Abstract
The underlying pathologies of psychiatric disorders, which cause substantial personal and social losses, remain unknown, and their elucidation is an urgent issue. To clarify the core pathological mechanisms underlying psychiatric disorders, in addition to laboratory‐based research that incorporates the latest findings, it is necessary to conduct large‐sample‐size research and verify reproducibility. For this purpose, it is critical to conduct multicenter collaborative research across various fields, such as psychiatry, neuroscience, molecular biology, genomics, neuroimaging, cognitive science, neurophysiology, psychology, and pharmacology. Moreover, collaborative research plays an important role in the development of young researchers. In this respect, the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium and Cognitive Genetics Collaborative Research Organization (COCORO) have played important roles. In this review, we first overview the importance of multicenter collaborative research and our target psychiatric disorders. Then, we introduce research findings on the pathophysiology of psychiatric disorders from neurocognitive, neurophysiological, neuroimaging, genetic, and basic neuroscience perspectives, focusing mainly on the findings obtained by COCORO. It is our hope that multicenter collaborative research will contribute to the elucidation of the pathological basis of psychiatric disorders.
Keywords: autism spectrum disorder, bipolar disorder, COCORO, major depressive disorder, schizophrenia
The World Health Organization (WHO) estimates that more than 264 million people of all ages suffer from depression worldwide. Twenty million people are estimated to have schizophrenia (SZ) worldwide. An estimated 46 million people in the world have bipolar disorder (BD). Much effort is devoted to the treatment of psychiatric disorders; however, there is a gap between clinical practice guideline evidence and clinical training that results in inadequate medical care for those with psychiatric disorders. Thus, the dissemination, education, and verification of clinical practice guidelines is required. 1 Psychiatric disorders have a significant economic impact, and the estimated cost to the global economy in lost productivity is enormous (https://ourworldindata.org/mental health). Therefore, it is necessary to conduct translational research to elucidate the pathophysiology of psychiatric disorders and to develop new therapeutic and diagnostic techniques. In general, translational research includes a wide range of research, from nonclinical to clinical research and development, to enable the actual clinical application of new findings found by researchers in academia through basic research. Psychiatric disorders are syndromes associated with social dysfunction caused by both genetic and environmental factors. Vulnerability genes for psychiatric disorders do not directly increase the risk of developing the disorder, but they are related to endophenotypes of psychiatric disorders (e.g. 2). Therefore, translational research in psychiatric disorders at the gene, amino acid/protein, cell, neural circuit, brain, and cognition/behavior levels is critical. Although many translational studies have been performed at each level, most of them are small‐scale studies and not reproducible, and there have been no large‐scale studies with high reproducibility with diagnostic or clinical usefulness.
To overcome this problem, in 2014, we established the Cognitive Genetics Collaborative Research Organization (COCORO) with the aim of elucidating the pathogenesis of psychiatric disorders and the molecular mechanisms of brain function through bidirectional translational research between basic neuroscience and clinical research (Fig. 1). Currently, 38 research institutes are participating in COCORO, including major Japanese research institutions (Fig. 2). Researchers in various fields, such as neuroscience, molecular biology, genomics, psychiatry, neuroimaging, cognitive science, neurophysiology, psychology and pharmacology, have discussed translational research in psychiatry and reported novel findings, as mentioned in the current review. The research resource database collected by COCORO is the largest in the Japanese psychiatry field and one of the best databases for psychiatry in the world. This research resource database includes over 10 000 subjects, including those with SZ, BD, major depressive disorder (MDD) and autism spectrum disorder (ASD), and healthy comparison subjects. There are multiple research resources, such as genomic DNA, blood RNA, plasma, serum, lymphoblastoid B‐cell lines and induced pluripotent stem cells (iPSCs), for more than 20 000 subjects, along with detailed clinical information, cognitive data, neurophysiological data, neuroimaging data and personality trait data for each individual.
Fig 1.
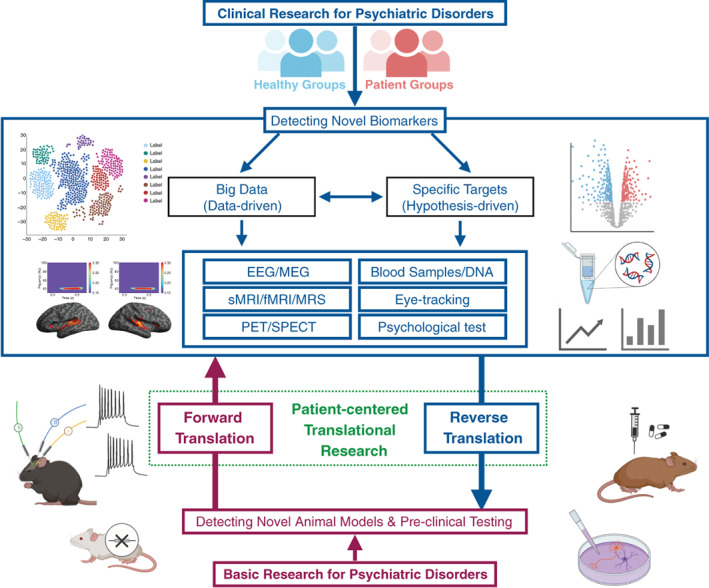
Schematic of bidirectional translational research (forward translation and reverse translation) between clinical research and basic neuroscience in psychiatry. Clinical research needs to detect novel biomarkers, by focusing on specific targets (hypothesis‐driven) or using big data (data‐driven), that are translatable to basic research. Basic research needs to detect novel animal models that would be useful for preclinical testing. These clinical and basic studies need to be performed bidirectionally through forward translation and reverse translation to achieve patient‐centered translational therapeutic research. (Figure made by Yoji Hirano)
Fig 2.
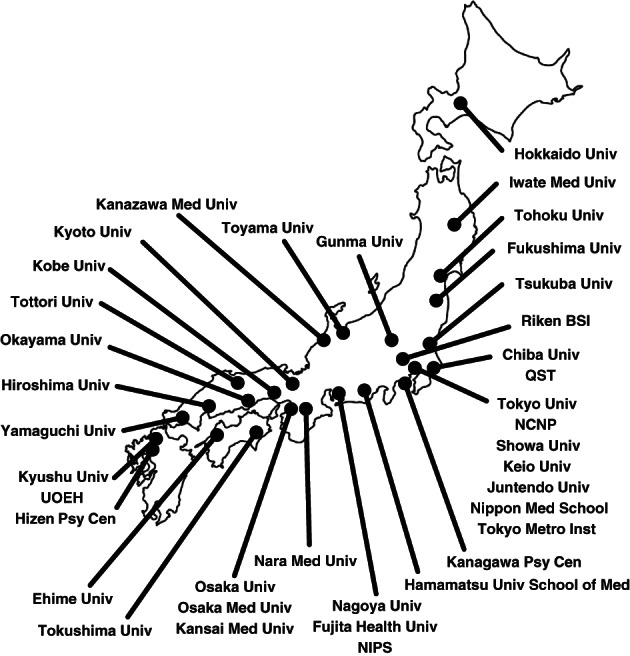
Institutions of the Cognitive Genetics Collaborative Research Organization (COCORO). The Cognitive Genetics Collaborative Research Organization (COCORO) consists of many psychiatric facilities in Japan. COCORO means “mind” in Japanese. There are 38 institutes participating in COCORO, which have been updated from Koshiyama et al 4 The purpose of COCORO is to elucidate the mechanisms of psychiatric disorders and brain functions. Abbreviations: Med, Medical; NCNP, National Center of Neurology and Psychiatry; NIPS, National Institute for Physiological Sciences; Psy Cen, Psychiatric Center; QST, National Institutes for Quantum and Radiological Science and Technology; Riken BSI, Riken Brain Science Institute; Tokyo Metro Inst, Tokyo Metropolitan Institute of Medical Science; Univ, University; UOEH, University of Occupational and Environmental Health.
COCORO is primarily focused on SZ research, since SZ is the most frequently investigated disease in psychiatry. In COCORO, using large samples of individuals with SZ, we have discovered novel findings and reproduced previous findings. Based on the established findings in SZ research, future studies will explore the heterogeneity among psychiatric disorders. In addition, we are conducting our research projects with as little burden on each institute as possible, as not all institutes collect data, such as cognitive function, eye movement, and structural MRI data, with all approaches. By taking into account the characteristics of each participating institute, COCORO has created a system that maximizes the sample size obtained at each institute.
In the following sections, after discussing multicenter collaborative research, we provide an overview of major psychiatric disorders and introduce a wide range of findings from genetic to cognitive studies, focusing on research results obtained by COCORO.
Multicenter Research System (System, Ethics, Education and Training)
Multicenter research is essential for the investigation of the pathophysiology of psychiatric disorders, and the establishment of an organized system is one of the most important issues to be addressed. In this regard, in COCORO, we have been working on (i) balancing interinstitutional research independence and complementarity; (ii) developing a collaborative research ethics system; and (iii) establishing a common measurement system, which is always an issue in multi‐institutional collaborative research. First, to secure the compatibility of research independence and complementarity among institutions in clinical research, it is critical to examine the reproducibility of data obtained from samples at one institution using data from other institutions. 3 , 4 For example, in COCORO, in addition to using data from other institutions to test reproducibility, we also conduct studies to confirm the generality of well‐known findings using a large sample from multiple institutions 5 and then use some of the collected data to conduct spin‐off studies for secondary analysis at various institutions (e.g. 6). Second, regarding the ethics system for joint research, to simplify complicated procedures, Osaka University plays the main role as the COCORO representative organization and unifies the ethical procedures for joint research within each institution. Finally, to establish a common measurement system in COCORO, we created an innovative standard protocol for brain magnetic resonance imaging (MRI), and each institution switched to this standard protocol. Regarding cognitive social function, since there was no simple battery of tests, a simple 15‐min method to measure cognitive dysfunction using a simplified version of the Wechsler Adult Intelligence Scale, Third Edition (WAIS‐III), intelligence test 7 was developed in COCORO. A presymptomatic intelligence test (Japanese Adult Reading Test [JART]) 8 , 9 has also been used in COCORO studies. Regarding tests of neurophysiological function, six facilities have conducted eye movement tests using a common protocol with the Eyelink system (SR Research, Ontario, Canada), and data were uploaded to a common server every month for quality control and data sharing.
To elucidate the pathogenesis of psychiatric disorders, it is very important to have close collaboration between researchers in the fields of basic and clinical research and to secure researchers in each field. Thus, the development of investigators in clinical and biological psychiatry is a matter of urgency. To this point, multicenter research provides a great opportunity for educating young researchers through new educational resources to integrate cutting‐edge research technology with several data sets in each field, which can lead to novel translational research. To accomplish these missions, (i) we must increase the engagement and participation of midcareer investigators; and (ii) we must help or provide educators with a robust neuroscience background for young researchers, medical students, and residents. In COCORO, we have tried hard to provide such opportunities for the development of young investigators. 10 , 11 For example, we have provided some MRI data to the Enhancing Neuroimaging Genetics through Meta‐Analysis (ENIGMA) consortium (see Koshiyama et al., 4 Thompson et al. 12 ). In turn, COCORO is developing research based on ideas from the ENIGMA consortium. Through these mutual international collaborations, we encourage young researchers and provide opportunities for them to be involved in cross‐institutional collaborations. As COCORO is composed of the parent organizations, the permission of the participation in COCORO by the responsive person in the parent organization is necessary for all young people. Thus, it is hard to manage the conflict between the parent organization and COCORO.
