Abstract
Background and purpose
Effectiveness of autologous haematopoietic stem cell transplantation (AHSCT) in relapsing–remitting multiple sclerosis (MS) is well known, but in secondary–progressive (SP)‐MS it is still controversial. Therefore, AHSCT activity was evaluated in SP‐MS using low‐dose immunosuppression with cyclophosphamide (Cy) as a comparative treatment.
Methods
In this retrospective monocentric 1:2 matched study, SP‐MS patients were treated with intermediate‐intensity AHSCT (cases) or intravenous pulses of Cy (controls) at a single academic centre in Florence. Controls were selected according to baseline characteristics adopting cardinality matching after trimming on the estimated propensity score. Kaplan–Meier and Cox analyses were used to estimate survival free from relapses (R‐FS), survival free from disability progression (P‐FS), and no evidence of disease activity 2 (NEDA‐2).
Results
A total of 93 SP‐MS patients were included: 31 AHSCT, 62 Cy. Mean follow‐up was 99 months in the AHSCT group and 91 months in the Cy group. R‐FS was higher in AHSCT compared to Cy patients: at Year 5, 100% versus 52%, respectively (p < 0.0001). P‐FS did not differ between the groups (at Year 5: 70% in AHSCT and 81% in Cy, p = 0.572), nor did NEDA‐2 (p = 0.379). A sensitivity analysis including only the 31 “best‐matched” controls confirmed these results. Three neoplasms (2 Cy, 1 AHSCT) and two fatalities (2 Cy) occurred.
Conclusions
This study provides Class III evidence, in SP‐MS, on the superior effectiveness of AHSCT compared to Cy on relapse activity, without differences on disability accrual. Although the suppression of relapses was observed in the AHSCT group only, AHSCT did not show advantages over Cy on disability, suggesting that in SP‐MS disability progression becomes based more on noninflammatory neurodegeneration than on inflammation.
Keywords: autologous hematopoietic stem cell transplantation, case–control study, disability progression, multiple sclerosis, progressive multiple sclerosis
Thirty‐one secondary–progressive multiple sclerosis (SP‐MS) patients treated with autologous haematopoietic stem‐cell transplantation (AHSCT) were matched 1:2 with cyclophosphamide (Cy)‐treated SP‐MS patients; outcomes were evaluated over long‐term follow‐up (mean = 96 months). AHSCT was superior to Cy in suppressing relapse activity, without differences in Expanded Disability Status Scale worsening, suggesting that in SP‐MS disability accrual becomes more based on noninflammatory neurodegeneration than on inflammation. A hint of superior efficacy of AHSCT was observed on progression‐free survival, suggesting a potential benefit of transplant in selected cases, and supporting the feasibility of randomized clinical trials comparing AHSCT with available treatments to provide conclusive evidence on this issue.

INTRODUCTION
The benefit–risk ratio of autologous haematopoietic stem cell transplantation (AHSCT) in secondary–progressive (SP) multiple sclerosis (MS) is controversial, despite its high effectiveness in relapsing–remitting MS [1, 2]. In SP‐MS, almost none of the disease‐modifying treatments (DMTs) currently available halt disability accrual [3], and the only DMT approved so far [4] showed a limited clinical effect [5].
Nonetheless, in the absence of better alternative therapeutic options, AHSCT has been widely used also for the treatment of aggressive SP‐MS, showing heterogeneous results [6, 7].
In the absence of controlled trials, it is difficult to estimate the effectiveness of AHSCT in this MS phenotype mostly because, in AHSCT studies, patients affected by particularly aggressive disease course and harbouring potentially unfavourable outcomes are usually enrolled [8].
To provide further insight into the benefit–risk ratio of AHSCT in SP‐MS, in this study the effectiveness and safety of AHSCT were compared in this MS phenotype with a control group of patients selected for similar characteristics at treatment baseline, and who received low‐dose immunosuppression with intravenous (IV) cyclophosphamide (Cy). Cy is an alkylating agent with an immunosuppressive effect approved in Italy for the treatment of “the autoimmune diseases of the nervous system” [9], administered mostly in progressive MS and considered as a therapeutic option also in countries with limited access to high‐efficiency therapies [10, 11, 12].
Hence, we present the comparative evaluation of effectiveness and safety of AHSCT and Cy in SP‐MS, providing Class III evidence of superiority of AHSCT with respect to Cy in suppressing relapses, without differences on disability.
METHODS
A retrospective monocentric matched study was carried out in SP‐MS patients treated with AHSCT (cases) or Cy (controls). The patients were exposed to the treatments between 1991 and 2018. Data were collected up to the last follow‐up available; the database was locked on 31 May 2021.
SP‐MS patients diagnosed according to the McDonald criteria [13, 14] who had been consecutively enrolled in an open‐label monocentric registry study on AHSCT in MS at the Careggi University Hospital in Florence (Italy), were included as cases. SP‐MS patients who had been treated with Cy at the same centre were included as potential controls from whom a subsample of 1:2 matched controls was selected, as detailed below.
The design of the observational study through matching was blind to the clinical information related to the posttreatment outcome variables, which was linked to the matched observations only once the design phase was completed; this procedure provides objectivity for causal inference [15].
AHSCT procedure
Patients received AHSCT at the Cellular Therapies, Transfusion Medicine, and Transplant Unit of the Careggi University Hospital in Florence, in collaboration with the Tuscan Region MS Referral Centre of the same hospital. Briefly, mobilization of haematopoietic stem cells was obtained with IV Cy (4 g/m2 body surface area [BSA]) and granulocyte colony‐stimulating factor. The intermediate intensity conditioning regimen BEAM (carmustine, etoposide, cytarabine, and melphalan) + anti‐thymocyte globulin (ATG) was then administered [16]. Supportive therapies and clinical monitoring after AHSCT were carried out according to local practice.
