Abstract
Background and Purpose
Angiocentric gliomas (AGs) are epileptogenic low‐grade gliomas in young patients. We aimed to investigate the MRI findings of AGs and systematically review previous publications and three new cases.
Methods
We searched PubMed, Elsevier's abstract and citation database, and Embase databases and included 50 patients with pathologically proven AGs with analyzable preoperative MRI including 3 patients from our institution and 47 patients from 38 publications (median age, 13 years [range, 2‐83 years]; 35 men). Two board‐certified radiologists reviewed all images. The relationships between seizure/epilepsy history and MRI findings were statistically analyzed. Moreover, clinical and imaging differences were evaluated between supratentorial and brainstem AGs.
Results
Intratumoral T1‐weighted high‐intensity areas, stalk‐like signs, and regional brain parenchymal atrophy were observed in 23 out of 50 (46.0%), 10 out of 50 (20.0%), and 14 out of 50 (28.0%) patients, respectively. Intratumoral T1‐weighted high‐intensity areas were observed significantly more frequently in patients with stalk‐like signs (positive, 9/10 vs. negative, 14/40, p = .0031) and regional atrophy (13/14 vs. 10/36, p = .0001). There were significant relationships between the length of seizure/epilepsy history and presence of intratumoral T1‐weighted high‐intensity area (median 3 years vs. 0.5 years, p = .0021), stalk‐like sign (13.5 vs. 1 year, p < .0001), and regional atrophy (14 vs. 0.5 years, p < .0001). Patients with brainstem AGs (n = 7) did not have a seizure/epilepsy history and were significantly younger than those with supratentorial AGs (median, 5 vs. 13 years, p < .0001, respectively).
Conclusions
Intratumoral T1‐weighted high‐intensity areas, stalk‐like signs, and regional brain atrophy were frequent imaging features in AG. We also found that affected age was different between supratentorial and brainstem AGs.
Keywords: angiocentric glioma, magnetic resonance imaging, systematic review
INTRODUCTION
Angiocentric gliomas (AGs) are rare tumors of the central nervous system (CNS) that tend to occur in the supratentorial superficial regions of young patients. AGs were first recognized as distinct World Health Organization (WHO) grade 1 tumors owing to their indolent nature in the 2007 WHO classification of CNS tumors. 1 Recently, revised WHO classification of CNS tumors in 2021 classified AG as one of the “Pediatric‐type diffuse low‐grade gliomas.” 2
Clinically, patients with AGs often present with pharmacoresistant seizures/epilepsies because AGs tend to be superficially located. Seizures/epilepsies improve in most cases where complete resection is performed. 3 Radiologically, two characteristic features of AGs have been reported—high intensity on T1‐weighted imaging (T1WI) and stalk‐like T2‐weighted imaging (T2WI)/fluid‐attenuated inverted recovery (FLAIR) high‐intensity lesions extending to the ventricle. 3 However, the frequency of these MRI findings is unknown, and several authors have reported cases without these “pathognomonic” features. 4 , 5 , 6 , 7 , 8
The purpose of this systematic review was to investigate the frequency of different MRI findings of AGs and to explore the yet unidentified features that may be characteristic. To the best of our knowledge, this systematic review presents the largest cohort (n = 50) with analyzable MR images, including three new cases from our hospital.
METHODS
Study selection
We searched PubMed, Elsevier's abstract and citation database (SCOPUS), and Embase databases using the following search terms on July 6, 2021, without any language or date limits:
(angiocentric glioma) AND ((radiology) OR (neuroradiology) OR (imaging) OR (magnetic resonance) OR (MRI)) for PubMed;
ALL ((angiocentric AND glioma) AND ((radiology) OR (neuroradiology) OR (imaging) OR (magnetic AND resonance) OR (MRI))) for SCOPUS;
angiocentric AND glioma AND ((radiology OR neuroradiology OR imaging) OR (magnetic AND resonance) OR MRI) for Embase.
Publications were considered eligible if they included all of the following criteria:
The tumors were pathologically proven AGs.
Preoperative T1WI (either pre‐contrast or post‐contrast enhanced) and T2WI or FLAIR images were available.
Each patient's demographic data were available.
Exclusion criterion was as follows:
The full text was unavailable.
Non‐English references were translated into English using Google Translate (www.translate.google.com) and reviewed. We also obtained our institutional review board exemption for the inclusion of three unpublished cases with pathologically proven AGs with preoperative MR images from our hospital. Data were acquired in compliance with all applicable Health Insurance Portability and Accountability Act regulations.
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 statement. 9
Data analyses
Two board‐certified radiologists independently reviewed all studies and MR images of the eligible exams. When discrepancies arose between the two reviewers, another board‐certified radiologist made the tiebreaker decisions.
Collected data
The following data were collected:
Demographic
Patient age at diagnosis
Sex
Clinical
Presenting complaint
Seizure/epilepsy history
Treatment strategy
Recurrence after complete resection
Period between the initial surgery and tumor recurrence
Survival status
Follow‐up duration
Imaging
Tumor size, laterality, and region
Tumor margin status
Involvement of both cortex and subcortex
Cystoid component: defined as a nonenhancing component with a cerebrospinal fluid‐like high intensity on T2WI and low intensity on T1WI.
