Abstract
A growing body of research details spatial representation in bat hippocampus, and experiments have yet to explore hippocampal neuron responses to sonar signals in animals that rely on echolocation for spatial navigation. To bridge this gap, we investigated bat hippocampal responses to natural echolocation sounds in a non‐spatial context. In this experiment, we recorded from CA1 of the hippocampus of three awake bats that listened passively to single echolocation calls, call‐echo pairs, or natural echolocation sequences. Our data analysis identified a subset of neurons showing response selectivity to the duration of single echolocation calls. However, the sampled population of CA1 neurons did not respond selectively to call‐echo delay, a stimulus dimension posited to simulate target distance in recordings from auditory brain regions of bats. A population analysis revealed ensemble coding of call duration and sequence identity. These findings open the door to many new investigations of auditory coding in the mammalian hippocampus.
Keywords: acoustic‐related activity, auditory coding, echolocating bats, echolocation, mammalian hippocampus
1. INTRODUCTION
Decades of research on the hippocampus have centered on two major topics: spatial representation and episodic or non‐spatial memory functions. With the discovery of place cells, it has been proposed that the hippocampus is the biological instantiation of a “cognitive map,” which enables spatial navigation (O'Keefe & Nadel, 1978). Studies of spatial representation in the mammalian hippocampus have supported this notion by showing that this brain structure encodes an animal's spatial location in allocentric coordinates and with reference to landmarks (Deshmukh & Knierim, 2013; O'Keefe & Dostrovsky, 1971). Many studies have also shown that the hippocampus is implicated in non‐spatial coding. For example, hippocampal‐inactivated rats failed to recognize an object they previously interacted with (Cohen et al., 2013). Further, Fortin et al. (2002) demonstrated that the hippocampus mediates the coding of the sequential order of events. Animals learned to remember the sequential order of olfactory stimuli, and performance was impaired in hippocampal‐lesioned rats. In electrophysiological experiments, it has been shown that rodent hippocampal neurons discriminate between sound stimuli when animals are rewarded for performance in non‐spatial tasks, but not under passive listening conditions (Itskov et al., 2012; Sakurai, 2002). While many studies have separately investigated spatial or non‐spatial coding in the hippocampus, some research has also shown that the hippocampus can conjunctively code both spatial and non‐spatial information (Ergorul & Eichenbaum, 2004; McKenzie et al., 2014), and it is thought that the hippocampus combines these two streams of information to form memories that can be stored and later retrieved (Knierim, 2015).
The majority of work on hippocampal function in both spatial and non‐spatial representations comes from studies in rodents; however, another mammalian model, the bat, has solidified and broadened our understanding of spatial representation in the hippocampal formation (Ulanovsky & Moss, 2007, 2011; Yartsev & Ulanovsky, 2013). Specifically, place cells have been identified in two different bat species and share similar properties to those found in rodents (Ulanovsky & Moss, 2007; Yartsev & Ulanovsky, 2013). By taking advantage of bats' natural ability to navigate in a 3D environment, researchers have further characterized 3D place cells in both species (Wohlgemuth et al., 2018; Yartsev & Ulanovsky, 2013). Because bats can use echolocation to guide their navigation, they offer the opportunity to study the influence of active sensing, the production of sonar calls, on spatial representation in the hippocampus. Ulanovsky and Moss (2011) reported that the spatial information carried by place cells in the big brown bat, Eptesicus fuscus, showed a linear decay over the time course of approximately 500 ms following each echolocation call. Similarly, Wohlgemuth et al. (2018) showed that the size of place fields is influenced by sensory sample rate, revealing larger place fields when the big brown bat probes the environment with a lower sonar call rate compared with a higher call rate. Geva‐Sagiv et al. (2016) demonstrated global hippocampal remapping in Egyptian fruit bats with changes in the animal's sensing modality (vision vs. echolocation) to guide its navigation in the same room.
While studies of echolocating bats have shed light on mammalian hippocampal function, current research has focused largely on spatial tasks in these model organisms. Bat hippocampal function in non‐spatial contexts has not been explored, and there are many important questions that remain unaddressed: What information does the bat hippocampus encode in non‐spatial tasks? Is non‐spatial information (e.g., objects, sounds, textures, etc.) encoded in the bat hippocampus? The current study is the first to begin addressing these questions by analyzing activity of hippocampal neurons in stationary, awake big brown bats (Eptesicus fuscus), passively listening to echolocation calls or call sequences broadcast through a loudspeaker. We selected echolocation signals as stimuli for this experiment for several reasons: (1) echolocation calls are behaviorally relevant to bats (Griffin, 1958; Schnitzler & Henson, 1980; Thomas et al., 2004); (2) bats naturally adapt the duration and the temporal patterning of echolocation signals in response to echoes from obstacles and targets as they navigate (Moss et al., 2006, 2011). Past studies of neurons in the bat auditory pathway have characterized neurons that respond selectively to echolocation call‐echo pairs and are the posited neural substrate of sonar ranging (Dear & Suga, 1995; Macías et al., 2018; O'Neill & Suga, 1979; Portfors & Wenstrup, 1999). Here we build on past work to explore hippocampal responses to navigation‐related signals in passively listening bats. We first broadcast single echolocation calls and call‐echo pairs (simulating the dimension of the target range) and systematically varied sonar call duration and echo delay. In addition, we played several pre‐recorded echolocation call sequences taken from bats engaged in a target tracking task to analyze hippocampal activity driven by natural call sequences containing dynamic spectro‐temporal structure. Our analysis of responses to echolocation sound sequences focused on the question of whether bat hippocampal neurons respond selectively to natural acoustic stimuli.
2. MATERIALS AND METHODS
2.1. Animal subjects
Three adult, wild‐caught big brown bats (two male and one female; Eptesicus fuscus) were used for the current study. The bats were collected in the state of Maryland under a permit (no. 55440) issued by the Department of Natural Resources. The animals were housed in a vivarium at the Johns Hopkins University, and all procedures were approved by the Institution of Animal Care and Use Committee (IACUC).
2.2. Procedures
In preparation for neural recordings, the bat was anesthetized with isoflurane (3%–5%). The muscle overlying the skull was retracted, and a custom‐made head‐post was attached to the skull surface. Then, a craniotomy was made over the right hippocampus for extracellular recordings in the CA1 (1.8 mm lateral to midline and 2.6 mm lambda; Ulanovsky & Moss, 2007). The bat was given 0.1 ml of Metacam and 0.1 ml Sulfatrim (sulfamethoxazole and trimethoprim oral suspension) every day to prevent pain and infection. We allowed at least 3 days for the bat to recover before the experiment.
