Abstract
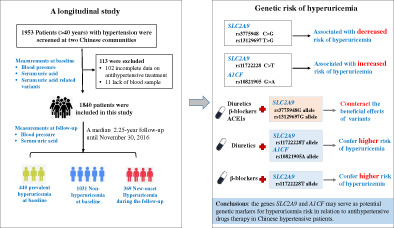
Elevated serum uric acid (UA) level has been shown to be influenced by multiple genetic variants, but it remains uncertain how UA‐associated variants differ in their influence on hyperuricemia risk in people taking antihypertensive drugs. We examined a total of 43 UA‐related variants at 29 genes in 1840 patients with hypertension from a community‐based longitudinal cohort during a median 2.25‐year follow‐up (including 1031 participants with normal UA, 440 prevalent hyperuricemia at baseline, and 369 new‐onset hyperuricemia). Compared with the wild‐type genotypes, patients carrying the SLC2A9 rs3775948G allele or the rs13129697G allele had decreased risk of hyperuricemia, while patients carrying the SLC2A9 rs11722228T allele had increased risk of hyperuricemia, after adjustment for cardiovascular risk factors and correction for multiple comparisons; moreover, these associations were modified by the use of diuretics, β‐blockers, or angiotensin converting enzyme inhibitors. The rs10821905A allele of A1CF gene was associated with increased risk of hyperuricemia, and this risk was enhanced by diuretics use. The studied variants were not observed to confer risk for incident cardiovascular events during the follow‐up. In conclusion, the genes SLC2A9 and A1CF may serve as potential genetic markers for hyperuricemia risk in relation to antihypertensive drugs therapy in Chinese hypertensive patients.
Keywords: antihypertensive drug therapy, genetic risk, hyperuricemia, serum uric acid
Genetic risk of hyperuricemia in hypertensive patients associated with antihypertensive drug therapy: A longitudinal study.
1. INTRODUCTION
Hypertension is one of the most important causes of cardiovascular mortality in China. About one‐third of Chinese adults aged from 35 to 74 years had hypertension, and only 29.6% of patients treated with antihypertensive drugs achieved the blood pressure (BP) goal. 1 Inter‐individual genetic variability is one of the reasons for adverse response to antihypertensive drugs. Recent efforts to personalized treatment have focused on the search for genetic predictors of antihypertensive drug response to minimize adverse effects.
Uric acid (UA) is the final breakdown product of purine metabolism, and elevated serum UA induces hyperuricemia and gout. 2 Epidemiological studies show that serum UA is positively associated with blood pressure levels and more than 20% of hypertensive patients have hyperuricemia. 3 , 4 As concentrations increase, UA loses its antioxidant capability and potentially becomes pro‐oxidant, which would further contribute to cardiovascular damage. 5 Decreased renal excretion of serum urate is thought to be the main cause of hyperuricemia. Serum UA level is influenced by both genetic and environmental factors, and the heritability of serum UA concentration is estimated at 25% ~ 63% in different ethnicities. 6 , 7 Previous Genome‐wide association studies (GWAS) have identified multiple serum UA‐related loci in European, American, and Asian populations. 8 , 9 , 10 , 11 Several genetic loci are known to involve in the renal urate‐transport system (SLC2A9, ABCG2, PDZK1, SLC22A11, and SLC17A1), but the biological mechanisms of most loci with regard to elevated serum UA level are currently unknown.
In addition, antihypertensive drugs affect serum UA levels in patients with hypertension. For example, thiazide diuretics can elevate serum UA through diverse mechanisms including impairment of UA secretion, inhibition of UA efflux and increase of UA reabsorption. 12 Several studies have investigated urate‐gene‐diuretic interactions on risk of hyperuricemia and gout, but the conclusions remain contrary. 13 , 14 , 15 It has been reported that β‐blockers are associated with high UA by reducing the estimated glomerular filtration rate (eGFR). 16 Regarding the effect of angiotensin converting enzyme inhibitors (ACEI) on serum UA, the conclusions are contradictory. 16 , 17 Thus, it remains uncertain how serum UA‐associated genetic variants differ in their influence on hyperuricemia risk in people taking antihypertensive drugs.
In this study, a total of 43 variants that have been identified to be associated with serum UA level in Caucasians and Asians in recent GWAS studies were comprehensively investigated in 1840 patients with hypertension from a community‐based longitudinal cohort, aiming to assess the genetic risk of hyperuricemia and its relationship with antihypertensive drugs treatment.
2. MATERIALS AND METHODS
2.1. Study population
This prospective study was conducted at two communities, the Benxi County, Liaoning Province and Hongxinglong County, Heilongjiang province in the northern region in China. A total of 1953 participants with hypertension were screened, including 463 patients from January to November in 2009 and 1490 patients from January 2012 to December 2014. Hypertension is defined as blood pressure ≥140/90 mm Hg, and/or current use of antihypertensive medications, and/or a history of hypertension. Enrolled patient was interviewed and completed a standardized questionnaire at baseline survey, including age, gender, education level, medical history, antihypertensive treatment, and lifestyle behaviors (smoking status and alcohol consumption). Body weight and height were measured by nurses, and body mass index was calculated as weight in kilograms divided by the square of height in meters.
BP was measured by trained nurses with validated oscillometric monitors with appropriately sized arm cuffs (regular adult, large, or small). Participants were required to rest for at least 5 min in a seated position, and then BP was measured three times at 1‐min intervals. The average of the second and third readings was used. The enrolled patients were provided with antihypertensive drugs, including calcium channel blockers (CCB), angiotensin receptor blockers (ARB), ACEI, thiazide‐type diuretics, or β‐blockers, unless intolerance was reported.
Blood samples were collected from the antecubital vein after an overnight fast at baseline. Biochemical variables, including serum blood glucose, total cholesterol, triglycerides, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), creatinine, and uric acid were examined by using an automatic analyzer (Hitachi 7060, Tokyo, Japan). All measurements were taken at Beijing FuWai Clinical Laboratory qualified by the Centers for Disease Control and Prevention. Hyperuricemia was defined as uric acid >420 μmol/L in men, or >360 μmol/L in women.