Description of Targeted Psychiatric Disorders
Schizophrenia is a psychiatric disorder characterized by positive symptoms (hallucinations, delusions, and disorganized speech), negative symptoms (blunted affect, avolition, anhedonia, and asociality) and impairments in various domains of cognition such as executive functions, memory, and processing speed. 13 The prevalence of SZ in the world population is approximately 1%, and SZ is among the top 10 global causes of disability. 14 Antipsychotics are effective for reducing psychotic symptoms in the acute phase of SZ and for preventing a relapse of the disease. Psychosocial interventions including social‐skills training, cognitive‐behavioral therapy, and supported employment are also important for the management of the negative symptoms or cognitive impairments associated with SZ. 15 A recent review revealed that approximately half of patients with SZ recovered or significantly improved over the long term. 16 Combinations of pharmacotherapy and psychosocial interventions can improve outcomes in individuals with SZ.
Major depressive disorder affects an individual's functional capacity through depressive symptoms such as sadness, emptiness, irritable mood, somatic changes, and cognitive impairments. It was reported that the 12‐month and lifetime prevalences of adult MDD based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), criteria were 10.4% and 20.6%, respectively, and the mean age at adult MDD onset was 29.1 years in the USA. 17 In contrast, in Japan, the 12‐month prevalence of MDD was lower (2.9%) than that in the USA. 18 Regarding sex differences, the lifetime prevalence of MDD in women (26.1%) is approximately twice that in men (14.7%). 17 BD is characterized by repeated depressive and manic episodes. The essential features of mania or hypomania are increased energy or activity along with elevated, expansive, or irritable mood. 19 BD is classified as BD type I (BD I), with severe manic and depressive episodes, and BD type II (BD II), with hypomanic and depressive episodes. The lifetime prevalence of BD I is approximately 1% worldwide. 20 It was reported that the median onset ages were 24.3 years for BD I and 30.1 years for BD II. 21 There is no clear sex difference in the prevalence of BD. 22 MDD and BD are multifactorial disorders that are caused by complex interactions of genetic and environmental factors. 23 , 24 The treatment of MDD is based on environmental adjustments and pharmacotherapy with antidepressants. In addition to pharmacotherapy, cognitive‐behavioral therapy and interpersonal therapy are effective in preventing relapse after the mood state has stabilized. 25 In contrast, since BD has a high relapse rate and often becomes chronic, it is important to promote an understanding of the disorder among patients and the people around them through psychological education. 26 Mood stabilizers with relapse‐preventing effects are the mainstay of pharmacotherapy for BD. 27 Individuals with MDD and BD have been reported to show declines in various measures of cognitive function (e.g. 28). Moreover, suicide prevention is extremely important, as MDD and BD are high‐risk factors for suicidal thoughts or behaviors in psychiatric patients. 19 , 29 , 30
Autism spectrum disorder is characterized by difficulty with social communication, repetitive behavior, and highly restricted interests or activities. 31 Four separate disorders, including autistic disorder, childhood disintegrative disorder, Asperger syndrome, and pervasive developmental disorder, were integrated into the single entity of ASD in DSM‐5. The estimated prevalence of ASD was reported to be 14.5 per 1000 children aged 8 years and was reported to be approximately 4–5 times higher in boys than in girls (23.4 vs. 5.2 per 1000) in 2012. 32 Nearly 75% of patients with ASD have comorbid psychiatric disorders, including attention‐deficit/hyperactivity disorder (ADHD), anxiety disorder, BD, sex dysphoria, obsessive‐compulsive disorder, SZ, and tic disorders. 33 Although medication is not available to treat the core features of ASD, medical treatments as well as psychoeducation can be effective for secondary symptoms such as difficulties with emotion regulation. 33 , 34
Neurocognition
Neurocognitive impairments have been reported in a variety of psychiatric disorders, including SZ, 35 BD 36 and MDD. 37 In patients with developmental disorders, substantial variation of cognitive domains, but not impairment, have been reported. 38 In many psychiatric disorders, neurocognitive impairment has been reported to be an independent predictor of social functioning 39 , 40 , 41 and recognized as an important therapeutic target. 42 Based on this background, COCORO has focused on cognitive impairment in psychiatric disorders.
First, Fujino and colleagues investigated the degree of estimated cognitive decline in patients with SZ. 43 A total of 446 patients with SZ (228 males, 218 females), consisting of three sample sets obtained from 11 psychiatric facilities, and 686 healthy controls, participated in this study. Fujino and colleagues found that the IQ decline, which is the mean difference between premorbid IQ estimated from the JART 25 and current IQ estimated from the full IQ (FIQ) from the WAIS‐III, was 10 points or greater in 70% and 20 points or greater in 40% of subjects with SZ. This report was the first to show changes in cognitive function from before to after illness in Japanese patients with SZ. A ten‐point decrease in IQ must have a significant impact on the daily life of subjects. The data also showed that the distribution of IQ decline in subjects with SZ followed a normal distribution and was not bimodal or distorted. The results from this study showed that it is important to assess not only the current cognitive impairment but also the change in cognitive function when considering whether to target cognitive dysfunction as a therapeutic target in clinical practice and how much recovery can be expected during treatment.
As a second approach to the study of cognitive dysfunction, Sumiyoshi and colleagues investigated whether IQ declines predicted the social functioning of patients. 44 One hundred forty patients with SZ and 156 healthy volunteers were enrolled in the study. The logistic regression analyses were conducted with work hours dichotomized into four categories (0, 10, 20, or 30 h per week) as dependent variables. Sumiyoshi and colleagues showed that the model that included IQ decline, social functioning and psychotic symptoms predicted the ability to work. The results of this study showed that IQ decline is a useful predictor of social functioning in patients with SZ. The results also showed that scores of only two tests on the WAIS‐III (similarities and symbol search) predicted full‐battery WAIS‐III scores with high precision. 7 In this study, WAIS‐III data were obtained from 150 patients with SZ and 221 healthy controls. First, exploratory factor analysis was used to test the representativeness of the IQ structure, and multiple regression analyses were conducted to test the predictability of the FIQ. Then, candidate tests were nominated based on the consistency of subtests across versions. Finally, the optimality of candidate tests was evaluated in terms of sensitivity to functional outcome measures and conciseness in administration time. To integrate cognitive dysfunction treatment into daily clinical practice, it is crucial to measure cognitive dysfunction with a concise and reliable method. The results of this study indicated that the IQ decline in patients, which may predict their ability to work, could be estimated in only 15 min in daily clinical practice. To implement this test in the real world, we held regular training sessions on this procedure at workshops of various scientific meetings.
The third approach to the study of cognitive dysfunction is a biological approach. Koshiyama and colleagues investigated the relationships between subcortical structure volumes and neurocognitive indices. Using subcortical structure volume data from 163 patients with SZ, they showed that the right nucleus accumbens (NAc) volume was significantly correlated with the digit symbol coding score from the WAIS‐III. 45 In another study, using the subcortical structure volumes from 173 patients with SZ and their scores on the Wechsler Memory Scale‐Revised (WMS‐R), it was found that the NAc volume, as well as hippocampal volume, was correlated with immediate and delayed recall scores from the verbal memory test on the WMS‐R. 46 A previous study showed an association between intrinsic motivation and cognitive test performance in patients with SZ. 47 Because the NAc is a part of the brain reward system, 48 our findings support the importance of motivation in the cognitive functioning of patients with schizophrenia in terms of brain structural abnormalities. More recently, Yasuda and colleagues compared brain structures and functional connectivity among 633 healthy controls, 54 patients with SZ without cognitive impairment, and 111 patients with SZ with cognitive impairment. 6 In this study, Yasuda and colleagues found a significant decrease in cortical thickness in the whole brain and in broad areas in the frontotemporal lobe and a significant increase in the volumes of the lateral ventricle, basal ganglia and hippocampus in patients with SZ with cognitive impairment compared to the controls and patients with SZ without cognitive impairment. They also found significant hyperconnectivity between the thalamus and a broad range of brain regions in patients with SZ with cognitive impairment compared to controls and significant hyperconnectivity between the accumbens and the superior and middle frontal gyri in patients with SZ without cognitive impairment compared with patients with SZ with cognitive impairment. The results of this study showed that both brain structure and functional connectivity were extensively impaired in patients with SZ with cognitive impairment, suggesting that cognitive function subgroups could be useful to elucidate brain pathophysiology in patients with SZ.
As we have shown in this section, COCORO has continued to take a comprehensive approach to cognitive dysfunction using a variety of research methods and techniques. It is noteworthy that the close network of diverse researchers and the smooth exchange of data among more than 23 000 individuals at multiple institutions in COCORO make these efforts possible.
Neurophysiology
Eye movement measures
Eye movement abnormalities are often reported in various psychiatric disorders, including SZ, BD, MDD, and ASD. Eye movement abnormalities are currently recognized as one of the endophenotypes of psychiatric disorders. 49 , 50 , 51 , 52 In SZ, several studies have attempted to distinguish patients with SZ from healthy controls and/or individuals with other mental illnesses. 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 Eye movement studies in COCORO began with a study to develop an integrated eye movement score as a neurophysiological marker of SZ based on relatively small data samples. 59 A subsequent study replicated this initial finding using twice the sample size and showed that a discriminant analysis using three eye movement measures, the scanpath length during a free viewing test, the horizontal position gain during a smooth pursuit test, and the duration of fixations during a fixation stability test, distinguished SZ patients from healthy controls with 82.5% accuracy. 60 The researchers proposed a newer and more sophisticated version of the score that can be obtained from only a few examinations taking approximately 15 min.
Eye movement performance (scanpath length and integrated eye movement score) is associated with cognitive functions measured using WAIS scores and social activities measured as work hours per week in SZ patients. 61 , 62 A potential mechanism has been suggested in which decreased eye movement performance leads to reduced cognitive performance (perceptual organization) that eventually results in reduced social activities. 61 Some research investigating biological mechanisms underlying eye movement abnormalities in patients with SZ has revealed genetic associations with the performance of smooth pursuit eye movements 63 and an association between cortical thickness and scanpath length during free viewing. 64 The detailed history and progress of eye movement research in COCORO through 2020 are summarized elsewhere. 65 The latest discoveries in the studies in COCORO have involved differences in eye movement performance between individuals with SZ and individuals with ASD, 66 the association between abnormal scanpath length during free viewing and a disruption of attentional control in SZ patients, 67 and abnormal eye movements in MDD patients. 68 In COCORO, a large database of eye movements, with over 800 subjects, including patients with various psychiatric disorders and healthy subjects, allows us not only to obtain novel findings but also to evaluate the reproducibility of findings. Takahashi et al. 69 provided robust and reproducible findings showing the influences of age on eye movements in healthy individuals. Thus, studies using databases in COCORO will contribute not only to psychiatry but also to neuroscience.
Electroencephalography and magnetoencephalography studies
Electroencephalography (EEG) and magnetoencephalography (MEG) are noninvasive neurophysiological methods with excellent temporal resolution in the millisecond range that have revealed novel insights into cognitive and sensory abnormalities in individuals with psychiatric disorders, especially in patients with SZ. 70 , 71 , 72 Auditory‐related symptoms (especially auditory hallucinations) are known to be prominent compared to other sensory modality symptoms in SZ, and auditory‐related deficits can be found even at the early sensory processing level (e.g. tone matching), which could contribute directly to impairments in cognitive and social functioning. 73 Accordingly, auditory‐related EEG/MEG indices such as mismatch negativity (MMN) 74 and gamma oscillations, including the auditory steady‐state response (ASSR), 74 have been used as highly reproducible biomarkers in SZ. MMN is a neurophysiological event‐related potential EEG/MEG measure of early auditory information processing that is hypothesized to reflect abnormal predictive coding in SZ. 74 , 75 , 76 Many studies have repeatedly shown that the amplitude of MMN, especially in the duration MMN paradigm, is reduced in first‐episode SZ and chronic SZ patients as well as in clinical high‐risk subjects. 77 , 78 Physiologically, since N‐methyl‐D‐aspartate (NMDA) receptor antagonists reduce the MMN amplitude 78 , 79 MMN has the potential to be a therapeutic biomarker for identifying abnormal NMDAR‐mediated neurotransmission in SZ.
