Abstract
Canine leptospirosis has not been reported in the Sydney dog population since 1976. However, between 2017 and 2020, leptospirosis was confirmed in 17 dogs, five of which were known to hunt rodents. Dogs infected between 2017 and 2019 lived within a 3 km radius in the Inner City of Sydney (n = 11). In 2020, cases emerged across a broader area of Sydney; Inner City (n = 1), Inner West (n = 3), Lower North Shore (n = 1) and Upper North Shore (n = 1). The disease was characterised by severe hepatorenal involvement resulting in an unusually high case fatality rate (88%). In conjunction with supportive clinical signs, diagnosis was confirmed by real‐time PCR on whole blood (n = 1), kidney (n = 1), urine (n = 4), whole blood and urine (n = 9) or by seroconversion (n = 3). Antibody titres determined by Microscopic Agglutination Test (MAT) to Leptospira serovars were measured in 12 dogs: seven were positive for serovar Copenhageni, one was positive for serovar Hardjo, three were negative for all serovars, likely due to insufficient time for seroconversion before death and one had a low positive titre (1/50) for serovars Australis and Robinsoni. This sudden emergence of a highly fatal disease in pet dogs in Sydney has led to the introduction of Leptospira vaccination protocols for dogs living in inner Sydney using a monovalent vaccine containing serovar Copenhageni. The success of this vaccination program will require ongoing research to understand the emergence of leptospirosis in this region and the serovars involved.
Keywords: Australia, canine, Leptospira, leptospirosis, outbreak
Abbreviations
- AKI
acute kidney injury
- aPTT
activated partial thromboplastin time
- DIC
disseminated intravascular coagulation
- IRIS
International Renal Interest Society
- IVF
intravenous fluid
- LPHS
leptospirosis pulmonary haemorrhage syndrome
- MAT
Microscopic Agglutination Test
- PT
prothrombin time
Leptospirosis is caused by a motile aerobic spirochete bacterium of the genus Leptospira. In dogs, the most pathogenic serovars cause vasculitis leading to tissue injury, including acute kidney and hepatic disease. Importantly, several serovars are zoonotic. Rodents are the most common reservoir hosts for Leptospira genotypes and shed leptospires in their urine. 1 , 2 Dogs become infected by contact with urine or indirectly via contaminated environments. 1 Following infection, bacteraemia occurs lasting around 10 days followed by vasculitis, organ ischaemia and invasion of the kidneys and liver, resulting in shedding of leptospires into urine. 3 Leptospirosis should be suspected in dogs with nonspecific clinical signs (lethargy, vomiting, diarrhoea, haemorrhages, conjunctivitis), consistent clinicopathological abnormalities (azotaemia, hyperbilirubinaemia, increased liver enzymes, glucosuria) and risk factors (rodent contact, exposure to contaminated environments). 1 Fatality rates of 18% to 55% have been previously described in cases from Australia, 4 , 5 Europe 6 , 7 , 8 , 9 , 10 and the USA. 11 , 12 Diagnosis can be achieved by PCR on blood or urine prior to antibiotic administration or markedly elevated antibody levels to specific Leptospira serovars (Microscopic Agglutination Test [MAT]) or seroconversion (4‐fold rise in antibody titre). 1 , 6 , 8 , 9 While case definition varies across jurisdictions, these criteria are used to confirm a diagnosis of human leptospirosis in countries with a high leptospirosis incidence (New Zealand, 13 USA 14 ).
Prevention is achieved by vaccination and limiting contact with sources of infection. 3 There is one registered vaccine in Australia (Protech® C2i, Boehringer Ingelheim) containing serovar Copenhageni. 15 Historically, dogs in Sydney have not been vaccinated due to apparent absence of disease. In the most recent limited serosurvey of 956 healthy dogs in Australian animal shelters in 2004, seroprevalence of 2.4% was estimated in New South Wales in 431 dogs. 16 Copenhageni was the most prevalent serovar (5/10 dogs). Earlier seroprevalence studies in dogs with no known history of exposure showed seroprevalence of 9.8% in dogs in New South Wales and Victoria (501 dogs) in 1990 17 and 6.8% in Sydney (600 dogs) in 1972, 18 and Copenhageni was the most prevalent (16/49 and 30/41, respectively).
No literature describing clinical leptospirosis in New South Wales has been published since 1976. 19 , 20 , 21 In this study, we characterise clinicopathological findings, diagnostic imaging and clinical outcomes in dogs diagnosed with leptospirosis in a recent Sydney outbreak with a high fatality rate.
Materials and methods
Medical records of cases presented between December 2017 and June 2019 were retrospectively reviewed. From July 2019, cases were enrolled prospectively. Cases were identified following referral or direct contact from referring veterinarians after a leptospirosis alert was issued across Sydney. Leptospirosis was suspected in dogs with consistent clinical and clinicopathological findings (azotaemia, hyperbilirubinaemia, elevated liver enzymes). Diagnosis was confirmed by positive PCR on blood, urine or kidney tissue (IDEXX or Vetnostics laboratories), the presence of Leptospira‐shaped organisms in the kidneys identified with silver stain (Warthin‐Starry) or via seroconversion (4‐fold increase in MAT titre) or a MAT titre ≥1/800 in nonvaccinated dogs (WHO Leptospirosis Laboratory, Public and Environmental Health, Department of Health, Cooper Plains, Queensland; 23 serovar assay testing for serovars Arborea, Australis, Bataviae, Bulgarica, Canicola, Celledoni, Copenhageni, Cynopteri, Djasiman, Grippothyphosa, Hardjo, Icterohaemorrhagiae, Javanica, Kremastos, Medanensis, Panama, Pomona, Robinsoni, Shermani, Swajizak, Tarassovi, Topaz, Zanoni). Real‐time PCR was used at both reference veterinary diagnostic laboratories. Both are National Association of Testing Authorities accredited with all tests run with quality controls.
Medical records were reviewed for signalment, history, vaccination status, physical examination findings, results of haematology, serum biochemistry, coagulation profiles (prothrombin time [PT], activated partial thromboplastin time [aPTT]), urinalysis, diagnostic imaging, urine output, blood pressure measurements, treatment regime, outcomes and postmortem findings.
