Abstract
Background and Aims
In 2015, Georgia began a hepatitis C virus (HCV) elimination programme. Although screening programmes have been decentralized for high‐risk groups, viraemic testing remains a bottleneck for people who inject drugs. Here, we describe two models of viraemic testing that aimed to address this gap.
Methods
We assigned eight harm reduction sites (HRS) to one of three arms (2,1:1): Xpert HCV viral load testing on‐site, blood draw on‐site with centralized HCV core antigen testing (HCVcAg), or standard‐of‐care (SOC) referral with viremia testing performed at treatment centres.
Results
1671 HCV‐seropositive participants were enrolled (Xpert, 37.1%; HCVcAg, 29.1%; referral, 33.8%). Participants were predominantly male (95.4%), mean age (IQR) 43 (37, 50) years and 1290 (77.2%) were currently injecting drugs. Significantly higher proportions of participants in the Xpert (100%) and HCVcAg (99.8%) arms received viraemia testing compared with the referral arm (91.3%) (Xpert vs referral, p < 0.0001; HCVcAg vs referral, p < 0.0001). Among viraemic participants, treatment uptake was similar (Xpert, 84.0%; HCVcAg, 79.5%; referral, 88.4%). The time between screening and sample collection for viraemia testing was significantly longer in the referral arm compared with both Xpert and HCVcAg arms (median 1 day compared with 0 days respectively), and the overall time between screening to treatment initiation was longer for the referral arm (median 67 days) compared with both Xpert and HCVcAg arms (median 57 and 50 days respectively).
Conclusions
Point‐of‐care viraemia testing and blood drawn on‐site for HCVcAg testing yielded more HCV‐seropositive patients receiving viraemic testing within a shorter timeframe compared with referrals.
Keywords: decentralized viremia testing, Hepatitis C, linkage to care, People who inject drugs
Abbreviations
- eCRFs
electronic case report forms
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HCVcAg
HCV core antigen
- HRS
harm reduction sites
- IQR
interquartile range
- LMICs
low‐ and middle‐income countries
- NAT
nucleic acid amplification testing
- POC
point‐of‐care
- PWID
people who inject drugs
- RDTs
rapid diagnostic tests
- SOC
Standard‐of‐care
- SVR
sustained virological response
- VL
viral load
Lay summary
The Georgian National Hepatitis Program has made strong progress in bringing hepatitis C screening to low levels of the health system, however, there is a drop off, especially among people who inject drugs, when people need to get the test to confirm if they are viraemic. This study looked at different ways to improve the retention of people who screened hepatitis C‐positive receiving a test to confirm if they have viremia and thus need treatment for hepatitis C. We found that both providing onsite RNA testing and onsite blood draw for sample referral for testing for viremia resulted in more people getting the viremia test than the standard of care method of referring people to another clinic after hepatitis C screening.
1. INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease globally, with an estimated 58 million individuals chronically infected as of 2019 1 and a disproportionately high burden in low‐ and middle‐income countries (LMICs). 2
Georgia is a middle‐income country with an estimated 3.7 million residents and a high prevalence of HCV infection, with 5.4% of the adult population chronically infected. 3 The burden of disease is largely among people who inject drugs (PWID), as well as a result of inadequate infection control measures in healthcare settings. 4 The number of PWID increased from 40 000 in 2009 5 to 52 500 in 2015. 6 The prevalence of HCV infection among PWID is reported to be between 50% and 92% in different regions. 7 , 8 , 9 , 10
In 2015, Georgia embarked on an HCV elimination programme to meet WHO HCV elimination targets. 3 Successful features of Georgia’s approach include high‐level political commitment, a funded national strategic plan, strong partnerships for operational research, and decentralization of care to the primary care level.
When this study began in 2018, 52 856 HCV‐infected individuals nationally had registered with the HCV programme. 11 Of these, 45 334 (85.8%) had started HCV treatment, of whom 40 946 (90.3%) completed treatment; 29 620 (79.5%) of those treated had a sustained virologic response (SVR) test 12 weeks after completion of treatment; 29 090 (98.2%) had no detectable virus.
A major barrier in the cascade of care is access to viral load (VL) confirmation and, therefore, uptake of treatment. In 2018, there were >20 000 HCV antibody‐positive individuals who had not been linked to care or had a confirmatory VL test. 12 , 13 , 14 HCV testing is currently undertaken at hospitals, harm reduction sites (HRS) and primary healthcare sites using rapid diagnostic tests (RDTs). Seropositive individuals are then referred to specialized treatment centres for HCV RNA testing and pre‐treatment assessment. Treatment of viraemic patients at designated treatment centres is initiated following authorization by the Social Service Agency. Prior to changes to the treatment committee in 2018, this resulted in a long wait between patients receiving a positive HCV‐RNA result and completing all pretreatment investigations and their treatment initiation (median 28 days, interquartile range [IQR] 21–38 days). 15
This study aimed to address key gaps and barriers identified in the care pathway, by simplifying and decentralizing HCV care into primary healthcare settings. The objectives of this prospective comparative study were to compare the feasibility and effectiveness of three different approaches to the confirmation of viraemia: Arm 1 (on‐site Xpert), on‐site point‐of‐care (POC) HCV VL testing using Xpert ® HV Viral Load assay; Arm 2 (centralized HCVcAg), blood collection on‐site for HCV core antigen (HCVcAg) testing at the Richard Lugar Center for Public Health Research (Lugar Center); Arm 3 (referral), patients referred to designated treatment centres for HCV nucleic acid amplification testing (NAT), the current standard of care (SOC). Key outcomes evaluated across the cascade of care included retention of patients and turn‐around time. Our hypothesis was that access to POC VL testing would increase uptake and reduce the time to VL confirmation compared with SOC.
