Summary
Patients with immune thrombocytopenia (ITP) may respond to one thrombopoietin receptor agonist (TPO‐RA) but not another. Limited data are available describing outcomes in patients who switched from romiplostim or eltrombopag to avatrombopag, a newer oral TPO‐RA. We performed a retrospective observational study of adults with ITP who switched from eltrombopag or romiplostim to avatrombopag at four US tertiary ITP referral centres. Forty‐four patients were included, with a mean ITP duration of 8.3 years and a median (range) of four prior ITP treatments. On avatrombopag, 41/44 patients (93%) achieved a platelet response (≥50 × 109/l) and 38/44 patients (86%) achieved a complete response (≥100 × 109/l). In all patients, the median platelet count on eltrombopag or romiplostim was 45 × 109/l vs 114 × 109/l on avatrombopag (p < 0.0001); in patients switched for ineffectiveness of romiplostim/eltrombopag, it was 28 × 109/l on romiplostim/eltrombopag vs 88 × 109/l on avatrombopag (p = 0.025). Fifty‐seven percent of patients receiving concomitant ITP medications before switching discontinued them after switching, including 63% of patients receiving chronic corticosteroids. In a heavily pretreated chronic ITP population, avatrombopag was effective following therapy with romiplostim or eltrombopag, with high response rates even in patients with inadequate response to a prior TPO‐RA.
Keywords: avatrombopag, eltrombopag, immune thrombocytopenia, romiplostim, thrombopoietin receptor agonist
INTRODUCTION
Primary immune thrombocytopenia (ITP) is an autoimmune condition characterized by thrombocytopenia and a bleeding tendency that occurs in approximately 3.3 per 100 000 people per year. 1 , 2 , 3 The pathogenesis of ITP involves antibody‐mediated platelet destruction and impaired production of platelets. 4 , 5 , 6
Initial treatment options include corticosteroids and immunoglobulin; thrombopoietin receptor agonists (TPO‐RAs) may be used after first‐line treatment. 7 , 8 Several TPO‐RAs have demonstrated a high rate of response in ITP trials, in excess of 60% for the three approved agents: romiplostim, eltrombopag and avatrombopag. 9 , 10 , 11 In some patients who do not have an adequate response to eltrombopag or romiplostim, switching to the other agent may reduce platelet count fluctuations and/or achieve a platelet response. 4 Available TPO‐RAs have differing molecular structures, mechanistic characteristics, toxicity profiles, binding sites, effects on receptors and differential downstream effects on signalling pathways. 12 , 13 These differences may contribute to the variable TPO‐RA response observed in individual patients. 14
While eltrombopag and romiplostim have been available for over a decade, avatrombopag is a newer oral TPO‐RA approved for ITP in 2019. In contrast to eltrombopag, avatrombopag does not require dietary restrictions around its administration and has no known signal for hepatotoxicity. The efficacy of avatrombopag in ITP was established following a 26‐week phase 3, randomized, double‐blind study of avatrombopag versus placebo in patients with chronic ITP (NCT01438840). 9
While several case series have described largely successful outcomes of patients with ITP who switched between eltrombopag and romiplostim (‘TPO‐RA switch therapy’), very limited data are available describing switching from eltrombopag or romiplostim to avatrombopag, particularly among patients who did not have an adequate response to eltrombopag or romiplostim. The aim of this multicentre, observational study was to evaluate outcomes in patients with ITP who switched from either eltrombopag or romiplostim to avatrombopag.
METHODS
Study design and patients
This was a multicentre, observational study of consecutive adult patients with primary or secondary ITP who switched from eltrombopag or romiplostim to avatrombopag for any reason between July 2019 and December 2020.
Data were collected retrospectively. Patients ≥18 years of age with a diagnosis of primary or secondary ITP were included if they had been on avatrombopag treatment for at least two months with no more than a one‐month gap between stopping eltrombopag or romiplostim and starting avatrombopag. Subjects were excluded if they were initiated on another TPO‐RA while on avatrombopag, had received any experimental therapy for ITP within 30 days of switch, or had developed thrombocytopenia unrelated to ITP.
Medical records were abstracted for demographic characteristics, type of ITP (primary versus secondary), ITP diagnostic and treatment history, use of concomitant chronic therapy for ITP before and after switching to avatrombopag, adverse events before and after switching, and reason for switching.
