Abstract
Aging is a nonmodifiable risk factor for cardiovascular disease associated with arterial stiffening and endothelial dysfunction. We hypothesized that sex differences exist in vascular aging processes and would be attenuated by global deletion of the G protein-coupled estrogen receptor. Blood pressure was measured by tail-cuff plethysmography, pulse wave velocity (PWV) and echocardiography were assessed with high-resolution ultrasound, and small vessel reactivity was measured using wire myography in adult (25 wk) and middle-aged (57 wk) male and female mice. Adult female mice displayed lower blood pressure and PWV, but this sex difference was absent in middle-aged mice. Aging significantly increased PWV but not blood pressure in both sexes. Adult female carotids were more distensible than males, but this sex difference was lost during aging. Acetylcholine-induced relaxation was greater in female than male mice at both ages, and only males showed aging-induced changes in cardiac hypertrophy and function. GPER deletion removed the sex difference in PWV and ex vivo stiffness in adult mice. The sex difference in blood pressure was absent in KO mice and was associated with endothelial dysfunction in females. These findings indicate that the impact of aging on arterial stiffening and endothelial function is not the same in male and female mice. Moreover, nongenomic estrogen signaling through GPER impacted vascular phenotype differently in male and female mice. Delineating sex differences in vascular changes during healthy aging is an important first step in improving early detection and sex-specific treatments in our aging population.
NEW & NOTEWORTHY Indices of vascular aging were different in male and female mice. Sex differences in pulse wave velocity, blood pressure, and large artery stiffness were abrogated in middle-aged mice, but the female advantage in resistance artery vasodilator function was maintained. GPER deletion abrogated these sex differences and significantly reduced endothelial function in adult female mice. Additional studies are needed to characterize sex differences in vascular aging to personalize early detection and treatment for vascular diseases.
Keywords: arterial stiffening, GPER, pulse wave velocity, sex differences, vascular aging
INTRODUCTION
Chronological aging and sex are nonmodifiable risk factors for cardiovascular disease (CVD) involving changes in arterial structure and function (1, 2). Arterial stiffening in large arteries accompanied by endothelial dysfunction in small arteries is collectively referred to as “vascular aging” (3). During aging, both elastic arteries and muscular arterioles demonstrate adverse remodeling characterized by marked changes in lumen size, wall thickness, and material stiffness (4, 5). In the Framingham Heart Study, arterial stiffness assessed by pulse wave velocity (PWV) increases with age and is higher in men than age-matched women, but this relationship is reversed after menopause (6, 7).
The etiology of arterial stiffening is complex and incorporates structural and functional changes in vascular beds that precedes end organ damage (8, 9). Certainly, extracellular matrix components such as collagen and elastin along with smooth muscle interplay with the viscoelastic components of the arterial wall to influence vascular structure and intercellular communication (10–12). It is still uncertain whether arterial stiffening precedes or follows hypertension, but increased inflammation, endothelial dysfunction, neurohormonal disorders, and metabolic syndrome are known risk factors (13). Arterial stiffening may also be hereditary as indicated in a twin cohort and the Bogalusa Heart Study, but the specific gene remains unknown (14–16). Importantly, early reflected waves due to arterial stiffening induce cardiac remodeling and dysfunction (17–19).
In addition to the stiffness component of vascular aging, endothelial dysfunction is also a key manifestation. Endothelial cells line the inner circumference of the entire vascular system and play a critical role in regulating vascular resistance. The endothelium’s role in vasodilation was first discovered in 1980 and was later attributed to the production of nitric oxide (NO) (20, 21). During vascular aging, arterial stiffness is frequently accompanied by endothelial dysfunction, characterized by a decrease in NO-mediated vasorelaxation (22, 23). Being young or female results in better performance in tests of flow-mediated dilation, a measurement of endothelial function (24, 25). NO bioavailability and the associated oxidative stress are most likely involved in this loss of function (26, 27).
Recently, we showed that PWV is more prognostic in female mice, whereas blood pressure is more informative in male mice during hypertension (28). This study also demonstrates that sex differences in PWV are absent in mice lacking the G protein-coupled estrogen receptor (GPER). This novel nongenomic estrogen receptor also exerts protective effects on vascular tone, blood pressure, arterial remodeling, and diastolic function (29–32). Given that chronological aging and sex hormones both contribute to vascular aging, the goal of the current study was to delineate sex differences and the role of GPER in both large elastic and small muscular arteries. We chose to compare vascular function at reproductive maturity with middle age to allow investigation of chronological aging in the absence of reproductive senescence, since the latter includes alterations in circulating sex hormones that may obscure the impact of GPER deletion. It is important to note that although mice do not experience menopause, estrous cyclicity declines around 12 mo of age and is followed by a period of maintained estradiol levels known as persistent estrous (33).
MATERIAL AND METHODS
Animals
Male and female wild-type (WT) and global GPER knockout (KO) mice on a C57BL/6 background were inbred at the Tulane animal facility with a temperature-controlled room and 12-h:12-h light/dark cycle (32, 34, 35). Animals had unlimited access to standard chow diet and water. All procedures were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved and monitored by the Tulane University Institutional Animal Care and Use Committee. Animals were randomized to either adult (21–25 wk) or middle-aged (52–57 wk) groups. Investigators were blinded during data collection, and data analysis was performed by a different investigator.
Tail-Cuff Plethysmography
Conscious blood pressure was measured using the CODA 4-Channel High-Throughput Noninvasive Blood Pressure System (Kent Scientific, Torrington, CT). Two days of acclimation to the environment and restrainer was followed by 8 days of blood pressure measurements that occurred at the same time each day. Tail temperature was warmed to 30°C before beginning the inflation protocol, which consisted of 10 cycles of cuff inflation to 250 mmHg followed by a 20-s deflation. Measurements without a definitive inflection point indicating the return of blood flow or with a blood flow volume of less than 30 µL were excluded. The average blood pressure was recorded for each day, and the final blood pressure was reported as the mean of the daily averages after excluding days that were ±2 SDs.
Pulse Wave Velocity and Echocardiography
The Vevo 1100 ultrasound (VisualSonics, Toronto, ON, Canada) was used for cardiovascular analysis. Anesthesia was induced using 3% isoflurane-oxygen mixture, and spontaneously breathing mice were maintained with 0.5%–1.5% isoflurane-oxygen mixture by nose cone in the supine position on a 37°C-heated EKG platform. Depilatory cream was applied to the chest, and abdomen and around the throat and wiped with wet gauze. Intracarotid pulse wave velocity (ic-PWV) was measured by ultrasound as previously described (28). Briefly, the right carotid artery was imaged from the aortic arch to the carotid bifurcation. Arrival of the pulse wave was measured at the proximal and distal ends of the vessel in M-mode and color Doppler. Distance was determined using the ultrasound built-in ruler and divided by the difference in pulse time arrival. Cardiac function was assessed in short-axis view in M-mode for left ventricular function, as well as subcoastal view in color Doppler for mitral flow as previously described (28).
Passive Biaxial Mechanical Testing
The carotid artery was dissected and cannulated onto 25-gauge needles with 9-0 black mononylon sutures on a pressure myograph system (Danish Myo Technology 114 P, Aarhus, Denmark) in Hank’s balanced salt solution as previously described (34). Pressure was applied to the lumen of the vessel and the outer diameter was optically tracked. Axial force was measured with an inline force transducer, and the vessel was extended axially (stretched) with a micrometer. Biaxial phenotyping was performed as previously described (36). On the unloaded configuration being found, the vessel was inflated to 100 mmHg and equilibrated for 20 min. The vessel was axially extended by a factor of 1.25 for the initial estimated in vivo length and preconditioned to obtain repeatable mechanical loading curves. The in vivo length was confirmed by determining where the axial force held nearly constant over a pressure range of 10–150 mmHg. Pressure-diameter preconditioning was performed by subjecting the vessel to three cycles of pressurization from 10 to 150 mmHg. Axial force-length preconditioning was next conducted by pressurizing to 50 mmHg and extending the axial length from 5% below to 5% above the in vivo length at a rate of 10 µm/s. The unloaded configuration was reestablished before pressure-diameter testing was performed at the in vivo length with three cycles of pressurization from 10 to 150 mmHg.
A parallel ring was placed on a microscope to measure unloaded dimensions. The stretch ratio was calculated by dividing the loaded to unloaded axial length and used to determine the adjusted wall thickness during pressurization. Distensibility was calculated as the percentage of starting external diameter. Stress was calculated as σ = [P (dyn/cm2)·Dint]/(2·WT), whereas strain ε = Dint − D10 mmHg/D10 mmHg.
