Abstract
New Findings
-
What is the topic of this review?
In this report, we summarize the latest clinical evidence linking developmental programming in the kidney to later life blood pressure and kidney disease.
-
What advances does it highlight?
Population‐level studies now show convincingly that low birth weight, fetal growth restriction and preterm birth are associated with and have a synergistic impact on the risk of kidney disease in later life. A new approach also considers how evolutionary selection pressure might fail to select for long‐term robustness of kidney function.
Abstract
The global burden of kidney disease is high and rising. The risk of kidney disease among individuals is highly variable, in part related to genetic and environmental factors, but also likely to be modulated by developmental programming of the number of nephrons and kidney function in fetal life. The number of nephrons varies widely across the population and is lower among those who were born small or preterm. Population registry evidence clearly shows an association between these birth circumstances and later‐life risk of hypertension and kidney disease, not only for chronic kidney disease but also for acquired kidney disease, demonstrating an inherent susceptibility to kidney disease in these individuals. Gestational stressors impact kidney development, a process that is likely to be layered upon the evolutionary history of the kidney and how the organ has developed in response to selection pressure to support reproductive capacity in early adulthood, but not to withstand multiple stresses later in life. Reducing the global burden of kidney disease in future generations will require both individual‐ and population/environment‐level risks to be addressed.
Keywords: birth weight, blood pressure, developmental programming, evolution, kidney, nephron number
1. INTRODUCTION
It is estimated that ∼10% of adults are living with chronic kidney disease (CKD) worldwide (GBD Chronic Kidney Disease Collaboration, 2020). Individual susceptibility to kidney disease is highly variable. This variability is hard to attribute solely to genetic or environmental factors. Conditions that impact development of the fetal kidney are increasingly being recognized as important modulators of this risk and are associated with greater odds of hypertension and kidney disease throughout the lifespan. Noting that hypertension and kidney disease tend to be more prevalent in populations with lower birth weights, Brenner et al. (1988) suggested that LBW, which is a marker for intrauterine stress, might be associated with a developmentally acquired reduction in the number of nephrons, which could contribute over the life course to hypertension and kidney disease. The concept, termed developmental programming, was described by Barker et al. (1989), who observed that adults born with a low birth weight (LBW; birth weight <2.5 kg) died prematurely from cardiovascular disease. Seminal observations by Bertram, Hoy and others have demonstrated a 13‐fold variation in the number of nephrons in human populations, in addition to significant correlations between the number of nephrons and birth weight and gestational age (Hinchliffe et al., 1993; Puelles et al., 2011). The variability in population‐level risk of kidney disease might, in part, reflect this variability in the number of nephrons.
2. LINKING EARLY DEVELOPMENT, BLOOD PRESSURE AND KIDNEY FUNCTION
Based on the known inverse relationship between kidney mass and blood pressure, exacerbated by high sodium intakes, Brenner et al. (1988) hypothesized that a kidney with fewer nephrons would be less able to excrete salt, which would contribute to higher blood pressure. Kidneys with fewer nephrons would also be less able to withstand superimposed kidney injury (because nephrons once lost do not regenerate), which would increase the risk of CKD (Brenner et al., 1988). These relationships have been demonstrated in several animal models, although other programmed factors, in addition to the number of nephrons, are also likely to contribute to the risk of hypertension, such as the activity of the renal–angiotensin–aldosterone system, sympathetic nervous system, tubule sodium transport etc. Several molecular mechanisms have been identified as mediators of programming in the kidney, including alterations in the expression of genes that govern branching morphogenesis, modulation of apoptosis, premature senescence, premature progenitor differentiation and sex effects (Bhunu et al., 2021).