Recent studies have shown that the brain is spontaneously active and periodically becomes activated in particular patterns, even without exposure to external stimuli. 80 External stimuli not only induce neural responses from the brain's static state but also play a role in selecting a specific phase of neural activity from the ever‐changing rhythmic dynamic state of the brain. As a result, brain activity is maintained and is continuously rewriting the intrinsic information in the brain. This intrinsic and rhythmic periodic activity of neurons is called neural oscillation. Neural oscillations in the gamma range (30–100 Hz) generated in the neocortex by interactions among neurons and interneurons in local circuits are reliable and important translatable neurophysiological biomarkers of SZ. 70 Aberrant gamma oscillations have been observed and studied extensively in SZ using evoked activity paradigms, especially with the ASSR task, which is highly reproducible. 81 , 82 Many studies have reported a decreased phase synchronization and evoked power of 40‐Hz ASSRs (elicited by 40‐Hz steady click sounds) in both the early and chronic states of SZ. 83 , 84 , 85 , 86 In addition to reduced stimulus‐locked 40‐Hz ASSR oscillations (phase‐locking factor and evoked power), recent studies have shown increased (non‐phase‐locked) spontaneous gamma oscillations (induced power) during click‐sound stimulation in patients with SZ, 85 along with lower primary auditory cortex volume. 87 Progressive reductions in auditory‐evoked gamma oscillations are also observed over time in first‐episode SZ patients but not in clinical high‐risk subjects, indicating that evoked gamma oscillations may manifest progressively abnormal neural function that occurs after the onset of psychosis. 88 When considering these neurophysiological phenomena, basic research has shown that the mutual balance between excitation and inhibition within local neural networks is crucial for generating gamma oscillations. 80 Accordingly, intact neuronal informational processes depend on a proper excitability/inhibitory balance (E/I balance), and the loss of this mutual balance has been hypothesized to induce gamma oscillation deficits in SZ 85 , 89 , 90 Importantly, our previous findings of increased spontaneous broadband gamma power during auditory stimulation in patients with SZ 85 resembled the increased spontaneous broadband gamma power often reported in NMDA‐R hypofunction (i.e. increased E/I imbalance) animal models of SZ (e.g. 91, 92). Therefore, it is proposed that spontaneous gamma oscillations have great potential as a translatable neurophysiological biomarker for SZ.
Given the noninvasiveness and versatility of clinical EEG systems, which can be found in most medical facilities, EEG is very suitable for multicenter studies to detect useful functional biomarkers of psychiatric disorders. For example, the Boston CIDAR study (https://bricweb.bidmc.harvard.edu/bostoncidar/) and Bipolar‐Schizophrenia Network on Intermediate Phenotypes (B‐SNIP: http://b-snip.org/) study have successfully confirmed some of the previous EEG findings in SZ patients and individuals at high clinical risk with larger sample EEG data (e.g. 88, 93, 94). In Asia, in addition to the Asian Consortium on MRI studies in Psychosis (ACMP) (http://asia-mri-consortium.net/), researchers have been working to organize the Asian Consortium on EEG studies in Psychosis (ACEP), which will consist of seven university hospitals and four psychiatric hospitals. ACEP's main focus is to detect novel oscillatory EEG functional biomarkers, which could be used to redefine the classifications of psychosis and be valuable for translational research. The consortium hopes that this multicenter EEG study can contribute to the understanding of the underlying pathology of psychosis and lead to novel neurofunction‐based treatments.
Neuroimaging
Recent neuroimaging evidence from international collaborative large‐scale MRI studies, particularly those of the ENIGMA consortium, has demonstrated structural and functional abnormalities in major neuropsychiatric disorders (e.g. SZ, mood disorders, and ASD). 12 COCORO has developed a framework of multisite neuroimaging studies in partnership with the ENIGMA consortium for standard imaging protocols and data sharing 95 , 96 and has successfully replicated and extended their work as detailed below. However, potential confounding factors that could affect brain morphology/function (e.g. illness chronicity and medication) and low disease specificity of the brain changes in these disorders prevent the clinical application (e.g. diagnosis or the prediction of treatment response on the basis of biological findings) of these findings. 97 Further research, including ongoing COCORO neuroimaging projects such as a study across major psychiatric disorders, a biotype study using brain‐based biomarkers, and a clustering of SZ heterogeneity on the basis of distinct brain morphological and functional patterns using a large database of more than 8000 patients with various psychiatric disorders and healthy controls, could provide neuroimaging evidence that could lead to a better understanding of the neurobiology of psychiatric disorders and to the clinical application of neuroimaging research findings.
Structural MRI
The COCORO structural MRI working group replicated the subcortical findings of the ENIGMA consortium on the basis of T1‐weighted MR images from 1680 healthy individuals and 884 patients with SZ, obtained with 15 imaging protocols at 11 sites within COCORO, using FreeSurfer; the patients were characterized by smaller volumes of the hippocampus, amygdala, thalamus, and accumbens but larger volumes of the caudate, putamen, and pallidum. 95 , 98 Hippocampal atrophy and pallidum expansion in SZ patients were partly attributable to antipsychotic medications regardless of medication type (i.e. typical or atypical), where the pallidum volume was also positively associated with illness duration. 99 Based on a subsequent study using the Multiple Automatically Generated Templates (MAGeT) brain segmentation algorithm, 100 thalamic volume reduction and its relation to cognitive deficits in SZ patients had a subdivision specificity (in particular, the medial dorsal and ventral lateral nuclei), whereas no thalamic reduction was exhibited in individuals with a high clinical risk for developing psychosis when compared with healthy subjects. These subcortical findings may partly reflect state abnormalities and could change during the course of SZ.
Regarding cortical changes in SZ, a voxel‐based morphometric study of 1252 healthy subjects and 541 patients with SZ with data from seven different scanners/parameters demonstrated that the patients exhibited gray matter reduction predominantly in the frontal and temporolimbic regions and that such a brain morphological characteristic could successfully differentiate SZ patients from control subjects despite the use of MRI data collected using different settings. 5 Interestingly, our genome‐wide association study (GWAS) focusing on the superior frontal gyrus volume in 158 patients with SZ and 378 healthy subjects revealed a significant contribution of genetic variants in the eukaryotic translation initiation factor 4 gamma, 3 (EIF4G3) gene on 1p36.12. 101 The gross brain morphology probably reflects prenatal brain development; sulcogyral patterns of the orbitofrontal cortex were significantly different between 155 SZ patients and 375 healthy subjects. 102 Because gyrification patterns generally remain stable after birth, this finding supports the early neurodevelopmental pathology of SZ.
Taken together, the findings from SZ structural MRI studies from the COCORO consortium demonstrated subcortical/cortical and gross morphological changes in a large multicenter cohort of SZ patients, which may reflect both neurodevelopmental and state‐related abnormalities associated with the illness as well as other influencing factors (e.g. medications). However, the causes, timing and course of these findings remain unclear. Cortical findings in SZ, which are at least partly genetically controlled, could contribute to the diagnosis with favorable classification accuracy, but it remains unknown whether these findings are specific to SZ or more generally observed across various neuropsychiatric disorders.
Diffusion tensor imaging
Recent large‐scale multisite studies from the ENIGMA consortium reported white matter microstructural alterations in neuropsychiatric diseases, including SZ, BD, depressive disorders, and 22q11 deletion syndrome. A meta‐analysis that was conducted with 1963 patients with SZ and 2359 healthy controls from 29 independent international studies identified widespread microstructural alterations, including alterations in the anterior corona radiata, corpus callosum, cingulum, fornix, and uncinate fasciculus, in patients with SZ. 103 Another meta‐analysis demonstrated altered white matter connectivity within the corpus callosum, cingulum, and fornix in BD patients. 104 Furthermore, a meta‐analysis found that alterations in the corpus callosum and corona radiata were reported in patients with MDD relative to healthy subjects. 105 Additionally, widespread white matter alterations in the corona radiata, corpus callosum, superior longitudinal fasciculus, posterior thalamic radiations, and sagittal stratum were identified in 22q11.2 deletion carriers. 106 The ENIGMA‐ADHD and ENIGMA‐ASD groups are currently working on diffusion tensor imaging (DTI) analyses. 107 However, the similarity and specificity of white matter microstructural alterations across these psychiatric disorders remain unclear.
In a COCORO study, a mega‐analysis of white matter microstructural alterations across four major psychiatric disorders in 696 patients with SZ, 211 with BD, 126 with ASD, 398 with MDD, and 1506 healthy comparison subjects from 12 sites in Japan was performed using the ENIGMA‐DTI imaging analysis protocol. 108 The results showed that patients with SZ demonstrated widespread alterations in white matter regions, including the anterior corona radiata, corpus callosum, fornix, and uncinate fasciculus; SZ and BD patients featured similar changes in the limbic system, such as the fornix and cingulum, compared to healthy subjects; and SZ, BD and ASD patients shared similar white matter microstructural differences in the body of the corpus callosum relative to healthy subjects. Regarding SZ and BD, data sets from both the ENIGMA consortium and COCORO showed similar results, and there were no significant differences in a direct comparison between SZ and BD in the COCORO study. 108 Given the confirmed findings from two sets of large‐scale studies from the ENIGMA consortium and COCORO as well as the cross‐disorder study by COCORO, 108 both patients with SZ and those with BD have similar regional alterations in brain white matter. Regarding MDD, whereas the COCORO study did not show significant differences in patients relative to healthy subjects, 108 van Velzen et al. 105 showed alterations in the anterior corona radiata and corpus callosum in MDD patients. The difference in sample size may have contributed to the inconsistencies. While van Velzen et al. 105 reported a Cohen's d effect size of −0.25 for fractional anisotropy (FA) in the anterior corona radiata in 921 MDD patients relative to 1265 healthy subjects, the COCORO study reported an effect size of −0.19. 108 While the ENIGMA groups are currently working on DTI analysis in patients with ASD, 107 the COCORO study showed white matter microstructural alterations in the body of the corpus callosum in those patients. 4 Additionally, Villalón‐Reina et al. 106 reported white matter alterations in the corpus callosum in 22q11.2 deletion carriers. Taken together, these findings show that while alterations in tracts connecting neocortical areas (e.g. the uncinate fasciculus) were observed only in patients with SZ, white matter alterations in the corpus callosum and the limbic system (i.e. the fornix and cingulum) may be common in patients with SZ and those with BD. Furthermore, white matter alterations in the corpus callosum may be widely common in individuals with psychiatric disorders, including 22q11.2 deletion carriers.
