Summary
Rhizosphere microbiome adapts their structural compositions to water scarcity and have the potential to mitigate drought stress of plants. To unlock this potential, it is crucial to understand community responses to drought in the interplay between soil properties, water management and exogenous microbes interference. Inoculation with dark septate endophytes (DSE) (Acrocalymma vagum, Paraboeremia putaminum) and Trichoderma viride on Astragalus mongholicus grown in the non‐sterile soil was exposed to drought. Rhizosphere microbiome were assessed by Illumina MiSeq sequencing of the 16S and ITS2 rRNA genes. Inoculation positively affected plant growth depending on DSE species and water regime. Ascomycota, Proteobacteria, Actinobacteria, Chloroflexi and Firmicutes were the dominant phyla. The effects of dual inoculation on bacterial community were greater than those on fungal community, and combination of P. putaminum and T. viride exerted a stronger impact on the microbiome under drought stress. The observed changes in soil factors caused by inoculation could be explained by the variations in microbiome composition. Rhizosphere microbiome mediated by inoculation exhibited distinct preferences for various growth parameters. These findings suggest that dual inoculation of DSE and T. viride enriched beneficial microbiota, altered soil nutrient status and might contribute to enhance the cultivation of medicinal plants in dryland agriculture.
Introduction
Drought is a major abiotic stress factor affecting plant growth and production (Bodner et al., 2015). Plants respond to such adverse environments both directly and indirectly, and indirect responses through altered interactions among species have recently received increased attention (Lata et al., 2018; deVries et al., 2020). Utilizing beneficial endophytic fungi such as arbuscular mycorrhizal (AM) fungi, dark septate endophytes (DSE) and Trichoderma spp. is one of the most promising strategies for improving plant growth and stress tolerance through modulating morphogenesis and physiological processes of their associated plants, thereby enhancing the ability of plants to cope with environmental stresses (Mona et al., 2017; He et al., 2019; Xu et al., 2020). Numerous studies have reported the roles of endophytic fungi in plant development under both normal conditions as well as in the presence of various abiotic stresses, such as drought, salinity and heavy metal exposure (Wani et al., 2015; Li et al., 2019a; Hou et al., 2020). Thus, the use of beneficial microbial species with the ability to promote plant growth to mitigate the adverse effects of drought on plants is an important component of sustainable agriculture (Lugtenberg et al., 2016; Ku′zniar et al., 2019).
DSE, a major group of endophytes within plants, are characterized by melanized septate hyphae and microsclerotia (Jumpponen, 2011). These fungi colonize the epidermis, cortex and even the intercellular space of vascular tissue of healthy plant roots and are found in the roots of > 600 different plant species (Kauppinen et al., 2014), especially plants that grow under extreme conditions, such as arid habitats (Xie et al., 2017; Knapp et al., 2018). Related reports have indicated that the interactions between DSE and host plants range from mutualistic to parasitic depending on the particular host‐symbiont combination (Li et al., 2019b). Previous studies have shown that DSE promote the growth of host plants by producing plant hormone substances, providing nutrients and decomposing complex carbohydrates to provide monosaccharides for the host plants, with the aim of protecting the host plant from various stresses (Surono and Narisawa, 2017; Yakti et al., 2019). Trichoderma spp. colonize the roots of many plants as opportunistic, avirulent plant symbionts and these fungi have been investigated as biological control agents, biofertilizers and soil amendments for application in agricultural systems (Velmourougane et al., 2017; Atieno et al., 2020). Trichoderma spp. enhance plant growth predominantly by solubilizing soil nutrients (Bayoumi et al., 2019), and increasing root length and secondary root number, and upregulating phytohormones such as indoleacetic acid, cytokinin, gibberellins and zeatin (Contreras‐Cornejo et al., 2009; Jaiswal et al., 2020). Furthermore, the interaction of both groups of microorganisms may be convenient for both plant physiology and nutrient content. For example, Metwally and Al‐Amri (2020) found that dual inoculation with AM fungi and Trichoderma viride (TV) could improve the biochemical parameters and mineral nutrient of onion plants. Liu et al. (2020) reported that the co‐inoculation with Epulorhiza repens and Umbelopsis nana increased the total dry weight of Cymbidium hybridum. Co‐inoculation with AM fungi and Trichoderma harzianum did not result in an additive effect on melon crop growth and nutritional status, but their combinations could control Fusarium wilt more effectively than each AM fungi applied alone (Martínez‐Medina et al., 2011). In addition, rhizosphere soil is an intense field of microbial activity and plant stress responses (Mendes et al., 2013). Rhizosphere‐associated microbes are instrumental in the decomposition of organic matter and maintenance of nutrients, and have beneficial effects on the adaptation of host plants to different environmental conditions (Bai et al., 2015; Henneron et al., 2020). Although the direct effects of beneficial microbial inoculants on plant growth and rhizosphere‐associated microbes have been widely reported (Santos et al., 2017; He et al., 2019), there is limited information available regarding the contribution of DSE, either alone or in combination with endophytic fungi, on the growth and native rhizosphere microbial community of medicinal plants under drought stress (DS).
Astragalus mongholicus Bunge is a widely distributed herbaceous perennial medicinal plant. The roots of this plant are important medicinal materials used as a common clinical tonic in China and other parts of the world because of their pharmacological effects and biological functions such as improving immunity, promoting body metabolism and lowering blood pressure (Qin et al., 2013). Therefore, considering the concept of sustainability and the need to enhance the growth status and drought resistance of medicinal plants, understanding the interaction between plants and beneficial microbes is crucial to receive benefit from the symbiotic mechanisms.
In a previous study, we investigated the influences of three DSE (Paraboeremia putaminum (PP), Scytalidium lignicola and Phoma herbarum) from the roots of Ophiopogon japonicus and Lonicera japonica on the performance of licorice (Glycyrrhiza uralensis) at different TV densities under sterilized conditions in a growth chamber. The combination of DSE and TV enhanced the root morphology and biomass more effectively than either agent alone (He et al., 2020). In the present study, we hypothesize that dual inoculation of DSE, either Acrocalymma vagum (AV) or PP and TV could either promote plant growth or change the rhizosphere microbiome of A. mongholicus, and that dual inoculation might have more positive effects under DS conditions than under control conditions. Therefore, we investigated the effects of dual inoculation under DS conditions on (i) plant height, leaf number, root surface area and root diameter, (ii) plant biomass, (iii) Soil physicochemical properties and (iv) soil microbial composition. Such data would display DSE and TV could withstand the drought conditions that affected host growth, and their potential for improving the stress tolerance and symbiotic performance of plants during A. mongholicus cultivation, in drought‐affected arid lands.
Results
Plant biomass and growth parameters
After 3 months of growth, all inoculated plants were green, alive and healthy under different water conditions, and DSE hyphal and microsclerotial structures were observed in all the tested roots of all inoculated plants (Fig. S1). Relative to the control plants, inoculation with DSE or TV increased plant biomass regardless of the water regime (Fig. 1). Under WW conditions, inoculation with AV increased plant height, while inoculation with PP decreased plant height; however, under DS conditions, inoculation with DSE or TV increased plant height compared with the control treatment (Fig. 1). The combination of DSE and TV, and DSE, TV and water had significantly interactive effects on plant biomass, height and leaf number, while the combination of TV and water had significantly interactive effects on plant biomass (Table 1).
Fig. 1.
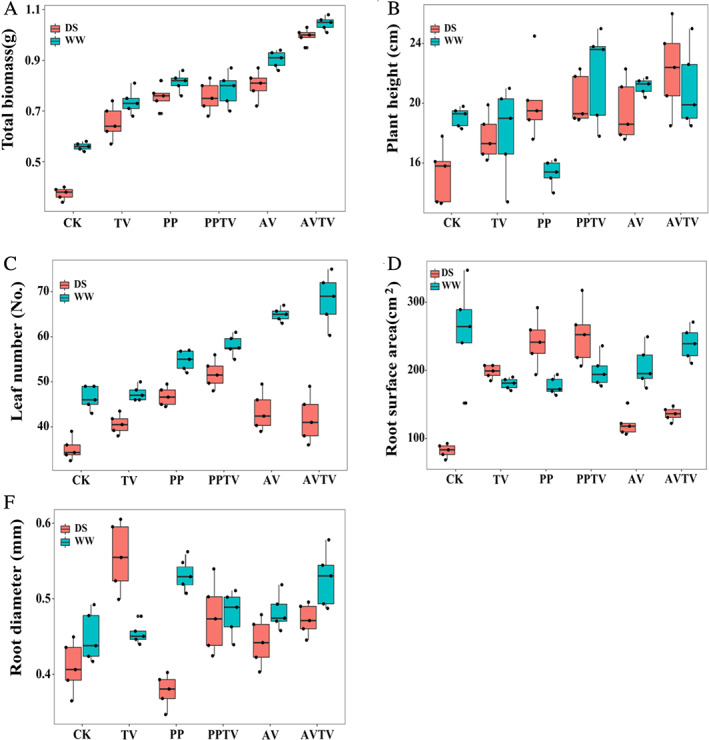
Effects of dark septate endophyte (DSE) and T. viride on the plant biomass and morphological parameters of A. mongholicus seedlings. CK, non‐inoculated plants; PP, plants inoculated with P. putaminum; AV, plants inoculated with A. vagum; TV, plants inoculated with TV; PP + TV, plants inoculated with P. putaminum and T. viride; AV + TV, plants inoculated with A. vagum and T. viride. WW, well‐watered; DS, drought stress treatment respectively.
Table 1.
Two‐way ANOVA of the effect of DSE, T. viride and water condition on plant growth parameters and soil physicochemical parameters of Astragalus mongholicus
| DSE | TV | Water condition | DSE*TV | DSE*Water | TV*Water | DSE*TV*Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Total biomass (g) | 1.96 | *** | 44.14 | *** | 92.35 | *** | 16.42 | *** | 7.29 | * | 6.33 | * | 21.85 | ** |
| Plant height (cm) | 0.99 | NS | 1.59 | NS | 7.44 | * | 5.28 | * | 0.71 | NS | 1.22 | NS | 6.79 | * |
| Leaf number (No.) | 29.84 | *** | 1.18 | NS | 6.08 | * | 13.85 | ** | 9.88 | * | 0.27 | NS | 7.48 | * |
| Root area surface (cm2) | 16.05 | ** | 7.36 | * | 1.72 | NS | 2.62 | NS | 3.03 | NS | 5.14 | * | 7.77 | * |
| Root diameter (mm) | 6.66 | * | 9.52 | * | 14.53 | ** | 2.87 | NS | 5.86 | * | 0.26 | NS | 1.84 | NS |
| Organic matter (mg g−1) | 7.05 | * | 0.09 | NS | 0.34 | NS | 7.02 | * | 8.32 | * | 0.72 | NS | 0.88 | NS |
| Available N (μg g−1) | 14.32 | ** | 1.03 | NS | 6.85 | * | 8.77 | * | 0.03 | NS | 0.32 | NS | 8.07 | * |
| Available P (μg g−1) | 6.88 | * | 0.04 | NS | 1.88 | NS | 6.21 | * | 0.61 | NS | 0.34 | NS | 11.29 | ** |
| Available K (μg g−1) | 9.27 | * | 0.34 | NS | 13.85 | ** | 6.83 | * | 6.24 | * | 0.29 | NS | 3.85 | NS |
Note: * means significant difference, P < 0.05; ** means that the significance level intervenes between P < 0.01. *** means the difference is very significant, P < 0.001.