Cases were classified based on organ involvement (renal, hepatic, pulmonary, haemorrhagic). 9 Acute kidney injury (AKI) was defined as documented AKI (historical, clinical, laboratory, imaging evidence), oliguria (<1 mL/kg/h) or anuria (no urine production) and progressive increase in creatinine concentration of >26.4 μmol/L within 48 h according to International Renal Interest Society (IRIS) guidelines. 22 Severity of AKI was based on the IRIS grading system. 22 The lowest urine output during hospitalisation was reported. Hepatic involvement was defined as presence of elevated liver enzymes and hyperbilirubinaemia and classified as mild (serum bilirubin 10‐20 μmol/L), moderate (bilirubin 20–30 μmol/L) or severe (bilirubin > 30 μmol/L). 9 Pulmonary involvement (leptospirosis pulmonary haemorrhage syndrome [LPHS]) was defined as disease‐causing laboured breathing, dyspnoea and haemoptysis or radiographic evidence of moderate to severe peribronchial, interstitial or alveolar infiltrates. 9 Haemorrhagic involvement was defined as evidence of haemorrhage (other than LPHS) or the presence of disseminated intravascular coagulation (DIC) (present if all criteria are fulfilled: thrombocytopaenia, prolonged PT and aPTT). 9 , 23
Results
Patient demographics and clinical presentation
Seventeen dogs were included (Table 1). One 14‐month‐old had completed its primary vaccination course, 10 months prior to onset of clinical signs.
Table 1.
Signalment of 17 dogs with leptospirosis
| Age |
| Puppy <1 year; n = 2 |
| Young adult, 1–4 years; n = 7 |
| Middle‐aged, 5–9 years; n = 7 |
| Geriatric, 15 years; n = 1 |
| Median age = 4 years |
| Sex |
| Male entire, n = 6 |
| Male neutered, n = 4 |
| Female entire, n = 3 |
| Female neutered, n = 4 |
| Breeds |
| American Staffordshire Terrier, n = 3 |
| Staffordshire Bullterrier, n = 2 |
| Cavoodle, n = 2 |
| Australian Kelpie, n = 1 |
| Australian Shepherd, n = 1 |
| Beagle, n = 1 |
| Bernese Mountain Dog, n = 1 |
| Fox Terrier, n = 1 |
| Fox Terrier Cross, n = 1 |
| Golden Retriever, n = 1 |
| Greyhound, n = 1 |
| Jack Russell Terrier, n = 1 |
| Miniature Schnauzer, n = 1 |
| Weight |
| 5.7–43.1 kg (median 19.6 kg) |
Five dogs hunted rodents and one was a working sheep dog in rural New South Wales. Dogs infected between 2017 and 2019 all lived within a 3 km radius in the Inner City of Sydney. In August 2020, cases occurred in the Inner City, Inner West and Lower North Shore. In October 2020, a case was identified in the Upper North Shore. In December 2020, a case was identified in the Inner West, in a dog obtained from a farm in the Northern Tablelands (Armidale) 12 days prior (Figure 1).
Figure 1.
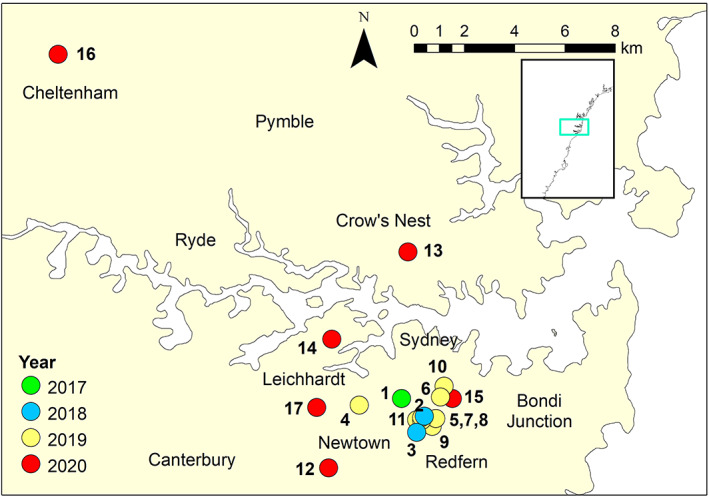
Location of 17 cases of canine leptospirosis between 2017 and 2020. 1 = Haymarket; 2, 5, 7, 8, 11 = Surry Hills; 3, 9 = Redfern; 4 = Glebe; 6, 10 = Darlinghurst; 12 = Newtown; 13 = Crows Nest; 14 = Balmain; 15 = Paddington; 16 = Cheltenham; 17 = Annandale.
Presenting complaints and physical examination findings are summarised in Table 2.
Table 2.
Presenting complaints and physical examination findings on admission in 17 dogs with leptospirosis
| Presenting complaints | Physical exam findings |
|---|---|
| Lethargy (n = 17) | Icteric mucous membranes (n = 13) |
|
Vomiting (n = 14) Regurgitation (n = 1) |
Abdominal pain (n = 12) |
| Inappetence (n = 14) | Dehydration (n = 11) |
| Diarrhoea (n = 9, haemorrhagic n = 1) | Mild lymphadenomegaly (n = 5) |
| Polydipsia (n = 2) | Pyrexia (T > 39°C) (n = 3) |
| Hypothermia (T < 38.0°C) (n = 3) | |
| Increased respiratory effort (n = 2) | |
| Increased lung sounds (n = 2) | |
| Soft pulmonary crackles (n = 2) | |
| Peripheral oedema (n = 1) (prior to fluid therapy) |
Confirmatory tests for leptospirosis
A diagnosis of leptospirosis was confirmed by positive PCR in blood (n = 1), urine (n = 4), kidney (n = 1), blood and urine (n = 9), seroconversion (n = 2) or a positive MAT titre ≥1/800 in a nonvaccinated dog (n = 1) (Table 3).
Table 3.