The findings of this study will inform decision making on how best to position and implement HRS‐based POC HCV viraemia testing as an alternative to SOC.
2. METHODOLOGY
2.1. Study design
A non‐randomized interventional study was conducted among PWID at eight HRS throughout Georgia (Figure 1), to evaluate two novel approaches (on‐site Xpert and centralized HCVcAg arms) to HCV viraemia testing and compare them with the current SOC (referral arm). The HRS were assigned to one of the three study arms (Figure 1). In all arms, patients were screened for HCV infection using an RDT; HCV‐seropositive patients were invited to enrol in the study. In the on‐site Xpert arm, participants at four HRS (Tbilisi New Vector, Zugdidi Xenon, Kutaisi New Way, Batumi Imedi) had venous blood collected for on‐site HCV RNA testing using the Xpert® HCV Viral Load assay. In the centralized HCVcAg arm, participants at two HRS (Tbilisi Akeso, Rustavi New Vector) had venous blood drawn on‐site; the blood was transported to Lugar Center for HCVcAg testing. In the referral arm, seropositive participants at two HRS (Gori Step to Future, Tbilisi New Way) were referred to treatment centres for venous blood collection and HCV RNA testing. Viraemic participants from all three arms were referred to designated treatment centres for pretreatment assessment and treatment initiation. To control for the effect of cost, consultations and additional tests (genotyping, fibrosis staging using the Fibrosis‐4 index, hepatitis B virus [HBV] screening and other biochemical tests) were provided free of charge for all participants.
FIGURE 1.
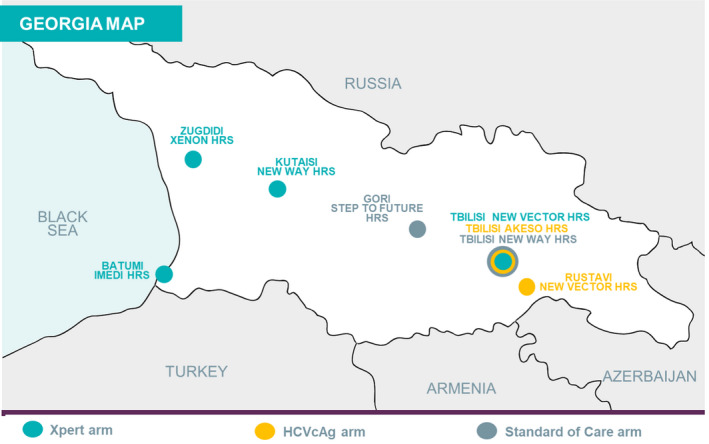
Setting and location of study sites
2.2. Settings and site selection
There are 14 HRS in Georgia (four in Tbilisi, the capital), providing healthcare and needle and syringe services. Eight HRS were selected, based on the following criteria: HCV antibody testing with an RDT available on‐site; on‐site Xpert arm, adequate space for the GeneXpert instrument; on‐site Xpert and centralized HCVcAg arms, a centrifuge and refrigerator available on‐site for sample processing and storage and a phlebotomist available on‐site to draw blood for HCV RNA testing. The limited number of HRS in Georgia precluded formal matching across the study arms; however, to the extent possible, sites were selected based on similarities, including the number of PWID screened for HCV infection each month, proportion of seropositive patients each month, distance from HRS to a treatment centre and settlement type (eg urban vs rural).
2.3. Study procedures
Trained research staff invited eligible, HCV‐seropositive patients to participate in the study and obtained their written informed consent. Participants in the on‐site Xpert and centralized HCVcAg arms had venous blood drawn on‐site for HCV viraemia testing. Venous whole blood (10 ml) was collected in K2‐EDTA, EDTA‐plasma preparation, or serum collection tubes, which were centrifuged on‐site to separate the plasma. In the on‐site Xpert arm, HCV RNA testing was performed on‐site using the Xpert® HCV Viral Load test. Results were made available to participants within 2 hours of blood being drawn. In the centralized HCVcAg arm, labelled plasma samples were shipped to the Lugar Center within 72 hours for HCVcAg testing. Results were returned to the HRS via the national hepatitis C screening database (Stop C) and made available to study participants in person or via telephone. In the referral arm, participants were referred for blood collection at treatment centres and results were returned to the sites via Stop C and communicated to participants either in person or by SMS (Figure 2).
FIGURE 2.
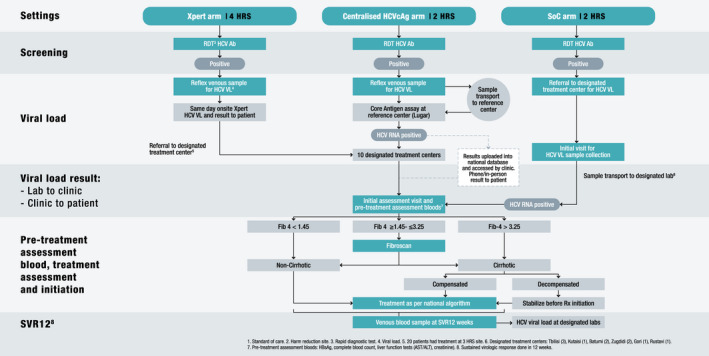
Clinical care pathways
To detect the proportion of anti‐HCV‐positive patients who receive viremia testing with precision of at least 10%, 90% power, and a significance level of 5%, and assuming a 5% rate of loss to follow‐up, a sample size of 620 participants per arm was chosen, for a total of 1860 participants.
At enrolment, all participants were invited to participate in face‐to‐face interviews to obtain demographic data (age, sex, level of education, socio‐economic status), medical history, co‐morbidities, substance and alcohol use, other potential risk factors for not linking into care, and perceptions around HCV viraemia testing.