T2WI, FLAIR, and T1WI signal intensity of noncystoid and cystoid components: signal intensities were compared with the cortices on each sequence.
Contrast enhancement pattern
Diffusion restriction: Mean apparent diffusion coefficient was calculated in the three cases from our hospital in a single slice while avoiding cystoid component.
Massive surrounding edema: determined when the area of the edema was equal to or larger than the tumor size.
Stalk‐like sign: defined as T2WI or FLAIR hyperintensity that tapers toward the lateral ventricle
Atrophy of the brain parenchyma near the tumor: We assumed that atrophy can occur in the brain parenchyma near AGs due to the epileptogenic nature of the tumor as is the case with low‐grade epilepsy associated tumors by Al‐Hajri et al. 10
MR spectroscopy findings
Perfusion MRI findings
The description of each study was extracted with respect to the presence or absence of contrast enhancement on post‐enhanced T1WI. In cases with high intensity on postcontrast T1WI, where the authors of each study stated that there was no contrast enhancement, intratumoral pre‐enhanced T1WI high intensity was considered present without analyzable pre‐enhanced T1WI images. When the signal intensity was not as hyperintense as vessels and subcutaneous fat, the tumors were deemed not to be enhanced on postcontrast T1WI without pre‐enhanced T1WI images.
Risk of bias assessment
We employed a tool to evaluate the methodological quality of case reports and case series proposed by Murad et al. 11 This tool comprises eight signaling questions in four domains: selection, ascertainment, causality, and reporting.
Statistical analysis
The frequency of high intensity on T1WI was compared between the presence and absence of the stalk‐like sign and atrophy of the tumor site using Fisher's exact tests. Age at diagnosis was compared between patients with supratentorial AGs and brainstem AGs using the Mann‐Whitney U‐test. To evaluate the relationship between seizure history and AG characteristic MRI findings, we performed Mann‐Whitney U‐tests, comparing the period since the initiation of seizure/epilepsy between the presence and absence of intratumoral T1WI high‐intensity area, stalk‐like sign, and atrophy of the surrounding brain parenchyma of the tumor, respectively. Family wise error‐corrected two‐sided p‐values <.05 using Bonferroni's method were considered statistically significant. All statistical analyses were performed using R software (version 4.0.0; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study selection
A database search using PubMed, SCOPUS, and Embase identified 501 abstracts. We screened these results according to the PRISMA 2020 guideline 9 and narrowed them down to 78 potentially eligible studies by removing duplications, irrelevant studies by title and abstract screening, and studies where full text was unavailable. After excluding an additional 40 studies based on the exclusion criteria, 38 studies with 47 patients with AGs satisfied the criteria for the systematic review. 4 , 5 , 6 , 7 , 8 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 In six studies, cases that did not meet the criteria were excluded. 4 , 21 , 30 , 34 , 38 , 39 The study selection process is summarized in the flow diagram in Figure 1. The studies in this systematic review were conducted between 2007 and 2020. In addition, we included three unpublished patients with AGs from our hospital (Table 1), resulting in a final study cohort of 50 patients.
FIGURE 1.
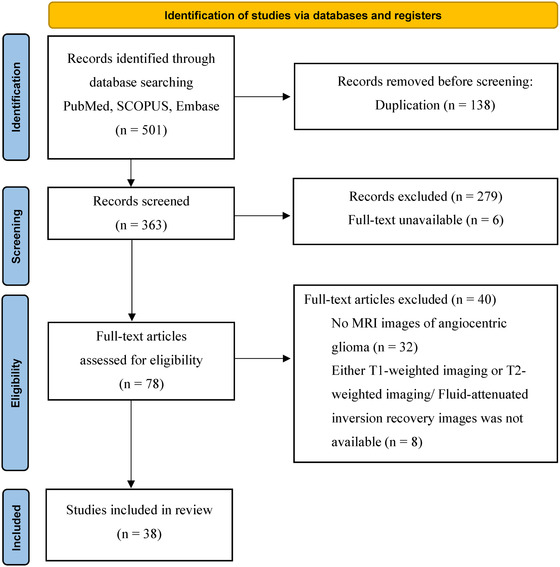
Flow diagram of study identification. SCOPUS is Elsevier's abstract and citation database. n, number
TABLE 1.