Outcomes
Treatment responses were defined as achievement of a given platelet count on at least one occasion and without requirement for rescue therapy. Achievement of platelet response was defined as a platelet count ≥50 000/μl and complete platelet response as a platelet count ≥100 000/μl. Patients requiring rescue therapy had their platelet counts disqualified for the purposes of response assessment for eight weeks (corticosteroids), four weeks [intravenous immunoglobulin (IVIG) or anti‐RhD immune globulin], or one week (platelet transfusion) from the time of receipt of rescue therapy. Median platelet count before and after switching was calculated separately as the median of the final three platelet counts on either romiplostim or eltrombopag and the most recent three platelet counts on avatrombopag. This approach was taken to minimize impacts from dose titration on measured platelet counts. Use of concomitant ITP medications (medications prescribed for long‐term use, including chronic corticosteroids) was evaluated both prior to switching and during treatment with avatrombopag. Rescue therapy was defined as acute administration of corticosteroids (either initiation or an increase from a prior stable chronic dose), IVIG, anti‐RhD immune globulin or platelet transfusion.
We additionally evaluated adverse events with TPO‐RA treatment before and after switching.
Subgroup analyses
We analysed the following subgroups: (1) patients who switched to avatrombopag because of insufficient effectiveness of prior TPO‐RA therapy, improved convenience with avatrombopag therapy or adverse events with prior TPO‐RA treatment; (2) patients with primary or secondary ITP; and (3) the TPO‐RA prior to the switch (romiplostim versus eltrombopag). We additionally performed a descriptive analysis of those patients receiving all three of eltrombopag, romiplostim and avatrombopag over the course of their disease as TPO‐RA monotherapies.
Statistical analyses
Quantitative parameters are shown with descriptive statistics. Median platelet counts on romiplostim or eltrombopag were compared with median platelet counts on avatrombopag using the Wilcoxon signed‐rank test. Statistical analysis was performed and graphs for figures were prepared using Microsoft Excel for Microsoft 365 (Microsoft, Redmond, WA, USA) and GraphPad Prism 9.0.2 (GraphPad, Inc., San Diego, CA, USA).
RESULTS
Study population
A total of 44 patients who switched to avatrombopag from a prior TPO‐RA were included. Participating institutions included Massachusetts General Hospital (n = 15), the University of Washington Medical Center (n = 14), the University of Southern California Medical Center (n = 10) and the Hospital of the University of Pennsylvania (n = 5). Baseline characteristics are presented in Table 1. Twenty‐five of 44 patients (57%) had primary ITP and 19 (43%) had secondary ITP. Forty‐eight percent were male, 68% were Caucasian, and the median (range) age was 61 years (21, 87). Patients had received a median (range) of 4 (2, 10) prior ITP therapies before receiving avatrombopag, including romiplostim (75%), eltrombopag (23%) and a combination of romiplostim and eltrombopag simultaneously in a single patient (2%). Time on a prior TPO‐RA before switching to avatrombopag was a median (range) of 9.7 (0.2, 179.2) months. Reasons for switching included greater convenience with avatrombopag (52%), insufficient effectiveness with prior TPO‐RA therapy (32%), and adverse event with prior TPO‐RA therapy (16%). Patient characteristics according to primary or secondary ITP, and aetiologies of secondary ITP, are given in Table S1. Patient characteristics according to reason for switching with additional subdivision by agent being switched from (romiplostim or eltrombopag) is given in Table S2.
TABLE 1.
Baseline characteristics of total population of patients
| Baseline characteristics | Total population | Effectiveness | Convenience | Adverse event |
|---|---|---|---|---|
| Total patients | N = 44 | n = 14 | n = 23 | n = 7 |
| Male, n (%) | 21 (48) | 9 (64) | 9 (39) | 3 (43) |
| White, n (%) | 30 (68) | 10 (71) | 15 (65) | 5 (71) |
| Primary ITP, n (%) | 25 (57) | 7 (50) | 13 (57) | 5 (71) |
| Age | ||||
| Median (range), years | 61 (21, 87) | 66.5 (39, 81) | 59 (21, 87) | 59 (34, 76) |
| Duration of ITP until AVA initiation | ||||
| Median (range), months | 49 (2, 550) | 73 (6, 404) | 43 (2, 550) | 85 (16, 124) |
| # Unique prior ITP therapies before AVA | ||||
| Median (range) | 4 (2, 10) | 7 (3, 10) | 4 (2, 8) | 4 (2, 8) |
| Previous TPO‐RA | ||||
| Romiplostim, ratio (%) | 33 (75) | 10 (71) | 21 (91) | 2 (29) |
| Eltrombopag, ratio (%) | 10 (23) | 4 (29) | 1 (4) | 5 (71) |
| Romiplostim/eltrombopag, ratio (%) | 1 (2) | 0 (0) | 1 (4) | 0 (0) |
Abbreviations: AVA, avatrombopag; ITP, immune thrombocytopenia; TPO‐RA, thrombopoietin receptor agonist.