Histology
Carotid samples were fixed in 10% formalin overnight followed by paraffin embedding. Paraffin blocks were serially cut into 5-µm sections and stained with Verhoeff Van Gieson (VVG) for elastin, Masson’s trichrome (MTC) for collagen, and picrosirius red (PSR) for type I and type III collagen. The images were obtained using an Olympus BX51 microscope, an Olympus DP27 Digital Camera, and cellSens software (Olympus Corporation, Center Valley, PA). VVG and MTC were imaged under bright field, whereas PSR was imaged with polarized dark field microscopy. A custom MATLAB (Mathworks, Natick, MA) code quantified the area fraction of elastin, smooth muscle, collagen, and different types of collagen fibers by determining the percentage of stained constituent pixels to total pixels as previously described (37, 38). All quantifications were performed on 20× images displaying the full cross section.
Vascular Reactivity
Animals were euthanized using isoflurane, and second-order mesenteric arteries were dissected and cleared of surrounding fascia. Four vessels from each animal were cut into ring segments and mounted on a wire myograph (DMT 620 M, Danish Myotechnology, Denmark). Normalization was performed as previously described (39). Vascular dynamics were assessed in increasing concentrations of two vasoconstrictors, phenylephrine (PE) and prostaglandin F2α (PGF2α), and two vasodilators, acetylcholine (ACh) and sodium nitroprusside (SNP). Vessels that relaxed less than 50% to ACh were considered denuded and excluded from the study.
Statistics
Data were analyzed using GraphPad Prism version 9.1 (GraphPad Software, San Diego, CA). Outliers were identified by ROUT method (Q = 1%). Two-way ANOVA and Sidak’s multiple comparison test was used to determine the impact of aging and sex with P < 0.05 being considered significant. Nonlinear regression was used to fit stress-strain curves with the extra sum-of-squares F test to determine whether datasets differed.
RESULTS
Sex Differences in Aging-Induced Changes in Cardiovascular Parameters and Tissue Weights
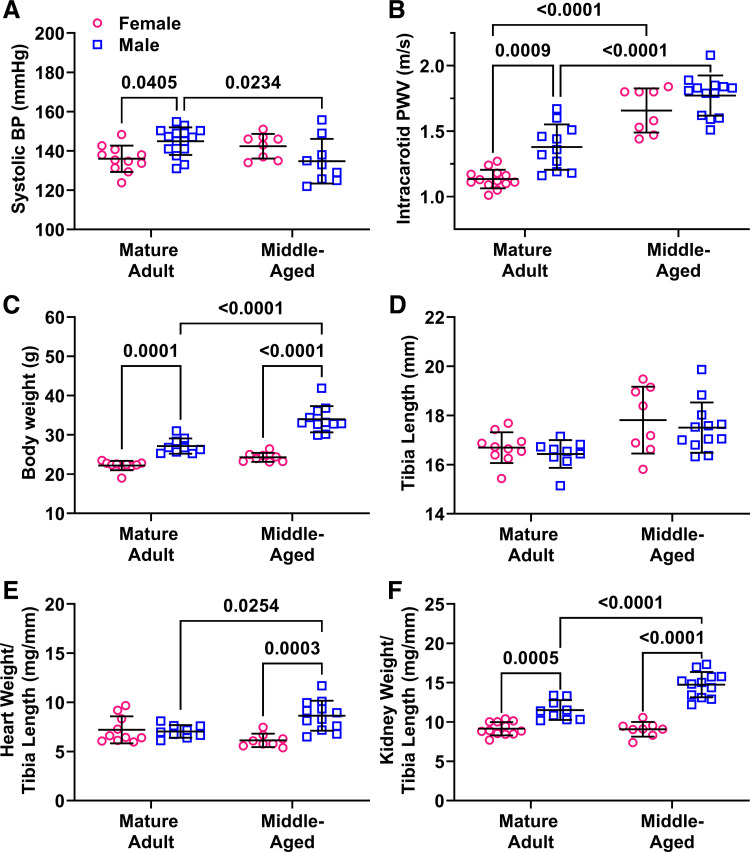
Systolic blood pressure measured by tail-cuff plethysmography was significantly higher in male adults (P = 0.04) but was not different across sex in middle-aged mice (P = 0.28) due to a decrease in BP in male mice during aging (P = 0.02; Fig. 1A). Similarly, intracarotid PWV was higher in adult males versus females (P = 0.0009) but not in middle-aged males compared with females (P = 0.41). Aging significantly increased ic-PWV in both male and female mice (P < 0.0001).
Figure 1.
Impact of sex and aging on cardiovascular parameters and tissue weights. Main effects from two-way ANOVA are listed in the legend and results of Sidak’s multiple comparison tests are indicated on each graph. n = 8–11 mice/group. A: systolic blood pressure (BP) measured by tail cuff was higher blood in male adults but not in middle-aged male mice. Pint = 0.002, Page = 0.44, Psex = 0.79. B: similarly, PWV was higher in adult male mice but not in middle-aged mice. Pint = 0.14, Page < 0.0001, Psex = 0.0002. C: total body weight was significantly greater in male adults and was increased by age in male but not female mice. Pint = 0.002, Page < 0.0001, Psex < 0.0001. D: tibia length was not impacted by sex or age. Pint = 0.94, Page = 0.0009, Psex = 0.36. E: heart weight normalized to tibia length was increased by aging only in male mice, resulting in a sex difference in middle age. Pint = 0.001, Page = 0.50, Psex = 0.004. F: normalized kidney weights were significantly higher in male mice of both age groups. Pint = 0.0001, Page = 0.0002, Psex < 0.0001. PWV, pulse wave velocity.
Total body weight was significantly greater in male adult mice (P = 0.0001) and was increased by age in male but not female mice (P < 0.0001), exacerbating the sex difference in middle-aged mice (P < 0.0001). In contrast, tibia length was not impacted by sex or age, indicating that the mice were at adulthood at the beginning of the study. Tissue weights were normalized to tibia length as is more appropriate for studies with aging-induced increases in body weight (40). Both heart and kidney weight were increased by aging only in male mice (P = 0.025 and P < 0.0001), resulting in higher normalized tissue weights in middle-aged male versus female mice (P = 0.0003 and P < 0.0001). The increased tissue weights in aging males can be attributed to the increased body weight, since when normalized there was no significant differences between groups (heart: 4.3 vs. 4.4 mg/g body wt, P = 0.58; kidney: 7.0 vs. 7.5 mg/g body wt, P = 0.06). As previously found by our group, uterine weight significantly increased from adulthood to middle age (86 vs. 163 mg, P = 0.0002) signifying a transition to reproductive senescence (41).