Demographic, clinical, and imaging data of the 3 patients with angiocentric gliomas in our hospital
| Demographic & clinical data | Patient | 1 | 2 | 3 | |
| Age at diagnosis (years) | 2 | 43 | 10 | ||
| Sex | Male | Male | Male | ||
| Seizure/epilepsy | No | Yes | Yes | ||
| Seizure/epilepsy started (years) | 8 | 10 | |||
| Surgery | No (biopsy‐proven) | Yes | Yes | ||
| Chemotherapy | Yes | No | No | ||
| Recurrence, period (from surgery) | No | No | |||
| Patient status | Survive | Survive | Survive | ||
| Follow up duration (month) | 39 | 72 | 38 | ||
| Imaging data | Size (mm: Anteroposterior × transverse × craniocaudal) | 38 × 40 × 41 | 8 × 9 × 10 | 24 × 28 × 21 | |
| Laterality | Middle | Left | Left | ||
| Tumor site | Pons, Medulla | Frontal lobe | Occipital lobe | ||
| Tumor margin | Well | Well | Well | ||
| Involvement of both cortex and subcortex | Yes | Yes | |||
| Massive surrounding edema (≥tumor size) | No | No | No | ||
| Morphology | Solid | Cystoid | Solid | ||
| MRI signal intensity | T2‐weighted image (compared with cortex) | High | High | High | |
| Fluid‐attenuated inversion recovery image (compared with cortex) | High | High & Low | High | ||
| T1‐weighted image (compared with cortex) | Low | High & Low | Low | ||
| Apparent diffusion coefficient (10–3mm2/s) | 1.6 | 1.04 | 1.34 | ||
| Stalk‐like sign | No | Yes | Yes | ||
| Atrophy of the brain parenchyma near the tumor site | No | Yes | No | ||
| Contrast enhancement | No | No | Nodular |
Risk of bias assessment
As we extracted data from case‐based studies, where the selection method was rarely mentioned, selection bias may have been introduced. Tumor size was available in only 21 out of 50 cases (42.0%). Treatment strategy and outcomes were ascertained in 48 out of 50 (96.0%) and 41 out of 50 (82.0%) patients, respectively. The follow‐up duration of the patients varied from 2 months to 7 years. Because we evaluated the cases with preoperative MR images, our research can be replicated by other investigators.
Demographic and clinical data
The demographic and clinical data of 50 patients are summarized in Table 2. The median age at diagnosis was 13 years (range, 2‐83 years). Patients 10‐19 years of age (22/50, 44.0%), followed by those under 10 years (17/50, 35.0%), were mainly affected; however, 11 out of 50 patients (22.0%) were over 20 years old (20‐29 years, 6/50 [12.0%]; 30‐39 years, 1/50 [2.0%]; 40‐49 years, 3/50 [6.0%], and an 83‐year‐old man). Male patients (35/50, 70.0%) were more frequently affected than female patients (14/50, 28.0%; the sex of one patient was not mentioned 40 ).
TABLE 2.
Demographic and clinical information of the 50 patients with angiocentric gliomas
| Demographic | |
|---|---|
| Median age at diagnosis (years [range]) | 13 [2‐83] |
| Sex | Male = 35, Female = 14, Not described = 1 |
| Clinical | |
| Seizure/epilepsy | 36/50 (72.0%) |
| Median length of seizure/epilepsy history (years [range])a | 2 [<1‐35] |
| Treatment strategy | |
| Surgery alone | 39/48 (81.3%) |
| Surgery and radiation | 1/48 (2.1%) |
| Surgery and chemotherapy | 2/48 (4.2%) |
| Chemotherapy alone | 3/48 (6.3%) |
| Chemotherapy and radiation | 1/48 (2.1%) |
| Follow‐up | 1/48 (2.1%) |
| Recurrence after complete resection | 2/29 (6.9%) |
| Patient status | Survive = 40/41 (97.6%), Death = 1/41 (2.4%) |
| Follow‐up duration (median [range]) (41 patients) | 16 months [2‐84] |
The length was calculated by subtracting the age of seizure/epilepsy onset from age at diagnosis. The length of the patients presenting with their first episode of seizure/epilepsy was calculated as zero (6 cases). Cases without a specific history length were excluded from the calculation (7 cases).
The majority of patients (36/50, 72.0%) had a history of seizure or epilepsy of 1‐35 years. None of the patients with brainstem AG had seizure/epilepsy history, but they presented with facial weakness (case 1), 17 , 18 recurrent pneumonia, 17 double vision, 18 irregular gait, 35 and headache with vomiting. 18 , 35 Surgery alone (39/48, 81.3%) was the most commonly employed treatment strategy, and the incidence of tumor recurrence after complete resection was low (2/29, 6.9%).
Neuroimaging data
The neuroimaging findings are summarized in Table 3. AGs were located in the brainstem in seven patients (7/50, 14.0%; pons, n = 3 [16, 17]; midbrain, n = 2 18 , 32 ; pons and medulla, n = 1 [case 1, Figure 2]; pons and cerebellum, n = 1 35 ). Most tumors involved both cortical and subcortical areas (39/50, 78.0%), followed by brainstem (7/50, 14.0%), subcortical area alone (2/50, 4.0%), diffuse (1/50, 2.0%), and thalamus (1/50, 2.0%). Cystoid components were observed in 28 out of 50 patients (56.0%). Contrast enhancement was observed in 11 out of 42 patients (26.8%); notably, brainstem AGs were not enhanced (0/7 patients, 0%). Intratumoral T1WI high‐intensity area, stalk‐like sign, and atrophy of the brain parenchyma near the tumor were observed in 23 out of 50 (46.0%), 10 out of 50 (20.0%), and 14 out of 50 (28.0%) patients, respectively. Three patients were reported to have adjacent focal cortical dysplasia (FCD), including 2 patients with intratumoral T1WI high‐intensity areas, stalk‐like signs, and atrophy of the surrounding brain parenchyma. 25 , 43 Perfusion MRI was performed in only one study, 14 in which elevated cerebral blood flow and volume were observed on dynamic susceptibility contrast perfusion MRI. On MR spectroscopy, decreased levels of N‐acetyl aspartate, elevated creatine and choline, and lactate peaks were observed in 4 out of 4 (100%), 2 out of 4 (50.0%), and 2 out of 4 (50.0%) patients, respectively. The MRI findings of the three patients from our hospital are shown in Figures 2, 3, 4.