Experiments were conducted in a sound‐attenuating chamber (Industrial Acoustics) whose walls and ceiling were lined with acoustic foam (Sonex). On each experimental day, we placed the awake bat in a custom holder and fixed its head in place with the head‐post attached to its skull. We used a 2 × 8 silicon probe (two shanks with 100 μm between shanks and 20 μm between two nearby recording sites on the same shank) from Neuronexus to record extracellular potentials from hippocampal neurons in the awake bat (Buz‐16, Neuronexus). We used sharp‐wave ripples and complex spike bursts as functional indicators of recording from CA1 layer (Figure 1). The neural signals were recorded at a sampling rate of 40 kHz using the Omniplex Neural Data Acquisition System (Plexon Inc.). The probe was moved to a new location for each recording session to sample different ensembles of neurons. After the experiment, the animal was perfused using saline and 4% paraformaldehyde (PFA). The brain was removed from the skull and fixed in 4% PFA for at least 2 days, and then cut into 40 μm sections using a cryostat (Leica Biosystems). The sections were stained with Cresyl Violet, and the recording sites were confirmed.
FIGURE 1.
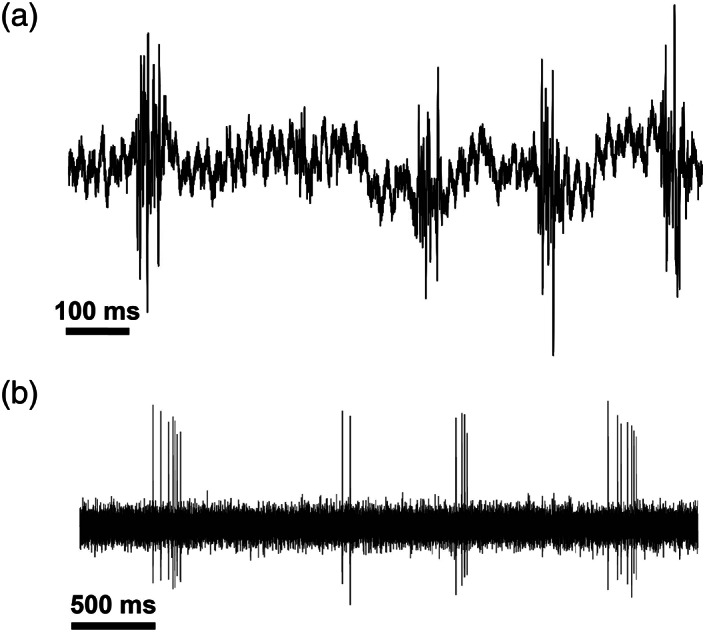
Example ripple events and complex spike bursts. Physiological indicators were used to verify recordings from CA1 of bat hippocampus
2.3. Acoustic stimuli
The big brown bat produces broadband, frequency modulated (FM) echolocation calls, adjusting signal bandwidth, duration, and temporal patterning as it encounters objects in the environment (Moss et al., 2006; Moss & Surlykke, 2001; Surlykke & Moss, 2000; Yu et al., 2019). The stimuli used in this experiment captured features of natural echolocation calls, which included the following: single FM sweeps (SFM), pulse‐echo pairs (PED), and natural call sequences (SEQ). Each echolocation call (SFM) is comprised of a two harmonic frequency‐modulated down sweep (100–40 kHz and 50–20 kHz; Figure 2a), mimicking a natural echolocation call of the big brown bat. The durations for SFM stimuli were either 1, 3, or 5 ms. We selected these sound durations because free‐flying big brown bats typically inspect objects with sonar calls of 1–5 ms. We paired SFM stimuli with attenuated and delayed replicas of the calls (pulses) to create PED stimuli. The delays ranged between 5 and 35 ms in 5 ms steps, simulating echoes from objects at distances ranging between 0.86 and 6 m (Figure 2b), and the amplitude of each simulated echo was 20 dB weaker than the call. The duration of the single FM sweeps and pulse‐echo pairs (SFM/PED) ranged from 1 to 40 ms due to the variable echo delays of 5–35 ms.
FIGURE 2.
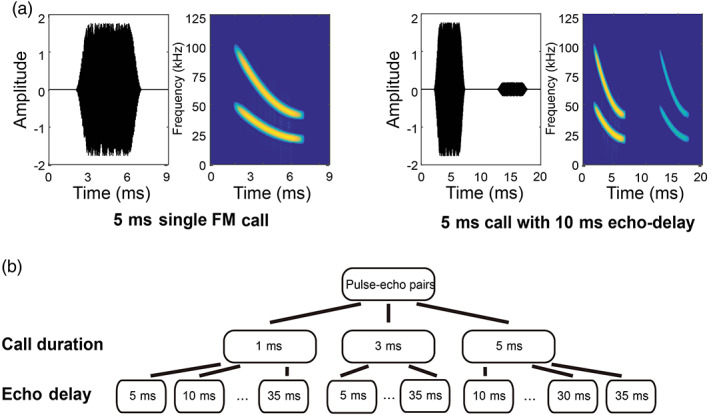
Single FM sweep (SFM) and pulse‐echo pairs (PED) stimuli. (a) Example stimuli in the SFM/PED set: The waveform and spectrogram of a single FM sweep with a duration of 5 ms (left), and a pulse‐echo pair with a duration of 5 ms and an echo delay of 10 ms (right). (b) schematic of the pulse‐echo pair stimuli. Three different durations and seven different echo delays were included. Note that there was no 5‐ms delay with a call duration of 5 ms to prevent overlap between the pulse and the echo
Recorded sequences were acquired as echolocating bats tracked approaching targets from a perch (Wohlgemuth & Moss, 2016). In all the recorded sequences, the bat showed adaptive changes in the features of its calls as the target approached. Figure 3 shows time waveforms of sonar tracking sequences. Note that the bat naturally calls at a lower rate when the target is far away and calls at a higher rate when it prepares to intercept the approaching target (Figure 3, green). We constructed the SEQ from the recorded sequences and introduced three modifications to the original sequences (Table 2). In the first modification, we introduced echoes that followed each call, decreasing in delay as the target approached (Figure 3, blue). Second, we reversed the temporal order of all the echolocation calls to create three reversed sequences in which the bat's call rate started high and decreased over the stimulus presentation (reversed sequences without echoes: Figure 3, red). The third manipulation was to substitute each call in the natural sequences with white noise pulses that matched the duration and timing of each corresponding call. The length of each stimulus sequence was 1.5 s, including approximately an 80 ms background noise before and after the first and last call in the sequence. The specific call durations and echo delays (in sequences with echoes) of the original three sequences is shown in Figure S1.
FIGURE 3.
