Abstract
Background
Infectious disease has a significant impact on livestock production. Availability of alternatives to antibiotics to prevent and treat disease is required to reduce reliance on antibiotics while not impacting animal welfare. Innate immune stimulants, such as mycobacterium cell wall fractions (MCWF), are used as alternatives to antibiotics for the treatment and prevention of infectious disease in a number of species including cattle, horses and dogs. This study aimed to evaluate the safety of Amplimune®, an MCWF‐based immune stimulant, for weaner Angus cattle.
Methods
On day −1 and 0, sixty mixed‐sex Angus weaner cattle were transported for 6 h before being inducted and housed in a large single pen, simulating feedlot induction conditions. The cattle were assigned to one of six treatment groups (n = 10 per group): 2 mL Amplimune intramuscularly (2IM); 2 mL Amplimune subcutaneously (2SC); 5 mL Amplimune intramuscularly (5IM); 5 mL Amplimune subcutaneously (5SC); 5 mL saline intramuscularly (SalIM) and 5 mL saline subcutaneously (SalSC) on day 0 following transportation. Body temperature, body weight, concentrations of circulating pro‐inflammatory cytokines (TNFα, IL‐1β, IL‐6 and IL‐12) and haematology parameters were measured at various times up to 96 h post‐treatment.
Results
No adverse effects from Amplimune treatment were observed. Amplimune induced an increase in circulating cytokine TNFα concentrations, total white blood cell count and lymphocyte counts indicative of activation of the innate immune system without causing an excessive inflammatory response.
Conclusions
Results confirm that Amplimune can be safely administered to beef cattle at the dose rates and via the routes of administration investigated here.
Keywords: Amplimune, cattle, respiratory disease, trained immunity
Abbreviations
- BRD
bovine respiratory disease
- DNA
deoxyribonucleic acid
- ELISA
enzyme‐linked immunosorbent assay
- h
hours
- HCT
haematocrit
- IL‐12
interleukin 12
- IL‐1β
interleukin 1 beta
- IL‐6
interleukin 6
- IM
intramuscular
- LYM
lymphocyte
- MCWF
mycobacterium cell wall fraction
- NEU
neutrophil
- NEU:LYM
neutrophil:lymphocyte ratio
- SC
subcutaneous
- SCFP
Saccharomyces cerevisiae fermentation products
- TNFα
tumour necrosis factor alpha
- WBC
white blood cell
Disease is a major cost to the beef cattle feedlot industry in Australia. 1 The Australian feedlot industry is investing in research to reduce disease incidence, reduce reliance on antibiotics and improve animal health and welfare outcomes. 2 Bovine respiratory disease (BRD) is responsible for the majority of veterinary treatments and health‐related mortalities in Australian feedlots. 3 It is estimated to reduce profits by between $67.10 and $1647.53 per animal if there are subclinical or clinical symptoms, or the animal dies. 3 Stress is well documented to have a suppressive effect on the immune response in livestock. 4 Consequently, stressors imposed by transportation, induction procedures at a feedlot, change in diet and surroundings, increased human handling and co‐habitation with unfamiliar animals are all classified as risk factors that predispose cattle to BRD. 4 , 5 , 6 , 7 In addition, factors such as breed, weight and season in which cattle are introduced to the feedlot can affect their risk of BRD. 8 Feedlot productivity and profitability could benefit from strategies to reduce the incidence and reduce reliance on antimicrobials to treat disease. Commercial vaccines are available in Australia to protect cattle against the major causal pathogens of BRD including Mannheimia haemolytica, bovine viral diarrhoea virus and bovine herpes virus type 1. Although these vaccines are effective at reducing the incidence of BRD associated with their target pathogens, 9 BRD is the result of a complex opportunistic infection with mixed aetiology. 5 , 6 Therefore, it is difficult to provide complete protection against BRD through vaccination. Furthermore, in 2010, Australian feedlot operators reported that only 7% of cattle entering feedlots were pre‐vaccinated against M. haemolytica and only 0.2% were pre‐vaccinated against bovine herpes virus‐1. 9 Though the proportion of animals pre‐vaccinated against BRD entering Australian feedlots has thought to have increased in the last decade, as suggested by a rise in seropositivity to bovine herpes virus‐1 at feedlot entry from 0.2% to 13.5%, it remains the case that a significant proportion of cattle entering Australian feedlots receive their first BRD vaccination at feedlot induction. 10 For cattle that are not pre‐vaccinated, it is common practice for feedlot operators to vaccinate against BRD at induction. However, there are limitations to the ability of vaccination at induction to provide full protection against BRD. Firstly, there is a variable time lapse between vaccination and the establishment of protective responses, leaving a gap in which cattle are not fully protected. Secondly, targeting every pathogen associated with BRD via vaccines has not yet been achieved and may not be achievable given the complexity of the infection.
Currently, Australian feedlot operators rely on antibiotics to treat BRD. 6 , 11 Although evidence to suggest any significant contribution of antibiotic use in agriculture to the rise in antibiotic resistance in human pathogens is lacking, the use of antibiotics in food‐producing animals is coming under increasing scrutiny. 12 Some consumers of animal protein are concerned by a perceived association between the use of antibiotics in livestock production and the rise in antibiotic resistance. 12 If consumers become dissatisfied with a component of a product, demand for that product decreases, damaging those markets. Despite this, there is increasing worldwide demand for animal‐based protein, which is expected to drive a predicted 67% increase in the use of antibiotics in livestock production systems by 2030. 12 Zelnate®, an immune stimulant based on bacterial DNA and cationic lipids, is registered for the treatment of and to reduce mortality and lung lesions associated with BRD in America, 13 but there is no current alternative to antibiotics registered for use in Australia to prevent or treat BRD. Additional alternatives to antibiotics to prevent and treat disease in livestock will help reduce the predicted increase in antibiotic use. Thus, reducing antibiotic use is a high priority for production animal industries worldwide. To achieve this goal suitable strategies will be required to ensure animal health and welfare are not compromised due to reduced antibiotic use.