Results of PCR (n = 17, submitted 1 to 6 days after the onset of clinical signs; IDEXX or Vetnostics laboratories), Microscopic Agglutination Test (MAT; n = 12, submitted 1 to 7 days after onset of clinical signs, Queensland Health Scientific Service Cooper Plains Queensland) and histopathology (n = 8, VPDS [Veterinary Pathology Diagnostic Services – the University of Sydney] or Vetnostics laboratories) in dogs with leptospirosis
| Dog number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR urine | pos | pos | pos | pos | pos | pos | pos | pos | neg | pos | neg | pos | pos | neg | pos | pos a | neg |
| PCR blood | pos | neg | neg | neg | pos | pos | pos | pos | neg | pos | pos | neg | pos | neg | pos | pos a | neg |
| PCR kidney | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | pos |
| Histopathology b | N/A | N/A | N/A | N/A | N/A | N/A | neg | pos | neg | pos | pos | neg | neg | N/A | N/A | N/A | neg |
| Serovar Arborea | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Australis | N/A | N/A | N/A | 50 | N/A | N/A | 50 | <50 | <50 | <50 | <50 | <50 | <50 | 400 | <50 | <50 | <50 |
| Serovar Bataviae | N/A | N/A | N/A | <50 | N/A | N/A | <50 | 200 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Bulgarica | N/A | N/A | N/A | <50 | N/A | N/A | <50 | 50 | <50 | <50 | 50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Canicola | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Celledoni | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Copenhageni | N/A | N/A | N/A | 800 | N/A | N/A | <50 | 800 | 200 | 100 | 200 | 100 | <50 | 1600 | <50 | <50 | <50 |
| Serovar Cynopteri | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Djasiman | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Gryppotyphosa | N/A | N/A | N/A | <50 | N/A | N/A | <50 | 50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Hardjo | N/A | N/A | N/A | <50 | N/A | N/A | <50 | 100 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | 1600 | <50 |
| Serovar Icterohaemorrhagiae c | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | <50 | <50 | 400 | <50 | <50 | <50 |
| Serovar Javanica | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | 400 | <50 | <50 | <50 |
| Serovar Kremastos | N/A | N/A | N/A | 50 | N/A | N/A | <50 | 100 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Medanensis | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | 100 | <50 |
| Serovar Panama | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Pomona | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | 100 | <50 | <50 | <50 |
| Serovar Robinsoni | N/A | N/A | N/A | <50 | N/A | N/A | 50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Shermani | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Swajizak | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Tarassovi | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Topaz | N/A | N/A | N/A | <50 | N/A | N/A | <50 | 50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| Serovar Zanoni | N/A | N/A | N/A | <50 | N/A | N/A | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
Note: Boldface has been used to make positive titres more clearly visible.
Tested positive for Leptospira species but not Leptospira interrogans.
Visualisation of spiral bacteria with silver stain.
Due to changes in the reference laboratories scope of testing, samples received after July 2020 were additionally tested for serovar Icterohaemorrhagiae.
MAT results for dog 14 are 24 days after initial presentation (convalescent titre). MAT results for dog 16 are 17 days after initial MAT titre (21 days after presentation).
neg, negative; pos, positive.
Antibody titres to Leptospira serovars were measured by MAT in 12 dogs; seven were positive for serovar Copenhageni and one was positive for serovar Hardjo. In one dog (9) (pretreated with metronidazole), diagnosis was established by seroconversion. In one unvaccinated dog (14), diagnosis was established by a positive MAT titre of 1/800 for serovar Copenhageni, which subsequently increased to 1/1600. One (16) had positive PCR results on blood and urine to Leptospira species, however, this case tested negative for Leptospira interrogans. This dog initially had a low positive titre (1/50) to serovars Hardjo and Zanoni and subsequently seroconverted to serovar Hardjo (1/1600) 17 days later. The 14‐month‐old (15), which received the primary vaccination course had a negative MAT, likely due to insufficient time for seroconversion.
In eight cases, histopathology of the kidneys (n = 1), kidneys and liver (n = 2) or a complete necropsy (n = 5) was performed. In three, Leptospira‐shaped organisms were identified in the kidneys with silver stain.
Clinicopathological findings
Haematology and serum biochemistry results were available in 15 dogs. In two retrospectively enrolled dogs, the medical record described severe azotaemia, elevated liver enzymes and icterus; however, numerical results were not recorded on the patient file. Ninety‐four percent had a mild leucocytosis with neutrophilia. A manual differential count was available in two cases, both with a mild left shift; 73% had mild to moderate thrombocytopaenia and 53% were anaemic. Anaemia was mild in most and severe in one (Table 4).
Table 4.
Results of haematology at the time of maximal deviation from reference interval in 15 dogs with leptospirosis
| Range | Median | IQR | Reference interval | |
|---|---|---|---|---|
| Haematocrit (%) | 19–55 | 36 | 32–47.5 | 37–55 |
| Thrombocytes (×109/L) | 31–312 | 90 | 52–218 | 200–600 |
| Leukocytes (×109/L) | 6.7–26.3 | 19.3 | 14.7–23.0 | 7–12 |
| Neutrophils (×109/L) | 5.6–24.2 | 14.8 | 11.9–20.0 | 4–9.3 |
| Monocytes (×109/L) | 0.3–3.5 | 1.4 | 0.9–1.8 | 0.2–0.9 |
| Lymphocytes (×109/L) | 0.6–2.4 | 1 | 0.8–2.05 | 0.9–3.6 |
| Eosinophils (×109/L) | 0–0.5 | 0 | 0–0.1 | 0.1–1.2 |
IQR, interquartile range.
Biochemistry results are summarised in Table 5. All dogs developed severe azotaemia and hyperphosphataemia, 94% had increased alkaline phosphatase‐activity and hyperbilirubinaemia and 87% had increased alanine transaminase. Creatine kinase was elevated in all five cases in which it was measured. All had increased lipase and 40% an increase in amylase. Fifty‐three percent developed hyperkalaemia. Ionised calcium was measured in nine with hypocalcaemia found in five. Importantly, on initial presentation, two cases were nonazotaemic and one had a normal bilirubin.
Table 5.
Results of serum biochemistry at the time of maximal deviation from reference interval in 15 dogs with leptospirosis
| Range | Median | IQR | Reference interval | |
|---|---|---|---|---|
| Creatinine (μmol/L) | 218–1039 | 621 | 480–799 | 40–120 |
| Urea (mmol/L) | 20.8–64.7 | 41.1 | 31.8–51.3 | 1–10 |
| Phosphate (mmol/L) | 2.4–6.0 | 3.4 | 2.9–4.7 | 0.8–1.6 |
| Bilirubin (μmol/L) | 8–491 | 245 | 107–446 | 1.2–8.1 |
| ALT (U/L) | 60–1716 | 196 | 102–527 | <60 |
| ALP (U/L) | 203–3356 | 1215 | 489–1766 | <110 |
| CK (U/L) | 452–4638 | 1521 | 515–3741 | <200 |
| Cholesterol (mmol/L) | 2–7.2 | 3.4 | 2.6–6.0 | 1.4–7.5 |
| Amylase (U/L) | 662–3394 | 1157 | 671–2486 | <1400 |
| Lipase (U/L) | 250–6000 | 1126 | 317–5481 | <60 |
| Protein (g/L) | 36–80 | 58 | 50–62 | 50–70 |
| Albumin (g/L) | 20–34 | 24 | 22–29 | 23–43 |
| Globulin (g/L) | 16–56 | 32 | 25–43 | 27–44 |
| Glucose (mmol/L) | 5.2–9 | 6.3 | 5.8–6.8 | 3.3–6.4 |
| Calcium total (mmol/L) | 2.2–2.9 | 2.6 | 2.4–2.7 | 2.1–2.9 |
| Calcium ion (mmol/L) | 0.97–1.37 | 1.18 | 1.14–1.34 | 1.2–1.4 |
| Sodium (mmol/L) | 124–158 | 142 | 133–146 | 137–150 |
| Potassium (mmol/L) | 3.2–7.9 | 4.7 | 4.5–5.7 | 3.3–4.8 |
| Chloride (mmol/L) | 89–126 | 99 | 97–107 | 105–120 |
ALP, alkaline phosphatase; ALT, alanine transaminase; CK, creatine kinase; IQR, interquartile range.