In humans, LBW, preterm birth and exposure to maternal diabetes and pre‐eclampsia during gestation have all been associated with increased blood pressure and/or risk of proteinuria and kidney dysfunction in later life (Low Birth Weight & Nephron Number Working Group, 2017). Low birth weight and small kidneys are associated with salt sensitivity (salt‐induced changes in blood pressure), suggesting altered sodium handling by these kidneys. The number of nephrons has been found to be lower in small cohorts of adults (birth weights unknown) with essential hypertension or CKD compared with normotensive adults (Hoy et al., 2008; Kanzaki et al., 2015). Inclusion of sclerosed glomeruli in nephron counts reduced the difference between hypertensive and non‐hypertensive subjects, suggesting exacerbation of nephron loss with hypertension (Hoy et al., 2008). National birth registry studies have shown that LBW, being small for gestational age (birth weight <10th percentile for gestational age) and preterm birth (birth before 36 weeks of gestation) are synergistic risk factors for end‐stage kidney disease in adulthood (Figure 1; Gjerde, Lillas, et al., 2020; Gjerde, Reisaeter, et al., 2020). Recent evidence also points to the impact of maternal obesity, maternal diabetes and pre‐eclampsia as additional programming risk factors (Low Birth Weight & Nephron Number Working Group, 2017). Gestational and birth circumstances, reflecting intrauterine exposures, are now robustly associated with the risk of kidney disease in later life at the population level. In the individual, however, LBW, small for gestational age or preterm birth are not always followed by hypertension and CKD. Changes induced by developmental programming might therefore constitute a ‘first hit’ in the kidney (i.e., conferring susceptibility to kidney disease or hypertension), but overt disease/dysfunction might then become manifest only after superimposition of a ‘second hit’, such as diabetes, ageing, genetics, primary kidney disease, catch‐up growth/overweight/obesity or environmental stresses imposed by climate change (Low Birth Weight & Nephron Number Working Group, 2017). This ‘second hit’ hypothesis has remained relatively theoretical until recently. New data show that LBW, small for gestational age and preterm birth are also associated with greater risks of acute kidney injury and glomerulonephritis (disorders unlikely to be impacted by developmental programming) in later life, supporting this concept (Gjerde, Reisaeter, et al., 2020).
FIGURE 1.
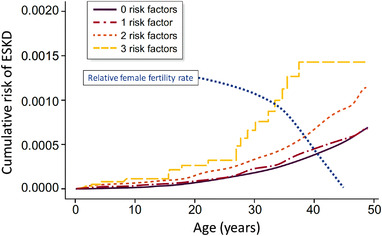
Risk of end‐stage kidney disease (ESKD) according to number of birth‐related risk factors (birth weight <10th percentile, birth weight for gestational age <10th percentile, and preterm birth). Reproduced with permission from Gjerde, Lillas, et al. (2020). The blue dotted line depicts the decrease in relative fertility rate for women from 20 to 45 years of age (Heffner, 2004). The risk of progression to ESKD for infants of low birth weight is largely delayed beyond peak reproductive years and has minimal effect on reproductive fitness
Currently, the number of nephrons in humans can only be measured post‐mortem through a painstaking process. The number of nephrons has therefore been counted only in small cohorts (Puelles et al., 2011). Existing evidence supports an average of ∼1,000,000 nephrons per kidney (range of 210,000 to 2.7 million), predominantly set at birth, with a predicted increase of 257,426 glomeruli per kilogram increase in birth weight (Hughson et al., 2003; Puelles et al., 2011). In addition to birth weight and gestational age, smaller kidney size, female sex, shorter adult height, Aboriginal Australian ethnicity and maternal vitamin A deficiency have been associated with a lower number of nephrons (Hoy et al., 2008).
Not all programming risks are associated with LBW or gestational age. These circumstances detectable at birth are therefore useful, but are not the only markers of programming risk in the kidney. Maternal health and the social determinants of health are important determinants of birth outcomes and therefore kidney development. Nutrition, infections, social stress, chronic illness, poverty, education level, age and other factors might therefore all impact the risk of hypertension and kidney disease in the next generation (Low Birth Weight & Nephron Number Working Group, 2017). In turn, women who experience developmental programming themselves, having had LBW or been born preterm, have a higher risk of developing pre‐eclampsia and having small or preterm offspring, potentiating a programming cycle across generations (Low Birth Weight & Nephron Number Working Group, 2017).
Human and animal studies therefore support a direct impact of environmental exposures during gestation on kidney development, supported further by recent evidence that the number of nephrons can be ‘reprogrammed’ through various interventions applied during pregnancies at risk (Nusken et al., 2018). The developmental programing has therefore long been regarded as an individual‐level maladaptation to fetal stress; however, a complementary explanation might lie in the evolutionary history of the human kidney (Chevalier, 2020).