TABLE 3.
Neuroimaging characteristics of the 50 patients with angiocentric gliomas
| Parameters | ||
|---|---|---|
| Size (median [range]) (21 tumors)a | 26 mm [10‐70] | |
| Laterality | ||
| Right | 14/50 (28.0%) | |
| Left | 28/50 (56.0%) | |
| Middle | 7/50 (14.0%) | |
| Diffuse | 1/50 (2.0%) | |
| Location | ||
| Frontal lobe | 22/50 (44.0%) | |
| Parietal lobe | 10/50 (20.0%) | |
| Temporal lobe | 15/50 (30.0%) | |
| Insula | 4/50 (8.0%) | |
| Basal ganglia | 4/50 (8.0%) | |
| Corpus callosum | 2/50 (4.0%) | |
| Brainstem | 7/50 (14.0%) | |
| Involvement of both cortex and subcortex | 39/50 (78.0%) | |
| Cystoid component | 28/50 (56.0%) | |
| T2‐weighted imaging signal intensity | Noncystoid component | Cystoid component |
| High intensity | 30/32 (93.8%) | 25/25 (100%) |
| Isointensity | 2/32 (6.2%) | |
| Fluid‐attenuated inversion recovery signal intensity | Noncystoid component | Cystoid component |
| High intensity | 27/29 (93.1%) | 2/16 (12.5%) |
| Isointensity | 2/29 (6.9%) | 0 |
| Low intensity | 0 | 14/16 (87.5%) |
| T1‐weighted imaging signal intensity | Noncystoid component | Cystoid component |
| High intensity | 15/40 (37.5%) | 4/28 (14.3%) |
| Isointensity | 4/40 (10.0%) | 1/28 (3.6%) |
| Low intensity | 13/40 (32.5%) | 23/28 (82.1%) |
| High and isointensity | 1/40 (2.5%) | 0 |
| High and low intensity | 5/40 (12.5%) | 0 |
| Iso‐ and low intensity | 2/40 (5.0%) | 0 |
| Intratumoral T1‐weighted imaging high‐intensity area | 23/50 (46.0%) | |
| Massive surrounding edema (≥tumor size) | 10/50 (20.0%) | |
| Stalk‐like sign | 10/50 (20.0%) | |
| Atrophy of the surrounding brain parenchyma of the tumor | 14/50 (28.0%) | |
| Contrast enhancement | ||
| Any | 11/41 (26.8%) | |
| Homogeneous | 1/41 (2.4%) | |
| Heterogeneous | 5/41 (12.2%) | |
| Nodular | 2/41 (4.9%) | |
| Rim | 2/41 (4.9%) | |
| Scarce | 1/41 (2.4%) | |
| Diffusion restriction | 1/8 (12.5%) | |
In cases where measurements in multiple directions were performed, the maximum value was used for the calculation of the tumor diameter.
FIGURE 2.
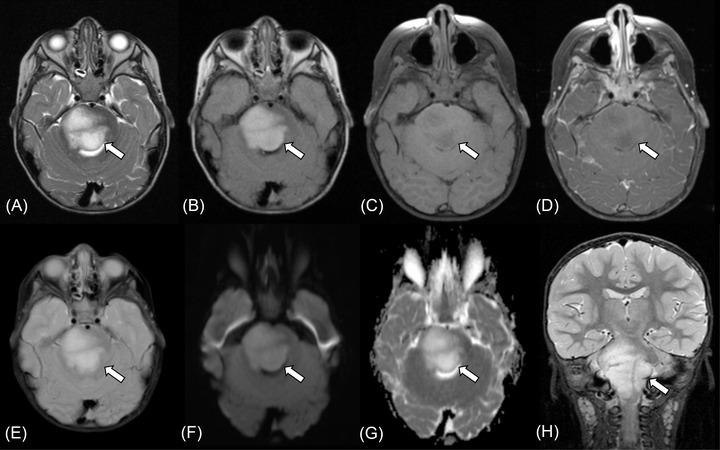
Brainstem angiocentric glioma in a 2‐year‐old boy presenting with right facial weakness (case 1). MRI shows a 38 × 40 × 41 mm mass in the pontomedullary region (A‐H, arrows). The tumor shows high intensity on T2‐weighted image (A) and fluid‐attenuated inversion recovery image (B), low intensity on T1‐weighted image (C) without contrast enhancement (D). T2*‐weighted image does not show intratumoral calcification or hemorrhage (E). Diffusion restriction is not observed with the mean apparent diffusion coefficient value of 1.60 × 10–3 mm2/s (F, G). Fat‐suppressed coronal T2‐weighted image shows infiltrating growth of the tumor with an ill‐defined margin (H)
FIGURE 3.