2.4. Treatment regimen and evaluation of cure
Eligible participants received treatment according to the national treatment guidelines. Twelve weeks after treatment completion, participants were requested to return to the treatment centre for a final venous blood sample to be collected (5 ml) for sustained viral response (SVR‐12) VL testing, using HCV NAT, to assess cure. Participants who had detectable VL (>10 IU/ml) were re‐treated, according to the national treatment guidelines (Figure 2).
2.5. Follow‐up visits
All participants completed a questionnaire 4 to 8 weeks after enrolment to assess their perceptions around HCV testing. Six months after enrolment, all participants were interviewed about their perception of the testing process, barriers to testing and treatment and steps in the HCV care cascade they completed.
2.6. Feasibility of decentralized HCV viraemia testing
The feasibility of decentralized HCV viraemia testing was assessed by determining operational aspects, including indeterminate rates and assay failure rates. Data from the GeneXpert instrument were downloaded for monthly monitoring. Operational characteristics were assessed at the four sites implementing decentralized testing (on‐site Xpert arm), through direct observation. The feasibility of HCVcAg testing was assessed by determining operational aspects at the Lugar Center and at HRS that implement HCVcAg testing.
2.7. Data collection and analysis
Data captured using electronic case report forms (eCRFs) were directly entered into OpenClinica Enterprise edition v 3.14. The final dataset was cleaned of errors. Data were analysed using SAS version 9.4 to provide descriptive statistics and perform bivariate analyses. Participants' characteristics were summarized by demographic, clinical, laboratory and treatment variables, as well as study arm, with medians and IQRs for quantitative data and frequencies and percentages for qualitative data. Bivariate analysis was used to examine patient‐level factors associated with attrition across the care cascade using Chi‐square tests, or Fisher’s exact test when cell counts were less than 5. Turn‐around time was calculated using date and time captured in the eCRF for clinical and laboratory steps in the care cascade; times (medians and IQRs [days]) were compared across study arms using Wilcoxon rank sum tests. A significance level of p < 0.05 was used for all statistical tests.
2.8. Ethical considerations
Permission to conduct the study was obtained from the Georgia Institutional Review Boards (NCDC IRB # 2017–046 and Health Research Union [HRU] IRB # 2017–12, IRB00009520). The study is registered at clinicaltrials.gov ID NCT03594838.
3. RESULTS
3.1. Participant characteristics
Of 1958 patients screened for study eligibility, 1671 (620, 486 and 565 in the on‐site Xpert, centralized HCVcAg and referral arms respectively) HCV‐seropositive adults were consecutively enrolled between May 2018 and September 2019; the full sample size was not reached in the latter two arms because of time constraints. The median (IQR) age was 43 (37, 50) years, with no differences across arms (on‐site Xpert, 43 [38, 50]; centralized HCVcAg, 43 [36, 50]; and referral arm, 43 [37, 50]; p > 0.05) (Table 1). Participants were predominantly male, with a lower proportion of males in the centralized HCVcAg arm (449 [92.4%]) compared with the proportions in the on‐site Xpert (595 [96.0%], p = 0.01) and referral arms (550 [97.3%], p = 0.0002). There was a significant difference in the distribution of ethnic groups, education level and employment status in the on‐site Xpert arm compared with both the centralized HCVcAg and referral arms and a significant difference in the proportion of participants with a self‐reported sexually transmitted infection (STI) in the centralized HCVcAg arm compared with both the on‐site Xpert and referral arms. There were significant differences between the use of HRS services, such as needle exchange programmes (NEP) and peer support, across all three study arms, as well as current injecting drug‐use, with the centralized HCVcAg arm having a significantly higher proportion of people who reported currently injecting than both the on‐site Xpert and referral arms (on‐site Xpert, 479 [77.3%]; centralized HCVcAg, 463 [95.3%] and referral arm, 348 [61.6%]; [all arms p < 0.0001]).
TABLE 1.
Patient characteristics
| Variables | Total | On‐site Xpert arm | Centralized HCVcAg arm | Referral arm | On‐site Xpert vs centralized HCVcAg arm | On‐site Xpert vs referral arm | Centralized HCVcAg arm vs referral arm | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 1671 | 620 | 486 | 565 | p‐valuesa | ||||||
| Age | |||||||||||
| Median (IQR) years | 43 (37, 50) | 43 (38, 50) | 43 (36, 50) | 43 (37, 50) | 0.42 | 0.76 | 0.58 | ||||
| n | % | n | % | n | % | n | % | ||||
| 18–30 | 102 | 6.1 | 38 | 6.1 | 36 | 7.4 | 28 | 5.0 | 0.04 | 0.25 | 0.31 |