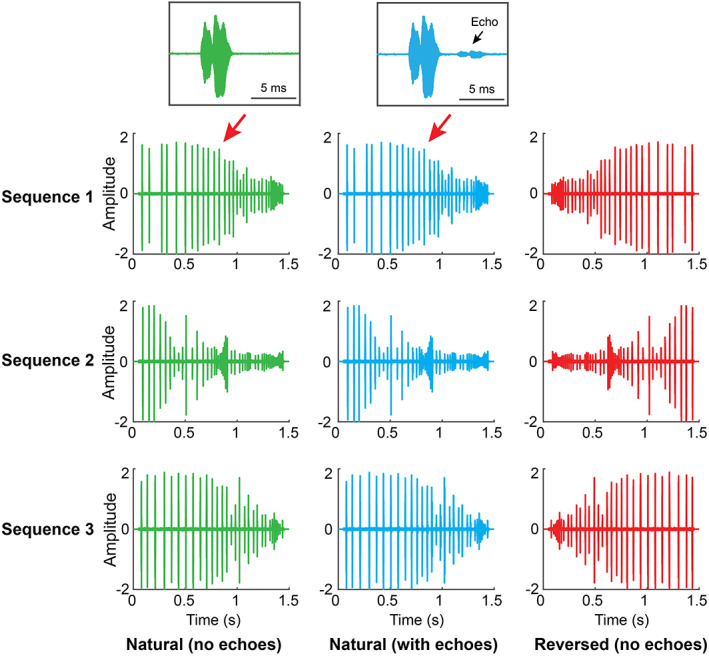
Example stimuli in SEQ. The left panel (green) plots the waveforms of three natural echolocation sequences recorded from a bat tracking an approaching target in a behavioral experiment. The middle panel (blue) are waveforms of the natural sequences with echoes. The right panel (red) shows the reversed sequences. The two insets on the top of the figure plot one single call from natural sequence 1, with (blue) or without (green) an echo
All acoustic stimuli were generated using a National Instrument card (PXIe 6358) at a sampling rate of 250 kHz. The output signals were amplified (Krohn‐Hite 7500) and then transmitted to a custom‐made loudspeaker that broadcasted the sound stimuli. The loudspeaker was placed directly facing the bat at a distance of 1 m. The loudspeaker was calibrated so that all simulated echolocation call stimuli were broadcast at 70 dB SPL and all simulated echo stimuli at 50 dB SPL at the bat's ear.
2.4. Stimulus presentation protocol
In each recording session, we first broadcast acoustic stimuli from the single FM sweep and pulse‐echo delay (SFM/PED) set. Each stimulus was repeated over 20 trials, thereby yielding a total of 460 trials (23 stimuli × 20 repetitions). All the stimuli in the SFM/PED set (a total of 23 stimuli) are summarized in Table 1. The order of all 460 trials of the SFM/PED presentation sets was randomized at the beginning of the recording session. The inter‐trial‐interval (ITI), or silent time in between two consecutive trials, was 300 ms. At the end of each stimulus presentation, a transistor‐transistor‐logic (TTL) pulse was generated by the National Instrument card and transmitted to the neural data acquisition system to synchronize the acoustic stimulus presentations and neural recordings. As soon as data collection with the SFM/PED set was completed, the SEQ presentation began. The procedures were identical to the single FM sounds and PED presentations described above. There was a total of 360 trials (18 stimuli × 20 repetitions) in the SEQ presentations, summarized in Table 2.
TABLE 1.
Stimuli included in SFM/PED broadcasts
| Echo delay/duration | 1 ms | 3 ms | 5 ms |
|---|---|---|---|
| SFM (call only) | 1 ms call | 3 ms call | 5 ms call |
| 5 ms delay | 1 ms call with 5 ms echo delay | 3 ms call with 5 ms echo delay | – |
| 10 ms delay | 1 ms call with 10 ms echo delay | 3 ms call with 10 ms echo delay | 5 ms call with 10 ms echo delay |
| 15 ms delay | 1 ms call with 15 ms echo delay | 3 ms call with 15 ms echo delay | 5 ms call with 15 ms echo delay |
| 20 ms delay | 1 ms call with 20 ms echo delay | 3 ms call with 20 ms echo delay | 5 ms call with 20 ms echo delay |
| 25 ms delay | 1 ms call with 25 ms delay | 3 ms call with 25 ms echo delay | 5 ms call with 25 ms echo delay |
| 30 ms delay | 1 ms call with 30 ms echo delay | 3 ms call with 30 ms echo delay | 5 ms call with 30 ms echo delay |
| 35 ms delay | 1 ms call with 35 ms echo delay | 3 ms call with 35 ms echo delay | 5 ms call with 35 ms echo delay |
TABLE 2.
Stimuli included in SEQ broadcasts
| Sequence 1 | Sequence 2 | Sequence 3 | |
|---|---|---|---|
| Natural (no echoes) | Natural 1 (no echoes) | Natural 2 (no echoes) | Natural 3 (no echoes) |
| Natural (with echoes) | Natural 1 (with echoes) | Natural 2 (with echoes) | Natural 3 (with echoes) |
| White noise (no echoes) | White noise 1 (no echoes) | White noise 2 (no echoes) | White noise 3 (no echoes) |
| White noise (with echoes) | White noise 1 (with echoes) | White noise 2 (with echoes) | White noise 3 (with echoes) |
| Reversed (no echoes) | Reversed 1 (no echoes) | Reversed 2 (no echoes) | Reversed 3 (no echoes) |
| Reversed (with echoes) | Reversed 1 (with echoes) | Reversed 2 (with echoes) | Reversed 3 (with echoes) |
2.5. Spike sorting
Spike data from each recording session (SFM, PED, and SEQ) were sorted together. We used a graphic‐clustering program in MatLab to perform spike sorting or cluster cutting across all channels (MClust, A.D. Reddish). Briefly, spike amplitude and other characteristics of the waveforms were plotted as a scatter diagram of two of the recording sites on the same shank of the silicon probe. Then we separated clusters of spikes that were well‐isolated from the noise as tentative single units. The quality of each isolated cluster was verified by inspecting its amplitude (and other waveform features) projections on different channels, inter‐spike‐interval, and was given a score of 1–5 for the cluster quality ranging from bad to excellent, respectively. We only included clusters with a score of 4 or higher in our analysis, and a total of 270 neurons from all three bats were used in our analysis.
2.6. Single‐unit analysis
Firing rates to SFM and PED stimuli for individual neurons were calculated for each trial and averaged across all 20 trials using a window of 80 ms following the offset of each stimulus presentation. Neurons that fired less than 30 spikes in the entire SFM/PED presentation set were excluded from the analysis. To test whether a given neuron showed any sound‐evoked activity, we performed a shuffle test. In this test, we randomly selected a time t and split the spike train into two parts (part I > t and part II < t). Then we exchanged the temporal order of two parts so that part II came before part I. This shuffling procedure was done to break the temporal relationship between the spikes and playbacks while preserving the temporal pattern of the spikes. After each shuffle, we recalculated the responses of individual neurons to each SFM/PED stimulus. The shuffle procedure was repeated 10,000 times to obtain distributions of shuffled responses to SFM/PED stimuli. A neural response exceeding 95% of the shuffled distribution was considered a significant response to the stimulus. We categorized neurons that showed a significant response to at least one of the stimuli in SFM‐PED as sound‐sensitive neurons and only included these neurons for all following SFM/PED analysis (including ensemble analysis).