DSE or TV inoculation decreased the root surface area under well‐watered (WW) conditions, but increased the root surface area under DS conditions, compared with the control treatment (Fig. 1). Root diameter was increased by PP inoculation compared with the control treatment under WW conditions, but under DS conditions, root diameter was increased by TV inoculation and decreased by PP inoculation (Fig. 1). The combination of TV and water, and DSE, TV and water significantly affected the root surface area, whereas the combination of DSE and water significantly affected the root diameter (Table 1).
Soil physicochemical properties
Compared with the control treatment, DSE inoculation increased soil organic matter under WW conditions, while PP inoculation decreased soil organic matter under DS conditions (Fig. 2). DSE inoculation increased soil available N under WW conditions, but there was no significant effect of TV inoculation on soil available N under different water conditions (Fig. 2). Soil available P was increased by DSE inoculation and decreased by TV inoculation under WW conditions, but inoculation with DSE or TV did not have a significant effect on soil available P under DS conditions (Fig. 2). AV inoculation increased soil available K under WW conditions, while PP inoculation decreased available K under DS conditions (Fig. 2). Interaction between DSE and TV was observed in soil organic matter, available N, P and K, while the combination of DSE and water significantly affected soil organic matter and available K, and DSE, TV and water significantly affected soil available N and P (Table 1).
Fig. 2.
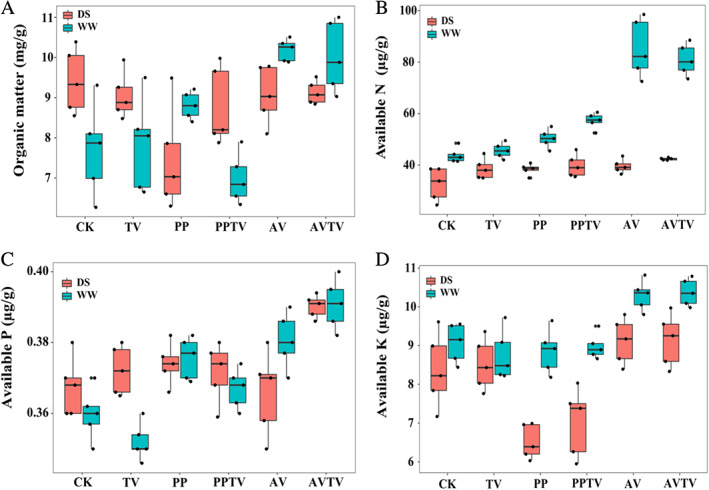
Effects of dark septate endophyte (DSE) and T. viride on rhizosphere soil physicochemical properties of A. mongholicus seedlings. CK, non‐inoculated plants; PP, plants inoculated with P. putaminum; AV, indicate plants inoculated with A. vagum; TV, plants inoculated with T. viride; PP + TV, plants inoculated with P. putaminum and T. viride; AV + TV, plants inoculated with A. vagum and T. viride; WW, well‐watered; DS, drought stress treatment respectively.
Characterization of Illumina sequencing data
A total of 2,320,031 fungal sequences and 2,007,161 bacterial sequences were obtained. Rarefaction curve analysis revealed high 16S rRNA gene sequencing depth and strong potential for observing community diversity in the Astragalus rhizosphere soil (Fig. S2A, B). Rank abundance curves showed that all treatments had high species evenness and homogeneity (Fig. S2C, D). The sequencing results covered the biological information of most of the fungi and bacteria in the soil samples.
After filtering 1,221,554 low‐quality sequences, 1,426,185 effective fungal and 1,126,498 effective bacterial sequences were clustered into 651 fungal and 3330 bacterial operational taxonomic units (OTUs) at 97% sequence similarity. Of 651 fungal OTUs, 187 occurred in all 12 treatments, while 37, 38, 18, 24, 55, 54, 43, 34, 6, 24, 111 and 20 OTUs were found only in the uninoculated condition (NCK), PP inoculation (NPP), AV inoculation (NAV), TV inoculation (NTV), TV and PP inoculation (NTVPP), and TV and AV inoculation (NTVAV) under WW conditions and in the DCK, DPP, DAV, DTV, DTVPP and DTVAV samples under DS conditions respectively (Fig. 3A). A total of 1818 bacterial OTUs were detected in all treatments, while 104, 104, 178, 100, 145, 104, 82, 64, 146, 92, 292 and 101 bacterial OTUs existed only in NCK, NPP, NAV, NTV, NTVPP, NTVAV, DCK, DPP, DAV, DTV, DTVPP and DTVAV samples respectively (Fig. 3B).
Fig. 3.
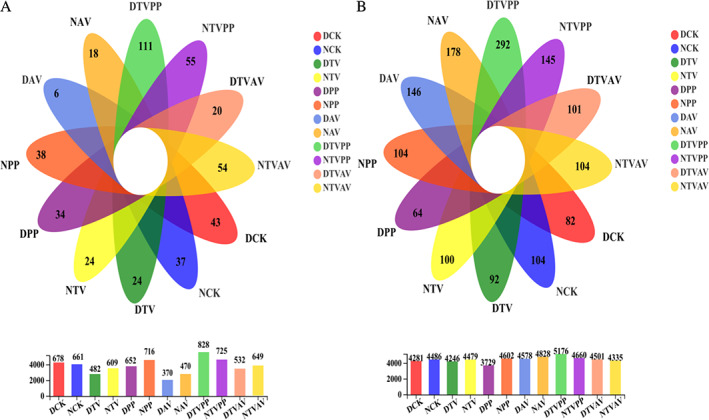
Venn diagram showing differences in composition of fungal (A) and bacterial (B) OTUs inoculated with DSE and T. viride under well‐watered and drought stress respectively. Each part in the Venn diagram represents one (group) treatment, and the number of circles and circles overlapped represents the number of OTUs shared between the samples (group), while the number without overlapped represents the number of OTUs unique to the samples (group). DCK, non‐inoculation under drought stress; NCK, non‐inoculation under well‐watered condition; DTV, inoculation with T. viride under drought stress; NTV, inoculation with T. viride under well‐watered condition; DPP, inoculation with P. putaminum under drought stress; NPP, inoculation with P. putaminum under well‐watered condition; DAV, inoculation with A. vagum under drought stress; NAV, inoculation with A. vagum under well‐watered condition; DTVPP, inoculation with T. viride and P. putaminum under drought stress; NTVPP, inoculation with T. viride and P. putaminum under well‐watered condition. DTVAV, inoculation with T. viride and A. vagum under drought stress; NTVAV, inoculation with T. viride and A. vagum under well‐watered condition.
Microbial community composition
A total of 651 fungal OTUs were found in the Astragalus rhizosphere and were classified as Ascomycota, Basidiomycota, Chytridiomycota, Zygomycota and certain unknown fungi. Ascomycota, Zygomycota and certain unknown fungi were identified in all treatments. Ascomycota was the dominant fungal phylum, with a relative abundance range of 92.7–99.3% across the various treatments (Fig. 4A). A total of 3330 bacterial OTUs were detected, and the dominant bacterial phyla were Actinobacteria, Chloroflexi, Firmicutes and Proteobacteria and the relative abundance of these phyla varied among treatments (Fig. 4B). Furthermore, the variation in fungal and bacterial community composition at the genus level under different inoculation treatments were significant (Fig. S3).
Fig. 4.
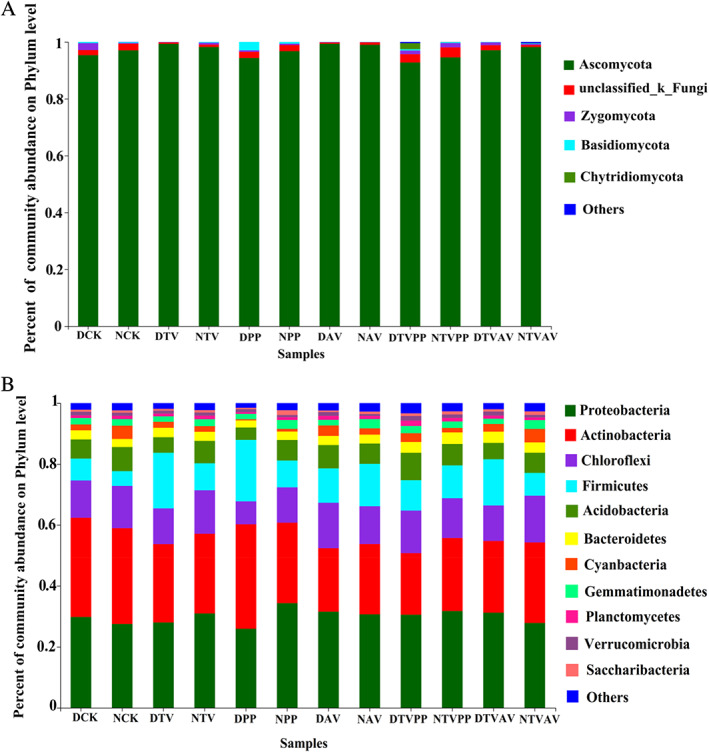
Relative abundance of fungi (A) and bacteria (B) at the phyla level in A. mongholicus rhizosphere. DCK, non‐inoculation under drought stress; NCK, non‐inoculation under well‐watered condition; DTV, inoculation with T. viride under drought stress; NTV, inoculation with T. viride under well‐watered condition; DPP, inoculation with P. putaminum under drought stress; NPP, inoculation with P. putaminum under well‐watered condition; DAV, inoculation with A. vagum under drought stress; NAV, inoculation with A. vagum under well‐watered condition; DTVPP, inoculation with T. viride and P. putaminum under drought stress; NTVPP, inoculation with T. viride and P. putaminum under well‐watered condition. DTVAV, inoculation with T. viride and A. vagum under drought stress; NTVAV, inoculation with T. viride and A. vagum under well‐watered condition.