| 31–40 | 548 | 32.8 | 196 | 31.6 | 166 | 34.2 | 186 | 32.9 | |||
| 41–50 | 631 | 37.8 | 238 | 38.4 | 170 | 35.0 | 223 | 39.5 | |||
| 51–60 | 328 | 19.6 | 134 | 21.6 | 89 | 18.3 | 105 | 18.6 | |||
| >60 | 62 | 3.7 | 14 | 2.3 | 25 | 5.1 | 23 | 4.1 | |||
| Sex | |||||||||||
| Male | 1594 | 95.4 | 595 | 96.0 | 449 | 92.4 | 550 | 97.3 | 0.01 | 0.19 | 0.0002 |
| Female | 77 | 4.6 | 25 | 4.0 | 37 | 7.6 | 15 | 2.7 | |||
| Ethnic group | |||||||||||
| Georgian | 1576 | 94.3 | 589 | 95.0 | 452 | 93.0 | 535 | 94.7 | 0.0002 | 0.0008 | 0.52 |
| Armenian | 34 | 2.0 | 21 | 3.4 | 7 | 1.4 | 6 | 1.1 | |||
| Other | 61 | 3.7 | 10 | 1.6 | 27 | 5.6 | 24 | 4.2 | |||
| Education level | |||||||||||
| Primary | 10 | 0.6 | 1 | 0.2 | 4 | 0.8 | 5 | 0.9 | 0.01 | 0.003 | 0.94 |
| Secondary | 989 | 59.2 | 337 | 54.4 | 229 | 61.5 | 353 | 62.5 | |||
| Tertiary | 672 | 40.2 | 282 | 45.5 | 183 | 37.7 | 207 | 36.6 | |||
| Employment status | |||||||||||
| Employed | 590 | 35.3 | 199 | 32.1 | 198 | 40.7 | 193 | 34.2 | 0.002 | 0.02 | 0.07 |
| Unemployed | 1062 | 63.6 | 419 | 67.6 | 282 | 58.0 | 361 | 63.9 | |||
| Other (Student, Retired) | 19 | 1.1 | 2 | 0.3 | 6 | 1.2 | 11 | 2.0 | |||
| Marital status | |||||||||||
| Married | 1115 | 66.7 | 429 | 69.2 | 311 | 64.0 | 375 | 66.4 | 0.19 | 0.13 | 0.21 |
| Divorced/Widowed | 195 | 11.7 | 61 | 9.8 | 57 | 11.7 | 77 | 13.6 | |||
| Single | 361 | 21.6 | 130 | 21.0 | 118 | 24.3 | 113 | 20.0 | |||
| Co‐morbidities (self‐reported)b | |||||||||||
| PLHIV | |||||||||||
| Yes | 12 | 0.7 | 4 | 0.7 | 6 | 1.2 | 2 | 0.4 | 0.58 | 0.27 | 0.15 |
| No | 1589 | 99.3 | 546 | 99.3 | 480 | 98.8 | 563 | 99.6 | |||
| On ART, ever, among PLHIV | |||||||||||
| Yes | 3 | 25.0 | 2 | 50.0 | 1 | 16.7 | 0 | 0.0 | 0.5 | 0.47 | 1.00 |
| No | 9 | 75.0 | 2 | 50.0 | 5 | 83.3 | 2 | 100.0 | |||
| HBV | |||||||||||
| Yes | 37 | 2.3 | 13 | 2.4 | 12 | 2.5 | 12 | 2.2 | 0.92 | 0.84 | 0.76 |
| No | 1546 | 97.7 | 534 | 97.6 | 474 | 97.5 | 538 | 97.8 | |||
| STI | |||||||||||
| Yes | 328 | 19.6 | 155 | 25.0 | 25 | 5.1 | 148 | 26.3 | <0.0001 | 0.17 | <0.0001 |
| No | 1340 | 81.4 | 465 | 75.0 | 461 | 94.9 | 414 | 73.7 | |||
| TB disease | |||||||||||
| Yes | 10 | 0.6 | 6 | 1.0 | 4 | 0.8 | 0 | 0.0 | 0.73 | 0.02 | 0.06 |
| No | 1659 | 99.4 | 614 | 99.0 | 481 | 99.2 | 564 | 100.0 | |||
| Harm reduction attendance | |||||||||||
| Currently Attending HRS | 732 | 43.8 | 432 | 69.7 | 73 | 15.0 | 227 | 40.2 | <0.0001 | <0.0001 | <0.0001 |
| Types of HRS attended, among currently attending participants | |||||||||||
| NSEP | 515 | 70.4 | 324 | 75.0 | 44 | 60.3 | 147 | 64.8 | 0.009 | 0.006 | 0.49 |
| OST | 222 | 30.3 | 88 | 20.4 | 53 | 72.6 | 81 | 35.7 | <0.0001 | <0.0001 | <0.0001 |
| NSEP & OST | 50 | 6.8 | 23 | 5.3 | 24 | 32.9 | 3 | 1.3 | <0.0001 | 0.01 | <0.0001 |
| Peer education/Case management programmes | 60 | 8.2 | 58 | 13.4 | 0 | 0 | 2 | 0.9 | 0.001 | <0.0001 | 0.42 |
| Risk behaviours | |||||||||||
| Currently injecting drugs | 1290 | 77.2 | 479 | 77.3 | 463 | 95.3 | 348 | 61.6 | <0.0001 | <0.0001 | <0.0001 |
| First age of injecting drug use (years), median (IQR) | 20 (18, 24) | 20 (18, 23) | 20 (19, 23) | 20 (18, 25) | 0.07 | 0.01 | 0.42 | ||||
| Drug use (past 6 months) | |||||||||||
| Opioids | 1261 | 75.5 | 454 | 73.2 | 449 | 92.4 | 358 | 63.4 | <0.0001 | 0.0003 | <0.0001 |
| Marijuana | 1096 | 65.6 | 391 | 63.1 | 364 | 74.9 | 341 | 60.4 | <0.0001 | 0.34 | <0.0001 |
| Amphetamine | 150 | 9.0 | 60 | 9.7 | 50 | 10.3 | 40 | 7.1 | 0.74 | 0.11 | 0.06 |
| Cocaine | 58 | 3.5 | 23 | 3.7 | 10 | 2.1 | 25 | 4.4 | 0.11 | 0.53 | 0.03 |
| Club drugs | 35 | 2.1 | 13 | 2.1 | 13 | 2.7 | 9 | 1.6 | 0.53 | 0.52 | 0.22 |
| Needle sharing (past 6 months), among all participants | |||||||||||
| Never | 969 | 58.0 | 383 | 61.8 | 300 | 61.7 | 286 | 50.6 | <0.0001 | <0.0001 | <0.0001 |
| Always | 10 | 0.6 | 6 | 1 | 4 | 0.8 | 0 | 0.0 | |||
| Sometimes | 295 | 17.6 | 93 | 15.0 | 163 | 33.5 | 39 | 6.9 | |||
| No Response or N/A | 397 | 23.8 | 138 | 22.3 | 19 | 3.9 | 240 | 42.5 | |||
| Cirrhosis, among participants enrolled in the treatment programme | |||||||||||
| Yes | 109 | 9.8 | 38 | 9.5 | 28 | 8.4 | 43 | 11.2 | 0.63 | 0.41 | 0.21 |
| No | 1006 | 90.5 | 364 | 90.5 | 304 | 91.6 | 340 | 88.8 | |||
| Decompensated (moderate or severe hepatic impairment; Child‐Pugh class B or C) | |||||||||||
| Yes | 10 | 0.9 | 4 | 1.0 | 2 | 0.6 | 4 | 1.0 | 0.7 | 1 | 0.69 |
| No | 1104 | 99.1 | 398 | 99.0 | 329 | 99.4 | 377 | 99.0 | |||
Abbreviations: ART, antiretroviral therapy; HBV, hepatitis B virus; HRS, harm reduction site; IQR, interquartile range; NSEP, needle syringe exchange program; OST, opioid substitution therapy; PLHIV, persons living with HIV; STI, sexually transmitted infection; TB, tuberculosis
p‐values for categorical variables derived from chi‐square or Fisher’s exact tests; for difference in medians, Wilcoxon rank sum tests were used.