In general, patients switching from eltrombopag transitioned immediately from eltrombopag on one day to avatrombopag the following day, and patients switching from romiplostim began avatrombopag approximately seven days from their final romiplostim dose. In patients switching from romiplostim, the median dose administered prior to switching was 4 μg/kg per week, and in patients switching from eltrombopag, the median dose administered prior to switching was 75 mg daily.
Switching outcomes in the full cohort
The median (range) duration of treatment with avatrombopag was 9.2 (2.8, 17.2) months. The median (range) weekly dose of avatrombopag was 140 (20, 280) mg. Among the total group of 44 patients, platelet response (platelet count ≥50 000/μl) was achieved in 41/44 patients (93%) and complete platelet response (platelet count ≥100 000/μl) was achieved in 38/44 patients (86%) after switching. The median platelet count on eltrombopag or romiplostim was 45 × 109/l vs 114 × 109/l on avatrombopag (p < 0.0001 by Wilcoxon signed‐rank test) (Figure 1A). Outcomes according to primary and secondary ITP are shown in Table S3.
FIGURE 1.
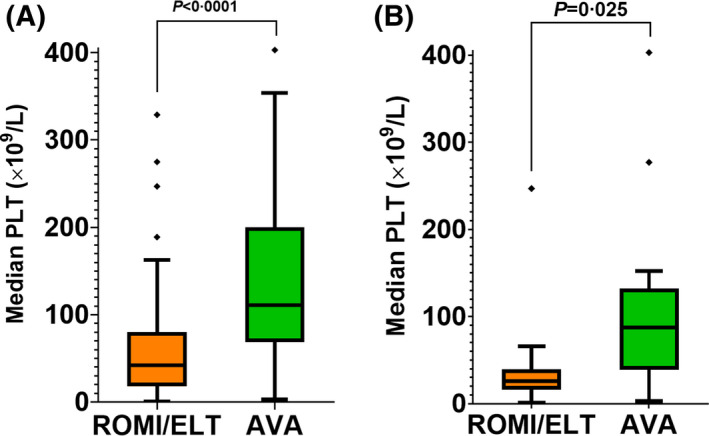
Median platelet counts for each patient prior to switch (during treatment with romiplostim or eltrombopag) versus following the switch to avatrombopag. For each patient, the median platelet count is the median of the most recent three platelet counts measured while receiving that agent. (A) All patients (N = 44). (B) Patients switched due to ineffectiveness of romiplostim or eltrombopag (N = 14). One patient with a median platelet count of 585 × 109/l on avatrombopag omitted from both graphs to preserve graph resolution. Abbreviations: AVA, avatrombopag; ELT, eltrombopag; PLT, platelet count; ROMI, romiplostim
Twenty‐eight of 44 patients (64%) were receiving concomitant ITP medications prior to switching to avatrombopag. Of these 28 patients, 16 (57%) were able to discontinue one or more concomitant medications after initiating avatrombopag (Table 2). A total of 19/44 patients (43%) received concomitant ITP medications after switching. Only 3/44 patients (7%) required addition of a concomitant ITP medication after starting avatrombopag.
TABLE 2.
Results in total population by reason for switch
| Results | Total population | Effectiveness | Convenience | Adverse event |
|---|---|---|---|---|
| Concomitant ITP meds after switching to AVA, ratio (%) | 19/44 (43) | 11/14 (79) | 6/23 (26) | 2/7 (29) |
| # Discontinued ≥1 concomitant ITP med after switch to AVA/# of patients on concomitant ITP meds before switch to AVA, ratio (%) | 16/28 (57) | 5/12 (42) | 7/12 (58) | 4/4 (100) |
| # Added concomitant ITP meds after switch to AVA/total population, ratio (%) | 3/44 (7) | 3/14 (21) | 0/23 (0) | 0/7 (0) |
| Concomitant steroids after switching to AVA, ratio (%) | 10/44 (23) | 8/14 (57) | 1/23 (4) | 1/7 (14) |
| # Able to reduce or discontinue steroids/# of patients on concomitant steroids before switch to AVA, ratio (%) | 18/19 (95) | 8/9 (89) | 7/7 (100) | 3/3 (100) |
| Discontinued, ratio (%) | 12/19 (63) | 3/9 (33) | 7/7 (100) | 2/3 (66) |
| Reduced, ratio (%) | 6/19 (32) | 5/9 (56) | 0/7 (0) | 1/3 (33) |
| Maintained same dose, ratio (%) | 1/19 (5) | 1/9 (11) | 0/7 (0) | 0/3 (0) |
| Increased dose, ratio (%) | 0/19 (0) | 0/9 (0) | 0/7 (0) | 0/3 (0) |
| # New to concomitant steroids with AVA, ratio (%) | 2/44 (5) | 2/14 (14) | 0/23 (0) | 0/7 (0) |