To achieve a reduction in the use of antibiotics in livestock industries whilst maintaining the highest level of welfare possible, a multi‐pronged approach is required, with a combined focus on improvements in genetics, management and the production environment. This entails breeding animals better able to resist and be resilient to disease; developing management strategies to improve the resilience of production animals; and modifying the environment to reduce pathogen exposure. Initiatives are already underway in each of these areas. Research is being done to improve the overall disease resistance of livestock by selecting animals with enhanced immune competence or general immune system performance 2 , 14 ; the efficacy of current vaccines is being improved through the use of new adjuvants like glycoprotein subunits 15 ; and livestock producers are adapting the way animals are handled in order to reduce pathogen exposure. 5 In addition, many different innate immune stimulants are being investigated for their ability to enhance immune system health, and growth and production. 16 , 17 The action of these immune stimulants has been linked with the concept of trained immunity. Trained immunity describes a recently discovered ability of innate immune cells to be ‘trained’ through repeated exposure to the same pathogen. 18 The ability of innate cells to be trained was thought to be restricted to adaptive immune cells. 18 Research has demonstrated the ability of innate immune cells to adapt to multiple exposures to various stimulants or infections via epigenetic changes, leading to a persistent, enhanced function. 18 In this trained state, the innate immune system has a heightened ability to deal with a wide range of pathogens that the host may encounter. 19 Armed with this new knowledge, researchers have been investigating the potential for innate immune stimulants, including Mycobacterium bovis and β‐glucan from Saccharomyces cerevisiae, to be used to provide short‐term protection against a broad range of diseases. 20 , 21 Indeed, trained immunity has been shown to be effective in stimulating the immune system to help prevent and treat a variety of diseases in farm animals. 21 , 22 , 23
Amplimune® is a commercially available innate immune stimulant, based on mycobacterium cell wall fractions (MCWF) of Mycobacterium phlei, a non‐pathogenic mycobacterium found in abundance in the natural environment. 24 The product is manufactured and marketed by NovaVive Inc. in the U.S., Canada and New Zealand 25 but is not commercially available in Australia. Amplimune has the potential to prevent and treat a variety of diseases in food‐producing animals, particularly during periods of stress when immune system function can be compromised. Specifically, Amplimune has the potential to be used in feedlot cattle to enhance protective responses to BRD pathogens and other diseases in the high‐risk period between induction and when protection from vaccination is achieved. Despite prior use in cattle, studies on safety, dose and route of administration have not been reported in cattle of a weight and age class typical of cattle entering Australian feedlots. Therefore, this study was designed to determine safe dose rates and routes of administration for Amplimune in weaned beef cattle. We hypothesised that administration of Amplimune would induce an innate immune response in weaners but would not induce any significant adverse side effects such as severe fever, excessive systemic inflammatory responses, or significant body weight loss.
Materials and methods
Overview
This study was conducted at the CSIRO, F.D McMaster research station located near Armidale in New South Wales, Australia. All experimental procedures were pre‐approved by the CSIRO, Chiswick Animal Ethics Committee (Animal Research Authority 18/26). Experimental conditions were designed to mimic those experienced by cattle during induction into a commercial feedlot, including exposure to transportation, common animal health treatments, introduction to a commercial starter ration and confinement to a pen. Changes in body weight, core body temperature, haematology and serum pro‐inflammatory cytokine parameters induced by administration of the innate immune stimulant Amplimune, via different routes (intramuscular or subcutaneous) were measured to assess safe doses and routes of administration of the product. A detailed timeline of experimental procedures is outlined (Figure 1).
Figure 1.
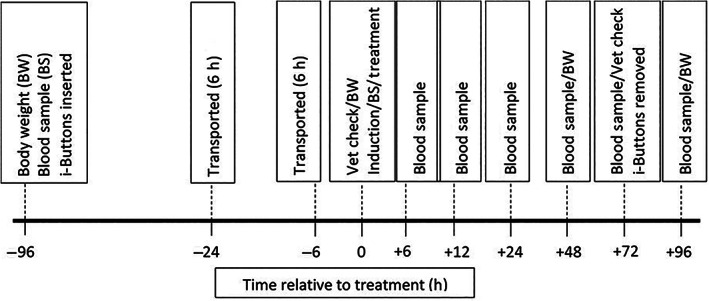
Timeline of procedures conducted including body weight (BW), blood sampling (BS), insertion and removal of i‐Buttons, transportation, veterinarian (Vet) checks, induction procedures and treatment.
Animals and experimental design
Sixty mixed sex Angus weaner cattle, (36 heifers and 24 steers) 6–8 months of age, were enrolled in the study. Cattle had an average body weight of 217 kg (SD = 29 kg). Cattle were grouped based on sex and body weight and then randomly allocated to six different treatment groups (n = 10). Treatments were applied high on the left side of the neck; 2 mL Amplimune (NovaVive Inc., Napanee, Ontario, Canada) via intramuscular (IM) injection (2IM), 2 mL Amplimune via subcutaneous (SC) injection (2SC), 5 mL Amplimune via IM injection (5IM), 5 mL Amplimune via SC injection (5SC), 5 mL of saline (Baxter Healthcare Pty Ltd, Old Toongabbie, NSW, Australia) via IM injection (SalIM) or 5 mL saline via SC injection (SalSC). Groups receiving saline acted as controls. To ensure treatments were not biased by sampling time, cattle were assigned a number (1–60) which was painted on their side for ease of identification and used to determine the order in which cattle were processed for sample collection. Two cattle from each of the six treatment groups were randomly assigned numbers 1–12, and so on up to 60 and were processed in numerical order for all experimental procedures.
Transport, induction and housing
All cattle were transported by road for 6 h, (−24 to −18 h relative to treatment) and then yarded overnight with access to lucerne hay and water. The following day animals were again transported by road for 6 h (−6 to 0 h relative to treatment). Following transportation, all cattle were assessed for body condition, respiration rate, rectal temperature and heart rate by a veterinarian to ensure they were in good health. Cattle received a series of health treatments commonly administered at an induction in a commercial feedlot. These included a clostridial vaccination (UltraVac® 7in1, Zoetis) and anthelmintic treatment (Genesis Ultra, Ancare). All health treatments were administered as per manufacturers' recommendations. Treatments (Amplimune or saline) were administered as part of the induction procedures (0 h) and cattle were weighed. Cattle were closely monitored by a veterinarian for 2 h following treatment for signs of acute adverse reactions to the treatment. For the duration of the trial cattle were housed in a single large pen at a stocking density of 5.3 m2 per head. Cattle had access to water ad libitum and were fed once daily with a commercial feedlot starter ration (Tullimba Feedlot, Torryburn, NSW, Australia) at an initial rate of 3 kg/head/day, increasing incrementally to 6 kg/head/day by the fourth day. Cattle were assessed for body condition, respiration rate, rectal temperature, evidence of injection site lesions and heart rate at 72 and 96 h post‐treatment to ensure they remained in good health.
Body temperature and body weight change
To assess changes in core body temperature induced by treatment, cattle were fitted with rectal probes, as described previously, 26 which housed i‐Button temperature loggers (Thermocron, Castle Hill, NSW, Australia) 4 days prior to treatment (−96 h). Loggers recorded core body temperature every 5 min from −96 h pre‐treatment to 72 h post‐treatment. Temperatures recorded whilst cattle grazed undisturbed in a small paddock prior to treatment (−72 to −48 h relative to treatment) were used to establish a baseline diurnal temperature profile for individual animals. Body weight was measured at −96 h pre‐treatment to provide a baseline, on day of treatment (0 h), 48 and 96 h post‐treatment. Body weights were recorded using a calibrated weighing platform (Tru‐Test, Datamars Ltd, Banyo, QLD, Australia).
Sample collection
Serial blood samples were collected via jugular venepuncture for haematology and to determine serum cytokine concentrations at 0, 6, 12, 24, 48, 72 and 96 h relative to treatment. Blood for haematology was collected into ethylenediaminetetraacetic acid (EDTA) vacutainers (Becton Dickinson, UK), mixed, and immediately placed on ice. In the laboratory, samples were equilibrated to room temperature, mixed well and immediately analysed on an automated haematology analyser (Cell‐Dyn 3500R, Abbott Diagnostics, Macquarie Park, NSW, Australia), using a veterinary package specifically designed for analysis of bovine samples. Parameters measured included total white blood cell counts (WBC), differential neutrophil (NEU) and lymphocyte (LYM) counts, haematocrit (HCT), and neutrophil:lymphocyte ratio (NEU:LYM) was calculated. Serum was prepared from blood collected in serum vacutainers by centrifugation at 700 g, for 20 min, at room temperature, and stored in multiple aliquots at −80°C for subsequent cytokine assays.