Responses of “do not know” are excluded.
3.2. HCV care cascade retention
Of 1671 anti‐HCV‐positive adults enrolled, 1621 (97.0%) participants received a confirmatory viraemia test. Significantly higher rates of viraemic testing were reported for the on‐site Xpert (620, 100.0%) and centralized HCVcAg arms (485, 99.8%) compared with rates in the referral arm (516, 91.3%, p < 0.0001 for both comparisons) (Figure 3). Of those confirmed positive, treatment uptake was similar (p = 0.08) for participants in the on‐site Xpert and referral arms (432 [84.0%] and 373 [88.4%] respectively) but was lower in the centralized HCVcAg arm (318 [79.5%]) compared with the referral arm (p = 0.0005).
FIGURE 3.
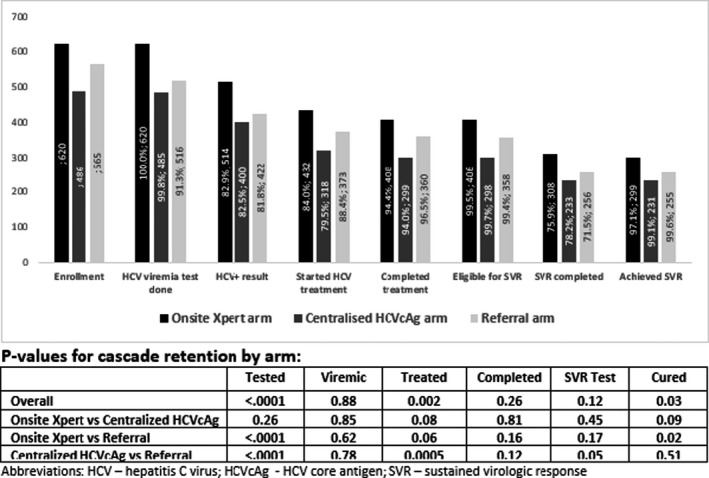
Retention of patients in the hepatitis C care cascade by study arm
Of the 1123 patients in whom treatment was initiated, the majority (1006 [90.2%]) were non‐cirrhotic and received a 12‐week regimen, while 109 (9.8%) were cirrhotic (10 [0.9%] were decompensated) (Table 1) and received treatment for 24 weeks, with no significant differences in rates of cirrhosis across any arms (data not shown). Similar proportions of participants completed treatment across all study arms (on‐site Xpert, 408 [94.4%]; centralized HCVcAg, 299 [94.0%] and referral arm, 360 [96.5%]; all p > 0.05). Overall, 49 (4.4%) participants discontinued treatment; 8 (0.7%) were lost to follow‐up, 4 (0.4%) were non‐compliant, 6 (0.5%) died and 16 (1.4%) had an unspecified reason for discontinuation. The proportion of participants who discontinued treatment was similar across all arms (all p > 0.05).
Of the participants eligible for SVR‐12 testing at study completion (on‐site Xpert, 406 [99.5%]; centralized HCVcAg, 298 [99.7%] and referral arm, 358 [99.4%]), similar proportions received SVR‐12 testing in all arms (on‐site Xpert, 308 [75.9%]; centralized HCVcAg, 233 [78.2%] and referral arm, 256 [71.5%]). Cure, defined as undetectable HCV VL, was achieved among 785 of the 797 [98.5%] participants who received SVR testing with similarly high rates across all arms (on‐site Xpert, 299 [97.1%]; centralized HCVcAg, 231 [99.1%] and referral arm, 255 [99.6%]). None of the following variables had an association with treatment failure: age, sex, cirrhotic status, genotype, Fib4 score, drug regimen, active drug‐use, frequency of alcohol use, or HRS use (data not shown). Of the 12 (1.5%) participants who experienced treatment failure, 4 (33.3%) were re‐treated. There was heterogeneity in cascade outcomes because of site‐to‐site variation within and between arms (supplementary Figure S1).