To analyze whether a neuron showed selectivity to a specific call duration, we averaged firing rates across all trials with the same call duration (e.g., 1 ms call with no echo; 1 ms call with 5–35 ms delay). Then we calculated the Selectivity Index (SI):
Max is the maximum firing rate across responses to all different call durations, and Min is the minimum firing rate for all call durations. There is no a priori assumption of the preferred duration in this index calculation. We tested the significance of the SI through a shuffle test. Specifically, we shuffled the order of trial labels to break the relationship between trial labels and the corresponding firing rates. We recalculated the averaged firing rate for each duration by calculating the mean firing rate across all trials with the same call duration according to the new trial labels. Then, we calculated the SI for the shuffled responses based on the maximum and minimum responses of the newly shuffled data. The shuffle procedures were repeated 10,000 times, and a distribution of shuffled SIs was obtained. Neurons with a SI exceeding 95% of the shuffled distribution are considered to be selective to call durations.
We also analyzed the pulse‐echo delay selectivity for each neuron by first averaging responses across all trials with the same pulse‐echo delay and then calculated the SI for this neuron. The same shuffling procedures described above were performed to test the significance of pulse echo delay selectivity. Additionally, we tested the selectivity for pulse echo delay at different call durations (comparing delay tuning with the same call duration) following the same steps above.
For SEQ stimuli, we analyzed the neural response for each stimulus in a window of 1500 ms after stimulus onset. We excluded neurons that fired less than 300 spikes in the entire SEQ presentation sets. We tested whether neurons showed any sound‐sensitive activity using a shuffle test that was the same for SFM/PED analysis. Only sound‐sensitive neurons with significant responses to at least one of the SEQ stimuli were included in all following SEQ analyses. For single‐unit analysis in SEQ, we tested whether any neuron showed selectivity to a given stimulus in SEQ stimuli. The procedure is similar to the SFM/PED duration selectivity analysis stated above. Briefly, we calculated the averaged firing rates for each stimulus in SEQ for a given neuron. Then a SI was calculated and compared to a shuffled distribution to determine response significance.
2.7. Ensemble similarity analysis
For both SFM/PED and SEQ analysis, firing rates for all neurons were Z‐score normalized across all stimuli presented. The entire dataset was separated into two half sets, with the first set contained neurons' firing rates to all stimuli in odd repetitions for every stimulus (1, 3, 5,…,19; 10 trials per stimulus in total), and the second set contained firing rates to the even repetitions (2, 4, 6, 8,…, 20; 10 trials per stimulus in total). This method of dividing the data allows the two sets to share roughly similar time spans during the recording session.
In the SFM/PED ensemble analysis, we created population vectors from each subset of data by averaging the firing rates of each neuron to every stimulus across 10 trials. Pairwise Pearson's correlation coefficients of the population vectors were calculated for all possible pairs between the first and second subsets of data. To quantify and test the strength of the ensemble coding dimension, we calculated a d′ metric for each coding dimension: call duration and pulse‐echo delay (McKenzie et al., 2014).
Here, is the mean correlation within a dimension (e.g., same duration with the same pulse‐echo delay), whereas is the mean correlation between dimensions (e.g., different duration with the same pulse‐echo delay). and are the corresponding variances. To test the significance of the d′ metric for each coding dimension, we shuffled the stimulus labels that corresponded to the population vectors and recalculated the correlations of all possible pairs between the two sets. New d′ values for duration and pulse‐echo delay were calculated based on the new correlation values. The shuffling procedures were repeated 10,000 times to obtain distributions of shuffled d′ for both duration and pulse‐echo delay. d′ that exceeds 95% of the shuffled d′ distribution is considered significant. For SEQ ensemble analysis, the steps were similar to SFM/PED ensemble analysis with the d′ calculated only for the dimension of identity (self vs. others).
3. RESULTS
In this experiment, we recorded from the right hippocampus in three awake, head‐fixed big brown bats listening passively to broadcasts of echolocation signals. A total of 270 single units from three bats were obtained in this experiment. We initially performed a shuffle test to determine whether a neuron showed significant responses to at least one of the stimuli presented (Tables 1 and 2). We characterized 121 sound‐sensitive neurons during SFM/PED playbacks (shuffle test, p < .05), and 98 sound‐sensitive during SEQ playbacks (shuffle test, p < .05). These neurons were included for further analysis of SFM/PED or SEQ data, respectively. There were 50 overlapping neurons that are sound‐sensitive to both SFM/PED and SEQ broadcasts.
3.1. Single FM/PED single‐unit analysis
Of the single hippocampal neurons isolated, 121 neurons showed sound‐evoked responses to SFM/PED broadcasts. We first determined if any of these neurons showed selectivity in their firing rates to specific call durations of SFM/PED stimuli. We calculated the Selectivity Index (SI) for each neuron based on its firing profile to stimuli with different call durations (see Section 2). There was a total of 14 (12%) neurons that showed selectivity to a specific call duration (shuffle test, p < .05; Figures 4a and 5a), significantly more than expected by chance (binominal distribution, p < .001). Specifically, a total of 5, 3, or 6 neurons showed a preference for a call duration of 1, 3, or 5 ms, respectively. This suggests that a fraction of hippocampal neurons can code call duration at the single neuron level.
FIGURE 4.
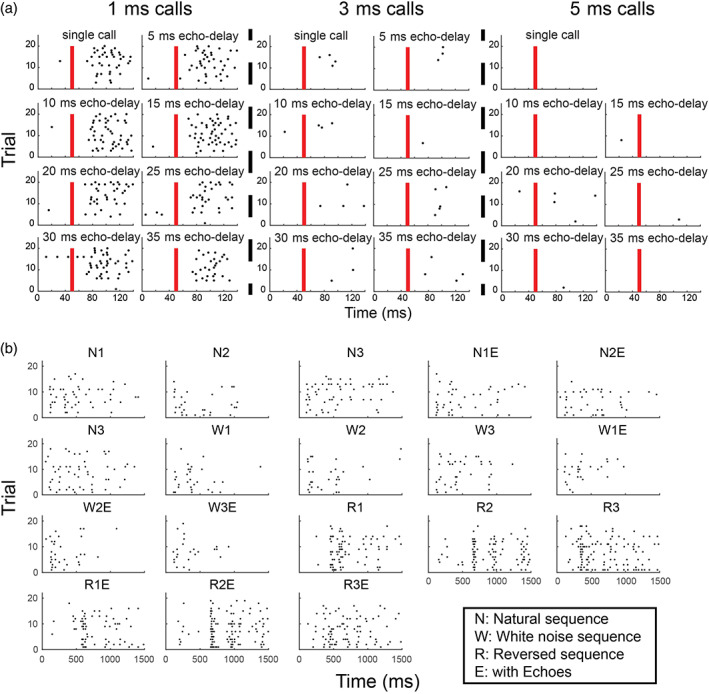
Example raster plots. (a) Example raster plots of a sound duration‐tuned neuron in the hippocampus. The red bar indicates the onset of the stimulus; each black dot indicates a spike event. This sound‐sensitive neuron showed preference to 1 ms calls, regardless of echo delay. (b) Raster plots of a neuron showing distinct responses to different natural sound sequences
FIGURE 5.