Regarding the clinical implications of white matter microstructural alterations, the role of structural connectivity in the anterior corona radiata and corpus callosum in social functioning was reported by another COCORO study from an archival subsample cohort of 149 patients with SZ. 109 The findings are consistent with the results from Kochunov et al., 110 who demonstrated associations of structural connectivity in the anterior corona radiata and corpus callosum with working memory and processing speed in 166 patients with SZ. However, associations of white matter microstructural alterations with clinical implications for other neuropsychiatric diseases are largely unknown. The associations can be widely investigated across neuropsychiatric disorders using pooled DTI data and clinical measures in COCORO. 4
Functional MRI
Neuroimaging research using functional MRI (fMRI) consists of task‐based fMRI and resting‐state fMRI (rsfMRI). Recent multicenter joint research in psychiatric diseases by fMRI mainly uses rsfMRI. RsfMRI is a very convenient method to evaluate brain function in various networks, for example, the visual, sensorimotor, executive control, salience, dorsal attention, auditory, and default mode networks. 111
A meta‐analysis of rsfMRI data from SZ patients and healthy controls indicated impaired functional connectivity in the limbic, frontoparietal executive, default mode, and salience networks, with reductions in gray matter volume in the insula, lateral postcentral cortex, striatum, and thalamus, in the SZ patients compared to the healthy controls. 112 An ENIGMA study of SZ patients demonstrated the effects of ketamine and midazolam on functional connectivity in rsfMRI. 113 Hyperconnectivity between the thalamus and a broad range of brain regions in SZ patients with deteriorated cognition has been reported; this hyperconnectivity was less evident in patients with SZ with preserved cognition. 6 These findings are consistent with those of another study that showed aberrant thalamocortical functional connectivity in chronic SZ. 114 In addition to SZ, the ENIGMA consortium reported impaired functional connectivity in various psychiatric disorders, such as ADHD/ASD 107 and epilepsy, 115 as well as in patients with a cyst compressing the cerebellum. 116 These findings suggested that evaluating the extent of impairment in various networks could contribute to elucidating the pathophysiology of psychiatric disorders.
Positron emission tomography studies
In neuropsychiatric disorders, an investigation of the living brain by high‐resolution positron emission tomography (PET) and magnetic resonance spectroscopy (MRS), combined with appropriate pharmacokinetic and physiological analyses, enabled us to provide important quantitative information on abnormal brain functions. PET has been used to examine the distribution of neurotransmitters in the human brain. Compared to other functional imaging methods, PET enables the qualitative and quantitative evaluation of changes in the distribution of tracers, making it possible to determine pathophysiology as well as diagnostic information in many psychiatric disorders. For example, in SZ patients, PET studies have demonstrated a hypofrontal pattern in patients with chronic SZ with a predominance of negative symptoms 117 , 118 but not in younger patients with predominantly positive symptoms, 119 suggesting that frontal lobe activity changes throughout the course of the disease and becomes more prominent in the chronic phase. In terms of neural circuits, Andreasen 120 showed dysfunctional prefrontal‐thalamic‐cerebellar circuitry in SZ subjects. Laterality differences in SZ are another investigated topic, and several PET studies have reported that SZ subjects have increased metabolism and perfusion in the left hemispheric brain structures relative to the right hemispheric brain structures in the resting state (e.g. 121). In addition, the severity of symptoms has been associated with the degree of the hyperactivation of the left hemisphere but not with the degree of the hypofrontal pattern.
Importantly, PET studies have also assessed the effects of therapeutic interventions (e.g. medication) on brain pathophysiology in SZ patients. Pharmacokinetic PET studies have shown that in early treatment with antipsychotics, there is high dopamine D2 receptor occupancy, particularly in the basal ganglia. This D2 receptor occupancy is dose dependent and associated not only with the therapeutic effects of antipsychotics but also with the incidence of side effects such as extrapyramidal syndromes. 122 Thus, it has been recognized that PET studies are essential for establishing the optimal dosage of antipsychotic drugs. Recent PET studies have also revealed that neuroinflammation appears to be one of the hallmarks of various psychiatric disorders. 123 Using the most commonly imaged biomarker of neuroinflammation, the 8 kDa translocator protein (TSPO), many PET studies have consistently found elevated TSPO binding in MDD patients 123 ; in contrast, a recent meta‐analysis demonstrated reduced TSPO binding in SZ patients. 124 This disease‐specific pattern highlights the relevance of validating neuroinflammation biomarkers for each psychiatric condition separately when targeting neuroinflammation treatments for each condition.
To our knowledge, no multicenter PET study has been conducted for psychiatric disorders except dementia, 125 which limits the pharmacokinetic and physiological findings in psychiatric disorders. Recently, an investigation of the glutamatergic neurotransmitter system has attracted substantial attention in the pathophysiology and treatment of neuropsychiatric diseases 126 ; however, clinical translation of knowledge on glutamate and AMPA that accumulated in a number of studies has been limited due to the inability to visualize AMPA receptors in the living human brain. As first reported, 127 a novel PET tracer for glutamate AMPA receptors in the human brain ([11C]K‐2) has been developed. Furthermore, in Japan, a multicenter cross‐disease AMPA‐PET study is in progress to investigate AMPA receptor abnormalities in various psychiatric disorders (SZ, BD, MDD and ASD) to elucidate the relationships between AMPA receptor density in the brain and psychiatric symptoms and their outcome. It is hoped that the findings of this multicenter cross‐disease AMPA‐PET study can contribute to the development of novel drugs and treatments for psychiatric disorders.
MRS studies
MRS has become a valuable tool for investigating the biochemical bases of psychiatric disorders such as SZ 128 , 129 1H MRS has revealed alterations in glutamate and glutamine levels in the brains of patients with SZ relative to those of healthy controls; glutamate and glutamine levels are generally interpreted as biomarkers of glutamatergic dysfunction in SZ. A recent comprehensive meta‐analysis showed that SZ is associated with elevations in glutamatergic metabolites across several brain regions, including the thalamus, basal ganglia and medial temporal lobe. It was also concluded that there were no regions showing a consistent reduction in glutamate metabolites in the patient group. 128 These findings supported the hypothesis that SZ is related to excess glutamatergic neurotransmission in several limbic regions and further suggested that chemical compounds that reduce glutamatergic transmission may have therapeutic potential, especially in the acute phase of the illness. 79 While the assessment of the excitatory neurotransmitter glutamate in vivo by 1 H MRS is straightforward due to its high cerebral concentration, the low level of the inhibitory neurotransmitter γ‐aminobutyric acid (GABA) makes it challenging to assess in vivo. Thus, the number of GABA‐MRS studies is limited, and most experiments were conducted by spectral editing methods such as Mescher‐Garwood point resolved spectroscopy (MEGA‐PRESS) at 3 T 130 , 131 , 132 and recently at 7 T. 133 A recent comprehensive meta‐analysis demonstrated that there were no significant SZ‐associated changes in GABA levels in the medial prefrontal cortex (MPFC) or dorsolateral prefrontal cortex (DLPFC). Interestingly, after excluding the outlier studies, GABA levels in the frontal cortex were lower in SZ patients than in controls. In the anterior cingulate cortex (ACC), a significant group difference was found in patients with lower GABA levels, which was more pronounced in first‐episode SZ patients. 129
To our knowledge, few multicenter MRS studies have been conducted for psychotic disorders (e.g. 134, 135), but the results of those that have enabled us to confirm previous evidence linking higher levels of glutamate in the ACC with a poor antipsychotic response by showing that the association is evident before the initiation of treatment. Further investigations to detect abnormal biochemical bases of psychosis by multicenter MRS studies are needed for possible improvements in outcomes associated with early intervention in psychosis.
Genetic and Other Biological Studies
Epidemiological studies have shown that genetic factors make substantial contributions to the etiology of ASD, SZ, and BD. Recent molecular genetic studies have provided evidence that many genetic variants, including rare variants with large effects and common variants with small effects, contribute to the risk of these disorders. 136 , 137 , 138 , 139 The results of these studies have also suggested that genetic overlap is common among psychiatric disorders. 140 In COCORO, GWASs of psychiatric disorders were performed in the Japanese population. The Japanese population is genetically homogeneous compared to other populations with recent admixture. Therefore, the COCORO sample has the advantage of a minimized risk of population bias, and these GWASs have contributed to the elucidation of the genetic basis of these disorders. Studies of rare copy number variations (CNVs) for ASD and SZ were performed using array comparative genomic hybridization. 141 , 142 Approximately 8% of patients with such disorders had clinically significant or pathogenic CNVs, which were previously associated with a risk for psychiatric disorders. Some of these CNVs showed a statistically significant association with disorders, for example, deletions at 1q21.1, 22q11.2, and 47,XXX/47,XXY (SZ) and duplication at 22q11.2 (ASD). Consistent with the genetic overlap among psychiatric disorders, pathogenic CNVs at approximately 30 loci were common to these disorders. Phenotypic analysis indicated an association between pathogenic CNVs and treatment resistance to antipsychotics in SZ patients or intellectual disability comorbidity in SZ or ASD patients. Enrichment analysis based on gene ontology suggested that multiple biological pathways (e.g. synapse/neuron projection, oxidative stress response, small GTPase signaling) are common to the two disorders. 141 Furthermore, in‐depth functional analysis of pathogenic CNVs using patient‐derived iPSCs, mouse models, and postmortem brains has provided important clues to the pathophysiology and led to the identification of drug targets 143 , 144 , 145 , 146 , 147 {Torii, 2020 #86}.
For common variants, GWASs have identified common single nucleotide polymorphisms (SNPs) for SZ and BD in the Japanese population. 148 , 149 The SZ study identified 15 novel loci, including a top‐hit SNP of SPHKAP (P = 6.3 × 10−9, odds ratio [OR] = 1.63). This gene encodes a family of A‐kinase anchor proteins that interact with a protein kinase, suggesting signal transduction implications in the pathophysiology of SZ. An analysis of the polygenic risk score (PRS), an aggregate measure of genetic variants (i.e. SNPs) with small effect sizes, revealed that the genetic risk of SZ is shared across populations (Japanese and European populations) and across diseases (SZ and BD). In the BD GWAS, a novel association signal was identified at a locus of the FADS1/2/3 gene (P = 6.4 × 10−9, OR = 1.18). 149 As of these genes are implicated in the regulation of plasma lipid levels, the results suggest the involvement of lipid abnormalities in the pathophysiology of BD. PRS analysis supported the polygenic nature of BD, which is shared between the Japanese and European populations.
Genetic variants contribute to interindividual differences in medication response and toxicity. The elucidation of the genetic basis of interindividual differences in medication response and toxicity will facilitate precision medicine in psychiatry. Among others, it is important to identify genetic variants associated with the risk for clozapine‐induced agranulocytosis or granulocytopenia, as they are potentially life‐threatening conditions observed in approximately 1% of patients on clozapine treatment. In COCORO, a pharmacogenomic study of clozapine‐induced agranulocytosis or granulocytopenia identified a significant association with HLA‐B*59:01 (P = 3.81 × 10−8, OR = 10.7). 150
In addition to genetic variants, epigenetic mechanisms, including DNA methylation and histone modification, are suggested to be involved in the pathogenesis of psychiatric disorders. 151 One of the most studied epigenetic alterations is DNA methylation at CpG sites of the SLC6A4 gene, which encodes the serotonin transporter (5‐HTT), which has been implicated to be involved in mood and anxiety disorders and amygdala reactivity. 152 Considering the high prevalence of depressive symptoms in SZ patients, one study in COCORO examined DNA methylation of SLC6A4 in SZ patients. 153 This study identified significant hypermethylation of a CpG site in SLC6A4 in three independent cohorts of male patients with SZ and demonstrated that DNA methylation at this CpG site diminished the promoter activity of SLC6A4. Low‐activity alleles of the 5‐HTT‐linked polymorphic region (5‐HTTLPR) were associated with hypermethylation, and there was a negative correlation between DNA methylation levels and left amygdala volumes. These results suggested a pathophysiological role of epigenetic alterations of SLC6A4 in SZ. There is accumulating evidence that metabolic abnormalities are implicated in the pathophysiology of psychiatric disorders. 154 Carbonyl stress, which results from an increase in reactive carbonyl compounds (RCCs) or a decrease in the detoxification of RCCs, is associated with SZ. 155 , 156 While patients with SZ have higher levels of advanced glycation end products (AGEs), which are markers of carbonyl stress, the biological underpinnings remain unclear. One study in COCORO found that patients with SZ have significantly lower endogenous secretory receptor for AGEs (esRAGE) levels, which alleviates the burden of carbonyl stress. 157 This study also identified functional variants in the AGER gene associated with a marked decrease in esRAGE levels. Another study showed that measuring blood metabolites may be a useful objective tool for evaluating the severity of MDD. 158 In this study, metabolomic analysis of blood plasma from patients with MDD was conducted using liquid chromatography mass spectrometry (LC–MS), and five metabolites were associated with the severity of MDD regardless of the presence or absence of medication and diagnostic differences. Furthermore, several metabolites were independently associated with symptoms of MDD, including suicidal ideation.