Cytokine assays
Enzyme‐linked immunosorbent assays (ELISAs) were conducted to determine changes in the serum concentration of the pro‐inflammatory cytokines tumour necrosis factor alpha (TNFα), interleukin 1 beta (IL‐1β), interleukin 6 (IL‐6) and interleukin 12 (IL‐12). Bovine‐specific commercial ELISA kits (Bovine TNFα DuoSet ELISA, R&D Systems, Minneapolis, MN; Bovine IL‐ 6 DuoSet ELISA, R&D Systems, Minneapolis, MN) or in‐house developed assays (IL‐1β, IL‐12) were used as described previously. 27 Circulating IL‐12 cytokine concentrations were only assessed in a subset of samples collected from cattle in the 2SC, 5SC and SalSC treatment groups at −96, 0, 6, 12, 24 and 48 h. For each animal, samples collected pre‐ and post‐treatment were run on the sample plate to minimise the effects of plate‐to‐plate variation. All assays were performed in triplicate with pooled bovine serum samples used as controls to standardise results across plates. Cytokine concentrations in samples were calculated using a standard curve generated using a series of known cytokine standards.
Statistical analysis
Statistical analysis was conducted using Rx64 3.5.0. 28 For parameters measured over time, repeated measures linear mixed models, fitting animal as a random effect, were used to estimate variance components using the REML method. For parameters measured only once, simple linear models were used to estimate variance components using the REML method. Residuals generated from models were tested for normality by assessing skewness and kurtosis and data transformed were required to improve normality. Data for WBC, LYM and NEU were log transformed. Data for TNFα were square‐root transformed. Tukey post hoc tests were used to evaluate multiple pairwise comparisons of group means where appropriate.
Haematology, cytokine and body weight
Fixed effects assessed in all models included treatment, body weight, sex and treatment*sex. For analysis of repeated measures, time and time*treatment were also fitted as fixed effects. Where appropriate, baseline parameters for body weight, cytokine concentration and haematology values measured at −96 h were fitted as covariates in statistical models. Fixed effects were removed from the final model when they were clearly not significant (P > 0.1 for single effects or P > 0.2 for interactions). Where significant treatment effects were observed, specific linear contrasts were undertaken to compare Amplimune versus saline (placebo) delivered via the same route of administration (ie. SC or IM). Specific treatment group contrasts investigated were: 2SC versus SalSC, 5SC versus SalSC, 2SC versus 5SC, 2IM versus SalIM, 5IM versus SalIM, 2IM versus 5IM. For the tabular representation of results, least squares means (LSMs) were generated from models using raw untransformed data. However, the significance of fixed effects was determined using transformed data.
Core body temperature
To investigate treatment effects on body temperature, the mean and maximum values logged over 6, 12, 24 and 48 h post‐treatment were calculated for individual animals. Fixed effects included treatment, body weight, sex and treatment × sex. Mean and maximum temperature data recorded during the corresponding 6, 12, or 24 h periods prior to treatment (baseline diurnal body temperature) were also calculated and values fitted as covariates in statistical models. Data from the 24 h baseline measurement period was also fitted as a covariate when analysing body temperature data recorded in the 48 h period post‐treatment. Therefore, body temperature parameters analysed were mean body temperature – 6 h (mean 6 h), 12 h (mean 12 h), 24 h (mean 24 h) and 48 h (mean 48 h) post‐treatment and maximum body temperature – 6 h (max 6 h), 12 h (max 12 h), 24 h (max 24 h), 48 h (max 48 h) post treatment.
Results
The significance of covariate, fixed effects and interactions for all parameters in each model are summarised in Table 1.
Table 1.
Significance (p‐values) of covariate, fixed effects and interactions for all parameters
| Parameter | Trans | Cov | Trt | Weight b | Sex | Time | Trt × time | Trt × sex | Trt × sex × time |
|---|---|---|---|---|---|---|---|---|---|
| CBT | |||||||||
| Mean 6 h | None | <0.001 | 0.296 | 0.563 | 0.340 | NA | NA | 0.187 | NA |
| Max 6 h | None | <0.001 | 0.498 | 0.312 | 0.043 | NA | NA | 0.059 | NA |
| Mean 12 h | None | <0.001 | 0.679 | 0.143 | 0.703 | NA | NA | 0.073 | NA |
| Max 12 h | None | <0.001 | 0.527 | 0.213 | 0.199 | NA | NA | 0.235 | NA |
| Mean 24 h | None | 0.002 | 0.384 | 0.181 | 0.234 | NA | NA | 0.794 | NA |
| Max 24 h | None | <0.001 | 0.118 | 0.041 c | 0.247 | NA | NA | 0.026 c | NA |
| Mean 48 h | None | <0.001 | 0.155 | 0.165 | 0.079 | NA | NA | 0.228 | NA |
| Max 48 h | None | <0.001 | 0.031 c | 0.016 c | 0.056 | NA | NA | 0.004 c | NA |
| Cytokines | |||||||||
| TNFα | Sq Rt | <0.001 | 0.605 | 0.891 | 0.617 | 0.692 | 0.003 c | 0.598 | 0.164 |
| IL‐1β | Log | <0.001 | 0.706 | 0.819 | 0.956 | <0.001 | 0.934 | 0.343 | 0.727 |
| IL‐6 | Log | <0.001 | 0.504 | 0.514 | 0.975 | 0.346 | 0.115 | 0.324 | 0.108 |
| IL‐12 a | Log | <0.001 | 0.531 | 0.902 | 0.536 | 0.317 | 0.579 | 0.503 | 0.848 |
| Haematology | |||||||||
| WBCs | Log | <0.001 | 0.429 | 0.302 | 0.307 | <0.001 | 0.776 | 0.024 c | 0.525 |
| NEU | Log | 0.072 | 0.100 | 0.753 | 0.184 | <0.001 | 0.608 | 0.007 c | 0.873 |
| LYM | Log | <0.001 | 0.131 | 0.618 | 0.527 | <0.001 | 0.761 | 0.009 c | 0.234 |
| NEU:LYM | None | <0.001 | 0.354 | 0.302 | 0.576 | <0.001 | 0.759 | 0.010 c | 0.549 |
| HCT | None | <0.001 | 0.521 | 0.427 | 0.207 | <0.001 | 0.934 | 0.686 | 0.328 |
| BW | |||||||||
| Weight | None | <0.001 | 0.177 | NA | 0.004 | <0.001 | 0.138 | 0.183 | 0.848 |
Serum cytokine IL‐12 assays were only conducted on blood samples collected from 0 to 48 h post‐treatment.
Body weight recorded at −96 h (relative to treatment) was used as a fixed effect for body temperature, serum cytokine concentration, haematology and body weight parameters.
Fixed effects and interactions of interest.
Body temperatures recorded between −72 to −48 h (relative to treatment) were used as covariate for the body temperature parameter. Serum cytokine concentrations and haematology parameters recorded at −96 h (relative to treatment) were used as covariates for serum cytokine concentration and haematology parameters.