| Rescue therapy with AVA, ratio (%) | 9/44 (21) | 8/14 (57) | 0/23 (0) | 1/7 (14) |
| # Pts requiring rescue pre and post AVA, ratio (%) | 4/44 (9) | 3/14 (21) | 0/23 (0) | 1/7 (14) |
| # New rescue pts post AVA, ratio (%) | 5/44 (11) | 5/14 (36) | 0/23 (0) | 0/7 (0) |
| # of patients who no longer required rescue therapy after AVA/# of patients who required rescue therapy before AVA, ratio (%) | 11/15 (73) | 2/5 (40) | 7/7 (100) | 2/3 (67) |
| Mean duration on AVA (SD), months | 9.2 (4.0) | 7.8 (4.4) | 10.1 (3.8) | 9.0 (2.3) |
| Median duration on AVA (range), months | 9.2 (2.8, 17.2) | 7.1 (2.8, 17.2) | 10.1 (3.4, 15.6) | 8.5 (5.6, 11.7) |
| Mean weekly AVA dose, mean dose in mg (SD) | 154.1 (81.8) | 213 (85) | 117 (52) | 157.1 (84.5) |
| Median weekly AVA dose, median dose in mg (range) | 140 (20, 280) | 280 (20, 280) | 140 (40, 280) | 140 (60, 280) |
| # Discontinued AVA, ratio (%) | 6/44 (14) | 1/14 (7) | 4/23 (17) | 1/7 (14) |
| Remission attempt, ratio (%) | 1/44 (2) | 0/14 (0) | 1/23 (4) | 0/7 (0) |
| Formulary during inpatient procedure, ratio (%) | 1/44 (2) | 0/14 (0) | 1/23 (4) | 0/7 (0) |
| Lack of response, ratio (%) | 1/44 (2) | 1/14 (7) | 0/23 (0) | 0/7 (0) |
| Adverse event (headache, PVT), ratio (%) | 1/44 (2) | 0/14 (0) | 0/23 (0) | 1/7 (14) |
| Patient preference, ratio (%) | 1/44 (2) | 0/14 (0) | 1/23 (4) | 0/7 (0) |
| Rituximab used for AIHA, ratio (%) | 1/44 (2) | 0/14 (0) | 1/23 (4) | 0/7 (0) |
Abbreviations: AIHA, autoimmune hemolytic anaemia; AVA, avatrombopag; ITP, immune thrombocytopenia; PVT, portal vein thrombosis; SD, standard deviation.
Included in the 28 patients receiving one or more concomitant ITP medication prior to switching were 19 patients receiving concomitant chronic corticosteroids. Of these 19 patients, 12/19 (63%) were able to discontinue corticosteroids, 6/19 (32%) were able to reduce their dose, and 1/19 (5%) maintained the same dose after switching to avatrombopag.
Rescue therapy was required in 9/44 patients (21%) after switching to avatrombopag, as compared with 15/44 patients (34%) who required rescue on eltrombopag or romiplostim in the year prior to switching. Of the nine patients requiring rescue after switching, 5/44 patients (11%) required new rescue therapy while receiving avatrombopag, and 4/44 patients (9%) required rescue treatment before and after the switch to avatrombopag. Eleven patients who required rescue on eltrombopag or romiplostim did not require it after switching to avatrombopag.
During the observation period, 6/44 patients (14%) discontinued avatrombopag, one patient each due to attempted remission, formulary limitations, lack of response, adverse event (headache, portal‐vein thrombosis), patient preference and initiation of rituximab for autoimmune haemolytic anaemia. The other 38 patients remained on avatrombopag at the end of the observation period.
Outcomes by reason for switch
Ineffectiveness with prior TPO‐RA therapy as a reason for switch
Fourteen of 44 patients (32%) switched to avatrombopag from another TPO‐RA due to ineffectiveness of the prior TPO‐RA. The median dose of the prior TPO‐RA at the time of switching was 8 μg/kg weekly of romiplostim or 75 mg daily of eltrombopag. Platelet response was achieved in 12/14 patients (86%) and complete platelet response was achieved in 10/14 patients (71%; Table 3). The median platelet count in these patients was 28 × 109/l on romiplostim or eltrombopag versus 88 × 109/L on avatrombopag (p = 0.025 by Wilcoxon signed‐rank test; Figure 1B).
TABLE 3.
