verbal Learning Test; LLT, Location Learning Test; WCST, Wisconsin Card Sorting Test; TEA, Test of everyday attention.
a,b,c,dConcerns two subtasks within one cognitive test. If both scores are considered impaired, it will be counted as one impaired test.
Additional clinical assessment included the subjective duration of motor complaints before PD diagnosis and the Hospital Anxiety and Depression Scale. 28 All PD subjects underwent motor examination using the MDS‐Revised Unified Parkinson's Disease Rating Scale, Part III (MDS‐UPDRS‐III). In addition, specific items from the MDS‐UPDRS, Parts II and III, were used for the classification of motor phenotype, using criteria previously formulated by Stebbins et al. 29 Patients were classified into tremor dominant (TD), postural instability and gait difficulty (PIGD), and indeterminate motor phenotypes.
Image Acquisition
All subjects underwent brain magnetic resonance imaging (MRI) and VAChT PET imaging using ([18F]FEOBV). MRI of PD subjects was acquired using Siemens Magnetom Prisma 3‐Tesla magnetic resonance imaging scanners (Best, Netherlands) equipped with SENSE‐8 channel head coils. For each subject, anatomical T1‐weighted images were obtained using a sagittal three‐dimensional gradient‐echo T1‐weighted sequence with 0.9 × 0.9 × 0.9 mm acquisition. HCs underwent a T1‐weighted MRI scan (3 T Intera, Philips, Best, the Netherlands) with 1.0 × 1.0 × 1.0 mm acquisition. [18F]FEOBV imaging was performed on the same day as MRI. After a low‐dose computed tomography (CT) for attenuation and scatter correction, participants were scanned using either a Biograph 40‐mCT or 64‐mCT (Siemens Healthcare, Malvern, PA, USA). Both these systems are EARL certified and identical in software version, acquisition‐ and reconstruction‐protocols and PET detectors, and differ only in the number of CT slices. Patients and controls were randomly divided over the two PET scanners. [18F]FEOBV was injected using an intravenous bolus, and delayed imaging was performed over 30 minutes (in six 5‐minute frames) starting 210 minutes after injection.
Image processing was performed using Statistical Parametric Mapping (SPM) software. 30 [18F]FEOBV PET imaging frames were spatially coregistered within subjects with a rigid‐body transformation. The cropped T1‐weighted MR scan was coregistered with the subject PET image. Freesurfer software package (Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, USA) was used to segment MRI into cortical and subcortical brain regions. We calculated the distributed volume ratio (DVR) of each gray matter target region using the summed six delayed imaging frames and the white matter reference region as previously described. 21 , 31 A parametric image for individual subjects was created by using the average of six delayed imaging frames divided by the mean of the white matter reference region. Partial volume correction on our parametric images was performed using the Muller‐Gartner method. 32
Imaging Analysis
Voxel‐based analysis was performed as previously described. 33 Parametric PET images were spatially normalized to the Montreal Neurological Institute stereotactic template and smoothed with a Gaussian kernel of 8‐mm full‐width half‐maximum. The relevant brain areas were displayed in Montreal Neurological Institute atlas coordinates (in millimeters) in the stereotactic space using the automated anatomical labeling toolbox.
Statistical Analyses
To evaluate brain cholinergic innervation in de novo patients with PD, we first compared HC, PD‐NC, and PD‐MCI groups on baseline demographic and clinical characteristics. Comparisons between all three groups were performed using one‐way analysis of variance for parametric variables and χ2 testing for dichotomous variables. Comparisons between the two PD groups were performed using an independent sample t test or a Mann‐Whitney U test, for normal and skewed distributed data, respectively. Statistical analysis was considered significant for α < 0.05. Statistical analyses were performed using SPSS Statistics for Windows, version 24.0 (IBM Statistics, Armonk, NY, USA), and SPM software.
Whole brain voxel‐based analyses were performed to assess the difference in cholinergic innervation between groups. Parametric [18F]FEOBV DVR images were used for a two‐sample voxel‐wise statistical comparison between groups, in both positive and negative directions, controlling for age. The minimum cluster size was set to 50. The false discovery rate (FDR) approach was used for correction for multiple testing effects in the voxel‐based analyses. Additional post hoc Freesurfer‐based volume‐of‐interest (VOI) analysis was performed on regions selected based on the voxel‐based analysis. The percentage change in these regions was calculated by dividing the difference between the mean PD and HC groups by the mean HC group.
Results
Demographic and Clinical Characteristics
The 57 de novo PD patients (32 men) had a mean (SD) age of 64.6 (8.2) years and a mean (SD) MDS‐UPDRS‐III score of 30.8 (11.4); 17 (29.8%) patients were classified as PD‐MCI. Demographic and clinical characteristics of the different groups are presented in Table 2. HCs had a significantly lower age and higher MoCA score compared to both PD groups (P < 0.001). The PD‐MCI group had higher MDS‐UPDRS‐III scores (P < 0.01) and Hoehn and Yahr stage (P = 0.05) compared to the PD‐NC group, reflecting more severe motor impairment in the PD‐MCI group. No significant difference was found between the PD groups on the motor phenotype (TD, PIGD, or indeterminate). Information on duration of motor complaints was not available for 11 patients. Available data showed no significant difference in the duration of motor symptom complaints before PD diagnosis.