Principal co‐ordinates analysis ordination revealed that bacterial community composition significantly differed between the WW and DS conditions, while fungal community composition was not significantly affected by DS (Fig. S4A, B). Different inoculations have similar effects on the distribution of fungal and bacterial communities. The distribution of fungal and bacterial communities following inoculation with PP or the combination of PP and TV was similar to that without inoculation, and the distribution of fungal and bacterial communities following AV inoculation or the combination of AV and TV inoculation was similar to that with TV only inoculation (Fig. S4C, D).
Microbial diversity and richness
Compared with the control treatments, under DS conditions, the Shannon and Chao 1 indices of soil fungi were higher in the presence of combined PP and TV inoculation compared with uninoculated treatments, while AV or TV inoculation alone decreased Shannon index of soil fungi, and AV inoculation or the combination of AV and TV decreased Chao1 index of soil fungi. Under WW conditions, except for the inoculation of AV, the Shannon and Chao1 indices were no significantly than the uninoculated treatment (Fig. 5A, C).
Fig. 5.
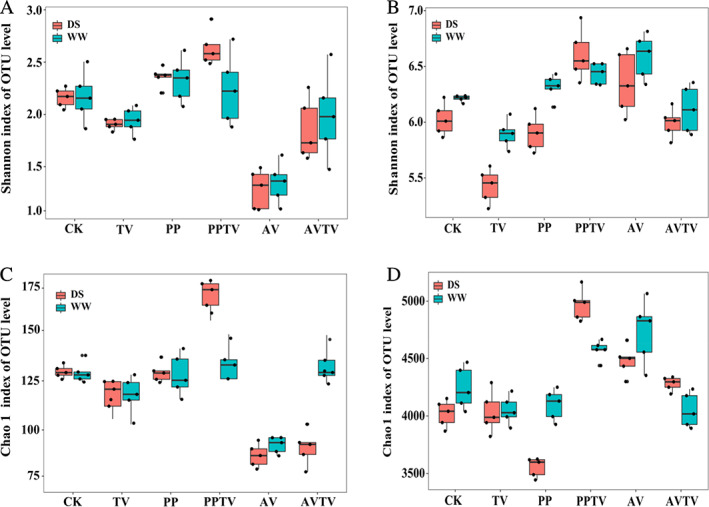
Diversity and richness index of A. mongholicus rhizosphere soil fungi and bacteria.
A. Shannon index of soil fungi.
B. Shannon index of soil bacteria.
C. Chao index of soil fungi.
D. Chao index of soil bacteria.
DSE inoculation significantly affected the diversity and richness of soil bacteria compared with the control treatment regardless of water regime. Under WW conditions, DSE and the combination of PP and TV increased the Shannon index of soil bacteria, while inoculation with TV reduced the value of this index. Under DS conditions, the combination of PP and TV inoculation increased the Shannon index, while inoculation with TV decreased the value of this parameter, compared with the control treatment. Under WW conditions, AV inoculation alone and the combination of PP and TV inoculation increased the Chao1 index, while there was no significant difference between the other treatments. Under DS conditions, PP inoculation decreased the Chao1 index, while AV inoculation alone or the combination of PP and TV increased the Chao1 index of soil bacteria, compared with the control treatment (Fig. 5B, D).
Linear discriminant analysis (LDA) showed that the inoculation effect for soil fungal communities was explained by 61 indicator species of fungi, and Stachybotrys, unclassified Ascomycota, unclassified Chaetomiaceae, Trichoderma, Chaetomium and Sporoboiomyces had the highest degree of enrichment in CK, PP, AV, TV, PP + TV and AV + TV treatments respectively (Fig. S5A). Moreover, there were 73 indicator bacterial taxa enriched across the various treatments, and Actinobacteria, Lysinibacillus, Propionibacteriales, Paenibacillaceae, Micrococcaceae and Chloroflexia had the highest degree of enrichment in CK, PP, AV, TV, PP + TV and AV + TV treatments respectively (Fig. S5B).
Correlation analyses
The Mantel test revealed significant relationships between DSE, TV, watering regime, total biomass, plant height, leaf number, root surface area, root diameter, soil organic matter, soil available N, soil available P, soil available K, soil fungi and soil bacteria (Table 2). The relative effects of cultivation age, soil type and soil properties on rhizospheric fungi and bacteria were quantitatively determined by using correlation coefficient (r value) and SEM (χ2 = 192.741, df = 24, P = 0.001, GFI = 0.869, AIC = 181.072 and RMSEA = 0.445). DSE positively affected soil organic matter, available N, available P, fungal abundance, total biomass, leaf number and root surface area. TV positively affected total biomass, root surface area and root diameter, whereas negatively affected soil bacterial abundance. Water conditions positively affected bacterial abundance, soil available N, total biomass, leaf number, plant height and root diameter and negatively affected soil available K. Soil organic matter positively affected root surface area and root diameter. Soil available N positively affected bacterial abundance and total biomass, while soil available P negatively affected bacterial abundance. Soil available K positively or negatively affected root diameter or root surface area respectively. Fungal abundance positively affected root surface area, while bacterial abundance positively or negatively affected leaf number or total biomass and plant height respectively (Fig. 6).
Table 2.
Mantel tests showing correlationships (R values) between DSE, T. viride, water condition, plant growth parameters, soil physicochemical parameters and microbial community composition
| Variable | DSE | TV | WC | TB | Leaf | PH | RSA | RD | SOC | SAN | SAP | SAK | Fungi | Bacterial |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSE | 1 | |||||||||||||
| TV | −0.03 | 1 | ||||||||||||
| WC | −0.04 | −0.03 | 1 | |||||||||||
| TB | 0.28** | 0.16* | 0.25** | 1 | ||||||||||
| Leaf | 0.21** | 0.06 | 0.14* | 0.32*** | 1 | |||||||||
| PH | 0.05 | 0.05 | 0.12* | 0.16* | 0.04 | 1 | ||||||||
| RSA | 0.16* | 0.11* | 0.05 | 0.22** | 0.08 | −0.05 | 1 | |||||||
| RD | 0.12* | 0.10* | 0.17* | 0.14* | −0.07 | −0.11* | 0.13* | 1 | ||||||
| SOM | 0.14* | 0.08 | −0.02 | −0.08 | 0.04 | −0.06 | 0.17* | 0.15* | 1 | |||||
| SAN | 0.15* | 0.06 | 0.04 | 0.34*** | −0.07 | 0.02 | 0.09 | 0.08 | 0.15* | 1 | ||||
| SAP | 0.18* | −0.02 | −0.05 | −0.06 | 0.01 | −0.03 | 0.02 | −0.06 | 0.21** | 0.11* | 1 | |||
| SAK | −0.04 | 0.03 | −0.42*** | 0.05 | 0.03 | −0.08 | −0.26** | 0.13* | −0.03 | 0.05 | −0.03 | 1 | ||
| Fungi | 0.25** | 0.06 | −0.08 | −0.06 | 0.05 | −0.03 | 0.24** | −0.07 | −0.04 | −0.02 | 0.02 | −0.11* | 1 | |
| Bacteria | −0.04 | −0.37*** | 0.27** | −0.15* | 0.31*** | −0.15* | 0.05 | −0.04 | 0.08 | 0.29** | −0.33*** | −0.04 | 0.17* | 1 |
Fig. 6.
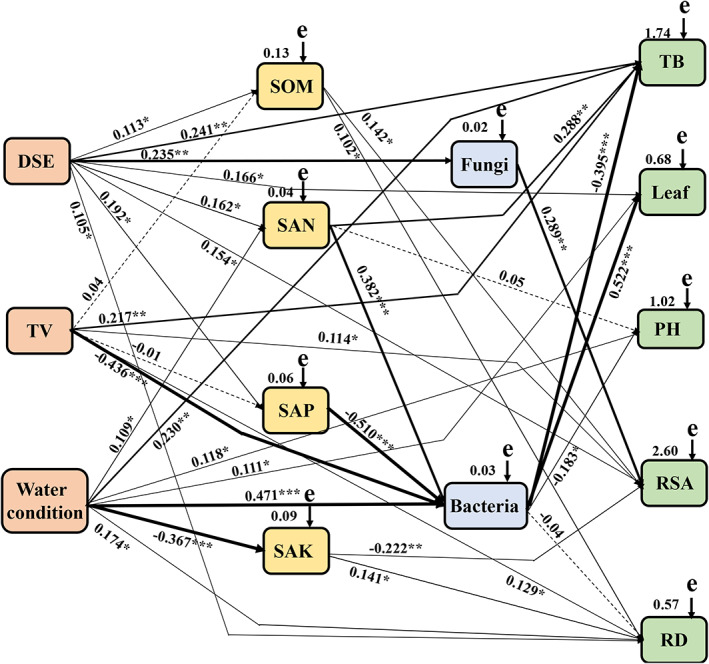
The causal relationships among DSE species, T. viride, water condition, plant growth parameter (total biomass, plant height, leaf number, root surface area, root diameter), soil parameter (organic matter, available N, available P, available K), soil fungi and bacteria based on structural equation model (SEM). The final model fitted the data well:maximum likelihood, χ2 = 192.741, df = 24, P = 0.001, goodness‐of‐fit index = 0.869, Akaike information criteria = 181.072 and root mean square error of approximation = 0.445. Solid lines and dashed lines indicate significant and non‐significant pathways respectively. The width of the solid lines indicates the strength of the causal effect, and the numbers near the arrows indicate the standardized path coefficients (*P < 0.05, **P < 0.01 and ***P < 0.001). The numbers in the upper‐right corner of the box indicate the R 2 values and represent the proportion of variance explained for each variable. TV, T. viride; SOC, soil organic carbon; SAN, soil available N; SAP, soil available P; SAK, soil available K; TB, total biomass; PH, plant height; RSA, root surface area; RD, root diameter.