Response results in total population and by reason for switching
| Response at least once in absence of rescue | Total population | Effectiveness | Convenience | Adverse event |
|---|---|---|---|---|
| PC ≥ 50 000, ratio (%) | 41/44 (93) | 12/14 (86) | 23/23 (100) | 6/7(86) |
| PC ≥ 100 000, ratio (%) | 38/44 (86) | 10/14 (71) | 22/23 (96) | 6/7 (86) |
Abbreviation: PC, platelet count.
Of these 14 patients, 12/14 (86%) were receiving concomitant ITP medications prior to switching; five of these 12 patients (42%) were able to discontinue one or more concomitant ITP medications after switching (Table 2). Only 3/14 patients (21%) required escalation of concomitant therapy after switching. Nine of 14 patients (64%) were on concomitant chronic corticosteroids prior to switching. Of those nine patients, 3/9 (33%) were able to discontinue steroids, 5/9 (56%) reduced their dose of steroids and 1/9 (11%) maintained the same dose. Although no patients previously on steroids required an increase in steroid dose, 2/14 patients (14%) initiated steroids after switching to avatrombopag. One of 14 patients (7%) discontinued avatrombopag due to a lack of response. Rescue therapy was required in 8/14 patients (57%): 5/14 patients (36%) required new rescue therapy after switching and 3/14 patients (21%) required rescue treatment before and after switching. Of note, 10/14 patients (71%) were receiving some additional ITP‐directed therapy (either chronic or rescue therapy) in the 30‐day period prior to switching, up to and including the date of switching.
Improved convenience with avatrombopag as a reason for switch
Improved convenience characteristics of avatrombopag, including the ability to take the medication with polyvalent cations in the diet, was the primary reason for switching in 23 patients. Patients switching from romiplostim were switched specifically to avoid the need for a weekly injection; those switching from eltrombopag did so to avoid the dietary restrictions present with eltrombopag. Table S2 gives additional baseline characteristics of these patients. The median dose of the prior TPO‐RA at the time of switching was 3 μg/kg weekly of romiplostim or 37.5 mg daily of eltrombopag. Response was achieved in all 23 patients (100%) and complete response was achieved in 22/23 patients (96%) (Table 3). The median platelet count in these patients on eltrombopag or romiplostim was 70 × 109/l vs 140 × 109/l on avatrombopag (p = 0.0004 by Wilcoxon signed‐rank test). Of these 23 patients, 12/23 patients (52%) were receiving concomitant ITP medications prior to use of avatrombopag. Of these 12 patients, 7/12 patients (58%) were able to discontinue one or more concomitant medications after switching to avatrombopag (Table 2).
Adverse events with prior TPO‐RA therapy as a reason for switch
A total of seven patients switched to avatrombopag due to adverse events. Two patients switched from romiplostim: one due to arthralgia and one due to arthralgia and venous thromboembolism (VTE). Five patients switched from eltrombopag due to headache (n = 2), cataracts (n = 1), liver function test abnormalities (n = 1), and VTE and superficial thrombophlebitis (n = 1). The median dose of the prior TPO‐RA at the time of switching was 6 μg/kg weekly of romiplostim or 75 mg daily of eltrombopag. After switching, 6/7 patients (86%) achieved a complete response with avatrombopag; one patient did not respond (Table 3). The median platelet count in these patients on eltrombopag or romiplostim was 56 × 109/l vs 121 × 109/l on avatrombopag (p = 0.039 by Wilcoxon signed‐rank test). One patient discontinued avatrombopag due to an adverse event of headache and portal‐vein thrombosis (while receiving enoxaparin 40 mg daily); this patient did not have a history of hepatitis C or antiphospholipid antibody syndrome, but did have a history of multiple prior venous thromboembolic events (including events occurring while receiving treatment with both romiplostim and eltrombopag, some of which occurred despite concurrent anticoagulation). Data regarding concomitant ITP medications and rescue therapy for these patients are shown in Table 2.
Outcomes after romiplostim to avatrombopag switch or eltrombopag to avatrombopag switch
In those switching from romiplostim, platelet response was observed in 31/33 patients (94%) and complete platelet response was observed in 28/33 patients (85%). Of the 14 patients receiving prior concomitant steroids with romiplostim, 10/14 (71%) were able to discontinue corticosteroid treatment and an additional 3/14 (21%) were able to reduce their dose. Two of 33 patients (6%) added a new corticosteroid in combination with avatrombopag.
In those switching from eltrombopag, both platelet response and complete platelet response were observed in 10/11 patients (91%). Of the five patients receiving prior concomitant steroids with eltrombopag, 2/5 (40%) were able to discontinue corticosteroid treatment and the remainder (3/5, 60%) were able to reduce their dose. No patients added a new corticosteroid while receiving treatment with avatrombopag.