Collaborative efforts in COCORO have resulted in the accumulation of more than 29 000 biological materials, including genomic DNA, blood RNA, immortalized lymphoblasts, serum and plasma. COCORO has been actively involved in collaborations with international genetics consortia. For instance, in collaboration with the Autism Sequencing Consortium (ASC), the largest exome sequencing analysis (N > 35,000) to date was conducted for ASD, and 102 risk genes were identified at a false discovery rate of 0.1. 159 Most of these genes were enriched in excitatory and inhibitory neuronal lineages, supporting the implications of an E/I imbalance in ASD. In another collaborative study with the ASC, postzygotic variants were analyzed in exome sequencing data from ASD trios (N = ~6,000). 160 While the contribution from de novo variants was already established, the study revealed that 7.5% of these variants are actually postzygotic variants, highlighting the importance of postzygotic variants in ASD.
Basic Neuroscience
Modeling psychiatric disorders using patient iPSC‐derived neurons
To date, a large number of studies have been conducted to elucidate the molecular and cellular etiology of psychiatric disorders using brain imaging and postmortem and animal model studies. Given the importance of the use of neurons that carry the genetic information of the patients, findings from studies with iPSC technology are expected to complement those of these existing studies. iPSC‐derived neurons from patients with SZ were first shown to have impaired neural function in 2011. 161 To date, dozens of SZ studies with iPSC‐derived neurons have been reported. 162 A series of these studies have elucidated potential disease‐relevant molecular and cellular phenotypes in patient iPSC‐derived neurons. 162 However, the details of the molecular and cellular etiology of these disorders remain unclear. Psychiatrists in COCORO have gathered detailed patient information, including genetic, cognitive function, personality trait, neurophysiological and brain imaging data, which enables targeted modeling of specific clinical features with patient iPSC‐derived neurons. Given the heterogeneity of psychiatric disorders, to simplify the interpretation of the experimental results, this patient information would be beneficial for selecting patients for iPSC reprogramming.
Although many SZ studies with iPSC technology have been published, 162 there are a few pharmacological studies using iPSC‐derived neurons from patients with SZ. 163 Recently, a pair of monozygotic twins with treatment‐resistant SZ and discordant responses to clozapine, a drug for treatment‐resistant SZ, was recruited by COCORO. Monozygotic twins with treatment‐resistant SZ and discordant responses to clozapine are extremely rare. The estimated incidence of such cases is only approximately 0.13 to 0.33 pairs per million patients with SZ. 164 iPSCs were generated from their lymphoblastoid B‐cell lines and differentiated into excitatory neurons by neurogenin 2 overexpression to analyze the molecular mechanisms underpinning the twins' discordant responses to clozapine. The expression level of genes encoding a cell adhesion molecule in iPSC‐derived neurons was different between these patients, suggesting differential cell adhesion function as a potential candidate for the molecular mechanism of discordant responses to clozapine. 164 In pharmacological studies using iPSC‐derived neurons from patients with BD, lithium treatment rescued the hyperexcitability of iPSC‐derived neurons from lithium responders but not lithium nonresponders. 165 , 166 Using iPSC‐derived neurons from pairs of monozygotic twins with schizoaffective disorder and SZ, lithium treatment rescued the increased density of inhibitory synapses in iPSC‐derived neurons from patients. 167 The findings from these studies clearly show that patients' clinical information is indeed indispensable for not only selecting patients for iPSC reprogramming but also interpreting the results, which contributes to the understanding of the molecular mechanisms behind the interindividual variability in drug response.
iPSC technology is emerging as a promising tool to analyze disease‐associated rare variants with high penetrance. One such strong genetic risk factor is 22q11.2 deletion. 141 Studies using iPSC‐derived neurons from patients with 22q11.2 deletion revealed that the deletion resulted in impaired balance from neurogenic to gliogenic competence, reduced tolerance to ER stress and impaired F‐actin dynamics. 168 , 169
In summary, iPSC technology provides an important approach for not only elucidating the molecular and cellular etiology of psychiatric disorders but also developing biomarkers for diagnosis and treatment efficacy, ultimately leading to molecular and cellular mechanism‐based stratification of patients with psychiatric disorders. One limitation of iPSC technology is that iPSC‐derived neurons do not provide insight into higher brain function, such as emotion and cognition. Comprehensive studies using patients' clinical information, iPSC‐derived neurons from patients and corresponding animal disease models are valuable for understanding the molecular and cellular etiology of psychiatric disorders. 170 , 171
Translational research on neural circuit function
Mental functions are controlled by neural circuits, and it is necessary to consider neural circuit mechanisms in the pathogenesis of psychiatric disorders. The basal ganglia, a subcortical structure, consists of four neuronal nuclei, namely, the striatum/NAc, globus pallidus, subthalamic nucleus, and substantia nigra, which form a loop circuit with the cerebral cortex and thalamus. 172 The pathway from the striatum/NAc to the substantia nigra reticulata (SNr), which is the output nucleus to the thalamus, can be divided into a direct pathway, which projects directly to the SNr, and an indirect pathway, which projects to the SNr via the globus pallidus and subthalamic nucleus. Neural circuit manipulation methods, such as optogenetics and reversible neurotransmission blocking, have been developed to specifically target the direct and indirect pathways of the basal ganglia in mice, and it has been shown that the direct and indirect pathways play different roles in decision making and cognitive learning. 172 , 173 It is assumed that circuit alterations occur in psychiatric pathologies, resulting in abnormalities in cognitive behavior and decision making. 174
Human imaging studies of subcortical structures in COCORO have revealed enlargement and asymmetry in the globus pallidus in SZ patients. 98 To investigate the biological significance of these findings, a neural circuit study of the globus pallidus was conducted as a COCORO basic research project. 175 The ventral pallidum, which is located in the ventral part of the globus pallidus, receives input from the NAc and has been suggested to be involved in cognitive learning and mental functions. 174 It has been reported that a population of neurons in the ventral pallidum expresses the neuropeptide enkephalin. 176 Selective inhibition of enkephalin‐positive neurons in the ventral pallidum by reversible neurotransmission blockade had no effect on reward/avoidance learning. In contrast, selective excitation of enkephalin‐positive neurons in the ventral pallidum by designer receptors exclusively activated by designer drugs (DREADDs) did not affect Pavlovian reward learning but impaired inhibitory avoidance learning. The direct pathway of the NAc is specifically involved in Pavlovian reward learning, while the indirect pathway of the NAc is specifically involved in inhibitory avoidance learning. 172 , 173 This suggests that enkephalin‐positive neurons in the ventral pallidum functions downstream of the indirect pathway of the NAc. Although the neural circuit mechanisms of the ventral pallidum in cognitive learning have been clarified, the role of the ventral pallidum in the pathogenesis of SZ remains unclear. Further neural circuit studies will elucidate the pathogenesis of psychiatric disorders. 174 It is necessary to understand the anatomical and functional characteristics of neural networks at the whole‐brain level. Whole‐brain imaging methods in animals, such as a high‐speed serial‐sectioning imaging system named FAST (block‐face serial microscopy tomography), will accelerate bidirectional translational research between imaging studies in human patients and neurocircuitry studies in animals. 177
Conclusions
Big data analysis with collaborative efforts is necessary for reliable and reproducible biological evidence in psychiatric disorders. Translational research to elucidate the pathologies of psychiatric disorders must be performed based on reliable and reproducible biological evidence in patients. More importantly, clinical, and basic research needs to be performed bidirectionally through forward translation and reverse translation to achieve patient‐centered translational therapeutic research (Fig. 1). We have introduced the activities of COCORO and highlighted the advantages of collaborative large‐scale data analyses for testing the reproducibility and robustness of the findings. COCORO is always open for collaboration using a large data set to contribute to the elucidation of the pathological basis of psychiatric disorders and overcoming their obstacles.
Disclosure statement
NO received grants from Dainippon‐Sumitomo, Eisai, Otsuka, Shionogi, Mochida, Nihon Medi‐Physics, KAITEKI, Takeda, Tanabe‐Mitsubishi, Eli Lilly and Daiichi Sankyo. NO received honorarium from Dainippon‐Sumitomo, Eisai, Otsuka, KAITEKI, Mochida, Takeda, Meiji Seika Pharma, EA Pharma, Pfizer, MSD and Lundbeck Japan. K.K. received grants from Eli Lily, MSD, Astellas, Takeda, Dainippon‐Sumitomo, Novardis, Tanabe‐Mitsubishi, Otsuka, Eisai, Shionogi and Ono Pharma. KK received honorarium from Astellas, Takeda, Dainippon‐Sumitomo, Kyowa, Otsuka, Fuji‐film‐Wako, Janssen, Yoshitomi and Meiji Seika Pharma.
Acknowledgments
Funding sources of this study are as follows: Japan Agency for Medical Research and Development (AMED) under grant number JP20dm0207069 (TO); Grant‐in‐Aid for Scientific Research B: JP21H02851 and JP19H03579, Grant‐in‐Aid for Scientific Research C: JP18K07604 and JP19H03579, the Fund for the Promotion of Joint International Research (Fostering Joint International Research B), JP20KK0193 from the Japan Society for the Promotion of Science (JSPS), and SIRS Research Fund Award from the Schizophrenia International Research Society (YH); JSPS KAKENHI grant number 21K12153 (KN); KAKENHI grant numbers 21K07543 and 21H00194 (IK); JSPS grant number 18K07550 (TT); Grant‐in‐Aid for Young Scientists (18K15375) and a Grant‐in‐Aid for Scientific Research (B) (21H02813) from JSPS, research grants from AMED, investigator‐initiated clinical study grants from TEIJIN PHARMA LIMITED and Inter Reha Co., Ltd., research grants from the Japan Health Foundation, Meiji Yasuda Mental Health Foundation, Mitsui Life Social Welfare Foundation, Takeda Science Foundation, SENSHIN Medical Research Foundation, Health Science Center Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Taiju Life Social Welfare Foundation, and Daiichi Sankyo Scholarship Donation Program, equipment‐in‐kind support for an investigator‐initiated study from Magventure Inc., Inter Reha Co., Ltd., BrainBox Ltd., and Miyuki Giken Co., Ltd. (YN); AMED under grant numbers (JP21uk1024002, JP21dm0207069, and JP20lm0203007j0004) and JSPS KAKENHI (20K06920) (KM); KAKENHI JP18H02542, Takeda Life Science Research Foundation (TH); JSPS KAKENHI grant numbers JP16H06280, JP16H06395, JP16H06399, JP16K21720, 20H03596 and 21H00451, AMED under grant numbers JP18dm0207004, JP21dm0307001, JP21dm0307004, and JP21dm0207069, UTokyo Center for Integrative Science of Human Behavior (CiSHuB), and the International Research Center for Neurointelligence (WPI‐IRCN) at The University of Tokyo Institutes for Advanced Study (UTIAS) (KK); Brain/MINDS and beyond studies (grant number JP20dm0307002) from the Japan Agency for Medical Research and Development, JSPS Grant‐in‐Aid for Specially Promoted Research JP19H05467, JSPS Grant‐in‐Aid for Scientific Research (B) Generative Research Field (JP18KT0022), JSPS Grant‐in‐Aid for Scientific Research (B) JP20H03611, SENSHIN Medical Research Foundation, AMED under grant number JP21dk0307103, AMED under grant number JP21uk1024002, Intramural Research Grant (3–1) for Neurological and Psychiatric Disorders of NCNP (RH).