3.3. Turn‐around time between HCV care cascade steps
The turn‐around time (median (IQR) [range] days) between VL testing to return of results to participants was significantly shorter in the on‐site Xpert arm (0.01 (0.01, 0.02), [0.0–3.0] days) compared with both the centralized HCVcAg (8.9 (6.0, 15.0), [0.04–118.1] days, p < 0.001) and referral arms (6.8 (3.9, 12.8), [0.1–376.7] days, p < 0.0001) (Table 2). The time between return of results to participants and initiation of treatment was longest for the on‐site Xpert arm (57 (38, 87), [9–776 days]) compared with both the centralized HCVcAg (31 (23, 61), [11–604 days] days, p < 0.0001) and referral arms (43 (29, 68), [1–636] days, p < 0.0001). The total time between HCV serological testing and treatment initiation was significantly longer for the referral arm (67 (45, 94), [18–776] days) compared with both the on‐site Xpert (57 (39, 87), [9–776] days, p = 0.0006) and centralized HCVcAg arms (50 (38, 80), [21–673] days, p < 0.0001) (Table 2).
TABLE 2.
Time between HCV care cascade steps
|
Time median (IQR) [range] |
HCV screening to sample collection for viraemia test | Sample collection to completion of sample testing | Test results to patient notification | Patient notification to treatment initiation | Total time (HCV screening to treatment initiation) |
|---|---|---|---|---|---|
| On‐site Xpert arm |
0 (0, 0) days [0–1 days] n = 620 |
0.07 (0.07, 0.08) days [0.06–0.97 days] n = 620 |
0.01 (0.01, 0.02) days [0.0–3.0 days] n = 620 |
57 (38, 87) days [9–776 days] n = 432 |
57 (39, 87) days [9–776 days] n = 432 |
| Centralized HCVcAg arm |
0 (0, 0) days [0–63 days] n = 483 |
5.9 (3.1, 8.0) days [0.2–65.2 days] n = 483 |
8.9 (6.0, 15.0) days [0.04–118.1 days] n = 483 |
31 (23, 61) days [11–604 days] n = 318 |
50 (38, 80) days [21–673 days] n = 318 |
| Referral arm |
1 (0, 4) days [0–483 days] n = 508 |
5.1 (1.2, 7.9) days [0.0–92.1 days] n = 505 |
6.8 (3.9, 12.8) days [0.1–376.7 days] n = 498 |
43 (29, 68) days [1–636 days] n = 366 |
67 (45, 94) days [18–776 days] n = 373 |
| Chi‐square p‐values | |||||
| On‐site Xpert vs. Centralized HCVcAg | 0.06 | <0.0001 | <0.0001 | <0.0001 | 0.58 |
| On‐site Xpert vs. Referral | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0006 |
| Centralized HCVcAg vs. Referral | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Times from viraemia testing to database entry: On‐site Xpert arm – 0 (0, 0) days, [0–21 days]; centralized HCVcAg arm – 3 (1, 6 days), [0–43 days]; referral arm – 2 (0, 4 days), [0–65 days].
Abbreviations: HCV, hepatitis C virus; IQR, interquartile range.
3.4. Feasibility of Xpert testing in HRS
Data were available for 619 samples for the on‐site Xpert arm; of those, 597 (96.4%) successfully provided a result, 514 (83.0%) were positive and none were indeterminate. There were 22 (3.6%) samples that resulted in errors, including 2 (0.3%) cartridge‐based errors, 3 (0.5%) machine‐based errors and 17 (2.7%) user‐based errors. Daily throughput was 4.7 samples per day.
Of the 485 participants who had samples taken for HCVcAg testing in the centralized HCVcAg arm, 4 (0.8%) participants' samples were rejected because of insufficient sample volume, these participants were recalled to the HRS to provide a second sample. All four participants returned to provide the second sample.
The national testing algorithm is used at the Lugar Center; samples that test HCVcAg‐negative or are HCVcAg‐indeterminate (≥3 to <10 fmol/L) are retested using a molecular platform for HCV RNA. 11 Therefore, if needed, the sample volume must be sufficient to complete both tests.
There were 16 (3.3%) samples that were HCVcAg‐indeterminate; of these 4 (25.0%) samples had insufficient sample volume to complete the HCV RNA test, these participants were requested to return to the HRS for resampling, 2 (50%) of whom returned. There were 14 samples tested, and 12 (85.7%) were positive, for HCV RNA. Of the 94 (19.4%) samples that tested negative for HCVcAg and required an HCV RNA test, 8 (8.5%) had insufficient sample volume, these participants were requested to return to their HRS for resampling, 5 (62.5%) of whom returned. In total, 91 samples underwent an HCV RNA test, of which 19 (20.9%) were positive for HCV RNA.
4. DISCUSSION
In this comparative evaluation, we report outcomes using two novel models of HCV VL testing. There are three key findings. First, we observed that the proportion of HCV‐seropositive participants who received viraemia testing was significantly higher in both intervention arms compared with the SOC. Second, the time between HCV screening and VL sample collection was significantly shorter for participants in the two intervention arms, because blood collection for viraemic testing was carried out at an HRS, unlike the SOC, in which patients are referred to treatment centres for RNA testing. Third, we observed that the time between VL sample collection and results being returned to patients was significantly shorter in the arm employing POC viraemic testing (Xpert) compared with the other two arms, where testing was carried out at centralized laboratories.