BW, body weight; CBT, core body temperature; Cov, covariate; HCT, haematocrit; IL‐1β, interleukin one beta; IL‐6, interleukin six; IL‐12, interleukin twelve; LYM, lymphocytes; Max, maximum; NEU, neutrophils; NEU:LYM, neutrophil:lymphocyte ratio; Sq Rt, square root; TNFα, tumour necrosis factor alpha; Trans, transformation; Trt, treatment; WBC, white blood cells.
Clinical observations
No changes in clinical parameters (body condition, respiration rate, rectal temperature or heart rate) were observed in any cattle following treatments administered in the trial. Further, there was no evidence of lesions at injection sites at 2, 72 and 96 h following treatment, irrespective of treatments administered.
Body weight
No significant differences in body weight change were detected between treatment groups over the course of the experimental period (P = 0.138).
Serum cytokine concentrations
There was a significant treatment effect on serum concentrations of TNFα, but this effect varied over time (time*treatment interaction, P = 0.003). Results from the analysis of specific linear contrasts between Amplimune treatments and their respective controls over time are presented in Table 2. Serum cytokine TNFα concentrations were higher in 2SC treatment group animals when compared to animals in the 5SC treatment group at 24, 72 and 96 h (P = 0.04, P = 0.01 and P = 0.01 respectively) and in the SalSC control group at 72 and 96 h (P = 0.02, P = 0.04 respectively). There was a trend suggesting serum TNFα concentrations were high in 2SC treatment group animals when compared to animals in the 5SC treatment group at 48 h (P = 0.07). No significant differences were observed in serum TNFα concentrations between any treatment groups receiving treatment via IM injection. No significant differences in serum cytokine concentrations of IL‐6 or IL‐12 were observed between treatment groups. There was an effect of time on concentrations of IL‐1β (P < 0.001), however this did not vary between treatments.
Table 2.
Treatment group LSMs (±SEM) for raw serum TNFα concentrations (ng/mL) post‐treatment
| TNFα | 2SC | 5SC | SalSC | 2IM | 5IM | SalIM |
|---|---|---|---|---|---|---|
| 0 h | 3.77 ± 0.57 | 3.63 ± 0.57 | 3.65 ± 0.57 | 4.48 ± 0.57 | 3.52 ± 0.57 | 3.53 ± 0.58 |
| 6 h | 3.02 ± 0.57 | 3.86 ± 0.57 | 4.22 ± 0.57 | 3.79 ± 0.57 | 4.05 ± 0.57 | 3.85 ± 0.58 |
| 12 h | 3.94 ± 0.57 | 3.86 ± 0.57 | 3.48 ± 0.57 | 3.69 ± 0.57 | 4.12 ± 0.57 | 4.04 ± 0.58 |
| 24 h | 4.92 ± 0.57a | 3.85 ± 0.57b | 4.13 ± 0.57ab | 3.21 ± 0.57 | 3.82 ± 0.57 | 3.54 ± 0.58 |
| 48 h | 5.16 ± 0.57 | 3.69 ± 0.57 | 3.57 ± 0.57 | 3.68 ± 0.57 | 4.02 ± 0.57 | 3.25 ± 0.58 |
| 72 h | 5.20 ± 0.57a | 2.52 ± 0.57b | 2.86 ± 0.57b | 4.27 ± 0.57 | 3.63 ± 0.57 | 3.31 ± 0.58 |
| 96 h | 5.46 ± 0.57a | 3.23 ± 0.57b | 3.38 ± 0.57b | 4.57 ± 0.57 | 3.12 ± 0.57 | 4.12 ± 0.58 |
Values in a row (within route of administration) with different superscripts are significantly different (P < 0.05). When calculating LSMs, baseline serum TNFα concentrations recorded at −96 h were fitted as a covariate in statistical models. Only treatments administered via the same route (subcutaneously (SC) or intramuscularly (IM)) were compared.
2IM, 2 mL IM injection of Amplimune; 2SC, 2 mL SC injection of Amplimune; 5IM, 5 mL IM injection of Amplimune; 5SC, 5 mL SC injection of Amplimune; h, hours; SalIM, IM injection of saline (placebo); SalSC, SC injection of saline (placebo); TNFα, tumour necrosis factor alpha.
Haematology
Total WBC count, LYM count, NEU count, HCT and the NEU:LYM ratio changed significantly over time, independent of treatment (P < 0.001). Differences in total WBC, LYM count, NEU count and the NEU:LYM ratio were observed between treatment groups, but the effect varied depending on sex (treatment × sex interaction, P = 0.02, P = 0.01, P = 0.01 and P = 0.01, respectively). Results from the analysis of specific linear contrasts between Amplimune and control treatments within sex are presented in Table 3.
Table 3.
Treatment group LSMs (±SEM) for raw haematology parameters, including: white blood cell, neutrophil, lymphocyte counts (×109/L), neutrophil:lymphocyte ratio and haematocrit (%)
| Haem | 2SC | 5SC | SalSC | 2IM | 5IM | SalIM |
|---|---|---|---|---|---|---|
| Heifer | ||||||
| WBC | 11.2 ± 0.57a | 10.0 ± 0.58ab | 9.17 ± 0.62b | 10.1 ± 0.57 | 10.4 ± 0.57 | 9.61 ± 0.57 |
| LYM | 4.75 ± 0.29ab | 5.18 ± 0.29a | 4.14 ± 0.31b | 4.42 ± 0.29a | 4.59 ± 0.29a | 3.66 ± 0.29b |
| NEU | 5.29 ± 0.51 | 4.13 ± 0.55 | 4.27 ± 0.52 | 4.48 ± 0.51 | 4.50 ± 0.51 | 4.99 ± 0.51 |
| NEU:LYM | 1.24 ± 0.19 | 0.82 ± 0.19 | 1.35 ± 0.18 | 1.12 ± 0.19 | 1.23 ± 0.19 | 1.48 ± 0.18 |
| HCT | 33.0 ± 0.40 | 33.5 ± 0.44 | 33.6 ± 0.40 | 32.5 ± 0.40 | 33.3 ± 0.44 | 32.8 ± 0.40 |
| Steer | ||||||
| WBC | 9.35 ± 0.70 | 10.5 ± 0.71 | 10.3 ± 0.70 | 10.2 ± 0.70a | 8.15 ± 0.71b | 9.46 ± 0.72ab |
| LYM | 3.98 ± 0.36 | 4.15 ± 0.36 | 3.94 ± 0.36 | 5.15 ± 0.36a | 4.05 ± 0.38b | 4.63 ± 0.37ab |
| NEU | 4.45 ± 0.62 | 5.23 ± 0.62 | 5.47 ± 0.62 | 4.00 ± 0.62 | 2.77 ± 0.63 | 3.69 ± 0.62 |
| NEU:LYM | 1.29 ± 0.23 | 1.60 ± 0.23 | 1.48 ± 0.23 | 0.85 ± 0.23 | 0.80 ± 0.23 | 0.83 ± 0.23 |
| HCT | 32.5 ± 0.45 | 33.1 ± 0.42 | 33.2 ± 0.44 | 32.1 ± 0.43 | 32.9 ± 0.42 | 32.4 ± 0.45 |
Values in a row (within routes of administration) with different superscripts are significantly different (P < 0.05). When calculating LSMs, baseline haematology parameters recorded at −96 h were fitted as a covariate in statistical models.