Cy procedure
Patients were treated at the Tuscan Region MS Referral Centre of the Careggi University Hospital in Florence. Cy was IV administered at the dosage of 0.75 g/m2 BSA according to the following schedule: monthly for the first year (12 administrations), then every other month in the second year of treatment (six administrations), and quarterly in the third year (four administrations). Ondansetron and 1 g IV methylprednisolone were administered before every Cy infusion; mesna was also consistently administered for the prevention of bladder toxicity.
Follow‐up and outcomes
Neurological follow‐up was performed at least yearly, or upon patient request according to clinical practice. Disability was assessed with the Expanded Disability Status Scale (EDSS) [17] by certified neurologists expert in MS. A relapse was defined as the exacerbation of neurological symptoms attributable to MS, lasting at least 24 h, in absence of fever or concomitant infection, associated with an objective modification at neurological examination.
EDSS worsening was defined as a confirmed increase of ≥1.0 or 0.5 EDSS points if baseline EDSS was <5.5 or ≥5.5, respectively. Disability progression was defined as the occurrence of at least two distinct episodes of EDSS worsening, that is, maintenance of a progressive disease course, as previously reported [18]. No evidence of disease activity 2 (NEDA‐2) was defined as the absence of both relapses and confirmed EDSS worsening.
Long‐term adverse events, including neoplasms, and deaths were reported up to the last follow‐up available.
Statistical methods
The design phase of the study was performed by an independent group of statisticians, who were masked to all the outcome variables. The subsample of 1:2 matched controls was selected from a cohort of SP‐MS patients treated with Cy using cardinality matching after trimming on the estimated propensity score (PS), using the following six covariates as confounders: sex, age, disease duration and EDSS at treatment start, number of relapses over the 2 years before treatment commencement, and the epoch of treatment (i.e., the year in which patients had received AHSCT or Cy, clustered in 5‐year epochs). First, to improve overlap in the selected observed confounders, we estimated the PS for all patients using a logistic regression model and trimmed the sample by removing the control patients with values of the estimated PS lower than the smallest estimated PS for the AHSCT patients [19]. The remaining control patients were used as a donor pool, from which we selected a 1:2 matched control subsample of patients using a two‐step procedure based on cardinality matching, a matching method that uses integer programming to find the largest matched control sample that is balanced with respect to the observed covariates according to predetermined criteria specified before matching [20]. We implemented cardinality matching without replacement with constraints on the standardized mean difference (SMD)—the difference in average covariate values, normalized by the square root of the average of the two within‐treatment group sample variances—for each included confounder. Specifically, we first used cardinality matching to select a subsample of 1:1 matched controls requiring that SMD for the EDSS at baseline was zero and SMDs for the other covariates were not greater than 0.1. We then removed the “best” matched controls and reapplied cardinality matching using data on the AHSCT patients and the remaining Cy patients to select another subsample of 1:1 matched controls (“second best” matched), by slightly relaxing the constraints on the SMDs; we required that SMD was not greater than 0.9 for all the covariates.
Baseline characteristics of the patients included are reported as mean and SD. Comparisons between the groups were performed using t‐test for unpaired samples. Survival free from the event was estimated using the Kaplan–Meier analysis; a Cox regression model was applied to adjust estimates of survival for age and progressive phase duration at treatment start, and number of DMTs received before AHSCT or Cy.
A sensitivity analysis including as controls only the “best” matches (Cy‐Sensitivity group) was also performed.
As discontinuation of treatment was observed in the Cy group only, following the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E9(R1) addendum [21], the outcomes in this group were analysed considering both the whole follow‐up period after the first dose, irrespective of treatment discontinuation (Intention‐To‐Treat–(ITT)/policy strategy), and the treatment epoch only, censoring cases at the time of the last administration of Cy (hypothetical strategy), assuming independent censoring conditional on the covariates. A two‐tailed p‐value of <0.05 was considered significant. The software programs used for statistics were SPSS and R.
Standard protocol approval and patient consent
The protocol was approved by the local ethics committee (Tuscan regional ethics committee for clinical experimentation; approval number 17696_oss); written informed consent was collected according to local regulations.
RESULTS
Patient characteristics
SP‐MS patients treated with AHSCT (n = 31) were matched with controls (n = 62) selected from a cohort of SP‐MS patients treated with Cy (n = 108), as detailed in the Methods section (Figure 1). Because of the matching design, baseline features did not differ between the two groups, except for age at treatment (mean = 39.3 years, SD = 7.27 in the AHSCT and 42.8 years, SD 7.09 in the Cy group, p = 0.029) and for the number of previous DMTs received (mean = 3.0 vs 1.3 in the AHSCT and Cy groups, respectively, p < 0.001; Table 1). The mean follow‐up duration was 99 months (range = 24–238) for the AHSCT group and 91 months (range = 7–285) for the Cy group (p = 0.346).
FIGURE 1.
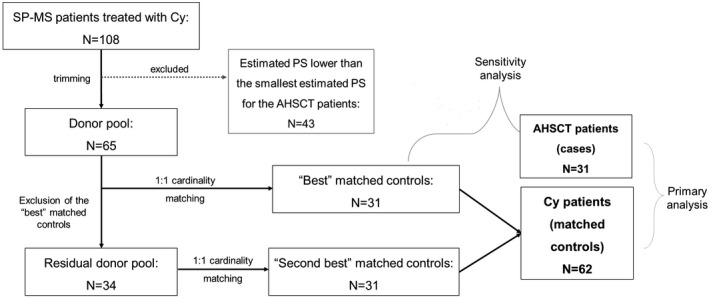
Patient inclusion diagram. Secondary–progressive multiple sclerosis (SP‐MS) patients treated with autologous haematopoietic stem cell transplantation (AHSCT; n = 31) were matched with control patients selected from a cohort of 108 SP‐MS patients treated with cyclophosphamide (Cy) using six covariates as confounders. After estimating propensity score (PS) for all patients, the sample of Cy patients was trimmed by removing 43 patients with values of the estimated PS lower than the smallest estimated PS for the AHSCT cases. The remaining 65 control patients were used as a donor pool, from which a subsample of 31 matched controls was selected with cardinality matching (“best” matched controls). These controls were then removed from the donor pool, and cardinality matching was reapplied to the residual donor pool to select another subsample of 31 matched controls, by slightly relaxing the constraints on the standardized mean differences (“second best” matched controls). All the 62 matched controls were included in the primary analysis; a sensitivity analysis was then performed including the 31 “best” matched controls only
TABLE 1.
