Abstract
Objective
This study aimed to evaluate microRNAs (miRNAs) as predictive biomarkers for type 2 diabetes (T2D) remission 12 months after sleeve gastrectomy (SG).
Methods
A total of 179 serum miRNAs were profiled, and 26 clinical variables were collected from 46 patients. Two patients were later excluded because of hemolysis, and six patients with unclear remission status were set aside to evaluate the prediction models. The remaining 38 patients were included for model building. Variable selection was done using different approaches, including Least Absolute Shrinkage and Selection Operator (LASSO). Prediction models were then developed using LASSO and assessed in the validation set.
Results
A total of 26 out of 38 patients achieved T2D remission 12 months after SG. The prediction model with only clinical variables misclassified two patients, which were correctly classified using miRNAs. Two miRNA‐only models achieved an accuracy of one but performed poorly for the validation set. The best miRNA model was a mixed model (accuracy: 0.974) containing four miRNAs (hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p) and four clinical variables (T2D medication, sex, age, and fasting blood glucose). These miRNAs are involved in pathways related to obesity and insulin resistance.
Conclusions
This study suggests that four serum miRNAs might be predictive biomarkers for T2D remission 12 months after SG, but further validation studies are needed.
Study Importance.
What is already known?
-
►
Sleeve gastrectomy (SG) is an effective weight loss surgery that may result in type 2 diabetes (T2D) remission.
-
►
Prediction models for T2D remission have been built for surgery types other than SG and have not included microRNAs (miRNAs).
What does this study add?
-
►
Four serum miRNAs (hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p) that might predict T2D remission 12 months after SG were identified.
-
►
These miRNAs are involved in pathways related to obesity and insulin resistance.
How might these results change the direction of research or the focus of clinical practice?
-
►
Biomarker research could focus on these miRNAs and validate them in larger cohorts to evaluate their predictive value.
-
►
The miRNAs could also be studied further to understand molecular subtypes of T2D patients with obesity.
INTRODUCTION
Sleeve gastrectomy (SG) is the most common bariatric surgery procedure in Poland and other countries, including the United States (1, 2, 3). It is a less complicated procedure and it has fewer surgery complications compared with other methods (4, 5, 6, 7). Although SG is comparable to other methods for weight loss and weight regain (8, 9, 10), SG has a lower success rate for type 2 diabetes (T2D) remission (4, 11). Therefore, identifying patients who can benefit the most from SG is valuable for effective treatment.
Different prediction models have been developed to predict T2D remission after bariatric surgery (12). However, most models were developed using cohorts of surgery methods other than SG or a limited number of SG patients (12). A 2019 study found that these models overestimated diabetes remission in SG patients with varying degrees (12). Better prediction models are needed for SG patients.
There is increasing interest in using biomarkers as predictive variables. A 2016 study used structural genetic variants as predictive biomarkers for T2D remission (13). We are interested in studying microRNA (miRNA), an epigenetic factor that regulates protein expression through destabilization of target mRNA (14). Epigenetic factors were reported to have a relationship with bariatric surgery outcomes (15, 16). However, the predictive value of miRNAs for surgery outcomes has not been explored before, to our knowledge.
We selected patients with diabetes and obesity from a larger cohort of the Białystok Bariatric Surgery Study (BBSS), an ongoing longitudinal study of Eastern Polish SG patients. Presurgery serum miRNA levels were collected for 46 diabetic patients with T2D remission status 12 months post surgery. We aim to explore whether miRNA information gives any added value to clinical data for predicting T2D remission after SG.
Additionally, we implemented machine learning approaches for variable selection and developed the prediction models. We profiled 179 serum miRNAs and collected 26 clinical variables, including those used in other T2D prediction models. To reduce data dimensionality, we chose Least Absolute Shrinkage and Selection Operator (LASSO) for variable selection and to develop classifiers.
Therefore, this pilot study aims to evaluate the added value of including miRNAs as predictive biomarkers for T2D remission after SG through machine learning approaches (Figure 1).
FIGURE 1.
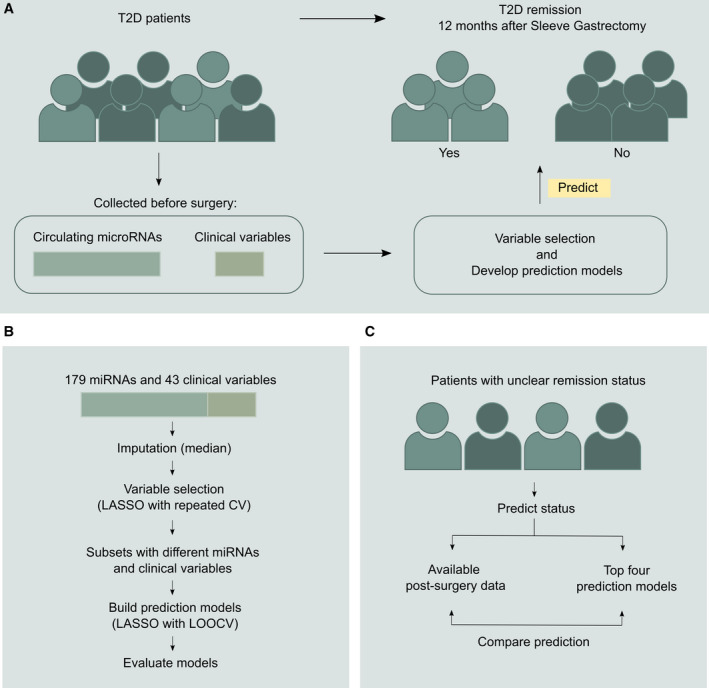
Overview of study design. (A) General framework of patient stratification based on miRNAs and clinical variables. (B) The study’s approach for variable selection and building prediction models with miRNAs and clinical variables. (C) The approach for evaluating the prediction models using patients with unclear remission status. CV, cross validation; LASSO, Least Absolute Shrinkage and Selection Operator; LOOCV, leave‐one‐out cross validation; miRNA, microRNA; T2D, type 2 diabetes [Color figure can be viewed at wileyonlinelibrary.com]
METHODS
Study participants
Patients were recruited from the BBSS (17), in which 321 Polish patients with obesity had undergone bariatric surgery, including SG, from 2016 to 2019. The inclusion criteria for surgery were BMI ≥ 40 kg/m2 or BMI ≥ 35 with comorbidities. The exclusion criteria included prior bariatric surgery, substance abuse, uncontrolled psychiatric illness, expected lack of compliance, or advanced cancer (17). A subset of the SG cohort had T2D based on the American Diabetes Association criteria and had T2D remission status 12 months post surgery (n = 46). Remission status was determined using the American Society of Metabolic and Bariatric Surgery (ASMBS) criteria, based on T2D medication status, hemoglobin A1c (HbA1c), and fasting glucose 12 months after surgery (18). The ASMBS criteria creates five remission groups (18). However, we regrouped patients into a binary remission status because of the sample size: patients with “complete” and “partial” remission were grouped into “remission.” “Improvement,” “unchanged,” and “recurrence” were grouped into “nonremission.” Six patients had unclear remission status due to missing information post surgery. We held out these six patients for model evaluation. All participants provided informed consent before the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Medical University of Białystok (project identification code: R‐I‐002/546/2015) (17).
