Abstract
In addition to forming symbiotic nodules on legumes, rhizobial strains are members of soil or rhizosphere communities or occur as endophytes, e.g., in rice. Two rhizobial strains which have been isolated from root nodules of the aquatic legumes Aeschynomene fluminensis (IRBG271) and Sesbania aculeata (IRBG74) were previously found to promote rice growth. In addition to analyzing their phylogenetic positions, we assessed the suitability of the 16S-23S ribosomal DNA (rDNA) intergenic spacer (IGS) sequences for the differentiation of closely related rhizobial taxa and for the development of PCR protocols allowing the specific detection of strains in the environment. 16S rDNA sequence analysis (sequence identity, 99%) and phylogenetic analysis of IGS sequences showed that strain IRBG271 was related to but distinct from Bradyrhizobium elkanii. Rhizobium sp. (Sesbania) strain IRBG74 was located in the Rhizobium-Agrobacterium cluster as a novel lineage according to phylogenetic 16S rDNA analysis (96.8 to 98.9% sequence identity with Agrobacterium tumefaciens; emended name, Rhizobium radiobacter). Strain IRBG74 harbored four copies of rRNA operons whose IGS sequences varied only slightly (2 to 9 nucleotides). The IGS sequence analyses allowed intraspecies differentiation, especially in the genus Bradyrhizobium, as illustrated here for strains of Bradyrhizobium japonicum, B. elkanii, Bradyrhizobium liaoningense, and Bradyrhizobium sp. (Chamaecytisus) strain BTA-1. It also clearly differentiated fast-growing rhizobial species and strains, albeit with lower statistical significance. Moreover, the high sequence variability allowed the development of highly specific IGS-targeted nested-PCR assays. Strains IRBG74 and IRBG271 were specifically detected in complex DNA mixtures of numerous related bacteria and in the DNA of roots of gnotobiotically cultured or even of soil-grown rice plants after inoculation. Thus, IGS sequence analysis is an attractive technique for both microbial ecology and systematics.
Rhizobia are classically defined as symbiotic bacteria capable of eliciting and invading root or stem nodules on leguminous plants, where they differentiate into N2-fixing bacteroids. Based on their 16S ribosomal DNA (rDNA) sequences, these nodule endosymbionts constitute a polyphyletic assemblage of bacteria grouped into four major phylogenetic branches of the α-2 subclass of the class Proteobacteria. Rhizobial strains are currently placed in the following genera: Allorhizobium (emended genus, Rhizobium), Mesorhizobium, Rhizobium, and Sinorhizobium constitute one of the rhizobial clades, whereas Azorhizobium, Bradyrhizobium, and Methylobacterium are each located on a different and well-resolved phylogenetic branch (45, 57). These legume symbionts are phylogenetically intertwined with several nonsymbiotic bacterial genera, including pathogenic, phototrophic, and denitrifying strains (for a review, see reference 48).
A remarkable ecological feature of rhizobia is their ability to thrive in very different environments. Many soils contain a rather large population of nonsymbiotic rhizobia that are found both in the bulk soil and in the rhizospheres of legumes and other plants (39, 40, 43). Some of these saprophytic or rhizospheric bacteria may become symbiotic by the horizontal acquisition of a symbiotic plasmid or a chromosomal symbiotic island (44), allowing them to synthesize and secrete strain-specific lipochitin-oligosaccharides for host nodulation and intracellular invasion. Rhizobia are also found as viable cells in water, where they are able to infect and nodulate aquatic legumes, such as Aeschynomene spp. and Sesbania spp. (8).
More recently, it has been recognized that these legume symbionts may also occur as endophytes in the roots of cereals, such as rice (Oryza breviligulata and Oryza sativa) (8, 14, 47, 55), wheat, and maize (39). These findings have stimulated research on rice growth promotion by rhizospheric and endophytic rhizobia (22). The isolation of plant growth-promoting rhizobia (PGPR) capable of enhancing rice yield under greenhouse and field conditions (55) is remarkable, since rice is the most important food crop produced in the world.
This study focuses on two rhizobial PGPR strains that were previously shown to promote rice growth (6, 7). These strains were isolated from root nodules of the aquatic legumes Aeschynomene fluminensis (IRBG271) (29, 41) and Sesbania aculeata (IRBG74) (J. K. Ladha, unpublished data), respectively, and had an uncertain taxonomic status. Therefore, one goal of this study was to determine their phylogenetic positions both by 16S rDNA and intergenic spacer (IGS) sequence analysis.
An important requirement for an agronomically useful rhizobium-graminea interaction is that the inoculated bacteria are able to establish a significant population on or in the host roots, particularly under competitive conditions in nonsterile substrates. Since the majority of bacteria cannot be easily cultured from their natural environments (35), a second objective of this study was to develop a culture-independent method for the rapid and easy detection of these rhizobial strains on rice roots. In microbial ecology, a wide range of methods for detection and identification of specific microorganisms in environmental samples has been developed. These include classical and molecular genetic methods. Traditional methods are mainly fluorescent-antibody and selective plating techniques, each of which is useful but limited in some respects (34, 39). Molecular biological techniques, such as phylogenetic probes or PCR-based approaches, allow the detection of particular DNA sequences and therefore can be used to trace their presence in target organisms directly in the environment (4, 9). The rDNA operon (rrn) is a particularly useful target for the development of nucleic acid hybridization- and PCR-based assays. In prokaryotes, the rDNA operon encodes the 16S (rrs), 23S (rrl), and 5S (rrf) rRNA genes. Although the 16S rRNA gene has been most widely used, the 16S-23S rDNA IGS region has received increased attention as a target in molecular detection and identification schemes (5, 30). In contrast to rRNA genes, which are remarkably well conserved throughout most bacterial species, the IGS regions exhibit a large degree of sequence diversity and length variation (24). Even within species, the IGS sequence variation may be very high, thus allowing intraspecies strain differentiation, as recently also shown for rhizobial strains (11, 30, 49, 51, 53).
Here we show that the presence of highly variable sequence stretches within IGS regions allows the development of a rapid, easy-to-perform, and sensitive PCR protocol for the specific detection of PGPR strains in the rhizospheres of rice plants cultivated in a gnotobiotic system as well as in nonsterile rice field soil. Phylogenetic sequence analysis of the 16S and IGS rDNA sequences of these PGPR strains consistently showed that IRBG271 is phylogenetically related to Bradyrhizobium elkanii, while strain IRBG74 is related to Agrobacterium tumefaciens bv. 1 (emended name, Rhizobium radiobacter [57]). Potential advantages and limitations of IGS sequence analyses for phylogenetic inference in rhizobia are discussed.
MATERIALS AND METHODS
Bacterial strains and cultural conditions.
The strains tested in this study are listed in Table 1. All the strains were routinely grown on YM medium, which consisted of SM medium (36) with the carbon source, potassium malate, replaced by 1% mannitol. All strains were grown at 28°C unless otherwise stated.
TABLE 1.