Coagulation was measured in nine cases (Table 6). Eighty‐three percent had prolonged aPTT, and 33% had prolonged PT. Three had prolonged PT, aPTT and thrombocytopenia fulfilling criteria for DIC. One case had petechiae and thrombocytopaenia; however, PT and aPTT were not measured. Overall 24% (4/17) showed clinical or laboratory evidence of haemorrhagic involvement.
Table 6.
Results of coagulation profile at the time of maximal deviation from reference interval in nine dogs with leptospirosis
| Range | Median | IQR | Reference interval | |
|---|---|---|---|---|
| PT (s) | 12 to >100 | 16 | 15–21 | 11–17 |
| aPTT (s) | 88 to >300 | 134 | 109–143 | 72–102 |
aPTT, activated partial thromboplastin time; IQR, interquartile range; PT, prothrombin time.
Urinalysis was performed in nine dogs (Table 7). Glucosuria was present in 3/9 (33%), proteinuria in 7/9 (78%), bilirubinuria in 5/9 (56%), pyuria in 4/6 (67%) and microscopic haematuria in 4/6 (67%). One had mild calcium oxalate crystalluria and one had tubular casts on sediment exam (unclassified). Urine culture was performed in six cases and was positive in one sample from the urinary catheter.
Table 7.
Urinalysis results in nine dogs with leptospirosis
| Range | Abnormal values | |
|---|---|---|
| USG (n = 8) | 1.010–1.050 | Isosthenuria (n = 3) |
| Minimally concentrated a (n = 4) | ||
| Hypersthenuria (n = 1) | ||
| Glucose (n = 9) | None to 2+ | Negative (n = 6) |
| Trace (n = 2) | ||
| 2+ (n = 1) | ||
| Protein (n = 9) | Negative to 2+ | Negative (n = 1) |
| Trace (n = 3) | ||
| 1+ (n = 3) | ||
| 2+ (n = 2) | ||
| Bilirubin (n = 9) | None to large | None (n = 4) |
| 1+ (n = 3) | ||
| 3+ (n = 1) | ||
| Large (n = 1) | ||
| Red cells (per HPF) (n = 6) | <5–>100 | <5 (n = 2) |
| 20 (n = 1) | ||
| >100 (n = 3) | ||
| Leukocytes (per HPF) (n = 6) | 3–20 | Neg (n = 1) |
| 3 (n = 1) | ||
| <5 (n = 3) | ||
| 20 (n = 1) | ||
| Crystals (n = 6) | Ca‐oxalate (n = 1) | |
| Casts (n = 6) | 3+ (unclassified) tubular casts (n = 1) | |
| Urine culture (n = 6) | Negative (n = 5) | |
| Light growth beta haemolytic streptococcus spp.(n = 1) – Catheter urine |
Minimally concentrated: USG 1.013–1.029; hypersthenuria >1.030.
HPF, high power field; USG, urine specific gravity.
Diagnostic imaging
Chest radiographs were taken in five dogs. In two dogs with respiratory signs, radiographic changes were consistent with LPHS (Table 8).
Table 8.
Radiographic findings and respiratory signs in five dogs with leptospirosis (interpreted by board‐certified specialist in diagnostic imaging)
| Dog number | Time radiographs taken | Respiratory signs | Imaging findings | Leptospiral haemorrhage syndrome |
|---|---|---|---|---|
| 1 | On admission | None | Marked diffuse mixed (bronchial, interstitial to alveolar) pulmonary pattern | Suspected, consistent clinical signs 2 days later and died, no necropsy |
| 4 | On admission | None | Unremarkable | Suspected, epistaxis, sublingual haematoma, no necropsy |
| 8 | On admission | Increased lung sounds and respiratory effort | Unremarkable | Suspected |
| 9 | 4 days after admission | Increased respiratory effort | Diffuse mild to moderate unstructured increase in pulmonary opacity, more severe in right middle and caudal lung lobes, hazy pulmonary markings and irregular ventral margination of the lung fields, more nodular increased pulmonary opacity caudo‐dorsally | Suspected |
| 14 | On admission | None | Unremarkable | No, complete recovery |
LPHS, leptospiral haemorrhage syndrome.
An abdominal ultrasound was performed in 14 dogs and findings are summarised in Table 9.
Table 9.
Abdominal ultrasound findings in 10 dogs in which the ultrasound was performed by a board‐certified specialist in diagnostic imaging
| Liver |
| Hepatomegaly (n = 4) |
| Hypoechoic parenchyma (n = 5) |
| Thickened gallbladder wall and common bile duct (n = 1) |
| Biliary sludge (n = 1) |
| Kidney |
| Pyelectasia (n = 1) |
| Hyperechoic renal cortex (n = 1) |
| Perirenal fluid (n = 6), extending into retroperitoneum (n = 3/6) |
| Lymphadenomegaly |
| Portal (n = 1) |
| Hepatic (n = 1) |
| Medial iliac (n = 3) |
| Mesenteric (n = 4) |
| Generalised (n = 1) |
| Other |
| Peritoneal effusion (n = 2) |
| Peritonitis (n = 3) |
| Mild pancreatitis (n = 1) |
| Mildly corrugated duodenum (n = 1) |
| Mild colonic wall thickening (n = 1) |
| Splenomegaly (n = 2) |
Organ manifestations
All dogs had renal involvement, 16 (94%) had hepatic involvement, five (29%) had pulmonary involvement and presumed LPHS and four (24%) showed bleeding tendencies (petechiae n = 1, haematemesis n = 1 and sublingual haematoma and bruising n = 1). The latter developed in a Greyhound after feeding tube placement – based on signalment; hyperfibrinolysis could not be excluded as the cause.
Treatment regime and outcomes
Twelve dogs were treated at specialist centres and five at their general practice veterinarian.
All dogs were treated with intravenous fluid (IVF) and antibiotics. Additional supportive treatments are summarised in Table 10.
Table 10.