TABLE 2.
Demographic and clinical characteristics of the study participants
| HC (n = 10) a | PD‐NC (n = 40) | PD‐MCI (n = 17) | P‐value | |
|---|---|---|---|---|
| Age (y) | 54.6 (6.0) | 63.4 (7.4) | 67.5 (9.6) | <0.001 * , b |
| Gender, male n (% male) | 5 (50%) | 19 (47.5%) | 13 (76.5%) | 0.124 |
| Educational level c | 5.0 (1.0) | 5.0 (2.0) | 6.0 (1.0) | 0.704 |
| Motor symptom duration (mo) | 20.2 (11.8) | 25.1 (17.2) | 0.273 | |
| MDS‐UPDRS‐III, total score | 28.2 (9.1) | 36.8 (14.1) | 0.008 * | |
| Motor phenotype, n TD/PIGD/indeterminate | 15/18/7 | 4/12/1 | 0.189 | |
| Hoehn and Yahr stage d | 2.0 (1.0); 26.7 | 2.0 (1.0); 34.5 | 0.050 | |
| MoCA, total score | 28.4 (0.8) | 26.1 (2.6) | 23.7 (3.2) | <0.001 * , e |
| HADS anxiety, total score | 4.5 (2.6) | 4.7 (2.4) | 0.778 | |
| HADS depression, total score | 3.4 (2.4) | 4.6 (2.6) | 0.085 |
Statistically significant (p < 0.05)
Data are presented as mean (standard deviation) unless otherwise indicated.
Post hoc analysis: HC < PD‐NC; HC < PD‐MCI.
Educational level according to the Dutch Verhage scale (Verhage, 1964), listed as median (interquartile range).
Hoehn and Yahr stage is listed as median (interquartile range); mean rank.
Post hoc analysis: HC > PD‐NC > PD‐MCI.
Abbreviations: HC, healthy control; PD‐NC, Parkinson's disease normal cognition; PD‐MCI, Parkinson's disease mild cognitive impairment; MDS‐UPDRS‐III, Movement Disorder Society‐Revised Unified Parkinson's Disease Rating Scale, Part III; TD, tremor dominant; PIGD, postural instability and gait difficulty; MoCA, Montreal Cognitive Assessment; HADS, Hospital Anxiety and Depression Scale.
Cognitive Functioning
Overall, 15 of 17 patients classified as PD‐MCI showed multi‐domain cognitive impairment, reflecting impaired scores in two or more cognitive domains. The most frequently affected domains were memory and executive functions, with 12 and 10 patients presenting impairments in these domains, respectively. The attention and visuospatial domains were affected in 7 of 17 PD‐MCI patients. Language was the least‐affected domain, with 4 PD‐MCI patients showing impaired scores. Detailed cognitive performance on the neuropsychological tests for both PD groups is presented in Appendix S1.
Cholinergic Innervation Compared to Controls
Whole brain voxel‐based analyses were performed to compare regional brain VAChT binding between the PD groups (PD‐MCI and PD‐NC) and the HCs in positive and negative directions while controlling for age. The PD groups showed evidence of bidirectional changes in different topographic patterns compared to the controls.
Significantly lower VAChT binding was found in both PD groups, primarily in the temporal and posterior cortical regions, compared to HC (Fig. 1; P < 0.05, FDR corrected). The topographic profiles for PD‐NC and PD‐MCI were comparable, with the main affected regions including the parietal, parieto‐occipital junction, and lateral temporal cortices. PD‐NC presented with additional lower binding in the frontal cortex, insula, and caudate (tail) and PD‐MCI with additional lower binding in the pons‐medulla regions. A detailed overview of significant clusters is provided in Appendix S1.
FIG. 1.