Passive Mechanical Testing of the Carotid Artery
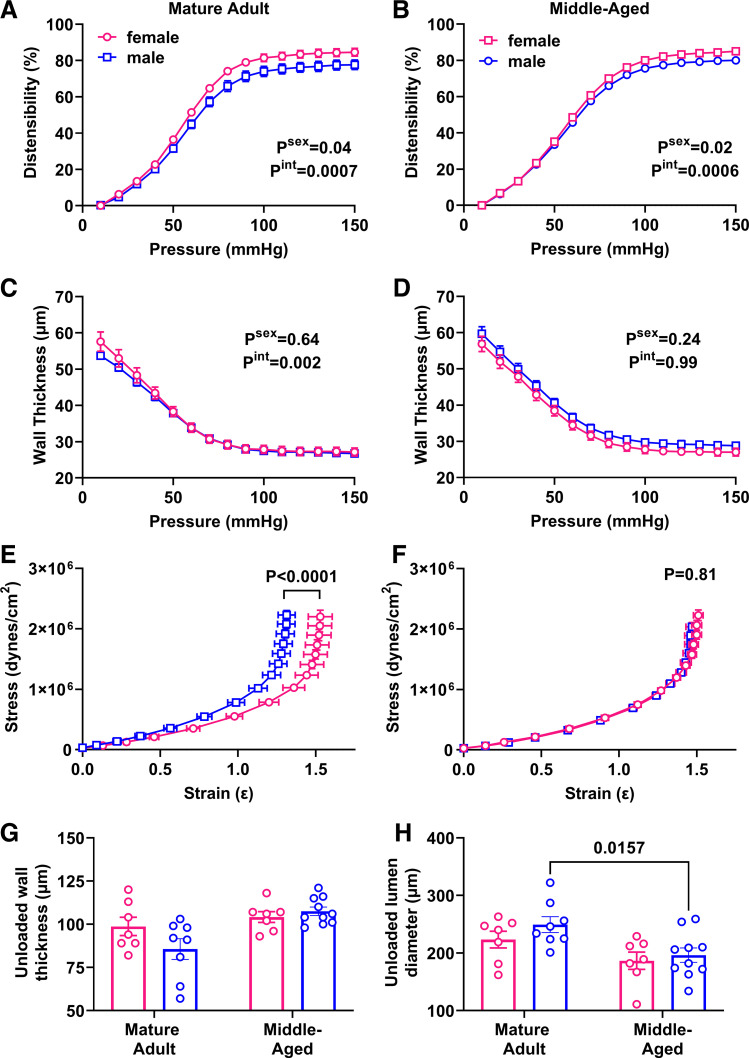
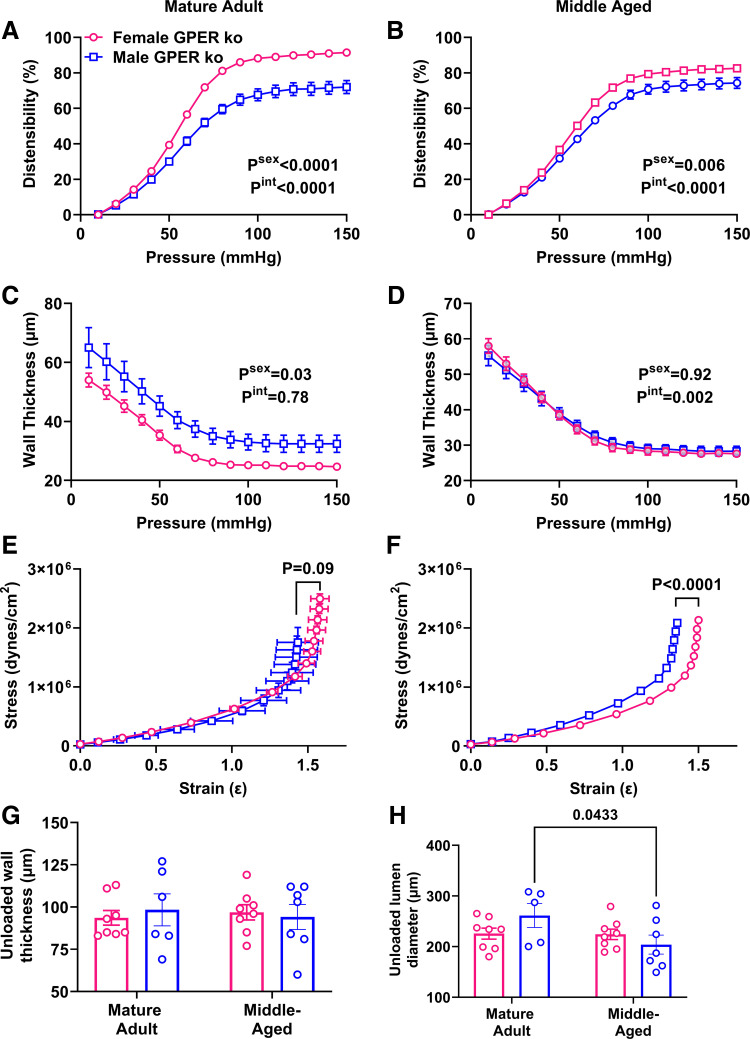
To determine whether increasing PWV is mediated by structural changes in the vasculature, we measured passive characteristics of carotid arteries in a pressure myograph system. Distensibility was greater in adult female mice (Psex = 0.04), especially at higher pressures (Pint = 0.0007; Fig. 2A). Surprisingly, this sex difference was maintained in middle-aged mice (Fig. 2B). Wall thickness did not show a main effect of sex (Psex = 0.64) but there was an interaction effect with pressure (Pint = 0.002; Fig. 2C). This interaction effect was lost in middle-aged mice (Fig. 2D). The stress-strain curve for adult males was significantly shifted to the left, indicating increased stiffness (Fig. 2E), and this sex difference was lost in middle age (P = 0.81; Fig. 2F). Analysis of unloaded geometry showed that wall thickness increased with aging in males but not females (Fig. 2G), because of a decrease in lumen diameter (Fig. 2H), whereas external diameter was maintained (adult male: 421 ± 11 µm, adult female: 421 ± 9 µm, middle-aged male: 411 ± 11 µm, middle-aged female: 395 ± 10 µm; two-way ANOVA: Pint = 0.46, Page = 0.12, Psex = 0.46).
Figure 2.
Impact of sex and aging on passive properties in the carotid artery. Main effects of sex and the interaction with pressure from two-way ANOVA are listed in the legend and results of Sidak’s multiple comparison tests are indicated on each graph. n = 7–9 mice/group. A and B: distensibility was greater in female mice in adulthood (A), Pint = 0.0007, Psex = 0.04, as well as in middle age (B). Pint = 0.0006, Psex = 0.02. C and D: wall thickness was not impacted by sex in either adulthood (C) or middle age (D). P > 0.05. E and F: stress-strain curves showed increased stiffness in male adult mice (E), but this sex effect was lost during aging (F). G: unloaded wall thickness increased with aging in males but not females. Pint = 0.07, Page = 0.004, Psex = 0.27. H: unloaded lumen diameter was decreased in males but not females with aging. Pint = 0.57, Page = 0.003, Psex = 0.21.
Vascular Reactivity in the Mesenteric Artery
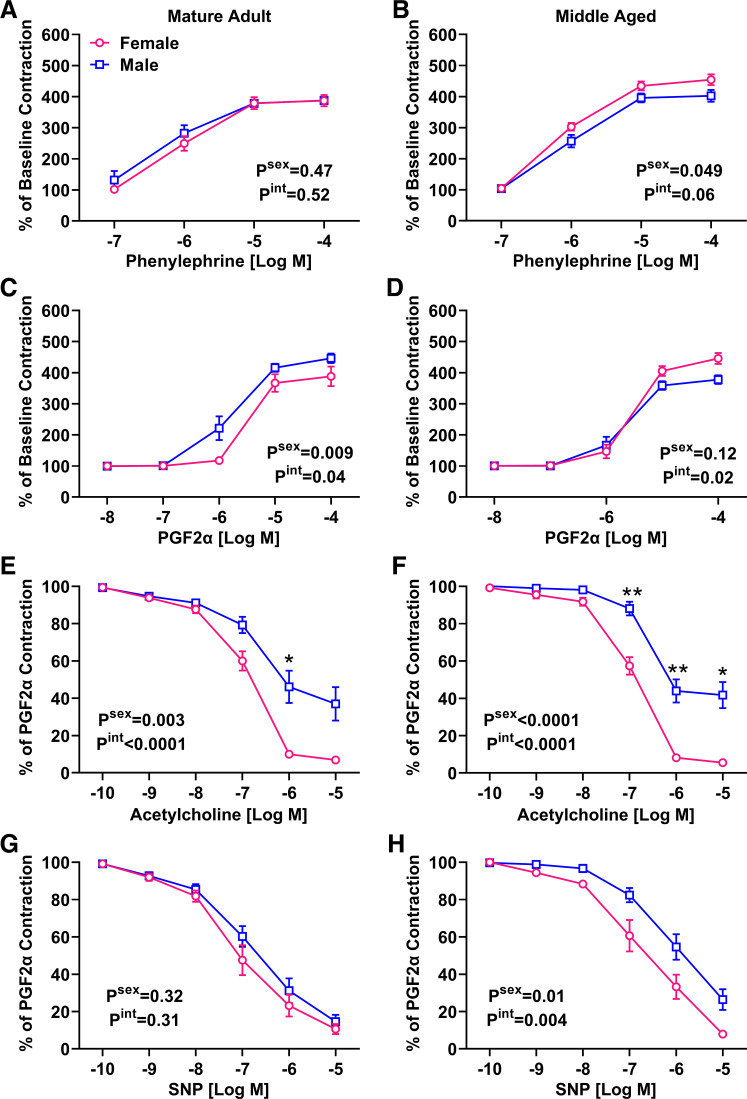
To determine whether sex differences in vascular aging also include changes in small vessel function, wire myography experiments were performed. Although increasing doses of phenylephrine (PE) increased vasoconstriction, there was no effect of sex or the interaction with concentration (Fig. 3A). However, a significant effect of sex was indicated in middle-aged mice, with females demonstrating significantly greater contraction especially at higher concentrations of PE (Fig. 3B). Prostaglandin F2α (PGF2α)-induced contraction was lower in adult female mice (Fig. 3C). The overall effect of sex was reduced with aging, although a significant interaction indicated that greater female contraction was still present at higher concentrations of PGF2α (Fig. 3D). Together these data indicate that in middle age, female vessels tend to have increased vasoconstrictor responses.
Figure 3.
Impact of sex and aging on mesenteric artery reactivity. Main effects from two-way ANOVA are listed on each graph and asterisks denote results of post hoc tests. n = 7–10 mice/group. A and B: no sex effect was detected in the PE response in adulthood (A), but middle-aged females had a significantly enhanced response (B). C and D: response to PGF2α was attenuated in female adults (C), but this pattern was switched in middle age (D). E and F: male mice had significantly blunted ACh-induced relaxation in adulthood (E), which was maintained in middle age (F). G and H: SNP-induced relaxation was not different by sex in adulthood (G) but was attenuated in middle-aged males (H). PE, phenylephrine; PWV, pulse wave velocity; SNP, sodium nitroprusside.