In the current study, 113 participants were excluded because of incomplete data on antihypertensive treatment or lack of blood samples. Of 1840 included patients with hypertension, 1400 had normal serum UA and 440 had prevalent hyperuricemia at baseline (Figure 1).
FIGURE 1.
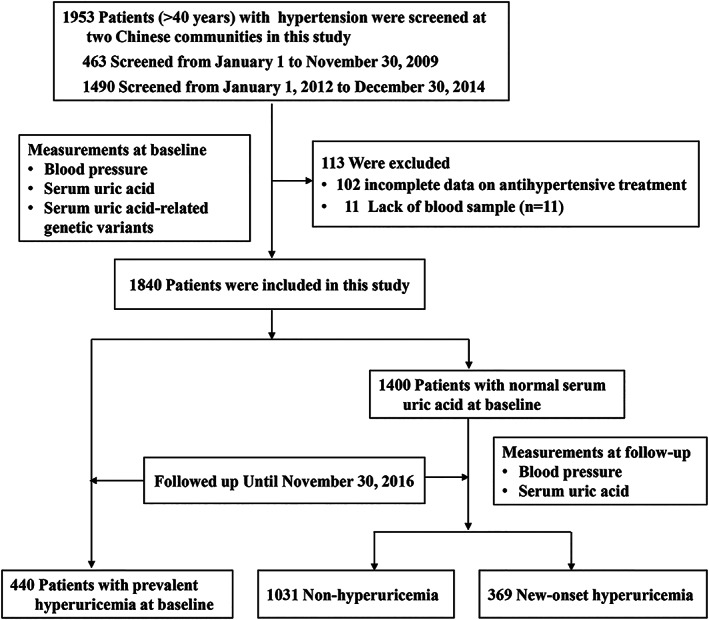
Flowchart of the current study
2.2. Follow‐up and outcome assessment
All patients were followed up for a 2‐year period by face‐to‐face interviews by trained physicians via structured questionnaires, and the last visit was from May to November in 2016. They underwent routine assessment of their blood pressure using the same standardized protocol as the baseline. Blood samples after a 12‐h overnight fast were collected again for measuring biochemical parameters including serum UA.
The main outcome was defined as a composite of myocardial infarction (MI), stroke (ischemic or hemorrhagic, fatal or nonfatal), hospitalization for unstable angina or acute decompensated heart failure, coronary revascularization, and deaths from cardiovascular causes. The endpoints were ascertained by local physicians primarily through self‐reports and review of medical records, and clinical medical records and imaging evidence were required to support all diagnosis. Deaths were reported by family members, work associates and/or obtained from death certificates and medical records. Definition of the endpoints was presented in the Supporting Information.
2.3. Gene variants selection and genotyping
We selected 43 genetic variants that have been identified to be associated with serum UA in Caucasians and Asians in recent GWAS studies, including 22 variants associated with low level of UA and 21 variants associated with high level of UA. 8 , 9 , 10 , 11 These variants were respectively located at the seven genes involving the urate‐transport or urate‐metabolism system (PDZK1, GCKR, SLC2A9, ABCG2, SLC17A1, SLC22A11, SLC22A12), and 22 genes whose mechanisms related to serum UA have not been confirmed yet (TRIM46, INHBB, ORC4L, LRP2, SFMBT1, TMEM171, RREB1, VEGFA, BAZ1B, STC1, A1CF, OVOL1, INHBC, ALDH2, HECTD4, UBE2Q2, IGF1R, NFAT5, MAF, HLF, BCAS3, QRICH2). Lists of genetic variants and functional annotations of the 29 genes were shown in Tables S1 and S2.
Genomic DNA was isolated from the peripheral white blood cell using FlexGen Blood DNA Kit (Cowin Biotech Co., Beijing, China). Variants were genotyped using the SNPscan™ Genotyping system (Genesky Biotechnologies Co., Shanghai, China) as previously described, 18 with details in the Supporting Information. For each variant, two allelic probes (one for wild allele and the other for mutant allele) and one common probe were designed (shown in Table S3).
2.4. Statistical analysis
The patients in this study were categorized into three groups: participants who had hyperuricemia at baseline were classified as the prevalent hyperuricemia group, those who developed hyperuricemia during the follow‐up period were classified as the new‐onset hyperuricemia group, and those who maintained normal serum UA were classified as the control group. Clinical characteristics between cases and control subjects were compared by the chi‐square test for categorical variables (expressed as numbers [percentages]) and the t test for quantitative variables (expressed as mean ± standard difference [SD]). The Mann–Whitney U test was used for triglycerides due to its skewed distribution.
The chi‐square test was used to examine the Hardy–Weinberg equilibrium for each variant and to compare the distribution of allele and genotype frequencies of studied variants between cases and control subjects. The allelic analysis was performed by using the PLINK 1.7 to calculate the odds ratio (OR) and 95% confidence interval (CI) for the association between alleles and the risk of hyperuricemia. 19 The additive genetic model (mm vs. Mm vs. MM) and the dominant genetic model (Mm + mm vs. MM) were used, in which M denotes the major allele and m as the minor allele. Logistic regression analysis was performed to calculate the OR and 95%CI for the association between genotypes and the risk of hyperuricemia. Multivariate analyses were adjusted for age, gender, body mass index, serum total cholesterol, HDL‐C, LDL‐C, triglycerides, serum creatine, fasting blood glucose, blood pressure, smoking status (current smoker, yes.no), alcohol intake (current drinker, yes/no), and medical history (coronary heart diseases, or diabetic mellitus, or stroke, yes/no). The genetic associations were corrected for multiple comparisons using the false discovery rate (FDR) derived from the Benjamin & Hochberg method. 20 The significance of multiplicative interactions between the variants and antihypertensive drugs was determined by the likelihood ratio test. Here, a statistical power analysis was performed using the Quanto program to estimate whether the samples could provide adequate power to identify the association with hyperuricemia risk. 21 The present study had >80% power to detect an association with ORs ≥1.20 or ORs ≤0.80 at an alpha level of 0.05 for genetic variants with minor allele frequency >0.20.