Baseline clinical and demographic characteristics of secondary–progressive multiple sclerosis patients included in AHSCT and matched Cy groups
| Characteristic | AHSCT, n = 31, mean (SD) | Cy, n = 62, mean (SD) | p |
|---|---|---|---|
| Age, years | 39.3 (7.27) | 42.8 (7.09) | 0.029 |
| Disease duration, years | 13.7 (5.28) | 13.8 (6.73) | 0.963 |
| Progressive phase duration, years | 3.6 (2.67) | 2.7 (3.55) | 0.225 |
| Previous treatment duration, years | 8.5 (5.43) | 6.9 (4.76) | 0.174 |
| Previous DMTs, n | 3.0 (1.30) | 1.3 (0.99) | <0.001 |
| Annualized relapse rate in the previous 2 years | 0.56 (0.63) | 0.46 (0.44) | 0.409 |
| Progression index pretreatment | 0.49 (0.18) | 0.64 (0.79) | 0.151 |
| EDSS | 5.9 (0.87) | 5.7 (1.01) | 0.392 |
| Year of treatment | 2009 (5.6) | 2007 (5.2) | 0.112 |
| n (%) | n (%) | p | |
|---|---|---|---|
| Sex, female | 23 (74%) | 38 (61%) | 0.217 |
| Cases with ≥1 relapse over the previous 2 years | 17 (55%) | 41 (66%) | 0.289 |
| Cases naïve to treatment | 0 (0%) | 11 (18%) | 0.013 |
| Cases who had received highly active DMTs | 13 (42%) | 3 (5%) | 0.0001 |
Highly active DMTs include the following: fingolimod, natalizumab, rituximab.
Abbreviations: AHSCT, autologous haematopoietic stem cell transplantation; Cy, cyclophosphamide; DMT, disease‐modifying treatment; EDSS, Expanded Disability Status Scale.
Cy treatment discontinuation
Treatment with Cy was discontinued in 26 of 62 (42%) patients in the Cy group before the completion of the 3‐year cycle. Reasons for discontinuation were lack of efficacy (15/26, 58%), adverse events (10/26, 38%), and pregnancy (1/26, 4%; data not shown).
Relapses
Following treatment, no relapses were reported in the AHSCT patients; relapse‐free survival (R‐FS) was higher in this group (100% at all the timepoints) compared to the Cy group, in both the treatment epoch (71% at Year 2, p < 0.0001; Figure 2a) and the whole follow‐up (52% at Year 5, p < 0.0001; Figure 2b.)
FIGURE 2.
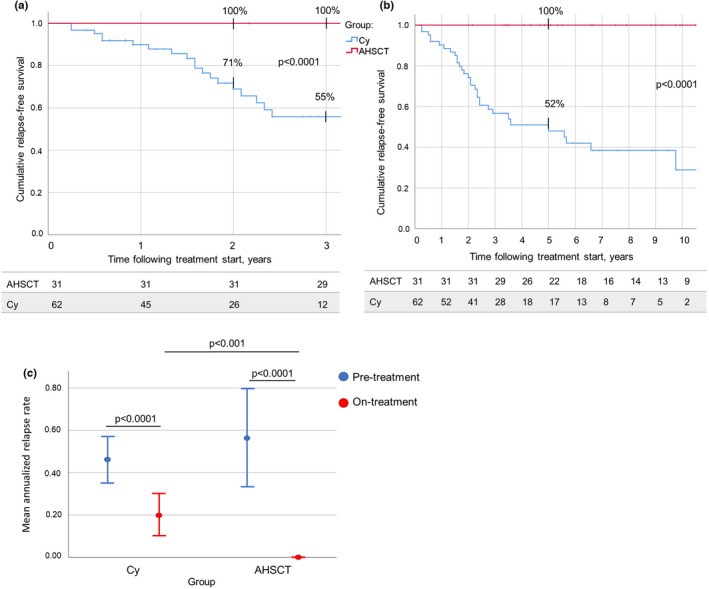
(a) Relapse‐free survival (R‐FS) following autologous haematopoietic stem cell transplantation (AHSCT; red) and treatment with cyclophosphamide (Cy; blue) during the treatment epoch was higher in AHSCT compared to Cy patients: 100% versus 55% at Year 3, respectively, p < 0.0001. (b) R‐FS over the whole follow‐up period following treatment start was higher in the AHSCT group compared to the Cy group: 100% and 52% at Year 5, respectively (p < 0.0001). The number of patients in observation at each timepoint is reported below the corresponding chart. (c) Annualized relapse rate (ARR) over 2 years prior to treatment start (blue) and during the first 2 years of treatment (red) in the AHSCT (right panel) and Cy (left panel) groups. ARR dropped in both groups (p < 0.0001) following treatment start. Pretreatment ARR did not differ between the two groups (p = 0.574), but ARR on treatment was lower in the AHSCT group compared to the Cy group (p < 0.001), suggesting that only AHSCT was able to induce the complete suppression of new inflammatory activity [Colour figure can be viewed at wileyonlinelibrary.com]
During the first 2 years following treatment start, annualized relapse rate (ARR) was reduced in both groups compared to the rate observed in the 2 years before treatment start: from 0.56 (SD = 0.63) to 0.00 (SD = 0.00) in AHSCT and from 0.46 (SD = 0.44) to 0.20 (SD = 0.37) in Cy patients (p < 0.0001), being the on‐treatment ARR lower in the AHSCT group compared to the Cy group (p < 0.001; Figure 2c).