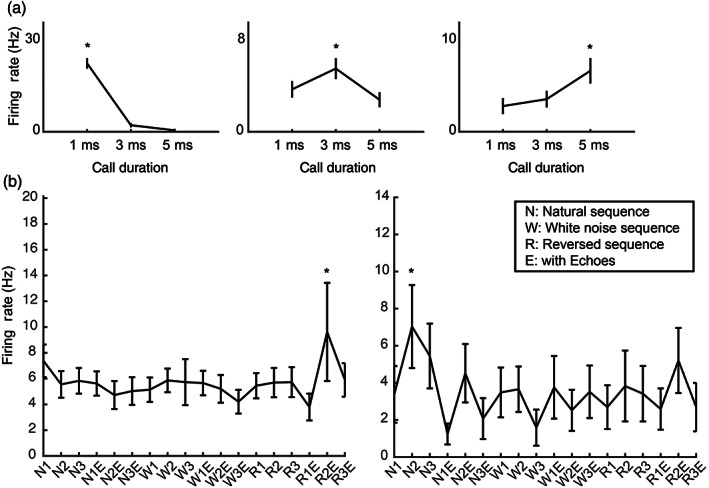
Examples of single unit data. (a) Duration‐tuned neurons. Three different units that are tuned to either 1‐, 3‐, or 5‐ms calls. The stars indicate the preferred durations of the neurons; (b) example units showed preference to selected stimuli in SEQ. The stars show the preferred stimuli of the neurons. The inset details the abbreviations of the tick labels for the stimuli
Similarly, we tested whether any neurons showed selectivity to a particular pulse‐echo delay during PED broadcasts. We found only three neurons that showed significant selectivity to a specific pulse‐echo delay, which is not more than expected by chance (binominal distribution, p = .86). In addition, we independently tested the pulse‐echo delay selectivity of calls at different durations. Only two neurons showed significant selectivity to pulse‐echo delay at a call duration of 1 ms, which is not more than expected by chance (binominal distribution, p = .94). Thus, hippocampal neurons did not show any pulse‐echo delay selectivity to the stimulus sets used in our experiments.
3.2. SEQ single‐unit analysis
A total of 98 neurons were sound‐responsive to at least one of the stimuli in the SEQ broadcasts. We tested if any of these neurons showed selectivity to a subset of the call sequences presented by calculating their SI. After comparing with the shuffled distribution of SIs, nine (9%) of neurons showed significant selectivity to some of the SEQ stimuli (Figures 4b and 5b), which exceeds that expected by chance (binomial distribution, p < .05). Out of the nine selective neurons, one also showed selectivity to SFM/PED call durations. This result suggests that a small number of neurons are selective to a subset of the SEQ playback stimuli.
3.3. Single FM/PED ensemble analysis
We separated the entire dataset into two subsets and created a population vector per subset using the mean firing rates for all 121 neurons to each of the SFM/PED stimuli. Then we calculated pairwise Pearson Correlation coefficients to obtain a similarity matrix (Figure 6a). To quantify the strength of coding in ensemble activity of a specific stimulus parameter or dimension, we calculated a d′ metric to contrast the correlations within a single dimension and correlations between different dimensions (see Section 2). Both call duration and pulse‐echo delay were tested by calculating the respective d′ metric and compared with a shuffled distribution (Figure 6b). Similar to single‐unit activity, the results of the shuffle test suggest that call duration is coded by ensemble activity (d′ = 1.02; p < .001) whereas pulse‐echo delay coding is not (d′ = 0.31; p = .06). The ensemble activity revealed a consistent pattern with single units that the hippocampal neurons encode call durations but not any of the pulse‐echo delays tested.
FIGURE 6.
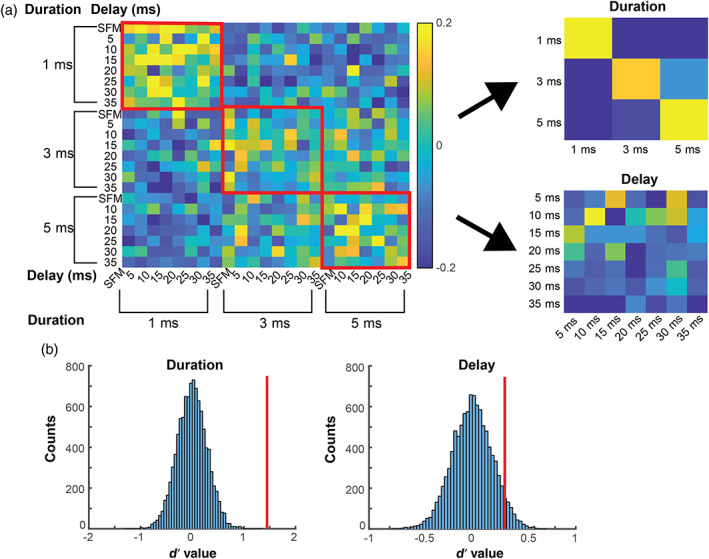
Ensemble analysis for single sweeps and pulse‐echo pairs. (a) Similarity matrix of correlation values between two subsets of data of neural responses to single FM/pulse‐echo pairs (SFM/PED) stimuli. The left panel shows the overall matrix. The axis labels represent echo delays (inner labels) and call duration (outer labels). The red squares enclosed correlation values between pairs that share the same durations. The right panel shows the condensed matrix by averaging the correlation values of the same call duration (top) or the same echo delay (bottom). (b) The shuffled distribution of d′ values for both duration (left) and delay (right). The vertical red lines represent the d′ value of the actual data
3.4. SEQ ensemble analysis
A similarity matrix was obtained based on the correlation coefficients between two subsets of data of the 98 neurons that responded to SEQ stimuli (Figure 7a). We observed high correlations along the diagonal in the matrix, suggesting that the hippocampus may differentiate stimulus sequence type at a population level. To test the strength of ensemble activity differentiating between stimulus types, we calculated the d′ metric and compared it to the shuffled distribution. The shuffle test revealed significantly higher correlations between the responses to the same stimulus than responses to different stimuli (d′ = 2.51; p < .001; Figure 7b), supporting the notion that the stimulus type can be differentiated at a population level. It is also noteworthy that the correlations between any sequence without echoes and the same sequence with echoes are very low compared with the correlation to the same sequence itself. This suggests that the hippocampus responds differentially at a population level to the sequences with and without echoes.
FIGURE 7.