Cox proportional‐hazards models were used to calculate the hazards ratio (HR) and 95% CI for the association between genotypes and the risk of cardiovascular outcomes, and traditional risk factors mentioned above were adjusted in the models. Person‐years of follow‐up started from the date of recruitment until the occurring date of cardiovascular outcomes, death, or the end of follow‐up (November 30, 2016), whichever came first. A two‐tailed probability value of ≤0.05 was considered significant. Analyses were performed with SPSS Statistics 20.0 (SPSS Inc, Chicago, USA).
3. RESULTS
3.1. Clinical characteristics of patients with hypertension
This study included 1031 control subjects with normal serum UA, 440 cases with the prevalent hyperuricemia at baseline, and 369 cases who developed hyperuricemia during the follow‐up period. The clinical characteristics of participants were shown in Table 1. The mean ± SD age was 63.1 ± 9.4 years in control subjects, 63.0 ± 9.8 years in cases with prevalent hyperuricemia, and 62.3 ± 9.8 years in cases with new‐onset hyperuricemia. As expected, patients with prevalent hyperuricemia or new‐onset hyperuricemia had a higher level of body mass index, systolic blood pressure, dyslipidemia, serum creatine, and serum UA compared with the control subjects. In addition, cases with prevalent hyperuricemia had a higher prevalence of coronary heart disease history but less frequency of current smoking. There were no significant differences in the use of CCB, ARB, and ACEI between the groups, whereas cases with hyperuricemia had a higher frequency of β‐blocker usage. The new‐onset hyperuricemia group had higher frequency of diuretic use compared to normal serum UA group.
TABLE 1.
Characteristics of patients with hyperuricemia and controls without hyperuricemia
| Characteristics | Non‐HUA (n = 1031) | Prevalent‐HUA (n = 440) | New‐onset HUA (n = 369) | p * | p † |
|---|---|---|---|---|---|
| Age, years | 63.1 ± 9.4 | 63.0 ± 9.8 | 62.3 ± 9.8 | 0.92 | 0.62 |
| Men, no. (%) | 386 (37.4) | 201 (45.7) | 142 (38.5) | 0.003 | 0.72 |
| Body mass index, kg/m2 | 25.8 ± 3.3 | 27.2 ± 3.3 | 26.9 ± 3.3 | <0.001 | <0.001 |
| Systolic BP, mm Hg | 155 ± 22 | 158 ± 21 | 158 ± 23 | 0.05 | 0.02 |
| Diastolic BP, mm Hg | 90 ± 13 | 90 ± 13 | 91 ± 13 | 0.25 | 0.05 |
| Lipids, mmol/L | |||||
| Total cholesterol | 5.4 ± 1.0 | 5.7 ± 1.0 | 5.6 ± 1.1 | <0.001 | 0.03 |
| Triglycerides | 1.4 (1.0–2.0) | 2.1 (1.1–2.3) | 1.7 (1.1–2.5) | <0.001 | <0.001 |
| HDL‐C | 1.41 ± 0.33 | 1.27 ± 0.28 | 1.36 ± 0.29 | <0.001 | 0.01 |
| LDL‐C | 3.5 ± 0.8 | 3.7 ± 0.9 | 3.6 ± 0.9 | <0.001 | 0.05 |
| Fasting serum glucose, mmol/L | 6.2 ± 1.8 | 6.1 ± 1.5 | 6.2 ± 1. 8 | 0.51 | 0.78 |
| Serum creatinine, μmol/L | 73.5 ± 16.6 | 84.2 ± 27.1 | 76.0 ± 18.4 | <0.001 | 0.05 |
| Serum uric acid, μmol/L | |||||
| Baseline | 293.1 ± 53.5 | 459.9 ± 73.6 | 334.4 ± 50.2 | <0.001 | <0.001 |
| Follow‐up | 314.3 ± 51.8 | 479.2 ± 81.5 | 433.8 ± 66.1 | <0.001 | <0.001 |
| Cigarette smoking, no. (%) | 346 (33.6) | 115 (26.1) | 128 (34.7) | 0.005 | 0.69 |
| Alcohol intake, no. (%) | 331 (32.1) | 125 (28.4) | 128 (34.7) | 0.16 | 0.36 |
| Medical history, no. (%) | |||||
| History of CHD | 274 (26.6) | 139 (31.6) | 101 (27.4) | 0.05 | 0.77 |
| History of Diabetes | 226 (21.9) | 84 (19.1) | 80 (21.7) | 0.22 | 0.92 |
| History of Stroke | 196 (19.0) | 84 (19.1) | 69 (18.7) | 0.97 | 0.90 |
| Usage of antihypertensive drugs, no. (%) | |||||
| Calcium channel blockers | 663 (64.3) | 227 (63.1) | 246 (66.7) | 0.67 | 0.42 |
| Angiotensin receptor blockers | 533 (51.7) | 242 (55.1) | 203 (55.0) | 0.22 | 0.27 |
| ACE inhibitors | 127 (12.3) | 43 (9.8) | 51 (13.8) | 0.16 | 0.42 |
| Diuretics | 207 (20.1) | 102 (23.2) | 105 (28.5) | 0.18 | 0.001 |
| β‐blockers | 188 (18.2) | 102 (23.2) | 91 (24.7) | 0.03 | 0.01 |
Note: Values were presented as mean ± SD, number (percentage), or median (interquartile range). The t‐test was used for comparison of continuous variables, the chi‐square test for categorical variables, and the Mann–Whitney U test for triglycerides.
Abbreviations: HUA, hyperuricemia; BP, blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; CHD, coronary heart disease; ACE, angiotensin converting enzyme.
p values were calculated between the prevalent‐HUA group and non‐HUA group;
p values were calculated between the new‐onset HUA group and non‐HUA group.