A significantly higher proportion of seropositive patients received viraemic testing when venous blood draw and/or POC testing were provided at an HRS compared with referral of patients between sites for blood collection for testing. Importantly, however, overall retention rates across the care cascade (including treatment initiation) remained comparably high for all study arms, indicating a strong cascade in this setting. The loss to follow‐up observed in the referral arm was probably associated with various practical barriers, including the distance participants had to travel from HRS/home to treatment centres and associated transportation costs, as well as perceptions of stigma for key high‐risk populations at higher‐tier health facilities. The statistically significant lower number of treatment initiation in centralized HCVcAg arm compared to referral arm may have been influenced by participant motivation as well as the difference in site population risk profiles. In all arms, most patients had to travel to a different clinic for treatment initiation. Referral participants additionally travelled to the clinic for the RNA testing, whereas centralized HCVcAg arm participants received the blood draw for RNA testing at the HRS. While less participants (91.3%) received RNA testing in the referral arm compared to the Xpert arm and centralized HCVcAg arm (100% and 99.8% respectively), those who completed RNA testing step‐in referral arm may have been more motivated to seek HCV care and treatment. Similar observations of the impact of patient referral on retention in the HCV care cascade have been reported, for example a recent systematic review by Oru et al. demonstrated that fully decentralized (‘one‐stop shop’) (n = 29 studies) compared with partially decentralized (patient referral) (n = 11 studies) HCV care models at Opioid Substitution Sites resulted in higher uptake of viremia testing (99% [95%CI 98%–100%] vs 87% [95%CI 81%–92%]). 16
As would be expected, there was a shorter time between HCV antibody RDT and blood collection for both the on‐site Xpert POC test and on‐site blood draw for centralized HCVcAg laboratory testing arms compared with the referral arm, consistent with a comparable study conducted by Iwamoto et al., which found similar shorter turn‐around times between HCV serological testing and confirmatory testing using GeneXpert on‐site compared with laboratory‐based confirmatory testing (0 vs 4 days). 17
We observed a significantly shorter time between VL test sample collection and return of results to patients in the on‐site Xpert arm (Xpert HCV RNA) compared with the centralized HCVcAg and referral arms (HCVcAg and HCV RNA respectively). POC VL testing is unique in this regard, providing a patient with same‐day results during a single visit. WHO currently promotes several good practice principles for simplified service delivery, including standardized algorithms, decentralized HCV testing and treatment, integration with harm‐reduction services and task‐shifting, 16 but these principles do not yet include the use of POC VL testing platforms. In addition, POC VL testing for HIV in adults and early infant diagnosis can provide same‐day results and significant reductions in result turn‐around times compared with centralized laboratory testing. 18 , 19
The time between testing and treatment initiation was longest in arm 1 compared to arm 2. This may have been caused by several factors. Arm 1 participants were recruited earlier and at a faster rate than Arm 2 and 3. Therefore, more participants were recruited to arm 1 before the simplification of the Georgian National Treatment Committee in August 2018. The time from notification of results to treatment was significantly longer before the change for all three arms. Other factors such as seasonal migration out of the country for employment might have also contributed to the longer time to treatment initiation in arm 1. Additionally, participants in arm 1 may have postponed treatment initiation as they had heard that changes to policy would soon enable them to receive treatment direct at the HRS.
There were several improvements made to the care pathway of the national HCV programme during the course of this project. First, VL confirmation and the cost of any additional tests required prior to treatment were made free‐of‐charge nationwide. Second, the practice of genotype testing prior to treatment was discontinued, removing an additional step in the cascade. Third, the process of central committee approval for treatment initiation was simplified and expedited. Finally, the capacity to initiate treatment at HRS was introduced in November 2019, with 20 participants receiving their treatment at on‐site Xpert arm HRS.
There were several challenges that impacted this study. Most notably, the COVID‐19 outbreak in Georgia affected all study arms, delaying treatment initiation and completion of SVR testing in some participants. Beyond the study cohort, COVID‐19 has had a major impact on HRS. HCV RDT screening rates decreased nationally by 48% between January and July 2020 in comparison with the same period in 2019 (168, 279 vs 270, 526), and the number of PWID who were enrolled in treatment during the same period also decreased, by 34% (202 vs 307). 20
Our findings have important implications for the future scale‐up of the Georgian national HCV programme, as well as for programmes in other eastern European countries where the PWID population is an important driver of HCV infections. The high level of participant retention and short turn‐around times observed in the on‐site Xpert and centralized HCVcAg arms have encouraged the national programme to introduce blood draw at all HRS that do not have HCV POC VL testing platforms. Utilizing sample transport mechanisms via National Centers for Disease Control Georgia Regional Centers, centralized confirmation can be performed at the Lugar Center under the state HCV programme. At three of the four HRS sites in the on‐site Xpert arm, the national programme now offers treatment integration (for non‐cirrhotic patients) at the HRS, to provide a fully decentralized package of HCV service delivery.
This study had some important limitations, including non‐random assignment of the HRS and heterogeneity between and within the study arms, such as the number of participants and sites, demographics, risk‐factor profiles and HRS use by participants (Table 1), which may have resulted in some bias in the outcomes, including cascade retention and turn‐around times. In addition, changes made to patient‐care procedures during the study, as outlined above, may have biased or confounded the outcomes observed (eg treatment of some participants in the on‐site Xpert arm at HRS towards the end of the study).
In conclusion, POC viraemia testing and blood draw on‐site for HCVcAg testing resulted in a higher proportion of HCV‐seropositive patients receiving viraemic testing within a shorter timeframe compared with a referral for blood collection using the standard of care.
AUTHORS CONTRIBUTIONS
S Shilton, VC, AG, IK and MJ conceptualized the study. VC led the protocol development. VC, S Shilton, AG, IK, MJ, KS, AA, MB, MA, MT and LG finalized the protocol. S Shadaker completed the statistical analysis. JM wrote the first draft of the manuscript. S Shilton, IK, MJ and RR provided study coordination and supervision. EA, PE and AG provided overall technical input and guidance. S Shilton, JM, VC, MJ, S Shadaker, LG, MT, MA, MB, KS, RR, AA, EA, PE, IK and AG edited the manuscript. All authors have read and approved the final manuscript.
DISCLAIMER
The findings and conclusions in this report are those of the authors and not necessarily the official position of the US Centers for Disease Control and Prevention.