Disability
During the whole follow‐up, EDSS worsening‐free survival (W‐FS) at Year 5 was 45% for AHSCT patients and 48% for Cy patients (p = 0.717; Figure 3a). During the treatment epoch, W‐FS at Year 2 was 55% in the AHSCT group and 73% in the Cy group (p = 0.084; data not shown). During the whole follow‐up, survival free from disability progression (P‐FS) at Years 3 and 5 was 84% and 70% in the AHSCT group, and 89% and 81% in the Cy group, respectively (p = 0.572; Figure 3b). P‐FS at Year 2 during the treatment epoch was 97% in the AHSCT group and 91% in the Cy group (p = 0.301; data not shown).
FIGURE 3.
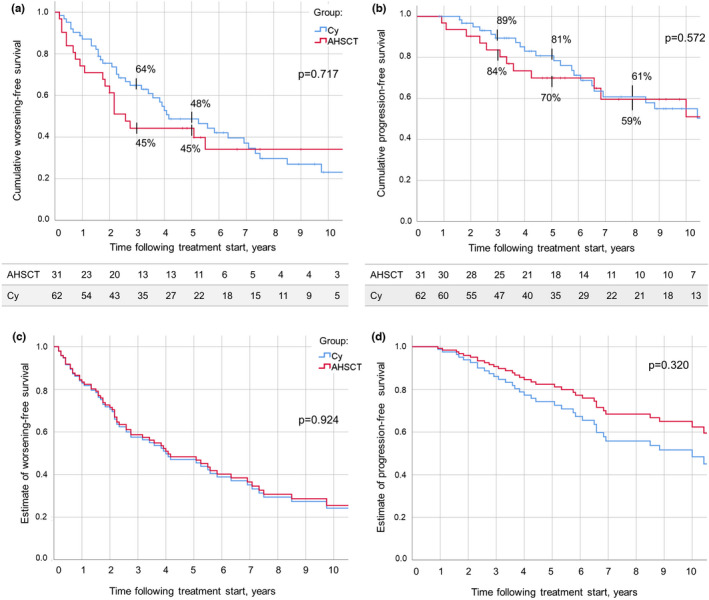
(a) Expanded Disability Status Scale worsening‐free survival (W‐FS) following autologous haematopoietic stem cell transplantation (AHSCT; red) and start of treatment with cyclophosphamide (Cy; blue) up to the last follow‐up available. W‐FS at Year 3 was 45% in the AHSCT and 64% in the Cy group; at Year 5, it was 45% and 48%, respectively (p = 0.717). (b) Disability progression‐free survival (P‐FS) over follow‐up did not differ between the AHSCT group and the Cy group, being at Year 5 following treatment commencement 70% and 81%, respectively (p = 0.572). The number of patients in observation at each timepoint is reported below the corresponding chart. (c, d) Cox estimated distribution of W‐FS (c) and P‐FS (d) in the AHSCT and Cy groups, adjusted for age at treatment, progressive phase duration, and number of previous disease‐modifying treatments received. No differences were observed between the two groups in both the analyses (p = 0.924 and p = 0.320, respectively). These data indicate no differences of effectiveness on disability worsening and on disability progression between the treatments. However, after applying the Cox model, a hint of higher effectiveness of AHSCT can be observed, suggesting insufficient power of this study for this outcome measure [Colour figure can be viewed at wileyonlinelibrary.com]
To assess the robustness of these results, a Cox regression analysis was conducted further adjusting the estimates of survival for the three variables that were not well balanced between the groups (Table 2). Cox estimates of W‐FS and P‐FS did not differ between the groups during both the treatment epoch (p = 0.441 and p = 0.419, respectively; data not shown) and the whole follow‐up (p = 0.924 and 0.320, respectively; Figure 3c,d).
TABLE 2.
Cox regression model for estimates of survival free from EDSS worsening and disability progression, and NEDA‐2 survival during the whole follow‐up
| Variable | EDSS worsening | Disability progression | NEDA‐2 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Group, AHSCT | 0.96 (0.46–2.03) | 0.924 | 0.65 (0.28–1.52) | 0.320 | 0.69 (0.34–1.41) | 0.311 |
| Age at treatment, years | 1.02 (0.98–1.06) | 0.229 | 1.01 (0.97–1.06) | 0.588 | 1.02 (0.98–1.05) | 0.357 |
| SP phase duration, years | 1.01 (0.93–1.09) | 0.729 | 0.99 (0.89–1.10) | 0.848 | 0.99 (0.92–1.07) | 0.936 |
| Previous DMTs, n | 1.17 (0.91–1.50) | 0.214 | 1.23 (0.93–1.64) | 0.150 | 1.15 (0.90–1.46) | 0.265 |
Abbreviations: AHSCT, autologous haematopoietic stem cell transplantation; CI, confidence interval; DMT, disease‐modifying treatment; EDSS, Expanded Disability Status Scale; HR, hazard ratio; NEDA‐2, no evidence of disease activity 2; SP, secondary progressive.
A suggestion for superior P‐FS in AHSCT compared to Cy patients was observed (hazard ratio [HR] = 0.65, 95% confidence interval = 0.28–1.52, p = 0.320), equivalent to a 35% reduction in the risk of progression.
EDSS deteriorated at Month 36 compared to baseline in the AHSCT group only (p = 0.017), whereas at the last follow‐up it increased in both groups (p < 0.001 for the AHSCT and <0.0001 for the Cy groups; Figure 4a). The progression index following treatment commencement and EDSS at last follow‐up did not differ between the groups (p = 0.509 and p = 0.064, respectively; data not shown). At last follow‐up, EDSS ≥ 8 was observed in two of 31 (6%) and in 11 of 62 (18%) cases in the AHSCT and Cy groups, respectively (p = 0.127; Figure 4b).