Sample preparation and miRNA extraction
Serum samples were obtained between 2 and 4 weeks before surgery from patients in the overnight fasting state. Blood samples were collected in Sarstedt S‐Monovette tubes (Sarstedt, Inc., Nümbrecht, North Rhine‐Westphalia, Germany) with separator gel. The samples were allowed to clot for at least 30 minutes and were then centrifuged for 10 minutes at 2,500 rpm. Serum samples were immediately stored at −80°C until use.
RNA was isolated using the miRNeasy Serum/Plasma Advanced Kit (QIAGEN, Hilden, Germany). Three RNA spike‐ins (UniSP2, UniSP4, and UniSP5) were added to the kit’s “RPL buffer” as RNA isolation controls. Serum volumes of 200 uL were used for isolation, and 20 uL of nuclease‐free water was used for elution. A no‐template sample (nuclease‐free water) was also included to evaluate RNA isolation quality.
Quality control of miRNA extraction
The miRCURY locked nucleic acid (LNA) miRNA QC PCR Panel (QIAGEN) was used to assess miRNA quality, monitor complementary DNA (cDNA) synthesis, evaluate hemolysis, and assess polymerase chain reaction (PCR) efficiency. For this quality control (QC) panel, 2 uL of miRNA elute was used for 10 uL of reverse transcription (RT) reaction using the miRCURY LNA RT Kit (QIAGEN). Two spike‐ins were used for cDNA synthesis (UniSp6 and cel‐miR‐39). A total of 1.5 uL of cDNA was used for the QC panel. Two samples were later excluded because of hemolysis (final n = 44), as indicated by a difference in cycle threshold (Ct) values between miR‐23a‐3p and miR‐451a of more than five (19). PCR was done using the Roche LightCycler 480 Instrument (Roche, Basel, Switzerland) with SYBR Green dye.
miRNA profiling
Using the Serum/Plasma miRCURY LNA miRNA Focus PCR Panel (QIAGEN), profiling was done with a 96‐well plate format (20) (Supporting Information Table S1). For this panel, 4 uL of miRNA elute was used for 20‐uL cDNA synthesis, along with the two spike‐ins for cDNA synthesis. The whole cDNA reaction was used for profiling. No‐template controls were also used to evaluate background miRNA levels. PCR was done using the Roche LightCycler 480 Instrument with SYBR Green dye.
Data preprocessing
Raw miRNA data were preprocessed using the GeneGlobe Data Analysis Center (QIAGEN; geneglobe.qiagen.com) to remove miRNAs below a Ct cutoff (Ct = 35) and to apply interplate calibration. The processed data were then normalized using a global mean normalization. There were no missing values for miRNAs.
A total of 43 baseline clinical variables were collected from patients, including blood biochemical parameters, blood morphology measures, and anthropometric measurements. We selected 26 clinical variables with missingness less than 10%. Median imputation was used for missing values. The total number of clinical and miRNA variables was 205.
Variable selection
Ten unique variable sets were created: six sets with only miRNA variables, two with only clinical variables, and two sets with miRNA and clinical variables. Variables were normalized to obtain z scores.
Out of the 44 patients with miRNA data, six patients with unclear remission status were set aside for model evaluation. Therefore, 38 patients with clear remission status were used for variable selection and building classifiers.
Selecting serum miRNA variables
Six miRNA‐only variable sets were created using different methods. One set contains all 179 miRNAs, another includes miRNAs from statistical testing, and four other sets contain LASSO‐selected miRNAs.
miRNA selection using statistical significance and fold change
Fold change is the ratio of relative normalized miRNA expression between remission groups. Unpaired t tests were used to calculate p values. Four miRNAs with p < 0.05 and fold regulations of at least 1.5 were selected in this variable set.
Variable selection with LASSO
LASSO (21) with repeated 10‐fold cross validation (500 repeats) was built using all 179 miRNAs. A total of 20 miRNAs had nonzero coefficients and they were ranked based on their importance. The top five, ten, fifteen, and all nonzero miRNAs were selected as four sets of LASSO‐selected miRNAs.
Selecting pre‐surgery clinical variables
Two sets of clinical variables were created: one set contains all 26 variables, and another has LASSO‐selected variables. The LASSO selection process is the same as that for miRNAs. Repeated cross validation with 10 folds and 500 repeats was done using all 26 clinical variables, and then the resulting nonzero variables were selected.
Selecting serum miRNA and clinical variables
Two sets of miRNA and clinical variables were created: one set contains all available variables (205 variables), and another has LASSO‐selected variables. The LASSO selection process was done using all variables with the same repeated cross‐validation approach. The nonzero variables were selected.
Prediction models
Ten LASSO models were built with each variable set. A leave‐one‐out cross‐validation approach was used. Model performances were obtained using caret and epiR in R (R Foundation, Vienna, Austria), and models were compared based on their accuracy.