Contributor Information
Toshiaki Onitsuka, Email: onitsuka.toshiaki.939@m.kyushu-u.ac.jp.
Ryota Hashimoto, Email: ryotahashimoto55@ncnp.go.jp.
REFERENCES
- 1. Takaesu Y, Watanabe K, Numata S et al. Improvement of psychiatrists' clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the 'Effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)' project: A nationwide dissemination, education, and evaluation study. Psychiatry Clin. Neurosci. 2019; 73: 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egan MF, Goldberg TE, Kolachana BS et al. Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001; 98: 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodman SN, Fanelli D, Ioannidis JP. What does research reproducibility mean? Sci. Transl. Med. 2016; 8: 341ps12. [DOI] [PubMed] [Google Scholar]
- 4. Koshiyama D, Miura K, Nemoto K et al. Neuroimaging studies within cognitive genetics collaborative research organization aiming to replicate and extend works of ENIGMA. Hum. Brain Mapp. 2020. 10.1002/hbm.25040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemoto K, Shimokawa T, Fukunaga M et al. Differentiation of schizophrenia using structural MRI with consideration of scanner differences: A real‐world multisite study. Psychiatry Clin. Neurosci. 2020; 74: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasuda Y, Okada N, Nemoto K et al. Brain morphological and functional features in cognitive subgroups of schizophrenia. Psychiatry Clin. Neurosci. 2020; 74: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sumiyoshi C, Fujino H, Sumiyoshi T et al. Usefulness of the Wechsler intelligence scale short form for assessing functional outcomes in patients with schizophrenia. Psychiatry Res. 2016; 245: 371–378. [DOI] [PubMed] [Google Scholar]
- 8. Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin. Neurosci. 2006; 60: 332–339. [DOI] [PubMed] [Google Scholar]
- 9. Hidese S, Matsuo J, Ishida I et al. Association between lower estimated premorbid intelligence quotient and smoking behavior in patients with schizophrenia. Schizophr Res. Cogn. 2019; 15: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters H, Zdravkovic M, João Costa M et al. Twelve tips for enhancing student engagement. Med. Teach. 2019; 41: 632–637. [DOI] [PubMed] [Google Scholar]
- 11. Ommering BWC, Wijnen‐Meijer M, Dolmans D, Dekker FW, van Blankenstein FM. Promoting positive perceptions of and motivation for research among undergraduate medical students to stimulate future research involvement: A grounded theory study. BMC Med. Educ. 2020; 20: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson PM, Jahanshad N, Ching CRK et al. ENIGMA and global neuroscience: A decade of large‐scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry 2020; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marder SR, Cannon TD. Schizophrenia. N. Engl. J. Med. 2019; 381: 1753–1761. [DOI] [PubMed] [Google Scholar]
- 14. Fleischhacker WW, Arango C, Arteel P et al. Schizophrenia–time to commit to policy change. Schizophr. Bull. 2014; 40: S165–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kern RS, Glynn SM, Horan WP, Marder SR. Psychosocial treatments to promote functional recovery in schizophrenia. Schizophr. Bull. 2009; 35: 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vita A, Barlati S. Recovery from schizophrenia: Is it possible? Curr. Opin. Psychiatry 2018; 31: 246–255. [DOI] [PubMed] [Google Scholar]
- 17. Hasin DS, Sarvet AL, Meyers JL et al. Epidemiology of adult DSM‐5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 2018; 75: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawakami N, Takeshima T, Ono Y et al. Twelve‐month prevalence, severity, and treatment of common mental disorders in communities in Japan: Preliminary finding from the world mental health Japan survey 2002‐2003. Psychiatry Clin. Neurosci. 2005; 59: 441–452. [DOI] [PubMed] [Google Scholar]
- 19. McIntyre RS, Berk M, Brietzke E et al. Bipolar disorders. Lancet 2020; 396: 1841–1856. [DOI] [PubMed] [Google Scholar]
- 20. Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther. Adv. Psychopharmacol. 2018; 8: 251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baldessarini RJ, Bolzani L, Cruz N et al. Onset‐age of bipolar disorders at six international sites. J. Affect. Disord. 2010; 121: 143–146. [DOI] [PubMed] [Google Scholar]
- 22. Kessing LV, Gonzalez‐Pinto A, Fagiolini A et al. DSM‐5 and ICD‐11 criteria for bipolar disorder: Implications for the prevalence of bipolar disorder and validity of the diagnosis ‐ a narrative review from the ECNP bipolar disorders network. Eur. Neuropsychopharmacol. 2021; 47: 54–61. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez V, Alameda L, Trotta G et al. Environmental risk factors in bipolar disorder and psychotic depression: A systematic review and meta‐analysis of prospective studies. Schizophr. Bull. 2021; 47: 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agerbo E, Trabjerg BB, Borglum AD et al. Risk of early‐onset depression associated with polygenic liability, parental psychiatric history, and socioeconomic status. JAMA Psychiat. 2021; 78: 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dunlop BW. Evidence‐based applications of combination psychotherapy and pharmacotherapy for depression. Focus (Am Psychiatr Publ) 2016; 14: 156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miklowitz DJ, Efthimiou O, Furukawa TA et al. Adjunctive psychotherapy for bipolar disorder: A systematic review and component network meta‐analysis. JAMA Psychiat. 2021; 78: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montgomery SA, Schatzberg AF, Guelfi JD et al. Pharmacotherapy of depression and mixed states in bipolar disorder. J. Affect. Disord. 2000; 59: S39–S56. [DOI] [PubMed] [Google Scholar]
- 28. Matsuo J, Hori H, Ishida I et al. Performance on the Wechsler adult intelligence scale (WAIS) in Japanese patients with bipolar and major depressive disorders in euthymic and depressed states. Psychiatry Clin. Neurosci. 2020; 75: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pini S, Abelli M, Costa B et al. Separation anxiety and measures of suicide risk among patients with mood and anxiety disorders. J. Clin. Psychiatry 2021; 82: 20m13299. [DOI] [PubMed] [Google Scholar]
- 30. Tondo L, Vazquez GH, Baldessarini RJ. Prevention of suicidal behavior in bipolar disorder. Bipolar Disord. 2021; 23: 14–23. [DOI] [PubMed] [Google Scholar]
- 31. Sanchack KE, Thomas CA. Autism Spectrum disorder: Primary care principles. Am. Fam. Physician 2016; 94: 972–979. [PubMed] [Google Scholar]
- 32. Christensen DL, Braun KVN, Baio J et al. Prevalence and characteristics of autism Spectrum disorder among children aged 8 years ‐ autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill. Summ. 2018; 65: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma SR, Gonda X, Tarazi FI. Autism Spectrum disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018; 190: 91–104. [DOI] [PubMed] [Google Scholar]
- 34. Reyes NM, Pickard K, Reaven J. Emotion regulation: A treatment target for autism spectrum disorder. Bull. Menninger Clin. 2019; 83: 205–234. [DOI] [PubMed] [Google Scholar]
- 35. Nuechterlein KH, Green MF, Kern RS et al. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 2008; 165: 203–213. [DOI] [PubMed] [Google Scholar]
- 36. Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: A meta‐analysis. Acta Psychiatr. Scand. Suppl. 2007; 116: 17–26. [DOI] [PubMed] [Google Scholar]
- 37. Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur. J. Pharmacol. 2010; 626: 83–86. [DOI] [PubMed] [Google Scholar]
- 38. Zwick GP. Neuropsychological assessment in autism spectrum disorder and related conditions. Dialogues Clin. Neurosci. 2017; 19: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nuechterlein KH, Subotnik KL, Green MF et al. Neurocognitive predictors of work outcome in recent‐onset schizophrenia. Schizophr. Bull. 2011; 37: S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duarte W, Becerra R, Cruise K. The relationship between neurocognitive functioning and occupational functioning in bipolar disorder: A literature review. Eur. J. Psychol. 2016; 12: 659–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shimizu Y, Kitagawa N, Mitsui N et al. Neurocognitive impairments and quality of life in unemployed patients with remitted major depressive disorder. Psychiatry Res. 2013; 210: 913–918. [DOI] [PubMed] [Google Scholar]
- 42. Harvey PD, Balzer AM, Kotwicki RJ. Training engagement, baseline cognitive functioning, and cognitive gains with computerized cognitive training: A cross‐diagnostic study. Schizophr. Res. Cogn. 2020; 19: 100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujino H, Sumiyoshi C, Yasuda Y et al. Estimated cognitive decline in patients with schizophrenia: A multicenter study. Psychiatry Clin. Neurosci. 2017; 71: 294–300. [DOI] [PubMed] [Google Scholar]
- 44. Sumiyoshi C, Fujino H, Yamamori H et al. Predicting work outcome in patients with schizophrenia: Influence of IQ decline. Schizophr. Res. 2018; 201: 172–179. [DOI] [PubMed] [Google Scholar]
- 45. Koshiyama D, Fukunaga M, Okada N et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci. Rep. 2018; 8: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koshiyama D, Fukunaga M, Okada N et al. Subcortical association with memory performance in schizophrenia: A structural magnetic resonance imaging study. Transl. Psychiatry 2018; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fervaha G, Zakzanis KK, Foussias G, Graff‐Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiat. 2014; 71: 1058–1065. [DOI] [PubMed] [Google Scholar]
- 48. Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron 2015; 86: 646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Calkins ME, Dobie DJ, Cadenhead KS et al. The consortium on the genetics of Endophenotypes in schizophrenia: Model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr. Bull. 2007; 33: 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tamminga CA, Ivleva EI, Keshavan MS et al. Clinical phenotypes of psychosis in the bipolar‐schizophrenia network on intermediate phenotypes (B‐SNIP). Am. J. Psychiatry 2013; 170: 1263–1274. [DOI] [PubMed] [Google Scholar]
- 51. Hashimoto R. Do eye movement abnormalities in schizophrenia cause praecox Gefühl? Psychiatry Clin. Neurosci. 2021; 75: 79–80. [DOI] [PubMed] [Google Scholar]
- 52. Wolf A, Ueda K, Hirano Y. Recent updates of eye movement abnormalities in patients with schizophrenia: A scoping review. Psychiatry Clin. Neurosci. 2021; 75: 82–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arolt V, Teichert HM, Steege D, Lencer R, Heide W. Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biol. Psychiatry 1998; 44: 448–458. [DOI] [PubMed] [Google Scholar]
- 54. Matsushima E, Kojima T, Ohta K et al. Exploratory eye movement dysfunctions in patients with schizophrenia: Possibility as a discriminator for schizophrenia. J. Psychiatr. Res. 1998; 32: 289–295. [DOI] [PubMed] [Google Scholar]
- 55. Campana A, Duci A, Gambini O, Scarone S. An artificial neural network that uses eye‐tracking performance to identify patients with schizophrenia. Schizophr. Bull. 1999; 25: 789–799. [DOI] [PubMed] [Google Scholar]
- 56. Kojima T, Matsushima E, Ohta K et al. Stability of exploratory eye movements as a marker of schizophrenia–a WHO multi‐center study. World Health Organization. Schizophr. Res. 2001; 52: 203–213. [DOI] [PubMed] [Google Scholar]
- 57. Suzuki M, Takahashi S, Matsushima E et al. Exploratory eye movement dysfunction as a discriminator for schizophrenia: A large sample study using a newly developed digital computerized system. Eur. Arch. Psychiatry Clin. Neurosci. 2009; 259: 186–194. [DOI] [PubMed] [Google Scholar]
- 58. Benson PJ, Beedie SA, Shephard E, Giegling I, Rujescu D, St Clair D. Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biol. Psychiatry 2012; 72: 716–724. [DOI] [PubMed] [Google Scholar]