Summary of drugs used for treatment in 17 dogs with leptospirosis
| Treatment | Drug |
|---|---|
| Fluid therapy | IV fluids (n = 17) |
| Antibiotics | Ampicillin IV (n = 9) |
| Amoxicillin–clavulanate IV (n = 6) | |
| Amoxicillin IV (n = 2) | |
| Cephazolin IV (n = 2) | |
| Doxycycline PO (n = 2) | |
| Enrofloxacin IV (n = 3) | |
| Metronidazole IV (n = 7) | |
| Antiemetics | Maropitant IV (n = 14) |
| Ondansetron IV (n = 6) | |
| Metoclopramide IV (n = 5, as CRI in n = 3) | |
| Gastroprotectants | Esomeprazole IV (n = 6) |
| Analgesia | Buprenorphine IV (n = 9) |
| Methadone IV (n = 3) | |
| Fentanyl IV (n = 1) | |
| Medication to improve urine output | Frusemide bolus IV (n = 4) |
| Frusemide bolus IV and CRI + mannitol bolus IV and CRI (n = 3) | |
| Frusemide bolus IV + mannitol bolus IV (n = 1) | |
| Frusemide bolus IV + mannitol CRI (n = 1) | |
| Dopamine CRI (n = 1) | |
| Noradrenaline CRI (n = 1) | |
| Treatment for hyperkalaemia | Calcium gluconate (n = 1) |
| Glucose bolus + neutral insulin CRI (n = 1) | |
| Antihypertensive medication | Amlodipine (n = 1) |
| Liver protectants | S‐adenosyl‐methionine PO (n = 4) |
| N‐acetylcysteine IV (n = 2) | |
| Ursodeoxycholic acid PO (n = 2) | |
| Miscellaneous | Lactulose PO (n = 1) |
| Vitamin K SC (n = 1) | |
| Blood products | Fresh frozen plasma (n = 1) |
| Fresh whole blood (n = 1) |
CRI, continuous rate infusion; IV, intravenously; PO, per os; SC, subcutaneous.
A urinary catheter was placed in seven to measure urine output, in the remainder urine output was estimated. Five dogs were oliguric then became anuric, four were oliguric throughout, three were anuric throughout and two had normal to increased output (1.6–2.4 mL/kg/hr). One of the latter fully recovered, the other developed stage 3 chronic kidney disease (CKD). Blood pressure was measured in nine dogs. One of three hypertensive dogs (systolic blood pressure 260, 240 and 200 mmHg) was treated with amlodipine.
Seven dogs developed peripheral oedema – in the eyes (chemosis) (n = 1), periorbital (n = 1), on peripheral limbs (n = 3), face (n = 3), skin (n = 1) and neck (n = 1) or as serous nasal discharge (n = 1).
Six dogs died, nine were euthanased and two survived. Reasons for euthanasia were anuria (n = 5), respiratory distress (n = 3), seizures (n = 2), nystagmus (n = 1), refractory hyperkalaemia (n = 1) and upper airway obstruction (n = 1). Causes of death were respiratory distress, hyperkalaemia and anuria (n = 1), LPHS and seizures (n = 1), respiratory distress and LPHS (n = 1). Three died for undetermined reasons.
Of the two dogs that survived, one was hospitalised for five days and treated with IVF, antibiotics (initially amoxicillin–clavulanate 20 mg/kg intravenously three times a day, then doxycycline 5 mg/kg twice a day), antiemetics (maropitant 1 mg/kg once a day (SID)) and liver protectants (S‐adenosyl‐methionine 30 mg/kg per os SID). There was hepatorenal disease with initial IRIS AKI grade 2 (creatinine 218 μmol/L) and severe hepatic involvement (bilirubin 107 μmol/L). It was polyuric throughout hospitalisation and showed complete recovery within 1 month based on normal biochemistry results. The second dog was hospitalised for 1 week. It was treated with IVF and antibiotics (cephazolin 22 mg/kg intravenously for 1 week, doxycycline 10 mg/kg SID for 2 weeks). It had hepatorenal disease with initial IRIS AKI grade 3 (creatinine 297 μmol/L) and severe hepatic involvement (bilirubin 71 μmol/L). This dog had normal to increased urine production and developed CKD stage 3 after discharge, which is nonprogressive at the time of writing, 3 months following.
Necropsy and histopathology findings
Necropsy was performed on five dogs and findings are summarised in Table 11; examination was limited in one given significant freeze–thaw artefact.
Table 11.
Summary of necropsy findings in five dogs with leptospirosis
| Dog number | 7 a | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|
| Jaundice | +++ | +++ | +++ | +++ | +++ |
| Multisystemic haemorrhage (variably affecting: cutaneous, subcutaneous, lungs, kidney, gastrointestinal, heart) | − | +++ | +++ | ++ | + |
| Ascites | + | +++ | ++ | ++ | +++ |
| Pleural effusion | ++ | ++ | ++ | ++ | ++ |
| Pulmonary oedema | − | ++ | ++ | ++ | ++ |
| Hepatomegaly | − | − | + | − | + |
| Splenomegaly | − | − | + | − | − |
Examination was limited by severe freeze–thaw artefact.
‘+, ++, +++’ mild, moderate, marked, respectively; ‘−’ not detected.
Histopathological examination of multiple organs was performed for seven, all of which included kidney (including silver staining) and liver. An additional dog only had kidney evaluated histologically. Histologic findings are summarised in Table 12.
Table 12.
Summary of histopathological findings in eight dogs with leptospirosis
| Dog number | 7 a | 8 | 9 | 10 | 11 | 12 | 13 | 17 |
|---|---|---|---|---|---|---|---|---|
| Multisystemic haemorrhage (variably affecting kidneys, lungs, gastrointestinal, heart, subcutaneous) | − | +++ | +++ | +++ | ++ | ++ | + | ++ |
| Tubulointerstitial nephritis (lymphoplasmacytic) | ++ | + | + | ++ | + | ++ | ++ | + |
| Tubular degeneration +/− necrosis | − | ++ | +++ | +++ | +++ | +++ | ++ | ++ |
| Renal tubular casts (protein, cellular) | ++ | +++ | +++ | +++ | ++ | +++ | ++ | + |
| Membranous glomerulonephritis | ++ | + | − | − | − | − | − | − |
| Renal tubular and lamina spirochete organisms (silver stain, Warthin‐Starry) | NP | ++ | − | + | + | NP | NP | − |
| Hepatocellular dissociation with Kupffer cell hypertrophy | − | +++ | +++ | +++ | +++ | ++ | X | +++ |
| Cholangiohepatitis (lymphoplasmacytic) | − | ++ | + | + | ++ | + | X | − |
| Pulmonary oedema | X | +++ | +++ | +++ | ++ | X | X | +++ |
| Pancreatitis | X | ++ | + | − | ++ | X | X | X |
| Lymph node follicular hyperplasia | X | ++ | +++ | ++ | + | X | X | X |
| Cystitis | X | − | + | X | + | X | X | X |
| Alzheimer type II astrogliosis | X | ++ | − | − | ++ | X | X | X |
Examination was limited by severe freeze–thaw artefact.