Model evaluation using six patients with unclear remission status
Remission labels were determined using available postsurgery clinical measures. The label decision was first made based on the discontinuation of T2D medicines. Then HbA1c and fasting glucose information was considered. For prediction, we first applied the same median imputation and z score scalar used for the model‐building data. Then prediction was made using four models: one clinical‐only model, one clinical and miRNA model, and two miRNA models (Supporting Information Table S2). We then compared the prediction with their remission labels.
Correlation analysis
Pearson correlation was done to evaluate the relationship between clinical variables and miRNAs hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p. Multiple testing correction was done using false discovery rate (FDR). Two plots for unadjusted and adjusted p values were made using ggcorrplot package in R (R Foundation).
Pathway analysis
Pathway analysis was done for miRNAs hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p. The DIANA miRPath version 3 software (http://www.microrna.gr/miRPathv3) was used to identify experimentally reported target genes and evaluate the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
RESULTS
Patient demographics and miRNA profiles
Six clinical variables were significantly associated with remission after SG: T2D medication; age; HbA1c; and fasting plasma glucose, as well as plasma glucose 30 and 60 minutes after oral glucose tolerance test (OGTT; Table 1). The remission group had a much lower proportion of patients taking diabetes medication before surgery (remission vs. nonremission: 12% vs. 83.3%, adjusted p = 0.003). The remission group was also significantly younger and had lower plasma glucose and lower HbA1c. Additionally, the remission group had higher plasma insulin and took fewer medications for chronic diseases, but the relationships were not significant after FDR (Table 1).
TABLE 1.
Baseline clinical data from patients measured before surgery
| Variable | Remission | Nonremission | p value | p value (adj) |
|---|---|---|---|---|
| No. of patients | 26 | 12 | ||
| Age at time of SG (y) | 45.5 (38.25;54) | 58 (56.25;65.25) | 0 | 0.004 |
| BMI before SG (kg/m2) | 46.87 (43.33;50.77) | 45.87 (43.65;52.75) | 0.975 | 0.975 |
| Percentage of body fat before SG (%) | 47.1 (44.77;50.58) | 49.4 (44.83;51.4) | 0.46 | 0.594 |
| Fasting blood insulin before SG (IU/mL) | 34.55 (29.53;53.57) | 34.72 (27.55;43.52) | 0.396 | 0.567 |
| Plasma insulin measured at 30 min during OGTT (n = 35; IU/ml) | 128.08 (109.6;173.73) | 74.08 (61.61;126.53) | 0.031 | 0.091 |
| Plasma insulin measured at 60 min during OGTT (n = 35; IU/mL) | 159.18 (146.12;231.63) | 123.27 (73.27;168.24) | 0.213 | 0.395 |
| Plasma insulin measured at 120 min during OGTT (n = 35; IU/mL) | 121.86 (82.54;243.84) | 90.56 (52.09;105.67) | 0.045 | 0.118 |
| Number of chronic diseases before SG (1 or more) | 19 (73%) | 12 (100%) | 0.084 | 0.546 |
| Number of chronic disease medications before SG (2 or more) | 12 (46%) | 12 (100%) | 0.017 | 0.216 |
| HbA1c before SG (%) | 6.4 (5.9;6.88) | 7.1 (6.65;8.25) | 0.005 | 0.021 |
| Fasting blood glucose before SG (mg/dL) | 132.5 (123.25;143.5) | 154.5 (146.75;178.75) | 0 | 0.004 |
| Plasma glucose measured at 30 min during OGTT (n = 35; mg/dL) | 232.5 (194.5;239) | 248 (235;271) | 0.011 | 0.042 |
| Plasma glucose measured at 60 min during OGTT (n = 35; mg/dL) | 248 (224.75;282.25) | 298 (283;315) | 0.002 | 0.012 |
| Plasma glucose measured at 120 min during OGTT (n = 35; mg/dL) | 194.5 (159.75;218.25) | 225 (183;243) | 0.186 | 0.395 |
| Bilirubin before SG (mg/dL) | 0.47 (0.36;0.59) | 0.38 (0.3;0.57) | 0.307 | 0.499 |
| C‐reactive protein before SG (mg/L) | 5.89 (2.62;10.53) | 3.92 (1.69;10.24) | 0.48 | 0.594 |
| Cholesterol before SG (mg/dL) | 190 (165.5;214) | 184 (152.5;203.25) | 0.387 | 0.567 |
| Triglyceride before SG (mg/dL) | 146 (131.25;231) | 163 (126;225) | 0.888 | 0.923 |
| High‐density lipoprotein before SG (mg/dL) | 39.5 (35;45) | 44.5 (37.75;53.5) | 0.209 | 0.395 |
| Low‐density lipoprotein before SG (mg/dL) | 118.5 (97.12;146) | 103.95 (82.83;133.75) | 0.272 | 0.471 |
| White blood cell count before SG (103/uL) | 7.95 (6.65;9.07) | 8.2 (7.5;8.62) | 0.753 | 0.879 |
| Red blood cell count before SG (106/uL) | 4.98 (4.7;5.26) | 5.07 (4.82;5.29) | 0.777 | 0.879 |
| Platelet blood count before SG (103/uL) | 224 (203.75;263) | 209.5 (190;283.75) | 0.414 | 0.567 |
| Hemoglobin cell count before SG (g/dL) | 14.35 (13.25;15.05) | 14.45 (13.3;15.05) | 0.826 | 0.895 |
| Male sex | 16 (61.5%) | 10 (83.3%) | 0.333 | 0.546 |
| Diabetes medication before SG (n = 37) | 3 (12%) | 10 (83.3%) | 0 | 0.003 |
Values show the median (first;third quartiles) or the number of patients and percentages. p values are shown for the χ2 test (categorical variables) and Kruskal‐Wallis test (continuous variables). Rows with p < 0.05 are shown in bold. Multiple testing correction was done using the false discovery method. If not otherwise stated, n = 38.
Abbreviations: adj, adjusted; OGTT, oral glucose tolerance test; sleeve gastrectomy.