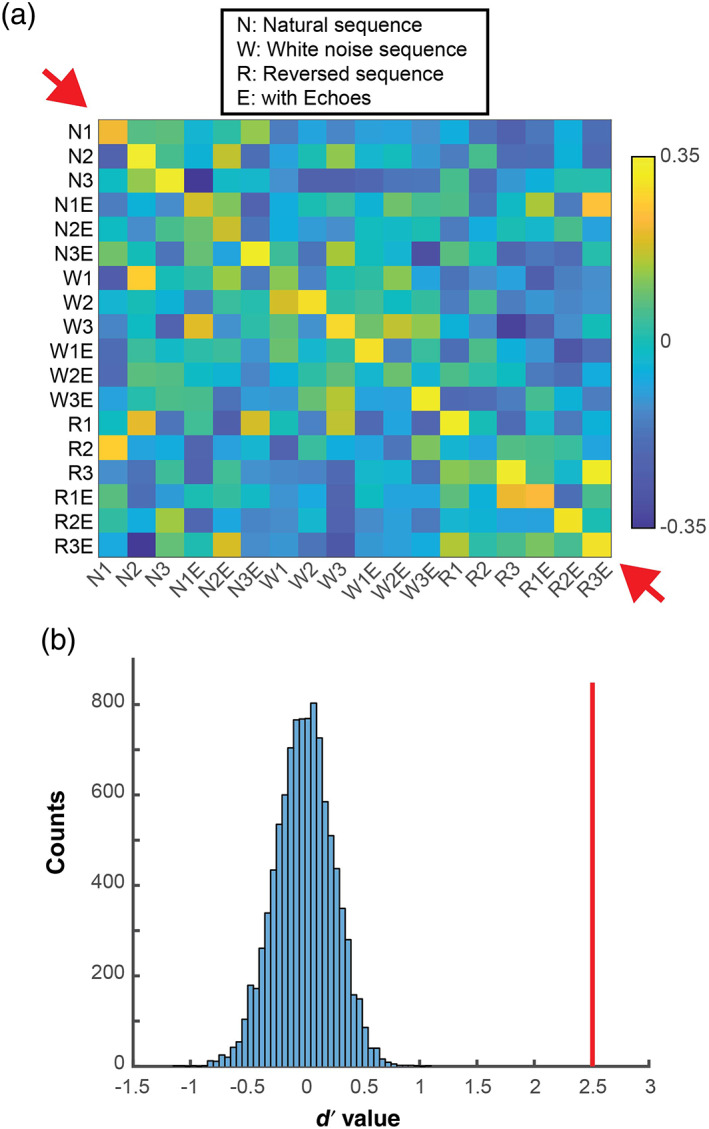
Ensemble analysis for SEQ data. (a) Similarity matrix of correlation values between two subsets of data of neural responses to SEQ stimuli. The correlation values along the diagonal (red arrows) are higher than other values in the matrix. The inset shows the abbreviations of the stimulus sets on the axis label. (b) The shuffled distribution of d′ values. The vertical red lines represent the d′ value of the actual data
4. DISCUSSION
The goal of the current study was to determine if hippocampal neurons in the bat respond to natural echolocation sounds. Past hippocampal studies in bats have been carried out using a variety of spatial navigation tasks, and little is known about bat hippocampal activity evoked by sound stimuli. Our study bridges this gap with the first report of hippocampal responses to species‐specific echolocation signals in passively listening bats.
Using SFM/PED stimuli, we discovered a population of hippocampal neurons that showed selectivity for sound duration. While sound duration tuning has been reported in the bat central auditory pathway (Ehrlich et al., 1997; Galazyuk & Feng, 1997), this is the first report of sound duration tuning in the hippocampus. Our findings align with earlier reports that rodent hippocampal neurons differentiate between sound stimuli of different durations (Sakurai, 2002); however, there are also noteworthy differences between our findings and the rodent data. Specifically, Sakurai (2002) trained rats to discriminate pure tones with durations of 1 and 3 s, an order of magnitude longer than the stimuli we used in our study (1, 3, and 5 ms). Additionally, Sakurai reported that the neurons did not code for sound duration in a passive listening condition, and responses were tied to task‐relevant behavior. Other rodent studies that investigated hippocampal responses to sound stimuli also reported that the neurons did not differentiate between stimuli under passive listening conditions (Itskov et al., 2012; Vinnik et al., 2012). By contrast, we found that bat hippocampal neurons responded differentially to echolocation call durations of only 1–5 ms under passive listening conditions, suggesting possible species differences between rodents and bats. Specifically, hippocampal neurons in the big brown bats respond selectively to the duration of very brief natural echolocation signals, which are biologically relevant to their navigation and orientation in space.
In contrast to sound duration selectivity, we found no evidence for pulse‐echo delay selectivity in bat hippocampal neurons. Pulse‐echo delay conveys spatial information (i.e., the distance to a sonar target), and the bat uses this information to guide navigation. It was initially surprising to us that the population of neurons sampled in the bat hippocampus, a brain structure implicated in spatial navigation, did not encode pulse‐echo delay, and this negative result prompts us to consider the following possible explanations. First, the pulse‐echo delay provides egocentric spatial information (i.e., the distance to a target relative to the bat), and hippocampal selectivity to this stimulus parameter may only emerge when the bat actively produces sounds that return echoes. It may also be that the 5 ms pulse‐echo delay steps used in the present study were too large to reliably capture pulse‐echo delay tuning in bat hippocampus if selectivity to this stimulus dimension is sharper than that reported in past studies of echo delay tuned neurons in bat auditory cortex, inferior colliculus, and superior colliculus (Dear et al., 1993; Dear & Suga, 1995). In this context, it is noteworthy that pulse‐echo delay tuning in the bat midbrain is sharpened when the bat actively produces calls that return echoes from physical objects (Kothari et al., 2018). Secondly, the current experiment used sound stimuli that may have omitted key natural features to evoke hippocampal activity. Even though we attempted to compensate for the energy loss in echoes by reducing its amplitude, natural echoes also contain additional spectral‐temporal information introduced by object size, material, and shape, which may shape neural responses (see Sanderson & Simmons, 2000). Thus, the echolocation sound stimuli in our experiment may have lacked acoustic features that would have otherwise evoked robust responses. To test this, future hippocampal studies could use stimulus sets that are recorded from a bat using sonar to inspect different objects (both calls and echoes). Further, neural telemetry recordings from a free‐flying bat could be synchronized with the reconstruction of the bat's sonar stimulus space to determine if the activity is modulated by echoes from objects at different distances. Lastly, the hippocampus may code pulse‐echo delay (object range) only when the information carried by pulse‐echo delay is task‐relevant and/or rewarded. Past studies have, for example, measured the bat's pulse‐echo delay discrimination performance in psychophysical tasks whereby the bat crawls down the arm of a platform to report which of two phantom sonar targets returns an echo at a shorter delay. The bat receives a food reward for each correct response and can discriminate echo delay differences of less than 60 μs (Moss & Schnitzler, 1995; Simmons, 1973). A similar task could be implemented while recording from the bat hippocampus to investigate whether hippocampal neurons encode pulse‐echo delay when this information is task‐relevant and rewarded.