Patients receiving treatment with eltrombopag, romiplostim and avatrombopag
Fourteen patients received TPO‐RA monotherapy with each of eltrombopag, romiplostim and avatrombopag over the course of their disease. The sequencing of these agents, reason for treatment change from the first and second agents used, and best response to avatrombopag for each patient is described in Table 4.
TABLE 4.
Patients treated with each of the three TPO‐RAs as TPO‐RA monotherapy. Note that patients switched directly from agent 2 to agent 3 (avatrombopag), but may have had intervening therapies between agent 1 and agent 2
| Patient no. | Agent 1 | Reason for change | Agent 2 | Reason for change | Agent 3 | Best response to AVA |
|---|---|---|---|---|---|---|
| 1 | ROMI | Ineffectiveness | ELT | Ineffectiveness | AVA | CR |
| 2 | ELT | Ineffectiveness | ROMI | Ineffectiveness | AVA | NR |
| 3 | ELT | Ineffectiveness | ROMI | Ineffectiveness | AVA | CR |
| 4 | ELT | Ineffectiveness | ROMI | Ineffectiveness | AVA | NR |
| 5 | ELT | Ineffectiveness | ROMI | Convenience | AVA | CR |
| 6 | ROMI | Ineffectiveness | ELT | Cataract | AVA | NR |
| 7 | ROMI | Patient choice | ELT | Ineffectiveness | AVA | CR |
| 8 | ELT | Hepatotoxicity | ROMI | Convenience | AVA | CR |
| 9 | ELT | Abdominal pain | ROMI | Musculoskeletal pain | AVA | CR |
| 10 | ELT | Abdominal pain | ROMI | Convenience | AVA | CR |
| 11 | ROMI | Convenience | ELT | Thrombosis | AVA | CR |
| 12 | ROMI | Convenience | ELT | Headache | AVA | CR |
| 13 | ELT | Labile Plt counts | ROMI | Convenience | AVA | CR |
| 14 | ELT | Perisurgical a | ROMI | Convenience | AVA | CR |
Abbreviations: ELT, eltrombopag; ROMI, romiplostim; AVA, avatrombopag; NR, no platelet response; CR, complete platelet response.
Treatment was of intentionally limited duration to raise platelet count in preparation for a surgical procedure.
DISCUSSION
This multicentre, observational study found that 93% of patients with ITP who switched from romiplostim or eltrombopag to avatrombopag achieved a platelet response (platelet count ≥50 000/μl) and 86% achieved a complete platelet response (platelet count ≥100 000/μl). Just over half of patients switched to avatrombopag because of convenience, followed by ineffectiveness of the prior treatment and adverse events on the prior treatment. Many patients experienced a reduction in concomitant ITP medication use, including discontinuation or dose reduction of corticosteroids after switching to avatrombopag. Only six patients discontinued avatrombopag during the study period.
The present study adds to the evidence that ineffectiveness of one TPO‐RA may not predict ineffectiveness of another. A review of pooled retrospective findings found that 78% of patients who switched from eltrombopag to romiplostim or vice versa achieved or maintained a platelet response after switching (romiplostim to eltrombopag, 76%; eltrombopag to romiplostim, 80%). 5 Though response criteria differed somewhat, the findings of the present study confirm an increased platelet response when switching between TPO‐RAs and provide evidence that this also applies when switching to avatrombopag (response rate, 93%; complete response rate, 86%). Response rates were similar regardless of whether patients switched from romiplostim (94%) or eltrombopag (91%). Our findings are also consistent with those of the phase 3 study of avatrombopag versus placebo in patients with chronic ITP. 15 In that trial, the median number of weeks with a platelet response on avatrombopag was similar in patients who had and had not been previously treated with romiplostim or eltrombopag (12.7 vs 12.4 weeks). 15 Additionally, achievement of platelet response between previously exposed and non‐exposed patients was similar at day 8 (58.3% vs 70.0%), day 28 (41.7% vs 45%) and month 6 (66.7% vs 53.9%). 15
Reasons for switching from one TPO‐RA to another vary. While lack of effectiveness was the primary reason for switching TPO‐RAs in the pooled analysis [58% (172/295)], 5 convenience was the primary reason in our study (52%), followed by lack of effectiveness (32%). Patients on romiplostim were most likely to switch due to convenience of oral dosing with avatrombopag (64%) followed by lack of effectiveness (30%); those on eltrombopag were most likely to switch due to associated adverse events (45%) or lack of effectiveness (36%). In the pooled analysis, 93% of patients who switched for reasons other than effectiveness issues and 65% of those who switched due to insufficient effectiveness had improved platelet counts. In comparison, 97% and 86% of patients, respectively, achieved a platelet count ≥50 000/μl at least once in the present study.