Isolates and reference strains used
| DNA pool | Strain or isolatea | Host plant | Geographic origin | Source or referenceb |
|---|---|---|---|---|
| Bradyrhizobium sp. strain IRBG271 | Aeschynomene fluminensis | Philippines | IRRI | |
| Rhizobium sp. strain IRBG74 | S. aculeata | Philippines | IRRI | |
| A | Azospira oryzae 6a3T = LMG 9096T | Leptochloa fusca | Pakistan | 37 |
| B. elkanii USDA76T | Glycine max | United States | USDA | |
| Bradyrhizobium sp. strain TAL 209∗ | Vigna radiata | Thailand | 41 | |
| Bradyrhizobium sp. strain TAL 289∗ | Indigofera endecaphylla | Mexico | 41 | |
| Bradyrhizobium sp. strain TAL 1037∗ | Indigofera brevicalix | Kenya | 41 | |
| Bradyrhizobium sp. strain TAL 1521∗ | Acacia manginum | Hawaii | 41 | |
| B | Bradyrhizobium sp. strain CIAT 109∗ | Desmodium intortum | Zaire | CIAT |
| Bradyrhizobium sp. strain CIAT 1502∗ | Desmodium incanum | Hawaii | CIAT | |
| Bradyrhizobium sp. strain CIAT 2335∗ | Desmodium ovalifolium | Brazil | CIAT | |
| B. elkanii USDA31, USDA46 | G. max | United States | USDA | |
| C | B. japonicum B 15 | G. max | China | CCBAU |
| B. japonicum USDA6T | G. max | Japan | USDA | |
| B. japonicum X1-3, X6-9 | G. max | China | 51 | |
| B. japonicum USDA62, USDA110, USDA123 | G. max | United States | USDA | |
| B. japonicum DSM 30131 | G. max | Japan | DSMZ | |
| D | Bradyrhizobium sp. strain BC-C1, BC-C2 | Chamaecytisus proliferus | Gran Canaria, Canary Islands | 51 |
| Bradyrhizobium sp. strain BC-P7, BTA-1 | C. proliferus | La Palma, Canary Islands | 51 | |
| Bradyrhizobium sp. strain TAL1000 | Arachis hypogaea | Hawaii | HAMBI | |
| Bradyrhizobium sp. strain FN13, CICS70 | Lupinus montanus | Mexico | 3 | |
| Bradyrhizobium sp. strain Spr7-9 | A. hypogaea | China | 58 | |
| E | Mesorhizobium loti NZP 2213T | Lotus corniculatus | New Zealand | NZP |
| M. loti NZP 2227, NZP 2234 | Lotus sp. | NZP | ||
| Mesorhizobium amorphae ACCC 19665T | Amorpha fruticosa | China | CCBAU | |
| Mesorhizobium sp. strain HL56 | A. fruticosa | China | CCBAU | |
| Mesorhizobium ciceri USDA 3378T (UPM-Ca7)T | Cicer arietinum | Spain | USDA | |
| F | Mesorhizobium huakuii A106, PL-52 | Astragalus sinicus | China | CCBAU |
| M. huakuii CCBAU 2609T | A. sinicus | China | CCBAU | |
| Mesorhizobium mediterraneum USDA3392T | Cicer arietinum | Spain | USDA | |
| Mesorhizobium plurifarium LMG 1892T | Acacia senegal | Senegal | LGM | |
| Mesorhizobium plurifarium USDA4413T | A. senegal | Senegal | USDA | |
| Mesorhizobium tianshanense A-1BST | Glycyrrhiza uralensis | China | CCBAU | |
| G | Rhizobium etli CFN42T | Phaseolus vulgaris | Mexico | CFN |
| R. galegae HAMBI 1185 | Galega sp. | United Kingdom | HAMBI | |
| R. galegae HAMBI 503 | Galega officinalis | United States | HAMBI | |
| R. galegae HAMBI 540T | Galega orientalis | Finland | HAMBI | |
| R. galegae 59A2 | United States | USDA | ||
| H | Rhizobium giardinii USDA2914T (H152T) | P. vulgaris | France | USDA |
| R. hainanense I12 | Centrosema pubescens | China | CCBAU | |
| R. hainanense 166T | Desmodium smuatum | China | CCBAU | |
| R. hainanense H14 | Desmodium heterophyllum | China | CCBAU | |
| Rhizobium huautlense S02T | Sesbania herbacea | Mexico | CIFN | |
| Rhizobium gallicum USDA2918T (R602spT) | P. vulgaris | France | USDA | |
| I | Rhizobium leguminosarum 162X68 | Trifolium sp. | United States | USDA |
| R. leguminosarum USDA2370T | United States | USDA | ||
| Rhizobium mongolense USDA1844T | Medicago ruthenica | China | USDA | |
| R. tropici type A CFN299 | P. vulgaris | Mexico | CFN | |
| R. tropici type A C-05-I | P. vulgaris | Brazil | CFN | |
| R. tropici type B BR853 | Leucaena leucocephala | Brazil | CFN | |
| R. tropici type B CIAT 899T | P. vulgaris | Columbia | CFN | |
| J | Sinorhizobium fredii 2048 | Glycine soja | China | CCBAU |
| S. fredii USDA194, USDA205T | G. soja | China | USDA | |
| Sinorhizobium meliloti USDA1002T | United States | USDA | ||
| S. meliloti 102F28 | Medicago sativa | |||
| S. meliloti H1 | Melilotus albus | China | CCBAU | |
| Sinorhizobium terangae LMG 7834T (ORS1009T) | Acacia laeta | Senegal | LGM | |
| Sinorhizobium xinjiangense CCBAU 110T | G. max | China | CCBAU | |
| S. xinjiangense CCBAU 108, Rx22 | G. max | China | CCBAU | |
| K | A. oryzae 6a3T = LMG 9096T | Leptochloa fusca | Pakistan | 37 |
| R. rubi HAMBI 1187T | HAMBI | |||
| R. radiobacter IAM 12048T | IAM | |||
| R. radiobacter IAM 13129 | IAM | |||
| R. rhizogenes IAM 13570T | IAM |
∗, Phylogenetically close to the B. elkanii rrn branch (P. Vinuesa, unpublished data).
CCBAU, Culture Collection of Beijing Agricultural University, Beijing, China; ACCC, Agricultural Center Culture Collection, Chinese Academy of Agriculture, Beijing, China; CIFN, Centro de Investigación sobre Fijación de Nitrógeno, Cuernavaca, Mexico; CIAT, Centro Internacional de Agricultura Tropical, Cali, Columbia; HAMBI, Culture Collection of the Department of Microbiology, University of Helsinki, Helsinki, Finland; IAM, Institute of Applied Microbiology, The University of Tokyo, Tokyo, Japan; LMG, Collection of Bacteria of the Laboratorium voor Microbiologie, University of Ghent, Ghent, Belgium; NZP, Culture Collection of the Department for Scientific and Industrial Research, Biochemistry Division, Palmerston North, New Zealand; ORS, ORSTOM Collection, Institut Français de Recherche Scientifique pour le Développement et Coopération, Dakar, Senegal; USDA, Rhizobium Culture Collection, Beltsville Agricultural Research Center, U.S. Department of Agriculture, Beltsville, Md.; IRRI, International Rice Research Institute, Los Banos Philippines; DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany.
Extraction of DNA and techniques for DNA manipulation.
Three milliliters of 3-day-old cultures (fast-growing bacteria) or 6-day-old cultures (slow-growing strains) was collected by centrifugation, and DNA was isolated after cell lysis with N-laurylsarcosin (5% [wt/vol]) and phenol-chloroform extraction as described previously (19). Rice roots were collected and vigorously washed by vortexing them in sterile distilled water with seven changes. The washed roots were frozen in liquid nitrogen and powdered in a mortar before being resuspended in 400 μl of DNA extraction buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0], 100 mM NaCl, 1.0% sodium dodecyl sulfate) and incubated at 60°C for 30 min. Cell debris was removed by centrifugation, and the supernatant was used for DNA extraction as described above. General techniques for DNA manipulations were carried out according to standard protocols (1).
Sequencing of 16S rRNA gene and 16S-23S rRNA IGS regions.