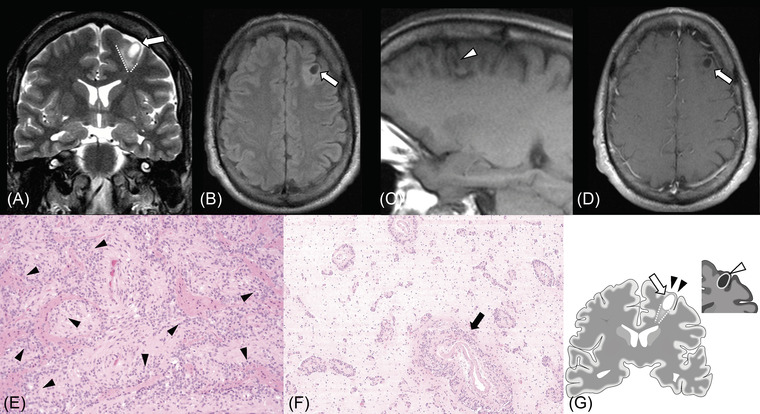
Supratentorial angiocentric glioma in a 43‐year‐old male presenting with refractory focal epilepsy, which started 35 years prior (case 2). MRI shows an 8 × 9 × 10 mm mass in the left frontal lobe (white arrows). The tumor contains a cystic area that is hyperintense on T2‐weighted image (A) and hypointense on fluid‐attenuated inversion recovery image (B). The stalk‐like sign (A, dotted lines) with focal atrophy of the surrounding brain parenchyma are observed on T2‐weighted coronal image. The rim of the tumor shows high intensity on precontrast enhanced T1‐weighted image (C, white arrowhead). No contrast enhancement is observed (D). Diffusion restriction is not observed with the mean apparent diffusion coefficient value of 1.04 × 10–3 mm2/s (not shown). Hematoxylin and eosin sections of the tumor demonstrate monomorphic low‐grade glial cells with an angiocentric growth pattern (E, black arrowheads; 40×) and extensive infiltration along blood vessels (F, black arrow; 10×). No calcification nor hemosiderin‐laden macrophages are observed. The main imaging features are represented in the illustration: The tumor (white arrow), the stalk‐like sign (dotted lines), focal atrophy (black arrowheads), and intratumoral T1‐weighted high intensity (white arrowhead) (G)
FIGURE 4.
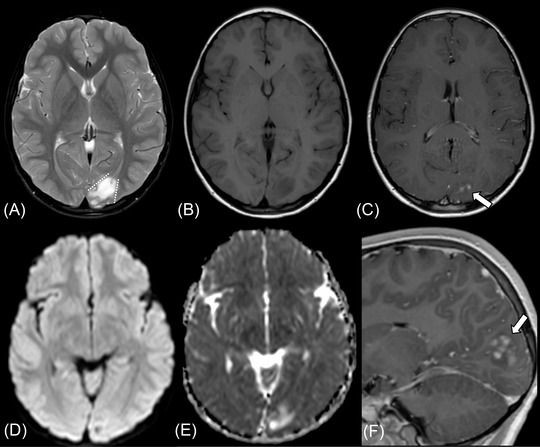
Supratentorial angiocentric glioma in a 10‐year‐old male presenting with the first episode of epilepsy (case 3). The tumor shows high intensity on fat‐suppressed T2‐weighted image (A) and fluid‐attenuated inversion recovery image (not shown) and low intensity on T1‐weighted image (B). The stalk‐like sign is observed without evidence of atrophy in the surrounding brain parenchyma (A, dotted lines). Diffusion restriction is not observed with the mean apparent diffusion coefficient value of 1.34 × 10–3 mm2/s (D, E). Nodular enhancement is observed in the postcontrast sagittal T1‐weighted image (C, F, thick arrows)
Statistical analyses
The results are summarized in the Table 4. Intratumoral T1WI high‐intensity areas were observed significantly more frequently in cases with stalk‐like signs (positive, 9/10 vs. negative, 14/40, p = .0031) and atrophy of the surrounding brain parenchyma of the tumor (positive, 13/14 vs. 10/36, p = .0001). Age at diagnosis was significantly lower in patients with brainstem AGs (median, 5 years [range, 2‐7 years]) than in those with supratentorial AGs (median, 13 years [range, 2‐83 years], p < .0001). Significant relationships were observed between the length of seizure/epilepsy and the presence of intratumoral T1WI high‐intensity area (median, 3 years [range, <1‐35 years] vs. absent, 0.5 [<1‐10], p = .0021), stalk‐like sign (13.5 years [<1‐35] vs. 1 year [<1‐14], p < .0001), and atrophy of the surrounding brain parenchyma of the tumor (14 years [1‐35] vs. 0.5 [0‐10], p < .0001).
TABLE 4.
Statistical analyses of clinical and imaging findings
| Relationship between MRI findings | Intratumoral T1WI high‐intensity area | p‐value |
|---|---|---|
| Stalk‐like sign | ||
| Positive (n = 10) | 9/10 (90.0%) | .0031* (Fisher's exact test) |
| Negative (n = 40) | 14/40 (35.0%) | |
| Atrophy of the surrounding brain parenchyma of the tumor | ||
| Positive (n = 14) | 13/14 (92.9%) | .0001* (Fisher's exact test) |
| Negative (n = 36) | 10/36 (27.8%) | |
| Tumor location (n = 50) | Age at diagnosis (median years [range]) | p‐value |
| Supratentorial angiocentric glioma (n = 43) | 13 [2‐83] | <.0001* (Mann‐Whitney U test) |
| Brainstem angiocentric glioma (n = 7) | 5 [2‐7] | |
| MRI findings in patients with seizure/epilepsy history (n = 29) | Seizure/epilepsy history length (median years [range])a | p‐value |
| Intratumoral T1WI high‐intensity area | ||
| Positive (n = 15) | 3 [<1‐35] | .0021* (Mann‐Whitney U‐test) |
| Negative (n = 14) | 0.25 [<1‐10] | |
| Stalk‐like sign | ||
| Positive (n = 8) | 13.5 [<1‐35] | <.0001* (Mann‐Whitney U‐test) |
| Negative (n = 21) | 1 [<1‐14] | |
| Atrophy of the surrounding brain parenchyma of the tumor | ||
| Positive (n = 9) | 14 [1‐35] | <.0001* (Mann‐Whitney U‐test) |
| Negative (n = 20) | 0.5 [<1‐10] | |
Abbreviations: n, number; T1WI, T1‐weighted image.