A total of 179 circulating miRNAs were profiled from serum samples collected before surgery. None of the miRNAs was significant between remission and nonremission groups after multiple testing correction using FDR (Supporting Information Table S1). However, eight miRNAs had unadjusted p ≤ 0.05, and four of them had a fold regulation of at least 1.5 (remission vs. nonremission group: upregulation = hsa‐miR‐382‐5p, hsa‐miR‐409‐3p; downregulation = hsa‐miR‐375, hsa‐miR‐1‐3p, respectively).
Variable selection and modeling results
Ten variable sets were created based on different variable selection processes (Table 2). One set for miRNAs contained the four significantly differentially expressed miRNAs (GeneGlobe miRNAs: hsa‐miR‐382‐5p, hsa‐miR‐409‐3p, hsa‐miR‐375, and hsa‐miR‐1‐3p). LASSO selected 20 out of 179 miRNAs after repeated cross validation, including three out of 4 significant miRNAs (hsa‐miR‐382‐5p, hsa‐miR‐375, and hsa‐miR‐1‐3p). For clinical variables, LASSO selected four out of twenty‐six variables: T2D medication, age, fasting plasma glucose, and sex. When all variables were provided, LASSO chose the same four clinical variables (T2D medication, sex, age, and fasting plasma glucose) and four miRNAs (hsa‐miR‐1‐3p, hsa‐miR‐21‐5p, hsa‐miR‐32‐5p, and hsa‐miR‐382‐5p; Table 2, set 4).
TABLE 2.
Prediction models using 10 different variable sets
| Variable set | Variables | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|
| 1 | Top 10 LASSO‐selected miRNAs | hsa‐miR‐382‐5p, hsa‐miR‐193a‐5p, hsa‐miR‐501‐3p, hsa‐miR‐21‐5p, hsa‐miR‐877‐5p, hsa‐miR‐141‐3p, hsa‐miR‐375, hsa‐miR‐32‐5p, hsa‐miR‐2110, hsa‐miR‐1260a | 1 (0.91‐1) | 1 (0.74‐1) | 1 (0.87‐1) |
| 2 | Top 15 LASSO‐selected miRNAs | hsa‐miR‐382‐5p, hsa‐miR‐193a‐5p, hsa‐miR‐501‐3p, hsa‐miR‐21‐5p, hsa‐miR‐877‐5p, hsa‐miR‐141‐3p, hsa‐miR‐375, hsa‐miR‐32‐5p, hsa‐miR‐2110, hsa‐miR‐1260a, hsa‐miR‐140‐5p, hsa‐miR‐543, hsa‐miR‐26a‐5p, hsa‐miR‐27b‐3p, hsa‐miR‐423‐3p | 1 (0.91‐1) | 1 (0.74‐1) | 1 (0.87‐1) |
| 3 | Top 20 LASSO‐selected miRNAs | hsa‐miR‐382‐5p, hsa‐miR‐193a‐5p, hsa‐miR‐501‐3p, hsa‐miR‐21‐5p, hsa‐miR‐877‐5p, hsa‐miR‐141‐3p, hsa‐miR‐375, hsa‐miR‐32‐5p, hsa‐miR‐2110, hsa‐miR‐1260a, hsa‐miR‐140‐5p, hsa‐miR‐543, hsa‐miR‐26a‐5p, hsa‐miR‐27b‐3p, hsa‐miR‐423‐3p, hsa‐miR‐151a‐5p, hsa‐miR‐29b‐3p, hsa‐miR‐1‐3p, hsa‐miR‐30e‐5p, hsa‐miR‐125a‐5p | 0.974 (0.86‐1) | 0.917 (0.62‐1) | 1 (0.87‐1) |
| 4 | 8 LASSO‐selected miRNAs and clinical variables | T2D medication, age, hsa‐miR‐382‐5p, hsa‐miR‐32‐5p, fasting blood glucose, sex, hsa‐miR‐1‐3p, hsa‐miR‐21‐5p | 0.974 (0.86‐1) | 0.917 (0.62‐1) | 1 (0.87‐1) |
| 5 | All clinical variables | All clinical variables (26) | 0.947 (0.82‐0.99) | 0.833 (0.52‐0.98) | 1 (0.87‐1) |
| 6 | 4 LASSO‐selected clinical variables | T2D medication, age, fasting blood glucose, sex | 0.947 (0.82‐0.99) | 0.833 (0.52‐0.98) | 1 (0.87‐1) |
| 7 | All available variables | All miRNAs and clinical variables (205) | 0.947 (0.82‐0.99) | 0.833 (0.52‐0.98) | 1 (0.87‐1) |
| 8 | Top 5 LASSO‐selected miRNAs | hsa‐miR‐382‐5p, hsa‐miR‐193a‐5p, hsa‐miR‐501‐3p, hsa‐miR‐21‐5p, hsa‐miR‐877‐5p | 0.921 (0.79‐0.98) | 0.917 (0.62‐1) | 0.923 (0.75‐0.99) |
| 9 | All miRNAs | All miRNAs (179) | 0.842 (0.69‐0.94) | 0.583 (0.28‐0.85) | 0.962 (0.8‐1) |
| 10 | GeneGlobe miRNAs | hsa‐miR‐409‐3p, hsa‐miR‐382‐5p, hsa‐miR‐375, hsa‐miR‐1‐3p | 0.789 (0.63‐0.9) | 0.5 (0.21‐0.79) | 0.923 (0.75‐0.99) |
Note: LASSO models ranked based on accuracy. Abbreviations: LASSO, Least Absolute Shrinkage and Selection Operator; miRNA, microRNA; T2D, type 2 diabetes.
Among the 10 prediction models, classifiers with miRNA variables performed best. Models with 10 or 15 miRNAs achieved an accuracy of 1 (95% CI: 0.91‐1; Table 2). Models with only clinical variables misclassified two nonremission patients, with an accuracy of 0.947 (95% CI: 0.82‐0.99; Tables 2 and 3). When four miRNAs were added into the clinical model, patient 1 was correctly predicted, but not patient 2 (Figure 2A; Table 3). Patient 2 was later correctly classified in the miRNA‐only models, and no other misclassifications were found (Figure 2B; Table 3).
FIGURE 2.