The methods for directly sequencing the PCR products of 16S rRNA genes were as described before (21), using an ALFexpress automated sequencer (Amersham Pharmacia Biotech). For the general IGS PCR, a forward primer, 926f (5′-GGT TAA AAC T[C/T]A AA[G/T] GAA TTG ACG G-3′, corresponding to a conserved region of the 3′ end of bacterial 16S rDNA, Escherichia coli sequence positions 901 to 926), and a reverse primer, 115r/23S (5′-CCG GGT T[T/G/C]C CCC ATT CGG-3′, corresponding to a conserved region of the 5′ end of 23S rDNA, E. coli sequence positions 97 to 115), were used to amplify the 16S-23S rDNA IGS region. The amplification was carried out by the following steps: initial denaturation at 94°C for 3 min; 30 cycles at 94°C for 1 min, 56°C for 1 min, and 72°C for 90 s; and then extension at 72°C for 10 min. The PCR products of the IGS were dialyzed and sequenced directly by using the Cy-5-end-labeled primer 1492fc (5′-AAG TCG TAA CAA GGT A[A/G]C CGT-3′, corresponding to E. coli 16S rDNA sequence positions 1471 to 1492) and the reverse primer 85rc/23S (5′-CCC CAC GGC TT[A/T] TCG CA[A/G] CGT ATC AC-3′, corresponding to E. coli 23S rDNA sequence positions 59 to 85). Sequencing reactions of 600 to 700 nucleotides (nt) yielded an almost-complete overlap only for short IGS sequences, which were thus sequenced from both strands. For large IGS fragments, the overlap was accordingly shorter.
Determination of the rrn operon copy number in rhizobial genomes.
To detect different copies of rRNA genes, Southern blots of chromosomal DNA (3 μg per lane digested with different restriction endonucleases) were hybridized with 16S rDNA-targeted gene probes or an oligonucleotide probe, respectively. Digoxigenin-labeled fragments of 16S rDNA were generated by PCR using the Dig-DNA labeling and detection kit (Roche) with the forward primer 342f (5′-CTC CTA CGG GAG GCA G-3′) and the reverse primer 926r (5′-YYC CGT CAA TTC CTT TAA GTT T-3′). The template for PCR was chromosomal DNA of strain IRBG74 or IRBG271, respectively. High-stringency hybridization was carried out at 65°C. Hybridization with a digoxigenin-labeled oligonucleotide (926f; 5′-AAA CTY AAA KGA ATT GA-3′) was carried out at 45°C (19).
For sequence analysis of different IGS copies in strain IRBG74, genomic DNA was digested with PstI, and fragments were separated by agarose gel electrophoresis in two different lanes. One lane was used for Southern blot hybridization with oligonucleotide 926f, and the other lane was used for excision of the corresponding fragments from the agarose gel. The gel slices were washed separately in Tris-EDTA buffer for 15 min and then disrupted by the freeze-thaw method in 50 μl of Tris-EDTA buffer. Five microliters of supernatant was used for PCR amplification of 16S rDNA with adjacent IGS sequences, using primers 25f (5′-AAC TKA AGA GTT TGA TCC TGG CTC-3′) and 115r/23S. Amplification products were cloned into the Topo TA vector (Invitrogen). Positive clones were used for plasmid sequencing.
Phylogenetic sequence analysis and design of strain-specific IGS-targeted primers.
The determined rDNA sequences together with reference sequences retrieved from GenBank were aligned by using the Ribosomal Database Project (31). The distances of aligned sequences (corresponding to E. coli 16S rDNA positions 57 to 1440) were calculated by the Jukes-Cantor method (25). The tree topology was inferred by the neighbor-joining method (38), and the phylogenetic tree was constructed with the Treecon software package (50). The sequences of IGS regions and known closely related sequences obtained were aligned by using CLUSTAL W, version 1.8 (46), and the tree was constructed as described above. Two sets of IGS-targeted primers were designed as follows: R2ssf (5′-CCT GGA TCA ACG CGG TAT-3′) and R2ssr (5′-CCA TAG CCG CTC CAA AGG A-3′) for strain IRBG74 and R3ssf (5′-GAG CGC TGT GCG ATG CAT CG-3′) and R3ssr (5′-GCT CAT CTT GCG ATG AAC GAG-3′) for strain IRBG271.
PCR conditions for specific IGS PCRs.
Strains from the rhizobia, sinorhizobia, mesorhizobia, bradyrhizobia, and agrobacteria were divided into 11 genomic DNA pools, 6 of which (A, B, C, D, E, and F) contained samples from slow-growing strains, while the other 5 (G, H, I, J, and K) contained samples from fast-growing strains. Each DNA pool consisted of 5 to 10 strains (Table 1). Pure genomic DNA of strain IRBG74 or IRBG271 was used as a positive control. The final concentration of each genomic DNA was 10 ng/μl, and 0.5 μl of DNA solution was used as a PCR template. PCRs were carried out in 25-μl volumes using Ready-To-Go PCR beads (Amersham Pharmacia Biotech) with 0.5 μl of each primer at 50 μM. Cycle conditions for direct and nested specific IGS amplifications were identical: initial denaturation at 94°C for 3 min; 30 cycles at 94°C for 1 min, 56°C for 1 min, and 72°C for 90 s; and then extension at 72°C for 10 min, with primers R2ssf-R2ssr or R3ssf-R3ssr, respectively. Five microliters of the amplification product was used for agarose (1.1%) gel electrophoresis. For nested PCR, the amplification products of general IGS PCRs (see above) were diluted 50-fold in sterile distilled water, and 1 μl was used for IGS-targeted specific PCR. In plant inoculation experiments, 70 ng of DNA extracted from rice roots per reaction mixture was used as a PCR template.
Rice germination and cultivation.
Seeds of O. sativa IR36 were dehusked, surface sterilized, and germinated on agar plates as previously described (20). The seedlings were aseptically transferred to glass tubes containing 5 ml of sterile plant medium (13) supplemented with 100 mg of glucose (instead of malate) per liter and 0.2% agar instead of quartz sand. Alternatively, the seedlings were transferred to 5 g of unsterilized dried rice field soil (unfertilized), which had been collected from the Camargue region of France and was saturated with distilled water prior to transplanting. Bacterial cells grown aerobically on liquid YM medium were washed twice in plant medium and inoculated into the rice test tubes at 2 × 107 cells of each strain per tube, with eight replicates per experiment. The rice plants were grown in a greenhouse for 15 days (at 30°C; 2,200 lx of light added for 14 h; 80% humidity); the lower parts of the tubes were immersed in ink-stained water to shade the roots.
Nucleotide sequence accession numbers.
The 16S rDNA sequence of Rhizobium sp. (Sesbania) strain IRBG74 and Bradyrhizobium sp. (Aeschynomene) strain IRBG271 have been deposited in GenBank under accession numbers AF364836 to AF364839 and AF271638, respectively. The accession numbers of IGS sequences were as follows: AF271639, Bradyrhizobium japonicum USDA123; AF271640, Bradyrhizobium. sp. (Arachis) strain TAL 1000; AF271641, B. japonicum X1-3; AF271642, B. japonicum USDA62; AF271643, B. japonicum DSM 30131; AF324182, B. elkanii USDA46; AF324181, Bradyrhizobium sp. strain BTA-1; AF271644, A. tumefaciens IAM 13129; AF271645, Rhizobium tropici CIAT 899; AF271646, Rhizobium galegae HAMBI 540; AF271647, Bradyrhizobium sp. strain IRBG271; AF271648, Rhizobium sp. strain IRBG74.
RESULTS
Phylogenetic position of rice growth-promoting rhizobial strains according to 16S rDNA sequence analysis.