Mesenteric arteries preconstricted with PGF2α were exposed to increasing doses of acetylcholine (ACh) and sodium nitroprusside (SNP) to test endothelium-dependent and -independent responses, respectively. The ACh response was significantly attenuated in adult male mesenteric arteries (Fig. 3E), and this response was maintained in middle age. In contrast, no effect of sex was observed in the SNP response during adulthood (Fig. 3G). A sex difference emerged in middle age, where females had greater SNP-induced vasodilation in comparison with males (Fig. 3H). These data indicate a female advantage in endothelial-dependent vasodilation that is independent of age, as well as a female advantage in endothelium-independent relaxation that emerges in middle age.
Impact of Global GPER Deletion on Sex Differences in Vascular Aging
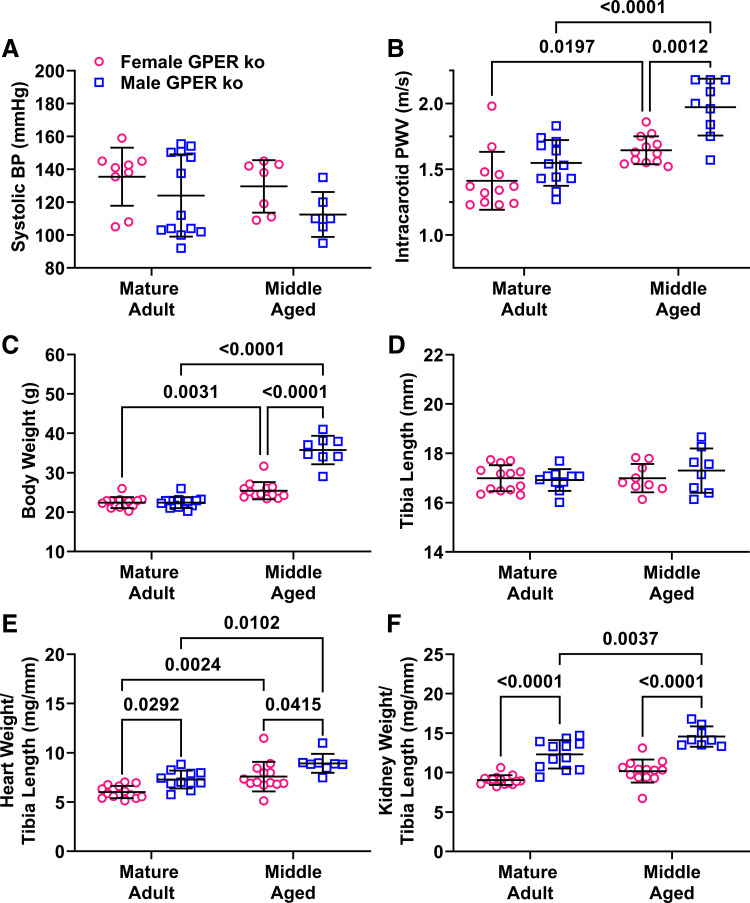
To determine the role of nongenomic estrogen signaling on sex differences in vascular aging, these studies were repeated in mice with global deletion of the G protein-coupled estrogen receptor (GPER) (32, 34, 35). No sex differences were detected in systolic blood pressure in either adults or middle-aged GPER KO mice (Fig. 4A). As previously reported, the sex difference in PWV in adulthood was absent in adult GPER KO mice (Fig. 4B) (34). Comparison of data with that from wild-type mice showed that the loss of the sex difference was due to higher PWV in both female GPER KO mice (KO: 1.4 vs. WT: 1.1 m/s, P = 0.0003, t test) and male GPER KO mice (KO: 1.5 vs. WT: 1.4 m/s, P = 0.03, t test). Values in middle age were similar in females (KO: 1.7 vs. WT: 1.6 m/s, P = 0.8, t test) but lower in male KO mice (KO: 1.8 vs. WT: 2.0, P = 0.02, t test). Aging increased PWV in both male and female KO mice, mirroring the effect of aging in wild-type mice. Interestingly, PWV was significantly higher in male versus female KO mice in middle age, a sex difference that was not observed in wild-type mice, because of higher values in KO versus wild-type males (1.8 vs. 2.0, P = 0.02, t test).
Figure 4.
Impact of sex and aging on systolic blood pressure and intracarotid PWV in global GPER knockout mice. Main effects from two-way ANOVA are listed in the legend and results of Sidak’s multiple comparison tests are indicated on each graph. n = 8–13 mice/group. A: systolic blood pressure (BP) measured by tail cuff was not different across sex or age. B: PWV was similar in adult mice but was significantly greater in male middle-aged mice. Pint = 0.09, Page < 0.0001, Psex = 0.0001. C: total body weight was not different in adults and was increased by age in both male and female mice. Pint < 0.0001, Page < 0.0001, Psex < 0.0001. D: tibia length was not impacted by sex or age. P < 0.05. E: normalized heart weight was increased by aging in both male and female mice, and a sex difference was seen in both age groups. Pint = 0.90, Page < 0.0001, Psex = 0.0002. F: normalized kidney weights were significantly higher in male mice of both age groups. Pint = 0.17, Page = 0.0001, Psex < 0.0001. PWV, pulse wave velocity.
GPER deletion removed the sex difference in body weight during adulthood because of decreased body weight in male knockout mice (WT: 27.1 vs. KO: 22.4 g, P < 0.0001; Fig. 4C). Both male and female KO mice gained weight with age, whereas female wild-type mice were protected from age-induced weight gain. Tibia length was similar across groups, mirroring the data from wild-type mice (Fig. 4D). A sex difference in heart weight was detected because of a decrease in GPER KO mice (102 vs. 120 mg, P = 0.02; Fig. 4E), but this effect was reversed in middle age with bigger hearts in GPER KO mice (133 vs. 109 mg, P = 0.049). Patterns of sex differences in kidney weight in GPER KO mice mirrored those seen in wild-type mice (Fig. 4F). The increase in uterine weight due to aging was also maintained in female mice with global GPER deletion (74 vs. 106 mg, P = 0.01).
Distensibility was greater in female versus male KO mice in adulthood (Fig. 5A). Area under the curve analysis showed that the distensibility of female KO mice was greater than that of wild-type mice (8,060 vs. 8,738, P = 0.0007). This sex difference was maintained in middle age (Fig. 5B). Wall thickness was significantly greater in male versus female KO mice and also was significantly greater than that of male wild-type mice (79.6 vs. 53.7 µm, P = 0.02), which was surprising considering that male KO mice were smaller (Fig. 5C). The impact of sex on wall thickness was reduced in middle age (Fig. 5D). Stress-strain curves for female and male adult KO mice were overlapping, although the maximum strain values were lower in males (Fig. 5E). Comparison of female KO versus WT stress-strain curves, however, showed no statistical difference (extra sum-of-squares F test, P = 0.36). This sex effect was maintained during aging (Fig. 5F). GPER deletion removed the increased wall thickness in males as seen in wild-type mice (Fig. 5G), whereas the impact of aging on unloaded lumen diameter in males was maintained (Fig. 5H).
Figure 5.
Impact of sex and aging on passive carotid properties in global GPER KO mice. Main effects from two-way ANOVA are listed in the legend and results of Sidak’s multiple comparison tests are indicated on each graph. n = 6 to 7 mice/group. A: distensibility was greater in female mice in adulthood. Pint < 0.0001, Page < 0.0001, Psex < 0.0001. B: greater female distensibility was maintained in middle age. Pint < 0.0001, Page < 0.0001, Psex = 0.006. C: wall thickness was greater in male KO mice. Pint = 0.78, Page < 0.0001, Psex = 0.03. D: impact of sex on wall thickness was absent in middle age. Pint = 0.002, Page < 0.0001, Psex = 0.92. E and F: stress-strain curves of male and female mice were overlapping in adulthood (E), but a sex effect emerged during aging (F). G: unloaded wall thickness was not impacted by sex or aging in GPER KO mice. P > 0.05. H: unloaded lumen diameter was decreased in aging males. Pint = 0.08, Page = 0.07, Psex = 0.62. KO, knockout.