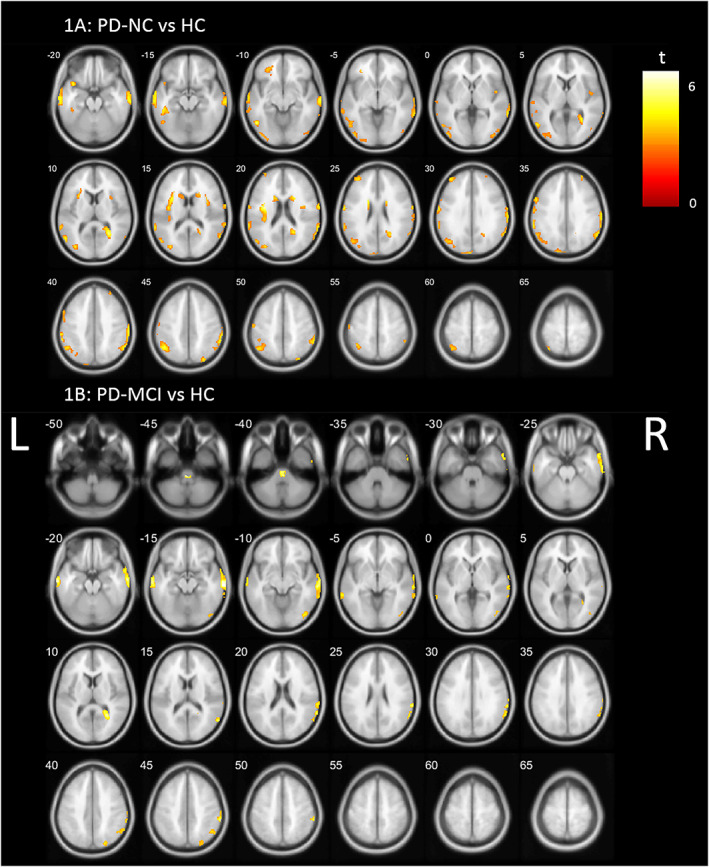
Whole brain voxel‐based analyses showing significant lower VAChT (vesicular acetylcholine transporter) binding (P < 0.05, FDR [false discovery rate] corrected) in (A) PD‐NC (Parkinson's disease normal cognition) and (B) PD‐MCI (Parkinson's disease mild cognitive impairment) compared to HCs (healthy controls), controlled for age. L, left; R, right. [Color figure can be viewed at wileyonlinelibrary.com]
Reverse direction voxel‐based comparison between HCs and both PD groups showed significantly higher VAChT binding in PD subjects compared to HCs (P < 0.05, FDR corrected). This higher cholinergic innervation was most prominently apparent in the PD‐NC group (Fig. 2A), including the cerebellum, cingulate cortex, gyrus rectus, anteroventral striatum, putamen, dorsal tegmentum, thalamus, metathalamus (lateral geniculate nucleus and medical geniculate nucleus), pulvinar, amygdala, hippocampus, and parahippocampal regions. The PD‐MCI groups showed a more limited topography of higher VAChT binding, including the cerebellar vermis, right gyrus rectus, amygdala, hippocampus and metathalamus, dorsal tegmentum, right inferior thalamus/pulvinarthalamus, and left precentral gyrus (Fig. 2B). A detailed overview of significant clusters is provided in Appendix S1.
FIG. 2.
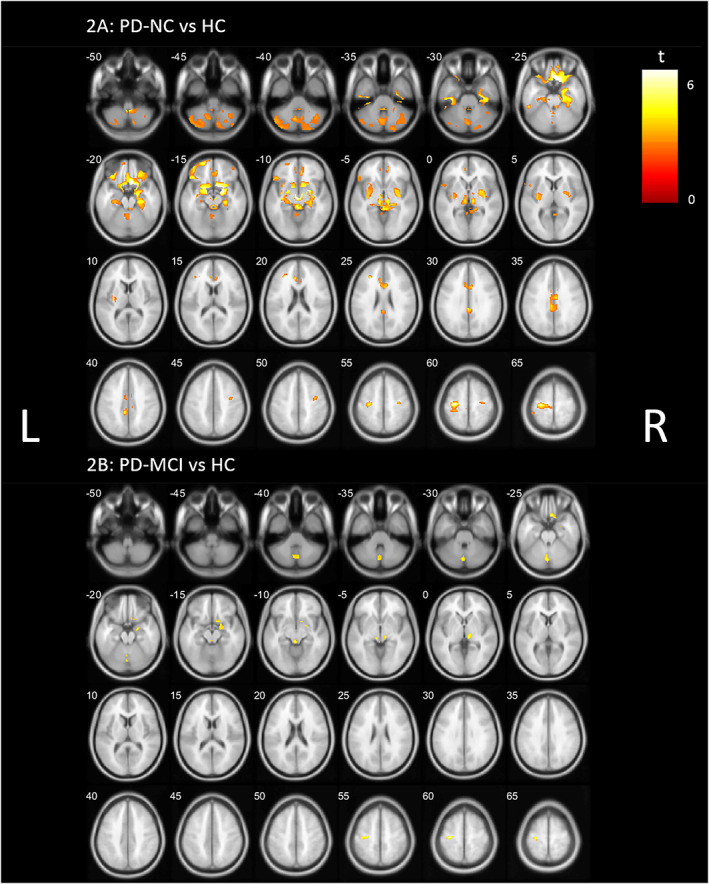
Whole brain voxel‐based analyses showing significant higher VAChT (vesicular acetylcholine transporter) binding (P < 0.05, FDR [false discovery rate] corrected) in (A) PD‐NC (Parkinson's disease normal cognition) and (B) PD‐MCI (Parkinson's disease mild cognitive impairment) compared to HCs (healthy controls), controlled for age. L, left; R, right. [Color figure can be viewed at wileyonlinelibrary.com]
Most prominent representative examples of the magnitude of absolute binding changes with HC based on VOI comparison include reduced binding in the lateral occipital gyri (−13.3%), the pericalcarine (−19.0%), and the transverse temporal gyri (−23.1%) and increase in the cerebellar cortex (+14.2%), medial and lateral orbitofrontal regions (respectively, +8.8% and + 6.5%), frontal pole (+6.1%), bilateral amygdala (+4.8%), and anterior cingulate (+3.8%) in the PD‐NC group. For the PD‐MCI group, representative examples include the lateral occipital gyri (−15.7%), cuneus (−19.6%), pericalcarine (−23.9%), middle temporal gyri (−14.2%), and inferior parietal gyri (−15.4%) with lower VAChT binding in PD‐MCI and increase in the cerebellar cortex (+13.2%), right medial and lateral orbitofrontal regions (respectively, +2.6% and + 3.9%), and right amygdala (+2.9%).