3. EVOLUTIONARY EXPLANATION FOR PREVALENCE OF A LOW NUMBER OF NEPHRONS AT BIRTH: INCREASED RISK FOR CHRONIC KIDNEY DISEASE
The wide variation in the number of nephrons in healthy populations is consistent with the operation of developmental plasticity, whereby epigenetic processes respond to environmental stimuli to alter the phenotype independent of genotype (Lea et al., 2017). Experimental studies in the mouse revealed that maternal micronutrient or macronutrient deficiency resulted in > 50% reduction in the number of nephrons of offspring, demonstrating a strong influence of fetal energy metabolism on nephrogenesis (Sampogna et al., 2015). A decreased the number of nephrons was also demonstrated in Japanese quail embryos subjected to undernutrition, consistent with an evolutionarily conserved adaptive trait (Nishimura et al., 2007).
Charles Darwin's evolutionary theory is based on three tenets: (1) existence of inter‐individual variation within a population; (2) selection by the environment for reproductive success (fitness); and (3) heritability of the variations (Darwin, 2009). Natural selection is constrained by conservation of energy, which must be allocated to competing demands that together determine fitness: growth, reproduction, immune response and maintenance. The mammalian kidney is the product of transitions from a marine environment to freshwater and to terrestrial life; a sequence recognized by Homer Smith as an explanation for a high‐filtering glomerulus coupled with an energy‐demanding tubule required to reclaim 99% of the filtrate (Smith, 1953). Thus, maternal nutritional deficiency favours allocation of energy to the brain over the kidney, a life‐history strategy that proved highly successful, with Homo sapiens migrating from Africa over the entire globe in 70,000 years (Wallace, 2013).
How were these evolutionary trade‐offs made, and how could they explain the rising epidemic of CKD today? The perinatologist and neonatologist are familiar with three potential fetal outcomes in a mother subjected to undernutrition (whose energy requirement is increased 20% through pregnancy and breastfeeding): slowed somatic growth (intrauterine growth restriction); accelerated maturation through cortisol release, with preterm birth; and death (Rylander et al., 2013). Balancing the energy resources and needs of both mother and fetus, the placenta is an extraordinary temporary organ that is produced by the fetus, but favours the mother. In the face of fetal stress and restricted maternal nutrition, having reached reproductive age and proved fertile, the mother is more likely than the stressed fetus to reproduce in the future (Haig, 2019). Modulating this balance, epigenetic responses to environmental exposures are operative in three generations during fetal life: the mother (reflecting her prior fetal exposure), the fetus and its germ cells (Barker et al., 2012). These considerations would favour selection for diversion of energy from nephrogenesis to fetal brain growth and a reduced number of nephrons in the undernourished fetus (Chevalier, 2020).
Recent advancements in understanding the metabolic regulation of nephrogenesis have provided a mechanistic framework for explaining the reduced number of nephrons in the stressed fetus. The population of nephron progenitor cells must be poised to proliferate to supply an increasing demand for new nephrons, but this must also be synchronized with differentiation and maturation of the resulting complement of nephrons, accompanied by exhaustion of most progenitor cells (Tortelote et al., 2021). This is a process regulated by epigenetic signalling. Environmental metabolic constraints signalled to the fetus via the placenta promote fetal DNA hypomethylation of nephron progenitor cells, thereby limiting the number of nephrons to preserve brain development but also meeting the metabolic demand of the fetus through postnatal maturation (Chevalier, 2020; Wanner et al., 2019).
Driven by natural selection, life‐history strategy favours reproductive fitness. Growth and development to sexual maturity place the greatest demands on energy consumption. Previously regarded as relatively static through the life course, a recent study of 6,400 individuals across the globe ranging in age between 8 days and 95 years revealed four distinct phases in metabolic status. There is a rapid 50% rise in adjusted basal energy expenditure from birth through the first year of life, followed by a decrease during adolescence to a new plateau at 20 years that is maintained through most of adulthood (60 years), ending with an additional 20% decrease with further ageing (Pontzer et al., 2021). An increased basal metabolic rate in early postnatal life is required for normal somatic growth, which increases the susceptibility of a reduced number of nephrons to nutritional restriction, a major risk factor for the rising prevalence of CKD in low‐income settings (Alderman & Headey, 2018).