Discussion
Effects of DSE and TV on the growth of Astragalus plants
As important root endophytes, DSE are reported to have negative, neutral or positive ecological roles in plant growth (He et al., 2019; Li et al., 2019a). These organisms also increase plant resistance to a wide range of environmental stressors (Zhang et al., 2017; Jin et al., 2018). In the present study, plants inoculated with AV and PP exhibited higher biomass and leaf number than the control plants, indicating positive effects of these organisms on the plant growth and formed a strain‐dependent symbiosis with Astragalus plants. For example, compared with AV inoculation PP inoculation had a greater impact on biomass under well‐watered (WW), while there was no significant difference between AV and PP on biomass under DS. Furthermore, the average root diameter of PP‐inoculated Astragalus plants decreased compared with the root diameter of the control plants under DS. Roots with small diameters have been reported to exhibit faster growth and allocation of more nutrients, which is beneficial for plants under drought conditions (Comas et al., 2013). TV inoculation in the present study increased plant biomass, height, leaf number, root surface area and root diameter under DS. Similarly, Guler et al. (2016) found that T. atroviride ID20G increased fresh and dry weight of maize roots and helped plants invert the adverse effects of DS. Estévez‐Gefriaud et al. (2020) reported that T. asperellum strain T34 improved the dry weight of maize plants, regardless of water regime and improved leaf relative water content, water use efficiency and photosystem II (PSII) maximum efficiency and photosynthesis under drought. TV inoculation in combination with water stress alleviated the effects of drought and these results were compatible with Khoshmanzar et al. (2020) dissecting this notion in wheat plants.
There is limited information regarding the interactions of DSE and TV on host plants, especially when water availability is considered. Our previous study demonstrated that DSE associated with TV augmented plant biomass and height, shoot branching and root surface area (He et al., 2020). In the present study, DSE × TV significantly affected plant biomass, height and leaf number and DSE × TV × watering regime significantly affected these three parameters and root surface area. Similarly, Parkash et al. (2011) and Commatteo et al. (2019) revealed that Dendrocalamus strictus plants and tomato plants bio‐inoculated with a consortium of AM fungi and TV showed enhancement in growth parameters. Kushwaha et al. (2019) also found that co‐inoculation of fungal endophytes and TV increased overall plant biomass and yield of Withania somnifera. The results of the present study revealed the synergistic and beneficial activity of DSE associated with TV in Astragalus growth.
Relationship between soil microbe and Astragalus plant growth mediated by dual inoculation
It was previously not known whether dual inoculation‐mediated changes in rhizosphere microbial communities would augment the growth and DS tolerance of Astragalus plants. The present study suggested that rhizosphere microbes associated with Astragalus were influenced by dual inoculation. For instance, soil fungi such as Stachybotrys, unclassified Ascomycota, unclassified Chaetomiaceae, Trichoderma and Chaetomium had the highest degree of enrichment in the rhizosphere of Astragalus plants exposed to different inocula under different water conditions. Certain Ascomycota, including DSE, form mycorrhizae in plant roots and enhance plant nutrient uptake and growth (Surono and Narisawa, 2018). The genus Stachybotrys is a marked producer of cellulose‐degrading enzymes (Fernandes et al., 2021). Trichoderma is an effective biofertilizer, soil amendment and biocontrol agent (Herrera‐Parra et al., 2017; Saxena et al., 2020). Chaetomium is a genus of the family ‘Chaetomiaceae’, and application of spores and methanol extracts of C. globosum, C. lucknowense and C. cupreum to pomelo seedlings inoculated with Phytophthora nicotianae reduced the extent of root rot and increased plant weight (Hung et al., 2015). Moreover, soil bacteria such as Actinobacteria, Lysinibacillus, Propionibacteriales, Paenibacillaceae, Micrococcaceae and Chloroflexia had the highest degree of enrichment in the rhizosphere of Astragalus plants treated with different inocula under different water conditions. Khan et al. (2019) reported that Actinobacteria was highly abundant in medicinal plant rhizosphere microbiomes in arid soil, and Actinobacteria can increase the amount of phosphate available to plants through P solubilization or mineralization (Soumare et al., 2021). Members of the genus Lysinibacillus enhanced the growth and yield of rice (Oryza sativa) under greenhouse conditions (Dhondge et al., 2021). Sun et al. (2020) reported that Propionibacteriales have drought, salt tolerance, alkali resistance and stress resistance, in addition to strong degradation capabilities (Ivanova et al., 2016), while Paenibacillaceae are plant growth promoter and biocontrol agents (Delgado‐Ramírez et al., 2021). Cui et al. (2018) found that Micrococcaceae had strong effects on microbial N metabolism, and a member of the family Micrococcaceae isolated from halophytic rangeland plants could improve wheat productivity, especially the attributes related to seed and forage quality, under salinity stress conditions (Hajiabadi et al., 2021). Chloroflexia, which has been investigated in association with a variety of crop species, is considered to play a positive role in crop growth (Visioli et al., 2018; Wei and Yu, 2018). In addition, the microbes colonizing the Astragalus rhizosphere exhibited distinct preferences for various growth parameters (Fig. S6), and the SEM analysis further demonstrated that soil fungi positively affected root surface area, while soil bacteria positively affected leaf number, and negatively affected plant height and biomass. Plants can recruit target microbial communities through signalling molecules, and then use the immune system to provide specific nutrients and habitat types to exert selective pressure, thereby enriching beneficial microbial communities (Foster et al., 2017; Martin et al., 2017; Cordovez et al., 2019). Thus, the enrichment of beneficial microbial communities is essential for the survival and development of plants, allowing them to thrive in diverse environments (Gagnon et al., 2020; Mengistu, 2020).
Effects of DSE and TV on soil nutrients and microbes of Astragalus plants
DSE increased organic matter, available N and available P in the soil and AV inoculation increased soil available K under WW conditions, while inoculation with PP decreased organic matter and available K in the soil under DS. Furthermore, DSE × TV significantly affected soil organic matter, available N, P and K contents and DSE × TV × watering regime significantly affected soil available N and P content. This was congruent with observations for W. somnifera after co‐inoculation with TV and native endophytic fungi (Kushwaha et al., 2019). Two possible reasons can explain the interactions of DSE and TV on soil nutrient properties. First, dual inoculation of DSE and TV could improve the root system and N and P absorption by plants, which consequently led to the depletion of these common nutrients in the soil (Halifu et al., 2019; Li et al., 2019a). Second, DSE and TV could act as decomposers, converting soil organic nutrients into available forms to promote the growth and tolerance of plants to stressful conditions (Schmoll, 2018; He et al., 2019; Alothman et al., 2020). Endophytic fungi increase the interactions between plants and soil and expand the amount of available N and P because they secrete several enzymes required for the mineralization of organic N and insoluble P in the soil into available forms. Thus, endophytic fungi provide a link between plant and soil environments that promotes the growth and tolerance of plants (Berthelot et al., 2016; Saravanakumar et al., 2016; Guo et al., 2020).
In addition, the plant rhizosphere contains a complex community of microorganisms, including bacteria, archaea and fungi. These microorganisms affect plant survival, growth and adaptability (Xiong et al., 2021). In the present study, the relative abundance of microbes colonizing the rhizosphere of Astragalus differed among treatments, and Ascomycota, Proteobacteria, Actinobacteria, Chloroflexi and Firmicutes were the dominant phyla across the various treatments. The predominance of Ascomycota in arid and semi‐arid regions was previously reported (Porras‐Alfaro et al., 2011). Latif et al. (2020) found that Proteobacteria, Actinobacteria, Acidobacteria, Bacteroidetes, Firmicutes, Chloroflexi and Gemmatimonadetes were the predominant bacterial phyla in the rhizosphere of Triticum aestivum, and Barraza et al. (2020) reported that the bacterial community structure of common bean roots was mainly composed of Actinobacteria, Proteobacteria, Bacteroidetes, Acidobacteria and Firmicutes. The major rhizosphere microbes may vary widely among plant species, but Actinobacteria and Proteobacteria might be the most common bacterial phyla in plant rhizospheres.
Biotic and abiotic stressors alter rhizosphere microbe community structures and may augment or diminish certain microbial populations (Santoyo et al., 2017; Achouak et al., 2019). In the present study, water stress had a more significant impact on the distribution of bacterial communities than on fungal communities. This might be explained by the formation of stable symbiotic relationships between some soil fungi and host plants (Bouasria et al., 2012; He et al., 2021). Here, the number of representative fungi and bacteria between different inoculation treatments was significantly different. Among them, the number of fungi and new OTUs in Astragalus rhizosphere soil under most of the treatments was lower under DS than under normal water conditions, but the opposite result was observed for combined inoculation with PP and TV. This phenomenon was not evident when PP or TV were inoculated separately, which indicated that dual inoculation with PP and TV had a favourable synergistic effect on microbial community composition in Astragalus rhizosphere soils (Ważny et al., 2018; He et al., 2020). Moreover, the microbes colonizing the Astragalus rhizosphere exhibited distinct preferences for various soil factors (Fig. S7). These findings were consistent with those previously reported that microbial inoculation broadly influences plant rhizosphere microbial communities by altering soil chemical properties and indirectly affecting host plant growth (Raklami et al., 2019; Ullah et al., 2019).
Conclusions and outlook
This study explored the associations between A. mongholicus roots and DSE derived from the roots and rhizosphere soil of licorice grown on farmlands of northern China. Two DSE species were effective colonizers of A. mongholicus roots. Dual inoculation with DSE and TV had a positive effect on the growth of A. mongholicus depending on the DSE species and water regime. In addition, dual inoculation and water regime also markedly affected the composition and diversity of the rhizosphere microbial communities, and such impact was greater on the bacterial community than on the fungal community. Moreover, under drought stress, combined inoculation of A. mongholicus with PP and TV exerted a stronger impact on the rhizosphere microbiome compared with combined inoculation with AV and TV. The soil factor changes caused by dual inoculation and water regime partially account for the observed variations in the rhizosphere microbiome. Furthermore, the rhizosphere microbes mediated by dual inoculation exhibited distinct preferences for various growth parameters. In this manner, dual inoculation promoted plant growth and drought tolerance, thereby facilitating survival of A. mongholicus under DS. The findings from this study may help develop efficient and eco‐friendly biofertilizers for the cultivation of medicinal plants, selecting symbiotic fungal consortia for the biofertilizers based on the soil characteristics and microbial community that such plants harbour in dryland agriculture.
Experimental procedures
Biological materials and growth substrates
Two DSE fungi (AV and P. putaminum) and TV isolated from the roots and rhizosphere soil of licorice (G. uralensis), which grows naturally in arid farmland of North China, were used in this study. These fungal species were identified based on morphological characters and internal transcribed spacer (ITS) phylogeny, and were deposited in the culture collection of the Laboratory of Endangered Species Breeding Engineering, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China (He et al., 2019). ITS sequences for the fungi are available in the GenBank database under accession numbers MK392024 (AV), MK601233 (P. putaminum) and MK396066 (TV). Five millilitres of sterile water was placed in a Petri dish containing mature TV and the suspension (spore concentration 1 × 107 CFU ml−1) was thoroughly mixed and transferred to a sterile conical flask on an ultraclean workbench (Al‐Hazmi and TariqJaveed, 2016). The TV spore inoculum density was measured using a haemocytometre. This TV spore concentration was used in the subsequent inoculation experiments. Seeds of A. mongholicus were acquired from the China National Traditional Chinese Medicine Corporation of Beijing.