3.2. Association between variants and hyperuricemia risk in patients with hypertension
The frequencies of 43 tested variants did not deviate significantly from the Hardy–Weinberg equilibrium in cases and control subjects (all p > 0.05). Three genetic models including the allelic association analysis, additive model, and dominant model were used to analyze the association between variants and hyperuricemia risk after adjustment for age, gender, and traditional vascular risk factors and after correction for multiple comparisons (Table 2). In an allelic analysis, the minor alleles of rs3775948G and rs13129697G of SLC2A9 gene were significantly associated with decreased level of serum UA, whereas rs11722228T of SLC2A9 gene and rs10821905A of A1CF gene were significantly associated with high level of serum UA in hypertensive patients who had prevalent hyperuricemia at baseline or who had new‐onset hyperuricemia during the follow‐up period. Consistently, there existed significantly additive genetic effects on the risk of hyperuricemia for the four variants.
TABLE 2.
Association between the studied variants and risk of hyperuricemia
| Allelic association | Genotype frequency, n (%) | Additive model (mm vs. mM vs. MM) | Dominant model (mm + Mm vs. MM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic variants | MAF | OR a (95% CI) | p a | FDR c | MM | Mm | mm | Adjusted OR (95% CI) b | p b | FDR c | Adjusted OR b (95% CI) b | p b | FDR c |
| SLC2A9 | |||||||||||||
| rs3775948 C>G | G | CC | CG | GG | |||||||||
| Non‐HUA | 44.8% | 1.00 | 314 (30.5%) | 510 (49.5%) | 206 (20.0%) | 1.00 | 1.00 | ||||||
| Prevalent‐HUA | 33.0% | 0.61 (0.51–0.72) | <0.001 | <0.001 | 198 (45.0%) | 194 (44.1%) | 48 (10.9%) | 0.55 (0.46–0.67) | <0.001 | <0.001 | 0.48 (0.37–0.62) | <0.001 | <0.001 |
| New‐onset HUA | 35.6% | 0.68 (0.57–0.81) | <0.001 | <0.001 | 152 (41.2%) | 171 (46.3%) | 46 (12.5%) | 0.66 (0.55–0.79) | <0.001 | <0.001 | 0.60 (0.46–0.77) | <0.001 | <0.001 |
| rs13129697 T>G | G | TT | TG | GG | |||||||||
| Non‐HUA | 49.2% | 1.00 | 265 (25.7%) | 517 (50.1%) | 248 (24.1%) | 1.00 | 1.00 | ||||||
| Prevalent‐HUA | 40.2% | 0.69 (0.59–0.81) | <0.001 | <0.001 | 155 (35.5%) | 213 (48.7%) | 69 (15.8%) | 0.61 (0.51–0.73) | <0.001 | <0.001 | 0.53 (0.41–0.70) | <0.001 | <0.001 |
| New‐onset HUA | 41.7% | 0.74 (0.62–0.88) | <0.001 | 0.007 | 123 (33.3%) | 184 (49.9%) | 62 (16.8%) | 0.71 (0.60–0.85) | <0.001 | <0.001 | 0.66 (0.50–0.86) | 0.002 | 0.003 |
| rs11722228 C>T | T | CC | CT | TT | |||||||||
| Non‐HUA | 26.8% | 1.00 | 558 (54.1%) | 393 (38.1%) | 80 (7.8%) | 1.00 | 1.00 | ||||||
| Prevalent‐HUA | 36.3% | 1.55 (1.31–1.84) | <0.001 | <0.001 | 177 (40.2%) | 207 (47.1%) | 56 (12.7%) | 1.64 (1.36–1.98) | <0.001 | <0.001 | 1.93 (1.49–2.48) | <0.001 | <0.001 |
| New‐onset HUA | 33.1% | 1.35 (1.12–1.62) | <0.001 | <0.001 | 158 (42.8%) | 178 (48.2%) | 33 (9.0%) | 1.38 (1.14–1.66) | <0.001 | 0.001 | 1.61 (1.26–2.05) | <0.001 | <0.001 |
| A1CF | |||||||||||||
| rs10821905 G>A | A | GG | GA | AA | |||||||||
| Non‐HUA | 4.9% | 1.00 | 930 (90.2%) | 100 (9.7%) | 1 (0.1%) | 1.00 | 1.00 | ||||||
| Prevalent‐HUA | 6.0% | 1.23 (0.88–1.73) | 0.23 | 0.23 | 389 (88.4%) | 49 (11.1%) | 2 (0.5%) | 1.44 (0.97–2.13) | 0.07 | 0.07 | 1.44 (0.96–2.17) | 0.08 | 0.08 |
| New‐onset HUA | 7.3% | 1.55 (1.09–2.20) | 0.01 | 0.01 | 315 (85.4%) | 54 (14.6%) | 0 (0.0%) | 1.66 (1.16–2.38) | 0.01 | 0.01 | 1.69 (1.17–2.42) | 0.01 | 0.01 |
Abbreviations: A1CF, APOBEC1 complementation factor; CI, confidence interval; FDR, false discovery rate; HUA, hyperuricemia; M, major allele; m, minor allele; MAF, minor allele frequency; OR, odds ratio; SLC2A9, solute carrier family 2 member 9.
The allelic analysis was performed by using the PLINK 1.7 to calculate the ORs (95% CI) for the association between alleles and the risk of hyperuricemia after adjustment for age, gender, body mass index, triglycerides, total cholesterol, HDL‐C, LDL‐C, serum creatine, blood glucose, blood pressure, smoking status, alcohol intake, medical history of coronary heart disease, diabetes, and stroke.
Adjusted ORs (95% CI) and p values were obtained by using multivariate logistic regression analysis after adjustment for covariates as same as the mentioned above.
FDR was obtained by Benjamin &Hochberg method for correction of multiple comparisons.