Glomerular filtration rate is dependent on kidney metabolic rate, determined largely by tubular reabsorption (Singer, 2001). Consequently, an elevated basal energy expenditure in childhood and adolescence requires optimal nephron function to match the metabolic load. For infants with LBW and a reduced number of nephrons, this is by necessity provided by an adaptive increase in nephron size, a response that is more robust in early development (Bhathena, 1996). A modest reduction in the number of nephrons in the LBW infant leads to compensatory nephron hypertrophy, with postponement of end‐stage kidney disease beyond peak reproductive years (Figure 1; Gjerde, Lillas, et al., 2020; Heffner, 2004).
The ageing kidney has reduced proliferative capacity, increased activation of cell death pathways and diminished immune and repair responses, all of which increase susceptibility to stressors, such as hypoxia and oxidative injury (Pomatto & Davies, 2017). As a consequence, the number of nephrons decreases by 50% in the ageing human kidney (Denic et al., 2017). These traits are consistent with a reduction in basal energy expenditure in the final decades of life (Pontzer et al., 2021). As predicted by the central role played by metabolism in the life course of CKD, untargeted plasma metabolic profiling revealed new metabolic associations with CKD progression in children that are independent of current clinical predictors (Denburg et al., 2021).
4. CONCLUSION
The development of the human kidney in utero echoes our evolution as a species. Hominid evolution was moulded by climate change over a period of > 30 million years, with Homo sapiens emerging 200,000 years ago. The current change in climate has been precipitated by the industrial revolution that began > 200 years ago and is now recognized to be accelerating at a rate that far exceeds our adaptive capacity (Barraclough et al., 2017). The impact of environmental factors on kidney disease risk is disproportionately higher in lower resource settings, also where maternal and child health are most precarious (Bowe et al., 2020). The greatest impact of climate change on our species is likely to fall on the pregnant mother, fetus and newborn, creating a perfect storm where evolutionary and individual programming impacts collide. For the individual, the microenvironment within which they develop will impact their number of nephrons and life‐long kidney health, but this is also impacted by the macroenvironment in which the mother was conceived, grew up and lives.
The world is at a crossroads, where it is clear that environmental risks impact each one of us on many levels. Broader recognition of this reality on an individual physiological level might serve to increase awareness that we need urgent action to improve individual and planetary health, which have always been inextricably intertwined.
COMPETING INTERESTS
VL and RC have no disclosures or conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
ACKNOWLEDGEMENTS
Open access funding provided by Universitat Zurich.
Luyckx, V. A. , & Chevalier, R. L. (2022). Impact of early life development on later onset chronic kidney disease and hypertension and the role of evolutionary trade‐offs. Experimental Physiology, 107, 410–414. 10.1113/EP089918
Edited by: Jeremy Ward
[Correction added on May 23, 2022, after first online publication: CSAL funding statement has been added.]
REFERENCES
- Alderman, H. , & Headey, D. (2018). The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes. PLoS One, 13, e0195904. 10.1371/journal.pone.0195904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, D. J. P. , Lampl, M. , Roseboom, T. , & Winder, N. (2012). Resource allocation in utero and health in later life. Placenta, 33(Suppl 2), e30–e34. 10.1016/j.placenta.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Barker, D. J. , Osmond, C. , Golding, J. , Kuh, D. , & Wadsworth, M. E. (1989). Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. British Medical Journal, 298, 564–567. 10.1136/bmj.298.6673.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough, K. A. , Blashki, G. A. , Holt, S. G. , & Agar, J. W. M. (2017). Climate change and kidney disease—threats and opportunities. Kidney International, 92, 526–530. 10.1016/j.kint.2017.03.047 [DOI] [PubMed] [Google Scholar]