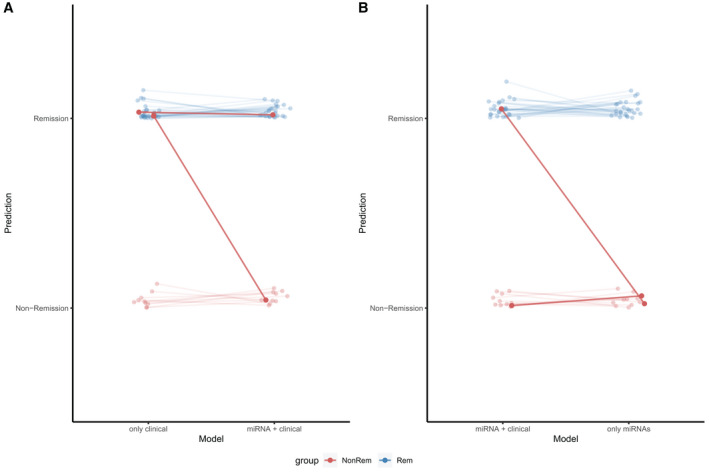
Adding miRNA information increases model accuracy. (A) Two nonremission patients (highlighted as dark red) were misclassified in a model with four clinical variables (accuracy = 0.947). One patient was correctly classified when four miRNAs were added (accuracy = 0.974). (B) The second patient was correctly classified in an miRNA‐only model (using 10 miRNAs, accuracy = 1). Other patients remained correctly classified. miRNA, microRNA; NonRem, nonremission; Rem, remission
TABLE 3.
Pre‐ and postsurgery characteristics of the two misclassified patients and predictions shown from LASSO models: with only clinical variables, with clinical and miRNA variables, and with 10 miRNAs
| Patient | Sex | Pre surgery | 12 months post surgery | Remission prediction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | T2D medication | Fasting plasma glucose | HbA1c | T2D medication | Fasting plasma glucose | HbA1c | Remission | Only clinical variables | Clinical and miRNAs | Only miRNAs | ||
| 1 | M | 63 | No | 193 | 8.1 | Yes | 117 | 6.3 | No | Yes | No | No |
| 2 | M | 66 | No | 135 | 6 | No | 128 | 5.9 | No | Yes | Yes | No |
Abbreviations: HbA1c, hemoglobin A1c; LASSO, Least Absolute Shrinkage and Selection Operator; M, male; miRNA, microRNA; T2D, type 2 diabetes.
Evaluating prediction models using six patients with unclear remission status
Four classifiers were selected for evaluation: a clinical‐only model, a mixed model with miRNA and clinical variables, and two miRNA‐only models (Table 4; Supporting Information Table S2). Models with clinical variables agreed the most with postsurgery data (Table 4). All models predicted patient A as nonremission, but postsurgery data suggested remission. All miRNA models predicted nonremission for patient C. Postsurgery values were within the remission group, but this patient had missing medication information. The miRNA‐only models had increasing disagreement with postsurgery data, indicating overfitting with the training data.
TABLE 4.
Pre‐ and postsurgery characteristics of six unclear patients and predictions shown from post‐surgery data and LASSO models: with only clinical variables, with clinical and miRNA variables, with 10 miRNAs, and with 15 miRNAs
| Patient | Sex | Pre surgery | 12 months post surgery | Remission prediction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | T2D medication | Fasting plasma glucose | HbA1c | T2D medication | Fasting plasma glucose | HbA1c | Based on postsurgery data | Only clinical variables | Clinical and miRNAs | Only 10 miRNAs | Only 15 miRNAs | ||
| A | F | 63 | Yes | 137 | NA | No | NA | 6.1 | Yes | No | No | No | No |
| B | F | 41 | Yes | 118 | 6.4 | No | NA | 5.7 | Yes | Yes | Yes | Yes | No |
| C | M | 43 | Yes | 127 | 6 | NA | 102 | 5.4 | Yes | Yes | No | No | No |
| D | F | 49 | Yes | NA | 7.6 | No | NA | NA | Yes | No | Yes | Yes | Yes |
| E | F | 54 | Yes | 135 | 7.5 | Yes | NA | 5.7 | No | No | No | No | No |
| F | M | 37 | No | 110 | 6.6 | NA | 95 | 4.9 | Yes | Yes | Yes | No | No |
Abbreviations: F, female; HbA1c, hemoglobin A1c; LASSO, Least Absolute Shrinkage and Selection Operator; M, male; miRNA, microRNA; NA, not available; T2D, type 2 diabetes.
Evaluating the four predictive miRNAs (hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, hsa‐miR‐21‐5p)
Four miRNAs that improved prediction for clinical models had significant correlations with glucose measures and HbA1c, but not with other clinical measures (Figure 3; Supporting Information Figure S1). The miRNA hsa‐miR‐382‐5p was significantly positively correlated with HbA1c (r = 0.432) and plasma glucose (r = 0.485 for fasting and r = 0.359 for 30 minutes during OGTT). The relationship with fasting plasma glucose was maintained after FDR (Figure 3). There were other significant correlations between miRNA and clinical variables, but they were not significant after FDR; for example, fasting plasma glucose with hsa‐miR‐32‐5p (r = −0.354) and hsa‐miR‐21‐5p (r = −0.346), as well as hemoglobin cell count with hsa‐miR‐21‐5p (r = −0.456). The miRNA hsa‐miR‐1‐3p was not significantly correlated with any of the selected clinical variables. The miRNA hsa‐miR‐32‐5p was positively correlated with hsa‐miR‐1‐3p (r = 0.393) and hsa‐miR‐21‐5p (r = 0.362) but was no longer significant after FDR.
FIGURE 3.