Under the dominant model analysis, patients carrying the rs3775948G allele (GG and CG genotype) in SLC2A9 had lower risk of prevalent hyperuricemia (OR: 0.48, 95% CI: 0.37–0.62; p < 0.001) or new‐onset hyperuricemia (OR: 0.60, 95% CI: 0.46–0.77; p < 0.001) compared with individuals with the wild genotype of rs3775948CC. Similarly, patients carrying rs13129697G allele (GG and TG genotypes) in SLC2A9 also had lower risk of hyperuricemia compared to the wild‐type genotype of rs13129697TT. In contrast, patients carrying SLC2A9 rs17122228T allele (TT and CT genotypes) or A1CF rs10821905A allele (AA and GA genotypes) had higher risk of hyperuricemia compared to patients carrying the wild genotypes during antihypertensive therapy. In further gender‐specific analysis, the data showed that SLC2A9 rs3775948G allele had a stronger association with lower risk of hyperuricemia in women (OR: 0.45, 95% CI: 0.34–0.59; p < 0.001) than in men (OR: 0.66, 95% CI: 0.48–0.92; p = 0.01), whereas A1CF rs10821905A allele was associated with higher risk of hyperuricemia in men but not in women (Table S4). As for other studied variants, we did not observe significant associations with serum UA in multivariate logistic regression analysis after adjustment for age, gender, and traditional vascular risk factors and after correction for multiple comparisons (Tables S5 and S6).
3.3. Correlation between variants and hyperuricemia risk in hypertensive patients with different antihypertensive drugs
Given that antihypertensive treatment might affect the serum UA level, we further analyzed the potential effect of antihypertensive drugs on the association between genetic variants and the risk of new‐onset hyperuricemia. The usage of diuretics and β‐blockers had significantly differential role in the genetic effects of UA‐related variants (Figures 2 and 3). When not receiving diuretics treatment, patients carrying SLC2A9 rs3775948G allele had decreased risk of new‐onset hyperuricemia (OR: 0.62, 95% CI: 0.47–0.83; p = 0.001) compared with patients carrying the wild‐type genotype of rs3775948CC; however, the protective effect of rs3775948G allele disappeared in patients who had diuretics treatment. Similarly, SLC2A9 rs13129697G allele contributed to decreased risk of new‐onset hyperuricemia when not using diuretics treatment compared with the wild‐type genotype of rs13129697TT (OR: 0.68, 95% CI: 0.51–0.92; p = 0.01). In addition, compared with patients who did not receive diuretics treatment, diuretics use significantly increased the risk of new‐onset hyperuricemia in patients who had the SLC2A9 rs11722228T allele (TT and CT genotypes) (OR: 2.44, 95% CI: 1.62–3.68; p < 0.001) or the A1CF rs10821905A allele (GA and AA genotypes) (OR: 3.19, 95% CI: 1.63–6.28; p = 0.001) (Figure 2).
FIGURE 2.
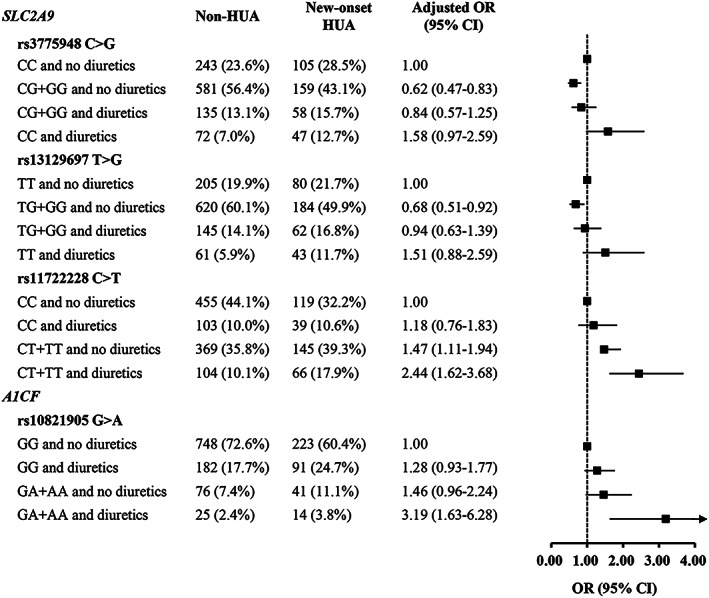
Association between UA‐related variants and hyperuricemia risk categorized by diuretics treatment. Adjusted OR (95% CI) were obtained by logistic regression model after adjusting for age, gender, body mass index, triglycerides, total cholesterol, HDL‐C, LDL‐C, serum creatine, blood glucose, blood pressure, smoking status, alcohol intake, and medical history of coronary heart disease, diabetes, or stroke. CI, confidence interval; HUA, hyperuricemia; OR, odds ratio; UA, uric acid
FIGURE 3.
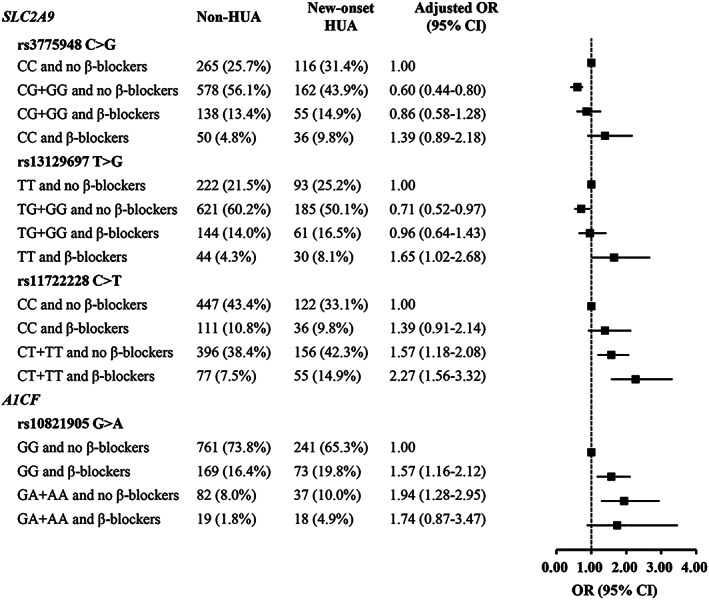
Association between UA‐related variants and hyperuricemia risk categorized by β‐blocker treatment. Adjusted OR (95% CI) were obtained by logistic regression model after adjusting for age, gender, body mass index, triglycerides, total cholesterol, HDL‐C, LDL‐C, serum creatine, blood glucose, blood pressure, smoking status, alcohol intake, and medical history of coronary heart disease, diabetes, or stroke. CI, confidence interval; HUA, hyperuricemia; OR, odds ratio; UA, uric acid
As shown in Figure 3, the β‐blocker treatment significantly increased the risk of hyperuricemia in patients with hypertension. When not receiving β‐blocker treatment, patients carrying SLC2A9 rs3775948G allele (GG and CG genotype) had decreased risk of new‐onset hyperuricemia (OR: 0.60, 95% CI: 0.44–0.80; p = 0.001) compared with patients carrying the wild‐type genotype of rs3775948CC; however, the protective effect of rs3775948G allele disappeared in patients who had β‐blocker treatment. Similarly, SLC2A9 rs13129697G allele contributed to decreased risk of new‐onset hyperuricemia when not using β‐blocker treatment compared with the wild‐type genotype of rs13129697TT (OR: 0.71, 95% CI: 0.52–0.97; P = 0.03). We also found β‐blocker treatment influenced the association between SLC2A9 rs11722228 and hyperuricemia risk. Compared with patients who did not receive β‐blocker treatment, β‐blocker treatment significantly increased the risk of new‐onset hyperuricemia in patients who had the SLC2A9 rs11722228T allele (TT and CT genotypes) (OR: 2.27, 95% CI: 1.56–3.32; p < 0.001). The β‐blocker treatment had no significant effect on the association between A1CF rs10821905 and hyperuricemia risk.