PD‐MCI versus PD‐NC Group Comparison
Lower VAChT binding was found in the PD‐MCI group in the fusiform and hippocampal regions, the anterior insula, and the left prefrontal cortex (uncorrected, P < 0.001). No significant differences were found after correction for multiple comparisons.
Post hoc: Controlling for Motor Impairment
Clinical characteristics comparing both PD groups demonstrated a significant higher MDS‐UPDRS‐III score in patients with PD‐MCI compared to PD‐NC (Table 2), indicating more severe motor impairment in the PD‐MCI group. Bidirectional whole brain voxel‐based analysis comparing both PD groups with controls was therefore repeated, controlling for both age and MDS‐UPDRS‐III.
Limited lower VAChT binding was found in PD‐NC and PD‐MCI compared to HCs (P < 0.001, uncorrected), including small regional differences in the occipital and temporal regions.
Reverse direction analysis showed higher VAChT binding in both PD‐NC and PD‐MCI compared to controls (Fig. 3; P < 0.001, uncorrected). PD‐NC showed most extensive higher binding when compared to controls, with a cholinergic topography, including the brainstem, gyrus rectus, posterior cingulate cortex, amygdala, and hippocampus and right parahippocampal regions. The PD‐MCI group demonstrated less widespread higher VAChT binding, limited to the right superior temporal region, left pulvinar, left optic radiation/occipital region, and precentral gyrus.
FIG. 3.
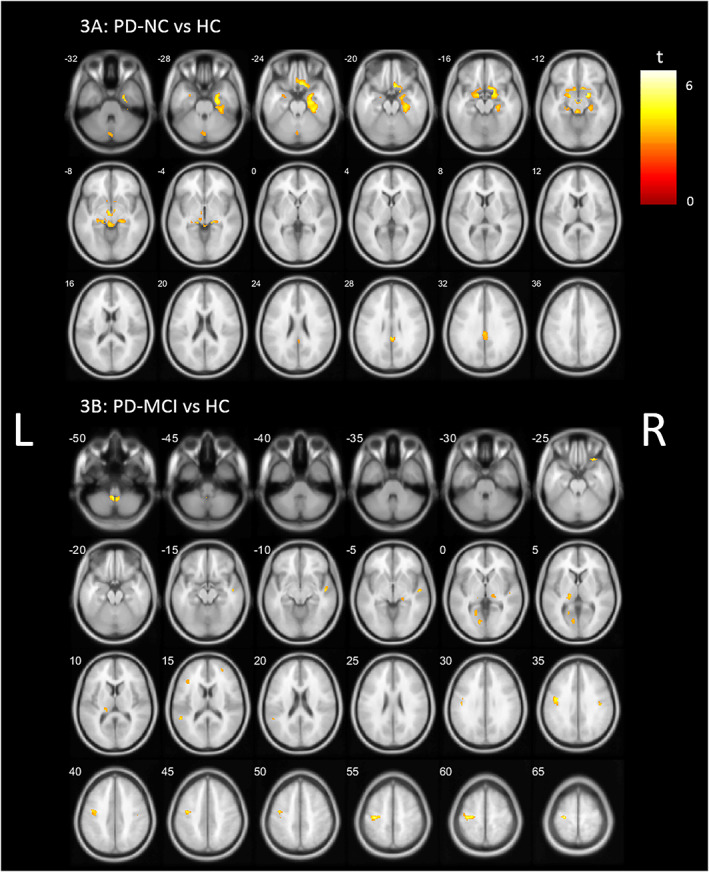
Post hoc whole brain voxel‐based analysis showing higher VAChT (vesicular acetylcholine transporter) binding (P < 0.001, uncorrected) in (A) PD‐NC (Parkinson's disease normal cognition) and (B) PD‐MCI (Parkinson's disease mild cognitive impairment) compared to HCs (healthy controls), controlling for age and MDS‐UPDRS‐III (Movement Disorder Society‐Revised Unified Parkinson's Disease Rating Scale, Part III) scores. L, left; R, right. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
We investigated the topography of regional cholinergic innervation status in newly diagnosed, treatment‐naive patients with PD, with and without cognitive impairment, compared to HCs. We found altered cholinergic innervation, demonstrated by both higher‐ and lower‐binding cholinergic brain regions. First, we demonstrated that cortical cholinergic denervation is already present at the start of the disease in both cognitively impaired and cognitively unaffected patients with PD. Second, we also found evidence of increased cholinergic binding in the PD groups. Cognitively unimpaired PD patients showed most robust evidence of higher‐than‐normal VAChT binding compared to the PD‐MCI group.