‘+, ++, +++’ mild, moderate, marked, respectively; ‘−’ not detected. ‘NP’ not performed. ‘X’ respective tissue not examined.
Discussion
This case series describes the re‐emergence of clinical canine leptospirosis with a high case fatality rate in urban Sydney. Prior to this, canine leptospirosis had not been reported in Sydney since 1976. 21 While disease awareness and subsequently testing increased after a leptospirosis alert was issued across Sydney, this alert was issued in July 2019 after seven cases of leptospirosis had been diagnosed by veterinarians in Sydney. Therefore, we believe that this case series represents true re‐emergence of disease and not previous under‐recognition.
Most cases of canine leptospirosis in Australia have been described in North Queensland, 4 , 5 , 24 with the first report in 1940. 25 In two case series describing 84 dogs between 1995 and 2006, antibodies to serovar Australis were most commonly identified. 4 , 5 Serovar Icterohaemorrhagiae was thought to be the causative serovar described in Tasmania in 1968. 26 Reservoir hosts for serovar Australis are likely native animals, including marsupials such as bandicoots and native rats and mice, whereas the potential reservoir host for serovar Icterohaemorrhagiae is the rat. 13 , 27 In 1962, a seroprevalence of 7.3% was reported with serovars Icterohaemorrhagiae and Esposito being the most common. 24 In Victoria, a seroprevalence of 10% was found in 1952 with serovar Icterohaemorrhagiae identified as the most common. 20
The highest antibody titre determined by MAT was found for serovar Copenhageni in most cases (7/12 dogs), the potential reservoir host for serovars Copenhageni and Icterohaemorrhagiae is the rat. 13 , 27 MAT was performed in three out of five dogs who were known to hunt rats and serovar Copenhageni had the highest titre in all. Previously published Sydney cases provide limited information, including four Greyhounds from a kennel in 1976, 21 five dogs with suspected leptospirosis in a boarding kennel in 1952 19 and three dogs with suspected leptospirosis in 1952. 20 Serovar Copenhageni was the serovar with the highest titre in most of these. Of note, cross‐reactivity between different serovars within the same serogroup (e.g. Icterohaemorrhagiae and Copenhageni) can occur and not all serovars were tested in all studies. In serosurveys of dogs in 2004, 16 1990 17 and 1972, 18 serovar Copenhageni was the most prevalent. Therefore, prior to the current cluster of cases, the monovalent vaccine containing bacterins of L. interrogans serovar Copenhageni (Protech C2i) has been used in dogs in New South Wales if deemed necessary, based on the knowledge of these prior cases. Based on these studies, the Sydney dog population might have been free from disease during the past several decades; however, exposure and subclinical infection are evident.
One dog showed seroconversion for serovar Hardjo, the reservoir hosts of which are sheep and cattle and this dog was used for herding in regional New South Wales (Young, Richmond, Hawkesbury). Clinical cases of leptospirosis showing an increase in antibody titre to serovar Hardjo are rarely reported with only two cases described in Queensland 4 , 5 and five in the USA 11 , 12 , 28 , 29 and none detail information about contact with reservoir hosts. The seroprevalence of Hardjo in dogs has previously been estimated to be low in New South Wales with 0.5% in 1972, 18 0.4% in 1990 17 and 0% in 2004, 16 however, extensive recent seroprevalence studies have not been published.
Predicting the infecting serovar based on a single MAT result is problematic due to serologic cross‐reactions, especially in acute stages 30 and ideally, a MAT titre should be repeated in 7–14 days. This was not always performed due to early fatalities or financial constraints. In most previous studies, only 6–8 serovars were included in the MAT panel. In our study, the panel contained 23 serovars, increasing sensitivity in detecting infection and standardly applied to all cases of suspect leptospirosis in humans and animals in Australia, via the WHO reference laboratory. Definitive identification of the causative serovar requires culture, which is technically difficult and takes several months, which is impractical in the clinical setting. 1
Diagnosis was confirmed with PCR testing of blood or urine in most dogs and PCR testing of kidney tissue in one, whereas diagnosis in previous studies was based on clinical presentation and antibody testing. 4 , 5 , 19 , 20 , 21 Samples for PCR should be collected prior to antibiotic administration. 2 , 3 Although several studies have shown positive urine PCR results in healthy dogs (shedders), 31 , 32 , 33 , 34 , 35 , 36 a positive result in a dog with consistent clinical signs and clinicopathologic changes suggest leptospirosis. 1 False‐negative PCR can be encountered due to low bacterial loads or after administration of antimicrobials. 1 False positive PCR results could occur due to contamination of the sample, which was considered unlikely in our cases due to the preventative measures in the reference laboratory setting. The positive PCR for Leptospira species but inability to detect L. interrogans in one case could be explained by very low levels of DNA. Infection with serovar Hardjo was later suspected following seroconversion. In another dog, PCR was negative in blood and urine, however, positive on kidney tissue. This could also be explained by low levels of shedding. In another two dogs, PCR results in blood and urine were also negative. One had been treated with metronidazole; the other did not receive antibiotics prior to presentation. The untreated dog with negative PCR had a high MAT titre to serovar Copenhageni of 1/800 initially with subsequent increase to 1/1600. This dog was seen carrying a rat 10 days prior to presentation. The negative PCR and full recovery of this dog may be due to exposure to a lower number of organisms. Metronidazole is not described for treatment of dogs with leptospirosis, but it cannot be excluded that administration resulted in a false‐negative PCR. This dog initially had negative MAT titres and developed a positive titre of 1/200 against serovar Copenhageni 4 days later. Subsequent MAT testing could not be performed.
Possible explanations for development of leptospirosis despite vaccination, which occurred in one dog, includes host factors leading to an inadequate immune response, vaccination failure or infection with a serovar other than serovar Copenhageni. It appears currently available vaccines elicit serogroup‐specific immunity and partial immunity to heterologous serogroups. 2
Clinical signs and physical exam findings were similar to those previously described. 5 , 6 , 7 , 8 , 10 , 11 , 37 Icteric mucus membranes were detected in a higher proportion of dogs in our study (76%) compared to what has been previously described (10%–45%). 6 , 7 Hyperbilirubinaemia (94%) was more common in our patients compared to what has been described in dogs overseas (17%–81%); 7 , 38 however, similar to dogs in Queensland. 5 Based on severity of hyperbilirubinaemia, hepatic involvement was classified as severe in all affected dogs. Liver involvement has been strongly associated with a negative outcome in a previous study. 9 While pancreatitis is a known complication in canine leptospirosis and could cause cholestasis, 3 , 37 mild pancreatitis (without any evidence of bile duct obstruction) was only found in 1/10 patients where abdominal ultrasound was performed, hence contribution of bile duct obstruction due to severe pancreatitis is unlikely.