As for ACEI treatment, rs3775948G allele and rs13129697G allele at SLC2A9 gene contributed to decreased risk of new‐onset hyperuricemia when not using ACEI, but the effects seemed to disappear under the ACEI treatment (Table S7). The treatment of CCB or ARB did not significantly affect the association between the variants rs3775948, rs13129697, rs11722228 at SLC2A9 gene and rs10821905 at A1CF gene in the risk of hyperuricemia.
3.4. Effects of hyperuricemia‐related risk variants and cardiovascular events
In this study, all patients were followed up for a median of 2.25 years, and a total of 160 events were documented. No significant associations were found between variants SLC2A9 rs3775948, rs13129697, rs11722228, or A1CF rs10821905 and the risk of incident cardiovascular events (Table S8).
4. DISCUSSION
In this study, we for the first time provided evidence in Chinese hypertensive patients that genetic variants rs3775948 and rs13129697 at SLC2A9 gene were associated with decreased risk of hyperuricemia whereas rs11722228 at SLC2A9 and rs10821905 at A1CF were associated with increased risk of hyperuricemia. Notably, these associations were influenced by antihypertensive drugs such as diuretics, β‐blocker and ACEI treatment. However, the 4 variants were not observed to contribute to the risk of incident cardiovascular events during a median 2.25‐year follow‐up period.
One strength of this study was a community‐based longitudinal study design comprised of 1400 participants with normal UA at baseline, 440 participants with prevalent hyperuricemia at baseline, and 369 participants with new‐onset hyperuricemia during the follow‐up period. A total of 43 variants that have been identified to be associated with serum UA level in Caucasians and Asians in recent GWAS studies were comprehensively investigated in Chinese patients with hypertension in this study.
In this hypertensive population, we found that patients carrying the SLC2A9 rs3775948G allele or the rs13129697G allele had decreased risk of hyperuricemia compared with patients carrying the wild‐type genotypes, whereas patients carrying the rs11722228T allele had increased risk of hyperuricemia; but these associations were modified by the use of diuretics, β‐blockers, and ACEIs. SLC2A9 encodes glucose transporter type 9 (GLUT‐9) which mainly expresses in the kidney, liver and intestines and plays an important role in the reabsorption of filtered urate at proximal tubules. SLC2A9 has been identified to confer the strongest association with UA levels in GWAS studies, accounting for 3.4% ~ 8.8% of the urate change in women and 0.5% ~ 2.0% in men. 22 , 23 , 24 Loss‐of‐function variants of SLC2A9 were associated with low UA level especially in women. Consistently, in this study, SLC2A9 rs3775948G allele had an association with decreased risk of hyperuricemia in women than in men.
The gain‐of‐function variants of SLC2A9 are reported to be associated with high UA level. Rs11722228 is a C/T polymorphism locating at intron 8 of the SLC2A9 gene, and the minor T allele has been reported to be associated with high UA level in healthy Chinese and Japanese population. 11 , 25 In this study, we found that rs11722228T allele was associated with higher risk of hyperuricemia in Chinese hypertensive patients, and the use of diuretics and β‐blockers further elevated this risk, indicating that hypertensive patients with rs11722228T allele may take considerate assessment on diuretics and β‐blockers use.
In addition to the SLC2A9 gene, we identified that the rs10821905A allele at A1CF gene was associated with increased risk of hyperuricemia. A1CF encodes apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1, complementation factor (A1CF), which plays a critical role in the production of apolipoprotein B (Apo B). Apo B has been reported to associate with serum UA levels in hypertensive patients. 26 The rs10821905 is located in the promoter region of A1CF gene. Bioinformatics analysis (JASPAR database, http://jaspar.genereg.net/) showed that the nucleotide G to A change of rs10821905 enables several transcription factors binding to the loci, such as forkhead box M1 (FOXM1), indicating that rs10821905 may affect the expression of A1CF and thereafter the production of Apo B. The rs10821905 has been reported to be related with UA level and gout in European population 10 and alcohol intake can influence this relationship. 27 In this study, our data showed that the association between A1CF rs10821905 and hyperuricemia risk was further modified by the use of diuretics, indicating that hypertensive patients with rs10821905A allele may take considerate assessment on diuretics use.