The length was calculated by subtracting the age of seizure/epilepsy onset from age at diagnosis. Length for the patients who presented with the first episode of seizure/epilepsy was calculated as zero (n = 6). Cases without mentioning the specific history length were excluded from the calculation (n = 7).
*Statistically significant.
DISCUSSION
In this systematic review of 50 patients with AGs, we found that AGs were most frequent in the supratentorial regions (43/50, 86.0%), with frequent involvement of both cortical and subcortical regions (39/50, 78.0%), and that patients under 20 years of age were the most affected (39/50, 78.0%). The majority of the patients had a seizure/epilepsy history (36/50, 72.0%) with a variety of lengths (median, 2 years; range, <1‐35 years). Other than these previously known features, we found the incidence of the characteristic neuroimaging features, including intratumoral T1WI high‐intensity area (23/50, 46.0%), stalk‐like sign (10/50, 20.0%), and atrophy of the surrounding brain parenchyma of the tumor (14/50, 28.0%). Furthermore, several unidentified clinical and imaging correlations were found: there were strong relationships between the length of seizure/epilepsy history and the presence of the aforementioned MRI findings; age at diagnosis was significantly lower in patients with brainstem AGs than in those with supratentorial AGs. To the best of our knowledge, this is the largest systematic review of neuroimaging findings in AGs.
Patients often have a long history of intractable seizures or epilepsy prior to surgical resection. 3 Surgical resection is the mainstay treatment strategy, and the majority of AG cases are curable by complete resection. 3 On the other hand, we found that patients with brainstem AGs did not have a history of seizure or epilepsy (n = 7). 17 , 18 , 32 , 35 This discrepancy indicates that the epileptogenesis of AG may be caused by its frequent location involving the cortex, not by its histopathological profile. There was also a significant difference in the age at diagnosis between patients with supratentorial and brainstem AGs (median, 13 years vs. 5 years, p < .0001, respectively). We suspect that patients with brainstem AGs were more likely to present with symptoms that required brain imaging, such as cranial nerve palsy and intracranial hypertension. To clarify this hypothesis, further studies with more cases of brainstem AG are necessary.
Neuroimaging features of AGs, such as high intensity on T1WI, nonenhancement, superficial location, and stalk‐like sign on T2WI/FLAIR images, have been noted in previous studies 8 , 27 , 30 , 31 , 44 , 45 ; however, information on the frequency of each finding has been limited. In this study, we demonstrated the frequency of each neuroimaging finding. For example, the well‐known stalk‐like sign was found not to be frequent (10/50, 20.0%). Contrast enhancement was observed in more than one‐fourth of patients (11/41, 26.8%), even though AGs are generally considered to have no contrast enhancement.
Intratumoral T1WI high‐intensity areas were significantly more common in patients with regional atrophy and stalk‐like signs. The length of seizure/epilepsy history was significantly longer in patients with intratumoral T1WI high‐intensity areas, stalk‐like signs, and regional atrophy. These findings suggest that T1WI high‐intensity areas in AGs may reflect some chronic process, although we were not able to confirm the correlation with pathological findings in the literature and our own cases. 8 Regarding the stalk‐like sign, we assume that it reflects several different pathologies, such as tumor infiltration along with the vessels extending from near the brain surface toward the ventricle, peritumoral gliosis caused by long‐standing tumor existence or seizure‐induced oxidative stress, and coexisting FCD, as is the case with polymorphous low‐grade neuroepithelial tumor of the young. 46 Indeed, the coexistence of AG and FCD was pathologically proven in three cases, 25 , 43 including two cases with stalk‐like signs. FCD may cause longstanding seizure/epilepsy, and conversely, a long seizure/epilepsy history may cause regional atrophy and brain parenchymal degeneration around the tumor. The longstanding presence of AG also explains the high incidence of tumor‐associated seizure/epilepsy‐induced brain atrophy and stalk‐like tumor extension. Further studies with a radiology‐pathology correlation may help elucidate the pathological background of the imaging findings.
There were some limitations to this study. First, the number of patients was small, although this study presented the largest cohort of AGs with analyzable neuroimaging findings. Second, neuroimaging findings were evaluated using limited images attached to each article and not by serial images. However, to mitigate the risk of inappropriate assessment, we performed an imaging investigation with the help of three board‐certified radiologists. Third, there was an uncertainty about the presence/absence of some signs in the missing sets of images in the literature cases. This may have introduced the bias on the frequency of each imaging finding. Fourth, there were missing data due to the heterogeneity of the collected studies, including tumor size and findings of advanced MRI sequences, such as perfusion MRI and MR spectroscopy. Further studies using these advanced sequences are required.