FIGURE 4.
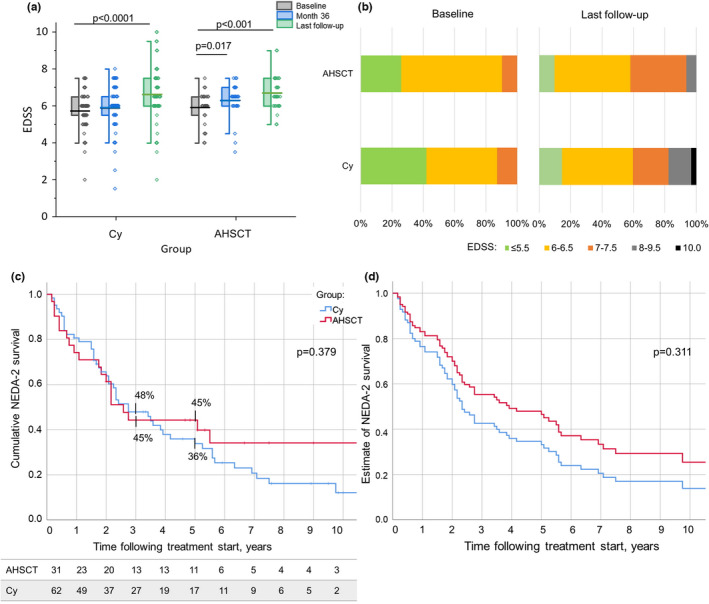
(a) Distribution of Expanded Disability Status Scale (EDSS) at baseline, at Month 36 following treatment start, and at last follow‐up available in the autologous haematopoietic stem cell transplantation (AHSCT; right panel) and cyclophosphamide (Cy; left panel) groups. EDSS deterioration was observed at Month 36 compared to baseline in the AHSCT group (p = 0.017) and at last follow‐up compared to baseline in both groups (p < 0.001 and <0.0001 for the AHSCT and Cy groups, respectively). (b) Proportion of patients with a definite EDSS at baseline and last follow‐up in the two groups. No differences in distribution were observed; at last follow‐up, the proportion of the patients with EDSS ≥ 8 was 6% in the AHSCT group and 18% in the Cy group, p = 0.127. (c) No evidence of disease activity 2 (NEDA‐2) survival (i.e., survival free from both relapses and EDSS worsening) did not differ between groups, being at Year 5 following treatment start 45% in the AHSCT and 36% in the Cy group (p = 0.379). (d) Cox estimated distribution of NEDA‐2 survival in the AHSCT and Cy groups, adjusted for age at treatment, progressive phase duration, and number of previous disease‐modifying treatments received. No differences were observed between the groups, p = 0.311 [Colour figure can be viewed at wileyonlinelibrary.com]
No evidence of disease activity 2
During the whole follow‐up, NEDA‐2 survival at Year 5 was 45% and 36% in the AHSCT and Cy groups, respectively (p = 0.379; Figure 4c). In the treatment epoch, NEDA‐2 survival at Year 2 was 55% in the AHSCT group and 59% in the Cy group (p = 0.815; data not shown). Cox estimates of NEDA‐2 survival did not differ between the groups during both the treatment epoch (p = 0.904; data not shown) and the whole follow‐up (p = 0.311; Figure 4d).
Sensitivity analysis
Thirty‐one AHSCT and 31 Cy‐Sensitivity controls were included (Table S1). No differences were observed between the two groups except for the number of previous DMTs used (p < 0.001). R‐FS was superior in the AHSCT cases compared to the Cy‐Sensitivity controls, being 100% versus 36%, respectively, at Year 3 (p < 0.0001; data not shown). W‐FS did not differ between the groups: in the AHSCT patients 45% at both Years 3 and 5, and in the Cy‐Sensitivity patients 55% at Year 3 and 39% at Year 5 (p = 0.795; Figure [Link], [Link]a). P‐FS was similar in the two groups: at Year 3, 84% in the AHSCT group and 89% in the Cy‐Sensitivity group; at Year 5, P‐FS was 70% in the AHSCT group and 77% in the Cy‐Sensitivity group (p = 0.397; Figure [Link], [Link]b) NEDA‐2 survival at Year 3 was 45% in the AHSCT group and 39% in the Cy group; at Year 5, it was 45% and 28%, respectively (p = 0.231; data not shown). After applying the Cox model, no differences were observed between groups in estimates of both W‐FS and P‐FS (p = 0.931 and 0.475, respectively; Figure [Link], [Link]c,d).
Long‐term adverse events and mortality
In the AHSCT group, early adverse events were aligned with those reported in the literature [22]; three autoimmune thyroiditis (10%) cases were diagnosed over follow‐up.
Three patients were diagnosed with a malignant neoplasm: one of 31 (3%) in the AHSCT group (myeloproliferative disorder at Year 12), two of 62 (3%) in the Cy group (one Hodgkin lymphoma at Month 10 and one kidney cancer at Year 15; p = 1.000). One additional patient in the Cy group showed a persistent increase of the neoplasm marker Ca‐125, but investigations for neoplasms were negative.
Two deaths occurred, both in the Cy group; a male aged 60 years died at Year 17 of follow‐up due to pneumonia, and a woman aged 45 years passed away at Year 8 of follow‐up for intracerebral haemorrhage with thrombocytopenia following splenectomy for spleen infarct. No fatalities were reported in the AHSCT group.
DISCUSSION
In this study, the effectiveness of AHSCT in SP‐MS was compared to that of low‐dose immunosuppression with IV Cy, using relapses and disability as main outcome measures. The hypothesis that AHSCT could modify the disease phenotype (i.e., interrupt progressive disease by the induction of a prolonged stabilization of disability) was also explored, adopting a different outcome measure of progression.