In addition to single echolocation pulse‐echo pairs, we also studied hippocampal responses to natural echolocation call sequences comprised of FM signals with dynamic spectro‐temporal features. Of the single hippocampal neurons studied here, some showed selective responses to one or more of the sequence stimuli. With the small number of neurons that showed selective responses, more work is needed to draw conclusions. That is, although each selective neuron showed a preferred stimulus, the data do not allow us to posit that a pool of neurons encoded each of the stimuli. Within the set of nine neurons that showed selective responses to natural sound sequences, one neuron also showed selectivity to call duration of the SFM/PED stimuli. However, we did not observe any duration‐related response selectivity within the sequences that could be tied directly to the duration selectivity of the SFM/PED stimuli. The sequences consist of fast repetition of calls with different durations, pulse intervals, and spectral content, and the combination of these stimulus features may have driven the activity of the neuron. Future studies will be designed to control acoustic parameters within dynamic sound sequences to directly investigate stimulus features that shape the selectivity of hippocampal neurons.
Past studies have shown that rodent hippocampal neurons show sound sensitivity when a stimulus is behaviorally relevant (Itskov et al., 2012; Sakurai, 2002). Future recordings of hippocampal activity in bats engaged in a behavioral sound‐sequence discrimination task could investigate if a larger population of neurons show sound‐sequence‐specific selectivity in the hippocampus and determine if stimulus parameters within a sequence are encoded at the population level.
We explored whether ensemble activity can distinguish between different sequences. Indeed, we discovered that population responses to different echolocation sequences could be reliably differentiated. This is seen in the higher correlation values along the diagonal of the correlation matrix presented in Figure 7. Many of the sequences contained apparently subtle spectro‐temporal differences (e.g., natural sequence 1 and natural sequence 3; Figure 3). Given that the bat was not required to discriminate between these sequences in a behavioral task, it is noteworthy that the population of bat hippocampal neurons discriminated between these natural call sequences under passive listening conditions. We predict that combining hippocampal recordings with a behavioral discrimination task would amplify this finding.
Interestingly, the ensemble activity appears to differentiate between the sequences with and without echoes (e.g., natural sequence 1 with echoes and natural sequence 1 without echoes). Figure 7 shows in the similarity matrix that the correlations between these pairs of stimuli are low. This suggests that these stimuli are processed differently. The sequences with echoes contain double the number of stimuli, with shorter time intervals between sounds. The white noise sequences were also differentiated from other sequences in the population analysis. One possible explanation is that the hippocampus of the passively listening bat primarily differentiates between the spectro‐temporal patterning of stimuli, independent of navigation context.
Past rodent studies have reported that hippocampal neurons can encode object or item identity, differentiate between sound duration or frequency, in situations that required the animal's active engagement in a behavioral task (Sakurai, 2002). The present experimental data reveal that bat hippocampal neurons can differentiate between stimuli (i.e., call sequences with spectro‐temporal pattern differences) at a population level in a passive listening context.
Why were the individual SFM/PED stimuli not differentiated at the population level? One possible explanation is that these sound stimuli were too short in duration to sufficiently drive hippocampal neuron activity. The entire duration of SFM/PED stimuli ranged from 1 and 40 ms. By contrast, the SEQ stimuli were 1500 ms in duration. The SFM/PED stimuli could have been too short to carry sufficient information for hippocampal discrimination, at least under passive listening conditions. Composite stimulus duration could be one of the acoustic features that the bat uses to differentiate between sound stimuli, but additional acoustic features may be necessary for successful discrimination. It is also possible that the PED stimuli in our experiments did not include specific echo delays that might have activated hippocampal neurons. These possibilities should be tested in future experiments that incorporate a wider range of stimuli.
In this study, we explored sound evoked activity in the hippocampus of the echolocating bat. Specifically, we played single FM echolocation calls (SFM), simulated call‐echo pairs (PED), and sound sequences (SEQ) while the awake bat listened passively. We discovered sound selective responses in the bat hippocampus, both at the single‐unit and population levels. Hippocampal responses to the SEQ stimuli were differentiated at a population level in a passive listening bat, a finding that differs from reports in rodents showing that sound‐related responses are strictly dependent on behavior‐relevance (Sakurai, 2002; Vinnik et al., 2012). These findings could be due to differences in species, stimulus sets, or other experimental details, which can be fully investigated in future studies. Notably, the stimuli we used in the present study were signals that echolocating bats produce to probe the environment and have biological relevance to the animal subjects of this experiment.
Here we report findings from the first study of natural sound selectivity in the hippocampus of the echolocating bat. Many open questions remain: What is the full catalog of sound features that the bat hippocampus distinguishes? Does the bat hippocampus code echolocation sound stimuli differently when the animal is actively engaged in behavioral tasks? Future work exploring these questions will broaden our understanding of general principles and species specializations of hippocampal coding.
Supporting information
Figure S1 Call durations and echo delays of natural sequences. Durations of calls in the three natural sequences and echo delays following calls in the three natural sonar sequences containing echo returns. Black circles indicate individual calls.
ACKNOWLEDGMENTS
We would like to thank Dr. James Knierim for providing feedback and support on the experiment and data analysis, and Dr. Janice Chen for suggestions on analysis for population neural activity. Dr. Noah Cowan and Dr. Mounya Elhilali also gave insightful advice on data analysis. Dr. Kate Allen and Dr. Jennifer Lawlor provided valuable comments on an earlier version of this manuscript. This work was supported by Air Force Office for Scientific Research Grant FA9550‐14‐1‐0398NIFTI, Office of Naval Research Grant N00014‐17‐1‐2736 and NIH NINDS R01 NS121413.