- 59. Miura K, Hashimoto R, Fujimoto M et al. An integrated eye movement score as a neurophysiological marker of schizophrenia. Schizophr. Res. 2014; 160: 228–229. [DOI] [PubMed] [Google Scholar]
- 60. Morita K, Miura K, Fujimoto M et al. Eye movement as a biomarker of schizophrenia: Using an integrated eye movement score. Psychiatry Clin. Neurosci. 2017; 71: 104–114. [DOI] [PubMed] [Google Scholar]
- 61. Morita K, Miura K, Fujimoto M et al. Abnormalities of eye movement are associated with work hours in schizophrenia. Schizophr. Res. 2018; 202: 420–422. [DOI] [PubMed] [Google Scholar]
- 62. Morita K, Miura K, Fujimoto M et al. Eye movement abnormalities and their association with cognitive impairments in schizophrenia. Schizophr. Res. 2019; 209: 255–262. [DOI] [PubMed] [Google Scholar]
- 63. Kikuchi M, Miura K, Morita K et al. Genome‐wide association analysis of eye movement dysfunction in schizophrenia. Sci. Rep. 2018; 8: 12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morita K, Miura K, Fujimoto M et al. Eye‐movement characteristics of schizophrenia and their association with cortical thickness. Psychiatry Clin. Neurosci. 2019; 73: 508–509. [DOI] [PubMed] [Google Scholar]
- 65. Morita K, Miura K, Kasai K, Hashimoto R. Eye movement characteristics in schizophrenia: A recent update with clinical implications. Neuropsychopharmacol. Rep. 2020; 40: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shiino T, Miura K, Fujimoto M et al. Comparison of eye movements in schizophrenia and autism spectrum disorder. Neuropsychopharmacol. Rep. 2020; 40: 92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Okada KI, Miura K, Fujimoto M et al. Impaired inhibition of return during free‐viewing behaviour in patients with schizophrenia. Sci. Rep. 2021; 11: 3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takahashi J, Hirano Y, Miura K et al. Eye movement abnormalities in major depressive disorder. Front. Psych. 2021; 12: 673443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Takahashi J, Miura K, Morita K et al. Effects of age and sex on eye movement characteristics. Neuropsychopharmacol. Rep. 2021; 41: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010; 11: 100–113. [DOI] [PubMed] [Google Scholar]
- 71. Hironaga N, Takei Y, Mitsudo T, Kimura T, Hirano Y. Prospects for future methodological development and application of magnetoencephalography devices in psychiatry. Front. Psych. 2020; 11: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Edgar JC. Identifying electrophysiological markers of autism spectrum disorder and schizophrenia against a backdrop of normal brain development. Psychiatry Clin. Neurosci. 2020; 74: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: Integrating clinical and basic features. Nat. Rev. Neurosci. 2015; 16: 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hamilton HK, Boos AK, Mathalon DH. Electroencephalography and event‐related potential biomarkers in individuals at clinical high risk for psychosis. Biol. Psychiatry 2020; 88: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kirihara K, Tada M, Koshiyama D et al. A predictive coding perspective on mismatch negativity impairment in schizophrenia. Front. Psych. 2020; 11: 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Smith R, Badcock P, Friston KJ. Recent advances in the application of predictive coding and active inference models within clinical neuroscience. Psychiatry Clin. Neurosci. 2021; 75: 3–13. [DOI] [PubMed] [Google Scholar]
- 77. Erickson MA, Ruffle A, Gold JM. A meta‐analysis of mismatch negativity in schizophrenia: From clinical risk to disease specificity and progression. Biol. Psychiatry 2016; 79: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Avissar M, Xie S, Vail B, Lopez‐Calderon J, Wang Y, Javitt DC. Meta‐analysis of mismatch negativity to simple versus complex deviants in schizophrenia. Schizophr. Res. 2018; 191: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Uno Y, Coyle JT. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019; 73: 204–215. [DOI] [PubMed] [Google Scholar]
- 80. Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012; 35: 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Thuné H, Recasens M, Uhlhaas PJ. The 40‐Hz auditory steady‐state response in patients with schizophrenia: A meta‐analysis. JAMA Psychiat. 2016; 73: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 82. Hirano Y, Nakamura I, Tamura S, Onitsuka T. Long‐term test‐retest reliability of auditory gamma oscillations between different clinical EEG systems. Front. Psych. 2020; 11: 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kwon JS, O'Donnell BF, Wallenstein GV et al. Gamma frequency‐range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry 1999; 56: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma‐band auditory steady‐state responses are impaired in first episode psychosis. Biol. Psychiatry 2008; 64: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous gamma activity in schizophrenia. JAMA Psychiat. 2015; 72: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tada M, Nagai T, Kirihara K et al. Differential alterations of auditory gamma oscillatory responses between pre‐onset high‐risk individuals and first‐episode schizophrenia. Cereb. Cortex 2016; 26: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 87. Hirano Y, Oribe N, Onitsuka T et al. Auditory cortex volume and gamma oscillation abnormalities in schizophrenia. Clin. EEG Neurosci. 2020; 51: 244–251. [DOI] [PubMed] [Google Scholar]
- 88. Oribe N, Hirano Y, Del Re E et al. Progressive reduction of auditory evoked gamma in first episode schizophrenia but not clinical high risk individuals. Schizophr. Res. 2019; 208: 145–152. [DOI] [PubMed] [Google Scholar]
- 89. Krystal JH, Anticevic A, Yang GJ et al. Impaired tuning of neural ensembles and the pathophysiology of schizophrenia: A translational and computational neuroscience perspective. Biol. Psychiatry 2017; 81: 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Grent‐'t‐Jong T, Gross J, Goense J et al. Resting‐state gamma‐band power alterations in schizophrenia reveal E/I‐balance abnormalities across illness‐stages. Elife 2018; 7: e37799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McNally JM, Aguilar DD, Katsuki F et al. Optogenetic manipulation of an ascending arousal system tunes cortical broadband gamma power and reveals functional deficits relevant to schizophrenia. Mol. Psychiatry 2020; 87: S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guyon N, Zacharias LR, Fermino de Oliveira E et al. Network asynchrony underlying increased broadband gamma power. J. Neurosci. 2021; 41: 2944–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Clementz BA, Sweeney JA, Hamm JP et al. Identification of distinct psychosis biotypes using brain‐based biomarkers. Am. J. Psychiatry 2016; 173: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Oribe N, Hirano Y, Del Re E et al. Longitudinal evaluation of visual P300 amplitude in clinical high‐risk subjects: An event‐related potential study. Psychiatry Clin. Neurosci. 2020; 74: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van Erp TG, Hibar DP, Rasmussen JM et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 2016; 21: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van Erp TGM, Walton E, Hibar DP et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. Psychiatry 2018; 84: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Takahashi T, Suzuki M. Brain morphologic changes in early stages of psychosis: Implications for clinical application and early intervention. Psychiatry Clin. Neurosci. 2018; 72: 556–571. [DOI] [PubMed] [Google Scholar]
- 98. Okada N, Fukunaga M, Yamashita F et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 2016; 21: 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hashimoto N, Ito YM, Okada N et al. The effect of duration of illness and antipsychotics on subcortical volumes in schizophrenia: Analysis of 778 subjects. Neuroimage Clin. 2018; 17: 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Takahashi T, Tsugawa S, Nakajima S et al. Thalamic and striato‐pallidal volumes in schizophrenia patients and individuals at risk for psychosis: A multi‐atlas segmentation study. Schizophr. Res. 2020. [DOI] [PubMed] [Google Scholar]
- 101. Hashimoto R, Ikeda M, Yamashita F et al. Common variants at 1p36 are associated with superior frontal gyrus volume. Transl. Psychiatry 2014; 4: e472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Isomura S, Hashimoto R, Nakamura M et al. Altered sulcogyral patterns of orbitofrontal cortex in a large cohort of patients with schizophrenia. NPJ Schizophr. 2017; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kelly S, Jahanshad N, Zalesky A et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Mol. Psychiatry 2018; 23: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Favre P, Pauling M, Stout J et al. Widespread white matter microstructural abnormalities in bipolar disorder: Evidence from mega‐ and meta‐analyses across 3033 individuals. Neuropsychopharmacology 2019; 44: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van Velzen LS, Kelly S, Isaev D et al. White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020; 25: 1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Villalón‐Reina JE, Martínez K, Qu X et al. Altered white matter microstructure in 22q11.2 deletion syndrome: A multisite diffusion tensor imaging study. Mol. Psychiatry 2020; 25: 2818–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hoogman M, van Rooij D, Klein M et al. Consortium neuroscience of attention deficit/hyperactivity disorder and autism spectrum disorder: The ENIGMA adventure. Hum. Brain Mapp. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Koshiyama D, Fukunaga M, Okada N et al. White matter microstructural alterations across four major psychiatric disorders: Mega‐analysis study in 2937 individuals. Mol. Psychiatry 2020; 25: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Koshiyama D, Fukunaga M, Okada N et al. Role of frontal white matter and corpus callosum on social function in schizophrenia. Schizophr. Res. 2018; 202: 180–187. [DOI] [PubMed] [Google Scholar]
- 110. Kochunov P, Coyle TR, Rowland LM et al. Association of White Matter with Core Cognitive Deficits in patients with schizophrenia. JAMA Psychiat. 2017; 74: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Barkhof F, Haller S, Rombouts SA. Resting‐state functional MR imaging: A new window to the brain. Radiology 2014; 272: 29–49. [DOI] [PubMed] [Google Scholar]
- 112. Brandl F, Avram M, Weise B et al. Specific substantial Dysconnectivity in schizophrenia: A Transdiagnostic multimodal meta‐analysis of resting‐state functional and structural magnetic resonance imaging studies. Biol. Psychiatry 2019; 85: 573–583. [DOI] [PubMed] [Google Scholar]
- 113. Adhikari BM, Dukart J, Hipp JF et al. Effects of ketamine and midazolam on resting state connectivity and comparison with ENIGMA connectivity deficit patterns in schizophrenia. Hum. Brain Mapp. 2020; 41: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li T, Wang Q, Zhang J et al. Brain‐wide analysis of functional connectivity in first‐episode and chronic stages of schizophrenia. Schizophr. Bull. 2017; 43: 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sisodiya SM, Whelan CD, Hatton SN et al. The ENIGMA‐epilepsy working group: Mapping disease from large data sets. Hum. Brain Mapp. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Guell X, Anteraper SA, Ghosh SS, Gabrieli JDE, Schmahmann JD. Neurodevelopmental and psychiatric symptoms in patients with a cyst compressing the cerebellum: An ongoing Enigma. Cerebellum 2020; 19: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Buchsbaum MS. The frontal lobes, basal ganglia, and temporal lobes as sites for schizophrenia. Schizophr. Bull. 1990; 16: 379–389. [DOI] [PubMed] [Google Scholar]