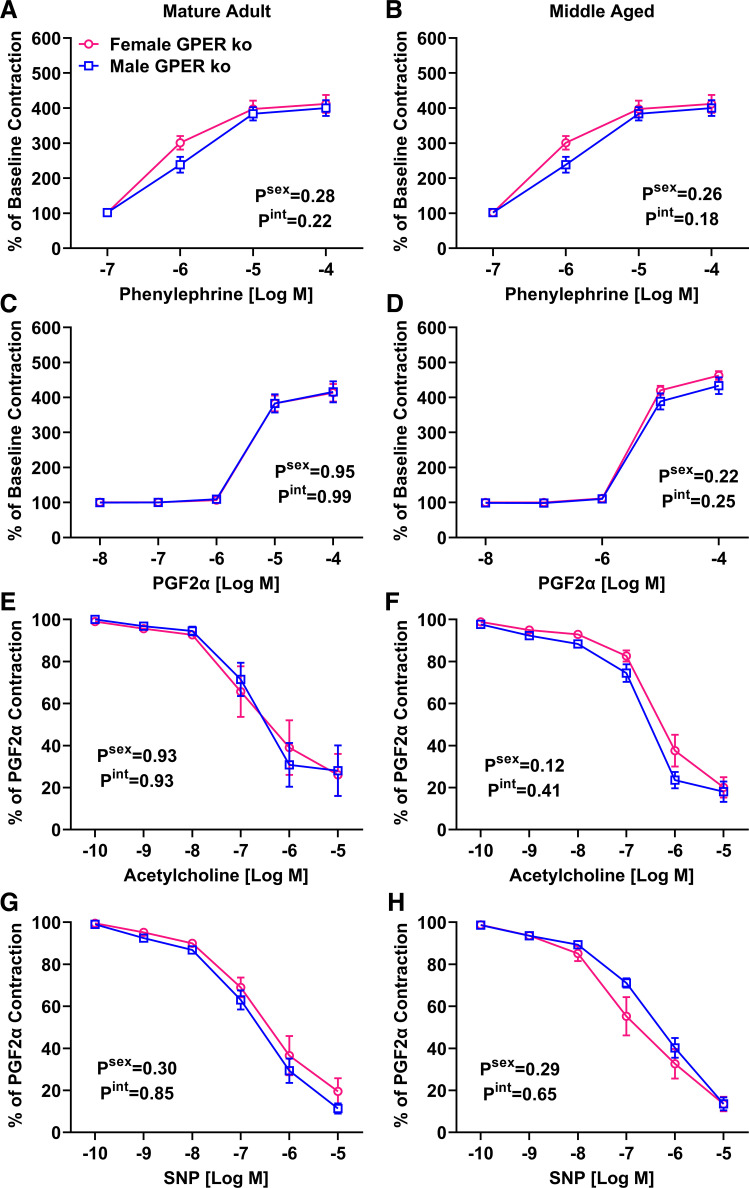
Wire myography of mesenteric arteries revealed no impact of sex in either adulthood or middle age on the vasoconstrictor response to PE (Fig. 6, A and B) or PGF2α (Fig. 6, C and D). This contrast data from wild-type mice showing that female vasoconstrictor responses are enhanced during aging. In addition, the sex difference in ACh-induced dilation was absent in GPER KO mice of both age groups (Fig. 6, E and F). The difference in KO mice could mainly be attributed to decreased vasodilation in female KO mice (6.8 versus 26%), although male KO mice had a slightly enhanced response (37% versus 28%). SNP responses in GPER KO mice lacked a sex effect in both age groups (Fig. 6, G and H). The attenuated response in middle-aged male wild-type mice was absent in GPER KO male mice.
Figure 6.
Impact of sex and aging on mesenteric artery reactivity in global GPER knockout mice. Main effects from two-way ANOVA are listed on each graph. Post hoc tests did not reach statistical significance. n = 6 to 7 mice/group. A and B: no sex effect was detected in the PE response in either adulthood (A) or middle age (B). C and D: response to PGF2α was attenuated in female adults (C), but this pattern was switched in middle age (D). E and F: male mice have significantly blunted ACh-induced relaxation in adulthood (E), which was maintained in middle age (F). G and H: SNP-induced relaxation was not different by sex in adulthood (G) but was attenuated in middle-aged males (H). PE, phenylephrine; SNP, sodium nitroprusside.
Associated Changes in Echocardiographic Indices
We evaluated echocardiographic indices of left ventricular structure and function to determine whether the changes in vascular parameters ranging from pulse wave velocity to mesenteric artery reactivity impact the heart’s function (Table 1). Neither sex nor age impacted relative wall thickness (RWT), ejection fraction (%EF), fractional shortening (%FS), cardiac output, (CO), and either diameter or volume in systole (s) or diastole (d). GPER KO mice showed an interaction effect for %EF and %FS, where larger indices in adult males were flipped in middle age to larger values in females. Female GPER KO mice had significantly lower CO independent of age. Another interaction effect was found in diastolic diameter, where male KO mice had an age-induced increased in diameter whereas females experienced a decrease during aging.
Table 1.
Echocardiographic indices captured in short-axis M-mode and mitral flow color Doppler
| Adult |
Middle Age |
P Value |
Adult Knockout |
Middle Age Knockout |
P Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Sex | Age | Int | Male | Female | Male | Female | Sex | Age | Int | |
| RWT | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.08 | 0.7 ± 0.09 | 0.2 | 0.4 | 0.7 | 1.0 ± 0.3 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.6 | 0.6 | 0.8 |
| %EF | 82 ± 7 | 68 ± 3 | 68 ± 4 | 69 ± 5 | 0.2 | 0.2 | 0.2 | 73 ± 4 | 67 ± 4 | 68 ± 4 | 78 ± 3 | 0.6 | 0.4 | 0.03 |
| %FS | 55 ± 8 | 38 ± 3 | 39 ± 4 | 39 ± 4 | 0.1 | 0.2 | 0.1 | 44 ± 3 | 38 ± 3 | 39 ± 3.5 | 47 ± 3 | 0.8 | 0.5 | 0.03 |
| CO, mL/min | 14 ± 1.5 | 14 ± 2.4 | 15 ± 1.0 | 13 ± 1.0 | 0.4 | 0.9 | 0.4 | 14 ± 1.0 | 12 ± 0.9 | 16 ± 1.6 | 13 ± 1.2 | 0.02 | 0.2 | 0.7 |
| Diameter; s | 1.6 ± 0.4 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 | 0.7 | 0.4 | 0.4 | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.2 | 1.7 ± 0.1 | 0.7 | 0.2 | 0.7 |
| Diameter; d | 3.4 ± 0.2 | 3.5 ± 0.3 | 3.5 ± 0.1 | 3.4 ± 0.2 | 0.9 | 0.9 | 0.6 | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.1 | 3.1 ± 0.1 | 0.03 | 0.7 | 0.04 |
| E max, mm/s | 639 ± 56 | 456 ± 26 | 588 ± 39 | 635 ± 49 | 0.2 | 0.2 | 0.02 | 628 ± 31 | 534 ± 32 | 594 ± 54 | 584 ± 45 | 0.2 | 0.8 | 0.3 |
| A max, mm/s | 271 ± 54 | 261 ± 27 | 381 ± 26 | 346 ± 18 | 0.5 | 0.01 | 0.7 | 374 ± 37 | 307 ± 24 | 370 ± 36 | 330 ± 39 | 0.1 | 0.8 | 0.7 |
| E/A ratio | 2.6 ± 0.4 | 1.9 ± 0.2 | 1.6 ± 0.1 | 1.9 ± 0.1 | 0.04 | 0.07 | 0.07 | 1.8 ± 0.2 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.1 | 0.1 | 0.5 | 0.1 |
| IVCT, ms | 16 ± 2.6 | 24 ± 1.4 | 16 ± 1.1 | 17 ± 3.3 | 0.3 | 0.002 | 0.4 | 20 ± 1.1 | 20 ± 1.7 | 16 ± 1.7 | 15 ± 1.4 | 0.7 | 0.004 | 0.9 |
| IVRT, ms | 19 ± 1.0 | 18 ± 1.7 | 17 ± 1.4 | 18 ± 1.6 | 0.9 | 0.5 | 0.5 | 20 ± 1.3 | 22 ± 1.5 | 22 ± 1.6 | 20 ± 1.2 | 0.4 | 0.3 | 0.02 |
| LVET, ms | 54 ± 3.3 | 54 ± 4.2 | 47 ± 2.4 | 48 ± 5.0 | 0.9 | 0.1 | 0.8 | 47 ± 2.3 | 53 ± 2.5 | 50 ± 3.4 | 50 ± 2.8 | 0.2 | 0.8 | 0.3 |
Values are means ± SE; n = 6–12 mice/group. P values are shown for the main effects analyzed by two-way ANOVA. Statistical analysis was performed by t test. P < 0.05 was considered significant, where shown in boldface. Amax, late filling velocity; CO, cardiac output; d, diastole; E/A, early to late filling velocity ratio; %EF, percent ejection fraction; Emax, early filling velocity; %FS, percent fractional shortening; Int, interaction effect; IVCT, isovolumetric contraction time; IVRT, isovolumetric relaxation time; LVET, left ventricular ejection time; RWT, relative wall thickness; s, systole.
Diastolic function assessed by mitral flow velocity showed an interaction effect for early filling (Emax), with lower velocities in adult females but higher in middle-aged females. Late filling (Amax) was increased by age independent of sex. E/A ratio indicated a sex difference with higher ratios in male mice. Isovolumetric contraction time (IVCT) significantly decreased with age whereas aortic diameter increased with age in both sexes. GPER deletion removed the interaction effect in Emax values, the aging effect on Amax, and the sex effect on E/A ratio. However, the aging-induced reduction in IVCT was maintained. Heart rate was similarly controlled across all groups while under anesthesia.