The variable response to TPO‐RAs observed in individual patients may be explained by differences in mechanisms of action. Romiplostim directly competes with endogenous TPO for binding at the TPO receptor, whereas avatrombopag and eltrombopag bind to the transmembrane domain of the TPO receptor and thus stimulate thrombopoiesis without affecting the binding of endogenous TPO. 16 Subtle differences in downstream signalling pathways following interaction with the TPO receptor exist between agents as well, with romiplostim inducing a greater degree of downstream AKT activation and less Janus‐kinase signal transducer and activator of transcription (JAK–STAT) activation than agents that bind to the transmembrane domain of the TPO receptor, such as eltrombopag and avatrombopag. 17 , 18 Ultimately, these differences may affect the quality of megakaryocyte activation and may explain both inter‐ and intra‐individual differences in response to different TPO‐RAs. 6 , 14 , 17 , 19 Together, these data provide a rationale for the existence of differential responses with different TPO‐RAs in different groups of patients, as observed in this study.
While this is one of the largest evaluations of switching from one TPO‐RA to another and the largest evaluation to date of switching to avatrombopag, our study has several limitations. First, our analysis is subject to the limitations typical of retrospective observational studies including selection bias and potential confounding, as well as the lack of a defined treatment protocol resulting in heterogeneity of dose adjustment and follow‐up frequency. Second, while the overall number of patients was high for a TPO‐RA switching study in ITP, there were relatively low patient numbers for each subgroup analysis. Third, we did not collect information on non‐platelet outcomes, including bleeding events and health‐related quality of life. Fourth, as this was not a randomized study, it is impossible to know if patients maintained on romiplostim or eltrombopag would have spontaneously improved their platelet counts (a ‘regression to the mean’ phenomenon) had those agents been continued rather than switched to avatrombopag. Strengths of the study include that it was a multicentre study involving four large US ITP tertiary referral centres and that detailed manual chart review was completed for all patients by ITP expert clinician‐investigators, facilitating high‐quality data collection and curation and allowing for collection of important clinical details (such as the reason for switching to avatrombopag).
CONCLUSIONS
In a heavily pretreated chronic ITP population, avatrombopag was effective following therapy with romiplostim or eltrombopag, with high response rates even in patients with inadequate response to a prior TPO‐RA. The effectiveness of avatrombopag following switching was such that use of concomitant ITP therapies, in particular concomitant steroids, declined considerably after switching to avatrombopag. These findings underscore the potential value of switching between TPO‐RAs when a prior TPO‐RA does not provide adequate effectiveness, convenience, or tolerability. Additional studies with a larger patient population are warranted to confirm the findings of the present study and to expand on observations in specific patient subgroups.
CONFLICT OF INTEREST
Hanny Al‐Samkari has served as consultant for Agios, Forma, Dova/Sobi, argenx, Rigel, Novartis and Moderna, and his institution has received research support on his behalf from Agios, Dova/Sobi and Amgen. Debbie Jiang has nothing to disclose. Terry Gernsheimer has received honoraria from Amgen, Dova Pharmaceuticals and Novartis; has acted as a consultant for the Platelet Disorder Support Association, Amgen, Dova Pharmaceuticals, Biogen, Momenta, Sanofi, Vertex, Cellphire, Fujifilm, Rigel, Shionogi and Principia; and has received research support from the National Heart, Lung and Blood Institute, Principia, Rigel and Cellphire. Howard Liebman has served as a consultant for Amgen, argenx, Dova/Sobi, Genzyme, Novartis and Sanofi and has received research funding from argenx, Momenta/Janssen and Principia/Sanofi. Susie Lee has nothing to disclose. Matthew Wojdyla is an employee of Swedish Orphan Biovitrum, Inc. Michael Vredenburg is an employee of Swedish Orphan Biovitrum, Inc. Adam Cuker has served as a consultant for Synergy; he has received royalties from UpToDate; and his institution has received research support on his behalf from Alexion, Bayer, Novartis, Novo Nordisk, Pfizer, Sanofi, Spark and Takeda.
AUTHOR CONTRIBUTIONS
Hanny Al‐Samkari and Adam Cuker contributed to concept and design, data collection, data analysis, writing of the manuscript, critical revisions of the intellectual content and final approval. Debbie Jiang, Terry Gernsheimer, Howard Liebman and Susie Lee contributed to data collection, critical revisions of the intellectual content and final approval. Matthew Wojdyla contributed to data analysis, critical revisions of the intellectual content and final approval. Michael Vredenburg contributed to critical revisions of the intellectual content and final approval.