Diuretics can increase the urate reabsorption by decreasing the renal clearance and thus have putatively adverse effects on UA level. 28 , 29 Several studies have investigated urate‐gene‐diuretic interactions on risk of gout, but the conclusions remain contrary. Vandell et al. identified several novel gene regions associated with hydrochlorothiazide‐induced serum UA elevation and hyperuricemia in Caucasian and African American participants in the pharmacogenomic evaluation of antihypertensive responses (PEAR) study, but not replicated in other cohorts. 13 A recent analysis based on the Atherosclerosis Risk in Communities (ARIC) Study reported that diuretic‐induced gout tends to occur among those with a genetic predisposition to hyperuricemia, 14 while data from the Nurses' Health Study (NHS) and the Health Professionals Follow‐up Study (HPFS) showed that SLC22A11 gene had a significant interaction only among women but in the opposite direction to the ARIC study. 15 Our study provided evidence that diuretics use differentially affected the risk of hyperuricemia according to certain genetic regions such as SLC2A9 and A1CF in Chinese hypertensive patients.
Emerging evidence suggest that antihypertensive drugs may affect the uric acid metabolism in patients with hypertension. The β‐blockers are reported to cause vasoconstriction in peripheral vessels, which may decrease eGFR in the nephrons and elevate serum UA level. 16 , 30 Uses of losartan and CCBs have been reported to decrease serum UA level in patients with hypertension or coronary artery disease, and exert a protective role from gout and cardiovascular events. 17 , 31 As for the effects of ACEIs on serum UA level, the results are inconsistent. It is reported that ACEIs can reduce the reabsorption of urate in renal paroxysmal tubules and thus decrease serum UA, 32 but a population‐based case–control study in patients with hypertension showed that ACEIs are associated with increased risk of gout. 17 However, there is lack of evidence regarding to the relationship between these antihypertensive drugs and genetic risk of hyperuricemia. In this study, our data for the first time showed that β‐blockers and ACEIs modified the effects of gene regions such as SLC2A9 and A1CF on the risk of hyperuricemia, while the use of CCBs and ARBs were not observed to affect the genetic risk of hyperuricemia. These associations were independent of the use of other antihypertensive drugs and vascular risk factors such as age, gender, body mass index, blood pressure, smoking, alcohol intake, and presence of coronary heart disease, diabetes, and stroke. The mechanisms are needed to clarify in future studies.
There are several pathophysiological mechanisms linking uric acid to inflammation and oxidative stress, which contribute to the development and progression of endothelial dysfunction and vascular damage. 33 In endothelial cells, elevated UA level stimulates the receptor for advanced glycation end products (RAGE) signaling pathway and activates nuclear factor kappa B (NF‐κB). This process leads to the extracellular release of high‐mobility group protein‐1 (HMGB1) and its interaction with RAGE contributes to the amplification of the inflammatory response, finally inducing endothelial dysfunction. 34 , 35 , 36 In vascular smooth muscle cells, uric acid induces proinflammatory cytokine production, apoptosis, and endothelial‐mesenchymal transition through activating MAPK signaling pathway, inducible cyclo‐oxygenase (COX‐2) and reactive oxygen species (ROS) production. 37 , 38 All these uric acid‐mediated proinflammatory responses play crucial roles in vascular remodeling, which contributes to cardiovascular risk, hypertension, atherosclerosis, and chronic kidney disease.
Some limitations of our study should be mentioned. Since the follow‐up period of patients was relatively short and variable, further more patients will be needed to verify the conclusion. However, we also included the patients with prevalent hyperuricemia at baseline (n = 440) and further assessed the association between variants and the presence of hyperuricemia, which showed similar results as in patients with new‐onset hyperuricemia during the follow‐up period. In addition, antihypertensive drugs investigated in our study included diuretics, β‐blockers, CCBs, ARBs and ACEIs, but some patients had taken two or three kinds of antihypertensive drugs. Although we adjusted this potential effect in the genetic model analysis, the bias cannot be totally avoided in the genetic association with hyperuricemia risk.
In summary, our study showed that the genes SLC2A9 and A1CF were associated with hyperuricemia risk in relation to antihypertensive drug therapy in Chinese hypertensive patients. These findings suggested that antihypertensive‐drug adverse effects on serum UA level may be predicted according to a certain genetic predisposition for hyperuricemia, which provides a possible antihypertensive drug selection in patients with hypertension.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/cge.14110.
ETHICS STATEMENT
This study has been approved by the Ethics Committee of FuWai Hospital. All participants reported themselves as Han nationality and provided written informed consent.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGMENTS
This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021‐1‐I2M‐011) and National Science and Technology Pillar Program during the Twelfth Five‐year Plan Period from the Ministry of Science and Technology, China (2011BAI11B04). We thank doctors and nurses at Benxi Railway Hospital, Liaoning, and Hongxinglong Center Hospital, Heilongjiang, China for data collection.
Chen Y, Yang Y, Zhong Y, et al. Genetic risk of hyperuricemia in hypertensive patients associated with antihypertensive drug therapy: A longitudinal study. Clinical Genetics. 2022;101(4):411-420. doi: 10.1111/cge.14110
Yu Chen and Yunyun Yang contributed equally to this study.
Funding information Chinese Academy of Medical Sciences, Grant/Award Number: 2021‐1‐I2M‐011; Ministry of Science and Technology of the People's Republic of China, Grant/Award Number: 2011BAI11B04
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study and supplementary files are available from the corresponding author on reasonable request.