The growth medium was a 1:2 (w/w) mixture of sand (< 2 mm) and soil collected from the farmland of North China where A. mongholicus plants were naturally planted. The growth medium had an organic matter content of 21.57 mg g−1, available nitrogen of 85.19 μg g−1 and available phosphorus of 7.90 μg g−1.
Inoculation assay
The inoculation experiment was conducted using a completely randomized factorial design (3 DSE inoculations × 2 TV inoculations × 2 water conditions) with five replicates. The three DSE inoculations were AV, P. putaminum (PP) and an uninoculated control (CK). The two TV inoculations were TV and an uninoculated control (sterilized TV spore fluid; CK). The two water conditions were well‐watered (WW, 70% field water capacity) and drought stress (DS, 30% field water capacity). There were two plants per pot/replicate, thus totalling 60 experimental pots.
Astragalus seeds were surface sterilized with 70% ethanol for 3 min and then treated with 2.5% sodium hypochlorite for 10 min, while providing agitation. The sterilized seeds were gently washed with sterile water several times, and aseptically planted onto water agar (10 g L−1) medium in Petri dishes for germination at 27°C. Following pre‐germination, the seedlings were transplanted to sterile plastic pots (13 cm diameter × 12 cm height, two seedlings per pot) containing 800 g non‐sterile growth medium. DSE inocula were prepared by aseptically growing DSE isolates in Petri dishes with potato dextrose agar (PDA) culture medium. For DSE inoculation, two 5‐mm plugs excised from the edge of an actively growing colony on culture medium were inoculated at a 1 cm range close to the roots of Astragalus seedlings. Specifically, each experimental pot was first filled with 600 g soil, on which the two 5‐mm DSE plugs were placed, followed by 200 g soil. After two weeks, the seedlings were irrigated with 30 ml of TV spore fluid; the control seedlings were irrigated with 30 ml of sterilized TV spore fluid. All inoculation procedures were conducted on an ultraclean workbench, and all experimental pots were kept in a growth chamber in a 14 h/10 h light/dark photoperiod, at 27°C/22°C (day/night), and 60% mean relative humidity. Seedling growth occurred for 3 months.
One month later, half of the seedlings (both control and inoculation treatments) were subjected to WW and DS treatment. Soil moisture was determined with a soil humidity recorder (L99‐TWS‐2, China). Water loss was supplemented daily with sterile distilled water to maintain the desired field capacity by regular weighing.
Plant growth parameters
Before harvesting, plant heights and leaf numbers of each replicate with two plants in each pot were measured. Shoots and roots from each pot were separately harvested, and roots were gently washed with tap water to remove sand. Individual root sections were allowed to float in water at a depth of approximately 1 cm in a lexiglass tray and were scanned using a desktop scanner (EPSON Perfection V800 Photo, Japan). The surface area and average diameter of the roots were determined using the WinRHIZO image analysis system (Chen et al., 2012). Root and shoot biomass were determined by oven drying at 70°C for 48 h. The root and shoot biomass and root growth index were conducted as the sum of the two plants per pot.
DSE root colonization
Fresh roots were washed with tap water and cut into segments of 0.5 cm length. These root segments were surface sterilized by dipping in 70% ethanol for 5 min, followed by 5% sodium hypochlorite for 5 min, and were then washed in sterile distilled water. A total of 30 randomly selected 0.5 cm‐long root segments in each sample were placed on slides and the DSE colonization status was observed under a biomicroscope (Phillips and Hayman, 1970).
Soil physicochemical properties
Rhizosphere soil samples from each replicate were passed through 2 mm sieves and divided into two subsamples: one subsample was dried at room temperature for soil physicochemical analyses, while the other subsample was frozen at −80°C for microbial community composition analysis. A 0.2 g dried soil sample was digested in a 10 ml of mixture of perchloric acid (12.7 mol L−1), sulfuric acid (18 mol L−1) and water in the ratio of 10:1:2 using the Mars 6 microwave reaction system (CEM Corporation, Matthews, NC, USA) until a clear liquid was obtained. Soil organic matter, available nitrogen (N), available phosphorus (P) and available potassium (K) contents were quantified via oxidization with dichromate in the presence of sulfuric acid (Rowell, 1994), and by using the alkaline hydrolysis diffusion, chlorostannous‐reduced molybdophosphoric blue method (Olsen et al., 1954), and flame photometer (Jackson, 1973) method.
Composition of soil microbial community
Total genomic DNA from 0.25 g soil samples was extracted with a Powersoil® DNA Extraction kit (Mo Bio, Carlsbad, CA, USA). DNA quality was tested by 0.1% (w/v) agarose gel electrophoresis and DNA purity and concentration were measured with a NanoDropTM 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Two pairs of universal primers, 338F (5′‐ACTCCTACGGGAGCAGCAG‐3′) and 806R (5′‐GGACTACHGGGTWTCTAAT‐3′) (Xu et al., 2016), and ITS1F 5′‐CTTGGTCATTTAGGAAGTAA‐3′) and ITS2R (5′‐GCTGCGTTCTTCATCATG ATGC‐3′) (Adams et al., 2013), were used to characterize the microbial communities by targeting the bacterial 16S rRNA genes v3–v4 and the fungal ITS1 and regions respectively. PCRs were conducted in triplicate in a 20 μl of reaction system containing 4 μl of 5 × FastPfu Buffer (for 16S v3–v4) or 2 μl of 10 × Buffer (for ITS), 2 μl of dNTPs, 0.8 μl of forward and reverse primers, 0.4 μl of FastPfu polymerase (for 16S v3–v4) or 0.2 μl of rTaq polymerase (for ITS), 0.2 μl of bovine serum albumin (BSA) and 10 ng template DNA. The PCR program comprised an initial denaturation at 95°C for 3 min, 28 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and elongation at 72°C for 45 s, followed by a final extension at 72°C for 10 min. PCR products were detected by gel electrophoresis (2%, w/v, agarose), purified with an AxyPrep™ DNA Gel Extraction kit (Axygen BioSciences Inc., Union City, CA, USA), and quantified in a QuantiFluor™ dsDNA system fitted with a QuantiFluor™‐ST fluorometer and a PCR tube adapter (Promega Corporation, Madison, WI, USA). Samples were sequenced using the paired‐end option (2 × 300 bp) of an Illumina MiSeq PE 300 platform (Illumina, San Diego, CA, USA) at the Environmental Genome Platform of Meiji Biomedical Technology Co. Ltd. (Shanghai, China).
Raw fastq files were demultiplexed, quality filtered and merged by Trimmomatic and fast length adjustment of short reads (FLASH; Johns Hopkins University, Baltimore, MD, USA) (Magoč and Salzberg, 2011). Sequences that were < 50 bp long and had an average quality score < 20 or ambiguous bases were removed. The filtered high‐quality sequences were merged according to the overlap sequences between read pairs. Sequences with mismatches along the primer region were removed before the downward process. Non‐chimeric sequences were dereplicated and singletons were discarded. The filtered non‐chimeric sequences were clustered into operational taxonomic units (OTUs) at 97% sequence level based on the UPARSE pipeline using USEARCH v. 8.0. The RDP Bayesian classifier algorithm was used to classify representative OTU sequences via the fungal (ITS) UNITE database v. 18.11.2018 and the Silva (SSU123) 16S rRNA gene reference database at a confidence threshold of 0.7. The RDP then collated the functional gene database from GenBank (Release 7.3; http://fungene.cme.msu.edu/) and obtained species annotation data. To eliminate potential bias caused by divergent sequence depth across samples, all samples were subsampled to the minimum sequencing depth. The dilution curve, Venn map and community composition analysis were conducted in R software package (R Core Team, Vienna, Austria; version 3.4.0) based on the OTU count and associated taxonomy tables. The alpha‐diversity index was calculated in mothur (v. 1.30.2). Other statistical analyses were performed in SPSS v. 22.0 (IBM Corp., Armonk, NY, USA) and the remaining graphs were strainted with Origin v. 9.0 (OriginLab, Northampton, MA, USA) (Chen and Boutros, 2011).
Abundance and diversity analyses
To characterize microbial diversity, Shannon and Chao1 indices were calculated based on the OTU data. The Chao1 index reflects community abundance while the Shannon index indicates community diversity (Maughan et al., 2012). Rank abundance and rarefaction curves strainted in QIIME estimate species evenness and evaluate species richness and sequence depth respectively (Chen et al., 2016). Fungal or bacterial OTU richness was defined as the number of fungal or bacterial OTUs per sample. The relative abundance of a specific fungal or bacterial OTU and class was defined as the ratio of corresponding sequences and class to the total reads per sample. Each representative OTU sequence in this study was used for taxonomic identification at the phylum, class, order, family and genus levels.
Statistical analysis
For statistical analysis of plant growth and rhizosphere soil parameters, nonparametric tests were performed because data were not normally distributed as determined by Shapiro–Wilk test. All boxplots display individual data points, median values, interquartile range and minimum and maximum values. Two‐way analysis of variance (ANOVA) was used to disclose the effects of DSE, TV, water regime and their interactions on fungal and bacterial OTU diversity. Data shown in the figures are means of ≥ 3 replicates. Variations among treatment means were compared using Tukey's honestly significant difference (HSD) tests at P < 0.05. Non‐metric multidimensional scaling (NMDS) was used to visualize compositional dissimilarities in the rhizosphere fungal and bacterial communities. The metaMDS command in the vegan package v. 2.4‐1 was used (Oksanen et al., 2016). To evaluate the interactions of DSE and TV on the rhizosphere fungal and bacterial communities, permutational multivariate analysis of variance (PerMANOVA) was performed using the adonis command in the vegan package with 999 permutations (Oksanen et al., 2016). Microbe preference analysis with growth parameters such as plant biomass, height, leaf number, root surface area and root diameter or edaphic factors such as water condition, soil organic matter, available N, available P and available K was performed according to Huang et al. (2018) and Yao et al. (2019). Mantel tests and the structural equation model (SEM) were used to assess the effects of DSE species, TV, water regime, and soil parameters on the growth and rhizosphere microbe using R‐3.2.2 packages ecodist (Goslee and Urban, 2007) and AMOS 21.0 (maximum likelihood). SPSS 21.0, Canoco 4.5, RStudio package vegan (Borcard et al., 2011), and Kaleida Graph 4.5 were used for statistical analyses and plotting of graphs.