All dogs had renal involvement, but two were nonazotaemic at initial presentation. Therefore, renal parameters should be rechecked within 24 h following initial testing, in nonazotaemic dogs with consistent clinical signs and known risk factors. Rechecking renal parameters every 48 h ongoing is recommended while hospitalised. Reports of leptospirosis without renal involvement are extremely rare in the literature. 9 , 12 , 37 Hyperkalaemia is a common complication of anuric/oliguric kidney failure and can cause severe bradyarrhythmias and cardiac arrest. Electrolytes should be checked at least twice daily to adjust fluid therapy. Treatment of severe hyperkalaemia was indicated in two dogs. Haemodialysis would have been helpful for these patients however was not available in New South Wales until 2021. 11
Thrombocytopaenia was present in 73% and is commonly found in dogs with leptospirosis. Proposed mechanisms include vasculitis due to circulating leptospires causing endothelial injury with subsequent platelet adhesion and activation of the coagulation cascade, 11 DIC, 6 , 7 immune‐mediated destruction 39 or splenic sequestration. 6 Results were consistent with DIC in three – all showed bleeding tendencies. Early aggressive treatment and supportive care are important to counteract development of DIC. Transfusion with fresh frozen plasma is recommended in dogs with DIC and signs of bleeding. 2
In dogs with LPHS, typical findings on thoracic radiographs include an interstitial (mild), reticulonodular (moderate) and alveolar (severe) lung pattern. 6 Radiographs are recommended even in the absence of respiratory signs to detect early lesions. Preventative measures include avoidance of stress, overhydration/hypervolaemia and control of systemic hypertension. 2 Radiographic changes consistent with LPHS were found in two dogs in our study. Overall, 29% had pulmonary involvement; however, radiographs were not taken in all patients at the time of respiratory distress. In other studies, pulmonary involvement has been described in up to 70%. 6 , 9 Severe lung involvement is associated with high mortality. 8 The pathogenesis of LPHS is unknown, however, systemic inflammatory, immune‐mediated and direct leptospiral effects have been proposed. 40 Treatment includes supportive care, oxygen therapy and in severe cases mechanical ventilation. Treatment with glucocorticoids, bronchodilators (theophylline) and frusemide has been attempted in earlier studies, but improved outcome was not demonstrated, 6 and further studies are needed before recommending these treatments. 2
The high fatality rate compared to published cases 6 , 8 , 11 could be explained by multiple factors. First, the urban Sydney dog population is considered immunonaïve with low reported levels of exposure, 16 therefore, more susceptible to infection. Second, it is possible that a more virulent strain of serovar Copenhageni is involved. Third, infection rates have risen during an episode of drought. This implies direct contact with reservoir hosts and inoculation with high numbers of organisms might be a more likely source than contact with contaminated soil or water. Finally, oliguric and anuric kidney failure was present in 13 dogs. Haemodialysis improves outcomes in dogs with leptospirosis 11 , 28 but unfortunately was not available in New South Wales.
In all but one dog with positive MAT titres, the highest was detected for serovar Copenhageni, however, in many dogs antibody titres were not measured or were negative likely due to insufficient time for seroconversion. This, and fatal leptospirosis in a vaccinated dog raises concern whether the currently available vaccine containing bacterins of L. interrogans serovar Copenhageni (Protech C2i) is protective in the current outbreak. Future studies are needed, including ongoing investigation of new leptospirosis cases, seroprevalence in the Sydney dog population prior to the current outbreak and characterisation of the immune response after vaccination to determine duration of immunity and presence of cross‐protection against other serovars.
Conflicts of interest
The authors declare no conflicts of interest for the work presented here.
Acknowledgments
This study was partially funded by the Margo Roslyn Flood Bequest. The authors would like to thank the following colleagues for their contribution of cases: Jody Braddock, Patrick Byrne, Amanda Taylor (Sydney Veterinary Emergency & Specialists, Rosebery, NSW 2018, Australia). Karim Chammas (Beecroft Vets, Beecroft, NSW 2119, Australia). Nima Rahmani (Vets on Crown, Surry Hills, NSW 2010, Australia). Max Yiu (Veterinary Specialist and Emergency Centre Northshore, Artarmon, NSW 2064, Australia). The authors would also like to thank Elaine Chew, Karen Barnes and Andrew Fortis (Veterinary Pathology Diagnostic Services, University of Sydney) for provision of excellent technical support. Open access publishing facilitated by the University of Sydney, as part of the Wiley – the University of Sydney agreement via the Council of Australian University Librarians.
Griebsch, C. , Kirkwood, N. , Ward, MP. , So, W. , Weerakoon, L. , Donahoe, S. and Norris, JM. , Emerging leptospirosis in urban Sydney dogs: a case series (2017–2020). Aust Vet J. 2022;100:190–200. 10.1111/avj.13148
References
- 1. Reagan KL, Sykes JE. Diagnosis of canine leptospirosis. Vet Clin Small Anim Pract 2019;49:719–731. [DOI] [PubMed] [Google Scholar]
- 2. Schuller S, Francey T, Hartmann K et al. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract 2015;56:159–179. [DOI] [PubMed] [Google Scholar]
- 3. Sykes JE, Hartmann K, Lunn K et al. 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med 2011;25:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tromp S. Report on leptospirosis between Cardwell and Babinda, Far North Queensland. 2006. [Google Scholar]
- 5. Miller RI, Ross SP, Sullivan ND et al. Clinical and epidemiological features of canine leptospirosis in North Queensland. Aust Vet J 2007;85:13–19. [DOI] [PubMed] [Google Scholar]
- 6. Kohn B, Steinicke K, Arndt G et al. Pulmonary abnormalities in dogs with leptospirosis. J Vet Intern Med 2010;24:1277–1282. [DOI] [PubMed] [Google Scholar]
- 7. Geisen V, Stengel C, Brem S et al. Canine leptospirosis infections ‐ clinical signs and outcome with different suspected Leptospira serogroups (42 cases). J Small Anim Pract 2007;48:324–328. [DOI] [PubMed] [Google Scholar]
- 8. Knopfler S, Mayer‐Scholl A, Luge E et al. Evaluation of clinical, laboratory, imaging findings and outcome in 99 dogs with leptospirosis. J Small Anim Pract 2017;58:582–588. [DOI] [PubMed] [Google Scholar]
- 9. Major A, Schweighauser A, Francey T. Increasing incidence of canine leptospirosis in Switzerland. Int J Environ Res Public Health 2014;11:7242–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mastrorilli C, Dondi F, Agnoli C et al. Clinicopathologic features and outcome predictors of Leptospira interrogans Australis serogroup infection in dogs: a retrospective study of 20 cases (2001‐2004). J Vet Intern Med 2007;21:3–10. [DOI] [PubMed] [Google Scholar]
- 11. Adin CA, Cowgill LD. Treatment and outcome of dogs with leptospirosis: 36 cases (1990–1998). J Am Vet Med Assoc 2000;216:371–375. [DOI] [PubMed] [Google Scholar]
- 12. Birnbaum N, Barr SC, Center SA et al. Naturally acquired leptospirosis in 36 dogs: serological and clinicopathological features. J Small Anim Pract 1998;39:231–236. [DOI] [PubMed] [Google Scholar]
- 13. NZ MoH . Ministry of Health NZ, Leptospirosis. 2017. Available at: https://health.govt.nz/our‐work/diseases‐and‐conditions/communicable‐disease‐control‐manual/leptospirosis. Accessed 28 June 2021.