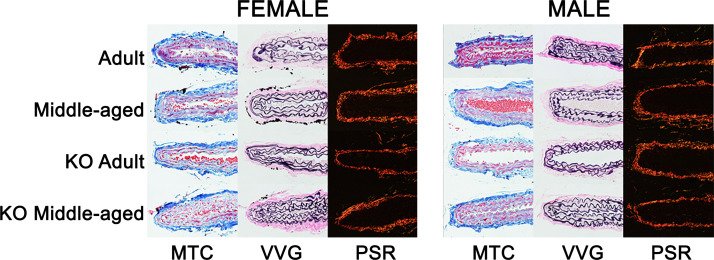
Histology of Carotid Artery Cross Sections
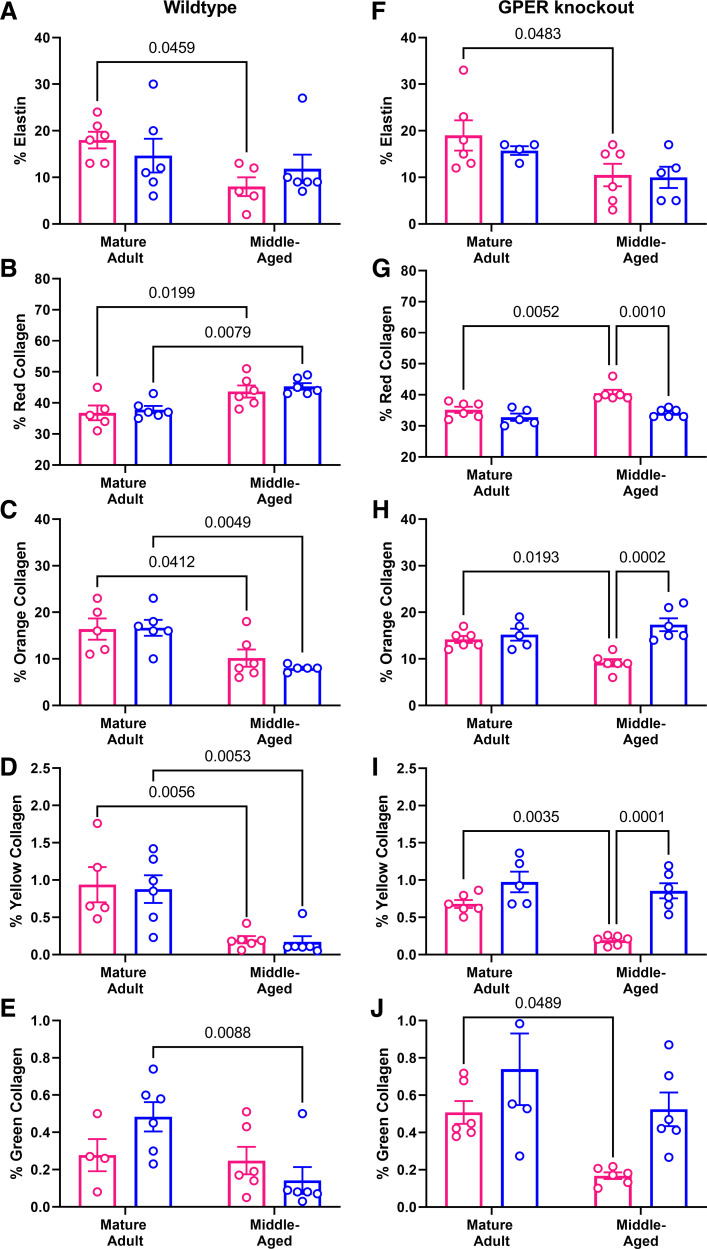
Histological staining was used to determine whether aging-induced stiffening in the carotid artery was associated with changes in the extracellular matrix (representative images in Fig. 7). Neither total collagen or smooth muscle content assessed by Masson’s trichrome was significantly different across sex or age in either wild-type or GPER KO mice (collagen male vs. female: adult WT: 34 ± 4 vs. 39 ± 4; middle-aged WT: 43 ± 4 vs. 39 ± 4; adult KO: 38 ± 2 vs. 32 ± 3; middle-aged KO: 41 ± 4 vs. 46 ± 3 and smooth muscle male vs. female: adult WT: 50 ± 4 vs. 46 ± 5; middle-aged WT: 41 ± 4 vs. 47 ± 5; adult KO: 52 ± 4 vs. 50 ± 5; middle-aged KO: 45 ± 3 vs. 41 ± 3). Elastin content was decreased by aging only in female mice (Fig. 8A). Picrosirius red staining was analyzed under polarized light, which reveals that a change in color from red to orange to yellow to green as collagen fibers decrease in thickness. Red (thick, fibrillar) collagen was significantly increased in both sexes with aging (Fig. 8B), whereas orange and yellow collagen was significantly decreased by aging independent of sex (Fig. 8, C and D). However, green (thin fibers) collagen showed a decrease in males but not females (Fig. 8E). Global GPER deletion mirrored the wild-type pattern for changes in elastin (Fig. 8F), but the patterns for red, orange, and yellow collagen were changes to alterations only in female mice (Fig. 8, G–I). Green collagen in GPER KO mice was decreased in female mice as opposed to a male-only effect in wild-type mice (Fig. 8J).
Figure 7.
Representative images of carotid artery histology. Formalin-fixed carotid cross sections were stained with Masson’s trichrome (MTC: blue, collagen; black, nuclei; red, smooth muscle), Verhoeff Van Gieson (VVG: black, elastin), and Picrosirius red (PSR: yellow/orange, thick collagen; green, thin collagen).
Figure 8.
Histological analysis of carotid cross sections by sex and age. Results from post hoc tests where P < 0.05 are indicated on each graph. n = 5 to 6 mice/group. In wild-type mice, elastin content (A) was decreased only in females. Picrosirius red staining revealed that red birefringent collagen (B) was similarly increased by age in both sexes, whereas orange (C) and yellow (D) collagen was decreased by aging in both sexes. Green (thin) collagen (E) was decreased only in males. In GPER knockout mice, elastin content (F) was reduced only in female mice. Red collagen (G) was increased with age only in females, whereas orange (H), yellow (I), and green (J) collagen was decreased with age only in females.
DISCUSSION
We found that sex differences in PWV, blood pressure, and large artery stiffness favoring females were abrogated in middle age. In contrast, the female advantage in endothelial-mediated vasodilation was preserved, and females also maintained endothelium-independent relaxation during aging whereas males experienced an aging-induced loss in this function. In contrast to these advantages, middle-aged females showed significantly greater vasoconstrictor responses than age-matched males. Vascular changes with middle age were associated with cardiac hypertrophy and decreased diastolic function (E/A ratio) in males with no significant changes in females. Our parallel study using global GPER knockout mice showed a loss of female advantage in PWV and ex vivo stiffness in adulthood. The sex difference in systolic blood pressure was also absent in KO mice and was associated with a loss of endothelium-dependent dilation in females. Taken together, these data show that the impact of vascular aging on large artery mechanics and small vessel reactivity is different across sex and that global GPER deletion impacts both PWV and endothelial function.
Pulse wave velocity (PWV) is the gold standard for assessing arterial stiffness (42). Arteries stiffen to accommodate the microstructural, mechanical, and genetic changes that accompany advancing age (6, 43). This study shows that female mice compared with male mice are protected from arterial stiffening in adulthood, but the sex difference is lost in middle age. These results are similar to our previous study of chronic Ang II infusion in mice suggesting that the female advantage of lower PWV is eliminated by both hypertension and aging (28). Some clinical studies support the premise that during aging women experience a greater decline in arterial compliance versus men despite similar blood pressures (44). However, the Baltimore Longitudinal Study of Aging showed the opposite trend, with similar PWV in men and women before age 50 but greater stiffening in men during the aging process to promote a sex difference (45). Clinical studies must account for a variety of factors that can be better controlled in animal studies, and our current findings indicate that during healthy aging females experience greater stiffening in large arteries.
Although some studies show changes in carotid artery dimensions during aging, our study including both sexes found this change only in male mice because of a decrease in internal diameter indicating inward remodeling (46). In contrast, female mice experienced no change in carotid dimensions despite the increase in PWV, indicating remodeling that is occurring independent of changes in geometry. This lack of wall thickening in female mice was also found in response to NG-nitro-l-arginine methyl ester + high-salt diet to induce arterial remodeling (47). Conceivably, this lack of significant changes in arterial dimensions in females may indicate a lack of mechano-adaptation to normalize wall stress. In contrast, our previous study using angiotensin II infusion found that female mice had a greater increase in unloaded thickness in comparison with male mice (28). A clinical study assessing carotid intima-media thickness (CIMT) shows that the rate of increase in women during aging is significantly less than that of men (48). Similarly, in patients with type 2 diabetes, the progression of CIMT is impacted significantly by aging only in men (49). Taken together with the current study, vascular aging is most likely sex specific and may not involve changes in geometry in females.