2IM, 2 mL IM injection of Amplimune; 2SC, 2 mL SC injection of Amplimune; 5IM, 5 mL IM injection of Amplimune; 5SC, 5 mL SC injection of Amplimune; Haem, Haematology parameters; HCT, haematocrit; LYM, lymphocyte count; NEU, neutrophil count; NEU:LYM, neutrophil:lymphocyte ratio; SalIM, IM injection of saline (placebo); SalSC, SC injection of saline (placebo); WBC, white blood cell count.
Following treatment, WBC counts were higher in 2SC heifers compared to control (SalSC) heifers (P = 0.02). Differences were also observed in WBC counts in steers from the 2IM and the 5IM groups with steers in 5IM having higher WBC counts post‐treatment (P = 0.04).
In contrast, following treatment LYM counts were higher in 2IM and 5IM heifers compared to control (SalIM) heifers (P = 0.03 and P = 0.02 respectively). Differences were also observed in LYM counts in 5SC heifers compared with control (SalSC) heifers, with 5SC heifers having higher LYM counts post‐treatment (P = 0.04). Differences were also observed in LYM counts in the 2IM and 5IM steers with the 2IM steers having higher LYM counts post‐treatment (P = 0.04).
As differences in LYM counts were observed in heifers receiving 5 mL Amplimune versus saline (control) when administered either IM or SC, specific contrasts were conducted to compare differences in LYM counts in heifers receiving Amplimune IM or SC at the 5 mL dose. No significant differences were observed (P = 0.25).
Temperature
Treatment group differences were observed in maximum core body temperature at 24 and 48 h post‐treatment, however, the effect of treatment varied depending on sex (treatment × sex interaction, P = 0.03 and P = 0.004 respectively). Results from the analysis of specific linear contrasts between Amplimune treatment groups and their respective control on mean and maximum core body temperature within sex are presented in Tables 4 and S1. Steers in 2SC had higher maximum core body temperatures at 24 and 48 h post‐treatment, compared to steers in the control group (SalSC) (P = 0.05, P = 0.02 respectively). No significant differences were observed in mean core body temperature between any treatments (Table S1). There was a significant effect of sex on maximum body temperature 6 h post‐treatment (P = 0.04) and there was a significant effect of body weight on maximum core body temperature at 24 and 48 h post‐treatment (P = 0.04 and P = 0.02 respectively).
Table 4.
Treatment group LSMs (±SEM) for maximum core body temperature (°C) within 6, 12, 24 or 48 h blocks post‐treatment
| Max body temp | 2SC | 5SC | SalSC | 2IM | 5IM | SalIM |
|---|---|---|---|---|---|---|
| Steer | ||||||
| 6 h block | 39.9 ± 0.18 | 39.6 ± 0.16 | 39.5 ± 0.16 | 39.4 ± 0.16 | 39.1 ± 0.17 | 39.5 ± 0.16 |
| 12 h block | 40.3 ± 0.25 | 39.8 ± 0.22 | 39.7 ± 0.21 | 39.5 ± 0.21 | 39.2 ± 0.22 | 39.7 ± 0.21 |
| 24 h block | 40.9 ± 0.26a | 40.1 ± 0.22ab | 39.9 ± 0.22b | 39.9 ± 0.22 | 39.5 ± 0.22 | 39.8 ± 0.22 |
| 48 h block | 41.2 ± 0.24a | 40.6 ± 0.21ab | 40.1 ± 0.21b | 40.2 ± 0.21 | 39.7 ± 0.21 | 39.8 ± 0.21 |
| Heifer | ||||||
| 6 h block | 39.5 ± 0.14 | 39.7 ± 0.18 | 39.5 ± 0.13 | 39.9 ± 0.15 | 39.6 ± 0.13 | 39.7 ± 0.14 |
| 12 h block | 39.8 ± 0.19 | 39.9 ± 0.25 | 39.8 ± 0.18 | 40.0 ± 0.20 | 39.8 ± 0.17 | 39.9 ± 0.19 |
| 24 h block | 39.8 ± 0.20 | 39.9 ± 0.25 | 39.8 ± 0.18 | 40.0 ± 0.20 | 40.0 ± 0.18 | 39.8 ± 0.20 |
| 48 h block | 39.8 ± 0.19 | 40.1 ± 0.24 | 40.0 ± 0.17 | 40.1 ± 0.19 | 40.2 ± 0.17 | 39.9 ± 0.19 |
Values in a row (within the route of administration) with different superscripts are significantly different (P < 0.05). When calculating LSMs, baseline body temperatures for individual cattle in 6, 12 or 24 h blocks recorded between −72 and −48 h were fitted as a covariate in statistical models. Baseline body temperatures (°C) for individual cattle in the 24 h block recorded between −72 and −48 h were fitted as a covariate in the 48 h block statistical model. Only treatments administered via the same route (subcutaneously (SC) or intramuscularly (IM)) were compared.
2IM, 2 mL IM injection of Amplimune; 2SC, 2 mL SC injection of Amplimune; 5IM, 5 mL IM injection of Amplimune; 5SC, 5 mL SC injection of Amplimune; Max, maximum; SalIM, IM injection of saline (placebo); SalSC, SC injection of saline (placebo); temp, temperature.
Discussion
The current study investigated the response of weaner Angus cattle to the administration of Amplimune, an innate immune stimulant based on MCWF, under conditions that mimicked feedlot induction. Changes in body temperature, body weight, circulating pro‐inflammatory cytokines and haematology parameters induced by treatment with Amplimune were investigated at varying dose rates and routes of administration. Results supported the hypothesis that administration of Amplimune to weaner beef cattle would induce an immune response that is safe and does not have negative impacts on health and performance outcomes in the 96 h following administration.