Supporting information
SUPPLEMENTARY APPENDIX
ACKNOWLEDGEMENTS
H. Al‐Samkari is the recipient of the Harvard KL2/CMeRIT Award and the American Society of Hematology Scholar Award. The authors thank Chelsea Bernheisel, an employee of Dova Pharmaceuticals, a Sobi company (Durham, NC, USA), for her assistance with data analysis. Editorial support in the form of medical writing assistance was provided by Vaidehee Deshpande, PhD; Sarah S. Bubeck, PhD; and Michael R. Page, PharmD, RPh, of BioCentric, Inc. (Collingswood, NJ, USA), and funded by Dova Pharmaceuticals, a Sobi company (Durham, NC, USA).
Al‐Samkari HP, Jiang D, Gernsheimer T, Liebman H, Lee S, Wojdyla M, Vredenburg M, Cuker A. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: A multicentre US study. Br J Haematol. 2022;197:359–366. 10.1111/bjh.18081
Funding information Editorial support for this study was funded by Dova Pharmaceuticals, a Sobi company (Durham, NC, USA).
[Corrections added on 18 March 2022, after first online publication: In the Summary section, “44 × 109/l vs 113 × 109/l” was corrected to “45 × 109/l vs 114 × 109/l”. In Table S3, “1/25 (4)” was corrected to “2/25 (8)” in this current version.]
REFERENCES
- 1. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174–80. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Samkari H, Kuter DJ. Immune thrombocytopenia in adults: modern approaches to diagnosis and treatment. Semin Thromb Hemost. 2020;46(3):275–88. [DOI] [PubMed] [Google Scholar]
- 3. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93. [DOI] [PubMed] [Google Scholar]
- 4. Cantoni S, Carpenedo M, Mazzucconi MG, De Stefano V, Carrai V, Ruggeri M, et al. Alternate use of thrombopoietin receptor agonists in adult primary immune thrombocytopenia patients: a retrospective collaborative survey from Italian hematology centers. Am J Hematol. 2018;93(1):58–64. [DOI] [PubMed] [Google Scholar]
- 5. González‐Porras JR, Godeau B, Carpenedo M. Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia. Ther Adv Hematol. 2019;10:2040620719837906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98(1):10–23. [DOI] [PubMed] [Google Scholar]
- 7. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jurczak W, Chojnowski K, Mayer J, Krawczyk K, Jamieson BD, Tian W, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–47. [DOI] [PubMed] [Google Scholar]
- 11. Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet. 2008;371(9610):395–403. [DOI] [PubMed] [Google Scholar]
- 12. Khellaf M, Viallard JF, Hamidou M, Cheze S, Roudot‐Thoraval F, Lefrere F, et al. A retrospective pilot evaluation of switching thrombopoietic receptor‐agonists in immune thrombocytopenia. Haematologica. 2013;98(6):881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6):1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Samkari H, Kuter DJ. Relative potency of the thrombopoietin receptor agonists eltrombopag, avatrombopag and romiplostim in a patient with chronic immune thrombocytopenia. Br J Haematol. 2018;183(2):168. [DOI] [PubMed] [Google Scholar]
- 15. McCrae KR, Allen LF, Aggarwal K, Vredenburg M, Tian W, Kuter D. 2019. Comparable avatrombopag (AVA) efficacy in patients with chronic immune thrombocytopenia (c‐ITP) who had received prior thrombopoietin receptor agonist (TPO‐RA) treatment. In: ISTH 2019 Annual meeting [conference proceedings on the internet]; 2019 July 6–10; Melbourne, Australia. Available from: https://academy.isth.org/isth/2019/melbourne/263602/
- 16. Cheloff AZ, Al‐Samkari H. Avatrombopag for the treatment of immune thrombocytopenia and thrombocytopenia of chronic liver disease. J Blood Med. 2019;10:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raslova H, Vainchenker W, Plo I. Eltrombopag, a potent stimulator of megakaryopoiesis. Haematologica. 2016;101(12):1443–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Buduo CA, Currao M, Pecci A, Kaplan DL, Balduini CL, Balduini A. Revealing eltrombopag's promotion of human megakaryopoiesis through AKT/ERK‐dependent pathway activation. Haematologica. 2016;101(12):1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Virk ZM, Kuter DJ, Al‐Samkari H. An evaluation of avatrombopag for the treatment of thrombocytopenia. Expert Opin Pharmacother. 2021;22(3):273–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY APPENDIX