Thirty‐one SP‐MS patients consecutively treated with intermediate‐intensity AHSCT in an open‐label monocentric study were therefore included as cases and matched using cardinality matching with 62 controls selected from a cohort of 108 SP‐MS patients treated with Cy, a drug used over the past 3 decades in SP‐MS supported by the evidence provided by previous studies [11, 23, 24, 25]. Controls were selected among patients who had received an active treatment rather than among those untreated for three main reasons: (1) this treated cohort was followed with a frequency and accuracy higher than any untreated one; (2) by definition, treated patients had a higher “propensity” to receive a treatment than the untreated ones; their choice can thus mitigate potential differences in baseline characteristics between the groups that could bias against the AHSCT arm; and (3) in our opinion, it is more appropriate to compare AHSCT patients with those receiving an alternative active treatment rather than with an untreated cohort of patients, also given the recent approval of a DMT for SP‐MS [4]; however, including a comparator group of patients treated with this latter DMT was not feasible, as the drug was licensed in Italy in 2021 and, to date, only a few patients with short follow‐up duration have received this treatment at our centre.
After matching, baseline characteristics were well balanced between the groups, except for age at treatment and the number of previous DMTs; this could be explained by a selection bias in the AHSCT cases, selected for the particularly aggressive disease course associated with a rate of treatment failure higher than the Cy patients, as this criterion was required for inclusion in the transplant program.
Complete suppression of relapses was observed in the AHSCT group only, to an extent similar to that reported by studies on high‐intensity regimen AHSCT [26], and R‐FS was higher in the AHSCT than in the Cy group. A remarkable reduction in ARR compared to pretreatment was observed in both the groups, which could in part be due to a regression toward the mean—especially in the Cy group. On the other hand, no differences were observed between the groups regarding W‐FS, with rates aligned with the literature [6, 27, 28].
Even if W‐FS is the most frequently used outcome measure for the evaluation of treatment failure in SP‐MS, it does not account for the occurrence of further disability accrual following the first episode of EDSS worsening, an event that might more properly describe the effect of treatments, perhaps optimally assessing whether the progressive disease course typical of SP‐MS is somehow modified by a DMT. The hypothesis that AHSCT might interrupt progressive disease course was therefore explored adopting a different outcome measure of progression, defined as the occurrence of at least two distinct episodes of confirmed disability accrual (P‐FS). No differences in this outcome were observed between the groups, and the proportion of cases showing stabilization of disability either at the baseline level or after a single‐step worsening was 70% in the AHSCT group and 81% in the Cy group at Year 5. In an aggressive MS population such as that selected in AHSCT studies, such an event might be considered a treatment response—although suboptimal—rather than a treatment failure. However, given the nonlinear nature of the EDSS and the variable time required for the transition from one EDSS step to another, this outcome could only be evaluated over a considerably long follow‐up. In this regard, we believe that the retrospective observation of a mean of 8 years in the present study allowed for a proper application of this outcome.
To exclude potential biases due to residual differences in baseline characteristics that persisted even after the matching, a Cox regression model was applied. After this correction, minimal—statistically nonsignificant—differences between groups emerged in the estimates of P‐FS. The size of the effectiveness of AHSCT versus Cy on P‐FS (HR = 0.65) was remarkable but did not reach statistical significance; this might be due to of the low power of the study due to the small sample size. Nonetheless, these data might be useful as an estimate for power calculation of future randomized clinical trials (RCTs) evaluating these outcomes in SP‐MS, an experiment that nowadays can be done with active comparators only because of approval of DMT for SP‐MS.
At last follow‐up, disability increased compared to baseline in both the groups without differences. However, suggestion of a higher frequency of the worse disability scores was observed in Cy patients compared to AHSCT.
To assess the robustness of the main results, a sensitivity analysis including the “best” matched controls only was performed, confirming the primary analysis.
These data indicate that in the patient population studied, AHSCT is remarkably superior to an immunosuppressive active comparator with a similar bioavailability in the central nervous system (CNS), on an inflammation‐associated outcome such as relapses, but on disability the difference—if present—seems marginal. The reason for this apparent paradox can be inferred by the current pathogenetic hypothesis that disability accrual in progressive MS seems to be more degeneration‐driven than inflammation‐driven [29]. However, the mutual relationship between neurodegeneration and inflammation is still debated [30, 31], and a chronic form of inflammation compartmentalized in the CNS seems to be the main driver for disability accrual, particularly in advanced disease [32, 33]. We can therefore speculate that the administration of high doses of lipophilic drugs such as those included in the BEAM protocol, or Cy, might cross the blood–brain barrier [34, 35] and act on compartmentalized inflammation, thus affecting the potential contribution of this latter to disability accrual. In this study, AHSCT did not show any advantages over Cy in disability worsening, but a hint of clinically meaningful superiority was observed in the estimates of P‐FS, with potential benefit in severe disability and mortality over long‐term follow‐up, although this observation warrants further investigations. In the absence of factors predictive of treatment response that allow the identification of SP‐MS patients who could gain benefit from the procedure, in our opinion AHSCT might be offered to selected SP‐MS patients who did not respond to alternative treatment options and who are at low risk of postprocedural complications, according to the judgment of the multidisciplinary team of the transplant centre. The lack of differences between groups in disability outcomes might be due either to ineffectiveness or to a possible, although mild, similar effectiveness of both the treatments on disability accrual. Supporting this latter hypothesis is the observation that Cy exerted a quite identical effect on both relapses and disability compared to mitoxantrone in an open‐labelled head‐to‐head study in SP‐MS [25], and that this latter treatment showed superior effectiveness compared to placebo in an RCT with SP‐MS [36]. However, a definite answer to this issue could be provided only by a matched untreated or “best‐treated” comparator group, and this was not feasible for the reasons stated above.