Clinical examinations of all animals were done pre‐treatment, to ensure animals were healthy when treated, and again post‐treatment to identify any potential adverse effects of treatments. Normal body condition, respiration rates, rectal temperatures and heart rates were observed pre‐treatment, post‐treatment (2 h) and again at the conclusion of the trial (96 h). Results showed that several of the response parameters measured in the study were unaffected by treatment throughout the trial. These included weight gain, circulating concentrations of the pro‐inflammatory cytokines IL‐1β, IL‐6, IL‐12 and the haematology parameters neutrophil count, haematocrit and neutrophil:lymphocyte ratio. In contrast, increases in circulating concentrations of the pro‐inflammatory cytokine TNFα, WBC and LYM counts and maximum body temperatures, were induced by treatment with Amplimune. Although treatment with Amplimune resulted in significant increases in these parameters, the maximum observed values only exceeded normal reference ranges up to 72 h post‐treatment and returned to normal values by 96 h. A normal temperature range of 37.8–39.2°C has been reported in adult cattle previously although young cattle and individuals can have higher temperature ranges. 29 Throughout the current trial, the maximum core body temperature in cattle treated with Amplimune ranged between 39.6–41.6°C, which dropped to 36–40.3°C on the final day of the trial. Similarly, cattle treated with saline (control) recorded a maximum range of 39.5–41.3°C, but by the conclusion of the trial that range had dropped to 38.8–40.6°C suggesting that treatment was not the cause of the change in temperature. Likewise, during the trial, 14 animals had lymphocyte counts which exceeded the reported normal range for bovines (2.5–7.5 × 109/L). 30 However, these animals were from all six treatment groups, including controls, and eight of the 14 had baseline lymphocyte counts outside the reference ranges prior to treatment. Lymphocyte counts, for all but one animal, returned to normal by 96 h post‐treatment. A similar trend was observed for WBC counts. In 36 animals, across all six treatment groups, WBC count increased beyond the normal reference range of 4–12 × 109/L, 30 however, 24 of these animals had baseline WBC counts outside the reference range prior to treatment. WBC counts, excluding one animal, returned to within the normal reference range by 96 h post‐treatment. To the authors' knowledge, an official normal range for concentrations of serum cytokines in cattle has not been reported, but many studies have tracked changes in concentrations of TNFα in serum from pre‐ to post‐challenge 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 (Table S2). The serum concentrations of TNFα across studies ranged from 0 to 140 ng/mL pre‐challenge, and 0.25 to 240 ng/mL post‐challenge. In the current study, the highest concentration of TNFα in serum observed was 22 ng/mL at 48 h post‐treatment. This indicates TNFα is within the range of previously reported baseline values and suggests that administration of Amplimune did not induce an excessive inflammatory response. Therefore, the current study suggests that MCWF is safe to administer to young beef cattle, while results from previous studies suggest it may play a role in enhancing immune system function and the ability of cattle to resist subsequent pathogen exposure. 17 , 25 , 41 , 42
Many of the same measures used to assess safety are also measures of innate immune system activation. The objective when using immune stimulants to help prevent disease is to stimulate the immune system to a level, which enhances responses to subsequent disease challenges without causing adverse effects, such as excessive inflammation. The challenge, therefore, is to choose a dose and route of administration that can enhance future immune responses without causing excessive inflammation. Examples of immune stimulants that have proven to induce excessive stimulation include recombinant bovine IL‐1β which reduced Staphylococcus uberis infection but induced sterile mastitis 43 and an intramammary loop for prevention of mastitis in dairy cows which significantly increased leukocyte concentrations. 44 Administration of Freund's complete adjuvant sometimes leads to severe lesions at the site of injection and in severe cases, mortality, 45 , 46 resulting in its withdrawal from widespread use as an adjuvant in laboratory animals. In the current study, MCWF in Amplimune did not cause lesions at the site of injection, nor did it induce excessive immune activation. However, there were indications of moderate immune activation including increases of the circulating pro‐inflammatory cytokine TNFα, when administered subcutaneously (SC) at a dose rate of 2 mL. Amplimune also induced increases in total WBC count in heifers when administered via SC or IM injection at a dose rate of 2 mL; and increases in lymphocyte counts in heifers when administered via SC or IM injection at a dose rate of 5 mL. In other studies investigating MCWF, from non‐Amplimune sources, in mice, 47 dogs 48 and horses, 49 changes have been observed in a variety of parameters. Activation of cytotoxic T‐cells by MCWF induced the reduction of three different cancer cell lines in mice tumours 47 ; clinical symptoms of bladder cancer, including dysuria, pollakiuria and haematuria, were reduced following administration of MCWF in dogs 48 ; and a reduction in the number of mares positive for endometritis, based on exfoliative cytology and bacteriology, when administered MCWF at 48 h post‐experimental challenge. 49 In these studies, immune activation was induced without excessive inflammation. Other studies have also investigated the ability of different types of innate immune stimulants to enhance immune system function in cattle. Liposome‐toll‐like receptor complexes delivered intranasally to beef calves induced significant increases in the percentage of CD14+ monocytes in nasal mucosal samples and upregulated expression of interferon‐gamma, interleukin‐8 and monocyte chemoattractant protein‐1 gene transcripts in nasopharyngeal samples. 16 Fermentation products from the yeast Saccharomyces cerevisiae (SCFP) have been fed to dairy calves, reducing the incidence of diarrhoea. 23 The effects of supplementation with SCFP in beef calves deliberately challenged with lipopolysaccharide from Escherichia coli have been reported with increased platelet concentrations and reduced serum TNFα concentrations observed in supplemented calves as compared with calves not fed SCFP. 33
The specific active ingredient of Amplimune is fragmented sections of M. phlei cell wall with nucleic acids conserved onto it. These fragments are adjuvanted in 2% squalane, a fully hydrogenated, digestible oil that is a precursor of cholesterols. The final product is an emulsion of MCWF in squalane in phosphate‐buffered saline. The lack of pathogenicity of M. phlei, particularly compared to other mycobacteria, such as M. bovis, 50 as well as its lack of cross‐reactivity with bovine tuberculosis and Johne's disease tests, 51 makes it ideal for inclusion in an immune stimulant formulation for cattle.
The efficacy of Amplimune to prevent and treat disease has been demonstrated in several breeds and age classes of cattle, through the assessment of pro‐inflammatory responses as an indication of innate immune induction, and also by assessing clinical symptoms of the disease. For example, Amplimune has been used to treat the symptoms of Escherichia coli infection in neonatal dairy calves 41 and has been shown to reduce morbidity and mortality, and increase weight gain in feedlot steers with an average weight of 115 kg, however, the influence of pen effects on observed results could not be assessed in the study. 25 Amplimune has also been successfully used to prevent mastitis and arthritis in adult dairy cows. 52 Although no significant difference in the likelihood of health treatments for both respiratory disease and/or diarrhoea was observed between calves in treatment groups receiving Amplimune or saline (control) during the first 9 weeks of life, more calves in the control group received disease treatments in the first 30 days following transportation. 17 Administration of Amplimune to neonatal dairy calves has also been shown to enhance immune system activation. 42 Subcutaneous injection of Amplimune in neonatal dairy calves increased the number of major histocompatibility complex Class II+ CD4+ T‐cells/mL (in peripheral blood); indicating an increase in the frequency of activated circulating lymphocytes. 42 Conversely, subcutaneous injection of Amplimune in dairy cows reduced clinical signs of metritis and mastitis but had no effect on white blood cells. 53 Other studies with different MCWFs have found increased production of a range of pro‐inflammatory cytokines including interleukin‐12 (IL‐12), interleukin‐6 (IL‐6), tumour necrosis factor‐alpha (TNFα) and interferon‐gamma (IFNγ), as well as increased neutrophils and primed macrophages in response to treatment. 54 , 55 The efficacy of MCWF from M. phlei to prevent and treat disease has also been investigated in horses and dogs. 48 , 49
Importantly, studies have also shown that treatment of cattle with M. phlei does not cause false‐positive results in bovine tuberculosis and Johne's disease tests, making it safe for use in food‐producing animals, without compromising disease surveillance. 51 Results of the current study suggest that Amplimune is safe to use in young beef cattle. Further studies will be required to evaluate the potential for Amplimune to be used as an alternative to antibiotics to prevent and treat disease in beef cattle via enhancement of immune system function and activation of trained immunity. A target application is in Australian feedlot cattle to reduce the incidence of BRD, where currently the only treatment option is the use of antibiotics and ancillary anti‐inflammatory therapy.
Conclusion and recommendations
The current study demonstrates that the innate immune stimulant Amplimune is safe to use in young feedlot cattle at 2 and 5 mL dose rates and SC and IM delivery routes. This was shown by a lack of any adverse side effects and no effect of treatment on weight gain, circulating concentrations of pro‐inflammatory cytokines IL‐1β, IL‐6 and IL‐12, and haematology parameters neutrophil count, haematocrit and neutrophil:lymphycyte ratio. Moderate innate immune induction triggered by treatment with Amplimune was demonstrated by increased total WBC count, lymphocyte count and circulating concentrations of the pro‐inflammatory cytokine TNFα. Future studies will assess the potential use of Amplimune as an antibiotic alternative and for the prevention of disease through stimulation of the innate immune response and trained immunity.