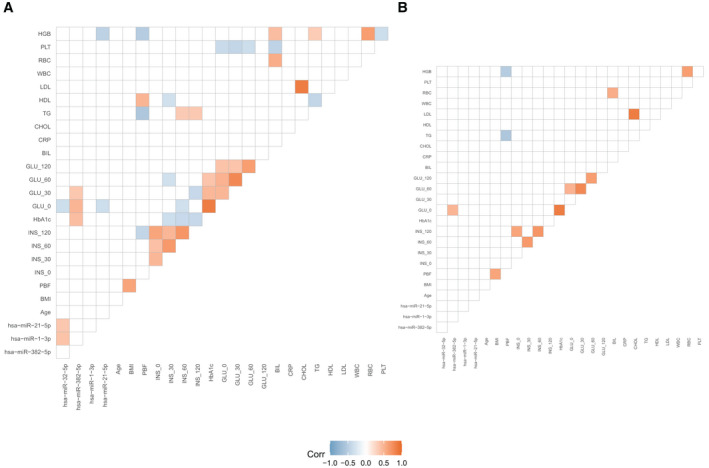
Significant Pearson correlations between selected miRNA and clinical variables. The analysis was done using R packages Hmisc and ggcorrplot. Nonsignificant correlations based on (A) p < 0.05 and (B) adjusted p < 0.05 are set to blank. Red boxes indicate positive correlations, whereas blue boxes represent negative correlations. BIL, bilirubin levels; CHOL, cholesterol levels; CRP, C‐reactive protein levels; GLU_0, fasting blood glucose levels; GLU_30, plasma glucose levels measured at 30 minutes during OGTT; GLU_60, plasma glucose levels measured at 60 minutes during OGTT; GLU_120, Plasma glucose levels measured at 120 minutes during OGTT; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein levels; HGB, hemoglobin cell count; INS_0, fasting blood insulin levels; INS_30, plasma insulin levels measured at 30 minutes during OGTT; INS_60, plasma insulin levels measured at 60 minutes during OGTT; INS_120, plasma insulin levels measured at 120 minutes during OGTT; LDL, low‐density lipoprotein levels; miRNA, microRNA; OGTT, oral glucose tolerance test; PBF, percentage of body fat; PLT, platelet blood count; RBC, red blood cell count; TG, triglyceride levels; WBC, white blood cell count
Pathway analysis was done for these miRNAs using the DIANA miRPath version 3 software. Three out of four miRNAs regulated 39 KEGG pathways, including 19 signaling pathways related to obesity and insulin resistance (Table 5). There was no information for hsa‐miR‐1‐3p in this database. Within these 19 pathways, hsa‐miR‐32‐5p regulated 253 genes, hsa‐miR‐21‐5p regulated 330 genes, and hsa‐miR‐382‐5p regulated 73 genes.
TABLE 5.
Obesity‐ and insulin resistance‐related pathways regulated by the four predictive miRNAs
| No. | KEGG pathway | p value | No. of genes | No. of miRNAs |
|---|---|---|---|---|
| 1 | Thyroid hormone signaling pathway | 9.22E‐05 | 33 | 3 |
| 2 | Lysine degradation | 2.04E‐04 | 15 | 2 |
| 3 | FoxO signaling pathway | 2.34E‐04 | 41 | 3 |
| 4 | Fatty acid elongation | 0.0012 | 7 | 3 |
| 5 | Prolactin signaling pathway | 0.0014 | 21 | 3 |
| 6 | Focal adhesion | 0.0021 | 52 | 3 |
| 7 | Adherens junction | 0.0024 | 20 | 2 |
| 8 | ECM‐receptor interaction | 0.0025 | 19 | 3 |
| 9 | Valine, leucine, and isoleucine biosynthesis | 0.0036 | 2 | 2 |
| 10 | Regulation of actin cytoskeleton | 0.0061 | 50 | 3 |
| 11 | MAPK signaling pathway | 0.0102 | 54 | 3 |
| 12 | p53 signaling pathway | 0.0102 | 21 | 3 |
| 13 | mTOR signaling pathway | 0.0133 | 18 | 3 |
| 14 | Protein processing in endoplasmic reticulum | 0.0140 | 39 | 3 |
| 15 | Hippo signaling pathway | 0.0157 | 32 | 3 |
| 16 | Fatty acid degradation | 0.0241 | 7 | 2 |
| 17 | Endocytosis | 0.0263 | 41 | 3 |
| 18 | PI3K‐Akt signaling pathway | 0.0370 | 68 | 3 |
| 19 | HIF‐1 signaling pathway | 0.0478 | 26 | 3 |
Abbreviations: ECM, extracellular matrix; FoxO, forkhead box protein O; HIF‐1, hypoxia‐inducible factor 1; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen‐activated protein kinase; miRNA, microRNA; mTOR, mechanistic target of rapamycin (serine/threonine kinase); PI3K‐Akt, phosphatidylinositol 3‐kinase‐protein kinase b.
DISCUSSION
This pilot study evaluated miRNAs as predictive biomarkers and used machine learning approaches to select the most potential miRNAs and for model building. We found that miRNAs might improve T2D remission prediction and that they are best used with clinical variables. We considered all miRNAs because statistically significant variables are not always good predictive variables (22).
Our clinical model, based on T2D medication, age, sex, and fasting plasma glucose, misclassified two nonremission patients. Both patients had similar presurgery conditions: they did not take any T2D medications before surgery, and they were in their 60s. Patient 1 needed T2D medicines after surgery; therefore, this patient had a nonremission status. In contrast, patient 2 seemed to be borderline partial remission after surgery. The second patient’s fasting blood glucose was only three points above the upper limit for partial remission (≤125 mg/dL). Therefore, the clinical models well predicted that patient 2 could achieve remission after surgery.
Adding miRNA information improved prediction for patient 1. When the miRNAs hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p were added into the clinical model, patient 1 was correctly predicted to have nonremission. Patient 2 was still predicted as remission. When 10 or 15 miRNAs were used instead of clinical variables, both patients were classified as nonremission. Considering that patient 2 seemed to be borderline remission, the model with both clinical variables and miRNAs appears to be most accurate.
Data from the six patients with unclear remission status also agree that clinical variables are essential in the prediction model. Models with clinical predictors matched the most with postsurgery information. Using only miRNAs increased the disagreement between prediction and postsurgery data. Although more samples are needed to confirm, this suggests that our miRNA‐only models are likely to be an overfit, and clinical variables should be kept in prediction models.
When available, miRNA information can help improve prediction for difficult patients and provide additional information to potentially imprecise clinical measures. Two out of four variables used in our clinical model can be inaccurate: fasting plasma glucose and T2D medication information. We requested for our patients to fast before the OGTT but we could not guarantee that they genuinely fasted. T2D medication was obtained through the patient questionnaire, which is subject to recall bias.