The safety profile of AHSCT was, overall, acceptable, and no fatalities were observed up to the last follow‐up, whereas two deaths occurred in the Cy group many years after treatment, which does not suggest direct toxicity of the medication. Three cases of malignancies were reported overall. The occurrence of haematological malignancies might be correlated with the administration of chemotherapy‐based treatments, including treatments administered before the transplant, but to our knowledge, no conclusive evidence is available so far on the increased risk of malignancies linked to DMTs or AHSCT for MS, and further investigations are needed to properly solve this relevant issue [6, 37].
Our study has several limitations. First of all, its observational design does not allow the complete exclusion of residual unknown confounders, and therefore potential inclusion biases might remain unbalanced between the two groups, despite the matching. However, potential confounders had been accurately selected. Moreover, the study was accurately designed, generating a dataset that was matched blind to the outcome variables. In addition, the sensitivity analyses performed confirm the robustness of the results. Given that the covariates that are still unbalanced or are unobserved should plausibly lead to an underestimation of the beneficial effect of AHSCT, the results that were obtained could be considered as a lower bound for the true effect.
In conclusion, this study shows that in SP‐MS AHSCT is superior to Cy in suppressing relapses, without statistically significant differences in disability progression. On this latter outcome, high‐intensity immunosuppression does not seem to give advantages over low‐dose immunosuppression, confirming that in SP‐MS progression may become independent from relapses [38], as it is probably based more on neurodegeneration than on new inflammation. However, the hint of superior efficacy of transplant detected on the P‐FS outcome does not exclude a potential benefit of the procedure in selected cases, and it supports the feasibility of RCTs comparing AHSCT with available DMTs, as these studies only can provide conclusive evidence on this issue.
CONFLICT OF INTEREST
A.Mar. reports nonfinancial support from Biogen Idec, Sanofi Genzyme, Novartis, Teva, and Roche, and personal fees from Merck Serono, outside the submitted work. A.M.R. has received personal compensation from Biogen Idec, Genzyme, Novartis, and Merck Serono for public speaking and advisory boards, outside the submitted work. R.S. reports honoraria from Jazz Pharmaceuticals and Sanofi Genzyme, outside the submitted work. L.M. has received educational grants and/or research funds from Fondazione Cassa di Risparmio di Firenze, Biogen, Merck Serono, Genzyme, and Roche and has received honoraria or consultation fees from Biogen, Roche, Mylan, Merck Serono, Genzyme, and Novartis, outside the submitted work. None of the other authors has any conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Alice Mariottini: Conceptualization (equal), data curation (equal), investigation (equal), resources (equal), visualization (lead), writing–original draft (lead). Giovanni Bulgarini: Data curation (lead), visualization (supporting), writing–original draft (supporting). Benedetta Forci: Conceptualization (equal), data curation (equal), investigation (equal), resources (equal). Chiara Innocenti: Data curation (equal), investigation (equal), resources (equal). Fabrizia Mealli: Conceptualization (equal), formal analysis (lead), methodology (lead), validation (equal), writing–review & editing (equal). Alessandra Mattei: Conceptualization (equal), formal analysis (equal), methodology (equal), software (equal), writing–original draft (equal). Chiara Ceccarelli: Formal analysis (lead), methodology (supporting), software (equal). Anna Maria Repice: Investigation (equal), resources (equal). Alessandro Barilaro: Resources (equal). Claudia Mechi: Resources (equal). Riccardo Saccardi: Investigation (equal), resources (equal), supervision (equal), writing–review & editing (equal). Luca Massacesi: Conceptualization (equal), investigation (equal), supervision (equal), writing–review & editing (lead).
Supporting information
ACKNOWLEDGEMENTS
The authors thank the haematological and neurological team that contributed to patient enrolment and caring. Open Access funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
Mariottini A, Bulgarini G, Forci B, et al. Autologous haematopoietic stem cell transplantation versus low‐dose immunosuppression in secondary–progressive multiple sclerosis. Eur J Neurol. 2022;29:1708–1718. doi: 10.1111/ene.15280
The present work was carried out at the Careggi University Hospital, Largo Brambilla 3, 50134 Florence, Italy.
DATA AVAILABILITY STATEMENT
Individual deidentified participant data will be shared upon written request.
REFERENCES
- 1. Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease‐modifying therapy on disease progression in patients with relapsing‐remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321:165‐174. 10.1001/jama.2018.18743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burt RK, Cohen BA, Russell E, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation–based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102:2373‐2378. DOI: 10.1182/blood-2003-03-0877 [DOI] [PubMed] [Google Scholar]
- 3. Ciotti JR, Cross AH. Disease‐modifying treatment in progressive multiple sclerosis. Curr Treat Options Neurol. 2018;20:12. DOI: 10.1007/s11940-018-0496-3 [DOI] [PubMed] [Google Scholar]
- 4. Kappos L, Bar‐Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double‐blind, randomised, phase 3 study. Lancet. 2018;391:1263‐1273. DOI: 10.1016/S0140-6736(18)30475-6 [DOI] [PubMed] [Google Scholar]
- 5. Burman J. Delaying the inevitable: are disease modifying drugs for progressive MS worthwhile? Mult Scler Relat Disord. 2021;54:103134. DOI: 10.1016/j.msard.2021.103134 [DOI] [PubMed] [Google Scholar]
- 6. Muraro PA, Pasquini M, Atkins HL, et al. Long‐term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74:459‐469. DOI: 10.1001/jamaneurol.2016.5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shevchenko JL, Kuznetsov AN, Ionova TI, et al. Long‐term outcomes of autologous hematopoietic stem cell transplantation with reduced‐intensity conditioning in multiple sclerosis: physician's and patient's perspectives. Ann Hematol. 2015;94:1149‐1157. DOI: 10.1007/s00277-015-2337-8 [DOI] [PubMed] [Google Scholar]
- 8. Daumer M, Griffith LM, Meister W, Nash RA, Wolinsky JS. Survival, and time to an advanced disease state or progression, of untreated patients with moderately severe multiple sclerosis in a multicenter observational database: relevance for design of a clinical trial for high dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation. Mult Scler J. 2006;12:174‐179. DOI: 10.1191/135248506ms1256oa [DOI] [PubMed] [Google Scholar]
- 9. Agency A‐IM . https://www.aifa.gov.it/documents/20142/1288746/Allegato‐4_Neurologia_04.01.2021.pdf. Accessed July 20, 2021. 2021.