Previous studies have established the importance of the cholinergic system in cognitive impairment in PD, even in the absence of dementia. 13 , 16 However, the majority of previous in vivo imaging studies are based on PET imaging using acetylcholinesterase tracers, which limit accurate estimates of high‐binding brain areas. The use of a VAChT PET tracer, like [18F]FEOBV, allows for more reliable and regionally specific cholinergic assessment, especially in high‐binding areas such as the striatum and cerebellum. 20 , 21 , 22 To our knowledge, this is the first assessment of regional cholinergic innervation in de novo patients with PD, with and without cognitive impairment, on a detailed cortical and subcortical level.
About 29.8% of patients in our study met PD‐MCI criteria, which is in line with previous research on the cognitive impairment in early PD. 3 In addition, the PD‐MCI group presented with more severe motor impairment. However, there was no difference between PD‐NC and PD‐MCI in terms of motor phenotype (TD, PIGD, or indeterminate) or duration of subjective motor complaints before PD diagnosis.
A striking finding in our study was the higher‐than‐normal cholinergic binding in de novo patients with PD compared to controls, most prominent in the PD‐NC group. The brain topography represented by higher VAChT binding included the cerebellum, parts of the thalamic complex, putamen, hippocampus and parahippocampal regions, amygdala, gyrus rectus, and cingulate cortex. Higher VAChT binding in the cerebellum and thalamus indicates a substantial role of the cholinergic projections from the MVN and PPN/LDTC, respectively. 10 , 11 In addition, the involvement of the hippocampus, parahippocampal region, amygdala, gyrus rectus, and cingulate cortex suggests the involvement of projections originating from the BF, including Ch1‐2 and Ch4 (nucleus basalis of Meynert) groups. 12 From the Ch4 cell group, two major pathways can be identified: the medial pathway joining the white matter of the gyrus rectus and projecting to the cingulum and the lateral pathway of which the capsular division projects to the amygdala and temporal lobe. 11 Finally, the altered [18F]FEOBV uptake in the putamen and caudate nucleus indicates the role of intrinsic cholinergic striatal interneurons or PPN/LDTC projections. 34 A possible role of the dopaminergic system should also be considered, as previous studies have shown an intricate interaction between the dopaminergic and cholinergic systems; it is the multiplicative and interacting effects of the two systems that lead to cognitive deficits. 17 , 35
The higher cholinergic innervation in de novo PD suggests a compensatory cholinergic upregulation in the early phase of the disease. A compensatory role of the cholinergic system has previously been suggested in Alzheimer's disease where cholinergic upregulation was found in prodromal patients with MCI. 36 , 37 Bohnen et al and Kim et al expanded on these findings by showing both independent and interactive roles of the cholinergic and dopaminergic systems in cognitive functioning in PD. 17 , 38 In the context of dopaminergic losses, preservation or even upregulation of cholinergic innervation may help preserve cognitive functioning. 39 In addition, Liu et al reported increased cortical acetylcholinesterase activity related to LRRK2 gene mutation in (premanifest) PD. 40 Bedard et al recently showed significantly higher [18F]FEOBV uptake in patients with idiopathic REM sleep behavior disorder (iRBD). 41 RBD is considered an important marker of prodromal PD 42 and associated with cognitive impairment conversion. 43 Our study is the first to demonstrate evidence of higher‐than‐normal cholinergic innervation in PD patients, substantiating the hypothesis of a compensatory cholinergic upregulation in very early PD.
Interestingly, the higher cholinergic VAChT binding was less profound in the PD‐MCI subgroup than in the cognitively unimpaired PD group, even though both groups consisted of newly diagnosed, treatment‐naive patients with PD with a similar duration of motor complaints before PD diagnosis. These findings suggest that a possible lack of compensatory cholinergic upregulation may be related to clinically evident cognitive impairment in early PD patients. In addition, the demonstrated denervation pattern in both PD‐NC and PD‐MCI groups provides further support for this hypothesis, as the relative extent of the denervation changes in the two PD subgroups had remarkable overlap and PD‐MCI did not show more profound cholinergic denervation than PD‐NC when compared to controls. In contrast, the PD‐NC group showed slightly more extant denervation changes, possibly related to the higher number of subjects in this group providing more statistical power.