- Bhathena, D. B. (1996). Focal glomerulosclerosis and maximal glomerular hypertrophy in human nephronopenia. Journal of the American Society of Nephrology, 7, 2600–2603. 10.1681/ASN.V7122600 [DOI] [PubMed] [Google Scholar]
- Bhunu, B. , Riccio, I. , & Intapad, S. (2021). Insights into the mechanisms of fetal growth restriction‐induced programming of hypertension. Integrated Blood Pressure Control, 14, 141–152. 10.2147/IBPC.S312868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe, B. , Artimovich, E. , Xie, Y. , Yan, Y. , Cai, M. , & Al‐Aly, Z. (2020). The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: A modelling study. BMJ Global Health, 5, e002063. 10.1136/bmjgh-2019-002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, B. M. , Garcia, D. L. , & Anderson, S. (1988). Glomeruli and blood pressure. Less of one, more the other? American Journal of Hypertension, 1, 335–347. 10.1093/ajh/1.4.335 [DOI] [PubMed] [Google Scholar]
- Chevalier, R. L. (2020). Bioenergetic evolution explains prevalence of low nephron number at birth: Risk factor for CKD. Kidney360, 1, 863–879. 10.34067/KID.0002012020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C. (2009). The annotated origin: A facsimile of the first edition of on the origin of species. Belknap Press of Harvard University Press. [Google Scholar]
- Denburg, M. R. , Xu, Y. , Abraham, A. G. , Coresh, J. , Chen, J. , Grams, M. E. , Feldman, H. I. , Kimmel, P. L. , Rebholz, C. M. , Rhee, E. P. , Vasan, R. S. , Warady, B. A. , & Furth, S. L. ; for the CKD Biomarkers Consortium (2021). Metabolite biomarkers of CKD progression in children. Clinical Journal of the American Society of Nephrology, 16, 1178–1189. 10.2215/CJN.00220121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic, A. , Lieske, J. C. , Chakkera, H. A. , Poggio, E. D. , Alexander, M. P. , Singh, P. , Kremers, W. K. , Lerman, L. O., & Rule, A. D. (2017). The substantial loss of nephrons in healthy human kidneys with aging. Journal of the American Society of Nephrology, 28, 313–320. 10.1681/ASN.2016020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Chronic Kidney Disease Collaboration (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet, 395, 709–733. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde, A. , Lillas, B. S. , Marti, H. P. , Reisaeter, A. V. , & Vikse, B. E. (2020a). Intrauterine growth restriction, preterm birth and risk of end‐stage renal disease during the first 50 years of life. Nephrology, Dialysis, Transplantation, 35, 1157–1163. 10.1093/ndt/gfaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde, A. , Reisaeter, A. V. , Skrunes, R. , Marti, H. P. , & Vikse, B. E. (2020b). Intrauterine growth restriction and risk of diverse forms of kidney disease during the first 50 years of life. Clinical Journal of the American Society of Nephrology, 15, 1413–1423. 10.2215/CJN.04080320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig, D. (2019). Cooperation and conflict in human pregnancy. Current Biology, 29, R455–R458. 10.1016/j.cub.2019.04.040 [DOI] [PubMed] [Google Scholar]
- Heffner, L. J. (2004). Advanced maternal age — How old is too old? New England Journal of Medicine, 351, 1927–1929. 10.1056/NEJMp048087 [DOI] [PubMed] [Google Scholar]
- Hinchliffe, S. A. , Howard, C. V. , Lynch, M. R. , Sargent, P. H. , Judd, B. A. , & van Velzen, D. (1993). Renal developmental arrest in sudden infant death syndrome. Pediatric Pathology, 13, 333–343. 10.3109/15513819309048221 [DOI] [PubMed] [Google Scholar]
- Hoy, W. E. , Bertram, J. F. , Denton, R. D. , Zimanyi, M. , Samuel, T. , & Hughson, M. D. (2008). Nephron number, glomerular volume, renal disease and hypertension. Current Opinion in Nephrology and Hypertension, 17, 258–265. 10.1097/MNH.0b013e3282f9b1a5 [DOI] [PubMed] [Google Scholar]
- Hughson, M. , Farris, A. B. , Douglas‐Denton, R. , Hoy, W. E. , & Bertram, J. F. (2003). Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney International, 63, 2113–2122. 10.1046/j.1523-1755.2003.00018.x [DOI] [PubMed] [Google Scholar]