Our prediction models can help decision‐making for newly diagnosed T2D patients who qualify for SG. Some of our patients were unaware of their T2D status and were diagnosed during their presurgery visit, which might explain the relatively low percentage of patients taking T2D medication. We found that most patients who did not report taking T2D medication achieved remission after SG, but not everyone. SG is a simpler surgery procedure but it has a lower T2D remission rate than Roux‐en‐Y gastric bypass (RYGB) (4, 11). Therefore, deciding on bariatric surgery for new T2D patients is not straightforward. Our prediction models might help predict whether SG would result in rapid T2D remission or not for these patients.
Previous prediction models, which used similar clinical variables, predicted remission in SG patients with sensitivity and specificity up to 0.92 and 0.83, respectively (12). Our clinical model with four variables achieved sensitivity and specificity of 0.83 and 1, respectively, and adding four miRNAs increased the sensitivity to 0.917. Confirmation in external cohorts is vital to confirm the usefulness of our models.
To our knowledge, these four serum miRNAs (hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p) have not been studied as predictive biomarkers for T2D remission after surgery. However, studies have reported associations between these miRNAs with obesity and T2D. The miRNA hsa‐miR‐382‐5p is involved in cholesterol homeostasis (23). Plasma and serum levels of hsa‐miR‐21‐5p are associated with T2D (24, 25, 26), as well as with obesity (27, 28). The miRNA hsa‐miR‐32‐5p is also associated with T2D (29) and obesity (29, 30). Our pathway analysis identified 19 obesity‐ and T2D‐related pathways regulated by these miRNAs, including mechanistic target of rapamycin (serine/threonine kinase) (mTOR), mitogen‐activated protein kinase (MAPK), phosphatidylinositol 3‐kinase‐protein kinase b (PI3K‐Akt), fatty acid elongation, and degradation pathways. The miRNA hsa‐miR‐1‐3p has regulatory roles in cardiac muscle tissues and tumor suppressors in various cancers (31). It is also dysregulated in pancreatic cancer patients (32).
These miRNAs have been studied in bariatric surgery patients to measure differential expression before and after surgery (16). An RYGB study reported that plasma hsa‐miR‐32‐5p and hsa‐miR‐21‐5p were significantly reduced 9 and 12 months after surgery (33). However, another RYGB study reported an increase of plasma hsa‐miR‐21‐5p 12 months after surgery (28). The miRNAs hsa‐miR‐1‐3p and hsa‐miR‐382‐5p were reported to be not significantly differentially expressed after RYGB (33). It appears that predictive miRNAs do not need to be differentially expressed after surgery. However, these studies were primarily done in RYGB patients, and more studies with SG patients are needed.
Our pilot study suggests that miRNAs could potentially predict T2D remission after the intervention. Our findings agree with a recent study that identified predictive miRNAs for T2D remission after diet intervention (34). The set of miRNAs are different from this study, which might reflect the study population. Our study focused on patients with T2D and obesity, whereas the other study’s patients had BMI around 30 as well as coronary heart disease. Nevertheless, our study has limitations, including the small number of participants and limited external validation. Owing to sample size limitations, we simplified T2D and remission groups as dichotomous traits. Future studies could also investigate T2D subtypes based on β‐cell function and insulin resistance measures (35) and include other diabetes‐related variables such as C‐peptide and T2D duration. Some of the patients were unaware of their T2D status, so we could not obtain an accurate T2D duration for these patients. Patients with differing risk profiles might have different remission rates after surgery. Another limitation is that we focused on SG without comparing other surgery types such as RYGB. RYGB has better long‐term T2D remission rates (4, 11), but only 8% of our BBSS patients underwent RYGB. Owing to study size limitations, we could not adequately compare miRNA’s predictive value between these two surgery types. It would also be interesting to see whether miRNAs can differentiate between the original ASMBS remission groups (“complete remission,” “partial remission,” “improvement,” “unchanged,” and “recurrence”). Additionally, we considered only 179 miRNAs that were included in the quantitative PCR profiling platform for serum samples. Using larger profiling platforms such as small RNA sequencing might uncover more or better predictive miRNAs.
In conclusion, we identified four miRNAs (hsa‐miR‐32‐5p, hsa‐miR‐382‐5p, hsa‐miR‐1‐3p, and hsa‐miR‐21‐5p) that might complement clinical models in predicting T2D remission after SG. Further studies in much larger data are needed to confirm the utility of these serum miRNAs as predictive biomarkers. The four serum miRNAs could also be studied further to understand molecular subtypes of T2D that separate remission and nonremission patients.O
CONFLICT OF INTEREST
The authors declared no conflict of interest.
Supporting information
Fig S1
Table S1‐S2
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Dr. Magdalena Niemira’s team (Medical University of Białystok, Poland) for helpful assistance during the microRNA profiling experiments. We also thank Dr. Marcin Czajkowski (Białystok University of Technology, Poland) for his valuable suggestions and comments regarding data analysis.
Wojciechowska G, Szczerbinski L, Kretowski M, Niemira M, Hady HR, Kretowski A. Exploring microRNAs as predictive biomarkers for type 2 diabetes mellitus remission after sleeve gastrectomy: A pilot study. Obesity (Silver Spring). 2022;30:435–446. doi: 10.1002/oby.23342
Funding information
This research was conducted within a project that has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 754432 and the Polish Ministry of Science and Higher Education, from financial resources for science in 2018‐2023 granted for the implementation of an international co‐financed project. This research was also supported by the Medical University of Bialystok funds through the subsidy grant no: SUB/1/DN/19/008/1196.