- 14. CDC ZaSALZBSPBDN . Zoonoses and Select Agent Laboratory (ZSAL) | Bacterial Special Pathogens Branch | DHCPP | NCEZID | CDC. 2020. Available at: https://www.cdc.gov/ncezid/dhcpp/bacterial_special/zoonoses_lab.html. Accessed 28 June 2021.
- 15. Public Chemical Registration Information System Search. Available at: portal.apvma.gov.au. Accessed 19 January 2021.
- 16. Zwijnenberg R, Smythe L, Symonds M et al. Cross‐sectional study of canine leptospirosis in animal shelter populations in mainland Australia. Aust Vet J 2008;86:317–323. [DOI] [PubMed] [Google Scholar]
- 17. Dickeson D, Love D. A serological survey of dogs, cats and horses in south‐eastern Australia for leptospiral antibodies. Aust Vet J 1993;70:389–390. [DOI] [PubMed] [Google Scholar]
- 18. Watson A, Wannan J, Porges W et al. Leptospiral agglutinins in dogs in Sydney. Aust Vet J 1976;52:425–426. [DOI] [PubMed] [Google Scholar]
- 19. Boon R. Some observations on cystitis, nephritis and leptospirosis in small animals. Aust Vet J 1952;28:81–84. [Google Scholar]
- 20. Forbes B, Lawrence J. A serological survey of dogs in Australia for leptospiral infection. Aust Vet J 1952;28:72–75. [Google Scholar]
- 21. Watson A, Davis P, Johnson J. Suspected leptospirosis outbreak in kennelled greyhounds. Aust Vet Pract 1976;6:84–88. [Google Scholar]
- 22. Cowgill, L Grading of acute kidney injury, (2016). http://www.iris-kidney.com. 2019. Accessed 20 January 2021.
- 23. Bateman SW, Mathews KA, Abrams‐Ogg AC et al. Diagnosis of disseminated intravascular coagulation in dogs admitted to an intensive care unit. J Am Vet Med Assoc 1999;215:798–804. [PubMed] [Google Scholar]
- 24. Spradbrow P. A serological survey of Brisbane dogs for leptospiral antibodies. Aust Vet J 1962;38:20–24. [Google Scholar]
- 25. Gray D. Canine leptospiral jaundice in Queensland. Aust Vet J 1940;16:200–203. [Google Scholar]
- 26. Corbould A. Leptospirosis icterohaemorrhagiae in dogs in Tasmania. Aust Vet J 1968;44:529. [DOI] [PubMed] [Google Scholar]
- 27. Health Q . 2020. https://www.health.qld.gov.au/healthsupport/businesses/forensic‐and‐scientific‐services/testing‐analysis/diseases/leptospirosis/fact‐sheets/copenhageni. Accessed 12 July 2021.
- 28. Goldstein RE, Lin RC, Langston CE et al. Influence of infecting serogroup on clinical features of leptospirosis in dogs. J Vet Intern Med 2006;20:489–494. [DOI] [PubMed] [Google Scholar]
- 29. Tangeman LE, Littman MP. Clinicopathologic and atypical features of naturally occurring leptospirosis in dogs: 51 cases (2000‐2010). J Am Vet Med Assoc 2013;243:1316–1322. [DOI] [PubMed] [Google Scholar]
- 30. Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis 2003;36:447–452. [DOI] [PubMed] [Google Scholar]
- 31. Miotto BA, Guilloux AGA, Tozzi BF et al. Prospective study of canine leptospirosis in shelter and stray dog populations: identification of chronic carriers and different Leptospira species infecting dogs. PLoS One 2018;13:e0200384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llewellyn J‐R, Krupka‐Dyachenko I, Rettinger AL et al. Urinary shedding of leptospires and presence of Leptospira antibodies in healthy dogs from Upper Bavaria. Berl Munch Tierarztl Wochenschr 2016;129:251–257. [PubMed] [Google Scholar]
- 33. Khorami N, Malmasi A, Zakeri S et al. Screening urinalysis in dogs with urinary shedding of leptospires. Comp Clin Pathol 2010;19:271–274. [Google Scholar]
- 34. Gay N, Soupé‐Gilbert M‐E, Goarant C. Though not reservoirs, dogs might transmit Leptospira in New Caledonia. Int J Environ Res Public Health 2014;11:4316–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harkin KR, Roshto YM, Sullivan JT et al. Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospires in dogs. J Am Vet Med Assoc 2003;222:1230–1233. [DOI] [PubMed] [Google Scholar]
- 36. Rojas P, Monahan A, Schuller S et al. Detection and quantification of leptospires in urine of dogs: a maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis 2010;29:1305–1309. [DOI] [PubMed] [Google Scholar]
- 37. Prescott JF, McEwen B, Taylor J et al. Resurgence of leptospirosis in dogs in Ontario: recent findings. Can Vet J 2002;43:955–961. [PMC free article] [PubMed] [Google Scholar]
- 38. Rentko VT, Clark N, Ross LA et al. A retrospective study of 17 cases. J Vet Intern Med 1992;6:235–244. [DOI] [PubMed] [Google Scholar]
- 39. Davenport A, Rugman FP, Desmond MJ et al. Is thrombocytopenia seen in patients with leptospirosis immunologically mediated? J Clin Pathol 1989;42:439–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Medeiros Fda R, Spichler A, Athanazio DA. Leptospirosis‐associated disturbances of blood vessels, lungs and hemostasis. Acta Trop 2010;115:155–162. [DOI] [PubMed] [Google Scholar]