Conflict of interest and sources of funding
The work was co‐funded by Meat and Livestock Australia (MLA) and the Commonwealth Scientific and Industrial Research Organisation (CSIRO). Author Annika Alexander was the recipient of the Ian McMaster Bequest scholarship and the QTAC Rural & Regional Enterprise scholarship. Authors McRae G, Alkemade S and Cervantes M were employees or are still employed by NovaVive (a company, which previously had or currently holds licensing rights for Amplimune™) at the time of study execution and manuscript preparation.
Supporting information
Table S1 Treatment group LSMs (±SEM) for Mean core body temperature (°C) within 6, 12, 24 or 48 h blocks post‐treatment.
Table S2 Experiments in cattle with recorded serum concentrations of pro‐inflammatory cytokine TNFα, pre‐ and post‐challenge.
Acknowledgments
The authors would like to acknowledge Dom Niemeyer, Duncan Elks, Graham Acton, Troy Kalinowski, Jim Lea, Amy Bell for their assistance in conducting trial work. We also gratefully acknowledge laboratory guidance and support provided by Tony Vuocolo, Suzie Briscoe and Sally Stockwell and care of animals provided by Justin Matthews.
Alexander, AL. , Doyle, E. , Ingham, AB. , Colditz, I. , McRae, G. , Alkemade, S. , Cervantes, MP. and Hine, BC. , The innate immune stimulant Amplimune® is safe to administer to young feedlot cattle. Aust Vet J. 2022;100:261–270. 10.1111/avj.13156
References
- 1. Lane J, Jubb T, Shephard R, Webb‐Ware J, Fordyce G. Priority list of endemic diseases for the red meat industries . North Sydney, NSW; 2015. 20 March 2015. Contract No.: B.AHE.0010.
- 2. Hine BC, Bell AM, Niemeyer DO et al. Immune competence traits assessed during the stress of weaning are heritable and favorably genetically correlated with temperament traits in Angus cattle. J Anim Sci 2019;97(10):4053–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blakebrough‐Hall C, McMeniman JP, González LA. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J Anim Sci 2020;98(2). 10.1093/jas/skaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blecha F. Immune system response to stress. In: Moberg GP, Mench JA, editors. The biology of animal stress. CAB International, Oxford, 2000;111–122. [Google Scholar]
- 5. Hay KE, Barnes TS, Morton JM et al. Risk factors for bovine respiratory disease in Australian feedlot cattle: use of a causal diagram‐informed approach to estimate effects of animal mixing and movements before feedlot entry.(clinical report). Prev Vet Med 2014;117(1):160–169. [DOI] [PubMed] [Google Scholar]
- 6. Cusack PMV, McMeniman NP, Lean IJ. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust Vet J 2003;81(8):480–487. [DOI] [PubMed] [Google Scholar]
- 7. Taylor JD, Fulton RW, Lehenbauer TW et al. The epidemiology of bovine respiratory disease: what is the evidence for predisposing factors? Can Vet J 2010;51(10):1095–1102. [PMC free article] [PubMed] [Google Scholar]
- 8. Hay KE, Morton JM, Mahony TJ et al. Associations between animal characteristic and environmental risk factors and bovine respiratory disease in Australian feedlot cattle. Prev Vet Med 2016;125:66–74. [DOI] [PubMed] [Google Scholar]
- 9. Perkins N. Animal health survey of the Australian feedlot industry (2010) . North Sydney, NSW: Meat and Livestock Australia ltd; 2013. Contract No.: P.PSH.0547.
- 10. Cusack PMV. Effects of vaccination in backgrounded feedlot cattle. Final report B.FLT.0235. Final report. North Sydney: Meat & Livestock Australia Limited; 2019. 15 March 2019.
- 11. Badger S, Sullivan K, Jordan D et al. Antimicrobial use and stewardship practices on Australian beef feedlots. Aust Vet J 2020;98(1–2):37–47. [DOI] [PubMed] [Google Scholar]
- 12. Lhermie G, Gröhn YT, Raboisson D. Addressing antimicrobial resistance: an overview of priority actions to prevent suboptimal antimicrobial use in food‐animal production. Front Microbiol 2017;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayer launches immunostimulant Zelnate® for animal health following authorisation in the US [press release] . Framingham: Questex LLC., 8 September 2015.
- 14. Hine BC, Ingham AB, Smith J, Colditz IG. Associations between immune competence, health and performance of sheep in the resource flock . Final report. 2017.
- 15. Nelson CS, Jenks JA, Pardi N et al. Human cytomegalovirus glycoprotein B nucleoside‐modified mRNA vaccine elicits antibody responses with greater durability and breadth than MF59‐adjuvanted gB protein immunization. J Virol 2020;94(9):e00186–e00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wheat W, Chow L, Rozo V et al. Non‐specific protection from respiratory tract infections in cattle generated by intranasal administration of an innate immune stimulant. PLoS One 2020;15(6):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Omontese BO, Caixeta LS, Machado VS et al. Effects of the administration of a non‐specific immune stimulant around transportation on health and performance of Jersey and Jersey‐cross heifer calves during the rearing period: randomized clinical trial. Front Vet Sci 2020;7:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011;9(5):355–361. [DOI] [PubMed] [Google Scholar]
- 19. Netea MG, Domínguez‐Andrés J, Barreiro LB et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020;20(6):375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byrne KA, Tuggle CK, Loving CL. Differential induction of innate memory in porcine monocytes by β‐glucan or bacillus Calmette‐Guerin. Innate Immun 2021;27(6):448–460. 10.1177/1753425920951607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerra‐Maupome M, Vang DX, McGill JL. Aerosol vaccination with Bacille Calmette‐Guerin induces a trained innate immune phenotype in calves. PLoS One 2019;14(2):e0212751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurley DJ, Barber CE, Adkins M et al. An immunomodulatory feed additive enhances in vitro viral vaccine recall antigen responses in dairy heifers. Res Vet Sci 2019;127:11–17. [DOI] [PubMed] [Google Scholar]
- 23. Alugongo GM, Xiao JX, Chung YH et al. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: performance and health. J Dairy Sci 2017;100(2):1189–1199. [DOI] [PubMed] [Google Scholar]
- 24. Filion MC, Lépicier P, Morales A et al. Mycobacterium phlei cell wall complex directly induces apoptosis in human bladder cancer cells. Br J Cancer 1999;79(2):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nosky B, Biwer J, Alkemade S et al. Effect of a non‐specific immune stimulant (Amplimune™) on the health and production of light feedlot calves. J Dairy Vet Anim Res 2017;6(3):302–306. [Google Scholar]
- 26. Lea JM, Niemeyer DDO, Reed MT et al. Development and validation of a simple technique for logging body temperature in free‐ranging cattle. Aust J Exp Agric 2008;48(7):741–745. [Google Scholar]
- 27. Lees AM, Wijffels G, McCulloch R et al. The influence of heat load on merino sheep. 3. Cytokine and biochemistry profiles. Anim Prod Sci 2020;60(16):1940–1948. [Google Scholar]
- 28. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2018. Available at: https://www.R-project.org/.