However, it should be noted that an alternative explanation for the difference in higher‐than‐normal VAChT binding between PD‐NC and PD‐MCI may be related to the severity of motor impairment. The PD‐MCI group presented with a higher MDS‐UPDRS‐III score than PD‐NC, indicating more severe motor impairment. Therefore, a post hoc analysis was performed, controlling for motor impairment. Our data showed that the higher cholinergic binding was still present in PD‐NC but less widespread and robust. The upregulation in the cerebellum and thalamic complex was less profound, as well as the striatal regions, suggesting an important role of increased PPN cholinergic innervation and cholinergic striatal interneurons in motor performance in early PD. This is in line with previous research showing cholinergic PPN projections and striatal interneurons strongly contributing to motor symptoms in PD, especially in the motor subtype presenting with postural instability and gait disorder. 31 , 44 In contrast, regions with BF cholinergic projections, including the posterior cingulate cortex, gyrus rectus, amygdala, and hippocampus and parahippocampal regions, remained present after correction for this confounder variable. Interestingly, these regions show partial overlap with the regional cholinergic topography we previously found to be related to cognitive functioning in PD on the level of multiple cognitive domains, 19 substantiating the cholinergic role of these regions in cognitive functioning in PD. On the contrary, controlling for MDS‐UPDRS‐III scores might cause overcorrection, as more severe motor and cognitive impairment often coincide 45 , 46 and MDS‐UPDRS‐III scores are significantly correlated with the majority of included cognitive tests (Appendix S1). The post hoc analysis controlling for motor performance may therefore provide an underestimation of the regional topography of higher cholinergic binding related to cognitive status.
In contrast to earlier findings, 13 , 14 , 15 we found only limited cortical cholinergic denervation in patients with PD‐MCI, without a substantial VAChT binding difference, versus PD‐NC. A possible explanation for this lack of difference may be the heterogeneity of cognitive performance in patients with PD. In our study, the majority of patients with PD‐MCI showed multidomain cognitive impairment, with a variety of domains affected. In addition, heterogeneity not only in cognitive functioning but also in other PD symptoms, including motor profile and nonmotor symptoms, may result in cholinergic heterogeneity. 47 The finding of a limited cholinergic denervation pattern may also contribute to the understanding why previous studies have found limited effectiveness of cholinesterase inhibitors in PD‐MCI. 48 , 49 We suggest that early cognitive decline is the result of failing cholinergic compensation rather than cholinergic denervation per se. A better stratification, including the specific profile of dopaminergic and cholinergic innervation, in addition to clinical cognitive performance, might therefore improve the effectiveness of cholinergic treatment in PD.
Limitations
One limitation of this study is the relatively small sample size of the PD subgroups. In addition, the PD‐MCI group presented with a heterogeneous cognitive profile, affecting multiple domains. The clinical heterogeneity of this group and the relatively small sample size of the subgroups may have contributed to the limited difference found between PD‐MCI and PD‐NC. Future studies with a larger sample size can allow for more detailed stratification and improve our understanding of possible compensatory mechanisms specific to cognitive functioning across different domains. Second, even though MDS guidelines were followed, the grouping of patients into PD‐NC and PD‐MCI is an arbitrary process, as cognitive changes occur along a continuous spectrum. This might add to the heterogeneity. Furthermore, the cross‐sectional design of our study does not allow the assessment of temporal changes. More detailed data on cognitive performance over time and the progression to PD‐MCI and PDD will enhance our understanding of the cholinergic role in the progression of the disease and the suggested compensatory mechanism. Finally, the HC group had a significantly lower age than both PD groups. We previously demonstrated an important role of age in the relationship between cognition and cholinergic innervation. 19 Although the analyses were corrected for age, a possible role of the age difference cannot be ruled out.
Conclusion
This study demonstrates evidence of bidirectional changes in cholinergic innervation in de novo patients with PD, with and without cognitive impairment, compared to HCs. Increased cholinergic binding in early PD, especially in the cognitively intact patients, suggests a compensatory cholinergic upregulation in this group. Overall, we postulate that in early, treatment‐naive patients with PD, the clinical syndrome of PD‐MCI may be related to limited or failing cholinergic upregulation instead of a more progressed (posterior cortical) cholinergic denervation.
Author Roles
Listing of each author and their contribution:
van der Zee: Conception, design, data acquisition, analysis, drafting, editing and revising of the manuscript.
Kanel: data analysis, critical revision of the manuscript.
Gerritsen: Study design, critical revision of the manuscript.
Boertien: Study design, data acquisition, critical revision of the manuscript.
Slomp: Data acquisition, critical revision of the manuscript.
Muller: Critical revision of the manuscript.
Bohnen: Critical revision of the manuscript.
Spikman: Study design, critical revision of the manuscript.
van Laar: Study concept and design, data acquisition, critical revision of the manuscript.