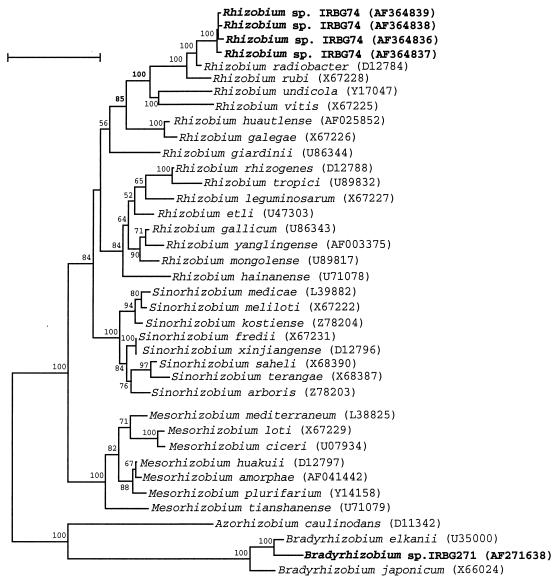
Almost-complete 16S rDNA sequences of isolates IRBG74 and IRBG271 were obtained and used for phylogenetic analysis together with published rhizobial reference sequences. The phylogenetic tree derived from the sequence distance values is shown in Fig. 1. The sequences of both PGPR strains appeared to be distinct from those of known species of rhizobia. That of strain IRBG271 was related, but not identical, to the sequence of B. elkanii USDA76T, the type strain of the species (28). The identity level of the 16S rDNA sequences of isolates IRBG271 and B. elkanii USDA76T or B. japonicum LMG 6138 was 99 or 98.2%, respectively. Isolate IRBG74 clustered in the Rhizobium-Agrobacterium branch, being more closely related to the former A. tumefaciens (emended name, R. radiobacter). The levels of 16S rDNA sequence identity between strain IRBG74 and R. radiobacter LMG 196, Rhizobium rubi LMG 156, and R. galegae IAM 13631 were 98.4, 97, and 95.4%, respectively. However, isolate IRBG74 differed from former Agrobacterium spp. in two major respects: it was isolated from nodules of S. aculeata, and it harbored a nifH gene (as detected by PCR and by Southern hybridization [not shown]). Thus, we refer to it as Rhizobium sp. (Sesbania).
FIG. 1.
Neighbor-joining dendrogram derived from a 16S rDNA sequence distance matrix (Jukes-Cantor) of the root nodule isolates IRBG74 and IRBG271 and known related Rhizobium (including former Agrobacterium), Sinorhizobium, Mesorhizobium, and Bradyrhizobium species. Bootstrap confidence levels greater than 50% are indicated at the internodes. GenBank accession numbers are shown in parentheses. Bar = 5% nucleotide divergence.
Comparison of rhizobial 16S-23S rDNA IGS regions.
Since only a few published sequences were available when the project was started, the 16S-23S rDNA IGS regions of several reference strains belonging to the Rhizobium-Agrobacterium cluster as well as to the Bradyrhizobium cluster were sequenced (for sequences and alignment, see http://www.institute.uni-bremen.de/∼reinhol/IGS.html). It has been reported that most 16S-23S IGSs lack tRNA sequences in Bacillus subtilis (16); however, in Rhizobium, former Agrobacterium, and Bradyrhizobium species, the presence of tandem tRNAIle and tRNAAla genes was observed, as previously (27) and more recently (49, 53) reported for Bradyrhizobium strains.
In fast-growing rhizobia and agrobacteria, the tRNA genes showed some sequence variability, while they were highly conserved among the slow-growing bradyrhizobia. In both groups, the IGS sequences read from the rrs to the rrl gene, contained a conserved region that was followed first by a highly variable region (region I), after which the genes for tRNAIle and tRNAAla were found, interrupted by a variable region (II). Downstream of the tRNA genes, a third highly variable region (III) was located preceding a conserved region.
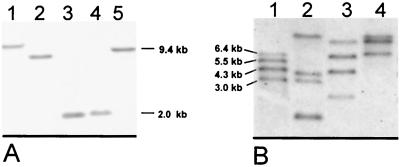
Copy numbers of rrn operons in rhizobial isolates.
Since some bacterial species may have multiple rDNA operons, the operon numbers in the strains under question were determined by Southern hybridization using a digoxigenin-labeled 16S rDNA fragment (Fig. 2A) or oligonucleotide (Fig. 2B). In Bradyrhizobium sp. strain IRBG271, only one hybridizing fragment was detected in chromosomal DNA when it was digested with five different restriction endonucleases (Fig. 2A), indicating that the genome contains only one copy. In contrast, in Rhizobium sp. strain IRBG74, three to four different hybridizing fragments were found with four restriction endonucleases (not shown). In order to exclude false positives due to putative internal restriction sites, hybridizations were repeated with an oligonucleotide probe targeted to a conserved site of the 16S rDNA gene (926f). Again, four hybridizing fragments were detected (Fig. 2B), indicating that this strain contains four different rrn operon copies. To detect putative sequence polymorphisms in these copies, the four different copies of 16S rDNA with the adjacent IGS region were amplified by PCR, cloned, and sequenced. The 16S rDNA genes diverged only slightly (4 to 6 nt [Fig. 1]), and sequence variation within the IGS was small (2 to 9 nt [see alignment]).
FIG. 2.
Southern blot hybridization of genomic DNA of Bradyrhizobium sp. strain IRBG271 (A) and Rhizobium sp. strain IRBG74 (B) for detection of the 16S rDNA copy number. Hybridization was performed with a homologous 16S rDNA gene probe (A) or a universal oligonucleotide probe (926f) (B). Each lane was loaded with 3 μg of DNA digested with BamHI (A, lane 1), EcoRI (A, lane 2), SmaI (A, lane 3; B, lane 2), Asp718 (A, lane 4), PstI (A, lane 5, and B, lane 1), PvuII (B, lane 3), or RsrII (B, lane 4).
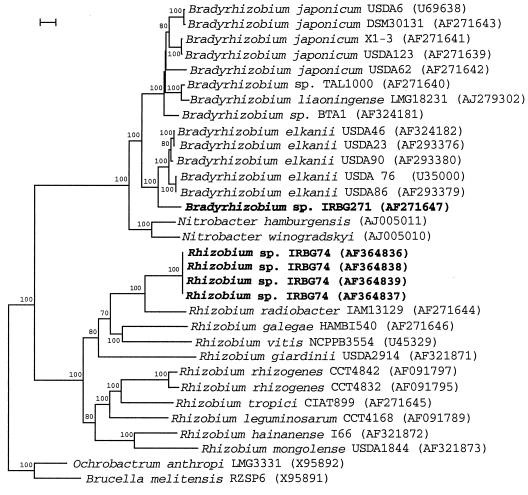
Phylogenetic analysis of rhizobial IGS sequences.
Aligned sequences checked carefully by hand using GeneDoc software (http://www.cris.com/∼ketchup/genedoc.shtml) were used to construct a phylogenetic tree (Fig. 3). The reference strains of the three validly described Bradyrhizobium species were well resolved, clustering with other representatives of each species. Strain IRBG271 was found to be most closely related to the B. elkanii cluster (sequence identity to B. elkanii USDA76T, 91.6%), as deduced also from the 16S rDNA analyses. The Chamaecytisus isolate BAT-1 was more closely related to but distinct from the B. japonicum lineage (sequence identity, 90.6 to 93.7%), which is consistent with previously published partial 16S rDNA sequence and IGS PCR-restriction fragment length polymorphism (RFLP) analyses (51, 52). Among the fast-growing strains of rhizobia and agrobacteria, strains of different species were clearly differentiated from each other. However, not all nodes were statistically significant (Fig. 3), and the tree topology partially differed from 16S rDNA analyses (Fig. 1). This is most likely due to the high IGS sequence variability in these strains, which was significantly greater than for the slow-growing rhizobia. Rhizobium sp. strain IRBG74 clustered again with R. radiobacter (formerly A. tumefaciens), displaying 86% sequence identity (Fig. 3).
FIG. 3.
Neighbor-joining dendrogram derived from a 16S-23S rDNA IGS sequence distance matrix (Jukes-Cantor) of Bradyrhizobium sp. and Rhizobium sp. clusters. Bootstrap confidence levels greater than 50% are indicated above the nodes. GenBank accession numbers are shown in parentheses. Bar = 5% nucleotide divergence.
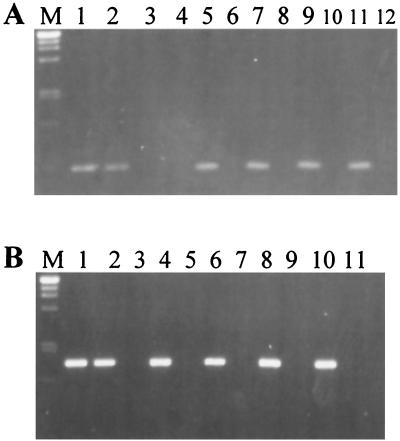
Specificity of the IGS-targeted primers.