REFERENCES
- 1. Lewington S, Lacey B, Clarke R, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176(4):524‐532. [DOI] [PubMed] [Google Scholar]
- 2. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol. 2017;44(3):388‐396. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Zhang M, Yu X, et al. Association of hypertension and hypertriglyceridemia on incident hyperuricemia: an 8‐year prospective cohort study. J Transl Med. 2020;18(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaudhary NS, Bridges SL Jr, Saag KG, et al. Severity of hypertension mediates the association of hyperuricemia with stroke in the REGARDS case cohort study. Hypertension. 2020;75(1):246‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sautin YY, Johnson RJ. Uric acid: the oxidant‐antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):608‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Q, Guo CY, Cupples LA, Levy D, Wilson PW, Fox CS. Genome‐wide search for genes affecting serum uric acid levels: the Framingham Heart study. Metabolism. 2005;54(11):1435‐1441. [DOI] [PubMed] [Google Scholar]
- 7. Nath SD, Voruganti VS, Arar NH, et al. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol. 2007;18(12):3156‐3163. [DOI] [PubMed] [Google Scholar]
- 8. Yang Q, Köttgen A, Dehghan A, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okada Y, Sim X, Go MJ, et al. Meta‐analysis identifies multiple loci associated with kidney function‐related traits in east Asian populations. Nat Genet. 2012;44(8):904‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Köttgen A, Albrecht E, Teumer A, et al. Genome‐wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang B, Mo Z, Wu C, et al. A genome‐wide association study identifies common variants influencing serum uric acid concentrations in a Chinese population. BMC Med Genomics. 2014;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ben Salem C, Slim R, Fathallah N, Hmouda H. Drug‐induced hyperuricaemia and gout. Rheumatology (Oxford). 2017;56(5):679‐688. [DOI] [PubMed] [Google Scholar]
- 13. Vandell AG, McDonough CW, Gong Y, et al. Hydrochlorothiazide‐induced hyperuricaemia in the pharmacogenomic evaluation of antihypertensive responses study. J Intern Med. 2014;276(5):486‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McAdams‐DeMarco MA, Maynard JW, Baer AN, Kao LW, Köttgen A, Coresh J. A urate gene‐by‐diuretic interaction and gout risk in participants with hypertension: results from the ARIC study. Ann Rheum Dis. 2013;72(5):701‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bao Y, Curhan G, Merriman T, Plenge R, Kraft P, Choi HK. Lack of gene‐diuretic interactions on the risk of incident gout: the Nurses' health study and health professionals follow‐up study. Ann Rheum Dis. 2015;74(7):1394‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ueno S, Hamada T, Taniguchi S, et al. Effect of antihypertensive drugs on uric acid metabolism in patients with hypertension: cross‐sectional cohort study. Drug Res (Stuttg). 2016;66(12):628‐632. [DOI] [PubMed] [Google Scholar]
- 17. Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case‐control study. BMJ. 2012;344:d8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du W, Cheng J, Ding H, Jiang Z, Guo Y, Yuan H. A rapid method for simultaneous multi‐gene mutation screening in children with nonsyndromic hearing loss. Genomics. 2014;104(4):264‐270. [DOI] [PubMed] [Google Scholar]
- 19. Purcell S, Neale B, Todd‐Brown K, et al. PLINK: a tool set for whole‐genome association and population‐based linkage analyses. Am J Hum Genet. 2007;81(3):559‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smeland OB, Frei O, Shadrin A, et al. Discovery of shared genomic loci using the conditional false discovery rate approach. Hum Genet. 2020;139(1):85‐94. [DOI] [PubMed] [Google Scholar]
- 21. Gauderman WJ, Morrison JM. Quanto 1.1: A Computer Program for Power and Sample Size Calculations for Genetic Epidemiology Studies. Available online: 2006. http://hydra.usc.edu/gxe. [Google Scholar]
- 22. Dehghan A, Köttgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome‐wide association study. The Lancet. 2008;372(9654):1953‐1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40(4):437‐442. [DOI] [PubMed] [Google Scholar]
- 24. Döring A, Gieger C, Mehta D, et al. SLC2A9 influences uric acid concentrations with pronounced sex‐specific effects. Nat Genet. 2008;40(4):430‐436. [DOI] [PubMed] [Google Scholar]
- 25. Hamajima N, Okada R, Kawai S, et al. Significant association of serum uric acid levels with SLC2A9 rs11722228 among a Japanese population. Mol Genet Metab. 2011;103(4):378‐382. [DOI] [PubMed] [Google Scholar]
- 26. Papavasileiou MV, Karamanou AG, Kalogeropoulos P, et al. Uric acid blood levels and relationship with the components of metabolic syndrome in hypertensive patients. J Hum Hypertens. 2016;30(7):414‐417. [DOI] [PubMed] [Google Scholar]
- 27. Rasheed H, Stamp LK, Dalbeth N, Merriman TR. Interaction of the GCKR and A1CF loci with alcohol consumption to influence the risk of gout. Arthritis Res Ther. 2017;19(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verbrugge FH, Dupont M, Steels P, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014;16(2):133‐142. [DOI] [PubMed] [Google Scholar]
- 29. McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population‐based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64(1):121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barrese V, Taglialatela M. New advances in beta‐blocker therapy in heart failure. Front Physiol. 2013;4:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyazaki S, Hamada T, Hirata S, et al. Effects of azelnidipine on uric acid metabolism in patients with essential hypertension. Clin Exp Hypertens. 2014;36(7):447‐453. [DOI] [PubMed] [Google Scholar]
- 32. Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther. 2003;17(5–6):397‐414. [DOI] [PubMed] [Google Scholar]
- 33. Russo E, Verzola D, Cappadona F, et al. The role of uric acid in renal damage ‐ a history of inflammatory pathways and vascular remodeling. Vessel Plus. 2021;5:15. [Google Scholar]
- 34. Yu MA, Sánchez‐Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin‐angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid‐induced endothelial dysfunction. J Hypertens. 2010;28(6):1234‐1242. [PubMed] [Google Scholar]
- 35. Cai W, Duan XM, Liu Y, et al. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. Biomed Res Int. 2017;2017:4391920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ko J, Kang HJ, Kim DA, et al. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019;33(12):13334‐13345. [DOI] [PubMed] [Google Scholar]
- 37. Chao HH, Liu JC, Lin JW, Chen CH, Wu CH, Cheng TH. Uric acid stimulates endothelin‐1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008;29(11):1301‐1312. [DOI] [PubMed] [Google Scholar]
- 38. Fu X, Niu N, Li G, et al. Blockage of macrophage migration inhibitory factor (MIF) suppressed uric acid‐induced vascular inflammation, smooth muscle cell de‐differentiation, and remodeling. Biochem Biophys Res Commun. 2019;508(2):440‐444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
The datasets used and/or analyzed during the current study and supplementary files are available from the corresponding author on reasonable request.