- 10. Brochet B, Deloire MS, Perez P, et al. Double‐blind controlled randomized trial of cyclophosphamide versus methylprednisolone in secondary progressive multiple sclerosis. PLoS One. 2017;12:e0168834. DOI: 10.1371/journal.pone.0168834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Canadian Cooperative Multiple Sclerosis Study Group . The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet. 1991;337:441‐446. [PubMed] [Google Scholar]
- 12. Gómez‐Figueroa E, Gutierrez‐Lanz E, Alvarado‐Bolaños A, et al. Cyclophosphamide treatment in active multiple sclerosis. Neurol Sci. 2021;42:3775‐3780. doi: 10.1007/s10072-021-05052-1 [DOI] [PubMed] [Google Scholar]
- 13. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840‐846. DOI: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 14. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol. 1983;13(3):227‐231. DOI: 10.1002/ana.410130302 [DOI] [PubMed] [Google Scholar]
- 15. Rubin DB. The designversus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26:20‐36. DOI: 10.1002/sim.2739 [DOI] [PubMed] [Google Scholar]
- 16. Sharrack B, Saccardi R, Alexander T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune‐mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. 2019;55:283‐306. Doi: 10.1038/s41409-019-0684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444‐1452. DOI: 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 18. Mariottini A, Filippini S, Innocenti C, et al. Impact of autologous haematopoietic stem cell transplantation on disability and brain atrophy in secondary progressive multiple sclerosis. Mult Scler. 2020;27(1):61‐70. [DOI] [PubMed] [Google Scholar]
- 19. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1‐21. DOI: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zubizarreta JR, Paredes RD, Rosenbaum PR. Matching for balance, pairing for heterogeneity in an observational study of the effectiveness of for‐profit and not‐for‐profit high schools in Chile. Ann Appl Stat. 2014;8:204‐231. [Google Scholar]
- 21. E9 I . Addendum on estimands and sensitivity analysis in clinical trials. 2019. [DOI] [PubMed]
- 22. Muraro PA, Martin R, Mancardi GL, et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13:391‐405. DOI: 10.1038/nrneurol.2017.81 [DOI] [PubMed] [Google Scholar]
- 23. Perini P, Gallo P. Cyclophosphamide is effective in stabilizing rapidly deteriorating secondary progressive multiple sclerosis. J Neurol. 2003;250:834‐838. DOI: 10.1007/s00415-003-1089-x [DOI] [PubMed] [Google Scholar]
- 24. Zephir H, de Seze J, Duhamel A, et al. Treatment of progressive forms of multiple sclerosis by cyclophosphamide: a cohort study of 490 patients. J Neurol Sci. 2004;218:73‐77. DOI: 10.1016/j.jns.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 25. Perini P, Calabrese M, Tiberio M, et al. Mitoxantrone versus cyclophosphamide in secondary‐progressive multiple sclerosis: a comparative study. J Neurol. 2006;253:1034‐1040. DOI: 10.1007/s00415-006-0154-7 [DOI] [PubMed] [Google Scholar]
- 26. Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem‐cell transplantation for aggressive multiple sclerosis: a multicentre single‐group phase 2 trial. Lancet. 2016;388:576‐585. DOI: 10.1016/S0140-6736(16)30169-6 [DOI] [PubMed] [Google Scholar]
- 27. Boffa G, Massacesi L, Inglese M, et al. Long‐term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology. 2021;96:e1215‐e1226. doi: 10.1212/WNL.0000000000011461 [DOI] [PubMed] [Google Scholar]
- 28. Nicholas RS, Rhone EE, Mariottini A, et al. Autologous haematopoietic stem cell transplantation in active multiple sclerosis: a real‐world case series. Neurology. 2021;97:e890‐e901. doi: 10.1212/WNL.0000000000012449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2019;9:3116. DOI: 10.3389/fimmu.2018.03116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutchinson M. Neurodegeneration in multiple sclerosis is a process separate from inflammation: no. Mult Scler J. 2015;21:1628‐1631. DOI: 10.1177/1352458515612244 [DOI] [PubMed] [Google Scholar]
- 31. Louapre C, Lubetzki C. Neurodegeneration in multiple sclerosis is a process separate from inflammation: yes. Mult Scler. 2015;21:1626‐1628. DOI: 10.1177/1352458515587598 [DOI] [PubMed] [Google Scholar]
- 32. Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 2019;76:1474. doi: 10.1001/jamaneurol.2019.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78:710‐721. DOI: 10.1002/ana.24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egorin MJ, Kaplan RS, Salcman M, et al. Cyclophosphamide plasma and cerebrospinal fluid kinetics with and without dimethyl sulfoxide. Clin Pharmacol Ther. 1982;32:122‐128. DOI: 10.1038/clpt.1982.135 [DOI] [PubMed] [Google Scholar]
- 35. Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295‐2305. DOI: 10.1200/JCO.2006.09.9861 [DOI] [PubMed] [Google Scholar]
- 36. Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo‐controlled, double‐blind, randomised, multicentre trial. Lancet. 2002;360:2018‐2025. DOI: 10.1016/S0140-6736(02)12023-X [DOI] [PubMed] [Google Scholar]
- 37. Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease‐modifying drugs. CNS Drugs. 2018;32:939‐949. DOI: 10.1007/s40263-018-0564-y [DOI] [PubMed] [Google Scholar]
- 38. Tremlett H, Yousefi M, Devonshire V, et al. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology. 2009;73:1616‐1623. DOI: 10.1212/WNL.0b013e3181c1e44f [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual deidentified participant data will be shared upon written request.