REFERENCES
- 1. American Society for Metabolic and Bariatric Surgery . Estimate of bariatric surgery numbers, 2011‐2019. Updated March 2021. Accessed February 17, 2019. https://asmbs.org/resources/estimate‐of‐bariatric‐surgery‐numbers
- 2. Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25:1822‐1832. [DOI] [PubMed] [Google Scholar]
- 3. Janik MR, Stanowski E, Paśnik K. Present status of bariatric surgery in Poland. Wideochir Inne Tech Maloinwazyjne. 2016;1:22‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Courcoulas AP, King WC, Belle SH, et al. Seven‐year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg. 2018;153:427‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang S‐H, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta‐analysis, 2003‐2012. JAMA Surg. 2014;149:275‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee W‐J, Chong K, Aung L, Chen S‐C, Ser K‐H, Lee Y‐C. Metabolic surgery for diabetes treatment: sleeve gastrectomy or gastric bypass? World J Surg. 2017;41:216‐223. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Lai D, Wu D. Laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity‐related comorbidities: a systematic review and meta‐analysis. Obes Surg. 2016;26:429‐442. [DOI] [PubMed] [Google Scholar]
- 8. Poirier P, Cornier M‐A, Mazzone T, et al. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683‐1701. [DOI] [PubMed] [Google Scholar]
- 9. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861‐877. [DOI] [PubMed] [Google Scholar]
- 10. Moshiri M, Osman S, Robinson TJ, Khandelwal S, Bhargava P, Rohrmann CA. Evolution of bariatric surgery: a historical perspective. AJR Am J Roentgenol. 2013;201:W40‐W48. [DOI] [PubMed] [Google Scholar]
- 11. Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266:650‐657. [DOI] [PubMed] [Google Scholar]
- 12. Shen S‐C, Wang W, Tam K‐W, et al. Validating risk prediction models of diabetes remission after sleeve gastrectomy. Obes Surg. 2019;29:221‐229. [DOI] [PubMed] [Google Scholar]
- 13. Pedersen HK, Gudmundsdottir V, Pedersen MK, Brorsson C, Brunak S, Gupta R. Ranking factors involved in diabetes remission after bariatric surgery using machine‐learning integrating clinical and genomic biomarkers. NPJ Genom Med. 2016;1:16035. doi: 10.1038/npjgenmed.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17:1712. doi: 10.3390/ijms17101712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izquierdo AG, Crujeiras AB. Obesity‐related epigenetic changes after bariatric surgery. Front Endocrinol (Lausanne). 2019;10:232. doi: 10.3389/fendo.2019.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langi G, Szczerbinski L, Kretowski A. Meta‐analysis of differential miRNA expression after bariatric surgery. J Clin Med. 2019;8:1220. doi: 10.3390/jcm8081220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szczerbinski L, Taylor MA, Citko A, et al. Clusters of glycemic response to oral glucose tolerance tests explain multivariate metabolic and anthropometric outcomes of bariatric surgery in obese patients. J Clin Med. 2019;8:1091. doi: 10.3390/jcm8081091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Related Dis. 2015;11:489‐506. [DOI] [PubMed] [Google Scholar]
- 19. Blondal T, Jensby Nielsen S, Baker A, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59:S1‐S6. [DOI] [PubMed] [Google Scholar]
- 20. QIAGEN . miRCURY LNA miRNA Focus PCR Panels. Accessed September 9, 2020. https://geneglobe.qiagen.com/pl/product‐groups/mircury‐lna‐mirna‐focus‐pcr‐panels/
- 21. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1‐22. doi:10.3390/jcm8081091. [PMC free article] [PubMed] [Google Scholar]
- 22. Lo A, Chernoff H, Zheng T, Lo S‐H. Why significant variables aren’t automatically good predictors. Proc Natl Acad Sci U S A. 2015;112:13892‐13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu Y‐W, Zhao J‐Y, Li S‐F, et al. RP5‐833A20.1/miR‐382‐5p/NFIA‐dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol. 2015;35:87‐101. [DOI] [PubMed] [Google Scholar]
- 24. Párrizas M, Brugnara L, Esteban Y, et al. Circulating miR‐192 and miR‐193b are markers of prediabetes and are modulated by an exercise intervention. J Clin Endocrinol Metab. 2015;100:E407‐E415. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Sundquist J, Zöller B, et al. Determination of 14 circulating microRNAs in Swedes and Iraqis with and without diabetes mellitus type 2. PLoS One. 2014;9:e86792. doi: 10.1371/journal.pone.0086792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial MiR‐126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810‐817. [DOI] [PubMed] [Google Scholar]
- 27. Murri M, Insenser M, Fernández‐Durán E, San‐Millán JL, Escobar‐Morreale HF. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA‐21, miRNA‐27b, miRNA‐103, and miRNA‐155 expression. J Clin Endocrinol Metab. 2013;98:E1835‐E1844. [DOI] [PubMed] [Google Scholar]
- 28. Ortega FJ, Mercader JM, Catalán V, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781‐792. [DOI] [PubMed] [Google Scholar]
- 29. Jones A, Danielson KM, Benton MC, et al. miRNA signatures of insulin resistance in obesity. Obesity (Silver Spring). 2017;25:1734‐1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang Z, Wei Z, Wu X, Yang H. Screening of exosomal miRNAs derived from subcutaneous and visceral adipose tissues: determination of targets for the treatment of obesity and associated metabolic disorders. Mol Med Rep. 2018;18:3314‐3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J‐Y, Huang J‐C, Chen G, Wei D‐M. Expression level and potential target pathways of miR‐1‐3p in colorectal carcinoma based on 645 cases from 9 microarray datasets. Mol Med Rep. 2018;17:5013‐5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng Q, Han L‐H, Zhao H‐J, Li H, Li J‐B. Abnormal alterations of miR‐1 and miR‐214 are associated with clinicopathological features and prognosis of patients with PDAC. Oncol Lett. 2017;14:4605‐4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alkandari A, Ashrafian H, Sathyapalan T, et al. Improved physiology and metabolic flux after Roux‐en‐Y gastric bypass is associated with temporal changes in the circulating microRNAome: a longitudinal study in humans. BMC Obes. 2018;5:20. doi: 10.1186/s40608-018-0199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rangel‐Zuñiga OA, Vals‐Delgado C, Alcala‐Diaz JF, et al. A set of miRNAs predicts T2DM remission in patients with coronary heart disease: from the CORDIOPREV study. Mol Ther Nucleic Acids. 2020;23:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahlqvist E, Prasad RB, Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69:2086‐2093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S2
Supplementary Material