Supporting information
Figure S1. Colonization of dark septate endophyte (DSE) strains in the roots of A. mongholicus plant after 3 months of inoculation. (A, C) Indicate roots inoculated with A. vagum. (B, D) Indicate roots inoculated with P. putaminum. Arrows indicate: Hy = DSE hyphae, Mi = DSE microsclerotia.
Figure S2. Rarefaction curves for the fungal (A) and bacterial (B) and ranking by the abundance of the fungal (C) and bacterial (D) operational taxonomic units (OTUs) in A. mongholicus rhizosphere. DCK, non‐inoculation under drought stress; NCK, non‐inoculation under well‐watered condition; DTV, inoculation with T. viride under drought stress; NTV, inoculation with T. viride under well‐watered condition; DPP, inoculation with P. putaminum under drought stress; NPP, inoculation with P. putaminum under well‐watered condition; DAV, inoculation with A. vagum under drought stress; NAV, inoculation with A. vagum under well‐watered condition; DTVPP, inoculation with T. viride and P. putaminum under drought stress; NTVPP, inoculation with T. viride and P. putaminum under well‐watered condition; DTVAV, inoculation with T. viride and A. vagum under drought stress; NTVAV, inoculation with T. viride and A. vagum under well‐watered condition.
Figure S3. Relative abundance of fungi (A) and bacteria (B) at the genus level in A. mongholicus rhizosphere. DCK, non‐inoculation under drought stress; NCK, non‐inoculation under well‐watered condition; DTV, inoculation with T. viride under drought stress; NTV, inoculation with T. viride under well‐watered condition; DPP, inoculation with P. putaminum under drought stress; NPP, inoculation with P. putaminum under well‐watered condition; DAV, inoculation with A. vagum under drought stress; NAV, inoculation with A. vagum under well‐watered condition; DTVPP, inoculation with T. viride and P. putaminum under drought stress; NTVPP, inoculation with T. viride and P. putaminum under well‐watered condition. DTVAV, inoculation with T. viride and A. vagum under drought stress; NTVAV, inoculation with T. viride and A. vagum under well‐watered condition.
Figure S4. Principal co‐ordinates analysis (PCoA) ordination of the community composition of fungi and bacteria in A. mongholicus rhizosphere under different water regimes. A, fungal community under well‐watered and drought stress condition; B, bacterial community under well‐watered and drought stress condition; C, fungi community under inoculation and non‐inoculation treatments; D, bacterial community under inoculation and non‐inoculation treatments. CK, non‐inoculation; TV, inoculation with T. viride; PP, inoculation with P. putaminum; AV, inoculation with A. vagum; TVPP, inoculation with T. viride and P. putaminum; TVAV, inoculation with T. viride and A. vagum.
Figure S5. Indicator fungi (A) and bacteria (B) with LDA scores of 2 or greater in A. mongholicus rhizosphere soil. LDA Effect Size (LEfSe) algorithm was used on OTUs level to determine taxa that differentially represented between different treatments. CK indicates non‐inoculated plants. PP, indicate plants inoculated with P. putaminum. AV, indicate plants inoculated with A.vagum. TV, indicate plants inoculated with T. viride. PP+TV, indicate plants inoculated with P. putaminum and T. viride. AV+TV, indicate plants inoculated with A.vagum and T. viride.
Figure S6. Preferences observed in plant growth parameter‐soil microbes associations under different water condition. (A) Preferences observed in plant growth parameter‐fungi under well‐watered conditions. (B) Preferences observed in plant growth parameter‐fungi under drought stress. (C) Preferences observed in plant growth parameter‐bacteria under well‐watered conditions. (D) Preferences observed in plant growth parameter‐bacteria under drought stress.
Figure S7. Preferences observed in edaphic factor‐soil microbes associations under different water condition. (A) Preferences observed in edaphic factor‐fungi under well‐watered conditions. (B) Preferences observed in edaphic factor‐fungi under drought stress. (C) Preferences observed in edaphic factor‐bacteria under well‐watered conditions. (D) Preferences observed in edaphic factor‐bacteria under drought stress.
Acknowledgments
This research was financially supported by the National Key R & D Program of China (2018YFC1706500). We additionally thank International Science Editing Ltd. for the language editing service.
Contributor Information
Wenquan Wang, Email: wwq57@126.com.
Xianen Li, Email: xeli@implad.ac.cn.
Data Availability Statement
Complete culture‐independent sequence data sets were submitted to the NCBI Short Read Archive (SRA) data‐base under the accession number PRJNA681470. All data generated or analysed during this study are included in this article and its supporting information files.
References
- Achouak, W. , Abrouk, D. , Guyonnet, J. , Barakat, M. , Ortet, P. , Simon, L. , et al. (2019) Plant hosts control microbial denitrification activity. FEMS Microbiol Ecol 95: fiz021. [DOI] [PubMed] [Google Scholar]
- Adams, R.I. , Miletto, M. , Taylor, J.W. , and Bruns, T.D. (2013). Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J 7: 1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Hazmi, A.S. , and TariqJaveed, M. (2016) Effects of different inoculum densities of Trichoderma harzianum and Trichoderma viride against Meloidogyne javanica on tomato. Saudi J Biol Sci 23: 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alothman, Z.A. , Bahkali, A.H. , Elgorban, A.M. , Al‐Otaibi, M.S. , Ghfar, A.A. , Gabr, S.A. , et al. (2020) Bioremediation of explosive TNT by Trichoderma viride . Molecules 25: 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atieno, M. , Herrmann, L. , Nguyen, H.T. , Phan, H.T. , Nguyen, N.K. , Srean, P. , et al. (2020) Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J Enviro Manag 275: 111300. [DOI] [PubMed] [Google Scholar]
- Bai, Y. , Muller, D.B. , Srinivas, G. , Garrido‐Oter, R. , Potthoff, E. , Rott, M. , et al. (2015). Functional overlap of the Arabidopsis leaf and root microbiota. Nature, 528: 364–369. [DOI] [PubMed] [Google Scholar]
- Barraza, A. , Vizuet‐de‐Rueda, J.C. , and Alvarez‐Venegas, R. (2020) Highly diverse root endophyte bacterial community is driven by growth substrate and is plant genotype independent in common bean (Phaseolus vulgaris L.). Peer J 8: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi, Y. , Taha, N. , Shalaby, T. , Alshaal, T. , and El‐Ramady, H. (2019) Sulfur promotes biocontrol of purple blotch disease via Trichoderma spp. and enhances the growth, yield and quality of onion. Appl Soil Ecol 134: 15–24. [Google Scholar]
- Berthelot, C. , Leyval, C. , Foulon, J. , Chalot, M. , and Blaudez, D. (2016) Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal‐polluted poplar phytomanagement sites. FEMS Microbiol Ecol 92: fifiw144. [DOI] [PubMed] [Google Scholar]
- Bodner, G. , Nakhforoosh, A. , and Kaul, H.P. (2015) Management of crop water under drought: a review. Agron Sustain Dev 35: 401–442. [Google Scholar]
- Borcard, D. , Gillet, F. , and Legendre, P. (2011) Numerical Ecology with R. New York, NY: Springer. [Google Scholar]
- Bouasria, A. , Mustafa, T. , De Bello, F. , Zinger, L. , Lemperiere, G. , Geremia, R.A. , et al. (2012) Changes in root‐associated microbial communities are determined by species‐specific plant growth responses to stress and disturbance. Eur J Soil Biol 52: 59–66. [Google Scholar]
- Chen, B. , Teh, B.S. , Sun, C. , Hu, S. , Lu, X. , Boland, W. , et al. (2016) Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis . Sci Rep 6: 29505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , and Boutros, P.C. (2011) VennDiagram: a package for the generation of highly customizable Venn and Euler diagrams in R. BMC Bioinform 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.L. , Dunbabin, V.M. , Diggle, A.J. , Siddique, K.H.M. , and Rengel, Z. (2012) Assessing variability in root traits of wild Lupinus angustifolius germplasm: basis for modelling root system structure. Plant Soil 354: 141–155. [Google Scholar]
- Commatteo, J.G. , Consolo, V.F. , Barbieri, P.A. , and Covacevich, F. (2019). Indigenous arbuscular mycorrhiza and Trichoderma from systems with soybean predominance can improve tomato growth. Soil Environ 38: 151–161. [Google Scholar]
- Comas, L.H. , Becker, S.R. , Cruz, V.M.V. , Byrne, P.F. , and Dierig, D.A. (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras‐Cornejo, H.A. , Macías‐Rodríguez, L. , Cortés‐Penagos, C. , and López‐Bucio, J. (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin‐dependent mechanism in Arabidopsis . Plant Physiol 149: 1579–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordovez, V. , Dini‐Andreote, F. , Carrión, V.J. , and Raaijmakers, J.M. (2019) Ecology and evolution of plant microbiomes. Annu Rev Microbiol 73: 69–88. [DOI] [PubMed] [Google Scholar]
- Cui, Y.X. , Fang, L.C. , Guo, X.B. , Wang, X. , Wang, Y.Q. , & Li, P.F. , et al. (2018). Responses of soil microbial communities to nutrient limitation in the desert‐grassland ecological transition zone. Sci Total Environ 642: 45–55. [DOI] [PubMed] [Google Scholar]
- Delgado‐Ramírez, C.S. , Hernández‐Martínez, R. , and Sepúlveda, E. (2021) Rhizobacteria associated with a native Solanaceae promote plant growth and decrease the effects of Fusarium oxysporum in tomato. Agronomy 11: 579. [Google Scholar]
- de Vries, F.T. , Griffiths, R.I. , Knight, C.G. , Nicolitch, O. , and Williams, A. (2020) Harnessing rhizosphere microbiomes for drought‐ resilient crop production. Science 368: 270–274. [DOI] [PubMed] [Google Scholar]
- Dhondge, H.V. , Pable, A.A. , Barvkar, V.T. , Dastager, S.G. , and Nadaf, A.B. (2021) Rhizobacterial consortium mediated aroma and yield enhancement in basmati and non‐basmati rice (Oryza sativa L.). J Biotechnol 328: 47–58. [DOI] [PubMed] [Google Scholar]
- Estévez‐Gefriaud, V. , Vicente, R. , Vergara‐Díaz, O. , Reinaldo, J.J.N. , and Trillas, M.I. (2020) Application of Trichoderma asperellum T34 on maize (Zea mays) seeds protects against drought stress. Planta 252: 8. [DOI] [PubMed] [Google Scholar]