Yu, C. , & Moss, C. F. (2022). Natural acoustic stimuli evoke selective responses in the hippocampus of passive listening bats. Hippocampus, 32(4), 298–309. 10.1002/hipo.23407
Funding information Air Force Office of Scientific Research, Grant/Award Number: FA9550‐14‐1‐0398NIFTI; National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01 NS121413; Office of Naval Research, Grant/Award Number: N00014‐17‐1‐2736
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Cohen, S. J. , Munchow, A. H. , Rios, L. M. , Zhang, G. , Ásgeirsdóttir, H. N. , & Stackman, R. W., Jr. (2013). The rodent hippocampus is essential for nonspatial object memory. Current Biology, 23(17), 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear, S. P. , Fritz, J. , Haresign, T. , Ferragamo, M. , & Simmons, J. A. (1993). Tonotopic and functional organization in the auditory cortex of the big brown bat, Eptesicus fuscus . Journal of Neurophysiology, 70(5), 1988–2009. [DOI] [PubMed] [Google Scholar]
- Dear, S. P. , & Suga, N. (1995). Delay‐tuned neurons in the midbrain of the big brown bat. Journal of Neurophysiology, 73(3), 1084–1100. [DOI] [PubMed] [Google Scholar]
- Deshmukh, S. S. , & Knierim, J. J. (2013). Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus, 23(4), 253–267. 10.1002/hipo.22101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich, D. , Casseday, J. H. , & Covey, E. (1997). Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus . Journal of Neurophysiology, 77(5), 2360–2372. [DOI] [PubMed] [Google Scholar]
- Ergorul, C. , & Eichenbaum, H. (2004). The hippocampus and memory for “what,”“where,” and “when.”. Learning & Memory, 11(4), 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, N. J. , Agster, K. L. , & Eichenbaum, H. B. (2002). Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience, 5(5), 458–462. 10.1038/nn834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazyuk, A. V. , & Feng, A. S. (1997). Encoding of sound duration by neurons in the auditory cortex of the little brown bat, Myotis lucifugus . Journal of Comparative Physiology A, 180(4), 301–311. [DOI] [PubMed] [Google Scholar]
- Geva‐Sagiv, M. , Romani, S. , Las, L. , & Ulanovsky, N. (2016). Hippocampal global remapping for different sensory modalities in flying bats. Nature Neuroscience, 19(7), 952–958. 10.1038/nn.4310 [DOI] [PubMed] [Google Scholar]
- Griffin, D. R. (1958). Listening in the dark: The acoustic orientation of bats and men. New Haven: Yale University Press. [Google Scholar]
- Itskov, P. M. , Vinnik, E. , Honey, C. , Schnupp, J. , & Diamond, M. E. (2012). Sound sensitivity of neurons in rat hippocampus during performance of a sound‐guided task. Journal of Neurophysiology, 107(7), 1822–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim, J. J. (2015). From the GPS to HM: Place cells, grid cells, and memory. Hippocampus, 25(6), 719–725. 10.1002/hipo.22453 [DOI] [PubMed] [Google Scholar]
- Kothari, N. B. , Wohlgemuth, M. J. , & Moss, C. F. (2018). Dynamic representation of 3D auditory space in the midbrain of the free‐flying echolocating bat. eLife, 7, 1–29. 10.7554/eLife.29053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías, S. , Luo, J. , & Moss, C. F. (2018). Natural echolocation sequences evoke echo‐delay selectivity in the auditory midbrain of the FM bat, eptesicus fuscus . Journal of Neurophysiology, 120(3), 1323–1339. 10.1152/jn.00160.2018 [DOI] [PubMed] [Google Scholar]
- McKenzie, S. , Frank, A. J. , Kinsky, N. R. , Porter, B. , Rivière, P. D. , & Eichenbaum, H. (2014). Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron, 83(1), 202–215. 10.1016/j.neuron.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, C. F. , Bohn, K. , Gilkenson, H. , & Surlykke, A. (2006). Active listening for spatial orientation in a complex auditory scene. PLoS Biology, 4(4), 615–626. 10.1371/journal.pbio.0040079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, C. F. , Chiu, C. , & Surlykke, A. (2011). Adaptive vocal behavior drives perception by echolocation in bats. Current Opinion in Neurobiology, 21(4), 645–652. 10.1016/j.conb.2011.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, C. F., & Schnitzler, H. U. (1995). Behavioral studies of auditory information processing. In: Popper, A. N., & Fay, R. R. (Eds) Hearing by Bats (Vol. 5, pp. 87–145). New York, NY: Springer Handbook of Auditory Research, Springer. [Google Scholar]
- Moss, C. F. , & Surlykke, A. (2001). Auditory scene analysis by echolocation in bats. The Journal of the Acoustical Society of America, 110(4), 2207–2226. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. , & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Research, 34(1), 171–175. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. , & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Oxford University Press. [Google Scholar]
- O'Neill, W. E. , & Suga, N. (1979). Target range‐sensitive neurons in the auditory cortex of the mustache bat. Science, 203(4375), 69–73. [DOI] [PubMed] [Google Scholar]
- Portfors, C. V. , & Wenstrup, J. J. (1999). Delay‐tuned neurons in the inferior colliculus of the mustached bat: Implications for analyses of target distance. Journal of Neurophysiology, 82(3), 1326–1338. [DOI] [PubMed] [Google Scholar]
- Sakurai, Y. (2002). Coding of auditory temporal and pitch information by hippocampal individual cells and cell assemblies in the rat. Neuroscience, 115(4), 1153–1163. [DOI] [PubMed] [Google Scholar]
- Sanderson, M. I. , & Simmons, J. A. (2000). Neural responses to overlapping FM sounds in the inferior colliculus of echolocating bats. Journal of Neurophysiology, 83(4), 1840–1855. [DOI] [PubMed] [Google Scholar]
- Schnitzler, H. , & Henson, O. (1980). Performance of airborne animal sonar systems: 1. Microchiroptera. In Animal sonar systems (Vol. 28, pp. 109–181). New York: Plenum Press. 10.10007/978-1-4684-7254-7_6 [DOI] [Google Scholar]
- Simmons, J. A. (1973). The resolution of target range by echolocating bats. The Journal of the Acoustical Society of America, 54(1), 157–173. 10.1121/1.2144101 [DOI] [PubMed] [Google Scholar]
- Surlykke, A. , & Moss, C. F. (2000). Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. The Journal of the Acoustical Society of America, 108(5), 2419–2429. 10.1121/1.1315295 [DOI] [PubMed] [Google Scholar]
- Thomas, J. A. , Moss, C. F. , & Vater, M. (2004). Echolocation in bats and dolphins. University of Chicago Press. [Google Scholar]
- Ulanovsky, N. , & Moss, C. F. (2007). Hippocampal cellular and network activity in freely moving echolocating bats. Nature Neuroscience, 10(2), 224–233. 10.1038/nn1829 [DOI] [PubMed] [Google Scholar]
- Ulanovsky, N. , & Moss, C. F. (2011). Dynamics of hippocampal spatial representation in echolocating bats. Hippocampus, 21(2), 150–161. 10.1002/hipo.20731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinnik, E. , Antopolskiy, S. , Itskov, P. M. , & Diamond, M. E. (2012). Auditory stimuli elicit hippocampal neuronal responses during sleep. Frontiers in Systems Neuroscience, 6, 49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth, M. J. , & Moss, C. F. (2016). Midbrain auditory selectivity to natural sounds. Proceedings of the National Academy of Sciences, 113(9), 2508–2513. 10.1073/pnas.1517451113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth, M. , Yu, C. , & Moss, C. (2018). 3D hippocampal place field dynamics in free‐flying echolocating bats. Frontiers in Cellular Neuroscience, 12, 270. 10.3389/fncel.2018.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartsev, M. M. , & Ulanovsky, N. (2013). Representation of three‐dimensional space in the hippocampus of flying bats. Science, 340(6130), 367–372. 10.1126/science.1235338 [DOI] [PubMed] [Google Scholar]
- Yu, C. , Luo, J. , Wohlgemuth, M. , & Moss, C. F. (2019). Echolocating bats inspect and discriminate landmark features to guide navigation. Journal of Experimental Biology, 222(8), 1–11. 10.1242/jeb.191965 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Call durations and echo delays of natural sequences. Durations of calls in the three natural sequences and echo delays following calls in the three natural sonar sequences containing echo returns. Black circles indicate individual calls.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