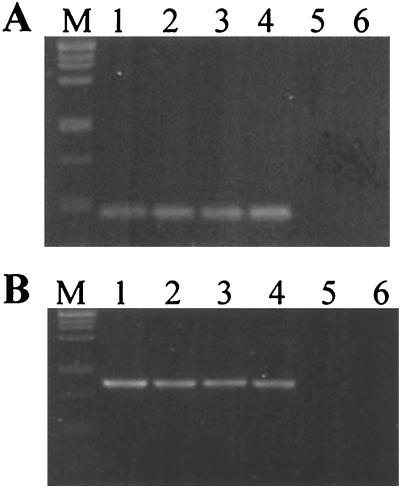
Strain-specific primers were developed from the highly variable regions I and III: R3ssf and R3ssr, amplifying a 451-bp fragment, for Bradyrhizobium sp. strain IRBG271 and R2ssf and R2ssr, yielding a 988-bp product, for Rhizobium sp. IRBG74. In order to test a wide range of strains for an assessment of primer specificity, pools of DNA samples from different strains were used. Eleven genomic DNA pools containing DNA from rhizobia, sinorhizobia, mesorhizobia, bradyrhizobia, and agrobacteria (shown in Table 1) were used as PCR templates. To assess whether the mixture of DNAs conferred any inhibitory effects on PCR, reactions were also carried out with an internal positive control, the DNA of the rhizobial isolates being added. Bradyrhizobium sp. strain IRBG271 was compared with slow-growing strains (mesorhizobia and bradyrhizobia) (Fig. 4A), while Rhizobium sp. strain IRBG74 was separately compared with fast-growing strains (rhizobia, sinorhizobia, and agrobacteria) (Fig. 4B). For the nested PCR, the IGS region was first amplified from the mixtures by using general primers, and the diluted PCR product was subsequently used as a template for the strain-specific PCR. The amplification products from DNAs of pure cultures were of the expected size, approximately 450 bp for strain IRBG271 and 1,000 bp for strain IRBG74 (Fig. 4, lanes 1). When DNA pools were used, a product of this size was amplified only when DNA of the specific strain, Bradyrhizobium sp. strain IRBG271 (Fig. 4A) or Rhizobium sp. strain IRBG74 (Fig. 4B), was added as a template. Thus, the PCR protocol was considered to be sufficiently specific to differentiate the rhizobial strains, even in DNA mixtures of closely related species.
FIG. 4.
Amplification products of nested PCR using IGS-targeted primers specific for Bradyrhizobium sp. strain IRBG271 (A) or Rhizobium sp. strain IRBG74 (B) in pure culture (lanes 1) or in the presence of a mixture of related bacteria (lanes 2 through 12). DNA pools of numerous reference strains (Table 1) of Bradyrhizobium spp. (A) or Rhizobium, Mesorhizobium, and Sinorhizobium (B) were used as templates. The specific IGS-targeted primers R3ssf-R3ssr (A) and R2ssf-R2ssr (B) were used in the second reaction mixture; 5 μl of the amplification product was loaded on a 1.1% agarose gel. The products were approximately 450 (A) or 1,000 (B) bp in size. M, size marker (λ DNA digested with PstI). (A) Lanes: 1, strain IRBG271; 2, strain pool A plus IRBG271; 3, strain pool A; 4, strain pool B; 5, strain pool C plus IRBG271; 6, strain pool C; 7, strain pool D plus IRBG271; 8, strain pool D; 9, strain pool E plus IRBG271; 10, strain pool E; 11, strain pool F plus IRBG271; 12, strain pool F. (B) Lanes: 1, IRBG74; 2, strain pool G plus IRBG74; 3, strain pool G; 4, strain pool H plus IRBG74; 5, strain pool H; 6, strain pool I plus IRBG74; 7, strain pool I; 8, strain pool J plus IRBG74; 9, strain pool J; 10, strain pool K plus IRBG74; 11, strain pool K.
Application of specific IGS-targeted PCR primers to detect rhizobial colonization of rice roots.
In order to assess whether the PCR protocol developed here was sufficiently sensitive and specific to detect colonization of roots by the rhizobial isolates, rice seedlings were inoculated either under gnotobiotic conditions or in complex soil. The total DNA isolated from the roots was subjected to PCR. In the nested IGS PCR using the specific primers R2ssf-R2ssr or R3ssf-R3ssr, single amplification products of the expected size (450 or 1,000 bp) were obtained (Fig. 5 and 6). In uninoculated control plants (Fig. 5 and 6, lanes 6), no amplification product was obtained, indicating that rice DNA (Fig. 5) or DNA of roots colonized by soil bacteria (Fig. 6) was not giving rise to false amplification products. The lack of an amplification product from soil-grown roots also indicated that the Asian rhizobial strains were not present in the rice field soil from France, or if present, they were not efficiently colonizing the rice cultivar.
FIG. 5.
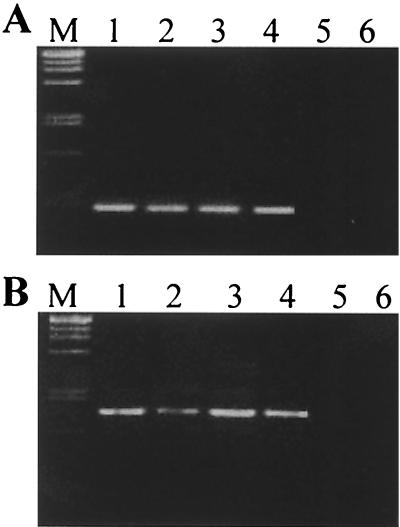
Detection of Bradyrhizobium sp. strain IRBG271 (A) or Rhizobium sp. strain IRBG74 (B) in association with roots of inoculated rice plants by nested IGS-targeted PCR, without and with the presence of other bacteria. The DNA of roots of rice seedlings grown in gnotobiotic culture in agar medium was used for amplification, with the specific IGS-targeted primers R3ssf-R3ssr (A) and R2ssf-R2ssr (B) used in the second reaction mixture; 5 μl of the amplification product was loaded on a 1.1% agarose gel. The products were approximately 450 (A) or 1,000 (B) bp in size. M, size marker (λ DNA digested with PstI). Lanes 1, pure culture of IRBG271 (A) or IRBG74 (B); lanes 2 to 4, rice inoculated with strain IRBG271 (A) or strain IRBG74 (B); lanes 3, rice inoculated with IRBG271 and IRBG74; lanes 4, rice inoculated with IRBG271, IRBG74, and A. oryzae 6a3; lanes 5, rice inoculated with A. oryza 6a3 only; 6, uninoculated rice.
FIG. 6.
Nested IGS-targeted PCR for detection of Bradyrhizobium sp. strain IRBG271 (A) or Rhizobium sp. strain IRBG74 (B) in association with inoculated rice roots grown in unsterilized soil. The plants were grown in unsterilized rice field soil from France inoculated with different bacterial strains, and total DNA was extracted after 15 days. For the PCR conditions and sample loading, see the legend to Fig. 5.
Seedlings inoculated with Rhizobium sp. strain IRBG74 or Bradyrhizobium sp. strain IRBG271 separately (Fig. 5 and 6, lanes 2) or in a mixture of both (Fig. 5 and 6, lanes 3), gave rise to amplification products of the appropriate size, indicating that the bacteria were colonizing the roots sufficiently well to be detected by our PCR assay. This was especially interesting for soil-grown roots (Fig. 6), where the rhizobial isolates had to compete with the microflora present in the rice field soil. As an additional competitive constraint, a grass-endophytic diazotroph Azospira oryzae 6a3, originally isolated from Kallar grass (37), was coinoculated (Fig. 5 and 6, lanes 4 and 5). In both experimental settings, the gnotobiotic agar system and soil, the rhizobia were still detectable in root DNA. An unnested PCR approach applying specific IGS PCR directly, without a prior general amplification of bacterial IGS fragments, led to a loss of specificity: DNA fragments of aberrant size were amplified from uninoculated roots and inoculated samples, especially when soil-grown plants were analyzed. The direct PCR protocol was specific, however, when applied to DNA of pure cultures (not shown). The protocol developed for nested IGS-targeted PCR, however, was sufficiently sensitive and specific to detect and identify the rhizobial isolates on roots.