- Fernandes, P.H.D. , de Souza, A.L.T. , Tanaka, M.O. , and Sebastiani, R. (2021) Decomposition and stabilization of organic matter in an old‐growth tropical riparian forest: effects of soil properties and vegetation structure. Forest Ecosyst 8: 13. [Google Scholar]
- Foster, K.R. , Schluter, J. , Coyte, K.Z. , and Rakoff‐Nahoum, S. (2017) The evolution of the host microbiome as an ecosystem on a leash. Nature 548: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, V. , Rodrigue‐Morin, M. , Tremblay, J. , Wasserscheid, J. , Champagne, J. , Bellenger, J.P. , et al. (2020) Vegetation drives the structure of active microbial communities on an acidogenic mine tailings deposit. PEER J 8: e10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslee, S.C. , and Urban, D.L. (2007) The ecodist package for dissimilarity‐based analysis of ecological data. J Stat Softw 22: 1–19. [Google Scholar]
- Guler, N.S. , Pehlivan, N. , Karaoglu, S.A. , Guzel, S. , and Bozdeveci, A. (2016) Trichoderma atroviride ID20G inoculation ameliorates drought stress‐induced damages by improving antioxidant defence in maize seedlings. Acta Physiol Plant 38: 132. [Google Scholar]
- Guo, K. , Sui, Y.H. , Li, Z. , Huang, Y.H. , Zhang, H. , and Wang, W.W. (2020) Colonization of Trichoderma viride Tv‐1511 in peppermint (Mentha × piperita L.) roots promotes essential oil production by triggering ROS mediated MAPK activation. Plant Physiol Biochem 151: 705–718. [DOI] [PubMed] [Google Scholar]
- Hajiabadi, A.A. , Arani, A.M. , Ghasemi, S. , Rad, M.H. , Etesami, H. , Manshadi, S.S. , et al. (2021) Mining the rhizosphere of halophytic rangeland plants for halotolerant bacteria to improve growth and yield of salinity‐stressed wheat. Plant Physiol Biochem 163: 139–153. [DOI] [PubMed] [Google Scholar]
- Halifu, S. , Deng, X. , Song, X.S. , and Song, R.Q. (2019) Effects of two Trichoderma strains on plant growth, rhizosphere soil nutrients, and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests 10: 758. [Google Scholar]
- He, C. , Wang, W.Q. , and Hou, J.L. (2019) Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front Microbiol 10: 2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C. , Wang, W.Q. , and Hou, J.L. (2020) Plant performance of enhancing licorice with dual inoculating dark septate endophytes and Trichoderma viride mediated via effects on root development. BMC Plant Biol 20: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C. , Zeng, Q. , Chen, Y.L. , Chen, C.X. , Wang, W.Q. , Hou, J.L. , et al. (2021) Colonization by dark septate endophytes improves the growth and rhizosphere soil microbiome of licorice plants under different water treatments. Appl Soil Ecol 166: 103993. [Google Scholar]
- Henneron, L. , Kardol, P. , Wardle, D.A. , Cros, C. , and Fontaine, S. (2020) Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol 228: 1269–1282. [DOI] [PubMed] [Google Scholar]
- Herrera‐Parra, E. , Cristóbal‐Alejo, J. , and Ramos‐Zapata, J.A. (2017) Trichoderma strains as growth promoters in Capsicum annuum and as biocontrol agents in Meloidogyne incognita . Chilen J Agr Res 77: 318–324. [Google Scholar]
- Hou, L.F. , Yu, J. , Zhao, L.L. , and He, X.L. (2020) Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front Microbiol 10: 3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Xiao, X. , Huang, H.Y. , Jing, J.Q. , Zhao, H.J. , Wang, L. , et al. (2018) Contrasting beneficial and pathogenic microbial communities across consecutive cropping fields of greenhouse strawberry. Appl Microbiol Biot 102: 5717–5729. [DOI] [PubMed] [Google Scholar]
- Hung, P.M. , Wattanachai, P. , Kasem, S. , and Poeaim, S. (2015) Efficacy of Chaetomium species as biological control agents against Phytophthora nicotianae root rot in citrus. Mycobiol 43: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, A.A. , Wegner, C.E. , Kim, Y. , Liesack, W. , and Dedysh, S.N. (2016) Identification of microbial populations driving biopolymer degradation in acidic peatlands by metatranscriptomic analysis. Mol Ecol 25: 4818–4835. [DOI] [PubMed] [Google Scholar]
- Jackson, M.L. (1973) Soil Chemical Analysis. New Delhi: Prentice Hall of India Pvt. Ltd, pp. 38–56. [Google Scholar]
- Jaiswal, A.K. , Mengiste, T.D. , Myers, J.R. , Egel, D.S. , and Hoagland, L.A. (2020) Tomato domestication attenuated responsiveness to a beneficial soil microbe for plant growth promotion and induction of systemic resistance to foliar pathogens. Front Microbiol 11: 604566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H.Q. , Liu, H.B. , Xie, Y.Y. , Zhang, Y.G. , Xu, Q.Q. , Mao, L.J. , et al. (2018) Effect of the dark septate endophytic fungus Acrocalymma vagum on heavy metal content in tobacco leaves. Symbiosis 74: 89–95. [Google Scholar]
- Jumpponen, A. (2011) Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 11: 207–211. [Google Scholar]
- Kauppinen, M. , Raveala, K. , WãLi, P.R. , and Ruosalainen, A.L. (2014). Contrasting preferences of arbuscular mycorrhizal and dark septate fungi colonizing boreal and subarctic Avenella flexuosa. Mycorrhiza, 24: 171–177. [DOI] [PubMed] [Google Scholar]
- Khan, N. , Bano, A. , and Babar, M.A. (2019) Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS One 14: e0213040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshmanzar, E. , Aliasgharzad, N. , Neyshabouri, M.R. , Khoshru, B. , and Arzanlou, M. (2020) Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water‐ deficit stress. Int J Environ Sci Te 17: 869–878. [Google Scholar]
- Knapp, D.G. , Németh, J.B. , Barry, K. , Hainaut, M. , Henrissat, B. , Johnson, J. , et al. (2018) Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Sci Rep 8: 6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku′zniar, A. , Włodarczyk, K. , and Woli′nska, A. (2019) Agricultural and other biotechnological applications resulting from trophic plant‐endophyte interactions. Agronomy 9: 779. [Google Scholar]
- Kushwaha, R.K. , Singh, S. , Pandey, S.S. , Venkata Rao, D.K. , Nagegowda, D.A. , Kalra, A. , et al. (2019) Compatibility of inherent fungal endophytes of Withania somnifera with Trichoderma viride and its impact on plant growth and with anolide content. J Plant Growth Regul 38: 1228–1242. [Google Scholar]
- Lata, R. , Chowdhury, S. , Gond, S.K. , and White, J.F., Jr. (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66: 268–276. [DOI] [PubMed] [Google Scholar]
- Latif, S. , Bibi, S. , Kouser, R. , Fatimah, H. , Farooq, S. , Naseer, S. , et al. (2020) Characterization of bacterial community structure in the rhizosphere of Triticum aestivum L. Genomics 112: 4760–4768. [DOI] [PubMed] [Google Scholar]
- Li, X. , He, C. , He, X.L. , Su, F. , Hou, L.F. , Ren, Y. , et al. (2019a) Dark septate endophytes improve the growth of host and non‐host plants under drought stress through altered root development. Plant Soil 439: 259–272. [Google Scholar]
- Li, X. , He, X.L. , Zhou, Y. , Hou, Y.T. , and Zuo, Y.L. (2019b) Effects of dark septate endophytes on the performance of Hedysarum scoparium under water deficit stress. Front Plant Sci 10: 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Liu, M. , Liao, Q.G. , Lü, F.B. , and Zhao, X.L. (2020) Effects of inoculated mycorrhizal fungi and nonmycorrhizal beneficial micro‐organisms on plant traits, nutrient uptake and root‐associated fungal community composition of the Cymbidium hybridum in greenhouse. J Appl Microbiol. 10.1111/jam.14967. [DOI] [PubMed] [Google Scholar]
- Lugtenberg, B.J.J. , Caradus, J.R. , and Johnson, L.J. (2016) Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol 92: fiw194. [DOI] [PubMed] [Google Scholar]
- Magoč, T. , and Salzberg, S.L. (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27: 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F.M. , Uroz, S. , and Barker, D.G. (2017) Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science 356: eaad4501. [DOI] [PubMed] [Google Scholar]
- Martínez‐Medina, A. , Roldán, A. , and Pascual, J. (2011) Interaction between arbuscular mycorrhizal fungi and Trichoderma harzianum under conventional and low input fertilization field condition in melon crops: growth response and fusarium wilt biocontrol. Appl Soil Ecol 47: 98–105. [Google Scholar]
- Maughan, H. , Wang, P.W. , Diaz Caballero, J. , Fung, P. , Gong, Y. , Donaldson, S.L. , et al. (2012) Analysis of the cystic fibrosis lung microbiota via serial Illumina sequencing of bacterial 16S rRNA hypervariable regions. PLoS One 7: e45791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes, R. , Garbeva, P. , and Raaijmakers, J.M. (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37: 634–663. [DOI] [PubMed] [Google Scholar]
- Mengistu, A.A. (2020) Endophytes: colonization, behaviour, and their role in defense mechanism. Int J Microbiol 2020: 6927219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally, R.A. , and Al‐Amri, S.M. (2020) Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett Appl Microbiol 70: 79–86. [DOI] [PubMed] [Google Scholar]
- Mona, S.A. , Hashem, A. , Abd_Allahm, E.F. , Alqarawi, A.A. , Soliman, D.W.K. , Wirth, S. , et al. (2017) Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J Integr Agric 16: 1751–1757. [Google Scholar]
- Oksanen, J. , Blanchet, F.G. , Friendly, M. , Kindt, R. , Legendre, P. , and McGlinn, D. (2016) Vegan: community ecology package. R package version 2: 4–1. [Google Scholar]
- Olsen, S.R. , Cole, C.V. , Watanabe, F.S. , and Dean, L.A. (1954) Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. In USDA Circular 939. Washington, DC: US Department of Agriculture, pp. 1–18. [Google Scholar]
- Parkash, V. , Sharma, S. , Aggarwal, A. (2011). Symbiotic and synergistic efficacy of endomycorrhizae with Dendrocalamus strictus L. Plant Soil Environ 57: 447–452. [Google Scholar]
- Phillips, J.M. , and Hayman, D.S. (1970) Improved procedures for clearing roots and staining parasitic and vesicular‐arbuscular fungi for rapid assessment of infection. Transt Br Mycol Soc 55: 158–163. [Google Scholar]