CONCLUSION
AGs are epileptogenic low‐grade gliomas that frequently occur in superficial supratentorial regions in patients under 20 years of age. Brainstem AGs were observed in younger patients and did not cause seizures or epilepsy. Characteristic imaging findings of AGs (i.e., intratumoral T1WI high‐intensity areas, stalk‐like signs, and atrophy of the surrounding brain parenchyma) may be induced by the indolent nature of AGs and associated longstanding seizure/epilepsy and/or coexisting FCD and gliosis.
ACKNOWLEDGMENTS AND DISCLOSURE
We would like to thank Editage (http://www.editage.com) for editing and reviewing this manuscript for English language.
Kurokawa R, Baba A, Emile P, Kurokawa M, Ota Y, Kim J, et al. Neuroimaging features of angiocentric glioma: A case series and systematic review. 2022;32:389–399. 10.1111/jon.12983
Funding informationNone.
REFERENCES
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 2021;23:1231‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ampie L, Choy W, DiDomenico JD, et al. Clinical attributes and surgical outcomes of angiocentric gliomas. J Clin Neurosci 2016;28:117‐22. [DOI] [PubMed] [Google Scholar]
- 4. Li JY, Langford LA, Adesina A, et al. The high mitotic count detected by phospho‐histone H3 immunostain does not alter the benign behavior of angiocentric glioma. Brain Tumor Pathol 2012;29:68‐72. [DOI] [PubMed] [Google Scholar]
- 5. Koral K, Koral KM, Sklar F. Angiocentric glioma in a 4‐year‐old boy: imaging characteristics and review of the literature. Clin Imaging 2012;36:61‐4. [DOI] [PubMed] [Google Scholar]
- 6. Kakkar A, Sharma MC, Suri V, et al. Angiocentric glioma: a treatable cause of epilepsy: report of a rare case. Neurol India 2012;62:677‐9. [DOI] [PubMed] [Google Scholar]
- 7. Harmsen H, Mobley BC, Davis LT. Angiocentric glioma mimicking encephalomalacia. Radiol Case Rep 2019;14:700‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amemiya S, Shibahara J, Aoki S, et al. Recently established entities of central nervous system tumors: review of radiological findings. J Comput Assist Tomogr 2008;32:279‐85. [DOI] [PubMed] [Google Scholar]
- 9. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al‐Hajri A, Al‐Mughairi S, Somani A, et al. Pathology‐MRI correlations in diffuse low‐grade epilepsy associated tumors. J Neuropathol Exp Neurol 2017;76:1023‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barman A, Nath A, Thakur D. Identification and characterization of fungi associated with blister blight lesions of tea (Camellia sinensis L. Kuntze) isolated from Meghalaya, India. Microbiol Res 2020;240:126561. [DOI] [PubMed] [Google Scholar]
- 13. Han G, Zhang J, Ma Y, et al. Clinical characteristics, treatment and prognosis of angiocentric glioma. Oncol Lett 2020;20:1641‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Halloran PJ, Amoo M, Dablouk MO, et al. Angiocentric glioma: drop metastases to the spinal cord. World Neurosurg 2020;136:110‐6. [DOI] [PubMed] [Google Scholar]
- 15. Gupta S, Rangari KV, Mehrotra A, et al. Temporal lobe angiocentric glioma with oligodendroglioma‐like areas: a rare association of an uncommon tumor. A case report with review of literature. Childs Nerv Syst 2020;36:641‐6. [DOI] [PubMed] [Google Scholar]
- 16. Taschner CA, Sankowski R, Scheiwe C, et al. Freiburg neuropathology case conference: hypersalivatory seizures in a 6‐year‐old child. Clin Neuroradiol 2019;29:581‐6. [DOI] [PubMed] [Google Scholar]
- 17. D'Aronco L, Rouleau C, Gayden T. Brainstem angiocentric gliomas with MYB‐QKI rearrangements. Acta Neuropathol 2017;134:667‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weaver KJ, Crawford LM, Bennett JA, et al. Brainstem angiocentric glioma: report of 2 cases. J Neurosurg Pediatr 2017;20:347‐51. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez‐Quarante LH, Fernández Carballal C, Agarwal V, et al. Angiocentric glioma in an elderly patient: case report and review of the literature. World Neurosurg 2017;97:755.e5‐10. [DOI] [PubMed] [Google Scholar]
- 20. McCracken JA, Gonzales MF, Phal PM, et al. Angiocentric glioma transformed into anaplastic ependymoma: review of the evidence for malignant potential. J Clin Neurosci 2016;34:47‐52. [DOI] [PubMed] [Google Scholar]
- 21. Adamek D, Siwek GP, Chrobak AA, et al. Angiocentric glioma from a perspective of A‐B‐C classification of epilepsy associated tumors. Folia Neuropathol 2016;54:40‐9. [DOI] [PubMed] [Google Scholar]
- 22. Cheng S, Lü Y, Xu S, et al. Cystoid angiocentric glioma: a case report and literature review. J Radiol Case Rep 2015;9:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitehead MT, Vezina G. MR spectroscopic profile of an angiocentric glioma. Anticancer Res 2015;35:6267‐70. [PubMed] [Google Scholar]