- 118. Schroder J, Buchsbaum MS, Siegel BV et al. Cerebral metabolic activity correlates of subsyndromes in chronic schizophrenia. Schizophr. Res. 1996; 19: 41–53. [DOI] [PubMed] [Google Scholar]
- 119. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br. J. Psychiatry 1992; 160: 179–186. [DOI] [PubMed] [Google Scholar]
- 120. Andreasen NC, O'Leary DS, Cizadlo T et al. Schizophrenia and cognitive dysmetria: A positron‐emission tomography study of dysfunctional prefrontal‐thalamic‐cerebellar circuitry. Proc. Natl. Acad. Sci. U. S. A. 1996; 93: 9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET Study. Brain 1992; 115: 367–382. [DOI] [PubMed] [Google Scholar]
- 122. Nord M, Farde L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci. Ther. 2011; 17: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 2020; 7: 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Plavén‐Sigray P, Matheson GJ, Collste K et al. Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: A meta‐analysis using individual participant data. Biol. Psychiatry 2018; 84: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shi Z, Fu LP, Zhang N et al. Amyloid PET in dementia syndromes: A Chinese multicenter study. J. Nucl. Med. 2020; 61: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 126. Zarate CA Jr, Manji HK. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp. Neurol. 2008; 211: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Miyazaki T, Nakajima W, Hatano M et al. Visualization of AMPA receptors in living human brain with positron emission tomography. Nat. Med. 2020; 26: 281–288. [DOI] [PubMed] [Google Scholar]
- 128. Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: A meta‐analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiat. 2016; 73: 665–674. [DOI] [PubMed] [Google Scholar]
- 129. Kumar V, Vajawat B, Rao NP. Frontal GABA in schizophrenia: A meta‐analysis of (1)H‐MRS studies. World J. Biol. Psychiatry 2020; 22: 1–13. [DOI] [PubMed] [Google Scholar]
- 130. Kegeles LS, Mao X, Stanford AD et al. Elevated prefrontal cortex γ‐aminobutyric acid and glutamate‐glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2012; 69: 449–459. [DOI] [PubMed] [Google Scholar]
- 131. Rowland LM, Krause BW, Wijtenburg SA et al. Medial frontal GABA is lower in older schizophrenia: A MEGA‐PRESS with macromolecule suppression study. Mol. Psychiatry 2016; 21: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marenco S, Meyer C, Kuo S et al. Prefrontal GABA levels measured with magnetic resonance spectroscopy in patients with psychosis and unaffected siblings. Am. J. Psychiatry 2016; 173: 527–534. [DOI] [PubMed] [Google Scholar]
- 133. Thakkar KN, Rösler L, Wijnen JP et al. 7T proton magnetic resonance spectroscopy of gamma‐aminobutyric acid, glutamate, and glutamine reveals altered concentrations in patients with schizophrenia and healthy siblings. Biol. Psychiatry 2017; 81: 525–535. [DOI] [PubMed] [Google Scholar]
- 134. Egerton A, Broberg BV, Van Haren N et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: A multicentre (1)H‐MRS study (OPTiMiSE). Mol. Psychiatry 2018; 23: 2145–2155. [DOI] [PubMed] [Google Scholar]
- 135. Dempster K, Jeon P, MacKinley M, Williamson P, Théberge J, Palaniyappan L. Early treatment response in first episode psychosis: A 7‐T magnetic resonance spectroscopic study of glutathione and glutamate. Mol. Psychiatry 2020; 25: 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, Kendler KS. Psychiatric genetics and the structure of psychopathology. Mol. Psychiatry 2019; 24: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gratten J, Wray NR, Keller MC, Visscher PM. Large‐scale genomics unveils the genetic architecture of psychiatric disorders. Nat. Neurosci. 2014; 17: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat. Rev. Genet. 2012; 13: 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nakatochi M, Kushima I, Ozaki N. Implications of germline copy‐number variations in psychiatric disorders: Review of large‐scale genetic studies. J. Hum. Genet. 2021; 66: 25–37. [DOI] [PubMed] [Google Scholar]
- 140. Doherty JL, Owen MJ. Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Med. 2014; 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kushima I, Aleksic B, Nakatochi M et al. Comparative analyses of copy‐number variation in autism Spectrum disorder and schizophrenia reveal etiological overlap and biological insights. Cell Rep. 2018; 24: 2838–2856. [DOI] [PubMed] [Google Scholar]
- 142. Kushima I, Aleksic B, Nakatochi M et al. High‐resolution copy number variation analysis of schizophrenia in Japan. Mol. Psychiatry 2017; 22: 430–440. [DOI] [PubMed] [Google Scholar]
- 143. Baba M, Yokoyama K, Seiriki K et al. Psychiatric‐disorder‐related behavioral phenotypes and cortical hyperactivity in a mouse model of 3q29 deletion syndrome. Neuropsychopharmacology 2019; 44: 2125–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sekiguchi M, Sobue A, Kushima I et al. ARHGAP10, which encodes rho GTPase‐activating protein 10, is a novel gene for schizophrenia risk. Transl. Psychiatry 2020; 10: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sawahata M, Mori D, Arioka Y et al. Generation and analysis of novel Reln‐deleted mouse model corresponding to exonic Reln deletion in schizophrenia. Psychiatry Clin. Neurosci. 2020; 74: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kimura H, Mori D, Aleksic B, Ozaki N. Elucidation of molecular pathogenesis and drug development for psychiatric disorders from rare disease‐susceptibility variants. Neurosci. Res. 2020; 170: 24–31. [DOI] [PubMed] [Google Scholar]
- 147. Torii Y, Iritani S, Marui T et al. Morphological alteration of myelin‐oligodendrocytes in a schizophrenic patient with 22q11.2 deletion syndrome: An autopsy study. Schizophr. Res. 2020; 223: 353–355. [DOI] [PubMed] [Google Scholar]
- 148. Ikeda M, Takahashi A, Kamatani Y et al. Genome‐wide association study detected novel susceptibility genes for schizophrenia and shared trans‐populations/diseases genetic effect. Schizophr. Bull. 2019; 45: 824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ikeda M, Takahashi A, Kamatani Y et al. A genome‐wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol. Psychiatry 2018; 23: 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Saito T, Ikeda M, Mushiroda T et al. Pharmacogenomic study of clozapine‐induced agranulocytosis/Granulocytopenia in a Japanese population. Biol. Psychiatry 2016; 80: 636–642. [DOI] [PubMed] [Google Scholar]
- 151. Kuehner JN, Bruggeman EC, Wen Z, Yao B. Epigenetic regulations in neuropsychiatric disorders. Front. Genet. 2019; 10: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kang HJ, Kim JM, Stewart R et al. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013; 44: 23–28. [DOI] [PubMed] [Google Scholar]
- 153. Ikegame T, Bundo M, Okada N et al. Promoter activity‐based case‐control association study on SLC6A4 highlighting Hypermethylation and altered amygdala volume in male patients with schizophrenia. Schizophr. Bull. 2020; 46: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008; 11: 851–876. [DOI] [PubMed] [Google Scholar]
- 155. Arai M, Miyashita M, Kobori A, Toriumi K, Horiuchi Y, Itokawa M. Carbonyl stress and schizophrenia. Psychiatry Clin. Neurosci. 2014; 68: 655–665. [DOI] [PubMed] [Google Scholar]
- 156. Arai M, Yuzawa H, Nohara I et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch. Gen. Psychiatry 2010; 67: 589–597. [DOI] [PubMed] [Google Scholar]
- 157. Miyashita M, Watanabe T, Ichikawa T et al. The regulation of soluble receptor for AGEs contributes to carbonyl stress in schizophrenia. Biochem. Biophys. Res. Commun. 2016; 479: 447–452. [DOI] [PubMed] [Google Scholar]
- 158. Setoyama D, Kato TA, Hashimoto R et al. Plasma metabolites predict severity of depression and suicidal ideation in psychiatric patients‐a multicenter pilot analysis. PLoS One 2016; 11: e0165267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Satterstrom FK, Kosmicki JA, Wang JB et al. Large‐scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 2020; 180: 568–584.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lim ET, Uddin M, De Rubeis S et al. Rates, distribution and implications of postzygotic mosaic mutations in autism spectrum disorder. Nat. Neurosci. 2017; 20: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Brennand KJ, Simone A, Jou J et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011; 473: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Balan S, Toyoshima M, Yoshikawa T. Contribution of induced pluripotent stem cell technologies to the understanding of cellular phenotypes in schizophrenia. Neurobiol. Dis. 2019; 131: 104162. [DOI] [PubMed] [Google Scholar]
- 163. Collo G, Mucci A, Giordano GM, Merlo Pich E, Galderisi S. Negative symptoms of schizophrenia and dopaminergic transmission: Translational models and perspectives opened by iPSC techniques. Front. Neurosci. 2020; 14: 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nakazawa T, Kikuchi M, Ishikawa M et al. Differential gene expression profiles in neurons generated from lymphoblastoid B‐cell line‐derived iPS cells from monozygotic twin cases with treatment‐resistant schizophrenia and discordant responses to clozapine. Schizophr. Res. 2017; 181: 75–82. [DOI] [PubMed] [Google Scholar]
- 165. Mertens J, Wang QW, Kim Y et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 2015; 527: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Stern S, Santos R, Marchetto MC et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub‐populations of neurons, predicting the patients' responsiveness to lithium. Mol. Psychiatry 2018; 23: 1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sawada T, Chater TE, Sasagawa Y et al. Developmental excitation‐inhibition imbalance underlying psychoses revealed by single‐cell analyses of discordant twins‐derived cerebral organoids. Mol. Psychiatry 2020; 25: 2695–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Toyoshima M, Akamatsu W, Okada Y et al. Analysis of induced pluripotent stem cells carrying 22q11.2 deletion. Transl. Psychiatry 2016; 6: e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Arioka Y, Shishido E, Kushima I et al. Chromosome 22q11.2 deletion causes PERK‐dependent vulnerability in dopaminergic neurons. EBioMedicine 2021; 63: 103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nakazawa T, Hashimoto R, Sakoori K et al. Emerging roles of ARHGAP33 in intracellular trafficking of TrkB and pathophysiology of neuropsychiatric disorders. Nat. Commun. 2016; 7: 10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Matsumura K, Seiriki K, Okada S et al. Pathogenic POGZ mutation causes impaired cortical development and reversible autism‐like phenotypes. Nat. Commun. 2020; 11: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Macpherson T, Morita M, Hikida T. Striatal direct and indirect pathways control decision‐making behavior. Front. Psychol. 2014; 5: 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Macpherson T, Hikida T. Nucleus Accumbens dopamine D1‐receptor‐expressing neurons control the Acquisition of Sign‐Tracking to conditioned cues in mice. Front. Neurosci. 2018; 12: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Macpherson T, Hikida T. Role of basal ganglia neurocircuitry in the pathology of psychiatric disorders. Psychiatry Clin. Neurosci. 2019; 73: 289–301. [DOI] [PubMed] [Google Scholar]
- 175. Macpherson T, Mizoguchi H, Yamanaka A, Hikida T. Preproenkephalin‐expressing ventral pallidal neurons control inhibitory avoidance learning. Neurochem. Int. 2019; 126: 11–18. [DOI] [PubMed] [Google Scholar]
- 176. Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 1993; 57: 1047–1060. [DOI] [PubMed] [Google Scholar]
- 177. Seiriki K, Kasai A, Hashimoto T et al. High‐speed and scalable whole‐brain imaging in rodents and primates. Neuron 2017; 94: 1085–1100 e6. [DOI] [PubMed] [Google Scholar]