- Porras‐Alfaro, A. , Herrera, J. , Natvig, D.O. , Lipinski, K. , and Sinsabaugh, R.L. (2011) Diversity and distribution of soil fungal communities in a semiarid grassland. Mycologia 103: 10–21. [DOI] [PubMed] [Google Scholar]
- Qin, X.M. , Li, Z.Y. , Sun, H.F. , Zhang, J. , Fang, X.S. , Yuan, C. , et al. (2013) Current situation and analysis of Astragalus medicinal resources in China. China J Chin Materia Medica 38: 61–66. [Google Scholar]
- Raklami, A. , Bechtaoui, N. , Tahiri, A. , Anli, M. , Meddich, A. , and Oufdou, K. (2019) Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front Microbiol 10: 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell, D.L. (1994) Soil Science: Methods and Applications. London: Longman Group. [Google Scholar]
- Santos, S.G.D. , Silva, P.R.A.D. , Garcia, A.C. , Zilli, J.É. , and Berbara, R.L.L. (2017) Dark septate endophyte decreases stress on rice plants. Braz J Microbiol 48: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoyo, G. , Hernandez‐Pacheco, C. , Hernandez‐Salmeron, J. , and Hernandez‐Leon, R. (2017) The role of abiotic factors modulating the plant‐microbe‐soil interactions: toward sustainable agriculture. A review. Spanish J Agr Res 15: e03R01. [Google Scholar]
- Saravanakumar, K. , Fan, L.L. , Fu, K.H. , Yu, C.J. , Wang, M. , Xia, H. , et al. (2016) Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci Rep 6: 35543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, A. , Mishra, S. , Ray, S. , Raghuwanshi, R. , and Singh, H.B. (2020) Differential reprogramming of defense network in Capsicum annum L. plants against Colletotrichum truncatum infection by phyllospheric and rhizospheric Trichoderma strains. J Plant Growth Regul 39: 751–763. [Google Scholar]
- Schmoll, M. (2018) Regulation of plant cell wall degradation by light in Trichoderma . Schmoll Fungal Biol Biotechnol 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumare, A. , Boubekri, K. , Lyamlouli, K. , Hafidi, M. , Ouhdouch, Y. , and Kouisni, L. (2021) Efficacy of phosphate solubilizing Actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere 17: 100284. [Google Scholar]
- Sun, X. , Zhang, L. , Pei, J. , and Huang, L.F. (2020) Regulatory relationship between quality variation and environment of Cistanche deserticola in three ecotypes based on soil microbiome analysis. Sci Rep 10: 6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surono, and Narisawa, K. (2017) The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol 28: 1–10. [Google Scholar]
- Surono, and Narisawa, K. (2018) The inhibitory role of dark septate endophytic fungus Phialocephala fortinii against Fusarium disease on the Asparagus officinalis growth in organic source conditions. Biol Control 121: 159–167. [Google Scholar]
- Ullah, A. , Akbar, A. , Luo, Q. , Khan, A.H. , Manghwar, H. , Shaban, M. , et al. (2019) Microbiome diversity in cotton rhizosphere under normal and drought conditions. Microb Ecol 77: 429–439. [DOI] [PubMed] [Google Scholar]
- Velmourougane, K. , Prasanna, R. , Singh, S. , Chawla, G. , Kumar, A. , and Saxena, A.K. (2017) Modulating rhizosphere colonisation, plant growth, soil nutrient availability and plant defense enzyme activity through Trichoderma viride‐Azotobacter chroococcum biofilm inoculation in chickpea. Plant Soil 421: 157–174. [Google Scholar]
- Visioli, G. , Sanangelantoni, A.M. , Vamerali, T. , Dal Cortivo, C. , and Blandino, M. (2018) 16S rDNA profiling to reveal the influence of seed‐applied biostimulants on the rhizosphere of young maize [DOI] [PMC free article] [PubMed]
- Wani, Z.A. , Ashraf, N. , Mohiuddin, T. , and Riyaz‐Ul‐Hassan, S. (2015) Plant‐endophyte symbiosis, an ecological perspective. Appl Microbiol Biotechnol 99: 2955–2965. [DOI] [PubMed] [Google Scholar]
- Ważny, R. , Rozpądek, P. , Jędrzejczyk, R.J. , Śliwa, M. , Stojakowska, A. , Anielska, T. , et al. (2018) Does co‐inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 28: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z. , and Yu, D. (2018) Analysis of the succession of structure of the bacteria community in soil from long‐term continuous cotton cropping in Xinjiang using high‐throughput sequencing. Arch Microbiol 200: 653–662. [DOI] [PubMed] [Google Scholar]
- Xie, L.L. , He, X.L. , Wang, K. , Hou, L.F. , and Sun, Q. (2017) Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in Northwest China and the influence of edaphic variables. Fungal Ecol 26: 135–143. [Google Scholar]
- Xiong, Q.Q. , Hu, J.L. , Wei, H.Y. , Zhang, H.C. , and Zhu, J.Y. (2021) Relationship between plant roots, rhizosphere microorganisms, and nitrogen and its special focus on rice. Agriculture‐Basel 11: 234. [Google Scholar]
- Xu, F.J. , Song, S.L. , Ma, C.Y. , Zhang, W. , Sun, K. , Tang, M.J. , et al. (2020) Endophytic fungus improves peanut drought resistance by reassembling the root‐dwelling community of arbuscular mycorrhizal fungi. Fungal Ecol 48: 100993. [Google Scholar]
- Xu, N. , Tan, G. , Wang, H. , and Gai, X.P. (2016). Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74: 1–8. [Google Scholar]
- Yakti, W. , Kovács, G.M. , Vági, P. , and Franken, P. (2019) Impact of dark septate endophytes on tomato growth and nutrient uptake. Plant Ecol Divers 11: 637–648. [Google Scholar]
- Yao, H. , Sun, X. , He, C. , Maitra, P. , Li, X.C. , and Guo, L.D. (2019) Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q.M. , Gong, M.G. , Yuan, J.F. , Hou, Y. , Zhang, H.M. , and Wang, Y. (2017) Dark septate endophyte improves drought tolerance in Sorghum . Int J Agric Biol 19: 53–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Colonization of dark septate endophyte (DSE) strains in the roots of A. mongholicus plant after 3 months of inoculation. (A, C) Indicate roots inoculated with A. vagum. (B, D) Indicate roots inoculated with P. putaminum. Arrows indicate: Hy = DSE hyphae, Mi = DSE microsclerotia.
Figure S2. Rarefaction curves for the fungal (A) and bacterial (B) and ranking by the abundance of the fungal (C) and bacterial (D) operational taxonomic units (OTUs) in A. mongholicus rhizosphere. DCK, non‐inoculation under drought stress; NCK, non‐inoculation under well‐watered condition; DTV, inoculation with T. viride under drought stress; NTV, inoculation with T. viride under well‐watered condition; DPP, inoculation with P. putaminum under drought stress; NPP, inoculation with P. putaminum under well‐watered condition; DAV, inoculation with A. vagum under drought stress; NAV, inoculation with A. vagum under well‐watered condition; DTVPP, inoculation with T. viride and P. putaminum under drought stress; NTVPP, inoculation with T. viride and P. putaminum under well‐watered condition; DTVAV, inoculation with T. viride and A. vagum under drought stress; NTVAV, inoculation with T. viride and A. vagum under well‐watered condition.
Figure S3. Relative abundance of fungi (A) and bacteria (B) at the genus level in A. mongholicus rhizosphere. DCK, non‐inoculation under drought stress; NCK, non‐inoculation under well‐watered condition; DTV, inoculation with T. viride under drought stress; NTV, inoculation with T. viride under well‐watered condition; DPP, inoculation with P. putaminum under drought stress; NPP, inoculation with P. putaminum under well‐watered condition; DAV, inoculation with A. vagum under drought stress; NAV, inoculation with A. vagum under well‐watered condition; DTVPP, inoculation with T. viride and P. putaminum under drought stress; NTVPP, inoculation with T. viride and P. putaminum under well‐watered condition. DTVAV, inoculation with T. viride and A. vagum under drought stress; NTVAV, inoculation with T. viride and A. vagum under well‐watered condition.
Figure S4. Principal co‐ordinates analysis (PCoA) ordination of the community composition of fungi and bacteria in A. mongholicus rhizosphere under different water regimes. A, fungal community under well‐watered and drought stress condition; B, bacterial community under well‐watered and drought stress condition; C, fungi community under inoculation and non‐inoculation treatments; D, bacterial community under inoculation and non‐inoculation treatments. CK, non‐inoculation; TV, inoculation with T. viride; PP, inoculation with P. putaminum; AV, inoculation with A. vagum; TVPP, inoculation with T. viride and P. putaminum; TVAV, inoculation with T. viride and A. vagum.
Figure S5. Indicator fungi (A) and bacteria (B) with LDA scores of 2 or greater in A. mongholicus rhizosphere soil. LDA Effect Size (LEfSe) algorithm was used on OTUs level to determine taxa that differentially represented between different treatments. CK indicates non‐inoculated plants. PP, indicate plants inoculated with P. putaminum. AV, indicate plants inoculated with A.vagum. TV, indicate plants inoculated with T. viride. PP+TV, indicate plants inoculated with P. putaminum and T. viride. AV+TV, indicate plants inoculated with A.vagum and T. viride.
Figure S6. Preferences observed in plant growth parameter‐soil microbes associations under different water condition. (A) Preferences observed in plant growth parameter‐fungi under well‐watered conditions. (B) Preferences observed in plant growth parameter‐fungi under drought stress. (C) Preferences observed in plant growth parameter‐bacteria under well‐watered conditions. (D) Preferences observed in plant growth parameter‐bacteria under drought stress.
Figure S7. Preferences observed in edaphic factor‐soil microbes associations under different water condition. (A) Preferences observed in edaphic factor‐fungi under well‐watered conditions. (B) Preferences observed in edaphic factor‐fungi under drought stress. (C) Preferences observed in edaphic factor‐bacteria under well‐watered conditions. (D) Preferences observed in edaphic factor‐bacteria under drought stress.
Data Availability Statement
Complete culture‐independent sequence data sets were submitted to the NCBI Short Read Archive (SRA) data‐base under the accession number PRJNA681470. All data generated or analysed during this study are included in this article and its supporting information files.