We found that sex differences in endothelial-mediated vasorelaxation were maintained even in middle age. A female advantage in endothelial function is reported in both humans, as well as laboratory rodents, whereas the impact of aging is not as clear (50–52). Some studies suggest that men experience this aspect of vascular aging earlier than women, but the timing of endothelial dysfunction with the onset of menopause clouds the underlying mechanisms (25, 53). Interestingly, this female advantage was lost in global GPER knockout mice, suggesting that indeed estrogen and this nongenomic receptor may play important roles in the female advantage. Indeed, we and others show that vasodilation in response to GPER activation includes both smooth muscle mechanisms as well as nitric oxide release from the endothelium (54, 55). Although studies in smooth muscle cells show that GPER signals through Gαs to promote the production of cAMP, the mechanism by which this receptor may influence endothelial-induced relaxation is not delineated (54). Endothelial dysfunction during aging is most commonly thought to be due to a reduction in nitric oxide bioavailability and an increase in cyclooxygenase-derived vasoconstriction, both of which can be impacted by increases in oxidative stress (56). Some knowledge has been gained on how sex impacts these mechanisms during aging but is obscured by the convergence of reproductive and chronological aging in women (52). The ability of GPER to decrease oxidative stress has been established but other potential mechanisms have not been excluded (32, 37, 57).
Females also gained an advantage with greater endothelium-independent dilation in middle age, which was abrogated in the GPER knockout mice. The loss of the female advantage in both endothelial-dependent and -independent mechanisms in middle age suggests a significant role for GPER in both endothelial and smooth muscle cells to provide protection from vascular aging. The involvement of multiple cells in the protective effects of GPER highlights one limitation of the current study, which was the use of global gene deletion. Newer cell-specific models are needed to provide additional insight into the role of GPER in the multiple cell types involved in cardiovascular regulation. This includes not only vascular cells but also the potential impact of GPER signaling in the central nervous system, as we found the highest levels of expression of this receptor in the brain (58). More work is also needed to understand how GPER interacts with other vascular receptors. GPER interacts with aldosterone, angiotensin type 1, and endothelin receptors and potentiates potassium channel activity, but whether it interacts with cholinergic or adrenergic mechanisms is not known (29, 59–62).
Importantly, arterial stiffening may be a greater risk factor for cardiac dysfunction in aging women (63, 64). Despite human data showing that diastolic dysfunction is more prevalent in women, our data showed a significant increase in cardiac hypertrophy and reduction in E/A ratio in middle-aged male mice (65). In contrast, both male and female GPER knockout mice experienced aging-induced cardiac hypertrophy and showed significantly lower E/A ratios across sex and age, supporting previous data on the role of GPER in diastolic function (66, 67). In humans, there is a strong relationship between arterial stiffening and diastolic dysfunction in aging populations (68). In the current study, a decrease in diastolic function was not noted in female middle-aged mice despite an increase in PWV, indicating a longer time may be needed to see the impact of arterial stiffening on the female heart.
Histological findings indicated that only the thick collagen indicated as red by polarized light imaging was increased in middle age. In contrast, both orange and yellow collagen was decreased in both sexes. The only significant sex differences noted here were a decrease in elastin to a greater extent in females and a decrease in green collagen to a greater extent in males. This “new” collagen tends to be loosely packed and disorganized fibers in the adventitia and is associated with fibroproliferation during maladaptive aortic remodeling (69, 70). The pattern in GPER KO mice was the same for elastin (decreased in females) but changes in collagen with aging were only seen in females and not males, suggesting that GPER deletion was protective regarding collagen levels in male mice. Previous work similarly suggests that GPER deletion may be protective in males, but the mechanism has not yet been determined (71).
The present experimental findings indicate that vascular aging was not the same across sexes and was not predictably impacted by loss of nongenomic estrogen signaling through GPER. In large conduit arteries, females experienced arterial stiffening through an increase in material stiffness but no change in vascular geometry. In the small arteries, females maintained endothelial function independent of aging, but this was lost with GPER deletion. In contrast, aging males adapted to arterial stiffening by increasing wall thickness but experienced a greater impact on cardiac function. It is important to note that these studies only assessed vascular aging in middle-aged mice, and it is expected that more dysfunction would be present if assessed later in the aging process. Additional studies are needed to more thoroughly understand how the aging process impacts vascular structure and function differently by sex to improve early detection and treatment for vascular disease in the clinic.
GRANTS
This work was funded by National Institutes of Health Grants HL155841 (to B.O.O.), HL133619 (to S.H.L.), HL116769 and EB026518 (to A.J.T.), and AG061588 (to L.G.); National Science Foundation Grant 1947770 (to K.S.M.); and American Heart Association Fellowships (to B.V. and I.K.-D.). Additional support was provided by the Tulane Center of Excellence in Sex-Based Biology and Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.G., A.J.T., K.S.M., and S.H.L. conceived and designed research; B.O.O., C.M.A., B.V., J.X.K., A.C.H., G.L.C-P., I.K.-D., Z.D., A.N.B., A.B.M., M.A.Z., and S.H.L. performed experiments; B.O.O., C.M.A., B.V., J.X.K., A.C.H., G.L.C-P., A.N.B., M.A.Z., A.J.T., K.S.M., and S.H.L. analyzed data; B.O.O., L.G., and S.H.L. interpreted results of experiments; B.O.O. and S.H.L. prepared figures; B.O.O. drafted manuscript; B.O.O., B.V., I.K.-D., A.J.T., and S.H.L. edited and revised manuscript; B.O.O., C.M.A., B.V., J.X.K., A.C.H., I.K.-D., Z.D., A.N.B., A.B.M., M.A.Z., L.G., A.J.T., K.S.M., and S.H.L. approved final version of manuscript.
REFERENCES
- 1.Lloyd-Jones DM, Leip EP, Larson MG, D'Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 113: 791–798, 2006. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 3.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension 71: 389–402, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 5.McCallinhart PE, Sunyecz IL, Trask AJ. Coronary microvascular remodeling in type 2 diabetes: synonymous with early aging? Front Physiol 9: 1463, 2018. doi: 10.3389/fphys.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 7.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, Hollenberg SM, El Khoudary SR. Arterial stiffness accelerates within 1 year of the final menstrual period: The SWAN Heart Study. Arterioscler Thromb Vasc Biol 40: 1001–1008, 2020. doi: 10.1161/ATVBAHA.119.313622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogola BO, Zimmerman MA, Clark GL, Abshire CM, Gentry KM, Miller KS, Lindsey SH. New insights into arterial stiffening: does sex matter? Am J Physiol Heart Circ Physiol 315: H1073–H1087, 2018. doi: 10.1152/ajpheart.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasan RS, Short MI, Niiranen TJ, Xanthakis V, DeCarli C, Cheng S, Seshadri S, Mitchell GF. Interrelations between arterial stiffness, target organ damage, and cardiovascular disease outcomes. J Am Heart Assoc 8: e012141, 2019. doi: 10.1161/JAHA.119.012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JZ, Sawada H, Moorleghen JJ, Weiland M, Daugherty A, Sheppard MB. Aortic strain correlates with elastin fragmentation in fibrillin-1 hypomorphic mice. Circ Rep 1: 199–205, 2019. doi: 10.1253/circrep.CR-18-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCallinhart PE, Cho Y, Sun Z, Ghadiali S, Meininger GA, Trask AJ. Reduced stiffness and augmented traction force in type 2 diabetic coronary microvascular smooth muscle. Am J Physiol Heart Circ Physiol 318: H1410–H1419, 2020. doi: 10.1152/ajpheart.00542.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension 65: 370–377, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension 65: 252–256, 2015. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley WA, Freedman DS, Higgs NA, Barnes RW, Zinkgraf SA, Berenson GS. Decreased arterial elasticity associated with cardiovascular disease risk factors in the young. Bogalusa Heart Study. Arteriosclerosis 6: 378–386, 1986. doi: 10.1161/01.atv.6.4.378. [DOI] [PubMed] [Google Scholar]
- 15.Snieder H, Hayward CS, Perks U, Kelly RP, Kelly PJ, Spector TD. Heritability of central systolic pressure augmentation: a twin study. Hypertension 35: 574–579, 2000. doi: 10.1161/01.hyp.35.2.574. [DOI] [PubMed] [Google Scholar]