TRIAL REGISTRATION
The study is registered at clinicaltrials.gov ID NCT03594838.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
Supporting information
Figure S1 Retention in the hepatitis C care cascade by site
ACKNOWLEDGEMENTS
We thank Maka Gogia, the Georgian Harm Reduction Network, and each of the Harm Reduction Sites and their staff, for the strong collaboration and support of this study. We are especially grateful to the study participants. Editorial support was provided by Adam Bodley.
Shilton S, Markby J, Japaridze M, . Feasibility and effectiveness of HCV viraemia testing at harm reduction sites in Georgia: A prospective three‐arm study. Liver Int. 2022;42:775–786. doi: 10.1111/liv.15191
Sonjelle Shilton and Jessica Markby contributed equally to this work.
Handling Editor: Alessio Aghemo
Funding informationThis study was funded by the Unitaid as part of the multi‐country HEAD‐Start (Hepatitis Elimination through Access to Diagnostics) project.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author.
REFERENCES
- 1. WHO . Hepatitis C: Key facts. 2020. https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐c.
- 2. WHO . Progress report on access to hepatitis C treatment: Focus on overcoming barriers in low‐ and middle‐income countries. CC BY‐NC‐SA 3.0 IGO. World Health Organization; 2018. [Google Scholar]
- 3. Gvinjilia L, Nasrullah M, Sergeenko D, et al. National Progress toward Hepatitis C Elimination – Georgia, 2015‐2016. MMWR Morb Mortal Wkly Rep. 2016;65(41):1132‐1135. [DOI] [PubMed] [Google Scholar]
- 4. Mitruka K, Tsertsvadze T, Butsashvili M, Gamkrelidze A., Sabelashvili P., Adamia E., Chokheli M., Drobeniuc J., Hagan L., Harris A.M., Jiqia T., Kasradze A., Ko S., Qerashvili V., Sharvadze L., Tskhomelidze I., Kvaratskhelia V., Morgan J., Ward J.W., Averhoff F. Launch of a Nationwide hepatitis C elimination program — Georgia, April 2015. 2015. MMWR Morb Mortal Wkly Rep 2015; 64(28): 753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sirbiladze T, Tavzarashvili L, Zabransky T, Sturua L. Estimating the prevalence of injection drug use in five cities in Georgia: Programme of assistance for the prevention of drug abuse and drug trafficking in the southern Caucasus (SCAD Programme), 2009.
- 6. Shengelia N, Chikovani I, Sulaberidze L, Sirbiladze T, Tavzarashvili L. HIV risk and prevention behaviors among people who inject drugs in seven cities of Georgia, bio‐behavioral surveillance survey study report. Curatio International Foundation; 2015. [Google Scholar]
- 7. Stvilia K, Tsertsvadze T, Sharvadze L, et al. Prevalence of hepatitis C, HIV, and risk behaviors for blood‐borne infections: a population‐based survey of the adult population of T’bilisi, Republic of Georgia. J Urban Health. 2006;83(2):289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karchava M, Sharvadze L, Gatserelia L, Badridze N, Tsertsvadze T. Prevailing HCV genotypes and subtypes among hiv infected patients in Georgia. Georgian Med News. 2009;177:51‐55. [PubMed] [Google Scholar]
- 9. Dershem L, Tabatadze M, Sirbiladze T, Tavzarashvili L, Tsagareli T. Characteristics, high‐risk behaviors and knowledge of STI/HIV/AIDS and prevalence of HIV, syphilis and hepatitis among injecting drug users in Kutaisi. 2009; 2009.
- 10. Bouscaillou J, Champagnat J, Luhmann N, et al. Hepatitis C among people who inject drugs in Tbilisi, Georgia: an urgent need for prevention and treatment. Int J Drug Policy. 2014;25(5):871‐878. [DOI] [PubMed] [Google Scholar]
- 11. Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, et al. Three years of Progress toward achieving hepatitis C elimination in the country of Georgia, April 2015‐march 2018. Clin Infect Dis. 2020;71(5):1263‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Georgia Ministry of Labour Health and Social Affairs (MoLHSA) . National Hepatitis C Virus Elimination Progress Report, Georgia 2015–2017, 2018.
- 13. Averhoff F, Lazarus JV, Sergeenko D, et al. Excellence in viral hepatitis elimination ‐ lessons from Georgia. J Hepatol. 2019;71(4):645‐647. [DOI] [PubMed] [Google Scholar]
- 14. Averhoff F, Shadaker S, Gamkrelidze A, et al. Progress and challenges of a pioneering hepatitis C elimination program in the country of Georgia. J Hepatol. 2020;72(4):680‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georgia Ministry of Labour Health and Social Affairs (MoLHSA) . National hepatitis C elimination program progress report, Georgia, 2018‐2019, 2020.
- 16. Oru E, Trickey A, Shirali R, Kanters S, Easteerbrook P. Decentralization, integration, and task‐shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta‐analysis. Lancet Global Health. 2021;9(4):e431‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwamoto M, Calzia A, Dublineau A, et al. Field evaluation of GeneXpert(®) (cepheid) HCV performance for RNA quantification in a genotype 1 and 6 predominant patient population in Cambodia. J Viral Hepat. 2019;26(1):38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drain PK, Dorward J, Violette LR, et al. Point‐of‐care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open‐label, non‐inferiority, randomised controlled trial. Lancet HIV. 2020;7(4):e229‐e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sacks E, Cohn J, Ochuka B, et al. Impact of routine point‐of‐care versus laboratory testing for early infant diagnosis of HIV: results from a multicountry stepped‐wedge cluster‐randomized controlled trial. J Acquir Immune Defic Syndr. 2020;84 Suppl 1(1):S5‐s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Georgian Harm Reduction Network . The impacts of COVID‐19 on harm reduction service provision: a comparative analysis. 2020. https://ghrn.ge/news.php?lang=eng2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Retention in the hepatitis C care cascade by site
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.