DISCUSSION
Strains of Azorhizobium, Bradyrhizobium, and Rhizobium have been identified as endophytes of different rice cultivars and species growing naturally or under cultivation in disjunct geographical regions around the world (8, 14, 47, 55). One objective of this study, therefore, was to determine the phylogenetic placement of the rice growth-promotig rhizobial strains IRBG74 and IRBG271, which are fast- and slow-growing isolates, respectively.
Our 16S rDNA phylogenetic analysis of rhizobial strains revealed the same overall topologies described by others (10, 57). The new S. aculeata isolate IRBG74 is placed in the former Agrobacterium-Allorhizobium clade, the closest phylogenetic neighbors being strains of A. tumefaciens bv. 1 and Agrobacterium rubi (now R. radiobacter and R. rubi, respectively). The high 16S rDNA sequence identity of strain IRBG74 to these strains (96.8 to 98.9 and 97%, respectively) strongly suggests its taxonomic placement in the genus Rhizobium according to Young (57). To our knowledge, this is the first report of a rhizobial strain from the R. radiobacter-Rhizobium undicola clade exhibiting a rice growth-promoting effect.
The A. fluminensis isolate IRBG271 was placed in the Bradyrhizobium clade in our analysis, being most closely related to B. elkanii USDA76T (99% sequence identity). This high similarity level is not sufficient, however, to classify isolate IRBG271 as a B. elkanii strain, since it is well documented for many bacterial groups, including rhizobia, that full-length rDNA sequence analysis provides insufficient taxonomic resolution to distinguish closely related (geno)species (3, 15, 42, 49).
Recently, several reports have shown the suitability of IGS PCR-RFLP analysis for the rapid genotypic characterization, identification, and grouping of large collections of Bradyrhizobium strains at much higher taxonomic resolution than rrs sequence or PCR-RFLP analysis (12, 49, 51). Therefore, sequencing of the IGS region would allow the full exploitation of this variable region for phylogenetic analysis of rhizobia, as frequently used in plant systematics and recently reported for several microbial groups (2, 17, 56). Two key studies that used IGS sequence analysis for inter- and intraspecific phylogenetic analysis of Bradyrhizobium strains were published during the review phase of this manuscript (49, 53). These studies revealed a very good correlation between groupings obtained by IGS sequence analysis, total DNA-DNA hybridization, and AFLP fingerprinting data. Willems et al. (53) reported that all the Bradyrhizobium genospecies identified by DNA-DNA hybridization were also resolved by IGS sequence analysis. However, IGS sequence similarity levels within the different genospecies were found to vary considerably, and therefore these authors found it impossible to use one particular level of IGS sequence similarity to delineate genospecies. A relatively low IGS sequence variation (94 to 100% sequence identity) was found within most of their AFLP clusters, whereas related genospecies displayed 81 to 89% IGS sequence similarity (53). The notable taxonomic resolution achieved by IGS sequencing, and the phylogenetic consistency of the delineated groups is also clearly illustrated in our analysis. The B. japonicum strains USDA6T, USDA123, and USDA62 have rrs sequences that diverge <1% and belong to the distinct serogroups denoted by their respective strain numbering. The first two strains belong to DNA homology group I of Hollis et al. (18), whereas USDA62 belongs to homology group Ia. This is reflected by the placement of USDA62 as a separate branch of the clade formed by strains USDA6T and USDA123 on our IGS dendrogram. The 16S rDNA sequence of B. liaoningense LMG18231 differs by only 3 nt from that of B. japonicum USDA6T (49, 54) but is well resolved on the IGS dendrogram as a distinct strain that is closely related to B. japonicum. The same applies for Bradyrhizobium sp. (Chamaecytisus) strain BTA-1, which is consistent with previous reports using 16S and IGS rDNA PCR-RFLP analyses and stable low-molecular-weight RNA fingerprints (23, 52). All these strains form a clade that is well resolved at highly significant bootstrap values from a cluster containing the B. elkanii reference strains, in good agreement with other recent reports (49, 53). Therefore, we conclude that IGS sequence analysis is a powerful tool to delineate inter- and intraspecific Bradyrhizobium groups at statistically significant levels. As discussed by Willems et al. (53), most of these clades will correspond to different genomic species. However, due to the the highly variable nature of the IGS region, in some instances this genetic marker might reveal infraspecific genotypic differences that are not apparent when studying overall genomic similarities.
The phylogenetic placement of strain IRBG271 on the IGS dendrogram as a sister branch to the cluster formed by B. elkanii strains (91.65% similarity with USDA76T) suggests, therefore, that it may correspond to a new genomic species. Strains IRBG271 and USDA76T were found to be related but distinguishable by their cellular fatty acid compositions (41), indicating that strain IRBG271 may eventually be classified as a new species related to B. elkanii. We refrain here from making a formal species proposal, since more isolates related to IRBG271 should be characterized using a polyphasic taxonomic approach.
A potential limitation of 16S-23S rDNA IGS sequences for phylogenetic analysis in prokaryotes is the fact that many bacterial species possess several copies of the rrn operon, which may differ from one another, as demonstrated unambiguously by several genome-sequencing projects. This applies also to rhizobia, particularly to the fast-growing strains like IRBG74, whereas most Bradyrhizobium sp. strains, like IRBG271, typically contain a single rrn operon (27, 53). Although strain IRBG74 was found to harbor four copies of rRNA genes, the sequence divergence among different copies was very low (2 to 9 nt) and therefore did not blur our analysis. Strain IRBG74, which was found to be related to R. radiobacter based on rrs sequence analysis, had a significantly different IGS region (only 86% identity), suggesting that it is genomically quite distinct from R. radiobacter and therefore may also represent a new rhizobial species. However, as discussed for strain IRBG271, the exact taxonomic affiliation of strain IRBG74 can only be ascertained by using a polyphasic taxonomic approach.
As a general conclusion, IGS sequences appeared to be more suitable for phylogenetic analyses in Bradyrhizobium than in the Rhizobium group. In the latter, at many nodes, the tree topology was statistically not well supported and differed from the 16S rDNA-based data. An illustrative example of this point is the clustering of R. tropici CIAT899 with Rhizobium hainanense I66 rather than with Rhizobium rhizogenes strains, as would be expected from the rrs sequence analysis. These inconsistencies are certainly a consequence of the high variability found in IGS sequences, including several gaps and insertions. However, this intrinsic sequence variability was very valuable for strain differentiation and identification in both bradyrhizobia and rhizobia, making IGS sequence analysis a very attractive technique for phylogenetic studies of these bacteria.
The second objective of this work was to develop a culture-independent, easy-to-perform, and sensitive technique to allow the specific detection of our rice growth-promoting strains in the rhizospheres of rice plants. At present, it is not known which mechanisms are responsible for the observed growth promotion that some rhizobia exert on rice. Since the reported N2-fixing activities measured in the rice rhizospheres is generally too low to account for the observed growth effect (8, 55), other factors, like phytohormone production, may be also involved, as is the case for other endophytic and rhizospheric associations (33). However, growth promotion on the field scale will only take place if the inoculated strains are able to establish themselves in the rhizosphere, on or inside the host roots. Therefore, it is of critical importance to be able to assess the colonizing abilities of potential inoculant strains, particularly under competitive conditions in nonsterile substrates. The high variability of the IGS region allowed the design of specific primers for the identification of streptococcal species or strains (5). Here we show the broad applicability of this strategy by using it to specifically detect our rice growth-promoting rhizobia colonizing rice roots.