- 29. gla.ac.uk Normal rectal temperatures. Glasgow, Scotland: University of Glasgow; 2020. Available at: https://www.gla.ac.uk/t4/~vet/files/teaching/clinicalexam/examination/info/temperatures.html#:~:text=The%20rectal%20temperature%20reference%20range,temperature%20outside%20of%20these%20ranges. Accessed 12 November 2020.
- 30. Kessell A. Bovine haematology and biochemistry. In: Cockcroft P, editor. Bovine medicine. 3rd edn. Hoboken, John Wiley & Sons, Incorporated, 2015;146–160. [Google Scholar]
- 31. Chen S, Wang J, Peng D et al. Exposure to heat‐stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci Rep 2018;8(1):14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abo‐Aziza FAM, Hendawy SHM, Oda SS et al. Cell‐mediated and humoral immune profile to hydatidosis among naturally infected farm animals. Vet World 2020;13(1):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burdick‐Sanchez NC, Carroll JA, Broadway PR et al. Some aspects of the acute phase immune response to a lipopolysaccharide (LPS) challenge are mitigated by supplementation with a Saccharomyces cerevisiae fermentation product in weaned beef calves. Transl Anim Sci 2020;4(3):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bordignon R, Volpato A, Glombowsky P et al. Nutraceutical effect of vitamins and minerals on performance and immune and antioxidant systems in dairy calves during the nutritional transition period in summer. J Therm Biol 2019;84:451–459. [DOI] [PubMed] [Google Scholar]
- 35. Ma FT, Shan Q, Jin YH et al. Effect of Lonicera japonica extract on lactation performance, antioxidant status, and endocrine and immune function in heat‐stressed mid‐lactation dairy cows. J Dairy Sci 2020;103(11):10074–10082. [DOI] [PubMed] [Google Scholar]
- 36. Reuter RR, Carroll JA, Dailey JW et al. Effects of dietary energy source and level and injection of tilmicosin phosphate on immune function in lipopolysaccharide‐challenged beef steers. J Anim Sci 2008;86(8):1963–1976. [DOI] [PubMed] [Google Scholar]
- 37. Safa S, Kargar S, Moghaddam GA et al. Heat stress abatement during the postpartum period: Effects on whole lactation milk yield, indicators of metabolic status, inflammatory cytokines, and biomarkers of the oxidative stress. J Anim Sci 2019;97(1):122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. El‐Deeb W, Elsohaby I, Fayez M et al. Use of procalcitonin, neopterin, haptoglobin, serum amyloid A and proinflammatory cytokines in diagnosis and prognosis of bovine respiratory disease in feedlot calves under field conditions. Acta Tropica 2020;204:105336. [DOI] [PubMed] [Google Scholar]
- 39. Burciaga‐Robles LO, Step DL, Krehbiel CR et al. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheima haemolytica on clinical signs and immune variables: model for bovine respiratory disease via viral and bacterial interaction. J Anim Sci 2010;88(6):2166–2178. [DOI] [PubMed] [Google Scholar]
- 40. Ibrahim HMM, El‐Seedy YY, Gomaa NA. Cytokine response and oxidative stress status in dairy cows with acute clinical mastitis. J Dairy Vet Anim Res 2016;3(1):1–6. [Google Scholar]
- 41. Romanowski R, Culbert R, Alkemade S et al. Mycobacterium cell wall fraction immunostimulant (Amplimune™) efficacy in the reduction of the severity of etec induced diarrhea in neonatal calves. Acta Vet 2017;67(2):222–237. [Google Scholar]
- 42. Griebel P. Evaluation of the ability of MCWF Immunostimulant to alter blood leucocyte populations in newborn calves . Experiment Report for Vetrepharm Research Inc.: Vaccine and Infectious Disease Organisation; 1999. 8 December 1999.
- 43. Wedlock DN, Denis M, Lacy‐Hulbert J et al. Interleukin‐1b infusion in bovine mammary glands prior to challenge with Streptococcus uberis reduces bacterial growth but causes sterile mastitis. Vet Res Commun 2008;32(6):439–447. [DOI] [PubMed] [Google Scholar]
- 44. Paape MJ, Schultze WD, Guidry AJ et al. Effect of an intramammary polyethylene device on the concentration of leukocytes and immunoglobulins in milk and on the leukocyte response to Escherichia coli endotoxin and challenge exposure with Staphylococcus aureus. Am J Vet Res 1981;42(5):774–783. [PubMed] [Google Scholar]
- 45. Broderson RJ. A retrospective review of lesions associated with the use of Freund's adjuvant. Lab Anim Sci 1989;39(5):400–405. [PubMed] [Google Scholar]
- 46. Haak T, Delverdier M, Amardeilh MF et al. Pathologic study of an experimental canine arthritis induced with complete Freund's adjuvant. Clin Exp Rheumatol 1996;14(6):633–641. [PubMed] [Google Scholar]
- 47. Shiga M, Miyazaki J, Tanuma K et al. The liposome of trehalose dimycolate extracted from M. bovis BCG induces antitumor immunity via the activation of dendritic cells and CD8+ T cells. Cancer Immunol Immunother 2021;70:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Filion MC, Rodrigues L, Johannes C et al. The in vitro and in vivo anti‐cancer potential of mycobacterium cell wall fraction (mcwf) against canine transitional cell carcinoma of the urinary bladder. Acta Vet 2017;67(4):477–494. [Google Scholar]
- 49. Rogan D, Fumuso E, Rodriguez E et al. Use of a mycobacterial cell wall extract (MCWE) in susceptible mares to clear experimentally induced endometritis with Streptococcus zooepidemicus . J Equine Vet Sci 2007;27(3):112–117. [Google Scholar]
- 50. Yuksel Z, Buber E, Kocagoz T et al. Mycobacterial strains that stimulate the immune system most efficiently as candidates for the treatment of bladder cancer. J Mol Microbiol Biotechnol 2011;20(1):24–28. [DOI] [PubMed] [Google Scholar]
- 51. Medellin‐Peña M. Understanding the role of a mycobacterium phlei immunostimulant in veterinary medicine. Napanee, Ontario, Canada: NovaVive Inc., 2017. [Google Scholar]
- 52. Mašić A, Prunić B, Bugarski D et al. Immunomodulator, mycobacterium cell wall fraction, an aid in control of persistent Mycoplasma bovis infection in dairy cows. Vet Glas 2017;71(1):58–68. [Google Scholar]
- 53. Solano‐Suarez KG. Novel strategies for peripartal health improvement in transition dairy cows [born digital]. Fort Collins, Colorado State University, 2019. [Google Scholar]
- 54. Korf J, Stoltz A, Verschoor J et al. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen‐associated host innate immune responses. Eur J Immunol 2005;35(3):890–900. [DOI] [PubMed] [Google Scholar]
- 55. Strohmeier GR, Fenton MJ. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect 1999;1(9):709–717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Treatment group LSMs (±SEM) for Mean core body temperature (°C) within 6, 12, 24 or 48 h blocks post‐treatment.
Table S2 Experiments in cattle with recorded serum concentrations of pro‐inflammatory cytokine TNFα, pre‐ and post‐challenge.