- Kanzaki, G. , Tsuboi, N. , Haruhara, K. , Koike, K. , Ogura, M. , Shimizu, A. , & Yokoo, T. (2015). Factors associated with a vicious cycle involving a low nephron number, hypertension and chronic kidney disease. Hypertension Research, 38, 633–641. 10.1038/hr.2015.67 [DOI] [PubMed] [Google Scholar]
- Lea, A. J. , Tung, J. , Archie, E. A. , & Alberts, S. C. (2017). Developmental plasticity: Bridging research in evolution and human health. Evolution, Medicine, and Public Health, 2017, 162–175. 10.1093/emph/eox019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low Birth Weight and Nephron Number Working Group (2017). The impact of kidney development on the life course: A consensus document for action. Nephron, 136, 3–49. 10.1159/000457967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, H. , Yang, Y. , Lau, K. , Kuykindoll, R. J. , Fan, Z. , Yamaguchi, K. , & Yamamoto, T. (2007). Aquaporin‐2 water channel in developing quail kidney: Possible role in programming adult fluid homeostasis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293, R2147–R2158. 10.1152/ajpregu.00163.2007 [DOI] [PubMed] [Google Scholar]
- Nusken, E. , Dotsch, J. , Weber, L. T. , & Nusken, K. D. (2018). Developmental programming of renal function and re‐programming approaches. Frontiers in Pediatrics, 6, 36. 10.3389/fped.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto, L. C. D. , & Davies, K. J. A. (2017). The role of declining adaptive homeostasis in ageing. The Journal of Physiology, 595, 7275–7309. 10.1113/JP275072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer, H. , Yamada, Y. , Sagayama, H. , Ainslie, P. N. , Andersen, L. F. , Anderson, L. J. , Arab, L. , Baddou, I. , Bedu‐Addo, K. , Blaak, E. E. , Blanc, S. , Bonomi, A. G. , Bouten, C. V. C. , Bovet, P. , Buchowski, M. S. , Butte, N. F. , Camps, S. G. , Close, G. L. , Cooper, J. A. , … IAEA DLW Database Consortium (2021). Daily energy expenditure through the human life course. Science, 373, 808–812. 10.1126/science.abe5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles, V. G. , Hoy, W. E. , Hughson, M. D. , Diouf, B. , Douglas‐Denton, R. N. , & Bertram, J. F. (2011). Glomerular number and size variability and risk for kidney disease. Current Opinion in Nephrology and Hypertension, 20, 7–15. 10.1097/MNH.0b013e3283410a7d [DOI] [PubMed] [Google Scholar]
- Rylander, C. , Odland, J. Ø. , & Sandanger, T. M. (2013). Climate change and the potential effects on maternal and pregnancy outcomes: An assessment of the most vulnerable – the mother, fetus, and newborn child. Global Health Action, 6, 19538. 10.3402/gha.v6i0.19538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampogna, R. V. , Schneider, L. , & Al‐Awqati, Q. (2015). Developmental programming of branching morphogenesis in the kidney. Journal of the American Society of Nephrology, 26, 2414–2422. 10.1681/ASN.2014090886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, M. A. (2001). Of mice and men and elephants: Metabolic rate sets glomerular filtration rate. American Journal of Kidney Diseases, 37, 164–178. 10.1016/S0272-6386(01)80073-1 [DOI] [PubMed] [Google Scholar]
- Smith, H. W. (1953). From fish to philosopher. Little. [Google Scholar]
- Tortelote, G. G. , Colon‐Leyva, M. , & Saifudeen, Z. (2021). Metabolic programming of nephron progenitor cell fate. Pediatric Nephrology, 36, 2155–2164. 10.1007/s00467-020-04752-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, D. C. (2013). Bioenergetics in human evolution and disease: Implications for the origins of biological complexity and the missing genetic variation of common diseases. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120267. 10.1098/rstb.2012.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner, N. , Vornweg, J. , Combes, A. , Wilson, S. , Plappert, J. , Rafflenbeul, G. , Puelles, V. G. , Rahman, R.‐U. , Liwinski, T. , Lindner, S. , Grahammer, F. , Kretz, O. , Wlodek, M. E. , Romano, T. , Moritz, K. M. , Boerries, M. , Busch, H. , Bonn, S. , Little, M. H. , … Huber, T. B. (2019). DNA methyltransferase 1 controls nephron progenitor cell renewal and differentiation. Journal of the American Society of Nephrology, 30, 63–78. 10.1681/ASN.2018070736 [DOI] [PMC free article] [PubMed] [Google Scholar]