To test the specificity of the PCR protocol, a large number of rhizobial and agrobacterial strains from different geographic locations and genera were used, including those found to be most closely related to IRBG74 and IRBG271 based on IGS sequence analyses. In complex mixtures of bacterial DNAs, as well as with the even more complex DNA mixtures extracted from roots grown in soil, the assay was remarkably specific. It is noteworthy that our PCR tests showed that the strains were even able to compete for colonization sites at rice roots when coinoculated with other grass endophytes like A. oryzae (37) or when rice seedlings were grown in unsterilized rice field soil, which should harbor plenty of other competing bacteria. This is in good agreement with the finding that these isolates promote plant growth by causing an enhanced seedling vigor in soil under greenhouse conditions (6, 7). However, 6 weeks after inoculation, the inoculated strains could not be isolated any longer from the rice roots (6). It is known that the majority of bacteria in a given ecosystem cannot be cultivated by conventional microbiological methods (35), and this has also been shown for diazotrophic endophytic bacteria occurring naturally in rice roots (14). Nonsporulating bacteria released into an environment might reach a physiological state in which they are viable but difficult to cultivate (26). Since the detection method that we have developed is solely based on the presence of bacterial DNA, it overcomes problems of cultivation, allowing us to prove the presence of rhizobia even in an unculturable state. Since IGS sequence variability can also be exploited to design strain-specific oligonucleotides for in situ hybridization, this rrn operon region is clearly of great relevance for both microbial systematics and ecology.
ACKNOWLEDGMENT
This project was funded by a collaborative grant to B.R.-H. and the International Rice Research Institute, Philippines, by the BMZ.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 2.Baldwin B G, Markos S. Phylogenetic utility of the external transcribed spacer (ETS) of 18S-26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae) Mol Phylogenet Evol. 1998;10:449–463. doi: 10.1006/mpev.1998.0545. [DOI] [PubMed] [Google Scholar]
- 3.Barrera L L, Trujillo M E, Goodfellow M, Garcia F J, Hernandez-Lucas I, Davila G, van Berkum P, Martinez-Romero E. Biodiversity of bradyrhizobia nodulating Lupinus spp. Int J Syst Bacteriol. 1997;47:1086–1091. doi: 10.1099/00207713-47-4-1086. [DOI] [PubMed] [Google Scholar]
- 4.Bej A K, Mahbubani M H, Dicesare J L, Atlas R M. Polymerase chain reaction-gene probe detection of microorganisms by using filter-concentrated samples. Appl Environ Microbiol. 1991;57:3529–3534. doi: 10.1128/aem.57.12.3529-3534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge B R, Fuller J D, De Azavedo J, Low D E, Bercovier H, Frelier P F. Development of specific nested oligonucleotide PCR primers for the Streptococcus iniae 16S-23S ribosomal DNA intergenic spacer. J Clin Microbiol. 1998;36:2778–2781. doi: 10.1128/jcm.36.9.2778-2781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas J C, Ladha J K, Dazzo F B. Rhizobial inoculation improves nutrient uptake and growth of lowland rice. Soil Sci Soc Am J. 2000;64:1644–1650. [Google Scholar]
- 7.Biswas J C, Ladha J K, Dazzo F B, Yanni Y G, Rolfe B G. Rhizobial inoculation influences seedling vigor and yield of rice. Agron J. 2000;92:880–886. [Google Scholar]
- 8.Chaintreuil C, Giraud E, Prin Y, Lorquin J, Ba A, Gillis M, de Lajudie P, Dreyfus B. Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl Environ Microbiol. 2000;66:5437–5447. doi: 10.1128/aem.66.12.5437-5447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry G R, Toranzos G A, Bhatti A R. Novel method for monitoring genetically engineered microorganisms in the environment. Appl Environ Microbiol. 1989;55:1301–1304. doi: 10.1128/aem.55.5.1301-1304.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lajudie P, Laurent-Fulele E, Willems A, Torck U, Coopman R, Collins M D, Kersters K, Dreyfus B, Gillis M. Allorhizobium undicola gen. nov., sp. nov., nitrogen-fixing bacteria that efficiently nodulate Neptunia natans in Senegal. Int J Syst Bacteriol. 1998;48:1277–1290. doi: 10.1099/00207713-48-4-1277. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira V M, Coutinho H L C, Sobral B W S, Gumaraes C T, Van Elsas J D, Manfio G P. Discrimination of Rhizobium tropici and R. leguminosarum strains by PCR-specific amplification of 16S-23S rDNA spacer region fragments and denaturing gradient gel electrophoresis (DGGE) Lett Appl Microbiol. 1999;28:137–141. doi: 10.1046/j.1365-2672.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- 12.Doignon-Bourcier F, Willems A, Coopman R, Laguerre G, Gillis M, de Lajudie P. Genotypic characterization of Bradyrhizobium strains nodulating small Senegalese legumes by 16S-23S rRNA intergenic gene spacers and amplified fragment length polymorphism fingerprint analyses. Appl Environ Microbiol. 2000;66:3987–3997. doi: 10.1128/aem.66.9.3987-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egener T, Hurek T, Reinhold-Hurek B. Endophytic expression of nif genes of Azoarcus sp. strain BH72 in rice roots. Mol Plant-Microbe Interact. 1999;12:813–819. doi: 10.1094/MPMI.1998.11.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Engelhard M, Hurek T, Reinhold-Hurek B. Preferential occurrence of diazotrophic endophytes, Azoarcus spp., in wild rice species and land races of Oryza sativa in comparison with modern races. Environ Microbiol. 2000;2:131–141. doi: 10.1046/j.1462-2920.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 15.Fox G E, Wisotzkey J D, Jurtshuk P., Jr How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 16.Fox K F, Fox A, Nagpal M, Steinberg P, Heroux K. Identification of Brucella by ribosomal-spacer-region PCR and differentiation of Brucella canis from other Brucella spp. pathogenic for humans by carbohydrate profiles. J Clin Microbiol. 1998;36:3217–3222. doi: 10.1128/jcm.36.11.3217-3222.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundmann G L, Neyra M, Normand P. High-resolution phylogenetic analysis of NO2−-oxidizing Nitrobacter species using the rrs-rrl IGS sequence and rrl genes. Int J Syst Evol Microbiol. 2000;50:1893–1898. doi: 10.1099/00207713-50-5-1893. [DOI] [PubMed] [Google Scholar]
- 18.Hollis A B, Kloos W E, Elkan G E. DNA:DNA hybridization studies of Rhizobium japonicum and related Rhizobiaceae. J Gen Microbiol. 1981;123:215–222. [Google Scholar]
- 19.Hurek T, Burggraf S, Woese C R, Reinhold-Hurek B. 16S rRNA-targeted polymerase chain reaction and oligonucleotide hybridization to screen for Azoarcus spp., grass-associated diazotrophs. Appl Environ Microbiol. 1993;59:3816–3824. doi: 10.1128/aem.59.11.3816-3824.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurek T, Reinhold-Hurek B, Van Montagu M, Kellenberger E. Root colonization and systemic spreading of Azoarcus sp. strain BH72 in grasses. J Bacteriol. 1994;176:1913–1923. doi: 10.1128/jb.176.7.1913-1923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurek T, Wagner B, Reinhold-Hurek B. Identification of N2-fixing plant- and fungus-associated Azoarcus species by PCR-based genomic fingerprints. Appl Environ Microbiol. 1997;63:4331–4339. doi: 10.1128/aem.63.11.4331-4339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James E K, Gyaneshwar P, Barraquio W L, Mathan N, Ladha J K. Endophytic diazotrophs associated with rice. In: Ladha J K, Reddy P M, editors. The quest for nitrogen fixation in rice. Manila, Philippines: IRRI; 2000. pp. 119–140. [Google Scholar]