Full financial disclosures for the previous 12 months
Dr. van der Zee, Dr. Kanel, Ms. Slomp, Dr. Gerritsen, and Dr. Spikman have nothing to disclose. J.M. Boertien has received writer's fee from Britannia Pharmaceuticals (Kinetic Magazine) and owns exchange traded funds that might include stocks from medically related fields. Dr. Müller has received research funding from the NIH, Department of Veterans Affairs, and The Michael J. Fox Foundation. Dr. Bohnen has received research funding from the NIH, Department of Veterans Affairs, Parkinson's Foundation, Farmer Family Foundation, The Michael J. Fox Foundation, Eisai, and EIP Pharma. He has been on the advisory board of Eisai and received in kind research support from Scion Neurostim, Expansion Therapeutics, and Innovative Health Solutions. Dr. van Laar has received research funding from Weston Brain Institute, Lysosomal Therapeutics, The Michael J. Fox Foundation, the Dutch Brain Institute, and the UMCG. He received lecture‐ and consultancy fees from AbbVie, Britannia, and PureIMS.
Supporting information
APPENDIX S1. Supporting Information
Acknowledgments
The authors thank all patients, caregivers, health‐care professionals, and students who have contributed to and collaborated in this project. The inclusion of patients was established with the help of the collaborative Parkinson Platform Northern Netherlands. The study was funded by the Weston Brain Institute.
Conflicts of interest/financial disclosures: This study was funded by the Weston Brain Institute.
[Correction added on 25 January 2022, after first online publication: The complete financial disclosures have been moved from the footnote to the online only section and the financial disclosure section in the footnote has been revised.]
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Post B, Muslimovic D, van Geloven N, Speelman JD, Schmand B, de Haan RJ, et al. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson's disease. Mov Disord 2011;26(3):449–456. [DOI] [PubMed] [Google Scholar]
- 2. Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, et al. Severity of mild cognitive impairment in early Parkinson's disease contributes to poorer quality of life. Parkinsonism Relat Disord 2014;20(10):1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aarsland D, Bronnick K, Williams‐Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010;75(12):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Domellöf ME, Ekman U, Forsgren L, Elgh E. Cognitive function in the early phase of Parkinson's disease, a five‐year follow‐up. Acta Neurol Scand 2015;132(2):79–88. [DOI] [PubMed] [Google Scholar]
- 5. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh‐Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8‐year prospective study. Arch Neurol 2003;60(3):387–392. [DOI] [PubMed] [Google Scholar]
- 6. Palavra NC, Naismith SL, Lewis SJG. Mild cognitive impairment in Parkinson's disease: a review of current concepts. Neurol Res Int 2013;2013:576091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord 2014;29(5):634–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res 2011;221(2):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016;91(6):1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang C, Zhou P, Yuan T. The cholinergic system in the cerebellum: from structure to function. Rev Neurosci 2016;27(8):769–776. [DOI] [PubMed] [Google Scholar]
- 11. Selden NR, Gitelman DR, Salamon‐Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998;121(Pt 1):2249–2257. [DOI] [PubMed] [Google Scholar]
- 12. Mesulam MM. The systems‐level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer's disease. Prog Brain Res 1996;109:285–297. [DOI] [PubMed] [Google Scholar]
- 13. Hilker R, Thomas AV, Klein JC, Weisenbach S, Kalbe E, Burghaus L, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 2005;65(11):1716–1722. [DOI] [PubMed] [Google Scholar]
- 14. Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010;74(11):885–892. [DOI] [PubMed] [Google Scholar]
- 15. Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 2009;73(4):273–278. [DOI] [PubMed] [Google Scholar]
- 16. Bohnen NI, Müller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, et al. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. J Cereb Blood Flow Metab 2012;32(8):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohnen NI, Albin RL, Müller MLTM, et al. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the Predemented cognitive Spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol 2015;72(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. J Neurol 2006;253(2):242–247. [DOI] [PubMed] [Google Scholar]
- 19. van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI. Cholinergic denervation patterns across cognitive domains in Parkinson's disease. Mov Disord 2021;36(3):642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petrou M, Frey KA, Kilbourn MR, PJH S, Raffel DM, Bohnen NI, et al. In vivo imaging of human cholinergic nerve terminals with (−)‐5‐18F‐Fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 2014;55(3):396–404. [DOI] [PubMed] [Google Scholar]
- 21. Nejad‐Davarani S, Koeppe RA, Albin RL, Frey KA, Müller MLTM, Bohnen NI. Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [18F]‐FEOBV. Mol Psychiatry 2019;24(3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Zee S, Vallez Garcia D, Elsinga PH, et al. [18F]Fluoroethoxybenzovesamicol in Parkinson's disease patients: quantification of a novel cholinergic positron emission tomography tracer. Mov Disord 2019. Jun;34(6):924–926. [DOI] [PubMed] [Google Scholar]
- 23. Boertien JM, van der Zee S, Chrysou A, Gerritsen MJJ, Jansonius NM, Spikman JM, et al. Study protocol of the DUtch PARkinson cohort (DUPARC): a prospective, observational study of de novo Parkinson's disease patients for the identification and validation of biomarkers for Parkinson's disease subtypes, progression and pathophysiology. BMC Neurol 2020;20(1):245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015. Oct;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 25. Schmand B, Bakker D, Saan R, Louman J. The Dutch Reading test for adults: a measure of premorbid intelligence level. Tijdschr Gerontol Geriatr 1991. Feb;22(1):15–19. [PubMed] [Google Scholar]
- 26. Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society task force guidelines. Mov Disord 2012;27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Diagnosing PD‐MCI by MDS task force criteria: how many and which neuropsychological tests? Mov Disord 2015;30(3):402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med 1997;27(2):363–370. [DOI] [PubMed] [Google Scholar]
- 29. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord 2013. May;28(5):668–670. [DOI] [PubMed] [Google Scholar]
- 30. Wellcome Trust Centre for Neuroimaging . University College, London, England. https://www.fil.ion.ucl.ac.uk/spm/software/spm12/.