- 16.Cecelja M, Jiang B, Keehn L, Hussain T, Silva Vieira M, Phinikaridou A, Greil G, Spector TD, Chowienczyk P. Arterial stiffening is a heritable trait associated with arterial dilation but not wall thickening: a longitudinal study in the twins UK cohort. Eur Heart J 39: 2282–2288, 2018. doi: 10.1093/eurheartj/ehy165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 45: 652–658, 2005. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 18.Bouthier JD, De Luca N, Safar ME, Simon AC. Cardiac hypertrophy and arterial distensibility in essential hypertension. Am Heart J 109: 1345–1352, 1985. doi: 10.1016/0002-8703(85)90364-3. [DOI] [PubMed] [Google Scholar]
- 19.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension 60: 362–368, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 21.Buga GM, Gold ME, Wood KS, Chaudhuri G, Ignarro LJ. Endothelium-derived nitric oxide relaxes nonvascular smooth muscle. Eur J Pharmacol 161: 61–72, 1989. doi: 10.1016/0014-2999(89)90180-5. [DOI] [PubMed] [Google Scholar]
- 22.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation 88: 2541–2547, 1993. doi: 10.1161/01.CIR.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 24.Skaug EA, Madssen E, Aspenes ST, Wisloff U, Ellingsen O. Cardiovascular risk factors have larger impact on endothelial function in self-reported healthy women than men in the HUNT3 Fitness study. PloS One 9: e101371, 2014. doi: 10.1371/journal.pone.0101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 26.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 28.Ogola BO, Clark GL, Abshire CM, Harris NR, Gentry KL, Gunda SS, Kilanowski-Doroh I, Wong TJ, Visniauskas B, Lawrence DJ, Zimmerman MA, Bayer CL, Groban L, Miller KS, Lindsey SH. Sex and the G protein-coupled estrogen receptor impact vascular stiffness. Hypertension 78, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer MR, Field AS, Kanagy NL, Barton M, Prossnitz ER. GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life Sci 91: 623–627, 2012. doi: 10.1016/j.lfs.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology 150: 3753–3758, 2009. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PloS One 5: e15433, 2010. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogola BO, Zimmerman MA, Sure VN, Gentry KM, Duong JL, Clark GL, Miller KS, Katakam PVG, Lindsey SH. G protein-coupled estrogen receptor protects from angiotensin II-induced increases in pulse pressure and oxidative stress. Front Endocrinol (Lausanne) 10: 586, 2019. doi: 10.3389/fendo.2019.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod 24: 784–794, 1981. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- 34.Ogola BO, Clark GL, Abshire CM, Harris NR, Gentry KL, Gunda SS, Kilanowski-Doroh I, Wong TJ, Visniauskas B, Lawrence DJ, Zimmerman MA, Bayer CL, Groban L, Miller KS, Lindsey SH. Sex and the G protein-coupled estrogen receptor impact vascular stiffness. Hypertension 78: e1–e14, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22: 636–648, 2008. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferruzzi J, Bersi MR, Humphrey JD. Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann Biomed Eng 41: 1311–1330, 2013. doi: 10.1007/s10439-013-0799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Kashyap S, Murphy B, Hutson DD, Budish RA, Trimmer EH, Zimmerman MA, Trask AJ, Miller KS, Chappell MC, Lindsey SH. GPER activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 310: H953–H961, 2016. doi: 10.1152/ajpheart.00631.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udelsman BV, Khosravi R, Miller KS, Dean EW, Bersi MR, Rocco K, Yi T, Humphrey JD, Breuer CK. Characterization of evolving biomechanical properties of tissue engineered vascular grafts in the arterial circulation. J Biomech 47: 2070–2079, 2014. doi: 10.1016/j.jbiomech.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmerman MA, Hutson DD, Mauvais-Jarvis F, Lindsey SH. Bazedoxifene-induced vasodilation and inhibition of vasoconstriction is significantly greater than estradiol. Menopause 26: 172–181, 2019. doi: 10.1097/GME.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol 243: H941–H947, 1982. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- 41.Gurrala R, Kilanowski-Doroh IM, Hutson DD, Ogola BO, Zimmerman MA, Katakam PVG, Satou R, Mostany R, Lindsey SH. Alterations in the estrogen receptor profile of cardiovascular tissues during aging. GeroScience 43: 433–442, 2021. doi: 10.1007/s11357-021-00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, Arteries E-I; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Jin C, Li S, Zheng X, Zhang X, Cui L, Gao X. Aging, arterial stiffness, and blood pressure association in Chinese adults. Hypertension 73: 893–899, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12396. [DOI] [PubMed] [Google Scholar]
- 44.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens 19: 2205–2212, 2001. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 45.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 62: 934–941, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gkousioudi A, Yu X, Ferruzzi J, Qian J, Wainford RD, Seta F, Zhang Y. Biomechanical properties of mouse carotid arteries with diet-induced metabolic syndrome and aging. Front Bioeng Biotechnol 10: 862996, 2022. doi: 10.3389/fbioe.2022.862996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spronck B, Ferruzzi J, Bellini C, Caulk AW, Murtada SI, Humphrey JD. Aortic remodeling is modest and sex-independent in mice when hypertension is superimposed on aging. J Hypertens 38: 1312–1321, 2020. doi: 10.1097/HJH.0000000000002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loboz-Rudnicka M, Jaroch J, Bociaga Z, Rzyczkowska B, Uchmanowicz I, Polanski J, Dudek K, Szuba A, Loboz-Grudzien K. Impact of cardiovascular risk factors on carotid intima-media thickness: sex differences. Clin Interv Aging 11: 721–731, 2016. doi: 10.2147/CIA.S103521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Liu Y, Zhang Y, Chen Y, Yang Z, Zhu Y, Zhan W. Gender difference in carotid intima-media thickness in type 2 diabetic patients: a 4-year follow-up study. Cardiovasc Diabetol 11: 51, 2012. doi: 10.1186/1475-2840-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther 292: 375–380, 2000. [PubMed] [Google Scholar]
- 51.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension 25: 517–523, 1995. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- 52.Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315: H1569–H1588, 2018. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 54.Lindsey SH, Liu L, Chappell MC. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81: 99–102, 2014. doi: 10.1016/j.steroids.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fredette NC, Meyer MR, Prossnitz ER. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol 176: 65–72, 2018. doi: 10.1016/j.jsbmb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152, 2010. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58: 665–671, 2011. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ 10: 4, 2019. doi: 10.1186/s13293-019-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogola B, Zhang Y, Iyer L, Thekkumkara T. 2-Methoxyestradiol causes matrix metalloproteinase 9-mediated transactivation of epidermal growth factor receptor and angiotensin type 1 receptor downregulation in rat aortic smooth muscle cells. Am J Physiol Cell Physiol 314: C554–C568, 2018. doi: 10.1152/ajpcell.00152.2017. [DOI] [PubMed] [Google Scholar]
- 60.Gohar EY, Daugherty EM, Aceves JO, Sedaka R, Obi IE, Allan JM, Soliman RH, Jin C, De Miguel C, Lindsey SH, Pollock JS, Pollock DM. Evidence for G-protein-coupled estrogen receptor as a pronatriuretic factor. J Am Heart Assoc 9: e015110, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choudhury N, Sikdar SK. 17β-estradiol potentiates TREK1 channel activity through G protein-coupled estrogen receptor. J Steroid Biochem Mol Biol 183: 94–105, 2018. doi: 10.1016/j.jsbmb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol 304: C532–C540, 2013. doi: 10.1152/ajpcell.00203.2012. [DOI] [PubMed] [Google Scholar]
- 63.Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, Lacolley P. Sex difference in cardiovascular risk: role of pulse pressure amplification. J Am Coll Cardiol 59: 1771–1777, 2012. doi: 10.1016/j.jacc.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol 61: 96–103, 2013[Erratum inJ Am Coll Cardiol61: 2573–2574, 2013]. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14: 591–602, 2017. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 66.Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res 94: 96–104, 2012. doi: 10.1093/cvr/cvs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Sun X, Lin MS, Ferrario CM, Van Remmen H, Groban L. G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Transl Res 199: 39–51, 2018. doi: 10.1016/j.trsl.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang S, Fan HM, Li J, Fan LY, Miao AY, Bao Y, Wu LZ, Zhu Y, Zhang DF, Liu ZM. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. Eur Heart J 31: 2799–2807, 2010. doi: 10.1093/eurheartj/ehq296. [DOI] [PubMed] [Google Scholar]
- 69.Bersi MR, Bellini C, Wu J, Montaniel KRC, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension 67: 890–896, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jussila T, Kauppila S, Bode M, Tapanainen J, Risteli J, Risteli L, Kauppila A, Stenback F. Synthesis and maturation of type I and type III collagens in endometrial adenocarcinoma. Eur J Obstet Gynecol Reprod Biol 115: 66–74, 2004. doi: 10.1016/S0301-2115(02)00406-2. [DOI] [PubMed] [Google Scholar]
- 71.Meyer MR, Rosemann T, Barton M, Prossnitz ER. GPER mediates functional endothelial aging in renal arteries. Pharmacology 100: 188–193, 2017. doi: 10.1159/000478732. [DOI] [PMC free article] [PubMed] [Google Scholar]