- 23.Jarabo-Lorenzo A, Velázquez E, Pérez-Galdona R, Vega-Hernández M C, Martínez-Molina E, Mateos P F, Vinuesa P, Martínez-Romero E, León-Barrios M. Restriction fragment length polymorphism analysis of PCR-amplified 16S rDNA and low molecular weight RNA profiling in the characterisation of rhizobial isolates from shrubby legumes endemic to the Canary Islands. Syst Appl Microbiol. 2000;23:418–425. doi: 10.1016/s0723-2020(00)80073-9. [DOI] [PubMed] [Google Scholar]
- 24.Jensen M A, Webster J A, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jukes T H, Cantor C R, editors. Evolution of protein molecules. New York, N.Y: Academic Press; 1969. [Google Scholar]
- 26.Kaprelyants A, Gottschal J C, Kell D K. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev. 1993;104:271–286. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuendig C, Beck C, Hennecke H, Goettfert M. A single rRNA gene region in Bradyrhizobium japonicum. J Bacteriol. 1995;177:5151–5154. doi: 10.1128/jb.177.17.5151-5154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuykendall L D, Saxena B, Devine T E, Udell S E. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol. 1992;38:501–505. [Google Scholar]
- 29.Ladha J K, So R B. Numerical taxonomy of photosynthetic rhizobia nodulating Aeschynomene species. Int J Syst Bacteriol. 1994;44:62–73. [Google Scholar]
- 30.Laguerre G, Mavingui P, Allard M R, Charnay M P, Louvrier P, Mazurier S I, Rigottiergois L, Amarger N. Typing of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions—application to Rhizobium leguminosarum and its different biovars. Appl Environ Microbiol. 1996;62:2029–2036. doi: 10.1128/aem.62.6.2029-2036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maidak B L, Cole J R, Parker C T, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nick G, de Lajudie P, Eardly B D, Suomalainen S, Paulin L, Zhang X, Gillis M, Lindström K. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. Int J Syst Bacteriol. 1999;49:1359–1368. doi: 10.1099/00207713-49-4-1359. [DOI] [PubMed] [Google Scholar]
- 33.Okon Y, Labandera-Gonzalez C A. Agronomic applications of Azospirillum—an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem. 1994;26:1591–1601. [Google Scholar]
- 34.Olsen P E, Rice W A. Rhizobium strain identification and quantification in commercial inoculants by immunoblot analysis. Appl Environ Microbiol. 1989;55:520–522. doi: 10.1128/aem.55.2.520-522.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace N R. A molecular view of the microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 36.Reinhold B, Hurek T, Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985;162:190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhold-Hurek B, Hurek T. Reassessment of the taxonomic structure of the diazotrophic genus Azoarcus sensu lato and description of three new genera and new species, Azovibrio restrictus gen. nov., sp. nov., Azospira oryzae gen. nov., sp. nov. and Azonexus fungiphilus gen. nov., sp. nov. Int J Syst Evol Microbiol. 2000;50:649–659. doi: 10.1099/00207713-50-2-649. [DOI] [PubMed] [Google Scholar]
- 38.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 39.Schloter M, Wiehe W, Assmus B, Steindl H, Becke H, Hoeflich G, Hartmann A. Root colonization of different plants by plant-growth-promoting Rhizobium leguminosarum bv. trifolii R39 studied with monospecific polyclonal antisera. Appl Environ Microbiol. 1997;63:2038–2046. doi: 10.1128/aem.63.5.2038-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segovia L, Pinero D, Palacios R, Martinez Romero E. Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum. Appl Environ Microbiol. 1991;57:426–433. doi: 10.1128/aem.57.2.426-433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.So R B, Ladha J K, Young J P W. Photosynthetic symbionts of the Aeschynomene species form a cluster with Bradyrhizobium on the basis of fatty acid and rRNA analyses. Int J Syst Bacteriol. 1994;44:392–403. doi: 10.1099/00207713-44-3-392. [DOI] [PubMed] [Google Scholar]
- 42.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 43.Sullivan J T, Eardly B D, Van Berkum P, Ronson C W. Four unnamed species of nonsymbiotic rhizobia isolated from the rhizosphere of Lotus corniculatus. Appl Environ Microbiol. 1996;62:2818–2825. doi: 10.1128/aem.62.8.2818-2825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan J T, Ronson C W. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc Natl Acad Sci USA. 1998;95:5145–5149. doi: 10.1073/pnas.95.9.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sy A, Giraud E, Jourand P, Garcia N, Willems A, de Lajudie P, Prin Y, Neyra M, Gillis M, Boivin-Masson C, Dreyfus B. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J Bacteriol. 2001;183:214–220. doi: 10.1128/JB.183.1.214-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueda T, Suga Y, Yahiro N, Matsuguchi T. Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J Bacteriol. 1995;177:1414–1417. doi: 10.1128/jb.177.5.1414-1417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Berkum P, Eardly B D. Molecular evolutionary systematics of the Rhizobiaceae. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1–24. [Google Scholar]
- 49.van Berkum P, Fuhrmann J J. Evolutionary relationships among the soybean bradyrhizobia reconstructed from 16S rRNA gene and internally transcribed spacer region sequence divergence. Int J Syst Evol Microbiol. 2000;50:2165–2172. doi: 10.1099/00207713-50-6-2165. [DOI] [PubMed] [Google Scholar]
- 50.Van de Peer Y, de Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 51.Vinuesa P, Rademaker J L W, de Bruijn F J, Werner D. Characterization of Bradyrhizobium spp. strains by RFLP analysis of amplified 16S rDNA and rDNA intergenic spacer regions. In: Martinez E, Hernandez M, editors. Highlights of nitrogen fixation research. New York, N.Y: Kluwer Academic/Plenum Publishers; 1999. pp. 275–279. [Google Scholar]
- 52.Vinuesa P, Rademaker J L W, Debruijn F J, Werner D. Genotypic characterization of Bradyrhizobium strains nodulating endemic woody legumes of the Canary Islands by PCR-restriction fragment length polymorphism analysis of genes encoding 16S rRNA (16S rDNA) and 16S-23S rDNA intergenic spacers, repetitive extragenic palindromic PCR genomic fingerprinting, and partial 16S rDNA sequencing. Appl Environ Microbiol. 1998;64:2096–2104. doi: 10.1128/aem.64.6.2096-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willems A, Coopman R, Gillis M. Comparison of sequence analysis of 16S-23S rDNA spacer regions, AFLP analysis and DNA-DNA hybridizations in Bradyrhizobium. Int J Syst Evol Microbiol. 2001;51:623–632. doi: 10.1099/00207713-51-2-623. [DOI] [PubMed] [Google Scholar]
- 54.Xu L M, Ge C, Cui Z, Li J, Fan H. Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int J Syst Bacteriol. 1995;45:706–711. doi: 10.1099/00207713-45-4-706. [DOI] [PubMed] [Google Scholar]
- 55.Yanni Y G, Rizk R Y, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Orgambide G, De Bruijn F, Stoltzfus J, Buckley D, Schmidt T M, Mateos P F, Ladha J K, Dazzo F B. Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil. 1997;194:99–114. [Google Scholar]
- 56.Yoon J H, Lee S T, Kim S B, Goodfellow M, Park Y H. Inter- and intraspecific genetic analysis of the genus Saccharomonospora with 16S to 23S ribosomal DNA (rDNA) and 23S to 5S rDNA internally transcribed spacer sequences. Int J Syst Bacteriol. 1997;47:661–669. doi: 10.1099/00207713-47-3-661. [DOI] [PubMed] [Google Scholar]
- 57.Young J M, Kuykendall L D, Martinez-Romero E, Kerr A, Sawada H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J Syst Evol Microbiol. 2001;51:89–103. doi: 10.1099/00207713-51-1-89. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Nick G, Kaijalainen S, Terefework Z, Paulin L, Tighe S W, Graham P H, Lindstrom K. Phylogeny and diversity of Bradyrhizobium strains isolated from the root nodules of peanut (Arachis hypogaea) in Sichuan, China. Syst Appl Microbiol. 1999;22:378–386. doi: 10.1016/S0723-2020(99)80046-0. [DOI] [PubMed] [Google Scholar]