- 31. Bohnen NI, Kanel P, Zhou Z, Koeppe RA, Frey KA, Dauer WT, et al. Cholinergic system changes of falls and freezing of gait in Parkinson's disease. Ann Neurol 2019;85(4):538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muller‐Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI‐based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12(4):571–583. [DOI] [PubMed] [Google Scholar]
- 33. Kanel P, Müller MLTM, van der Zee S, Sanchez‐Catasus CA, Koeppe RA, Frey KA, et al. Topography of cholinergic changes in dementia with Lewy bodies and key neural network hubs. J Neuropsychiatry Clin Neurosci 2020;32(4):370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dautan D, Huerta‐Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci 2014;34(13):4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ztaou S, Amalric M. Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson's disease. Neurochem Int 2019;126:1–10. [DOI] [PubMed] [Google Scholar]
- 36. Ikonomovic MD, Abrahamson EE, Isanski BA, Wuu J, Mufson EJ, DeKosky ST. Superior frontal cortex cholinergic axon density in mild cognitive impairment and early Alzheimer disease. Arch Neurol 2007;64(9):1312–1317. [DOI] [PubMed] [Google Scholar]
- 37. DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 2002;51(2):145–155. [DOI] [PubMed] [Google Scholar]
- 38. Kim K, Bohnen NI, Muller MLTM, Lustig C. Compensatory dopaminergic‐cholinergic interactions in conflict processing: evidence from patients with Parkinson's disease. Neuroimage 2019;190:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bohnen NI, Grothe MJ, Ray NJ, Müller MLTM, Teipel SJ. Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep 2018;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu S‐Y, Wile DJ, Fu JF, Valerio J, Shahinfard E, McCormick S, et al. The effect of LRRK2 mutations on the cholinergic system in manifest and premanifest stages of Parkinson's disease: a cross‐sectional PET study. Lancet Neurol 2018;17(4):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bedard M‐A, Aghourian M, Legault‐Denis C, Postuma RB, Soucy J‐P, Gagnon J‐F, et al. Brain cholinergic alterations in idiopathic REM sleep behaviour disorder: a PET imaging study with (18)F‐FEOBV. Sleep Med 2019;58:35–41. [DOI] [PubMed] [Google Scholar]
- 42. Postuma RB. Prodromal Parkinson's disease‐‐using REM sleep behavior disorder as a window. Parkinsonism Relat Disord 2014;20(Suppl 1):S1–S4. [DOI] [PubMed] [Google Scholar]
- 43. Rahayel S, Postuma RB, Montplaisir J, Mišić B, Tremblay C, Vo A, et al. A prodromal brain‐clinical pattern of cognition in Synucleinopathies. Ann Neurol 2021;89(2):341–357. [DOI] [PubMed] [Google Scholar]
- 44. Gilman S, Koeppe RA, Nan B, Wang C‐N, Wang X, Junck L, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 2010;74(18):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wojtala J, Heber IA, Neuser P, Heller J, Kalbe E, Rehberg SP, et al. Cognitive decline in Parkinson's disease: the impact of the motor phenotype on cognition. J Neurol Neurosurg Psychiatry 2019;90(2):171–179. [DOI] [PubMed] [Google Scholar]
- 46. Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G, et al. Mild cognitive impairment and cognitive‐motor relationships in newly diagnosed drug‐naive patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83(6):601–606. [DOI] [PubMed] [Google Scholar]
- 47. Müller MLTM, Bohnen NI. Cholinergic dysfunction in parkinson's disease. Curr Neurol Neurosci Rep 2013;13(9):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grace J, Amick MM, Friedman JH. A double‐blind comparison of galantamine hydrobromide ER and placebo in Parkinson disease. J Neurol Neurosurg Psychiatry 2009;80(1):18–23. [DOI] [PubMed] [Google Scholar]
- 49. Mamikonyan E, Xie SX, Melvin E, Weintraub D. Rivastigmine for mild cognitive impairment in Parkinson disease: a placebo‐controlled study. Mov Disord 2015;30(7):912–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