- 24. Ersen A, Canda MS, Men S, et al. Angiocentric glioma: the infiltrative glioma with ependymal differentiation. Turk Patoloji Derg 2017;33:251‐5. [DOI] [PubMed] [Google Scholar]
- 25. Ni H‐C, Chen S‐Y, Chen L, et al. Angiocentric glioma: a report of nine new cases, including four with atypical histological features. Neuropathol Appl Neurobiol 2015;41:333‐46. [DOI] [PubMed] [Google Scholar]
- 26. Chen G, Wang L, Wu J, et al. Intractable epilepsy due to angiocentric glioma: a case report and minireview. Exp Ther Med 2014;7:61‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguilar HN, Hung RW, Mehta V, et al. Imaging characteristics of an unusual, high‐grade angiocentric glioma: a case report and review of the literature. J Radiol Case Rep 2012;6:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar M, Ramakrishnaiah R, Samant R. Angiocentric glioma, a recently added WHO grade‐I tumor. Radiol Case Rep 2015;8:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu J‐Q, Patel S, Wilson BA, et al. Malignant glioma with angiocentric features. J Neurosurg Pediatr 2013;11:350‐5. [DOI] [PubMed] [Google Scholar]
- 30. Miyata H, Ryufuku M, Kubota Y, et al. Adult‐onset angiocentric glioma of epithelioid cell‐predominant type of the mesial temporal lobe suggestive of a rare but distinct clinicopathological subset within a spectrum of angiocentric cortical ependymal tumors. Neuropathology 2012;32:479‐91. [DOI] [PubMed] [Google Scholar]
- 31. Hu X‐W, Zhang Y‐H, Wang J‐J, et al. Angiocentric glioma with rich blood supply. J Clin Neurosci 2010;17:917‐8. [DOI] [PubMed] [Google Scholar]
- 32. Covington DB, Rosenblum MK, Brathwaite CD, et al. Angiocentric glioma‐like tumor of the midbrain. Pediatr Neurosurg 2009;45:429‐33. [DOI] [PubMed] [Google Scholar]
- 33. Shakur SF, McGirt MJ, Johnson MW, et al. Angiocentric glioma: a case series. J Neurosurg Pediatr 2009;3:197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Preusser M, Hoischen A, Novak K, et al. Angiocentric glioma: report of clinico‐pathologic and genetic findings in 8 cases. Am J Surg Pathol 2007;31:1709‐18. [DOI] [PubMed] [Google Scholar]
- 35. Almubarak AO, Alahmari A, Al Hindi H, et al. Angiocentric glioma of brainstem. Neurosciences (Riyadh) 2020;25:416‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cavalheiro S, da Costa MDS, Schaurich CG, et al. An 8‐year‐old girl with blepharospasm and left thalamic tumor. Brain Pathol 2019;29:457‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chatterjee D, Gupta K, Singla N, et al. Angiocentric glioma of hippocampus‐report of a rare intractable epilepsy‐related tumor. Neurol India 2016;64:340‐43. [DOI] [PubMed] [Google Scholar]
- 38. Grajkowska W, Matyja E, Daszkiewicz P, et al. Angiocentric glioma: a rare intractable epilepsy‐related tumour in children. Folia Neuropathol 2014;52:253‐9. [DOI] [PubMed] [Google Scholar]
- 39. Liu CQ, Zhou J, Qi X, et al. Refractory temporal lobe epilepsy caused by angiocentric glioma complicated with focal cortical dysplasia: a surgical case series. J Neurooncol 2012;110:375‐80. [DOI] [PubMed] [Google Scholar]
- 40. Taschner CA, Staszewski O, Zentner J, et al. Freiburg neuropathology case conference: a mass lesion of the mesial temporal lobe in a child. Clin Neuroradiol 2011;21:171‐6. [DOI] [PubMed] [Google Scholar]
- 41. Buccoliero AM, Castiglione F, Degl'innocenti DR, et al. Angiocentric glioma: clinical, morphological, immunohistochemical and molecular features in three pediatric cases. Clin Neuropathol 2013;32:107‐13. [DOI] [PubMed] [Google Scholar]
- 42. Kadak MT, Demirel A, Demir T. Angiocentric glioma manifesting as psychotic symptoms in an adolescent: a case report. Neurol Psychiatry Brain Res 2013;19:197‐200. [Google Scholar]
- 43. Takeda M, Kasai T, Morita K, et al. Cytopathological features of mammary analogue secretory carcinoma–review of literature. Diagn Cytopathol 2015;43:131‐7. [DOI] [PubMed] [Google Scholar]
- 44. Rosenzweig I, Bodi I, Selway RP, et al. Paroxysmal ictal phonemes in a patient with angiocentric glioma. J Neuropsychiatry Clin Neurosci 2010;22:123.E18‐20. [DOI] [PubMed] [Google Scholar]
- 45. Ma X, Ge J, Wang L, et al. A 25‐year‐old woman with a mass in the hippocampus. Brain Pathol 2010;20:503‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kurokawa M, Kurokawa R, Capizzano AA, et al. Neuroradiological features of the polymorphous low‐grade neuroepithelial tumor of the young: five new cases with a systematic review of the literature. Neuroradiology 2022. 10.1007/s00234-021-02879-5. [DOI] [PubMed] [Google Scholar]


